Recent Advances in the Synthesis and Applications of m-Aryloxy Phenols
Abstract
1. Introduction
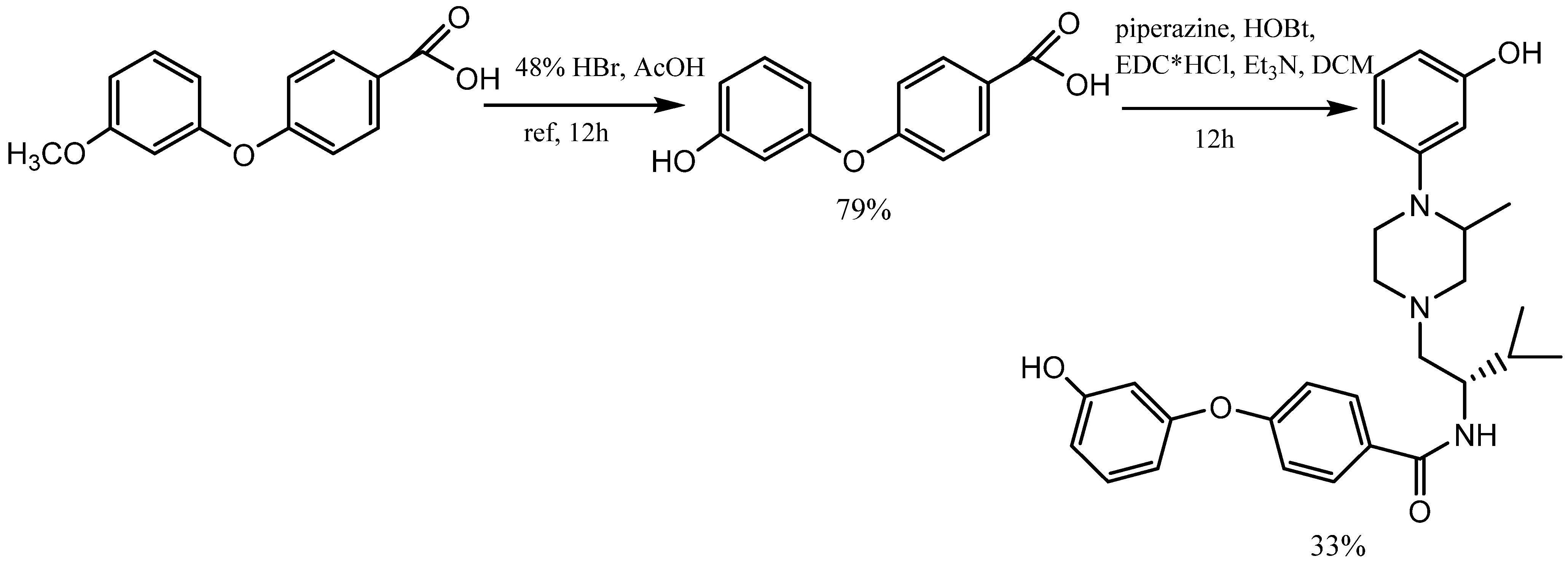
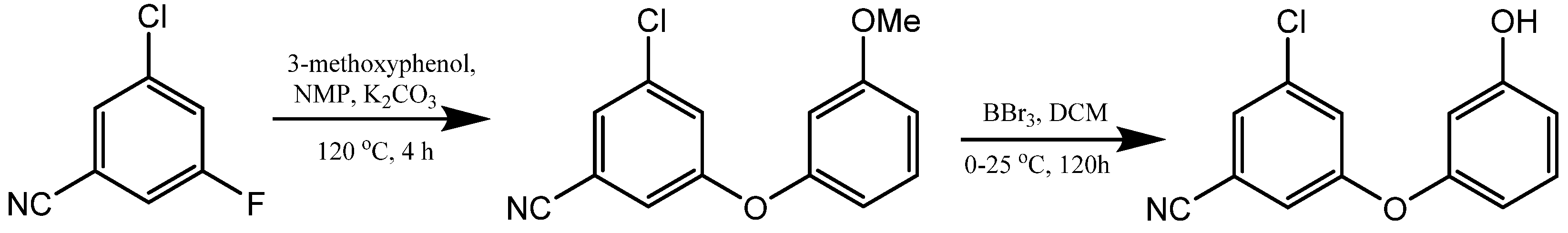
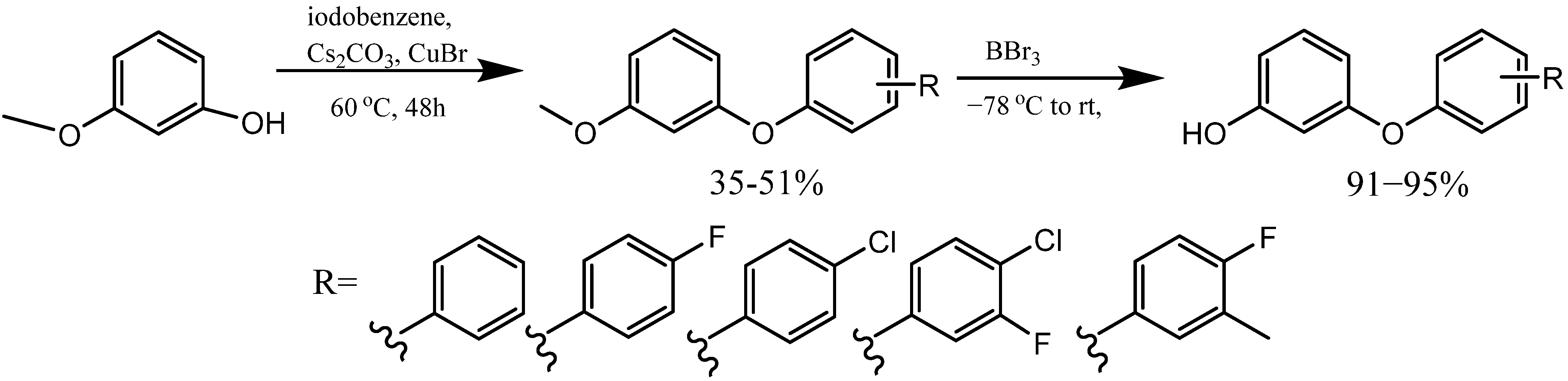
2. Synthesis of m-Aryloxy Phenols by Demethylation of m-Methoxy Phenols
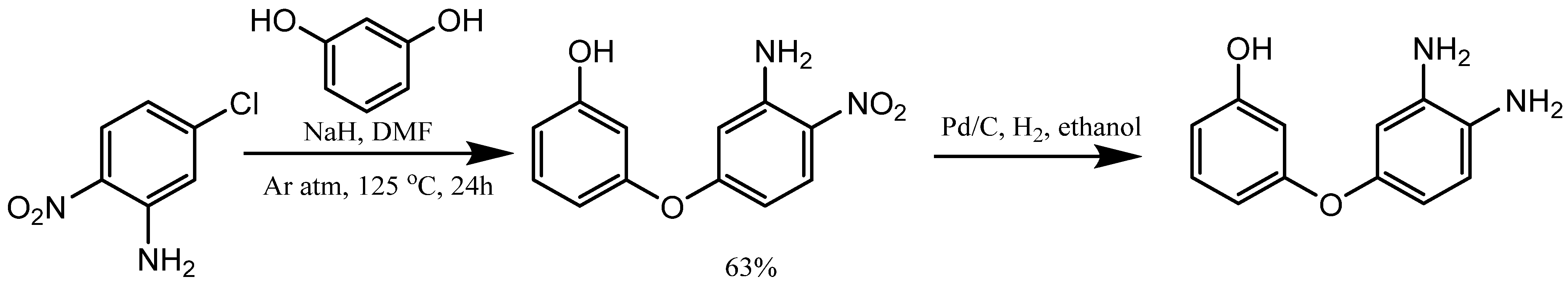
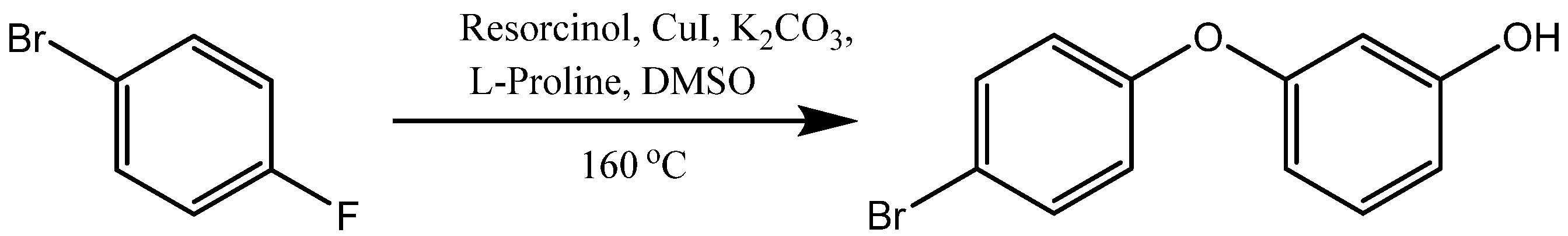
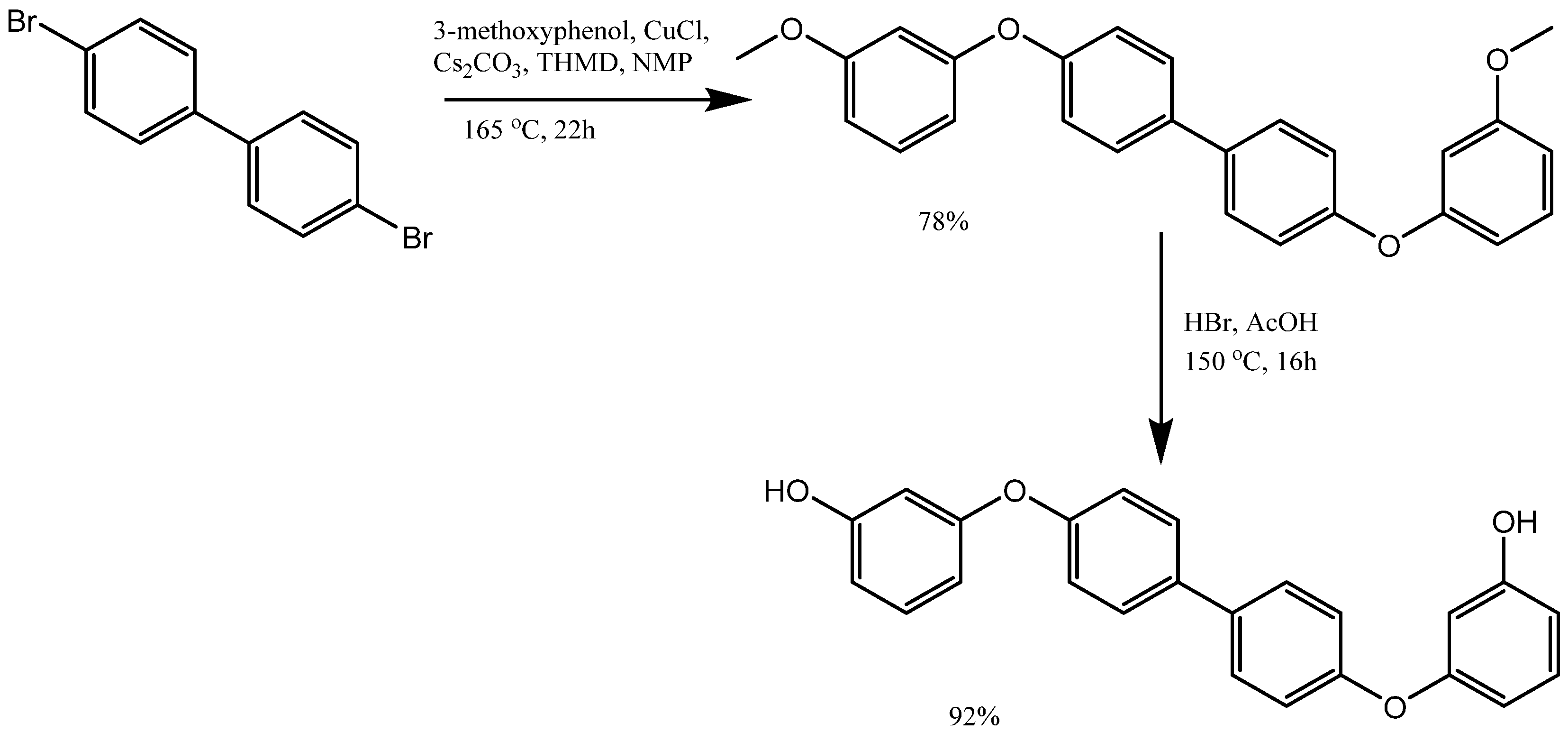
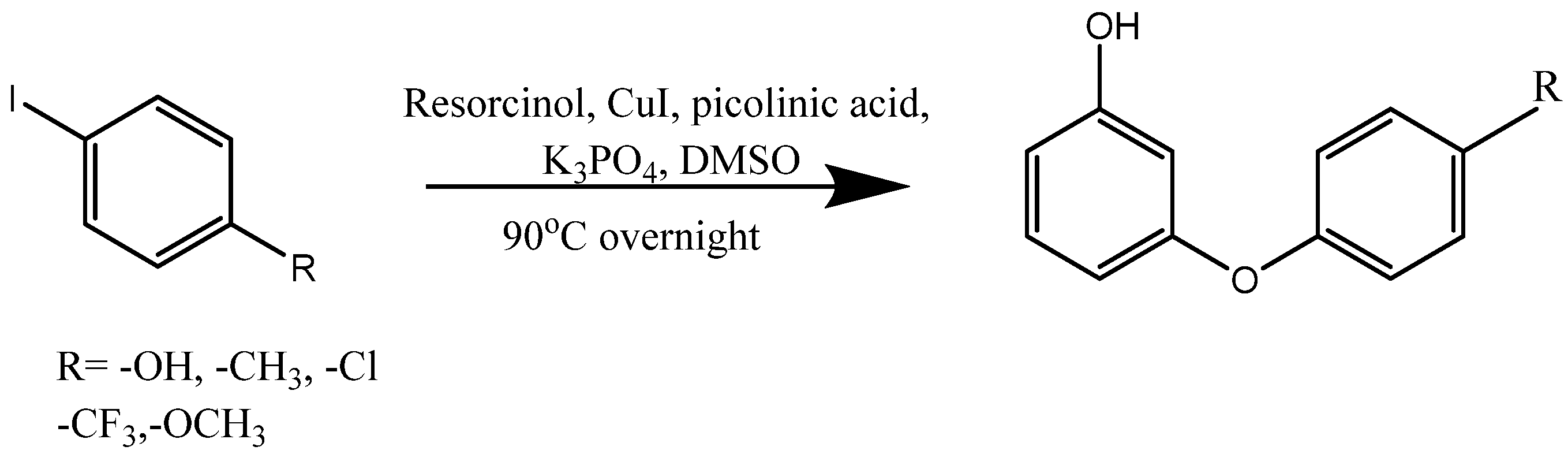
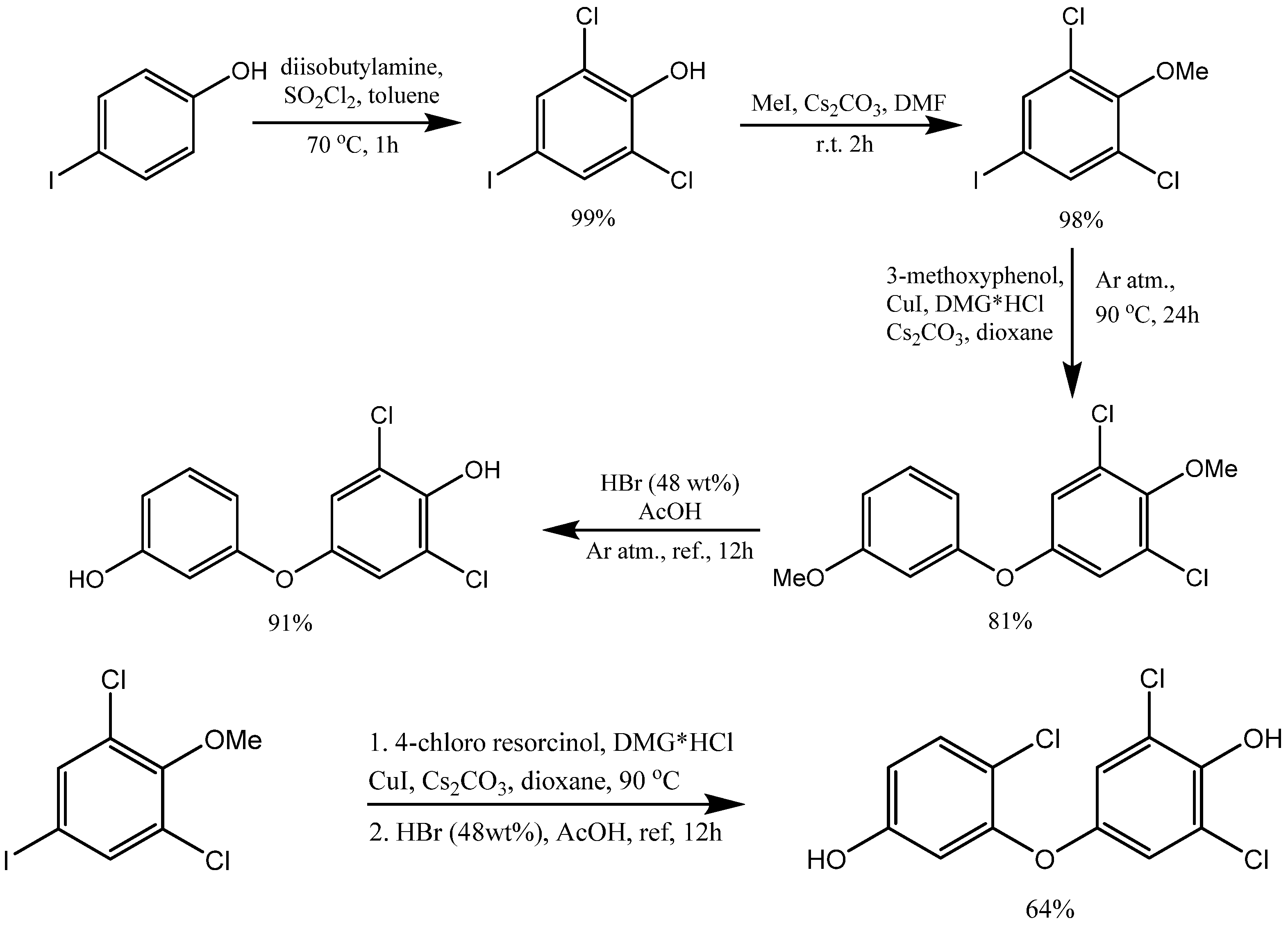
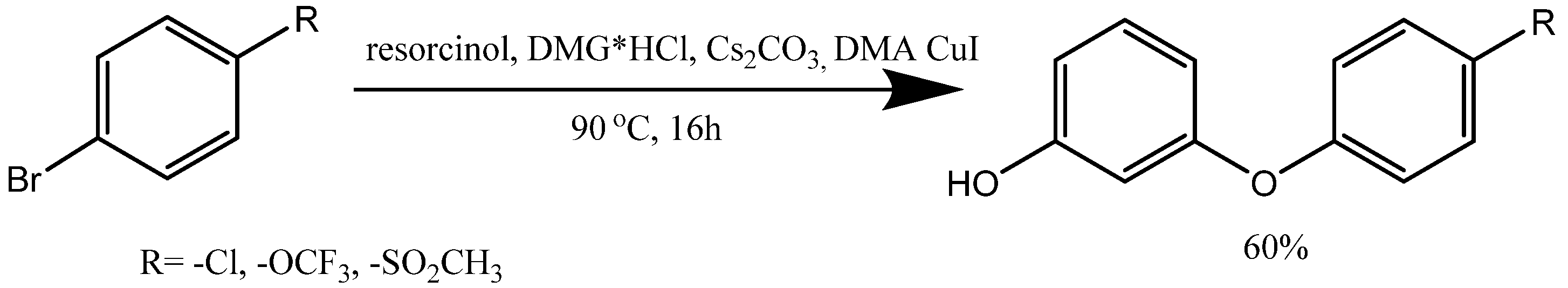
3. Synthesis of Aryloxy Phenols by Reactions between Aryl Halides and Resorcinol
4. Sonogashira Coupling: A Copper-Catalyzed Method for Biaryl Synthesis
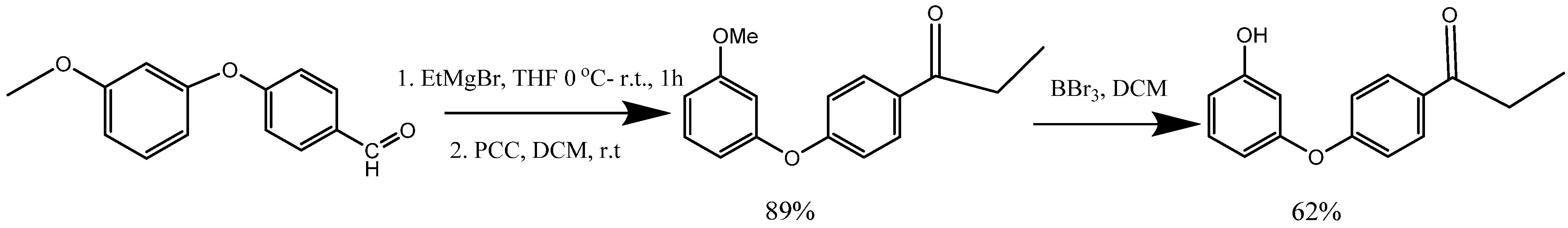
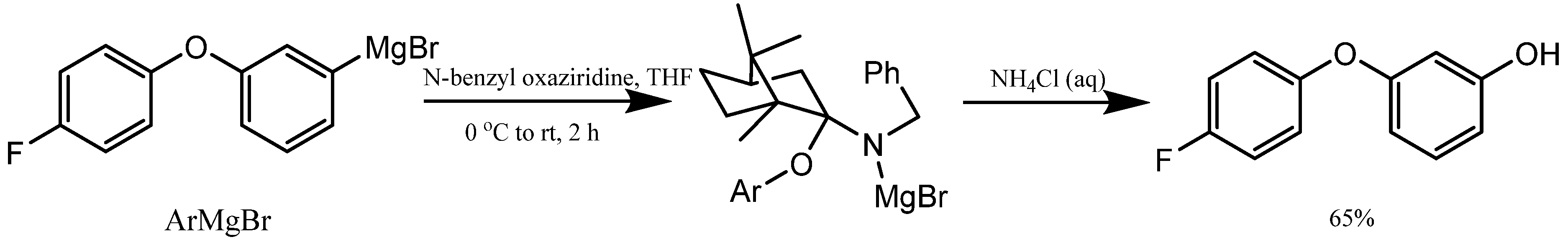
5. Synthesis of m-Aryloxy Phenols Using Grignard Reagents
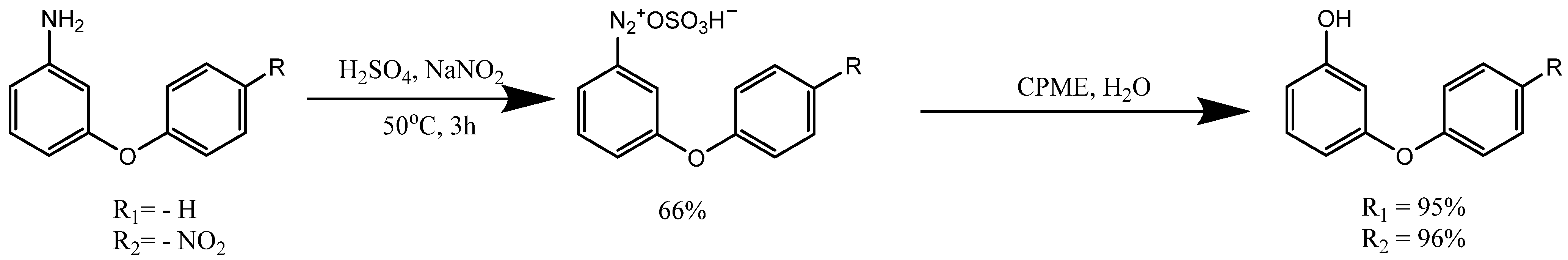
6. Hydrolysis of Diazonium Salts Using a Two-Phase System
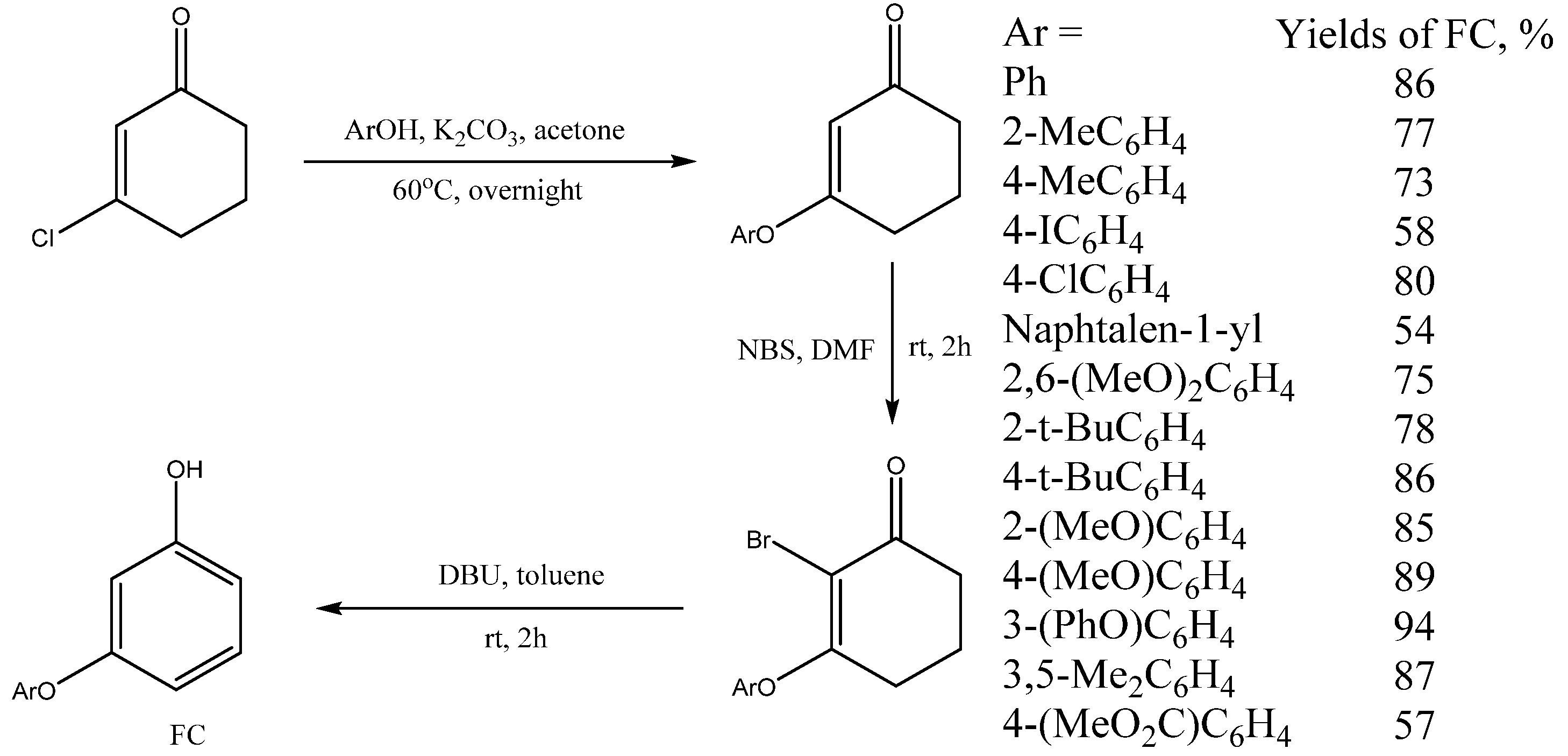
7. Synthesis of 3-Aryloxyphenols from 3-Chlorocyclohex-2-en-1-one
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Latha, G.; Devarajan, N.; Karthik, M.; Suresh, P. Nickel-Catalyzed Oxidative Hydroxylation of Arylboronic Acid: Ni(HBTC)BPY MOF as an Efficient and Ligand-Free Catalyst to Access Phenolic Motifs. Catal. Commun. 2020, 136, 105911. [Google Scholar] [CrossRef]
- Wang, S.K.; Chen, M.T.; Zhao, D.Y.; You, X.; Luo, Q.L. Iodine-Catalyzed Oxidative Aromatization: A Metal-Free Concise Approach to meta-Substituted Phenols from Cyclohex-2-enones. Adv. Synth. Catal. 2016, 358, 4093–4099. [Google Scholar] [CrossRef]
- Ullmann, F. Ueber eine neue Bildungsweise von Diphenylaminderivaten. Ber. Der Dtsch. Chem. Ges. 1903, 36, 2382–2384. [Google Scholar] [CrossRef]
- Ullmann, F. Ueber eine neue Darstellungsweise von Phenyläthersalicylsäure. Ber. Der Dtsch. Chem. Ges. 1904, 37, 853–854. [Google Scholar] [CrossRef]
- Hassan, J.; Sévignon, M.; Gozzi, C.; Schulz, E.; Lemaire, M. Aryl-aryl Bond Formation One Century after the Discovery of the Ullmann Reaction. Chem. Rev. 2002, 102, 1359–1470. [Google Scholar] [CrossRef] [PubMed]
- Zuo, L.; Yao, S.; Wang, W.; Duan, W. An Efficient Method for Demethylation of Aryl Methyl Ethers. Tetrahedron Lett. 2008, 49, 4054–4056. [Google Scholar] [CrossRef]
