Synergistic Multisystem Photocatalytic Degradation of Anionic and Cationic Dyes Using Graphitic Phase Carbon Nitride
Abstract
1. Introduction
2. Results and Discussion
2.1. Structural Characterization
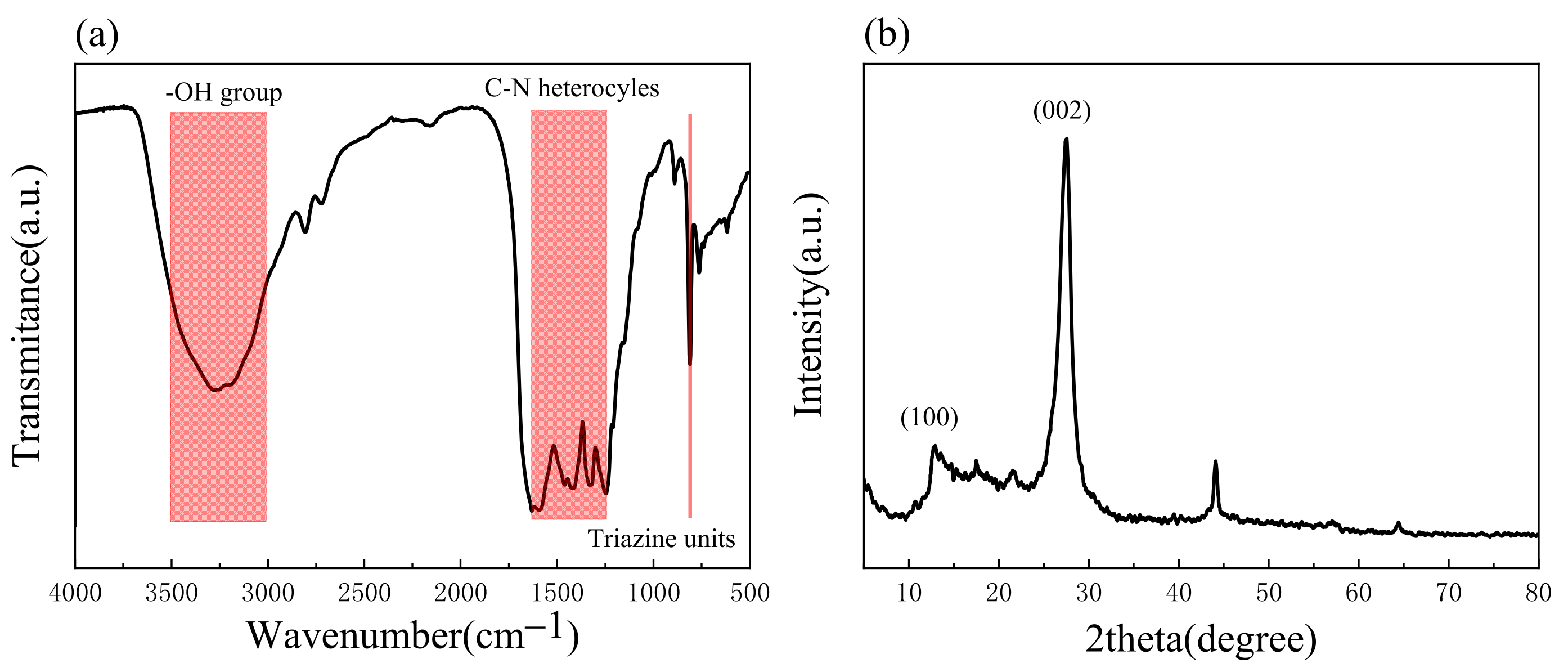
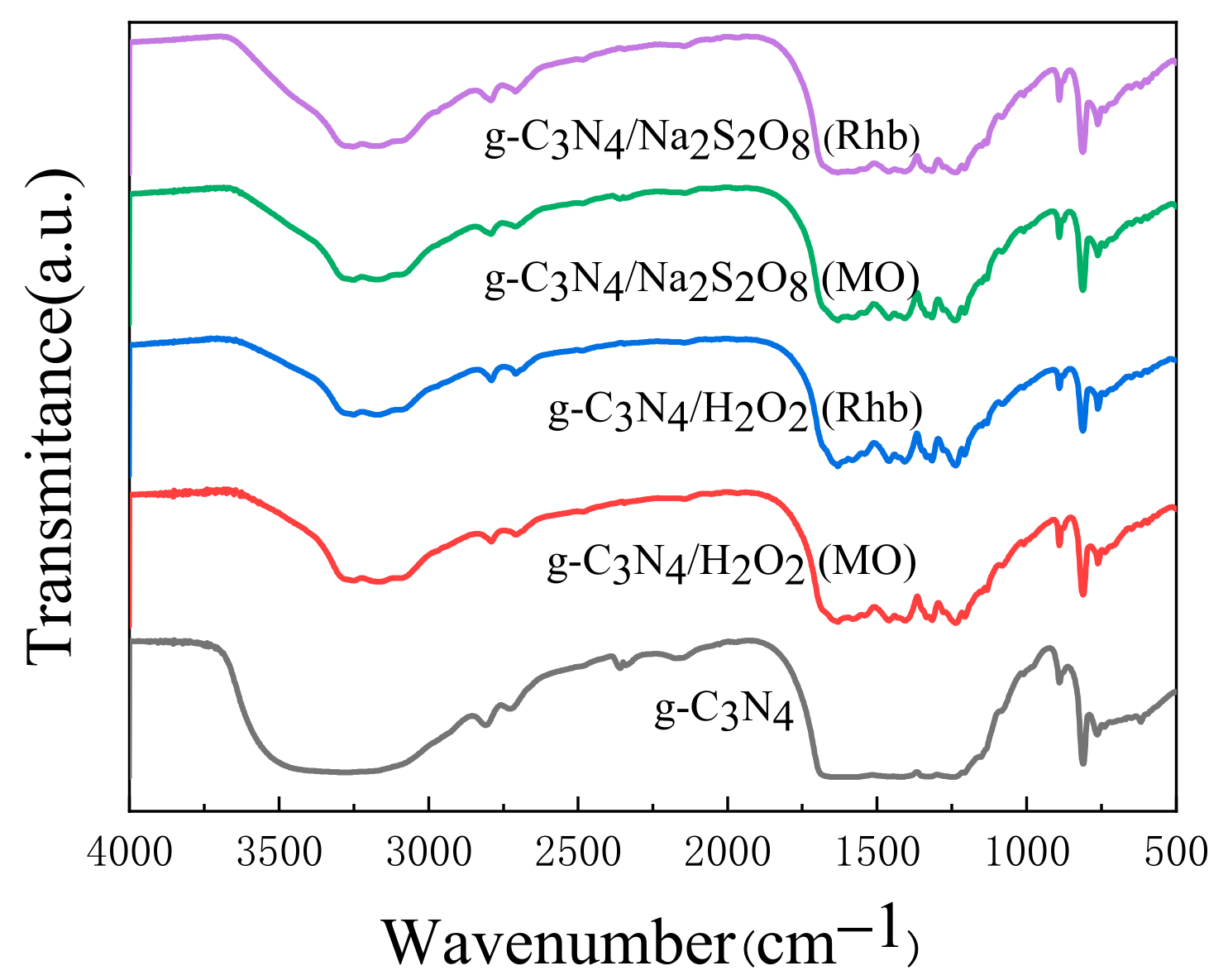
2.1.1. XRD and FTIR Analysis
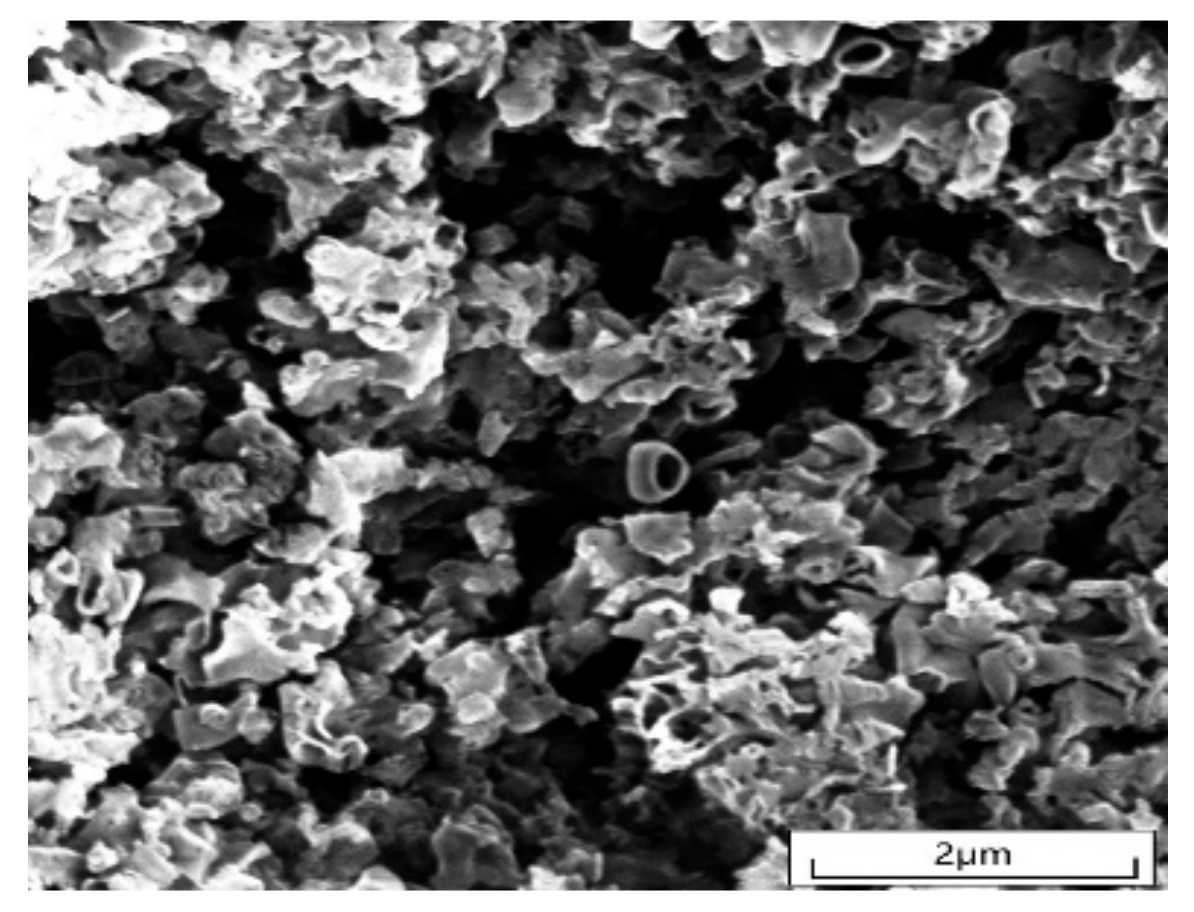
2.1.2. SEM Analysis
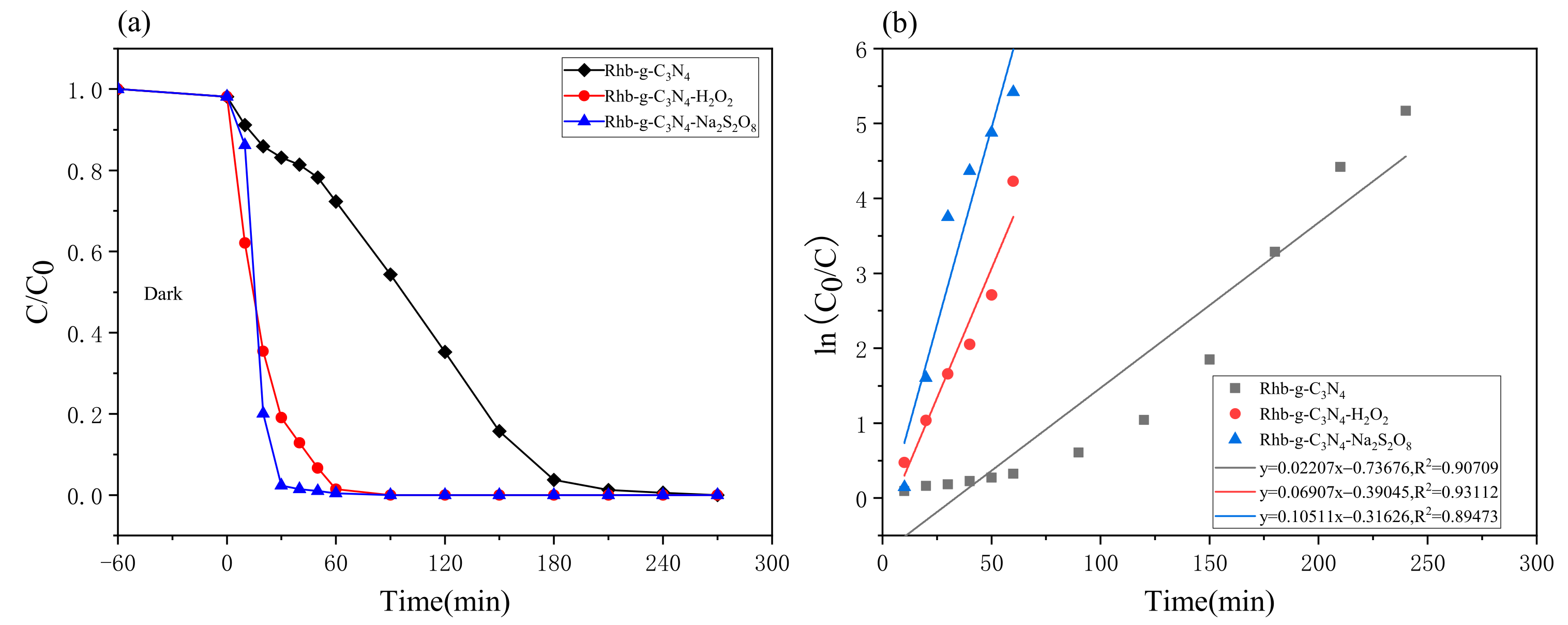
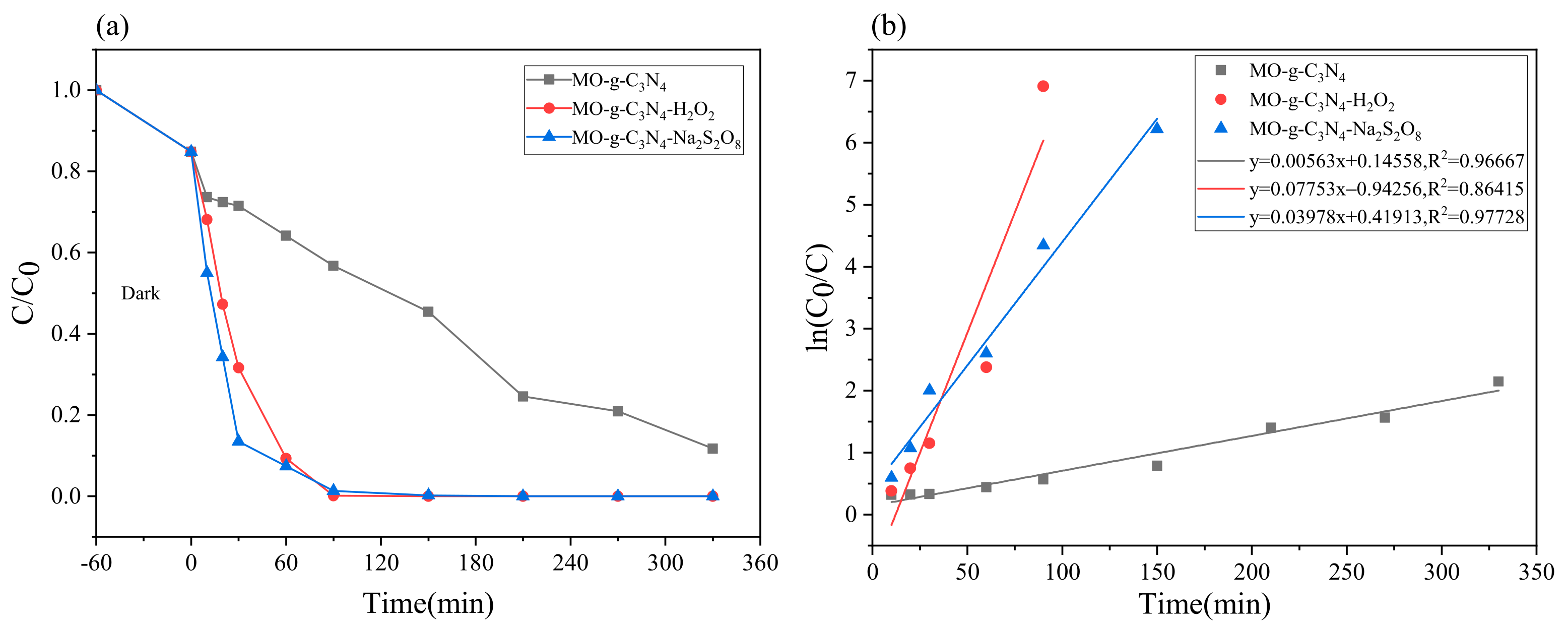
2.2. Evaluation of the Degradation Performance of Dyes
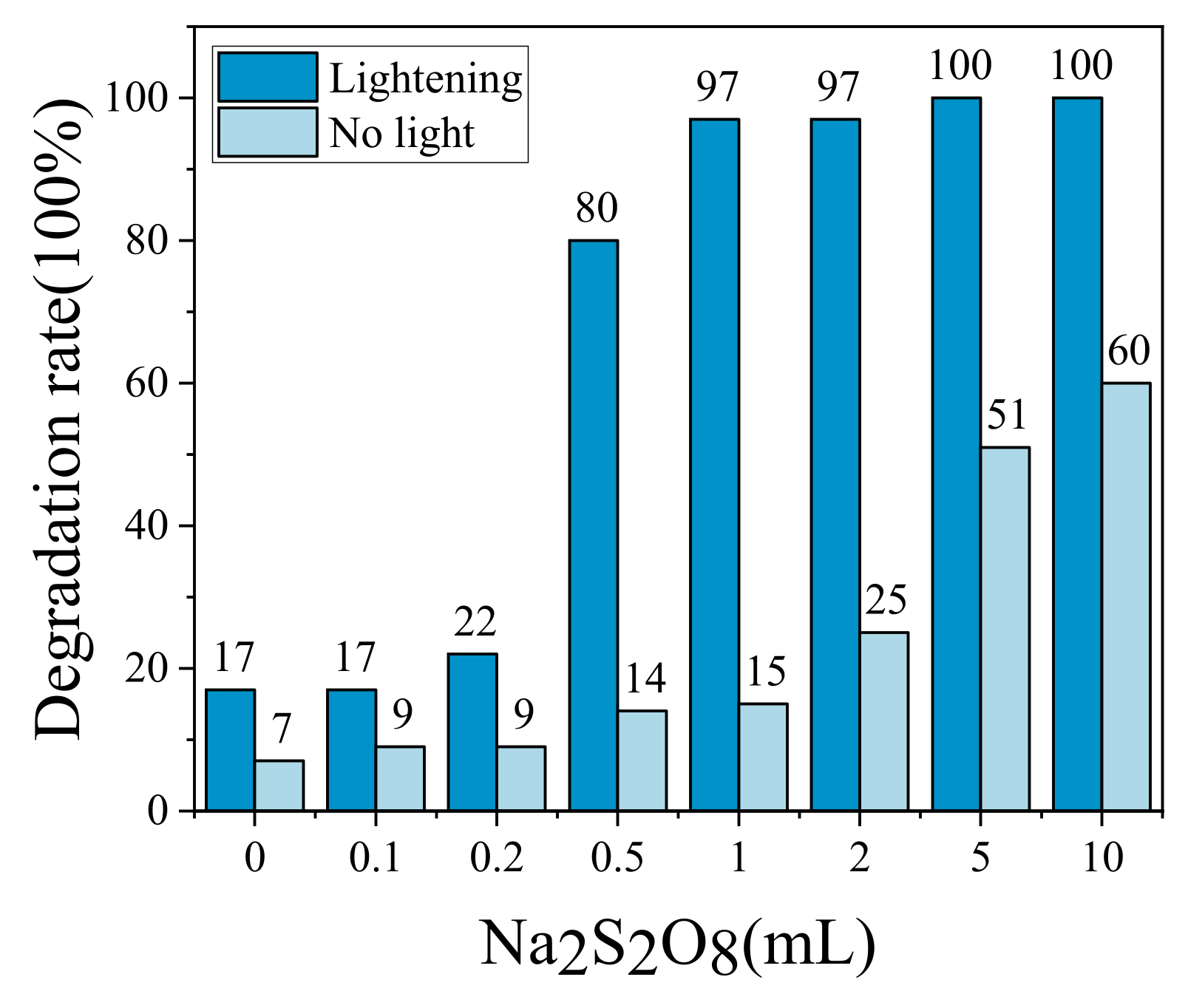
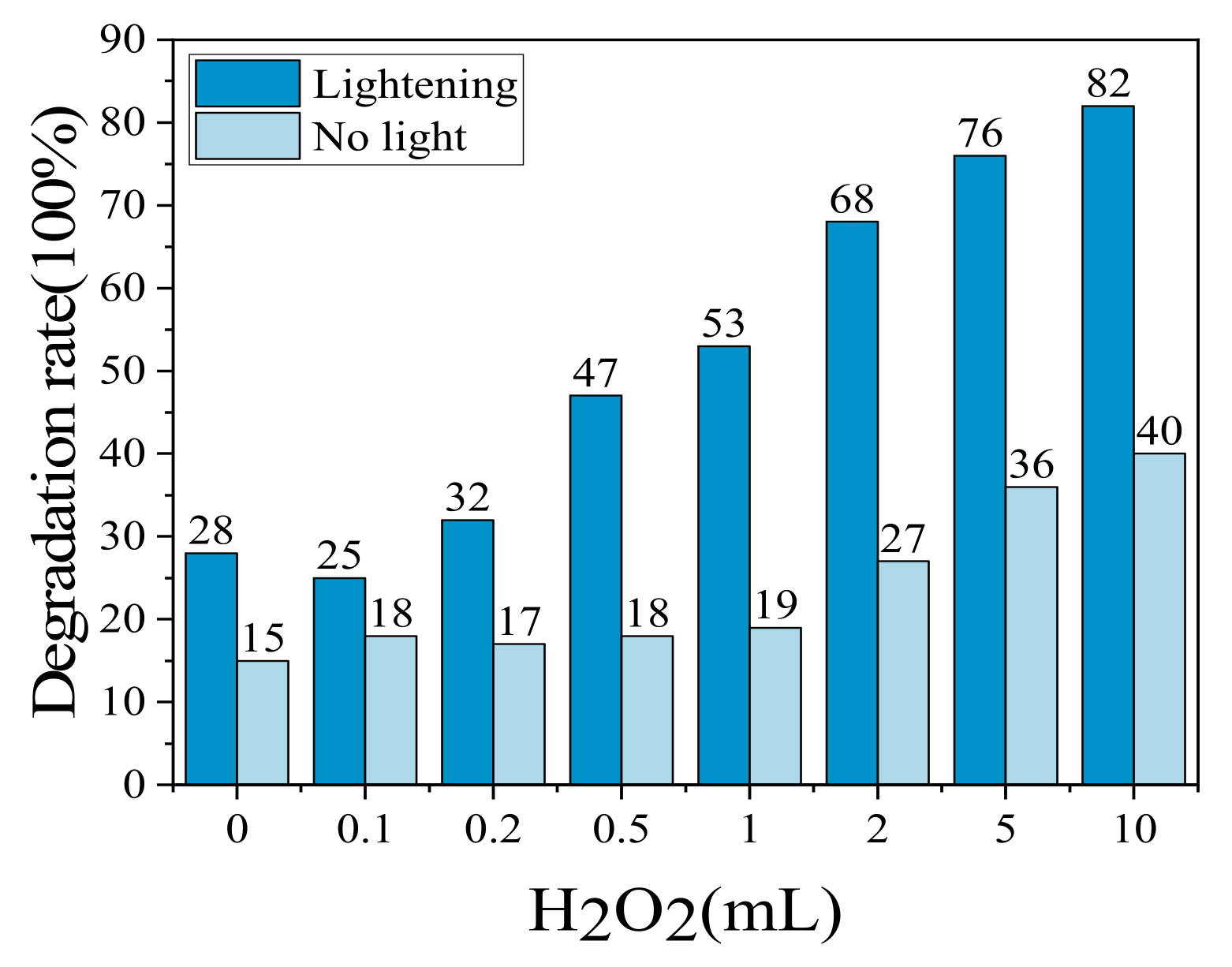
2.3. Effect of Volume Content
2.3.1. Effect of Na2S2O8 Levels on Rhb Degradation
2.3.2. Effect of H2O2 Volume on MO Degradation
2.4. Investigation of the Mechanism of Different Systems
2.4.1. Detection of Zeta Potential
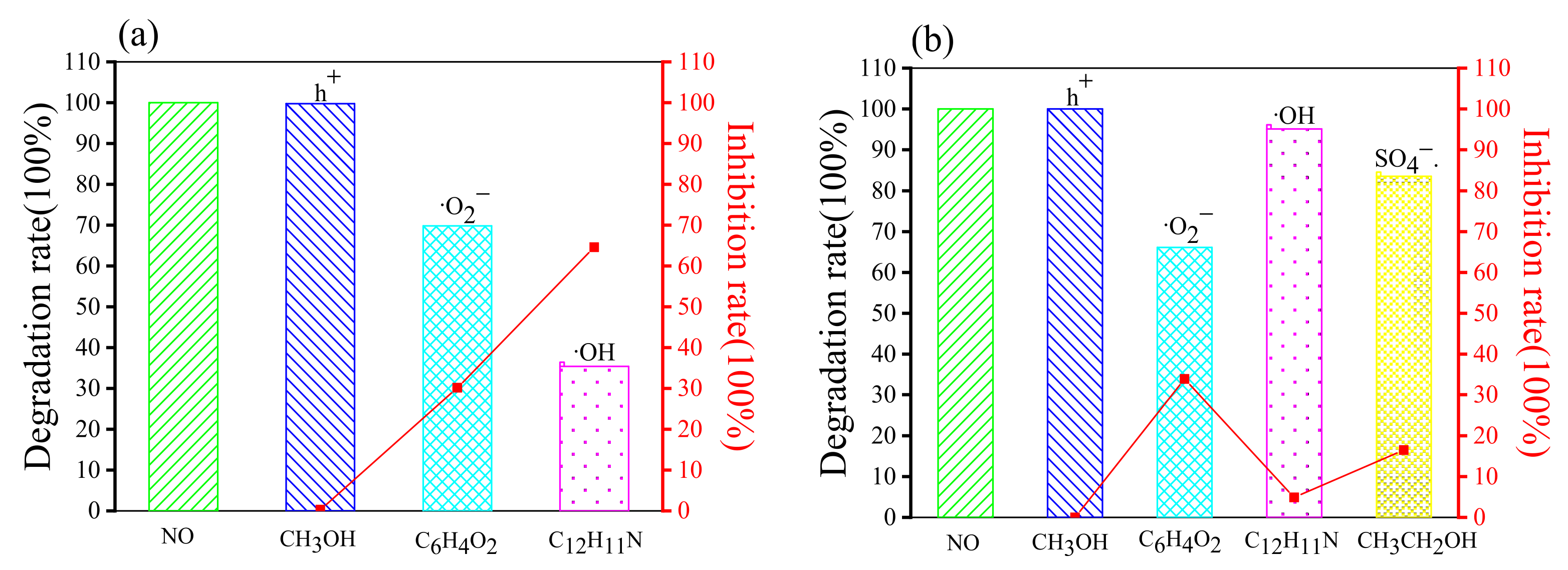
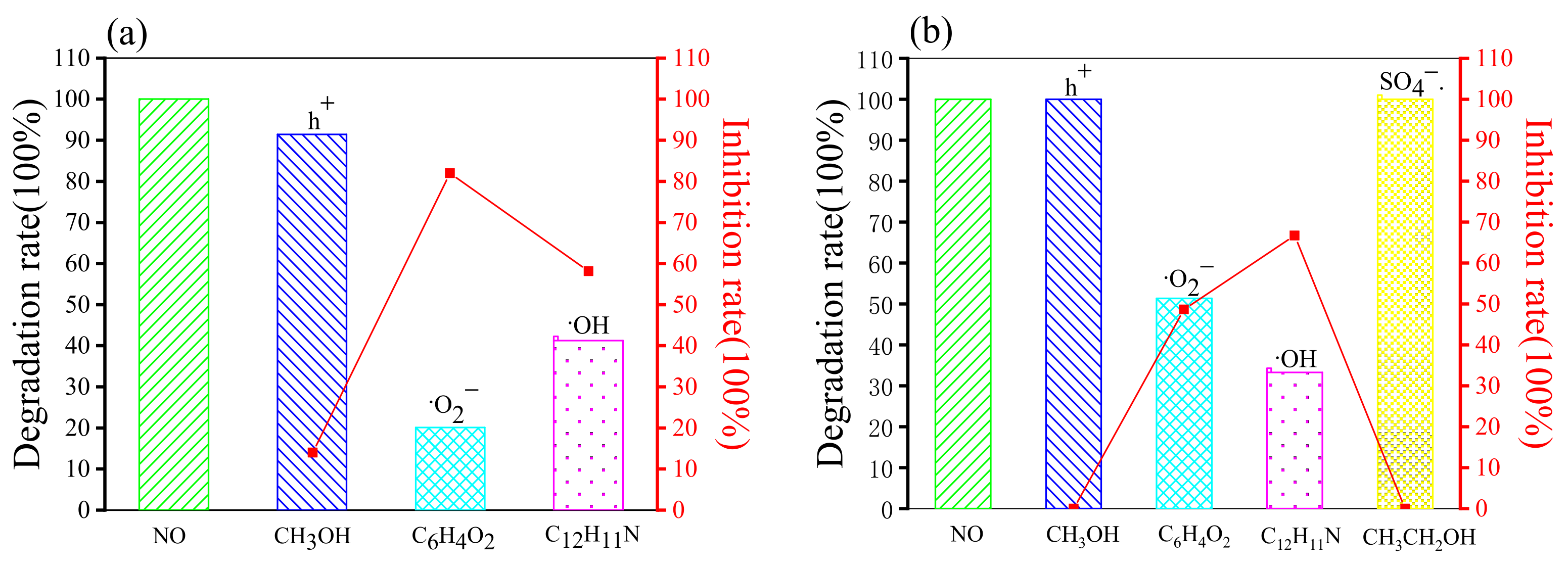
2.4.2. Free Radical Capture Experiments
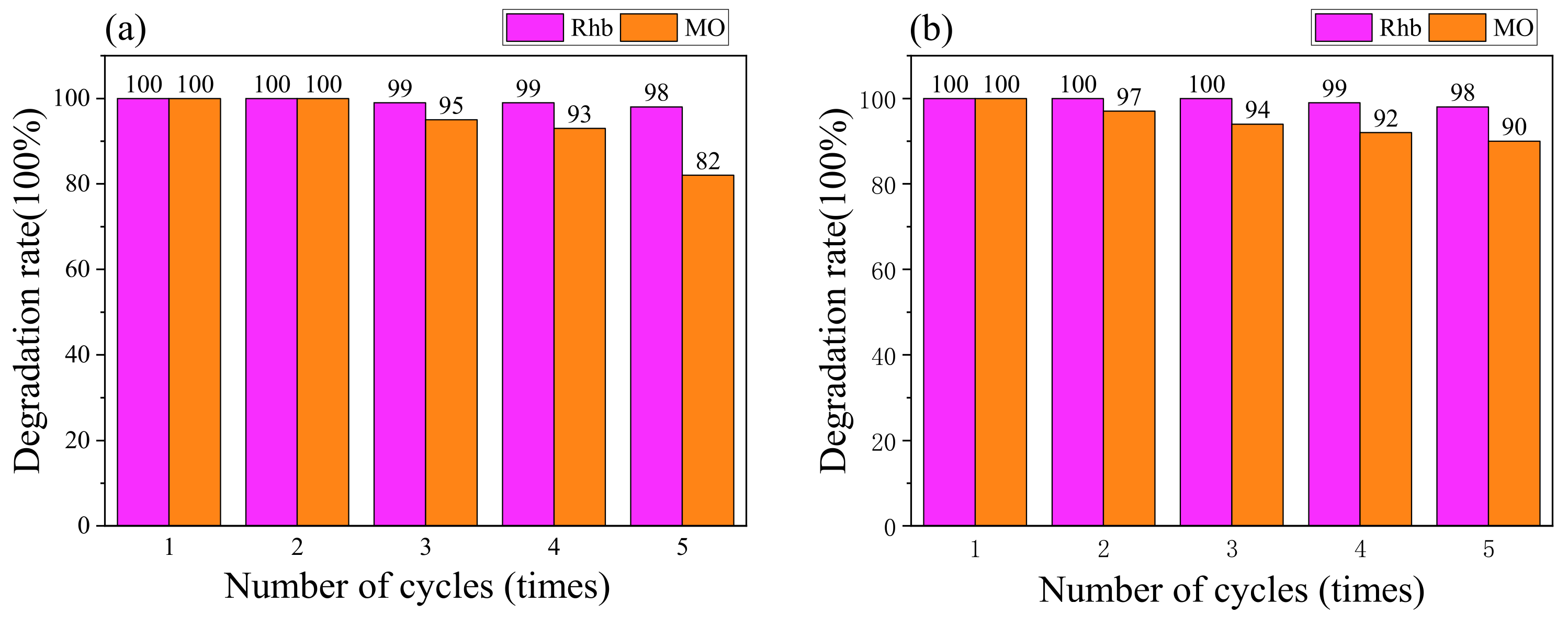
2.4.3. Stability Testing
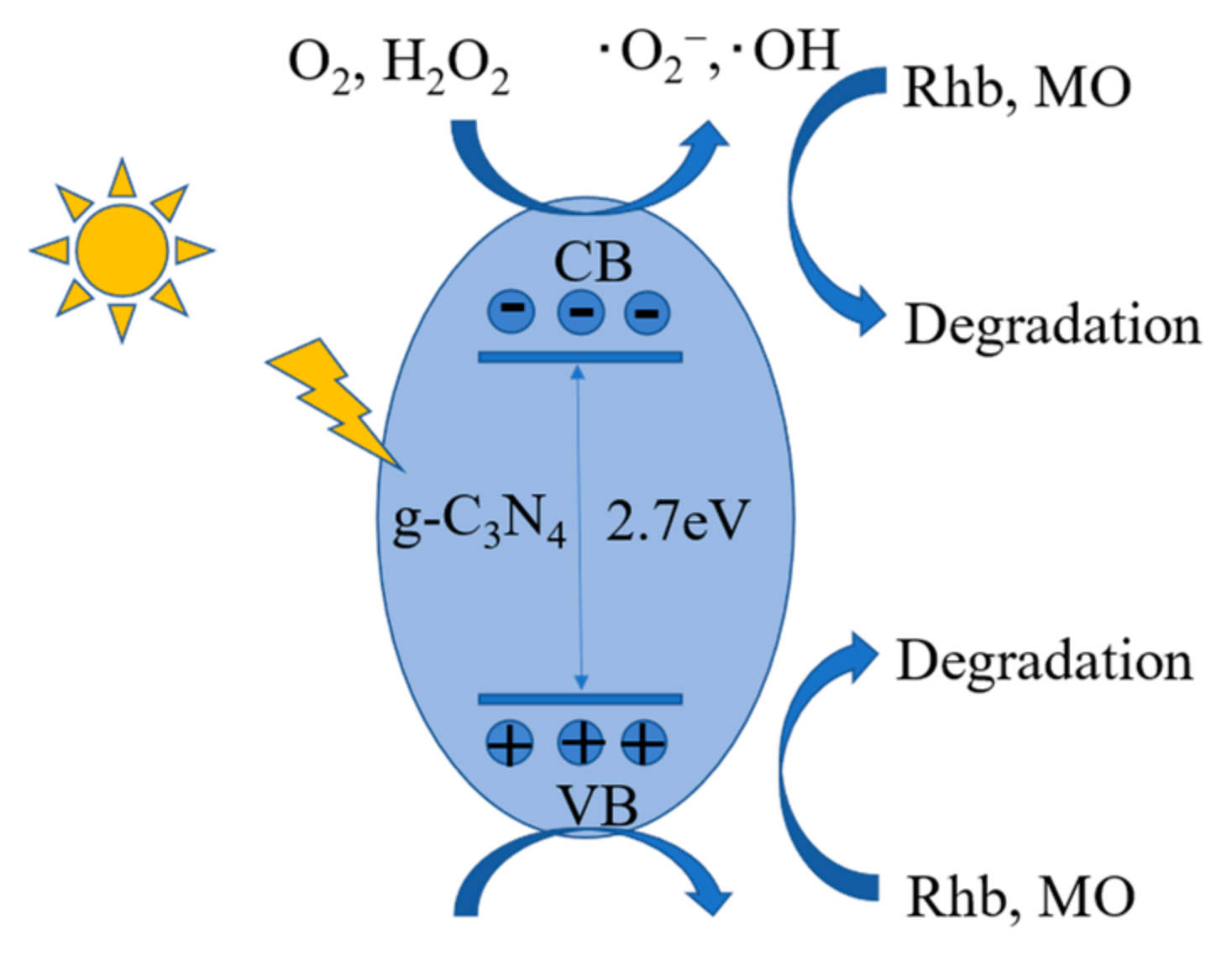
2.4.4. Principle of Photocatalysis
3. Experimental Part
3.1. Reagents and Apparatus
3.2. Preparation of Catalyst
3.3. Structural Characterization and Performance Testing
3.3.1. Structural Characterization
3.3.2. Photocatalytic Performance Testing
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Li, X.; Huang, G.H.; Chen, X.J.; Huang, J.; Li, M.G.; Yin, J.; Liang, Y.; Yao, Y.; Li, Y.P. A review on graphitic carbon nitride (g-C3N4) based hybrid membranes for water and wastewater treatment. Sci. Total Environ. 2021, 792, 148462. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.Y.; Zhang, F.; Li, Y.; Ding, P.H.; Li, P.P.; Xu, H.; Li, H.M. Partial Oxidation of Sn2+ Induced Oxygen Vacancy Overspread on the Surface of SnO2−x/g-C3N4 Composites for Enhanced LED-Light-Driven Photoactivity. J. Inorg. Organomet. Polym. Mater. 2019, 29, 765–775. [Google Scholar] [CrossRef]
- Marimuthu, S.; Antonisamy, A.J.; Malayandi, S.; Rajendran, K.; Tsai, P.C.; Pugazhendhi, A.; Ponnusamy, V.K. Silver nanoparticles in dye effluent treatment: A review on synthesis, treatment methods, mechanisms, photocatalytic degradation, toxic effects and mitigation of toxicity. J. Photochem. Photobiol. B Biol. 2020, 205, 111823. [Google Scholar] [CrossRef] [PubMed]
- Sutar, S.; Patil, P.; Jadhav, J. Recent advances in biochar technology for textile dyes wastewater remediation: A review. Environ. Res. 2022, 209, 112841. [Google Scholar] [CrossRef] [PubMed]
