The Protective Effects of Goitrin on LPS-Induced Septic Shock in C57BL/6J Mice via Caspase-11 Non-Canonical Inflammasome Inhibition
Abstract
1. Introduction
2. Results
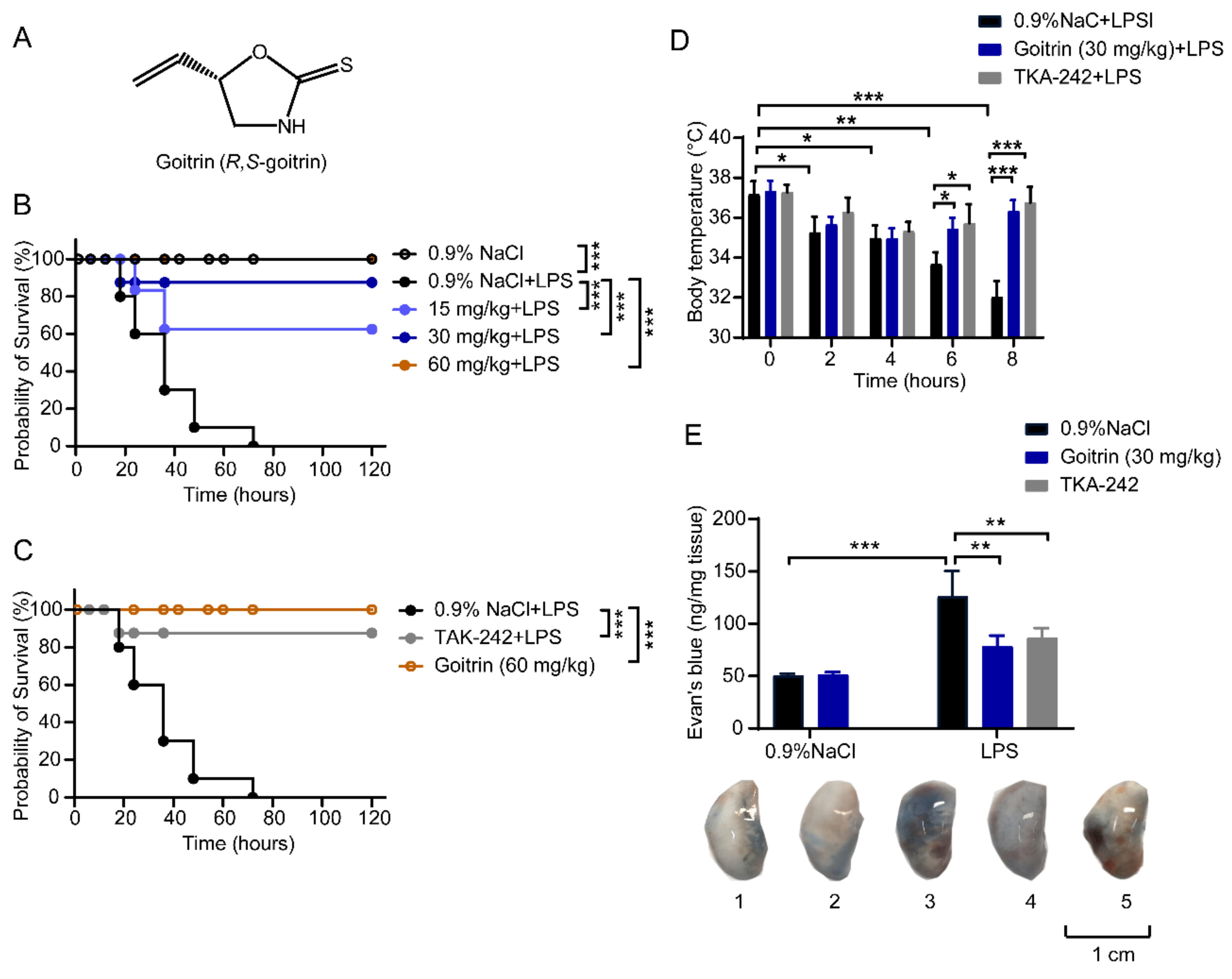
2.1. Protective Effects of Goitrin in LPS-Induced Septic Shock
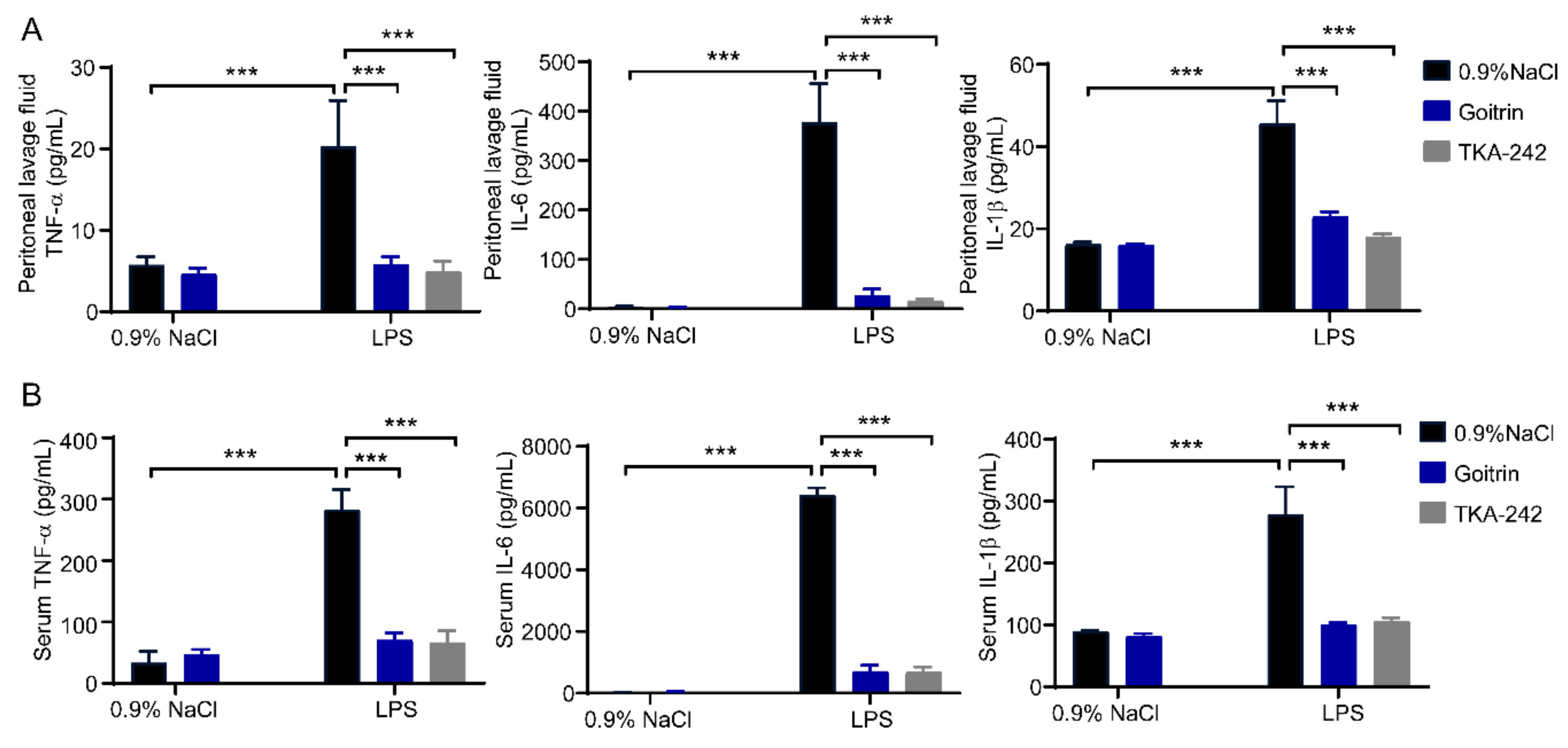
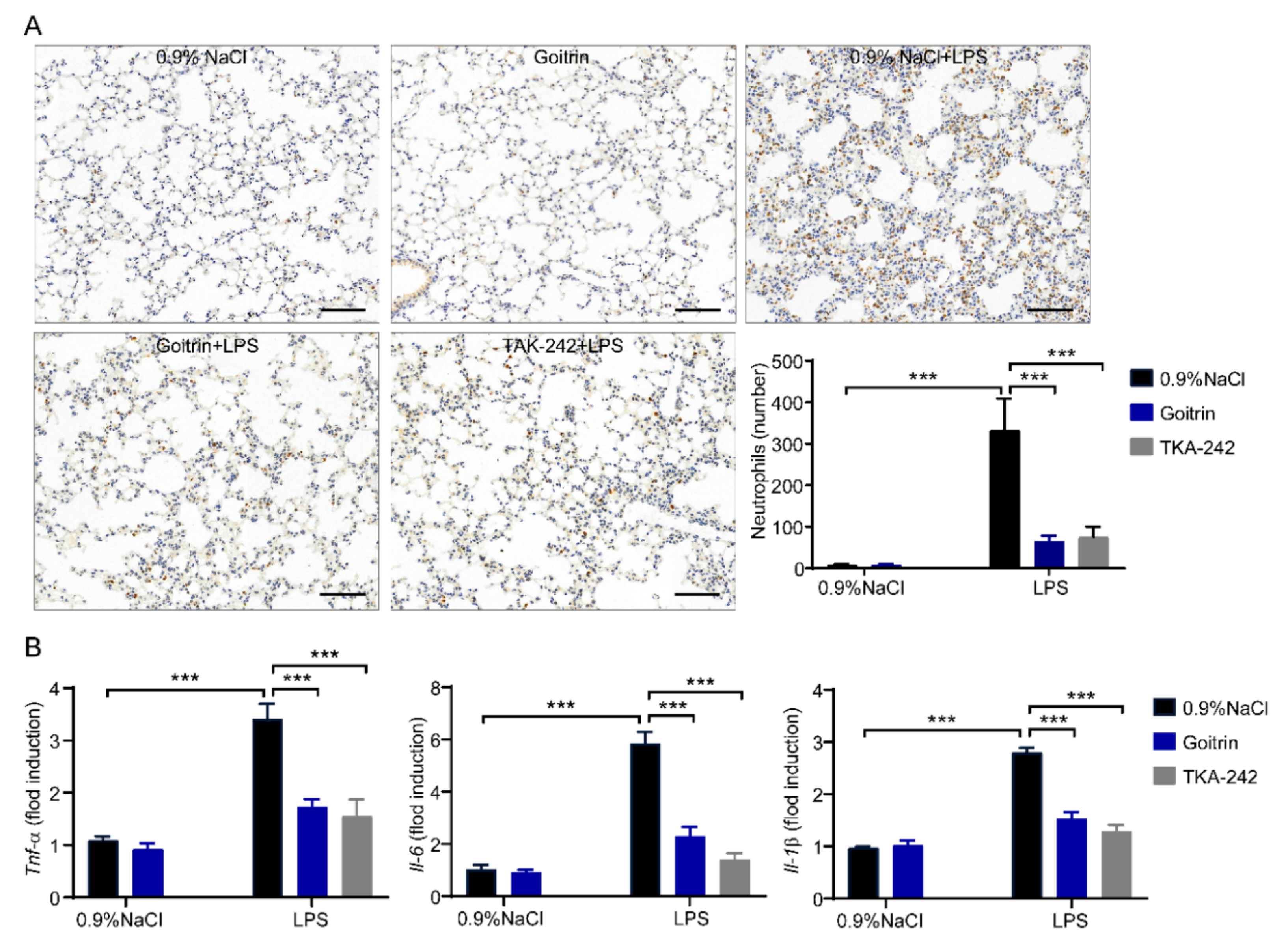
2.2. Goitrin Alleviates LPS-Induced Systemic Inflammation
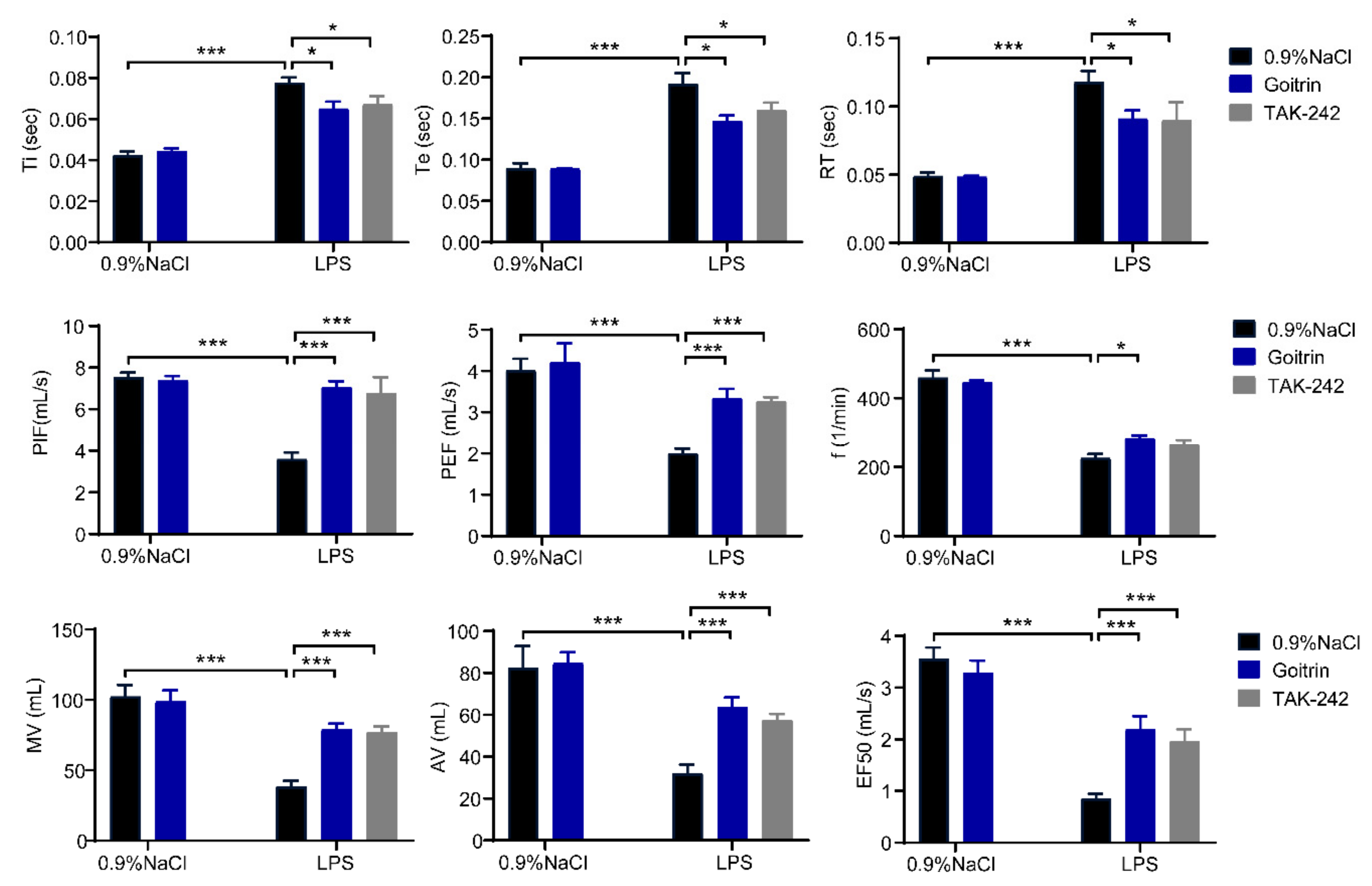
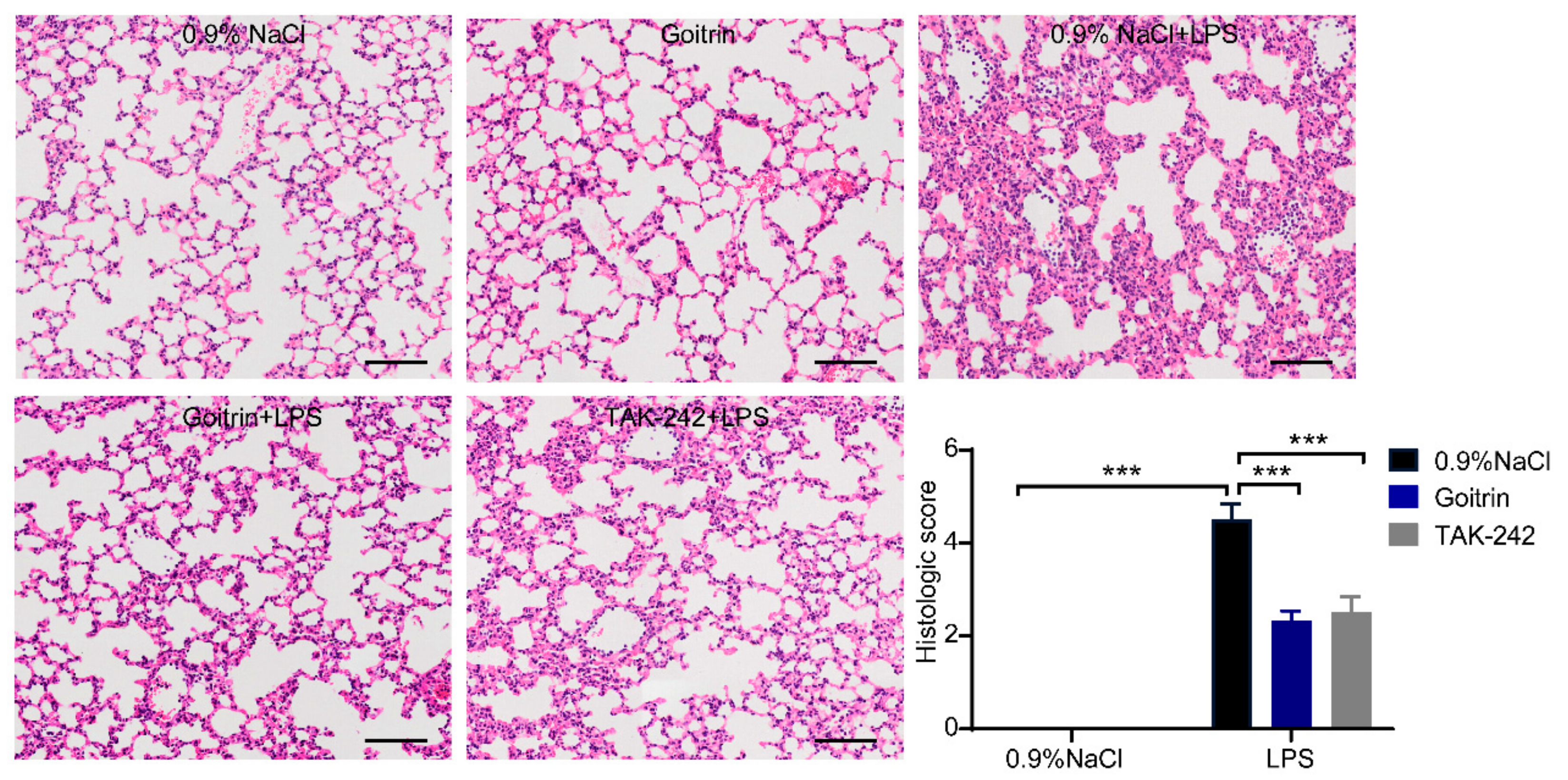
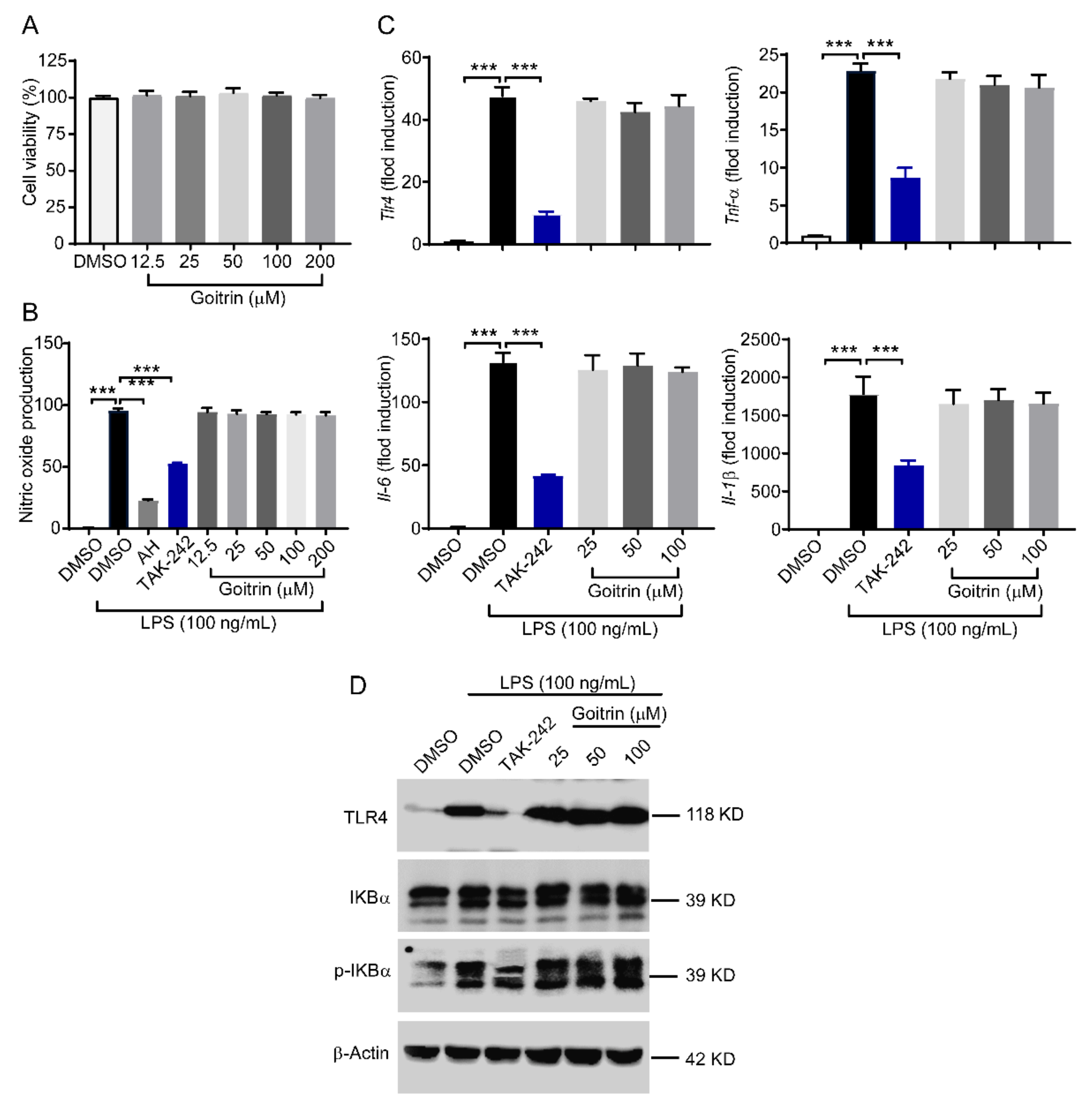
2.3. Goitrin Does Not Affect the LPS-Activated TRL4 Receptor
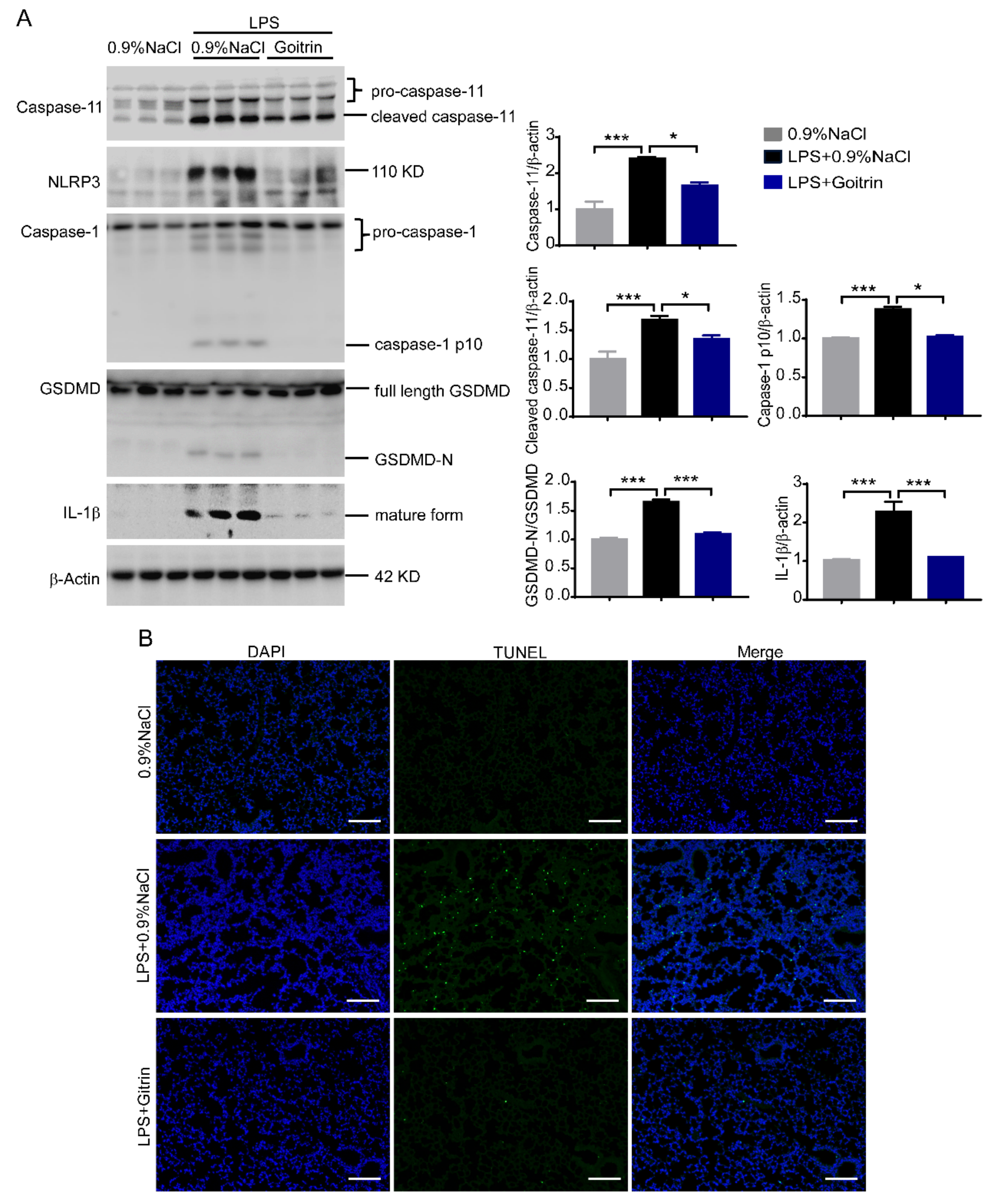
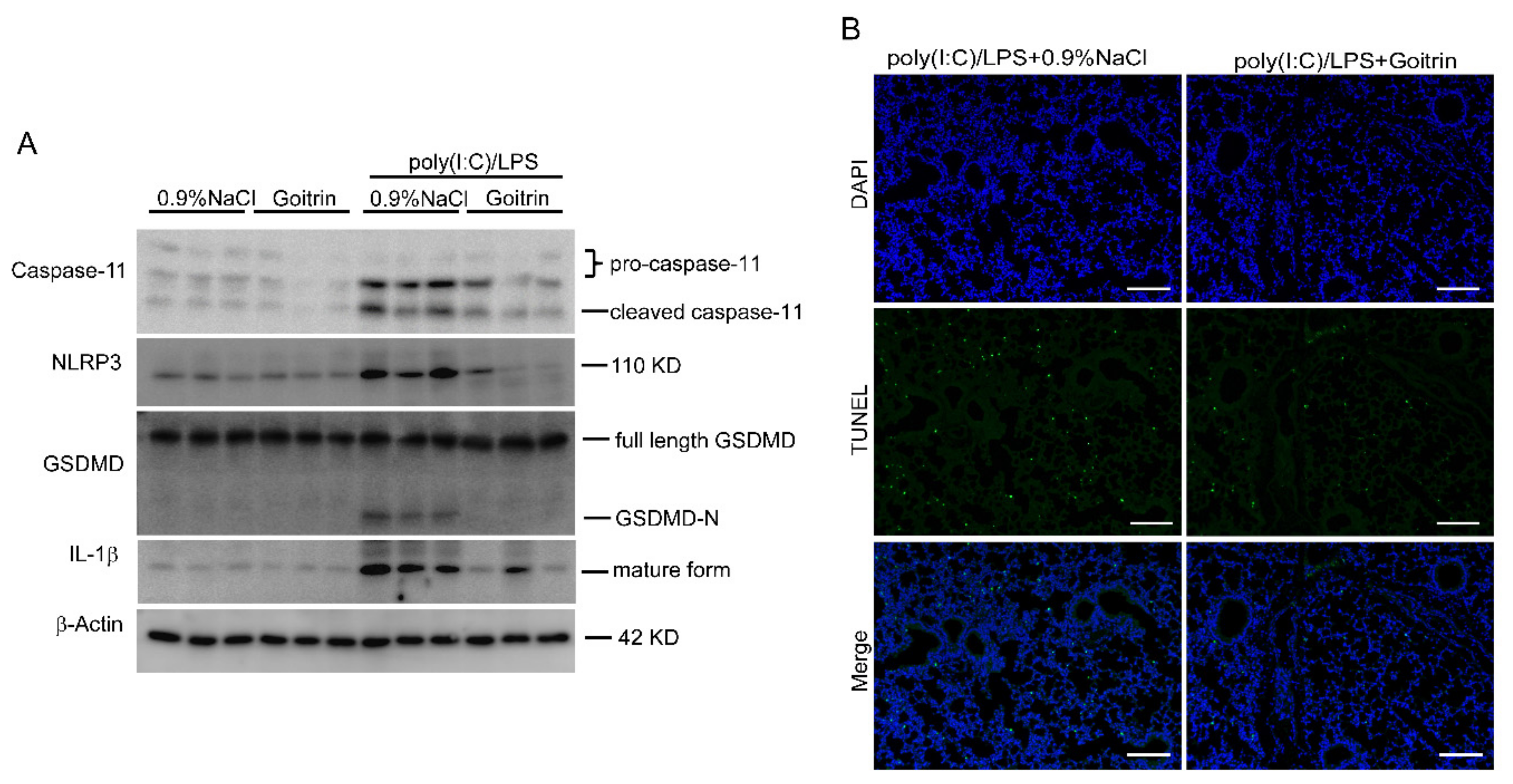
2.4. Goitrin Inhibits Caspase-11 Non-Canonical Inflammasome Activation
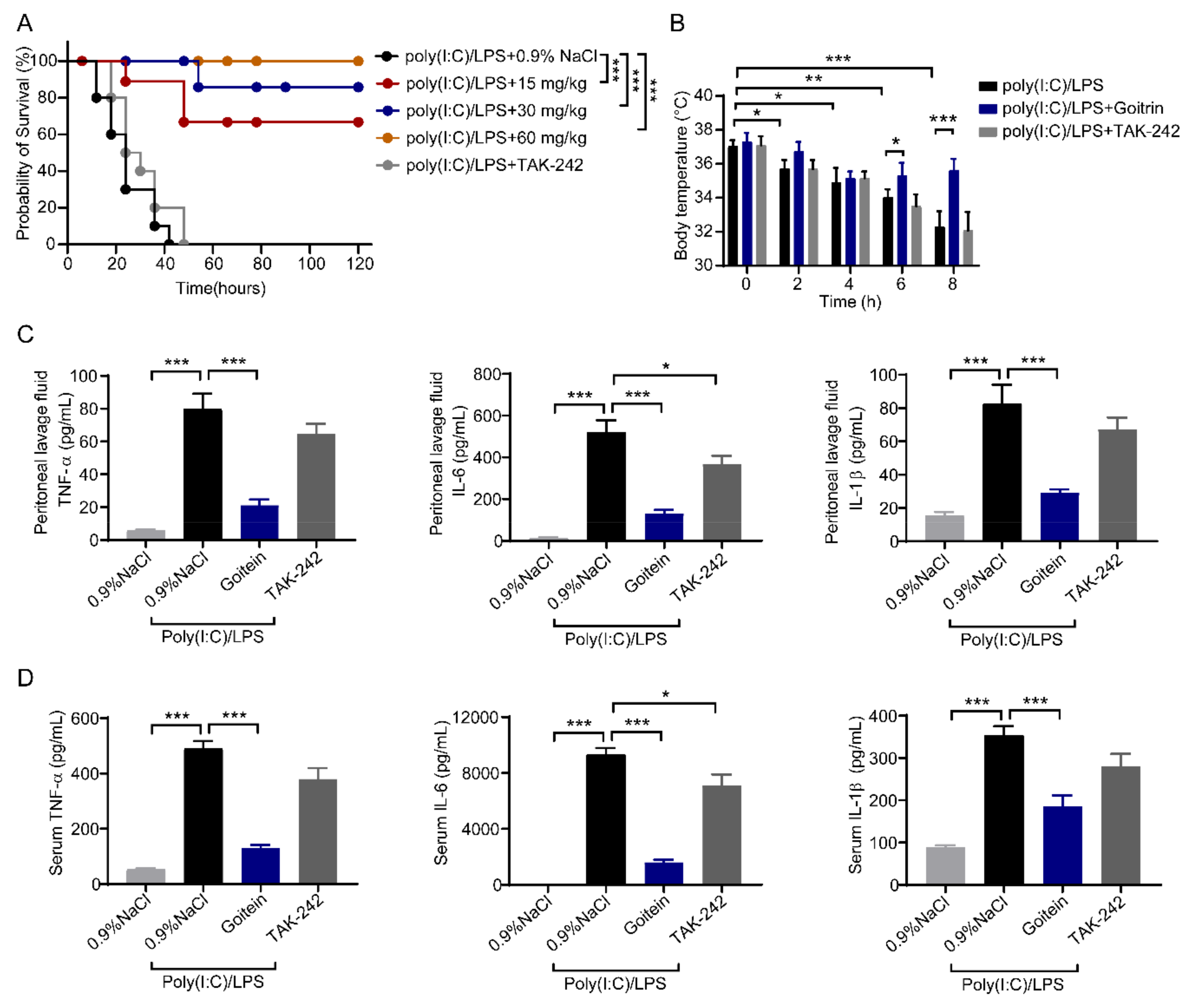
2.5. Goitrin Attenuates Caspase-11-Mediated Lethal Septic Shock
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Animals and Experimental Establishment
4.3. Survival Analysis and Measurement of Body Temperature
4.4. Cell Culture and Stimulation
4.5. Measurement of Lung Vessel Permeability
4.6. Lung Function Test
4.7. Histological and Immunohistochemistry Analysis and the TUNEL Assay
4.8. Quantitative Real-Time Polymerase Chain Reaction Assay
4.9. Enzyme-Linked Immunosorbent Assay
4.10. Western Blot Analysis
4.11. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef]
- Herrán-Monge, R.; Muriel-Bombín, A.; García-García, M.M.; Merino-García, P.A.; Martínez-Barrios, M.; Andaluz, D.; Ballesteros, J.C.; Domínguez-Berrot, A.M.; Moradillo-Gonzalez, S.; Macías, S.; et al. Epidemiology and Changes in Mortality of Sepsis After the Implementation of Surviving Sepsis Campaign Guidelines. J. Intensive Care Med. 2019, 34, 740–750. [Google Scholar] [CrossRef]
- Cecconi, M.; Evans, L.; Levy, M.; Rhodes, A. Sepsis and septic shock. Lancet 2018, 392, 75–87. [Google Scholar] [CrossRef]
- Mira, J.C.; Gentile, L.F.; Mathias, B.J.; Efron, P.A.; Brakenridge, S.C.; Mohr, A.M.; Moore, F.A.; Moldawer, L.L. Sepsis Pathophysiology, Chronic Critical Illness, and Persistent Inflammation-Immunosuppression and Catabolism Syndrome. Crit. Care Med. 2017, 45, 253–262. [Google Scholar] [CrossRef]