- Bradsher, C.K.; Brown, F.C.; Porter, H.K. Synthesis and Fungistatic Activity of Some 3-Hydroxybiphenyl Derivatives. J. Am. Chem. Soc. 1954, 76, 2357–2362. [Google Scholar] [CrossRef]
- McOmie, J.F.W.; Watts, M.L.; West, D.E. Demethylation of Aryl Methyl Ethers by Boron Tribromide. Tetrahedron 1968, 24, 2289–2292. [Google Scholar] [CrossRef]
- Kormos, C.M.; Jin, C.; Cueva, J.P.; Runyon, S.P.; Thomas, J.B.; Brieaddy, L.E.; Mascarella, S.W.; Navarro, H.A.; Gilmour, B.P.; Carroll, F.I. Discovery of N-{4-[(3-hydroxyphenyl)-3-methylpiperazin-1-yl] methyl-2-methylpropyl}-4-phenoxybenzamide analogues as selective kappa opioid receptor antagonists. J. Med. Chem. 2013, 56, 4551–4567. [Google Scholar] [CrossRef]
- Carroll, F.I.; Carlezon Jr, W.A. Development of κ Opioid Receptor Antagonists. J. Med. Chem. 2013, 56, 2178–2195. [Google Scholar] [CrossRef]
- Puls, K.; Olivé-Marti, A.L.; Pach, S.; Pinter, B.; Erli, F.; Wolber, G.; Spetea, M. In Vitro, In Vivo and In Silico Characterization of a Novel Kappa-Opioid Receptor Antagonist. Pharmaceuticals 2022, 15, 680. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Tago, T.; Toyohara, J.; Saito, Y.; Yamamoto, F. Radiosynthesis and in Vivo and ex Vivo Evaluation of Isomeric [11C] methoxy Analogs of Nimesulide as Brain Cyclooxygenase-2-Targeted Imaging Agents. Biol. Pharm. Bull. 2022, 45, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Hisa, T.; Arai, J.; Saito, Y.; Yamamoto, F.; Mukai, T.; Ohshima, T.; Maeda, M.; Ohkubo, Y. Isomeric Methoxy Analogs of Nimesulide for Development of Brain Cyclooxygenase-2 (COX-2)-Targeted Imaging Agents: Synthesis, In Vitro COX-2-Inhibitory Potency, and Cellular Transport Properties. Bioorg. Med. Chem. 2015, 23, 6807–6814. [Google Scholar] [CrossRef]
- Yang, F.; Zhao, J.; Li, Y.; Zhang, S.; Shao, Y.; Shao, H.; Ma, T.; Gong, C. Synthesis and Properties of Novel Organosoluble Polyimides Derived from Bis [3-(4-Amino-2-trifluoromethylphenoxy)phenyl] Ether. Eur. Polym. J. 2009, 45, 2053–2059. [Google Scholar] [CrossRef]
- Van Rossom, W.; Robeyns, K.; Ovaere, M.; Van Meervelt, L.; Dehaen, W.; Maes, W. Odd-Numbered Oxa-Calix[n]arenes (n= 5, 7): Synthesis and Solid-State Structures. Org. Lett. 2011, 13, 126–129. [Google Scholar] [CrossRef]