- Ahmaruzzaman, M.; Mishra, S.R. Photocatalytic performance of g-C3N4 based nanocomposites for effective degradation/removal of dyes from water and wastewater. Mater. Res. Bull. 2021, 143, 111417. [Google Scholar] [CrossRef]
- Alias, N.H.; Jaafar, J.; Samitsu, S.; Ismail, A.F.; Mohamed, M.A.; Othman, M.H.D.; Rahman, M.A.; Othman, N.H.; Nor, N.A.M.; Yusof, N.; et al. Mechanistic insight of the formation of visible-light responsive nanosheet graphitic carbon nitride embedded polyacrylonitrile nanofibres for wastewater treatment. J. Water Process. Eng. 2020, 33, 101015. [Google Scholar] [CrossRef]
- Yan, X.; Wang, Y.Y.; Kang, B.B.; Li, Z.; Niu, Y.H. Preparation and Characterization of Tubelike g-C3N4/Ag3PO4 Heterojunction with Enhanced Visible-Light Photocatalytic Activity. Crystals 2021, 11, 1373. [Google Scholar] [CrossRef]
- Guo, R.T.; Wang, J.; Bi, Z.X.; Chen, X.; Hu, X.; Pan, W.G. Recent advances and perspectives of g–C3N4–based materials for photocatalytic dyes degradation. Chemosphere 2022, 295, 133834. [Google Scholar] [CrossRef]
- Liu, J.B.; Bao, Y.; Zheng, W.T. Analyses of some structural properties on a class of hierarchical scale-free networks. Fractals 2022, 30, 2250136. [Google Scholar] [CrossRef]
- Anwer, H.; Mahmood, A.; Lee, J.; Kim, K.H.; Park, J.W.; Yip, A.C.K. Photocatalysts for degradation of dyes in industrial effluents: Opportunities and challenges. Nano Res. 2019, 12, 955–972. [Google Scholar] [CrossRef]
- Shabir, M.; Yasin, M.; Hussain, M.; Shafiq, I.; Akhter, P.; Nizami, A.S.; Jeon, B.H.; Park, Y.K. A review on recent advances in the treatment of dye-polluted wastewater. J. Ind. Eng. Chem. 2022, 112, 1–19. [Google Scholar] [CrossRef]
- Liu, J.B.; Bao, Y.; Zheng, W.T.; Hayat, S. Network coherence analysis on a family of nested weighted n-polygon networks. Fractals 2021, 29, 2150260. [Google Scholar] [CrossRef]
- Singh, P.; Mohan, B.; Madaan, V.; Ranga, R.; Kumari, P.; Kumar, S.; Bhankar, V.; Kumar, P.; Kumar, K. Nanomaterials photocatalytic activities for waste water treatment: A review. Environ. Sci. Pollut. Res. 2022, 29, 69294–69326. [Google Scholar] [CrossRef]
- Qamar, M.A.; Shahid, S.; Javed, M.; Shariq, M.; Fadhali, M.M.; Madkhali, O.; Ali, S.K.; Syed, I.S.; Awaji, M.Y.; Khan, M.S.; et al. Accelerated Decoloration of Organic Dyes from Wastewater Using Ternary Metal/g-C3N4/ZnO Nanocomposites: An Investigation of Impact of g-C3N4 Concentration and Ni and Mn Doping. Catalysts 2022, 12, 1388. [Google Scholar] [CrossRef]
