Application of Biomass Corrosion Inhibitors in Metal Corrosion Control: A Review
Abstract
1. Introduction
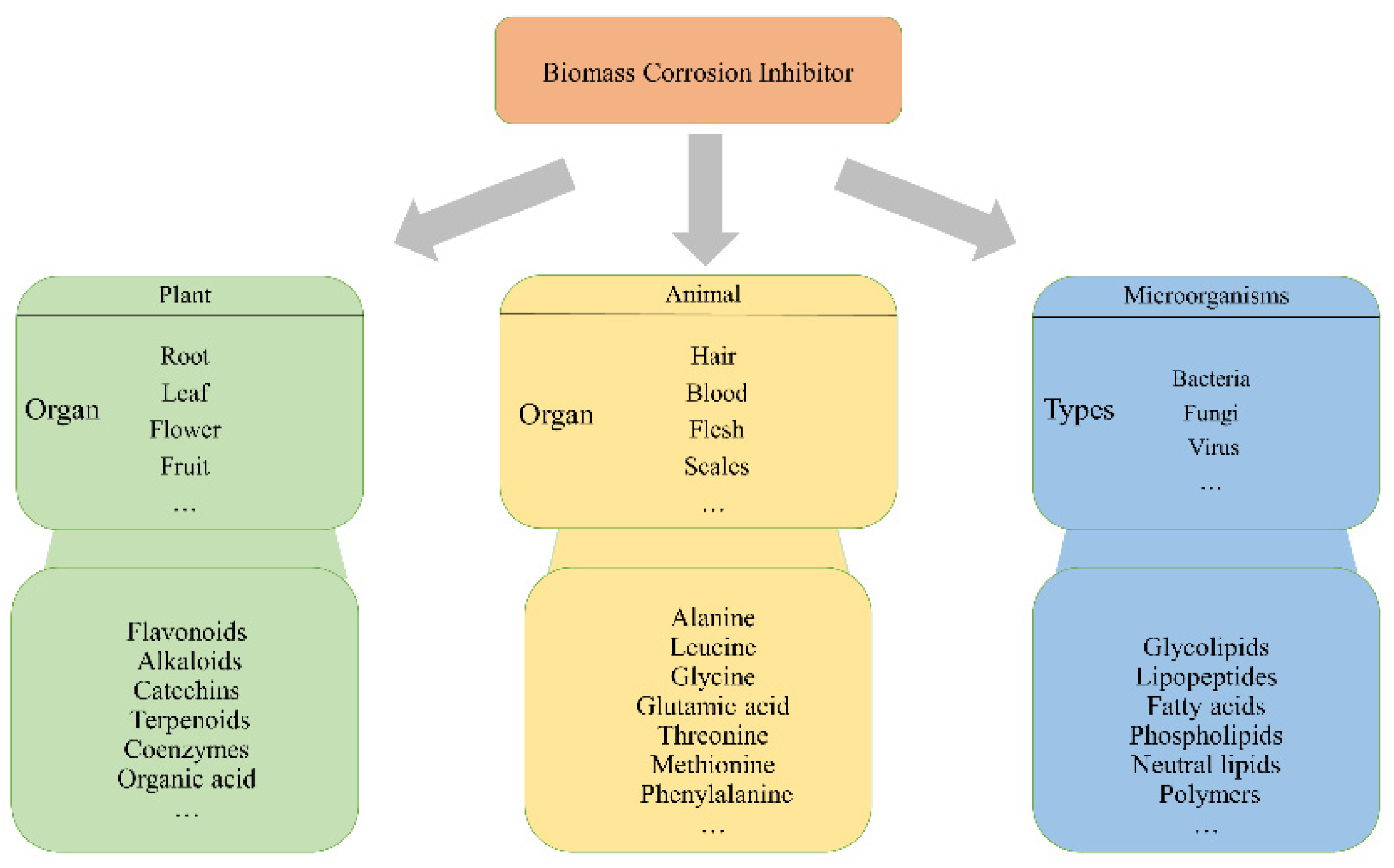
2. Classification of Biomass Corrosion Inhibitors
2.1. Corrosion Inhibitors Extracted from Plants
2.2. Amino-Acid-Based Corrosion Inhibitors Extracted from Protein
2.3. Extraction of Surfactants from Microorganisms as Corrosion Inhibitors
3. Preparation Method of Biomass Corrosion Inhibitor
3.1. Immersion Method
3.2. Enzymatic Digestion
3.3. Heating Reflux Method
3.4. Microwave Extraction Method
4. Inhibition Mechanism of Metal Corrosion by Biomass Corrosion Inhibitors
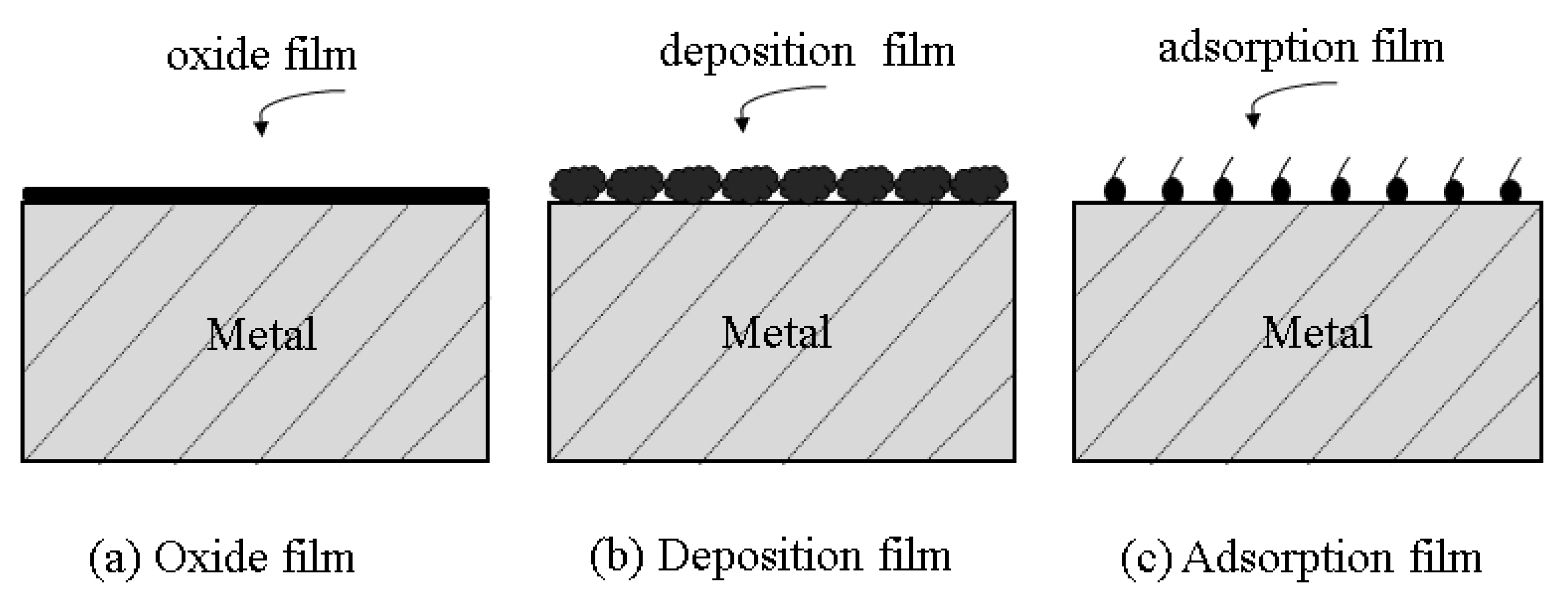
4.1. Physical Adsorption
4.2. Chemisorption
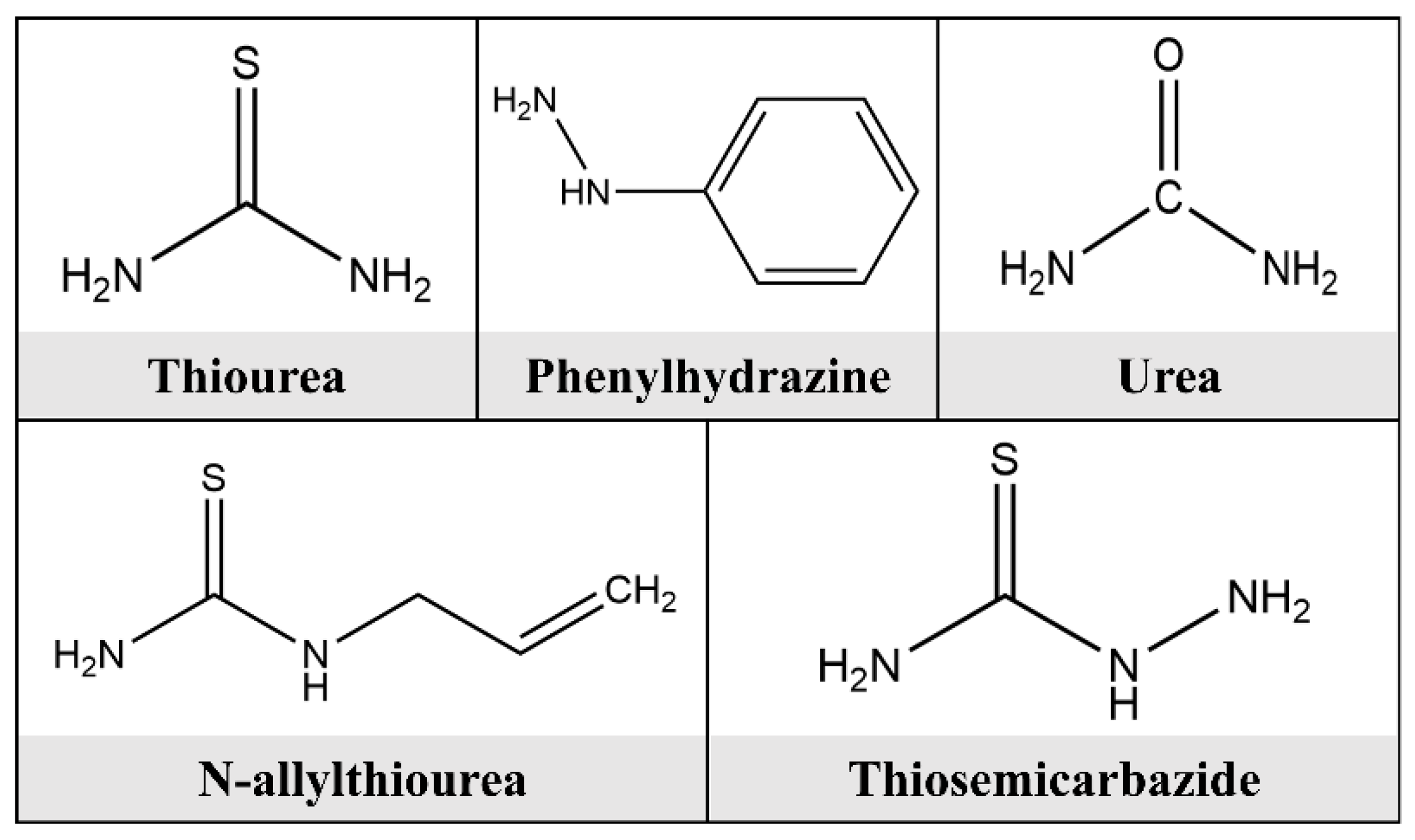
4.3. Film-Forming Adsorption Type
5. Evaluation Methods for Green Biomass Corrosion Inhibitors
5.1. Weight Loss Measurement
5.2. Electrochemical Methods
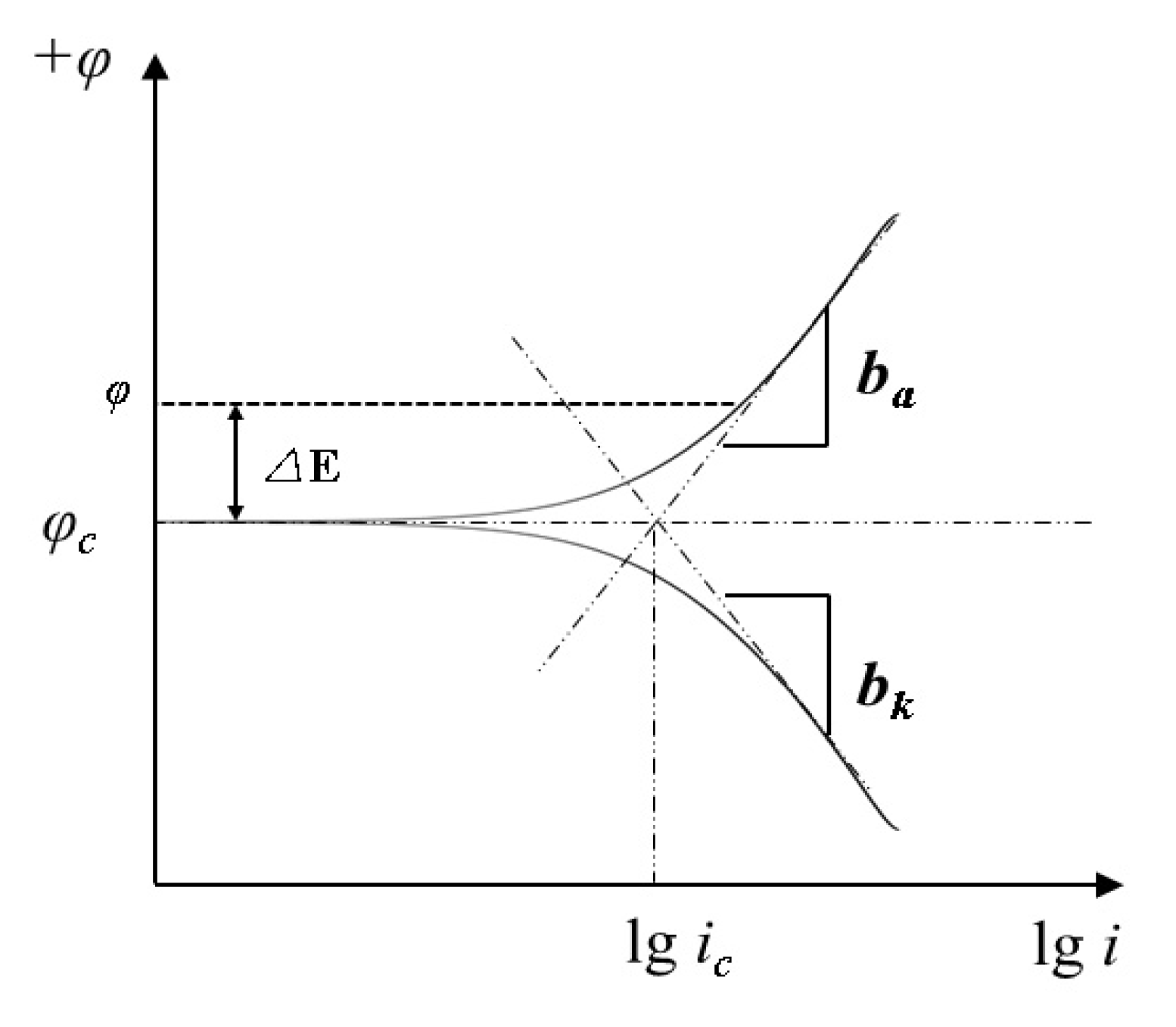
5.2.1. Potentiodynamic Polarization Technique (Tafel)
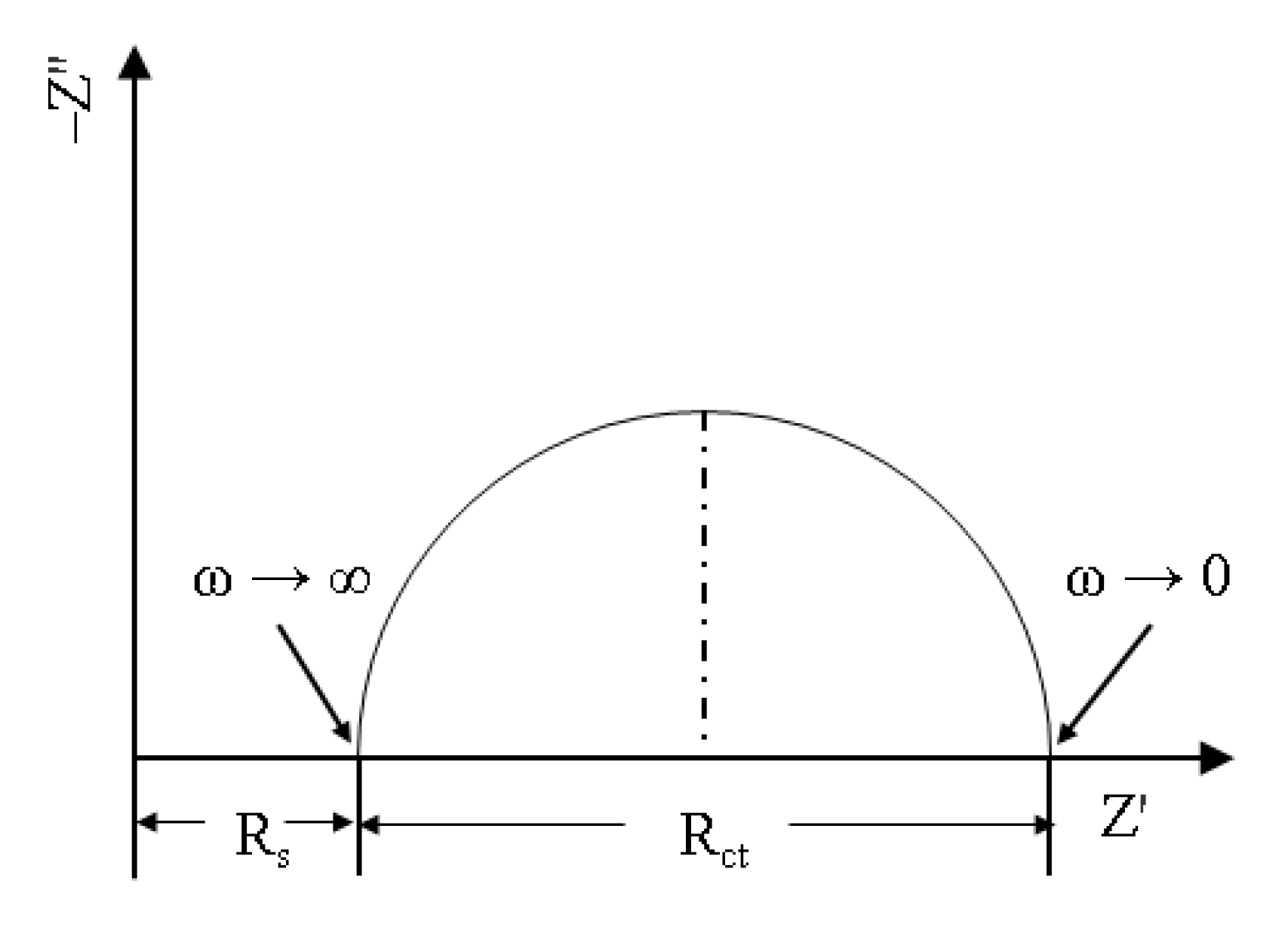
5.2.2. Electrochemical Impedance Spectroscopy (EIS)
5.3. Metal Surface Analysis Technology
5.4. Computer Simulation Study
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tan, B.; Xiang, B.; Zhang, S.; Qiang, Y.; Xu, L.; Chen, S.; He, J. Papaya leaves extract as a novel eco-friendly corrosion inhibitor for Cu in H2SO4 medium. J. Colloid Interface Sci. 2021, 582, 918–931. [Google Scholar] [CrossRef] [PubMed]
- Hossain, N.; Asaduzzaman Chowdhury, M.; Kchaou, M. An overview of green corrosion inhibitors for sustainable and environment friendly industrial development. J. Adhes. Sci. Technol. 2020, 35, 673–690. [Google Scholar] [CrossRef]
- Brycki, B.; Szulc, A. Gemini surfactants as corrosion inhibitors. A review. J. Mol. Liq. 2021, 344, 117686. [Google Scholar] [CrossRef]
- Kadhim, A.; Al-Amiery, A.; Alazawi, R.; Al-Ghezi, M.; Abass, R. Corrosion inhibitors. A review. Int. J. Corros. Scale Inhib. 2021, 10, 54–67. [Google Scholar] [CrossRef]
- Ma, I.A.W.; Ammar, S.; Kumar, S.S.A.; Ramesh, K.; Ramesh, S. A concise review on corrosion inhibitors: Types, mechanisms and electrochemical evaluation studies. J. Coat. Technol. Res. 2021, 19, 241–268. [Google Scholar] [CrossRef]
- Ahamad, I.; Prasad, R.; Quraishi, M. Inhibition of mild steel corrosion in acid solution by Pheniramine drug: Experimental and theoretical study. Corros. Sci. 2010, 52, 3033–3041. [Google Scholar] [CrossRef]
- Kalaiselvi, P.; Chellammal, S.; Palanichamy, S.; Subramanian, G. Artemisia pallens as corrosion inhibitor for mild steel in HCl medium. Mater. Chem. Phys. 2010, 120, 643–648. [Google Scholar] [CrossRef]
- Verma, C.; Ebenso, E.E.; Bahadur, I.; Quraishi, M.A. An overview on plant extracts as environmental sustainable and green corrosion inhibitors for metals and alloys in aggressive corrosive media. J. Mol. Liq. 2018, 266, 577–590. [Google Scholar] [CrossRef]
- El Ibrahimi, B.; Jmiai, A.; Bazzi, L.; El Issami, S. Amino acids and their derivatives as corrosion inhibitors for metals and alloys. Arab. J. Chem. 2020, 13, 740–771. [Google Scholar] [CrossRef]
- Lou, Y.; Chang, W.; Cui, T.; Wang, J.; Qian, H.; Ma, L.; Hao, X.; Zhang, D. Microbiologically influenced corrosion inhibition mechanisms in corrosion protection: A review. Bioelectrochemistry 2021, 141, 107883. [Google Scholar] [CrossRef]
