Stability of Graphene/Intercalated Oxygen/Ru(0001) as Studied by Thermal Desorption of CO and CO2 Molecules
Abstract
1. Introduction
2. Results and Discussion
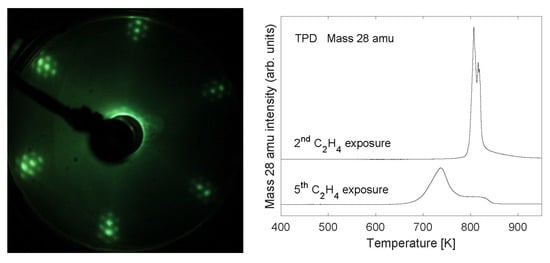
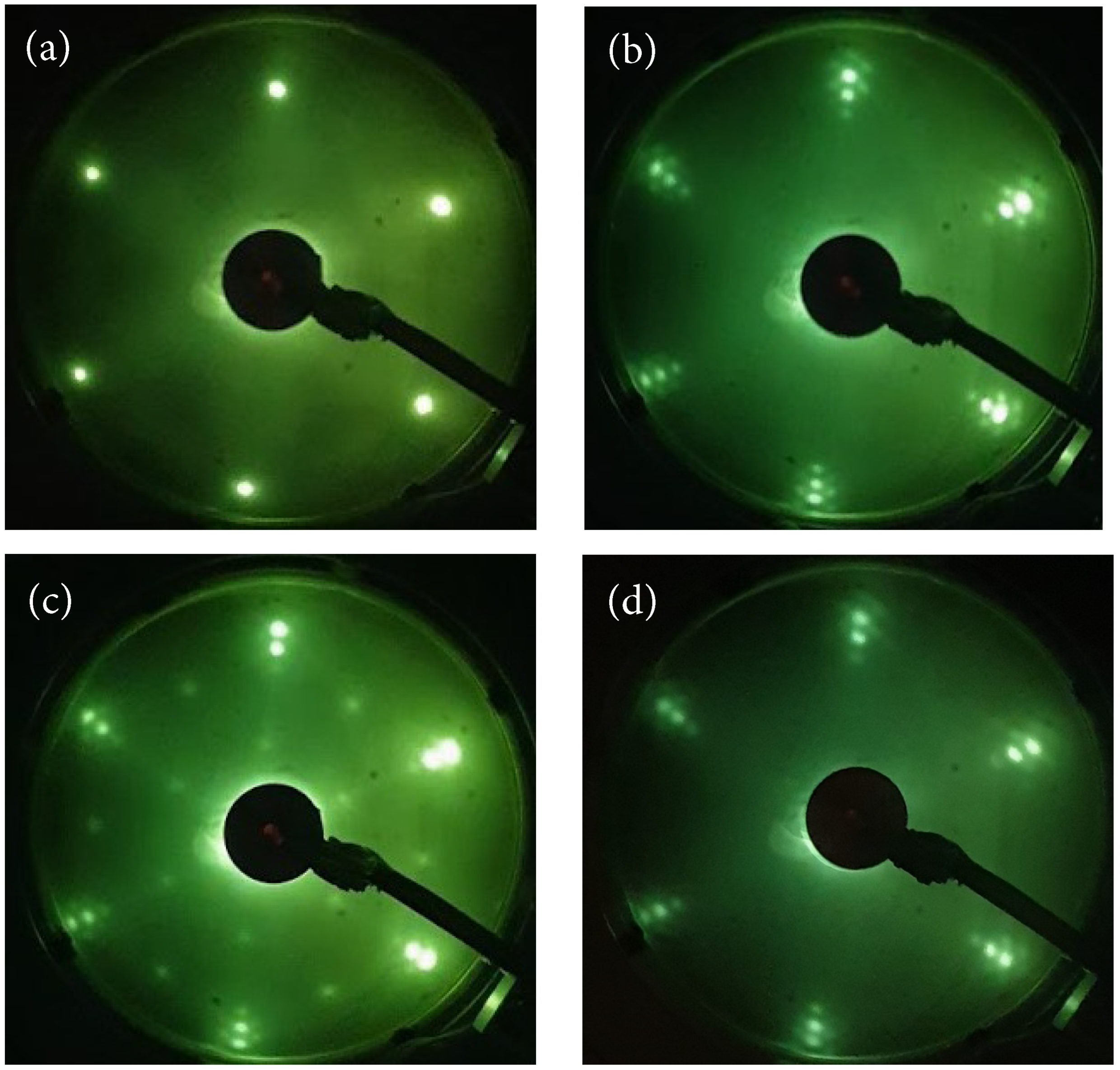
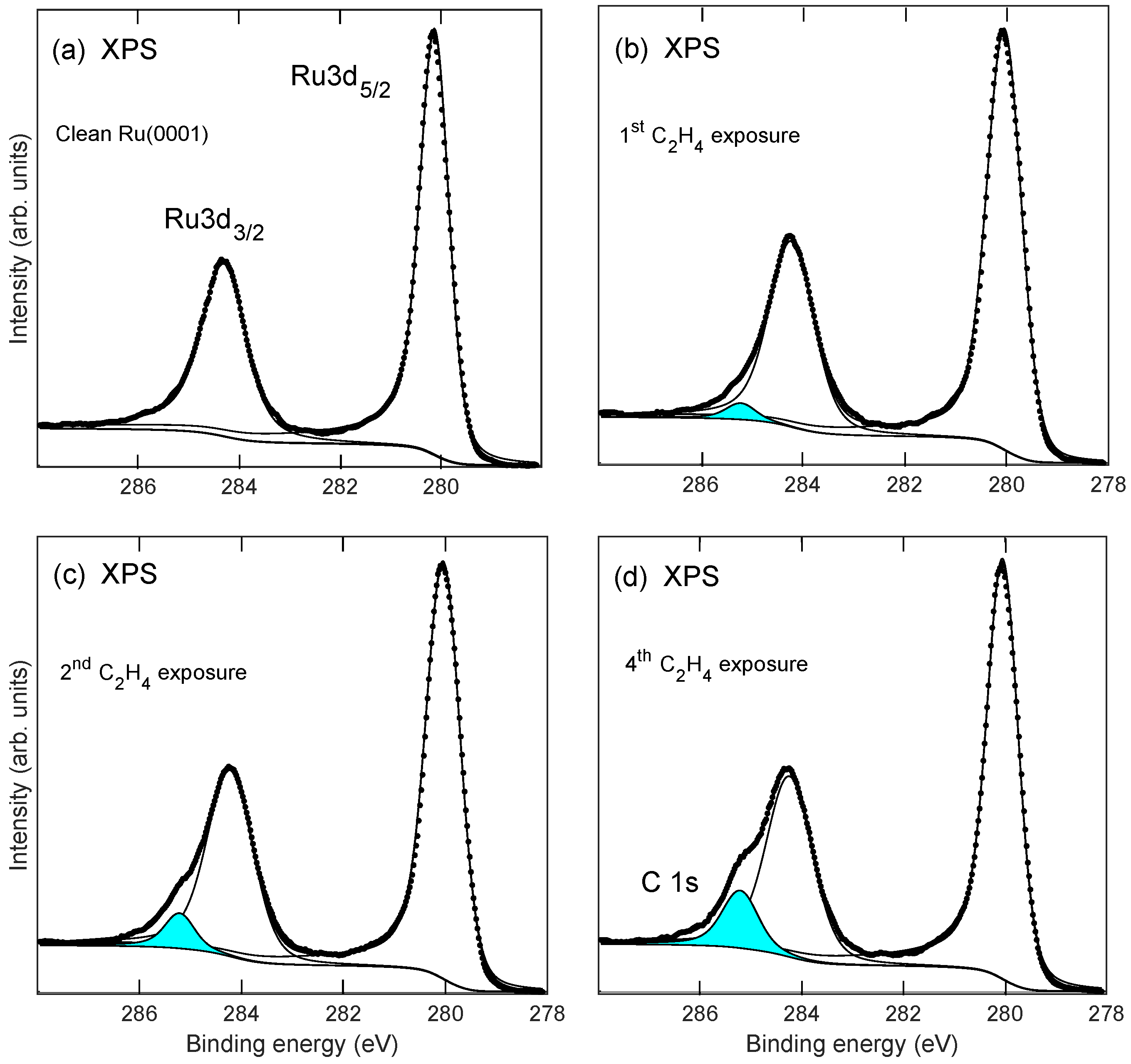
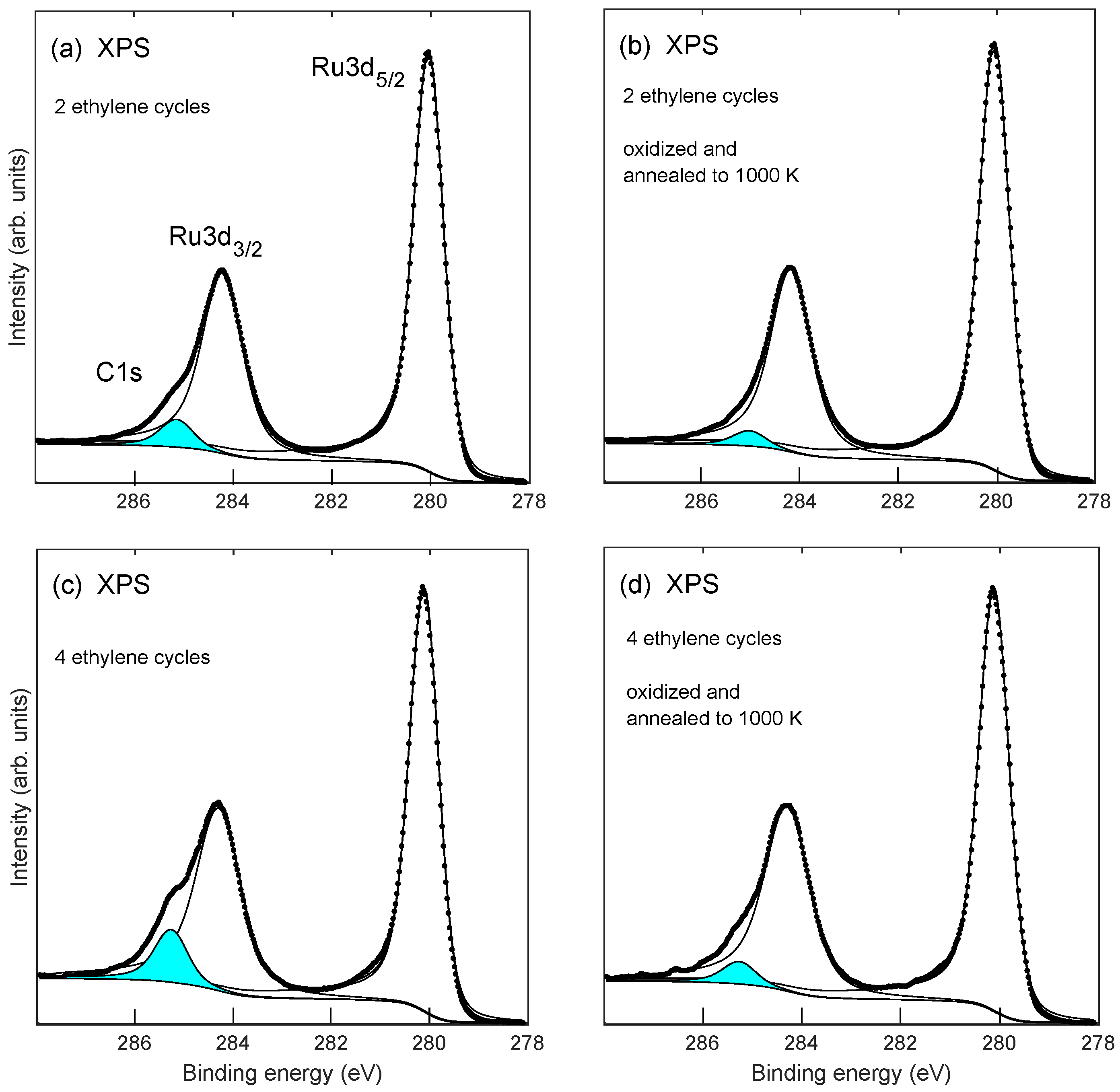
2.1. Growth of the Graphene/Oxygen/Ruthenium Layer
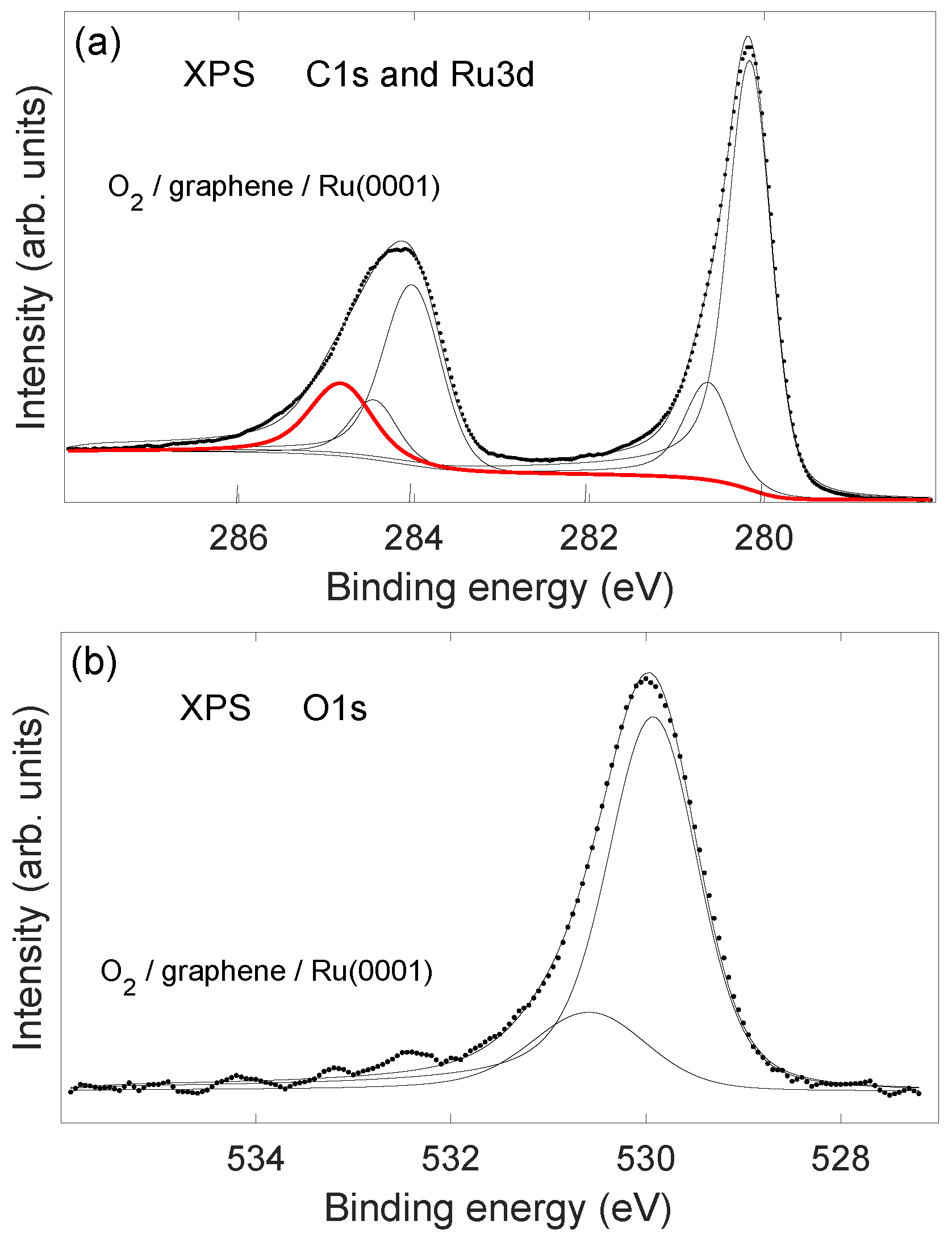
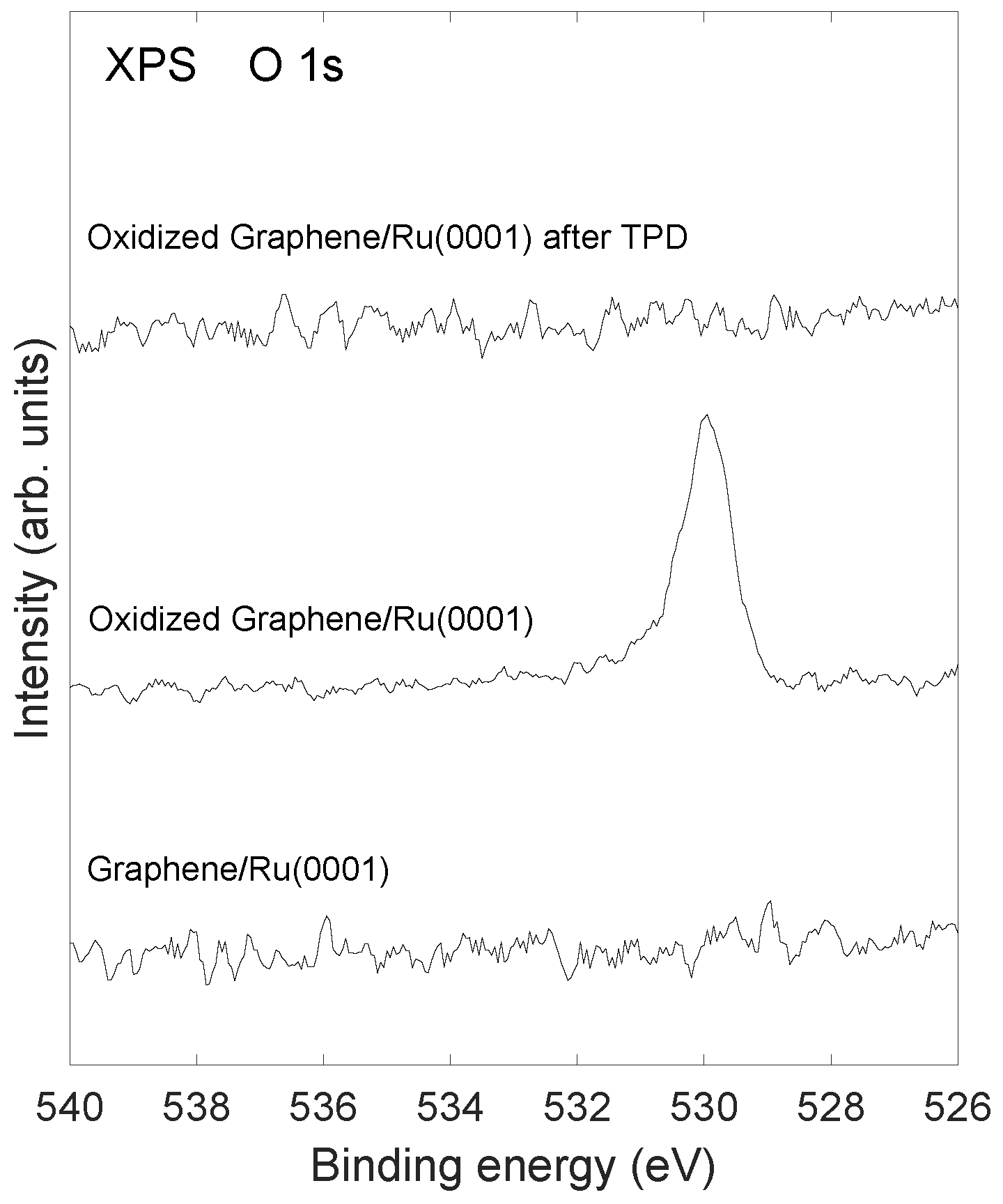
2.2. Stability of Layer
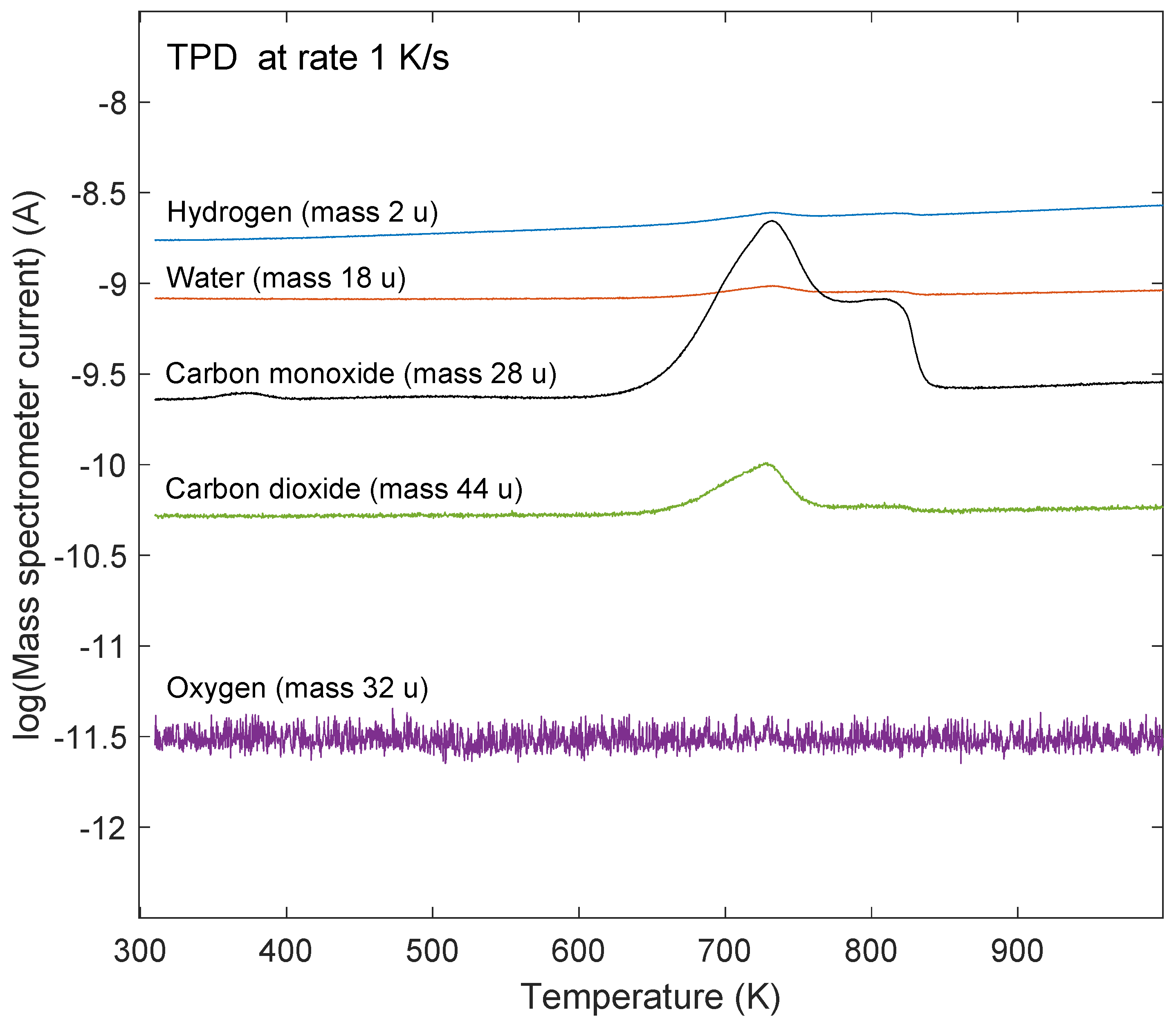
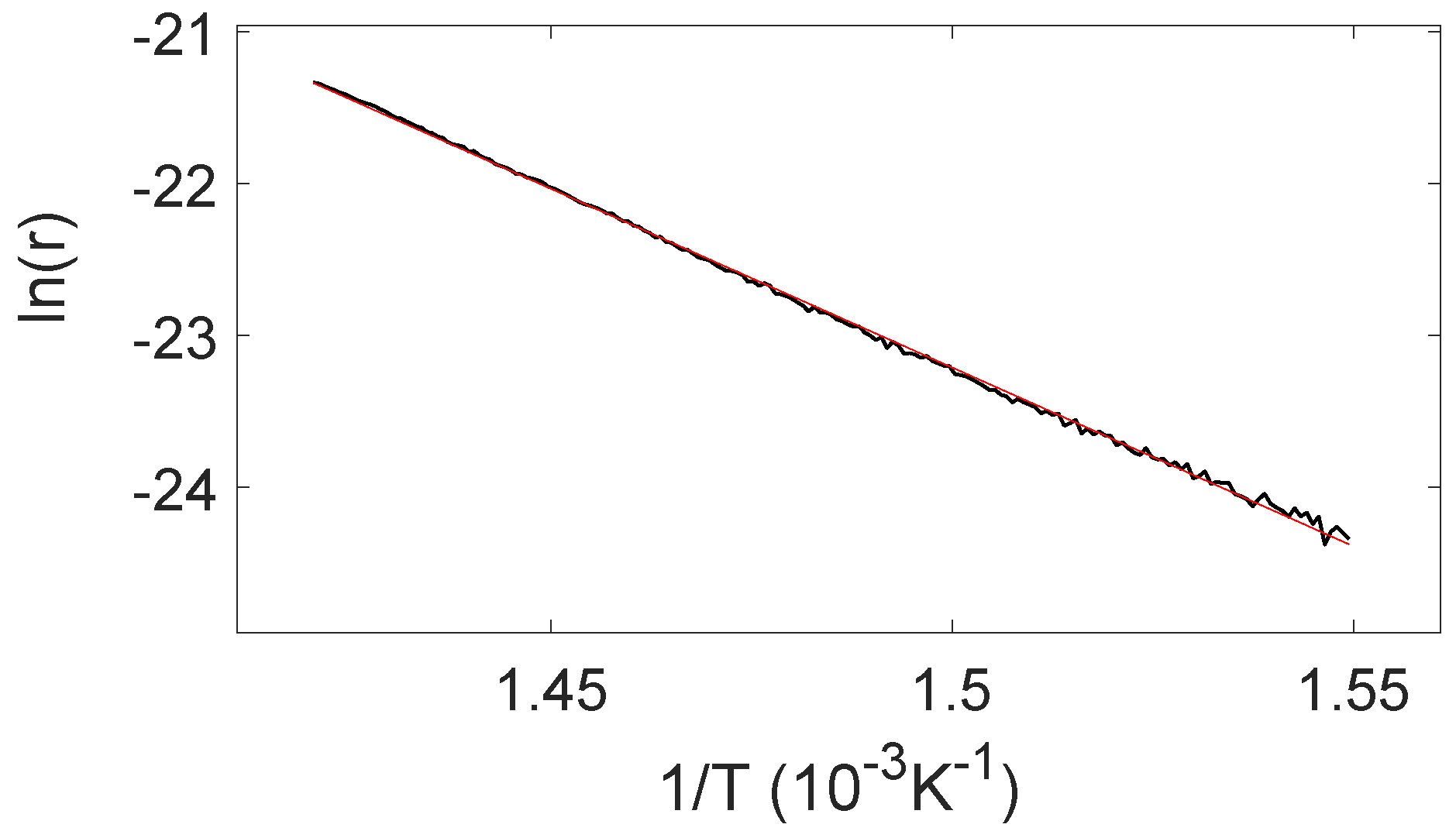
2.3. TPD Analysis
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Katsnelson, M.I.; Grigorieva, I.V.; Dubonos, S.V.; Firsov, A.A. Two-dimensional gas of massless Dirac fermions in graphene. Nature 2005, 438, 197–200. [Google Scholar] [CrossRef] [PubMed]
- Wintterlin, J.; Bocquet, M.L. Graphene on metal surfaces. Surf. Sci. 2009, 603, 1841–1852. [Google Scholar] [CrossRef]
- Dong, A.; Fu, Q.; Wei, M.; Liu, Y.; Ning, Y.; Yang, F.; Bluhm, H.; Bao, X. Facile oxygen intercalation between full layer graphene and Ru(0001) under ambient conditions. Surf. Sci. 2015, 634, 37–43. [Google Scholar] [CrossRef]
- Dedkov, Y.S.; Fonin, M.; Rüdiger, U.; Laubschat, C. Graphene-protected iron layer on Ni(111). Appl. Phys. Lett. 2008, 93, 022509. [Google Scholar] [CrossRef]