- Vignesh, S.; Eniya, P.; Srinivasan, M.; Sundar, J.K.; Li, H.T.; Jayavel, S.; Pandiaraman, M.; Manthrammel, M.A.; Shkir, M.; Palanivel, B. Fabrication of Ag/Ag2O incorporated graphitic carbon nitride based ZnO nanocomposite for enhanced Z-scheme photocatalytic performance of various organic pollutants and bacterial disinfection. J. Environ. Chem. Eng. 2021, 9, 105996. [Google Scholar] [CrossRef]
- Liu, J.B.; Zhao, J.; Min, J.; Cao, J. The Hosoya index of graphs formed by a fractal graph. Fractals 2019, 27, 1950135. [Google Scholar] [CrossRef]
- Song, J.H.; Zhao, K.; Yin, X.B.; Liu, Y.; Khan, I.; Liu, S.Y. Boosting the visible-light activity of ZrO2/g-C3N4 by controlling the crystal structure of ZrO2. J. Mater. Res. Technol. 2021, 36, 3086–3095. [Google Scholar]
- Lv, K.; Zhang, Q.Z. Nano-TiO2 photocatalytic technology and atmospheric pollution control. China Environ. Sci. 2018, 38, 852–861. [Google Scholar]
- Zeng, P.; Wang, Q.Y.; Zhang, H.J.; Wang, J.Q.; Chen, X.Y. Application of Photocatalysis techniques on treatment of wastewater with medium and low concentration of ammonium nitrogen. Sci. China Chem. 2019, 4, 78–82. [Google Scholar]
- Li, Y.J.; Cao, T.P.; Sun, D.W. A bismuth-rich Bi4O5Br2/TiO2 composites fibers photocatalyst enables dramatic CO2 reduction activity. Acta Mater. Compos. Sin. 2023, 41, 1–10. [Google Scholar]
- Meng, A.Y.; Zhang, L.Y.; Cheng, B.; Yu, J.G. Dual Cocatalysts in TiO2 Photocatalysis. Adv. Mater. 2019, 31, 1807660. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.F.; Yuan, J.Y.; Shen, M.; Du, B.; Xing, R. Synthesis and Photocatalytic Performance of ZnO Micro/Nano Materials Induced by Amphiphilic Calixarene. Chin. J. Inorg. Chem. 2022, 38, 261–273. [Google Scholar]
- Dong, J.Q.; Zhang, Y.; Hussain, M.I.; Zhou, W.J.; Chen, Y.Z.; Wang, L.N. g-C3N4: Properties, Pore Modifications, and Photocatalytic Applications. Nanomaterials 2022, 12, 121. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Wang, Z.; Li, Y.H.; Li, R.Q.; Li, X.Y.; Wang, K.Y. Establishing WO3/g-C3N4 Composite for “Memory” Photocatalytic Activity and Enhancement in Photocatalytic Degradation. Catal. Lett. 2019, 149, 1167–1173. [Google Scholar] [CrossRef]
- Tan, L.; Yu, C.F.; Wang, M.; Zhang, S.Y.; Sun, J.Y.; Dong, S.Y.; Sun, J.H. Synergistic effect of adsorption and photocatalysis of 3D g-C3N4-agar hybrid aerogels. Appl. Surf. Sci. 2019, 467, 286–292. [Google Scholar] [CrossRef]
- Palanivel, B.; Lallimathi, M.; Arjunkumar, B.; Shkir, M.; Alshahrani, T.; Al-Namshah, K.S.; Hamdy, M.S.; Shanavas, S.; Venkatachalam, M.; Ramalingam, G. rGO supported g-C3N4/CoFe2O4 heterojunction: Visible-light-active photocatalyst for effective utilization of H2O2 to organic pollutant degradation and OH radicals production. J. Environ. Chem. Eng. 2021, 9, 104698. [Google Scholar] [CrossRef]