- Danil de Namor, A.F.; Aparicio-Aragon, W.; Nwogu, N.; El Gamouz, A.; Piro, O.E.; Castellano, E.E. Calixarene and Resorcarene Based Receptors: From Structural and Thermodynamic Studies to the Synthesis of a New Mercury (II) Selective Material. J. Phys. Chem. B 2011, 115, 6922–6934. [Google Scholar] [CrossRef]
- Uysal Akku¸, G.; Al, E.; Korcan, S.E. Selective Extraction of Toxic Heavy Metals and Biological Activity Studies Using Pyrimidylthioamide Functionalised Calix[4]arene. Supramol. Chem. 2015, 27, 522–526. [Google Scholar] [CrossRef]
- Patra, S.; Maity, D.; Gunupuru, R.; Agnihotri, P.; Paul, P. Calixarenes: Versatile Molecules as Molecular Sensors for Ion Recognition Study. J. Chem. Sci. 2012, 124, 1287–1299. [Google Scholar] [CrossRef]
- Raynal, M.; Ballester, P.; Vidal-Ferran, A.; Van Leeuwen, P.W. Supramolecular Catalysis. Part 2: Artificial Enzyme Mimics. Chem. Soc. Rev. 2014, 43, 1734–1787. [Google Scholar] [CrossRef]
- Baldini, L.; Cacciapaglia, R.; Casnati, A.; Mandolini, L.; Salvio, R.; Sansone, F.; Ungaro, R. Upper Rim Guanidinocalix[4]arenes as Artificial Phosphodiesterases. J. Org. Chem. 2012, 77, 3381–3389. [Google Scholar] [CrossRef] [PubMed]
- da Silva, D.L.; Fernandes, S.A.; Sabino, A.A.; de Fátima, Â. p-Sulfonic Acid Calixarenes as Efficient and Reusable Organocatalysts for the Synthesis of 3,4-Dihydropyrimidin-2(1H)-ones/-thiones. Tetrahedron Lett. 2011, 52, 6328–6330. [Google Scholar] [CrossRef]
- Lindley, J. Copper Assisted Nucleophilic Substitution of Aryl Halogen. Tetrahedron 1984, 40, 1433–1456. [Google Scholar] [CrossRef]
- Vagin, S.I.; Reichardt, R.; Klaus, S.; Rieger, B. Conformationally flexible dimeric salphen complexes for bifunctional catalysis. J. Am. Chem. Soc. 2010, 132, 14367–14369. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Ren, B.H.; Lu, X.B. Trinuclear Salphen–Chromium (III) Chloride Complexes as Catalysts for the Alternating Copolymerization of Epoxides and Cyclic Anhydrides. J. Polym. Sci. 2021, 59, 1821–1828. [Google Scholar] [CrossRef]
- Wang, J.; Hu, J.; Zeng, K.; Yang, G. Preparation of self-promoted hydroxy-containing phthalonitrile resins by an in situ reaction. RSC Adv. 2015, 5, 105038–105046. [Google Scholar] [CrossRef]
- Zu, Y.; Zong, L.; Wang, J.; Jian, X. Enhanced Thermal Property via Tunable Bisphenol Moieties in Branched Phthalonitrile Thermoset. Polymer 2019, 172, 372–381. [Google Scholar] [CrossRef]