- Fateh, A.; Aliofkhazraei, M.; Rezvanian, A.R. Review of corrosive environments for copper and its corrosion inhibitors. Arab. J. Chem. 2020, 13, 481–544. [Google Scholar] [CrossRef]
- Izadi, M.; Shahrabi, T.; Ramezanzadeh, B. Electrochemical investigations of the corrosion resistance of a hybrid sol–gel film containing green corrosion inhibitor-encapsulated nanocontainers. J. Taiwan Inst. Chem. Eng. 2017, 81, 356–372. [Google Scholar] [CrossRef]
- Da Rocha, J.C.; Gomes, J.A.d.C.P.; D’Elia, E. Corrosion inhibition of carbon steel in hydrochloric acid solution by fruit peel aqueous extracts. Corros. Sci. 2010, 52, 2341–2348. [Google Scholar] [CrossRef]
- Ji, G.; Anjum, S.; Sundaram, S.; Prakash, R. Musa paradisica peel extract as green corrosion inhibitor for mild steel in HCl solution. Corros. Sci. 2015, 90, 107–117. [Google Scholar] [CrossRef]
- Krishnaveni, K.; Ravichandran, J. Influence of aqueous extract of leaves of Morinda tinctoria on copper corrosion in HCl medium. J. Electroanal. Chem. 2014, 735, 24–31. [Google Scholar] [CrossRef]
- Da Rocha, J.; Gomes, J.P.; D’elia, E.; Cruz, A.G.; Cabral, L.; Torres, A.; Monteiro, M. Grape pomace extracts as green corrosion inhibitors for carbon steel in hydrochloric acid solutions. Int. J. Electrochem. Sci. 2012, 7, 11941–11956. [Google Scholar]
- Amin, M.A.; Khaled, K.; Mohsen, Q.; Arida, H. A study of the inhibition of iron corrosion in HCl solutions by some amino acids. Corros. Sci. 2010, 52, 1684–1695. [Google Scholar] [CrossRef]
- Lima, K.C.d.S.d.; Paiva, V.M.; Perrone, D.; Ripper, B.; Simões, G.; Rocco, M.L.M.; Veiga, A.G.d.; D’Elia, E. Glycine max meal extracts as corrosion inhibitor for mild steel in sulphuric acid solution. J. Mater. Res. Technol. 2020, 9, 12756–12772. [Google Scholar] [CrossRef]
- Zerfaoui, M.; Oudda, H.; Hammouti, B.; Kertit, S.; Benkaddour, M. Inhibition of corrosion of iron in citric acid media by aminoacids. Prog. Org. Coat. 2004, 51, 134–138. [Google Scholar] [CrossRef]
- Abd El Aal, E.; Abd El Wanees, S.; Farouk, A.; Abd El Haleem, S. Factors affecting the corrosion behaviour of aluminium in acid solutions. II. Inorganic additives as corrosion inhibitors for Al in HCl solutions. Corros. Sci. 2013, 68, 14–24. [Google Scholar] [CrossRef]
- Abd El Haleem, S.M.; Abd El Wanees, S.; Abd El Aal, E.E.; Farouk, A. Factors affecting the corrosion behaviour of aluminium in acid solutions. I. Nitrogen and/or sulphur-containing organic compounds as corrosion inhibitors for Al in HCl solutions. Corros. Sci. 2013, 68, 1–13. [Google Scholar] [CrossRef]
- Bobina, M.; Kellenberger, A.; Millet, J.-P.; Muntean, C.; Vaszilcsin, N. Corrosion resistance of carbon steel in weak acid solutions in the presence of l-histidine as corrosion inhibitor. Corros. Sci. 2013, 69, 389–395. [Google Scholar] [CrossRef]
- Hamadi, L.; Mansouri, S.; Oulmi, K.; Kareche, A. The use of amino acids as corrosion inhibitors for metals: A review. Egypt. J. Pet. 2018, 27, 1157–1165. [Google Scholar] [CrossRef]
- Płaza, G.; Achal, V. Biosurfactants: Eco-friendly and innovative biocides against biocorrosion. Int. J. Mol. Sci. 2020, 21, 2152. [Google Scholar] [CrossRef]