- Guo, Y.G.; Sun, X.H.; Chen, Q.Q.; Liu, Y.J.; Lou, X.Y.; Zhang, L.; Zhang, X.J.; Li, Y.S.; Guan, J. Photocatalytic Applications of g-C3N4 Based on Bibliometric Analysis. Catalysts 2022, 12, 1017. [Google Scholar] [CrossRef]
- Ismael, M. A review on graphitic carbon nitride (g-C3N4) based nanocomposites: Synthesis, categories, and their application in photocatalysis. J. Alloys Compd. 2020, 846, 156446. [Google Scholar] [CrossRef]
- Saeed, M.; Muneer, M.; Haq, A.U.; Akram, N. Photocatalysis: An effective tool for photodegradation of dyes—A review. Environ. Sci. Pollut. Res. 2022, 29, 293–311. [Google Scholar] [CrossRef]
- Zhou, Y.M.; Elchalakani, M.; Liu, H.F.; Briseghella, B.; Sun, C.Z. Photocatalytic concrete for degrading organic dyes in water. Environ. Sci. Pollut. Res. 2022, 29, 39027–39040. [Google Scholar] [CrossRef]
- Pandey, S.K.; Mishra, P.K.; Tiwary, D. Enhanced photocatalytic performance of NiS/ZnO nanocomposite for the remediation of PNP and RhB dye. J. Environ. Chem. Eng. 2022, 10, 107459. [Google Scholar] [CrossRef]
- Ogata, F.; Yasuda, S.; Saenjum, C.; Nakamura, T.; Kawasaki, N. Interactions of Cationic and Anionic Dyes with Activated Carbons. e-J. Surf. Sci. Nanotechnol. 2020, 18, 269–274. [Google Scholar] [CrossRef]
- Asaithambi, S.; Sakthivel, P.; Karuppaiah, M.; Balaji, V.; Yuvakkumar, R.; Ravi, G. Visible light induced photocatalytic performance of Mn-SnO2@ZnO nanocomposite for high efficient cationic dye degradation. J. Mater. Sci. Mater. Electron. 2021, 32, 22168–22186. [Google Scholar] [CrossRef]
- Maruthupandy, M.; Muneeswaran, T.; Vennila, T.; Vaishali, C.V.; Anand, M.; Cho, W.S.; Quero, F. Photocatalytic efficiency of graphene/nickel oxide nanocomposites towards the degradation of anionic and cationic dye molecules under visible light. J. Photochem. Photobiol. A 2022, 427, 113819. [Google Scholar] [CrossRef]
- Ivanenko, I.; Hutsul, K.; Krymets, G. The precipitation synthesis of zinc (II) oxide for photocatalytic degradation of anionic and cationic dyes. Appl. Nanosci. 2022, 12, 755–759. [Google Scholar] [CrossRef]
- Gupta, T.; Chauhan, R.P. Enhanced photocatalytic degradation of cationic dye using Cu-doped ZnSe. Opt. Mater. 2023, 135, 113295. [Google Scholar] [CrossRef]
- Lu, E.J.; Tao, J.Q.; Yang, C.; Hou, Y.D.; Zhang, J.S.; Wang, X.C.; Fu, X.Z. Carbon-Encapsulated Pd/TiO2 for Photocatalytic H2 Evolution Integrated with Photodehydrogenative Coupling of Amines to Imines. Acta Phys. Chim. Sin. 2023, 39, 2211029. [Google Scholar] [CrossRef]