- Borca, B.; Calleja, F.; Hinarejos, J.J.; de Parga, A.L.V.; Miranda, R. Reactivity of periodically rippled graphene grown on Ru(0001). J. Phys. Condens. Matter 2009, 21, 134002. [Google Scholar] [CrossRef]
- Chen, S.; Brown, L.; Levendorf, M.; Cai, W.; Ju, S.Y.; Edgeworth, J.; Li, X.; Magnuson, C.W.; Velamakanni, A.; Piner, R.D.; et al. Oxidation Resistance of Graphene-Coated Cu and Cu/Ni Alloy. ACS Nano 2011, 5, 1321–1327. [Google Scholar] [CrossRef]
- Topsakal, M.; Şahin, H.; Ciraci, S. Graphene coatings: An efficient protection from oxidation. Phys. Rev. B 2012, 85, 155445. [Google Scholar] [CrossRef]
- Nilsson, L.; Andersen, M.; Balog, R.; Lægsgaard, E.; Hofmann, P.; Besenbacher, F.; Hammer, B.; Stensgaard, I.; Hornekær, L. Graphene coatings: Probing the limits of the one atom thick protection layer. ACS Nano 2012, 6, 10258–10266. [Google Scholar] [CrossRef]
- Sutter, E.; Albrecht, P.; Camino, F.E.; Sutter, P. Monolayer graphene as ultimate chemical passivation layer for arbitrarily shaped metal surfaces. Carbon 2010, 48, 4414–4420. [Google Scholar] [CrossRef]
- Prasai, D.; Tuberquia, J.C.; Harl, R.R.; Jennings, G.K.; Bolotin, K.I. Graphene: Corrosion-Inhibiting Coating. ACS Nano 2012, 6, 1102–1108. [Google Scholar] [CrossRef]
- Kang, D.; Y-Kwon, J.; Cho, H.; Sim, J.H.; Hwang, H.S.; Kim, C.S.; Kim, Y.J.; Ruoff, R.S.; Shin, H.S. Oxidation Resistance of Iron and Copper Foils Coated with Reduced Graphene Oxide Multilayers. ACS Nano 2012, 6, 7763–7769. [Google Scholar] [CrossRef]