- Bollini, M.; Domaoal, R.A.; Thakur, V.V.; Gallardo-Macias, R.; Spasov, K.A.; Anderson, K.S.; Jorgensen, W.L. Computationally-guided optimization of a docking hit to yield catechol diethers as potent anti-HIV agents. J. Med. Chem. 2011, 54, 8582–8591. [Google Scholar] [CrossRef]
- Pauwels, R. New Non-Nucleoside Reverse Transcriptase Inhibitors (NNRTIs) in Development for the Treatment of HIV Infections. Curr. Opin. Pharmacol. 2004, 4, 437–446. [Google Scholar] [CrossRef]
- Frey, K.M.; Gray, W.T.; Spasov, K.A.; Bollini, M.; Gallardo-Macias, R.; Jorgensen, W.L.; Anderson, K.S. Structure-Based Evaluation of C5 Derivatives in the Catechol Diether Series Targeting HIV-1 Reverse Transcriptase. Chem. Biol. Drug Des. 2014, 83, 541–549. [Google Scholar] [CrossRef]
- Huang, B.; Chen, W.; Zhao, T.; Li, Z.; Jiang, X.; Ginex, T.; Vilchez, D.; Luque, F.J.; Kang, D.; Gao, P.; et al. Exploiting the Tolerant Region I of the Non-Nucleoside Reverse Transcriptase Inhibitor (NNRTI) Binding Pocket: Discovery of Potent Diarylpyrimidine-Typed HIV-1 NNRTIs against Wild-Type and E138K Mutant Virus with Significantly Improved Water Solubility and Favorable Safety Profiles. J. Med. Chem. 2019, 62, 2083–2098. [Google Scholar] [CrossRef]
- Xia, Y.; Cao, K.; Zhou, Y.; Alley, M.R.K.; Rock, F.; Mohan, M.; Meewan, M.; Baker, S.J.; Lux, S.; Ding, C.Z.; et al. Synthesis and SAR of novel benzoxaboroles as a new class of β-lactamase inhibitors. Bioorg. Med. Chem. Lett. 2011, 21, 2533–2536. [Google Scholar] [CrossRef]
- Xiao, Y.C.; Chen, X.P.; Deng, J.; Yan, Y.H.; Zhu, K.R.; Li, G.; Yu, J.L.; Brem, J.; Chen, F.; Schofield, C.J.; et al. Design and Enantioselective Synthesis of 3-(α-Acrylic Acid) Benzoxaboroles to Combat Carbapenemase Resistance. Chem. Commun. 2021, 57, 7709–7712. [Google Scholar] [CrossRef]
- Kobayashi, O.; Niikura, N.; Inoue, T.; Mizuta, S.; Takatsuna, R.; Hirai, K.; Shirouzu, K.; Kaken Pharmaceutical Co., Ltd. Sagami Chemical Research Institute (Sagami CRI). Polycyclic Pyrazolinone Derivative and Herbicide Comprising Same as Effective Component Thereof. U.S. Patent 9580444, 28 February 2017. [Google Scholar]