- Tan, F.X.; Chen, Y.K.; Wang, R.; Luo, C.W.; Wu, D.J.; Yang, L.B.; Zhang, Z. Performance and mechanism of g-C3N4/PDS for photocatalytic degradation of atrazine. China Water Wastewater 2023, 39, 91–98. [Google Scholar]
- Qu, Y.H.; Chen, Z.Y.; Duan, Y.K.; Liu, L.F. H2O2 assisted photocatalysis over Fe-MOF modified BiOBr for degradation of RhB. J. Chem. Technol. Biotechnol. 2022, 97, 2881–2888. [Google Scholar] [CrossRef]
- Su, H.L.; Christodoulatos, C.; Smolinski, B.; Arienti, P.; O’Connor, G.; Meng, X.G. Advanced Oxidation Process for DNAN Using UV/H2O2. Engineering 2019, 5, 849–854. [Google Scholar] [CrossRef]
- Liu, J.F.; Ran, Z.Z.; Cao, Q.Y.; Ji, S.F. Preparation of MIL-88B (Fex, Co1−x) catalysts and their application in one-step liquid-phase methanol oxidation to methyl formate using H2O2. Chin. J. Catal. 2021, 42, 2254–2264. [Google Scholar] [CrossRef]
- Shvalagin, V.V.; Kompanets, M.O.; Kutsenko, S.; Kuchmy, S.Y.; Skoryk, M.A. Photocatalytic Activity of g-C3N4 in the Partial Oxidation of Benzyl Alcohol Under Visible Light. Theor. Exp. Chem. 2020, 56, 111–116. [Google Scholar] [CrossRef]
- Yu, Y.T.; Huang, H.W. Coupled adsorption and photocatalysis of g-C3N4 based composites: Material synthesis, mechanism, and environmental applications. Chem. Eng. J. 2023, 453, 139755. [Google Scholar] [CrossRef]
- Liu, H.J.; Zhang, H.; Chen, M.; Zhang, C.N.; Du, C.W.; Peng, J.B.; Jiang, K. Peroxymonosulfate-assisted photocatalysis with g-C3N4/BiOCOOH nanocomposites for the synergistic removal of organic pollutants. J. Water Process. Eng. 2020, 38, 101580. [Google Scholar] [CrossRef]
- Lin, M.X.; Li, F.Y.; Wang, W.; Cheng, W.Y.; Zhou, C.L. Synthesis of biochar-supported P-doped g-C3N4 photocatalyst and its photocatalytic degradation mechanism to naphthalene. Huanjing Kexue Xuebao 2021, 41, 3200–3210. [Google Scholar]
- Ke, P.; Zeng, D.L.; Cui, J.W.; Li, X.; Chen, Y. Improvement in Structure and Visible Light Catalytic Performance of g-C3N4 Fabricated at a Higher Temperature. Catalysts 2022, 12, 247. [Google Scholar] [CrossRef]
- Qi, S.Y.; Liu, X.T.; Zhang, R.Y.; Zhang, Y.M.; Xu, H.Y. Preparation and photocatalytic properties of g-C3N4/BiOCl heterojunction. Inorg. Chem. Commun. 2021, 133, 108907. [Google Scholar] [CrossRef]
- Zhang, S.W.; Gao, H.H.; Liu, X.; Huang, Y.S.; Xu, X.J.; Alharbi, N.S.; Hayat, T.; Li, J.X. Hybrid 0D-2D Nanoheterostructures: In Situ Growth of Amorphous Silver Silicates Dots on g-C3N4 Nanosheets for Full-Spectrum Photocatalysis. ACS Appl. Mater. Interfaces 2020, 12, 3274. [Google Scholar] [CrossRef]