- Zin, I.M.; Pokhmurskii, V.I.; Korniy, S.A.; Karpenko, O.V.; Lyon, S.B.; Khlopyk, O.P.; Tymus, M.B. Corrosion inhibition of aluminium alloy by rhamnolipid biosurfactant derived from Pseudomonas sp. PS-17. Anti-Corros. Methods Mater. 2018, 65, 517–527. [Google Scholar] [CrossRef]
- Parthipan, P.; Sabarinathan, D.; Angaiah, S.; Rajasekar, A. Glycolipid biosurfactant as an eco-friendly microbial inhibitor for the corrosion of carbon steel in vulnerable corrosive bacterial strains. J. Mol. Liq. 2018, 261, 473–479. [Google Scholar] [CrossRef]
- Shubina, V.; Gaillet, L.; Chaussadent, T.; Meylheuc, T.; Creus, J. Biomolecules as a sustainable protection against corrosion of reinforced carbon steel in concrete. J. Clean. Prod. 2016, 112, 666–671. [Google Scholar] [CrossRef]
- Mobin, M.; Aslam, R.; Zehra, S.; Ahmad, M. Bio-/environment-friendly cationic gemini surfactant as novel corrosion inhibitor for mild steel in 1 M HCl solution. J. Surfactants Deterg. 2017, 20, 57–74. [Google Scholar] [CrossRef]
- Gadow, H.S.; Motawea, M.M. Investigation of the corrosion inhibition of carbon steel in hydrochloric acid solution by using ginger roots extract. RSC Adv. 2017, 7, 24576–24588. [Google Scholar] [CrossRef]
- Suleiman, I.Y.; Salihu, S.A.; Emokpaire, O.S.; Ogheneme, O.C.; Shuaibu, L. Evaluation of Grewa Venusta (Wild Jute Tree) Extract as Corrosion Inhibitor for Mild Steel in Acidic Environment. Port. Electrochim. Acta 2017, 35, 143–158. [Google Scholar] [CrossRef]
- Zhao, A. Electrochemical Studies of Bitter Gourd (Momordica charantia) fruits as Ecofriendly Corrosion Inhibitor for Mild Steel in 1 M HCl Solution. Int. J. Electrochem. Sci. 2019, 14, 6814–6825. [Google Scholar] [CrossRef]
- Thomas, A.; Prajila, M.; Shainy, K.M.; Joseph, A. A green approach to corrosion inhibition of mild steel in hydrochloric acid using fruit rind extract of Garcinia indica (Binda). J. Mol. Liq. 2020, 312, 113369. [Google Scholar] [CrossRef]
- Tan, B.; He, J.; Zhang, S.; Xu, C.; Chen, S.; Liu, H.; Li, W. Insight into anti-corrosion nature of Betel leaves water extracts as the novel and eco-friendly inhibitors. J. Colloid Interface Sci. 2021, 585, 287–301. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Song, Z.; Han, H.; Donkor, S.; Jiang, L.; Wang, W.; Chu, H. A novel green reinforcement corrosion inhibitor extracted from waste Platanus acerifolia leaves. Constr. Build. Mater. 2020, 260, 119695. [Google Scholar] [CrossRef]
- Palaniappan, N.; Cole, I.; Caballero-Briones, F.; Manickam, S.; Justin Thomas, K.R.; Santos, D. Experimental and DFT studies on the ultrasonic energy-assisted extraction of the phytochemicals of Catharanthus roseus as green corrosion inhibitors for mild steel in NaCl medium. RSC Adv. 2020, 10, 5399–5411. [Google Scholar] [CrossRef]
- Haldhar, R.; Prasad, D.; Saxena, A. Armoracia rusticana as sustainable and eco-friendly corrosion inhibitor for mild steel in 0.5M sulphuric acid: Experimental and theoretical investigations. J. Environ. Chem. Eng. 2018, 6, 5230–5238. [Google Scholar] [CrossRef]
- Saxena, A.; Prasad, D.; Haldhar, R. Use of Asparagus racemosus extract as green corrosion inhibitor for mild steel in 0.5 M H2SO4. J. Mater. Sci. 2018, 53, 8523–8535. [Google Scholar] [CrossRef]