- Gotterbarm, K.; Zhao, W.; Höfert, O.; Gleichweit, C.; Papp, C.; Steinrück, H.P. Growth and oxidation of graphene on Rh(111). Phys. Chem. Chem. Phys. 2013, 15, 19625–19631. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Fu, Q.; Cui, Y.; Tan, D.; Bao, X. Growth Mechanism of Graphene on Ru(0001) and O2 Adsorption on the Graphene/Ru(0001) Surface. J. Phys. Chem. C 2009, 113, 8296–8301. [Google Scholar] [CrossRef]
- Lyon, H.B.; Somorjai, G.A. Low-Energy Electron-Diffraction Study of the Clean (100), (111), and (110) Faces of Platinum. J. Chem. Phys. 1967, 46, 2539–2550. [Google Scholar] [CrossRef]
- Shelton, J.; Patil, H.; Blakely, J. Equilibrium segregation of carbon to a nickel (111) surface: A surface phase transition. Surf. Sci. 1974, 43, 493–520. [Google Scholar] [CrossRef]
- Hamilton, J.; Blakely, J. Carbon segregation to single crystal surfaces of Pt, Pd and Co. Surf. Sci. 1980, 91, 199–217. [Google Scholar] [CrossRef]
- Himpsel, F.; Christmann, K.; Heimann, P.; Eastman, D.; Feibelman, P.J. Adsorbate band dispersions for C on Ru(0001). Surf. Sci. 1982, 115, L159–L164. [Google Scholar] [CrossRef]
- Rosei, R.; De Crescenzi, M.; Sette, F.; Quaresima, C.; Savoia, A.; Perfetti, P. Structure of graphitic carbon on Ni(111): A surface extended-energy-loss fine-structure study. Phys. Rev. B 1983, 28, 1161–1164. [Google Scholar] [CrossRef]