- Chen, P.; Shi, M.; Niu, M.; Zhang, Y.; Wang, R.; Xu, J.; Wang, Y. Effects of HPPD Inhibitor Herbicides on Soybean Root Exudates: A Combination Study of Multispectral Technique and 2D-COS Analysis. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2023, 289, 122241. [Google Scholar] [CrossRef]
- Kazui, Y.; Fujii, S.; Yamada, A.; Ishigami-Yuasa, M.; Kagechika, H.; Tanatani, A. Structure-activity relationship of novel (benzoylaminophenoxy) phenol derivatives as anti-prostate cancer agents. Bioorg. Med. Chem. 2018, 26, 5118–5127. [Google Scholar] [CrossRef] [PubMed]
- Elancheran, R.; Kabilan, S.; Kotoky, J.; Ramanathan, M.; Bhattacharjee, A. In Silico Molecular Docking, Synthesis of 4-(4-Benzoylaminophenoxy) Phenol Derivatives as Androgen Receptor Antagonists. Comb. Chem. High Throughput Screen. 2019, 22, 307–316. [Google Scholar] [CrossRef]
- Silva, M.E.T.D.; Martins, M.A.; Leite, M.D.O.; Milião, G.L.; Coimbra, J.S.D.R. Microalga Scenedesmus obliquus: Extraction of Bioactive Compounds and Antioxidant Activity. Rev. Ciência Agronômica 2021, 52, e20196848. [Google Scholar] [CrossRef]
- Frączek, T.; Kamiński, R.; Krakowiak, A.; Naessens, E.; Verhasselt, B.; Paneth, P. Diaryl ethers with carboxymethoxyphenacyl motif as potent HIV-1 reverse transcriptase inhibitors with improved solubility. J. Enzym. Inhib. Med. Chem. 2018, 33, 9–16. [Google Scholar] [CrossRef]
- Huang, B.; Kang, D.; Yang, J.; Zhan, P.; Liu, X. Novel Diarylpyrimidines and Diaryltriazines as Potent HIV-1 NNRTIs with Dramatically Improved Solubility: A Patent Evaluation of US20140378443A1. Expert Opin. Ther. Pat. 2016, 26, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Prener, L.; Baszczyňski, O.; Kaiser, M.M.; Dračínský, M.; Stepan, G.; Lee, Y.J.; Brumshtein, B.; Yu, H.; Jansa, P.; Lansdon, E.B.; et al. Design and Synthesis of Novel HIV-1 NNRTIs with Bicyclic Cores and with Improved Physicochemical Properties. J. Med. Chem. 2023, 66, 1761–1777. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.L.; Ji, Y.; Wang, H.; Wang, X.; Gauthier Jr, D.R. Highly Enantioselective Rhodium-Catalyzed Transfer Hydrogenation of Tetrasubstituted Olefins: Application toward the Synthesis of GPR40 Agonist MK-2305. Org. Lett. 2022, 24, 3254–3258. [Google Scholar] [CrossRef] [PubMed]