- Kaukonen, K.M.; Bailey, M.; Pilcher, D.; Cooper, D.J.; Bellomo, R. Systemic inflammatory response syndrome criteria in defining severe sepsis. N. Engl. J. Med. 2015, 372, 1629–1638. [Google Scholar] [CrossRef]
- Venet, F.; Rimmelé, T.; Monneret, G. Management of Sepsis-Induced Immunosuppression. Crit. Care Clin. 2018, 34, 97–106. [Google Scholar] [CrossRef]
- Rathinam, V.A.K.; Zhao, Y.; Shao, F. Innate immunity to intracellular LPS. Nat. Immunol. 2019, 20, 527–533. [Google Scholar] [CrossRef]
- Tisoncik, J.R.; Korth, M.J.; Simmons, C.P.; Farrar, J.; Martin, T.R.; Katze, M.G. Into the eye of the cytokine storm. Microbiol. Mol. Biol. Rev. 2012, 76, 16–32. [Google Scholar] [CrossRef]
- Hoshino, K.; Takeuchi, O.; Kawai, T.; Sanjo, H.; Ogawa, T.; Takeda, Y.; Takeda, K.; Akira, S. Cutting edge: Toll-like receptor 4 (tlr4)-deficient mice are hyporesponsive to lipopolysaccharide: Evidence for tlr4 as the lps gene product. J. Immunol. 2016, 197, 3749–3752. [Google Scholar] [CrossRef]
- Fitzgerald, K.A.; Kagan, J.C. Toll-like Receptors and the Control of Immunity. Cell 2020, 180, 1044–1066. [Google Scholar] [CrossRef]
- Kayagaki, N.; Wong, M.T.; Stowe, I.B.; Ramani, S.R.; Gonzalez, L.C.; Akashi-Takamura, S.; Miyake, K.; Zhang, J.; Lee, W.P.; Muszyński, A.; et al. Noncanonical inflammasome activation by intracellular LPS independent of TLR4. Science 2013, 41, 1246–1249. [Google Scholar] [CrossRef] [PubMed]
- Kayagaki, N.; Warming, S.; Lamkanfi, M.; Vande Walle, L.; Louie, S.; Dong, J.; Newton, K.; Qu, Y.; Liu, J.; Heldens, S.; et al. Non-canonical inflammasome activation targets caspase-11. Nature 2011, 479, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Kayagaki, N.; Stowe, I.B.; Lee, B.L.; O’Rourke, K.; Anderson, K.; Warming, S.; Cuellar, T.; Haley, B.; Roose-Girma, M.; Phung, Q.T.; et al. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature 2015, 526, 666–671. [Google Scholar] [CrossRef] [PubMed]