- McConville, C.; Woodruff, D.; Kevan, S. The electronic structure of graphitic overlayers on Ni100. Surf. Sci. 1986, 171, L447–L453. [Google Scholar] [CrossRef]
- Land, T.; Michely, T.; Behm, R.; Hemminger, J.; Comsa, G. STM investigation of single layer graphite structures produced on Pt(111) by hydrocarbon decomposition. Surf. Sci. 1992, 264, 261–270. [Google Scholar] [CrossRef]
- Røst, H.I.; Chellappan, R.K.; Strand, F.S.; Grubišić-Čabo, A.; Reed, B.P.; Prieto, M.J.; Tǎnase, L.C.; de Souza Caldas, L.; Wongpinij, T.; Euaruksakul, C.; et al. Low-Temperature Growth of Graphene on a Semiconductor. J. Phys. Chem. C 2021, 125, 4243–4252. [Google Scholar] [CrossRef]
- Marchini, S.; Günther, S.; Wintterlin, J. Scanning tunneling microscopy of graphene on Ru(0001). Phys. Rev. B 2007, 76, 075429. [Google Scholar] [CrossRef]
- Yi, P.; Dong-Xia, S.; Hong-Jun, G. Formation of graphene on Ru(0001) surface. Chin. Phys. 2007, 16, 3151–3153. [Google Scholar] [CrossRef]
- Martoccia, D.; Willmott, P.R.; Brugger, T.; Björck, M.; Günther, S.; Schlepütz, C.M.; Cervellino, A.; Pauli, S.A.; Patterson, B.D.; Marchini, S.; et al. Graphene on Ru(0001): A 25 × 25 Supercell. Phys. Rev. Lett. 2008, 101, 126102. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Xu, W.Y.; Que, Y.D.; Mao, J.H.; Meng, L.; Pan, L.D.; Li, G.; Wang, Y.L.; Du, S.X.; Liu, Y.Q.; et al. Intercalation of metals and silicon at the interface of epitaxial graphene and its substrates. Chin. Phys. B 2013, 22, 096803. [Google Scholar] [CrossRef]