- Haldhar, R.; Prasad, D.; Saxena, A. Myristica fragrans extract as an eco-friendly corrosion inhibitor for mild steel in 0.5 M H2SO4 solution. J. Environ. Chem. Eng. 2018, 6, 2290–2301. [Google Scholar] [CrossRef]
- Ahanotu, C.C.; Onyeachu, I.B.; Solomon, M.M.; Chikwe, I.S.; Chikwe, O.B.; Eziukwu, C.A. Pterocarpus santalinoides leaves extract as a sustainable and potent inhibitor for low carbon steel in a simulated pickling medium. Sustain. Chem. Pharm. 2020, 15, 100196. [Google Scholar] [CrossRef]
- Kalaiselvi, K.; Chung, I.-M.; Kim, S.-H.; Prabakaran, M. Corrosion resistance of mild steel in sulphuric acid solution by Coreopsis tinctoria extract: Electrochemical and surface studies. Anti-Corros. Methods Mater. 2018, 65, 408–416. [Google Scholar] [CrossRef]
- Makarenko, N.V.; Kharchenko, U.V.; Zemnukhova, L.A. Effect of amino acids on corrosion of copper and steel in acid medium. Russ. J. Appl. Chem. 2011, 84, 1362–1365. [Google Scholar] [CrossRef]
- Zapata-Loría, A.D.; Pech-Canul, M.A. Corrosion Inhibition of Aluminum in 0.1 m HCl Solution by Glutamic Acid. Chem. Eng. Commun. 2014, 201, 855–869. [Google Scholar] [CrossRef]
- Hamadi, L.; Kareche, A.; Mansouri, S.; Benbouta, S. Corrosion inhibition of Fe-19Cr stainless steel by glutamic acid in 1M HCl. Chem. Data Collect. 2020, 28, 100455. [Google Scholar] [CrossRef]
- Migahed, M.A.; Rashwan, S.M.; Kamel, M.M.; Habib, R.E. Synthesis, characterization of polyaspartic acid-glycine adduct and evaluation of their performance as scale and corrosion inhibitor in desalination water plants. J. Mol. Liq. 2016, 224, 849–858. [Google Scholar] [CrossRef]
- Fu, J.-J.; Li, S.-N.; Cao, L.-H.; Wang, Y.; Yan, L.-H.; Lu, L.-D. l-Tryptophan as green corrosion inhibitor for low carbon steel in hydrochloric acid solution. J. Mater. Sci. 2010, 45, 979–986. [Google Scholar] [CrossRef]
- Abdel-Fatah, H.T.M.; Abdel-Samad, H.S.; Hassan, A.A.M.; El-Sehiety, H.E.E. Effect of variation of the structure of amino acids on inhibition of the corrosion of low-alloy steel in ammoniated citric acid solutions. Res. Chem. Intermed. 2013, 40, 1675–1690. [Google Scholar] [CrossRef]
- Zarrok, H.; Zarrouk, A.; Salghi, R.; Hammouti, B.; Elbakri, M.; Ebn Touhami, M.; Bentiss, F.; Oudda, H. Study of a cysteine derivative as a corrosion inhibitor for carbon steel in phosphoric acid solution. Res. Chem. Intermed. 2013, 40, 801–815. [Google Scholar] [CrossRef]
- Khan, M.S.; Yang, C.; Zhao, Y.; Pan, H.; Zhao, J.; Shahzad, M.B.; Kolawole, S.K.; Ullah, I.; Yang, K. An induced corrosion inhibition of X80 steel by using marine bacterium Marinobacter salsuginis. Colloids Surf. B Biointerfaces 2020, 189, 110858. [Google Scholar] [CrossRef]
- Ornek, D.; Jayaraman, A.; Syrett, B.C.; Hsu, C.H.; Mansfeld, F.B.; Wood, T.K. Pitting corrosion inhibition of aluminum 2024 by Bacillus biofilms secreting polyaspartate or gamma-polyglutamate. Appl. Microbiol. Biotechnol. 2002, 58, 651–657. [Google Scholar] [CrossRef]
- Fawzy, A.; Abdallah, M.; Alfakeer, M.; Altass, H.M.; Althagafi, I.I.; El-Ossaily, Y.A. Performance of unprecedented synthesized biosurfactants as green inhibitors for the corrosion of mild steel-37-2 in neutral solutions: A mechanistic approach. Green Chem. Lett. Rev. 2021, 14, 488–499. [Google Scholar] [CrossRef]