- Hagar, J.A.; Powell, D.A.; Aachoui, Y.; Ernst, R.K.; Miao, E.A. Cytoplasmic LPS activates caspase-11: Implications in TLR4-independent endotoxic shock. Science 2013, 341, 1250–1253. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Z.; Ruan, J.; Pan, Y.; Magupalli, V.G.; Wu, H.; Lieberman, J. Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature 2016, 535, 153–158. [Google Scholar] [CrossRef]
- Rice, T.W.; Wheeler, A.P.; Bernard, G.R.; Vincent, J.; Angus, D.C.; Aikawa, N.; Demeyer, I.; Sainati, S.; Amlot, M.; Cao, C.; et al. A randomized, double-blind, placebo-controlled trial of TAK-242 for the treatment of severe sepsis. Crit. Care Med. 2010, 38, 1685–1694. [Google Scholar] [CrossRef]
- Opal, S.M.; Laterre, P.F.; Francois, B.; LaRosa, S.P.; Angus, D.C.; Mira, J.P.; Wittebole, X.; Dugernier, T.; Perrotin, D.; Tidswell, M.; et al. Effect of eritoran, an antagonist of MD2-TLR4, on mortality in patients with severe sepsis: The ACCESS randomized trial. JAMA 2013, 309, 1154–1162. [Google Scholar] [CrossRef]
- Wang, K.; Sun, Q.; Zhong, X.; Zeng, M.; Zeng, H.; Shi, X.; Li, Z.; Wang, Y.; Zhao, Q.; Shao, F.; et al. Structural Mechanism for GSDMD Targeting by Autoprocessed Caspases in Pyroptosis. Cell 2020, 180, 941–955.e20. [Google Scholar] [CrossRef]
- Hu, J.J.; Liu, X.; Xia, S.; Zhang, Z.; Zhang, Y.; Zhao, J.; Ruan, J.; Luo, X.; Lou, X.; Bai, Y.; et al. FDA-approved disulfiram inhibits pyroptosis by blocking gasdermin D pore formation. Nat. Immunol. 2020, 21, 736–745. [Google Scholar] [CrossRef]
- Chinese Pharmacopoeia Commission. Chinese Pharmacopoeia, 2020 ed.; (English); Part I; China Medical Science and Technology Press: Beijing, China, 2020; p. 246. [Google Scholar]
- Wang, R.; Yang, H.; Yang, Q.; Huang, S.; Wang, Z. Study on quality standard of Isatidis Radix. Chin. Tradit. Herb Drugs 2010, 41, 478–480. [Google Scholar]
- Shi, Y.; Xie, Z.; Wu, Y.; Li, Y.; Wang, R.; Wang, Z. Determination of R,S-goitrin of Banlangen Preparations by RP-HPLC. Chin. J. Exp. Tradit. Med. Formulae. 2011, 17, 128–130. [Google Scholar] [CrossRef]