- Li, L.; Wang, Y.; Meng, L.; Wu, R.t.; Gao, H.J. Hafnium intercalation between epitaxial graphene and Ir(111) substrate. Appl. Phys. Lett. 2013, 102, 093106. [Google Scholar] [CrossRef]
- Silva, C.C.; Iannuzzi, M.; Duncan, D.A.; Ryan, P.T.P.; Clarke, K.T.; Küchle, J.T.; Cai, J.; Jolie, W.; Schlueter, C.; Lee, T.L.; et al. Valleys and Hills of Graphene on Ru(0001). J. Phys. Chem. C 2018, 122, 18554–18561. [Google Scholar] [CrossRef]
- Voloshina, E.; Berdunov, N.; Dedkov, Y. Restoring a nearly free-standing character of graphene on Ru(0001) by oxygen intercalation. Sci. Rep. 2016, 6, 20285. [Google Scholar] [CrossRef]
- Martoccia, D.; Björck, M.; Schlepütz, C.M.; Brugger, T.; Pauli, S.A.; Patterson, B.D.; Greber, T.; Willmott, P.R. Graphene on Ru(0001): A corrugated and chiral structure. New J. Phys. 2010, 12, 043028. [Google Scholar] [CrossRef]
- Ulstrup, S.; Lacovig, P.; Orlando, F.; Lizzit, D.; Bignardi, L.; Dalmiglio, M.; Bianchi, M.; Mazzola, F.; Baraldi, A.; Larciprete, R.; et al. Photoemission investigation of oxygen intercalated epitaxial graphene on Ru(0001). Surf. Sci. 2018, 678, 57–64. [Google Scholar] [CrossRef]