- De Assunção Araújo Pereira, S.S.; Pêgas, M.M.; Fernández, T.L.; Magalhães, M.; Schöntag, T.G.; Lago, D.C.; de Senna, L.F.; D’Elia, E. Inhibitory action of aqueous garlic peel extract on the corrosion of carbon steel in HCl solution. Corros. Sci. 2012, 65, 360–366. [Google Scholar] [CrossRef]
- Jyothi, S.; Ravichandran, J. Luffa aegyptiaca leaves extract as corrosion inhibitor for mild steel in hydrochloric acid medium. J. Adhes. Sci. Technol. 2014, 28, 2347–2363. [Google Scholar] [CrossRef]
- Abou-Elseoud, W.S.; Abdel-karim, A.M.; Hassan, E.A.; Hassan, M.L. Enzyme- and acid-extracted sugar beet pectin as green corrosion inhibitors for mild steel in hydrochloric acid solution. Carbohydr. Polym. Technol. Appl. 2021, 2, 100072. [Google Scholar] [CrossRef]
- Yadav, M.; Goel, G.; Hatton, F.L.; Bhagat, M.; Mehta, S.K.; Mishra, R.K.; Bhojak, N. A review on biomass-derived materials and their applications as corrosion inhibitors, catalysts, food and drug delivery agents. Curr. Res. Green Sustain. Chem. 2021, 4, 100153. [Google Scholar] [CrossRef]
- Haldhar, R.; Prasad, D.; Bhardwaj, N. Extraction and experimental studies of Citrus aurantifolia as an economical and green corrosion inhibitor for mild steel in acidic media. J. Adhes. Sci. Technol. 2019, 33, 1169–1183. [Google Scholar] [CrossRef]
- Soltani, N.; Tavakkoli, N.; Kashani, M.K.; Mosavizadeh, A.; Oguzie, E.; Jalali, M. Silybum marianum extract as a natural source inhibitor for 304 stainless steel corrosion in 1.0 M HCl. J. Ind. Eng. Chem. 2014, 20, 3217–3227. [Google Scholar] [CrossRef]
- Suedile, F.; Robert, F.; Roos, C.; Lebrini, M. Corrosion inhibition of zinc by Mansoa alliacea plant extract in sodium chloride media: Extraction, Characterization and Electrochemical Studies. Electrochim. Acta 2014, 133, 631–638. [Google Scholar] [CrossRef]
- Singh, P.; Quraishi, M.; Ebenso, E.E. Microwave assisted green synthesis of bis-phenol polymer containing piperazine as a corrosion inhibitor for mild steel in 1M HCl. Int. J. Electrochem. Sci. 2013, 8, 890-10. [Google Scholar]
- Akinbulumo, O.A.; Odejobi, O.J.; Odekanle, E.L. Thermodynamics and adsorption study of the corrosion inhibition of mild steel by Euphorbia heterophylla L. extract in 1.5 M HCl. Results Mater. 2020, 5, 100074. [Google Scholar] [CrossRef]
- Hanini, K.; Merzoug, B.; Boudiba, S.; Selatnia, I.; Laouer, H.; Akkal, S. Influence of different polyphenol extracts of Taxus baccata on the corrosion process and their effect as additives in electrodeposition. Sustain. Chem. Pharm. 2019, 14, 100189. [Google Scholar] [CrossRef]
- Gerengi, H.; Sahin, H.I. Schinopsis lorentzii Extract As a Green Corrosion Inhibitor for Low Carbon Steel in 1 M HCl Solution. Ind. Eng. Chem. Fundam. 2011, 51, 780–787. [Google Scholar] [CrossRef]
- Wei, H.; Heidarshenas, B.; Zhou, L.; Hussain, G.; Li, Q.; Ostrikov, K. Green inhibitors for steel corrosion in acidic environment: State of art. Mater. Today Sustain. 2020, 10, 100044. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, M.; Liu, C.; Fang, Z. The Corrosion Inhibition Performance and Mechanism of Rhamnolipid for X65 Steel in CO2-Saturated Oilfield-Produced Water. J. Surfactants Deterg. 2021, 24, 809–819. [Google Scholar] [CrossRef]