- Pio, B.; Chobanian, H.R.; Guo, Y.; Josien, H.; Hagmann, W.K.; Miller, M.; Trujillo, M.E.; Kirkland, M.; Kosinski, D.; Mane, J.; et al. Design, Synthesis and Biological Evaluation of Indane Derived GPR40 AgoPAMs. Bioorg. Med. Chem. Lett. 2019, 29, 1842–1848. [Google Scholar] [CrossRef] [PubMed]
- Joseph, P.J.A.; Priyadarshini, S. Copper-Mediated C–X Functionalization of Aryl Halides. Org. Process Res. Dev. 2017, 21, 1889–1924. [Google Scholar] [CrossRef]
- Sperotto, E.; van Klink, G.P.M.; van Koten, G.; de Vries, J.G. The Mechanism of the Modified Ullmann Reaction. Dalton Trans. 2010, 39, 10338–10351. [Google Scholar] [CrossRef]
- Lin, H.; Sun, D. Recent synthetic developments and applications of the ullmann reaction. Rev. Org. Prep. Proced. Int. 2013, 45, 341–394. [Google Scholar] [CrossRef]
- Khan, F.; Dlugosch, M.; Liu, X.; Banwell, M.G. The Palladium-Catalyzed Ullmann Cross-Coupling Reaction: A Modern Variant on a Time-Honored Process. Acc. Chem. Res. 2018, 51, 1784–1795. [Google Scholar] [CrossRef]
- Xue, F.; Huang, J.; Ji, H.; Fang, J.; Li, H.; Martásek, P.; Roman, L.J.; Poulos, T.L.; Silverman, R.B. Structure-based design, synthesis, and biological evaluation of lipophilic-tailed monocationic inhibitors of neuronal nitric oxide synthase. Bioorganic Med. Chem. 2010, 18, 6526–6537. [Google Scholar] [CrossRef]
- Mukherjee, P.; Cinelli, M.A.; Kang, S.; Silverman, R.B. Development of Nitric Oxide Synthase Inhibitors for Neurodegeneration and Neuropathic Pain. Chem. Soc. Rev. 2014, 43, 6814–6838. [Google Scholar] [CrossRef] [PubMed]
- Sapkota, K.; Lee, E.Y.; Yang, J.H.; Kwon, Y.J.; Choi, J.W.; Na, Y.H. 4-Hydroxy-2’-nitrodiphenyl ether analogues as novel tyrosinase inhibitors. Bull. Korean Chem. Soc. 2010, 31, 1319–1325. [Google Scholar] [CrossRef]
- Sholehvar, F.; Asadzadeh, A.; Seyedhosseini, H. Molecular Docking Studies of Some Hydroxy Nitrodiphenyl Ether Analogues as Tyrosinase Inhibitors. J. Fasa Univ. Med. Sci. 2017, 6, 548–555. [Google Scholar]
- Bouey, E.; Masson, C.; Bertrand, K. Substituted Imidazolone Derivatives, Preparations and Uses. U.S. Patent 2010/0004159 A1, 7 January 2010. [Google Scholar]
- Takada, I.; Makishima, M. Peroxisome Proliferator-Activated Receptor Agonists and Antagonists: A Patent Review (2014-Present). Expert Opin. Ther. Pat. 2020, 30, 1–13. [Google Scholar] [CrossRef]