- Yang, H.H.; Wang, L.G.; Shuang Xu, S.; Cao, Y.; He, P.; Chen, J.Q.; Zheng, Z.; Li, H.Q. Green and selective hydrogenation of aromatic diamines over the nanosheet Ru/g-C3N4-H2 catalyst prepared by ultrasonic assisted impregnation-deposition method. Green Energy Environ. 2022, 77, 1361–1376. [Google Scholar] [CrossRef]
- Yao, G.Y.; Liu, Y.Q.; Liu, J.C.; Xu, Y. Facile Synthesis of Porous g-C3N4 with Enhanced Visible-Light Photoactivity. Molecules 2022, 27, 1754. [Google Scholar] [CrossRef]
- Liu, Y.F.; Ma, Z. TiOF2/g-C3N4 composite for visible-light driven photocatalysis. Colloids Surf. A Physicochem. Eng. Asp. 2021, 618, 126471. [Google Scholar] [CrossRef]
- Chen, Y.H.; Wang, B.K.; Hou, W.C. Graphitic carbon nitride embedded with graphene materials towards photocatalysis of bisphenol A: The role of graphene and mediation of superoxide and singlet oxygen. Chemosphere 2021, 278, 130334. [Google Scholar] [CrossRef]
- Ren, W.W.; Yang, J.K.; Zhang, J.X.; Li, W.; Sun, C.Y.; Zhao, H.L.; Wen, Y.T.; Sha, O.; Liang, B. Recent progress in SnO2/g-C3N4 heterojunction photocatalysts: Synthesis, modification, and application. J. Alloys Compd. 2022, 906, 164372. [Google Scholar] [CrossRef]
- Kumar, N.; Jung, U.J.; Jung, B.M.M.; Park, J.; Naushad, M. Zinc hydroxystannate/zinc-tin oxide heterojunctions for the UVC-assisted photocatalytic degradation of methyl orange and tetracycline. Environ. Pollut. 2023, 316, 120353. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, H.; Li, H.P.; Cai, T.F. Photocatalytic degradation of dyes by sulfur- and chlorine-co-doped g-C3N4 nanosheets. China Environ. Sci. 2021, 41, 4662–4669. [Google Scholar]
- Li, Z.Q.; Chen, X.W.; Wang, Y.; Cheng, T.; Huang, T.; Dong, P.Y.; Wang, W.Y.; Zhang, B.B.; Xi, X.G. Preparation of CeTiO4/g-C3N4 Composite with Efficient Photocatalytic Activity for Dye-Degradation. Chin. J. Inorg. Chem. 2022, 38, 53–62. [Google Scholar]
- Zhao, W.Y.; Yi, F.T.; Gan, H.H.; Zhang, H.N.; Qing, Y.X.; Jin, H.X.; Zhang, K.F. Synergistic Photocatalytic Dye Oxidation and Hexavalent Chromium Reduction by Chlorine Doped g-C3N4 Nanosheets. Mater. Rep. 2019, 33, 3377–3382. [Google Scholar]
- Cao, X.Y.; Tang, X.N.; Ma, H.; Chen, Y.Y. Study on TiO2@SiO2 Photocatalytic Degradation of Different Dyes and Its Mechanism. Rare Met. Mater. Eng. 2022, 51, 3001–3012. [Google Scholar]
- Yuan, H.; Shang, P.; Yang, J.; Huang, Q.; Song, L.; Jiang, X.F. Anion-Directed Self-Assembly of Calix [4] arene-Based Silver(I) Coordination Polymers and Photocatalytic Degradation of Organic Pollutants. Inorg. Chem. 2023, 62, 2652–2662. [Google Scholar] [CrossRef]