- Dong, Z.H.; Liu, T.; Liu, H.F. Influence of EPS isolated from thermophilic sulphate-reducing bacteria on carbon steel corrosion. Biofouling 2011, 27, 487–495. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, Q.; Liu, L.; Zheng, H.; Wu, X.; Li, Z.; Gao, P.; Sun, Y.; Yan, Z.; Li, X. Experimental, DFT and MD evaluation of Nandina domestica Thunb. extract as green inhibitor for carbon steel corrosion in acidic medium. J. Mol. Struct. 2022, 1265, 133367. [Google Scholar] [CrossRef]
- Mourya, P.; Banerjee, S.; Rastogi, R.B.; Singh, M.M. Inhibition of Mild Steel Corrosion in Hydrochloric and Sulfuric Acid Media Using a Thiosemicarbazone Derivative. Ind. Eng. Chem. Res. 2013, 52, 12733–12747. [Google Scholar] [CrossRef]
- Mazumder, M.A.J.; Al-Muallem, H.A.; Faiz, M.; Ali, S.A. Design and synthesis of a novel class of inhibitors for mild steel corrosion in acidic and carbon dioxide-saturated saline media. Corros. Sci. 2014, 87, 187–198. [Google Scholar] [CrossRef]
- Okafor, P.C.; Ikpi, M.E.; Uwah, I.E.; Ebenso, E.E.; Ekpe, U.J.; Umoren, S.A. Inhibitory action of Phyllanthus amarus extracts on the corrosion of mild steel in acidic media. Corros. Sci. 2008, 50, 2310–2317. [Google Scholar] [CrossRef]
- Lin, B.; Tang, J.; Wang, Y.; Wang, H.; Zuo, Y. Study on synergistic corrosion inhibition effect between calcium lignosulfonate (CLS) and inorganic inhibitors on Q235 carbon steel in alkaline environment with Cl−. Molecules 2020, 25, 4200. [Google Scholar] [CrossRef]
- Stern, M. The Mechanism of Passivating-Type Inhibitors. J. Electrochem. Soc. 1958, 105, 638. [Google Scholar] [CrossRef]
- Shamnamol, G.; Sreelakshmi, K.; Ajith, G.; Jacob, J.M. In Effective utilization of drugs as green corrosion inhibitor—A review. AIP Conf. Proc. 2020, 2225, 070006. [Google Scholar] [CrossRef]
- Yanhua, Z.; ZHUANG, J.; Yongsheng, Y.; Xianguang, Z. Research on anti-corrosion property of rare earth inhibitor for X70 steel. J. Rare Earths 2013, 31, 734–740. [Google Scholar] [CrossRef]
- Alvarez, P.E.; Fiori-Bimbi, M.V.; Neske, A.; Brandan, S.A.; Gervasi, C.A. Rollinia occidentalis extract as green corrosion inhibitor for carbon steel in HCl solution. J. Ind. Eng. Chem. 2018, 58, 92–99. [Google Scholar] [CrossRef]
- Bui, H.T.; Dang, T.-D.; Le, H.T.; Hoang, T.T. Comparative study on corrosion inhibition of vietnam orange peel essential oil with urotropine and insight of corrosion inhibition mechanism for mild steel in hydrochloric solution. J. Electrochem. Sci. Technol. 2019, 10, 69–81. [Google Scholar] [CrossRef]
- Aejitha, S.; Kasthuri, P.K.; Jyothi, S. Corrosion inhibitory action of Commiphora caudataextract on the mild steel corrosion in 1 M H2SO4 acid medium. J. Adhes. Sci. Technol. 2015, 30, 784–802. [Google Scholar] [CrossRef]
- Sangeetha, T.; Fredimoses, M. Inhibition of mild copper metal corrosion in HNO3 medium by acid extract of Azadirachta indica seed. J. Chem. 2011, 8, S1–S6. [Google Scholar] [CrossRef]
- Popoola, L.T. Organic green corrosion inhibitors (OGCIs): A critical review. Corros. Rev. 2019, 37, 71–102. [Google Scholar] [CrossRef]
- Li, H.; Zhang, S.; Qiang, Y. Corrosion retardation effect of a green cauliflower extract on copper in H2SO4 solution: Electrochemical and theoretical explorations. J. Mol. Liq. 2021, 321, 114450. [Google Scholar] [CrossRef]