- Li, J.; Shi, Y.; Xu, Y.; Yang, L.; Wang, Z.; Han, H.; Wang, R. Metabolic profiles and pharmacokinetics of goitrin in rats through liquid chromatography combined with electrospray ionization-tandem mass spectrometry. Biomed. Chromatogr. 2019, 33, e4606. [Google Scholar] [CrossRef]
- Zheng, C.; Wang, R.; Wang, Z. Improvement of the quality standard for Compound Banlangen Granules. Chin. Tradit. Herb. Drugs. 2015, 37, 1477–1482. [Google Scholar]
- Shi, Y.; Zheng, C.; Li, J.; Yang, L.; Wang, Z.; Wang, R. Separation and Quantification of Four Main Chiral Glucosinolates in Radix Isatidis and Its Granules Using High-Performance Liquid Chromatography/Diode Array Detector Coupled with Circular Dichroism Detection. Molecules 2018, 23, 1305. [Google Scholar] [CrossRef]
- Luo, Z.; Liu, L.F.; Wang, X.H.; Li, W.; Jie, C.; Chen, H.; Wei, F.Q.; Lu, D.H.; Yan, C.Y.; Liu, B.; et al. Epigoitrin, an Alkaloid from Isatis indigotica, Reduces H1N1 Infection in Stress-Induced Susceptible Model in vivo and in vitro. Front. Pharmacol. 2019, 10, 78. [Google Scholar] [CrossRef] [PubMed]
- Ruan, D.; Liu, W.; Shi, Y.; Tan, M.; Yang, L.; Wang, Z.; Zhou, Y.; Wang, R. Protective Effects of Aqueous Extract of Radix Isatidis on Lipopolysaccharide-Induced Sepsis in C57BL/6J Mice. J. Med. Food 2020, 23, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Diebner, H.H.; Reinke, S.; Rösen-Wolff, A.; Winkler, S. A Kinetic Response Model for Standardized Regression Analyses of Inflammation-Triggered Hypothermic Body Temperature-Time Courses in Mice. Front. Physiol. 2021, 12, 634510. [Google Scholar] [CrossRef]
- Radu, M.; Chernoff, J. An in vivo assay to test blood vessel permeability. J. Vis. Exp. 2013, 16, e50062. [Google Scholar] [CrossRef]
- Smith, P.; Jeffers, L.A.; Koval, M. Measurement of Lung Vessel and Epithelial Permeability In Vivo with Evans Blue. Methods Mol. Biol. 2021, 2367, 137–148. [Google Scholar] [CrossRef]
- Vaickus, L.J.; Bouchard, J.; Kim, J.; Natarajan, S.; Remick, D.G. Assessing pulmonary pathology by detailed examination of respiratory function. Am. J. Pathol. 2010, 177, 1861–1869. [Google Scholar] [CrossRef]