- Lee, J.; Kim, S.; Ko, S.; Kim, H.; Shin, S.; Ahn, E.; Lee, E.; Lee, H.; Jeon, M.; Han, J. Organometallic Compound and Organic Light-Emitting Device Including the Same. U.S. Patent 2021/36228, 4 February 2021. [Google Scholar]
- Xiao, L.; Chen, Z.; Qu, B.; Luo, J.; Kong, S.; Gong, Q.; Kido, J. Recent Progresses on Materials for Electrophosphorescent Organic Light-Emitting Devices. Adv. Mater. 2011, 23, 926–952. [Google Scholar] [CrossRef]
- Li, X.F.; Paoloni, F.P.; Weiber, E.A.; Jiang, Z.H.; Jannasch, P. Fully aromatic ionomers with precisely sequenced sulfonated moieties for enhanced proton conductivity. Macromolecules 2012, 45, 1447–1459. [Google Scholar] [CrossRef]
- Khomein, P.; Ketelaars, W.; Lap, T.; Liu, G. Sulfonated Aromatic Polymer as a Future Proton Exchange Membrane: A Review of Sulfonation and Crosslinking Methods. Renew. Sustain. Energy Rev. 2021, 137, 110471. [Google Scholar] [CrossRef]
- Bartholomäus, R.; Dommershausen, F.; Thiele, M.; Karanjule, N.S.; Harms, K.; Koert, U. Total synthesis of the postulated structure of fulicineroside. Chem. Eur. J. 2013, 19, 7423–7436. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, X.; Yu, B. O-Glycosylation Methods in the Total Synthesis of Complex Natural Glycosides. Nat. Prod. Rep. 2015, 32, 1331–1355. [Google Scholar] [CrossRef] [PubMed]
- Varley, R.J.; Dao, N.B.; Tian, W.W.; Christensen, S.; Tucker, S.; Wiggins, J. Epoxy Resin. International Patent WO 2018/018070 AI, 1 February 2018. [Google Scholar]
- Wang, L.; Xi, H.; Sun, X.; Shen, Y.; Yang, Y.; Pan, Y.; Hu, H. Synthesis of Functionalized p-Phenylene Oxide Oligomers. Synth. Commun. 2000, 30, 227–234. [Google Scholar] [CrossRef]
- Greene, T.W.; Wuts, P.G.M. Protecting Groups in Organic Synthesis, 2nd ed.; John Wiley & Sons: New York, NY, USA, 1991. [Google Scholar]
- Gim, H.J.; Li, H.; Jeong, J.H.; Lee, S.J.; Sung, M.K.; Song, M.Y.; Park, B.H.; Oh, S.J.; Ryu, J.H.; Jeon, R. Design, synthesis, and biological evaluation of a series of alkoxy-3-indolylacetic acids as peroxisome proliferator-activated receptor γ/δ agonists. Bioorg. Med. Chem. 2015, 23, 3322–3336. [Google Scholar] [CrossRef] [PubMed]