- Liu, F.; Nguyen, T.; Wang, Q.; Massuyeau, F.; Dan, Y.; Jiang, L. Construction of Z-scheme g-C3N4/Ag/P3HT heterojunction for enhanced visible-light photocatalytic degradation of tetracycline (TC) and methyl orange (MO). Appl. Surf. Sci. 2019, 496, 143653. [Google Scholar] [CrossRef]
- Song, Y.H.; Dai, W.L.; Xu, H.; Zhao, J.Z. Preparation and Photocatalytic Properties of g-C3N4/Bi12O17Cl2 Composites. Chin. J. Mater. Res. 2021, 35, 911–917. [Google Scholar]
- Ying, L.W.; Liu, Y.; Dong, W.B.; Yuan, H.X.; Ma, L.M. Effect of bromide ions on the UV/H2O2 oxidation of alanine in aqueous solutions. Chem. Eng. J. 2016, 285, 172–179. [Google Scholar] [CrossRef]
- Xu, H.; Ye, Q.; Wang, Q.G.; Zhou, P.; Huo, X.W.; Wang, Y.Q.; Huang, X.; Zhou, G.Y.; Zhang, J. Enhancement of organic contaminants degradation at low dosages of Fe (III) and H2O2 in g-C3N4 promoted Fe (III)/H2O2 system under visible light irradiation. Sep. Purif. Technol. 2020, 251, 117333. [Google Scholar] [CrossRef]
- Zhang, L.; Lin, Z.Y.; Zhang, W.J. Degradation of dyeing wastewater containing Rhodamine B by CuO-activated Persulfate with UV strengthening. Environ. Sci. Technol. 2020, 43, 82–89. [Google Scholar]
- Yang, L.H.; Ren, X.C.; Zhang, Y.J.; Chen, Z.Y. Heterogeneous activation of peroxymonosulfate by Cu+-decorated g-C3N4 under sunlight for degradation of organic pollutants. J. Environ. Chem. Eng. 2021, 9, 106596. [Google Scholar] [CrossRef]
- Zhang, Y.Q.; Li, L.F.; Zhou, F.; Yuan, X.Z.; Hu, S.Z.; Zhang, J. Zr-doped g-C3N4 photocatalytic degradation of organic pollutants. Fine Chem. 2022, 39, 2112–2121. [Google Scholar]
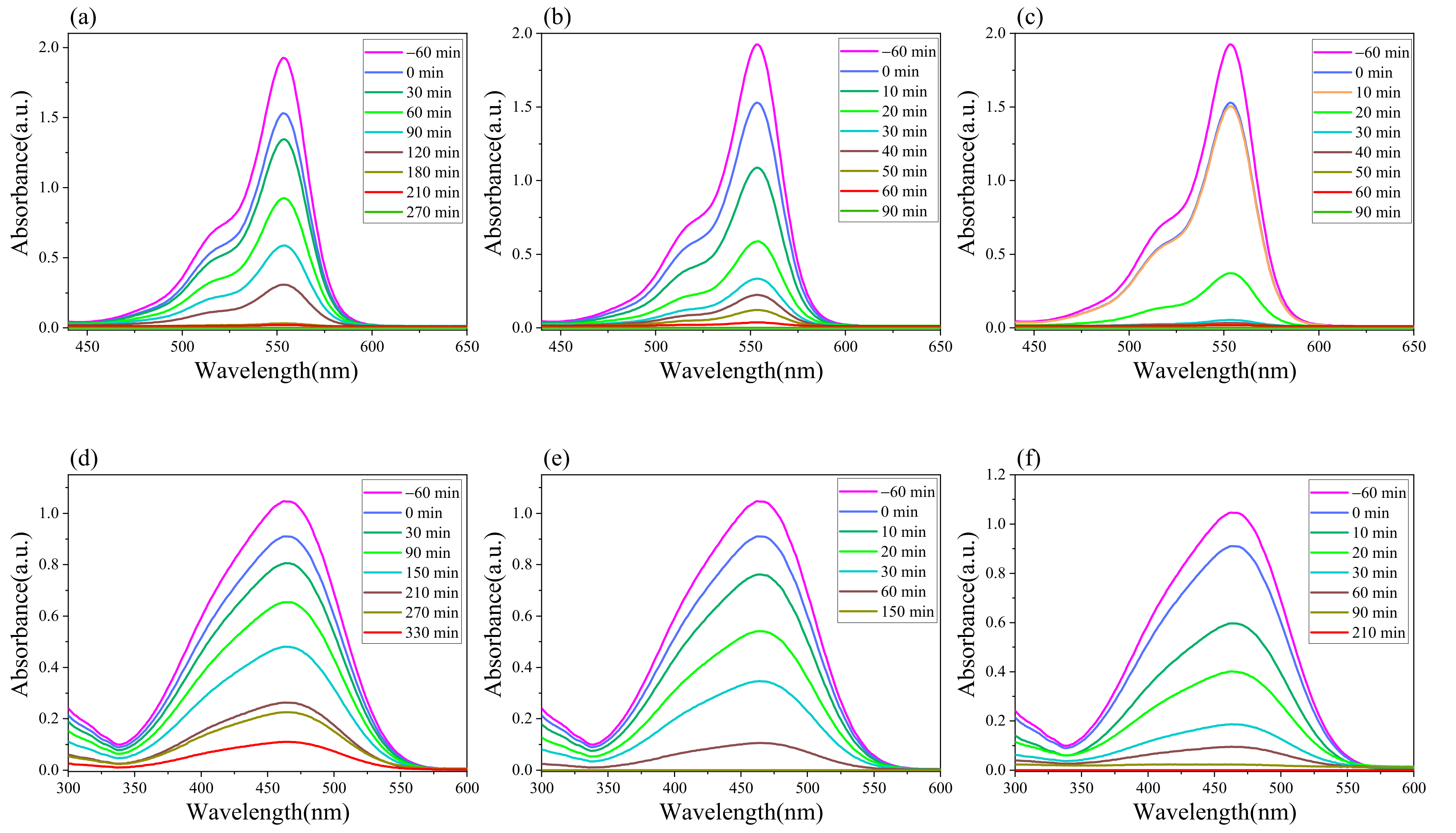
| Time (min) | Degradation Rate of g-C3N4/H2O2 (100%) | Degradation Rate of g-C3N4/Na2S2O8 (100%) |
|---|---|---|
| −60 | 0 | 0 |
| 0 | 1.84 | 1.84 |
| 10 | 43.57 | 13.81 |
| 20 | 64.60 | 79.92 |
| 30 | 80.94 | 97.66 |
| 40 | 87.14 | 98.73 |
| 50 | 93.35 | 99.24 |
| 60 | 98.54 | 99.56 |
| 90 | 100 | 100 |
| Time (min) | Degradation Rate of g-C3N4/H2O2 (100%) | Degradation Rate of g-C3N4/Na2S2O8 (100%) |
|---|---|---|
| −60 | 0 | 0 |
| 0 | 15.18 | 15.18 |
| 10 | 31.87 | 44.96 |
| 20 | 52.75 | 65.73 |
| 30 | 68.33 | 86.51 |
| 60 | 90.71 | 92.61 |
| 90 | 99.90 | 98.70 |
| 150 | 100 | 99.80 |
| 210 | 100 | 100 |
| Catalyst | Quality (mg) | Contaminants | Concentration (mg/L) | Volume (mL) | Time (h) | Degradation Rate (100%) | Speed (min−1) |
|---|---|---|---|---|---|---|---|
| ZHS/ZTO | 50 | MO | 10 | 200 | 2 | 68.45 | - |
| Sulfur/chlorine/g-C3N4 | 50 | Rhb | 10 | 50 | - | - | 0.01683 |