- Cheng, K.T.; Xiong, S.; Ye, Z.; Hong, Z.; Di, A.; Tsang, K.M.; Gao, X.; An, S.; Mittal, M.; Vogel, S.M.; et al. Caspase-11-mediated endothelial pyroptosis underlies endotoxemia-induced lung injury. J. Clin. Investig. 2017, 127, 4124–4135. [Google Scholar] [CrossRef]
- Karki, R.; Sharma, B.R.; Tuladhar, S.; Williams, E.P.; Zalduondo, L.; Samir, P.; Zheng, M.; Sundaram, B.; Banoth, B.; Malireddi, R.K.S.; et al. Synergism of TNF-α and IFN-γ Triggers Inflammatory Cell Death, Tissue Damage, and Mortality in SARS-CoV-2 Infection and Cytokine Shock Syndromes. Cell 2021, 184, 149–168.e17. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Zeng, L.; Zhu, S.; Liu, J.; Zeh, H.J.; Kroemer, G.; Wang, H.; Billiar, T.R.; Jiang, J.; Tang, D.; et al. cAMP metabolism controls caspase-11 inflammasome activation and pyroptosis in sepsis. Sci. Adv. 2019, 5, eaav5562. [Google Scholar] [CrossRef] [PubMed]
- Chu, L.H.; Indramohan, M.; Ratsimandresy, R.A.; Gangopadhyay, A.; Morris, E.P.; Monack, D.M.; Dorfleutner, A.; Stehlik, C. The oxidized phospholipid oxPAPC protects from septic shock by targeting the non-canonical inflammasome in macrophages. Nat. Commun. 2018, 9, 996. [Google Scholar] [CrossRef] [PubMed]
- Pålsson-McDermott, E.M.; O’Neill, L.A. Signal transduction by the lipopolysaccharide receptor, Toll-like receptor-4. Immunology 2004, 113, 153–162. [Google Scholar] [CrossRef]
- Schappe, M.S.; Szteyn, K.; Stremska, M.E.; Mendu, S.K.; Downs, T.K.; Seegren, P.V.; Mahoney, M.A.; Dixit, S.; Krupa, J.K.; Stipes, E.J.; et al. Chanzyme TRPM7 Mediates the Ca2+ Influx Essential for Lipopolysaccharide-Induced Toll-Like Receptor 4 Endocytosis and Macrophage Activation. Immunity 2018, 48, 59–74.e5. [Google Scholar] [CrossRef]
- Roger, T.; Froidevaux, C.; Roy, D.L.; Reymond, M.K.; Chanson, A.L.; Mauri, D.; Burns, K.; Riederer, B.M.; Akira, S.; Calandra, T. Protection from lethal gram-negative bacterial sepsis by targeting toll-like receptor 4. Proc. Natl. Acad. Sci. USA 2009, 106, 2348–2352. [Google Scholar] [CrossRef]