- Günther, S.; Menteş, T.; Reichelt, R.; Miniussi, E.; Santos, B.; Baraldi, A.; Locatelli, A. Au intercalation under epitaxial graphene on Ru(0001): The role of graphene edges. Carbon 2020, 162, 292–299. [Google Scholar] [CrossRef]
- Li, T.; Yarmoff, J.A. Intercalation and desorption of oxygen between graphene and Ru(0001) studied with helium ion scattering. Phys. Rev. B 2017, 96, 155441. [Google Scholar] [CrossRef]
- Cui, Y.; Fu, Q.; Zhang, H.; Bao, X. Formation of identical-size graphene nanoclusters on Ru(0001). Chem. Commun. 2011, 47, 1470–1472. [Google Scholar] [CrossRef]
- Sutter, P.W.; Flege, J.I.; Sutter, E.A. Epitaxial graphene on ruthenium. Nat. Mater. 2008, 7, 406–411. [Google Scholar] [CrossRef]
- Xu, W.Y.; Huang, L.; Que, Y.D.; Li, E.; Zhang, H.G.; Lin, X.; Wang, Y.L.; Du, S.X.; Gao, H.J. High quality sub-monolayer, monolayer, and bilayer graphene on Ru(0001). Chin. Phys. B 2014, 23, 098101. [Google Scholar] [CrossRef]
- Preobrajenski, A.B.; Ng, M.L.; Vinogradov, A.S.; Mårtensson, N. Controlling graphene corrugation on lattice-mismatched substrates. Phys. Rev. B 2008, 78, 073401. [Google Scholar] [CrossRef]
- Jiang, D.e.; Du, M.H.; Dai, S. First principles study of the graphene/Ru(0001) interface. J. Chem. Phys. 2009, 130, 074705. [Google Scholar] [CrossRef]
- Förster, G.D.; Rabilloud, F.; Calvo, F. Atomistic modeling of epitaxial graphene on Ru(0001) and deposited ruthenium nanoparticles. Phys. Rev. B 2015, 92, 165425. [Google Scholar] [CrossRef]