| CeTiO4/g-C3N4 | 100 | Rhb | 10 | 100 | 2.4 | 95.7 | 0.0202 |
| Chlorine/g-C3N4 | 50 | Rhb | 10 | 100 | - | - | 0.049 |
| TiO2@SiO2 | 100 | Rhb | 20 | 100 | 12 | 86 | - |
| TiO2@SiO2 | 100 | MO | 20 | 100 | 12 | 38 | - |
| {[Ag2(mu-NO3) L1]} n | 10 | Rhb | 49.6 | 20 | 6 | 85.2 | 0.00747 |
| {[Ag2(mu-NO3) L1]} n | 10 | MO | 18.68 | 20 | 6 | 70.6 | 0.00354 |
| This study (g-C3N4) | 100 | Rhb | 50 | 150 | 1.5 | 100 | 0.06907 0.10511 |
| This study (g-C3N4) | 50 | MO | 20 | 100 | 2.5 3.5 | 100 | 0.07753 0.03978 |
| Materials | Zeta Potential (mV) | Materials | Zeta Potential (mV) |
|---|---|---|---|
| g-C3N4 | −14.4 | g-C3N4 (MO) | −19.6 |
| g-C3N4 (Rhb) | −27 | g-C3N4 (MO, H2O2) | −12.6 |
| g-C3N4 (Rhb, H2O2) | −20.3 | g-C3N4 (MO, Na2S2O8) | −29.1 |
| g-C3N4 (Rhb, Na2S2O8) | −27.5 |
| Degradation Rate (100%) | Inhibition Rate (100%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No | h+ | ·O2− | ·OH | SO4−· | No | h+ | ·O2− | ·OH | SO4−· | |
| Rhb (g-C3N4/H2O2) | 100 | 99.75 | 69.85 | 35.4 | - | 0 | 0.25 | 30.15 | 64.6 | - |
| Rhb (g-C3N4/Na2S2O8) | 100 | 100 | 66.12 | 95.12 | 83.53 | 0 | 0 | 33.88 | 4.88 | 16.47 |
| MO (g-C3N4/H2O2) | 100 | 91.41 | 20.08 | 41.26 | - | 0 | 8.59 | 79.92 | 58.74 | - |
| MO (g-C3N4/Na2S2O8) | 100 | 100 | 51.35 | 33.27 | 100 | 0 | 0 | 48.65 | 66.73 | 0 |
| Type | Target Pollutant | Synergistic Multisystem | Main Active Radical |
|---|---|---|---|
| cationic dyes (alkaline dye) | Rhb | H2O2 | ·OH |
| Na2S2O8 | ·O2− | ||
| anionic dyes (acid dyes) | MO | H2O2 | ·O2− |
| Na2S2O8 | ·OH |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, W.; Ding, K.; Chen, G.; Wang, H.; Deng, X. Synergistic Multisystem Photocatalytic Degradation of Anionic and Cationic Dyes Using Graphitic Phase Carbon Nitride. Molecules 2023, 28, 2796. https://doi.org/10.3390/molecules28062796
Yang W, Ding K, Chen G, Wang H, Deng X. Synergistic Multisystem Photocatalytic Degradation of Anionic and Cationic Dyes Using Graphitic Phase Carbon Nitride. Molecules. 2023; 28(6):2796. https://doi.org/10.3390/molecules28062796
Chicago/Turabian StyleYang, Wen, Kun Ding, Guangzhou Chen, Hua Wang, and Xinyue Deng. 2023. "Synergistic Multisystem Photocatalytic Degradation of Anionic and Cationic Dyes Using Graphitic Phase Carbon Nitride" Molecules 28, no. 6: 2796. https://doi.org/10.3390/molecules28062796
APA StyleYang, W., Ding, K., Chen, G., Wang, H., & Deng, X. (2023). Synergistic Multisystem Photocatalytic Degradation of Anionic and Cationic Dyes Using Graphitic Phase Carbon Nitride. Molecules, 28(6), 2796. https://doi.org/10.3390/molecules28062796