- Wenzel, R.P.; Edmond, M.B. Septic shock—Evaluating another failed treatment. N. Engl. J. Med. 2012, 31, 2122–2124. [Google Scholar] [CrossRef]
- Von Moltke, J.; Ayres, J.S.; Kofoed, E.M.; Chavarría-Smith, J.; Vance, R.E. Recognition of bacteria by inflammasomes. Annu. Rev. Immunol. 2013, 31, 73–106. [Google Scholar] [CrossRef]
- Kelley, N.; Jeltema, D.; Duan, Y.; He, Y. The NLRP3 Inflammasome: An Overview of Mechanisms of Activation and Regulation. Int. J. Mol. Sci. 2019, 20, 3328. [Google Scholar] [CrossRef]
- Takeuchi, O.; Akira, S. Pattern recognition receptors and inflammation. Cell 2010, 140, 805–820. [Google Scholar] [CrossRef] [PubMed]
- Pfalzgraff, A.; Weindl, G. Intracellular Lipopolysaccharide Sensing as a Potential Therapeutic Target for Sepsis. Trends Pharmacol. Sci. 2019, 40, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Reinke, S.; Linge, M.; Diebner, H.H.; Luksch, H.; Glage, S.; Gocht, A.; Robertson, A.A.B.; Cooper, M.A.; Hofmann, S.R.; Naumann, R.; et al. Non-canonical Caspase-1 Signaling Drives RIP2-Dependent and TNF-α-Mediated Inflammation In Vivo. Cell Rep. 2020, 30, 2501–2511.e5. [Google Scholar] [CrossRef]
- Russo, A.J.; Vasudevan, S.O.; Méndez-Huergo, S.P.; Kumari, P.; Menoret, A.; Duduskar, S.; Wang, C.; Pérez Sáez, J.M.; Fettis, M.M.; Li, C.; et al. Intracellular immune sensing promotes inflammation via gasdermin D-driven release of a lectin alarmin. Nat. Immunol. 2021, 22, 154–165. [Google Scholar] [CrossRef]
- Tang, Y.; Wang, X.; Li, Z.; He, Z.; Yang, X.; Cheng, X.; Peng, Y.; Xue, Q.; Bai, Y.; Zhang, R.; et al. Heparin prevents caspase-11-dependent septic lethality independent of anticoagulant properties. Immunity 2021, 54, 454–467.e6. [Google Scholar] [CrossRef] [PubMed]
- Hotchkiss, R.S.; Monneret, G.; Payen, D. Immunosuppression in sepsis: A novel understanding of the disorder and a new therapeutic approach. Lancet Infect Dis. 2013, 13, 260–268. [Google Scholar] [CrossRef]