- Wang, B.; Bocquet, M.L.; Marchini, S.; Günther, S.; Wintterlin, J. Chemical origin of a graphene moiré overlayer on Ru(0001). Phys. Chem. Chem. Phys. 2008, 10, 3530–3534. [Google Scholar] [CrossRef]
- Doniach, S.; Sunjic, M. Many-electron singularity in X-ray photoemission and X-ray line spectra from metals. J. Phys. C Solid State Phys. 1970, 3, 285–291. [Google Scholar] [CrossRef]
- Crist, B.V. Volume 1—The elements and native oxides. In Handbooks of Monochromatic XPS Spectra; John Wiley & Sons: Hoboken, NJ, USA, 2000. [Google Scholar]
- Simonov, P.; Likholobov, V. Physicochemical Aspects of Preparation of Carbon-Supported Noble Metal Catalysts; CRC Press: Boca Raton, FL, USA, 2003; Chapter 12; pp. 409–454. [Google Scholar] [CrossRef]
- Kaga, Y.K.Y.; Abe, Y.A.Y.; Yanagisawa, H.Y.H.; Sasaki, K.S.K. Formation Process and Electrical Property of RuO2 Thin Films Prepared by Reactive Sputtering. Jpn. J. Appl. Phys. 1998, 37, 3457. [Google Scholar] [CrossRef]
- Al-Gaashani, R.; Najjar, A.; Zakaria, Y.; Mansour, S.; Atieh, M. XPS and structural studies of high quality graphene oxide and reduced graphene oxide prepared by different chemical oxidation methods. Ceram. Int. 2019, 45, 14439–14448. [Google Scholar] [CrossRef]
- Polanyi, M.; Wigner, E. Über die Interferenz von Eigenschwingungen als Ursache von Energieschwankungen und chemischer Umsetzungen. Z. Für Phys. Chem. 1928, 139A, 439–452. [Google Scholar] [CrossRef]