- Şahin, E.A.; Solmaz, R.; Gecibesler, İ.H.; Kardaş, G. Adsorption ability, stability and corrosion inhibition mechanism of phoenix dactylifera extrat on mild steel. Mater. Res. Express 2020, 7, 016585. [Google Scholar] [CrossRef]
- Zhang, K.; Xu, B.; Yang, W.; Yin, X.; Liu, Y.; Chen, Y. Halogen-substituted imidazoline derivatives as corrosion inhibitors for mild steel in hydrochloric acid solution. Corros. Sci. 2015, 90, 284–295. [Google Scholar] [CrossRef]
- Rodrigues, L.S.; do Valle, A.F.; D’Elia, E. Biomass of microalgae spirulina maxima as a corrosion inhibitor for 1020 carbon steel in acidic solution. Int. J. Electrochem. Sci. 2018, 13, 6169–6189. [Google Scholar] [CrossRef]
- Khiati, Z.; Othman, A.A.; Sanchez-Moreno, M.; Bernard, M.C.; Joiret, S.; Sutter, E.M.M.; Vivier, V. Corrosion inhibition of copper in neutral chloride media by a novel derivative of 1,2,4-triazole. Corros. Sci. 2011, 53, 3092–3099. [Google Scholar] [CrossRef]
- Njoku, D.I.; Onuoha, G.N.; Oguzie, E.E.; Oguzie, K.L.; Egbedina, A.A.; Alshawabkeh, A.N. Nicotiana tabacum leaf extract protects aluminium alloy AA3003 from acid attack. Arab. J. Chem. 2019, 12, 4466–4478. [Google Scholar] [CrossRef]
- Gomes, M.P.; Costa, I.; Pébère, N.; Rossi, J.L.; Tribollet, B.; Vivier, V. On the corrosion mechanism of Mg investigated by electrochemical impedance spectroscopy. Electrochim. Acta 2019, 306, 61–70. [Google Scholar] [CrossRef]
- Gerengi, H.; Jazdzewska, A.; Kurtay, M. A comprehensive evaluation of mimosa extract as a corrosion inhibitor on AA6060 alloy in acid rain solution: Part I. Electrochemical AC methods. J. Adhes. Sci. Technol. 2014, 29, 36–48. [Google Scholar] [CrossRef]
- Qiang, Y.; Zhang, S.; Tan, B.; Chen, S. Evaluation of Ginkgo leaf extract as an eco-friendly corrosion inhibitor of X70 steel in HCl solution. Corros. Sci. 2018, 133, 6–16. [Google Scholar] [CrossRef]
- Pustaj, G. Olive Leaf Extract as a Corrosion Inhibitor of Carbon Steel in CO2-Saturated Chloride–Carbonate Solution. Int. J. Electrochem. Sci. 2016, 11, 7811–7829. [Google Scholar] [CrossRef]
- Patel, N.; Jauhari, S.; Mehta, G. Mild steel corrosion inhibition by Bauhinia purpurea leaves extract in 1 N sulphuric acid. Arab. J. Sci. Eng. 2009, 34, 61. [Google Scholar]
- Miralrio, A.; Espinoza Vázquez, A. Plant extracts as green corrosion inhibitors for different metal surfaces and corrosive media: A review. Processes 2020, 8, 942. [Google Scholar] [CrossRef]
- Chaubey, N.; Savita; Singh, V.K.; Quraishi, M. Corrosion inhibition performance of different bark extracts on aluminium in alkaline solution. J. Assoc. Arab Univ. Basic Appl. Sci. 2017, 22, 38–44. [Google Scholar] [CrossRef]
- Chung, I.-M.; Malathy, R.; Kim, S.-H.; Kalaiselvi, K.; Prabakaran, M.; Gopiraman, M. Ecofriendly green inhibitor from Hemerocallis fulva against aluminum corrosion in sulphuric acid medium. J. Adhes. Sci. Technol. 2020, 34, 1483–1506. [Google Scholar] [CrossRef]
- Sharma, S.; Kumar, A. Recent advances in metallic corrosion inhibition: A review. J. Mol. Liq. 2021, 322, 114862. [Google Scholar] [CrossRef]