- Paoli, C.J.; Reynolds, M.A.; Sinha, M.; Gitlin, M.; Crouser, E. Epidemiology and Costs of Sepsis in the United States-An Analysis Based on Timing of Diagnosis and Severity Level. Crit. Care Med. 2018, 46, 1889–1897. [Google Scholar] [CrossRef]
- Schroder, K.; Tschopp, J. The inflammasomes. Cell 2010, 140, 821–832. [Google Scholar] [CrossRef]
- Rathkey, J.K.; Zhao, J.; Liu, Z.; Chen, Y.; Yang, J.; Kondolf, H.C.; Benson, B.L.; Chirieleison, S.M.; Huang, A.Y.; Dubyak, G.R.; et al. Chemical disruption of the pyroptotic pore-forming protein gasdermin D inhibits inflammatory cell death and sepsis. Sci. Immunol. 2018, 3, eaat2738. [Google Scholar] [CrossRef]
- Yang, D.; He, Y.; Muñoz-Planillo, R.; Liu, Q.; Núñez, G. Caspase-11 Requires the Pannexin-1 Channel and the Purinergic P2X7 Pore to Mediate Pyroptosis and Endotoxic Shock. Immunity 2015, 43, 923–932. [Google Scholar] [CrossRef]
- Vanden Berghe, T.; Hulpiau, P.; Martens, L.; Vandenbroucke, R.E.; Van Wonterghem, E.; Perry, S.W.; Bruggeman, I.; Divert, T.; Choi, S.M.; Vuylsteke, M.; et al. Passenger Mutations Confound Interpretation of All Genetically Modified Congenic Mice. Immunity 2015, 43, 200–209. [Google Scholar] [CrossRef]
- Aachoui, Y.; Leaf, I.A.; Hagar, J.A.; Fontana, M.F.; Campos, C.G.; Zak, D.E.; Tan, M.H.; Cotter, P.A.; Vance, R.E.; Aderem, A.; et al. Caspase-11 protects against bacteria that escape the vacuole. Science 2013, 339, 975–978. [Google Scholar] [CrossRef] [PubMed]
- Moretti, J.; Jia, B.; Hutchins, Z.; Roy, S.; Yip, H.; Wu, J.; Shan, M.; Jaffrey, S.R.; Coers, J.; Blander, J.M. Caspase-11 interaction with NLRP3 potentiates the noncanonical activation of the NLRP3 inflammasome. Nat. Immunol. 2022, 23, 705–717. [Google Scholar] [CrossRef] [PubMed]
- Rathinam, V.A.; Vanaja, S.K.; Waggoner, L.; Sokolovska, A.; Becker, C.; Stuart, L.M.; Leong, J.M.; Fitzgerald, K.A. TRIF licenses caspase-11-dependent NLRP3 inflammasome activation by gram-negative bacteria. Cell 2012, 150, 606–619. [Google Scholar] [CrossRef] [PubMed]