- Raaen, S.; Ramstad, A. Monte-Carlo simulations of thermal desorption of adsorbed molecules from metal surfaces. Energy 2005, 30, 821–830. [Google Scholar] [CrossRef]
- Miller, J.B.; Siddiqui, H.R.; Gates, S.M.; Russell, J.N.; Yates, J.T.; Tully, J.C.; Cardillo, M.J. Extraction of kinetic parameters in temperature programmed desorption: A comparison of methods. J. Chem. Phys. 1987, 87, 6725–6732. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, X.; Raaen, S. Stability of Graphene/Intercalated Oxygen/Ru(0001) as Studied by Thermal Desorption of CO and CO2 Molecules. Molecules 2023, 28, 2670. https://doi.org/10.3390/molecules28062670
Yu X, Raaen S. Stability of Graphene/Intercalated Oxygen/Ru(0001) as Studied by Thermal Desorption of CO and CO2 Molecules. Molecules. 2023; 28(6):2670. https://doi.org/10.3390/molecules28062670
Chicago/Turabian StyleYu, Xiaofeng, and Steinar Raaen. 2023. "Stability of Graphene/Intercalated Oxygen/Ru(0001) as Studied by Thermal Desorption of CO and CO2 Molecules" Molecules 28, no. 6: 2670. https://doi.org/10.3390/molecules28062670
APA StyleYu, X., & Raaen, S. (2023). Stability of Graphene/Intercalated Oxygen/Ru(0001) as Studied by Thermal Desorption of CO and CO2 Molecules. Molecules, 28(6), 2670. https://doi.org/10.3390/molecules28062670