- El Aoufir, Y.; Sebhaoui, J.; Lgaz, H.; El Bakri, Y.; Guenbour, A.; Bentiss, F.; Zarrouk, A.; Essassi, E.M.; Oudda, H. Theoretical Prediction and Experimental Study of Benzimidazole Derivate as a Novel Corrosion Inhibitor for Carbon Steel in 1.0 M HCl. Prot. Met. Phys. Chem. Surf. 2020, 56, 1027–1038. [Google Scholar] [CrossRef]
- Guo, L.; Zhang, R.; Tan, B.; Li, W.; Liu, H.; Wu, S. Locust Bean Gum as a green and novel corrosion inhibitor for Q235 steel in 0.5 M H2SO4 medium. J. Mol. Liq. 2020, 310, 113239. [Google Scholar] [CrossRef]

| Name | Metal | Corrosive Medium | Optimum η (%) | Ref. |
|---|---|---|---|---|
| Plant | ||||
| Ginger root | Carbon steel | HCl | 95.00 | [29] |
| Wild jute tree | Mild steel | HCl | 96.90 | [30] |
| Bitter gourd fruits | Mild steel | HCl | 96.00 | [31] |
| Binda rind | Mild steel | HCl | 97.33 | [32] |
| Betel leaves extracts | Carbon steel | HCl | 94.90 | [33] |
| Platanus acerifolia leaf | Carbon steel | NaCl | 99.86 | [34] |
| Catharanthus roseus | Mild steel | NaCl | 84.00 | [35] |
| Armoracia rusticana root | Mild steel | H2SO4 | 95.74 | [36] |
| Asparagus racemosus fruits | Mild steel | H2SO4 | 93.25 | [37] |
| Myristica fragrans fruit | Mild steel | H2SO4 | 87.81 | [38] |
| Mutiti leaf | Low-carbon steel | H2SO4 | 86.23 | [39] |
| Coreopsis tinctoria plant | Mild steel | H2SO4 | 80.62 | [40] |
| Animal | ||||
| Phenylalanine | Steel | HCl | 74.80 | [41] |
| Glutamic acid | Aluminum | HCl | 81.50 | [42] |
| Glutamic acid | Fe-19Cr stainless steel | HCl | 68.36 | [43] |
| Polyaspartic acid–glycine | Carbon steel | NaCl | 83.80 | [44] |
| Tryptophan | Low-carbon steel | HCl | 91.00 | [45] |
| Tryptophan | Low-alloy steel | Citric acid | 88.32 | [46] |
| L-tryptophan | Low-carbon steel | HCl | 92.70 | [45] |
| Cysteine | Carbon steel | H3PO4 | 93.00 | [47] |
| Microorganisms | ||||
| Glycolipid | Carbon steel | HCl | 87.00 | [26] |
| Marine bacterium | X80 steel | NaCl | 91.16 | [48] |
| Bacillus subtilis | Aluminum 2024 | 90.00 | [49] | |
| Sodium N-dodecyl asparagine (AsS) and sodium N-dodecyl arginine (ArS) | Mild steel alloy | NaCl | 90.00 | [50] |
| Ethane-1,2-diylbis(N,Ndimethyl-N-hexadecylammoniu-macetoxy)dichloride | Mild steel | HCl | 98.00 | [28] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Wang, R.; Zhang, Q.; Zhao, C.; Zhou, X.; Zheng, H.; Zhang, R.; Sun, Y.; Yan, Z. Application of Biomass Corrosion Inhibitors in Metal Corrosion Control: A Review. Molecules 2023, 28, 2832. https://doi.org/10.3390/molecules28062832
Wang Q, Wang R, Zhang Q, Zhao C, Zhou X, Zheng H, Zhang R, Sun Y, Yan Z. Application of Biomass Corrosion Inhibitors in Metal Corrosion Control: A Review. Molecules. 2023; 28(6):2832. https://doi.org/10.3390/molecules28062832
Chicago/Turabian StyleWang, Qihui, Ruozhou Wang, Qi Zhang, Chongkang Zhao, Xing Zhou, Huahao Zheng, Rui Zhang, Yi Sun, and Zhitao Yan. 2023. "Application of Biomass Corrosion Inhibitors in Metal Corrosion Control: A Review" Molecules 28, no. 6: 2832. https://doi.org/10.3390/molecules28062832
APA StyleWang, Q., Wang, R., Zhang, Q., Zhao, C., Zhou, X., Zheng, H., Zhang, R., Sun, Y., & Yan, Z. (2023). Application of Biomass Corrosion Inhibitors in Metal Corrosion Control: A Review. Molecules, 28(6), 2832. https://doi.org/10.3390/molecules28062832