- Gurung, P.; Malireddi, R.K.; Anand, P.K.; Demon, D.; Vande Walle, L.; Liu, Z.; Vogel, P.; Lamkanfi, M.; Kanneganti, T.D. Toll or interleukin-1 receptor (TIR) domain-containing adaptor inducing interferon-β (TRIF)-mediated caspase-11 protease production integrates Toll-like receptor 4 (TLR4) protein- and Nlrp3 inflammasome-mediated host defense against enteropathogens. J. Biol. Chem. 2012, 287, 34474–34483. [Google Scholar] [CrossRef] [PubMed]
- Moitra, J.; Sammani, S.; Garcia, J.G. Re-evaluation of Evans Blue dye as a marker of albumin clearance in murine models of acute lung injury. Transl. Res. 2007, 150, 253–265. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhao, J.; Xu, M.; Li, M.; Wang, B.; Qu, X.; Yu, C.; Hang, H.; Xia, Q.; Wu, H.; et al. Blocking GSDMD processing in innate immune cells but not in hepatocytes protects hepatic ischemia-reperfusion injury. Cell Death Dis. 2020, 11, 244. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruan, D.; Yang, J.; Luo, Q.; Shi, Y.; Ding, L.; Wang, Z.; Wang, R.; Yang, L. The Protective Effects of Goitrin on LPS-Induced Septic Shock in C57BL/6J Mice via Caspase-11 Non-Canonical Inflammasome Inhibition. Molecules 2023, 28, 2883. https://doi.org/10.3390/molecules28072883
Ruan D, Yang J, Luo Q, Shi Y, Ding L, Wang Z, Wang R, Yang L. The Protective Effects of Goitrin on LPS-Induced Septic Shock in C57BL/6J Mice via Caspase-11 Non-Canonical Inflammasome Inhibition. Molecules. 2023; 28(7):2883. https://doi.org/10.3390/molecules28072883
Chicago/Turabian StyleRuan, Deqing, Jingyi Yang, Qianfei Luo, Yanhong Shi, Lili Ding, Zhengtao Wang, Rui Wang, and Li Yang. 2023. "The Protective Effects of Goitrin on LPS-Induced Septic Shock in C57BL/6J Mice via Caspase-11 Non-Canonical Inflammasome Inhibition" Molecules 28, no. 7: 2883. https://doi.org/10.3390/molecules28072883
APA StyleRuan, D., Yang, J., Luo, Q., Shi, Y., Ding, L., Wang, Z., Wang, R., & Yang, L. (2023). The Protective Effects of Goitrin on LPS-Induced Septic Shock in C57BL/6J Mice via Caspase-11 Non-Canonical Inflammasome Inhibition. Molecules, 28(7), 2883. https://doi.org/10.3390/molecules28072883