- Gim, H.J.; Choi, Y.S.; Li, H.; Kim, Y.J.; Ryu, J.H.; Jeon, R. Identification of a Novel PPAR-γ Agonist through a Scaffold Tuning Approach. Int. J. Mol. Sci. 2018, 19, 3032. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Hu, J.; Wong, N.; Bai, X. Diarylether-Based Fluorogenic Probes for Detection of Hypochlorous Acid or Hydroxyl Radical. U.S. Patent 20160312033A1, 27 October 2016. [Google Scholar]
- Hu, J.J.; Wong, N.K.; Lu, M.Y.; Chen, X.; Ye, S.; Zhao, A.Q.; Gao, P.; Kao, R.Y.T.; Shen, J.; Yang, D. HKOCl-3: A fluorescent hypochlorous acid probe for live-cell and in vivo imaging and quantitative application in flow cytometry and a 96-well microplate assay. Chem. Sci. 2016, 7, 2094–2099. [Google Scholar] [CrossRef]
- Bai, X.; Huang, Y.; Lu, M.; Yang, D. HKOH-1: A highly sensitive and selective fluorescent probe for detecting endogenous hydroxyl radicals in living cells. Angew. Chem. Int. Ed. 2017, 56, 12893–12897. [Google Scholar] [CrossRef]
- Li, M.D.; Wong, N.K.; Xiao, J.; Zhu, R.; Wu, L.; Dai, S.Y.; Chen, F.; Huang, G.; Xu, L.; Bai, X.; et al. Dynamics of Oxygen-Independent Photocleavage of Blebbistatin as a One-Photon Blue or Two-Photon Near-Infrared Light-Gated Hydroxyl Radical Photocage. J. Am. Chem. Soc. 2018, 140, 15957–15968. [Google Scholar] [CrossRef]
- Breitenbucher, G.J.; Tichenor, M.S.; Merit, J.E.; Hawryluk, N.A.; Chambers, A.L.; Keith, J.M. Aryl-Substituted Heterocyclic Urea Modulators of Fatty Acid Amide Hydrolase. U.S. Patent WO 2010/141809 A1, 9 December 2010. [Google Scholar]
- Thomas, A.M.; Sujatha, A.; Anilkumar, G. Recent Advances and Perspectives in Copper-Catalyzed Sonogashira Coupling Reactions. RSC Adv. 2014, 4, 21688–21698. [Google Scholar] [CrossRef]
- Min, H.; Palani, T.; Park, K.; Hwang, J.; Lee, S. Copper-Catalyzed Direct Synthesis of Diaryl 1, 2-Diketones from Aryl Iodides and Propiolic Acids. J. Org. Chem. 2014, 79, 6279–6285. [Google Scholar] [CrossRef] [PubMed]
- Bryan, J.M.; George, W.K. Copper mediated formation of carbon-heteroatom bonds using organoboron reagents and ultrasound. Heterocycles Int. J. Rev. Commun. Heterocycl. Chem. 2015, 90, 271–297. [Google Scholar]
- Calderone, V.; Minutolo, F.; Tuccinardi, T.; Testai, L.; Granchi, C.; Martelli, A.; Citi, V.; De Lorenzo, C.V.; Lenzi, G.; Leo, F.; et al. New Activators of SIRT1 Enzyme for the Treatment of Cardiovascular and Cardiometabolic Pathologies. International Patent WO 2019/162911, 29 August 2019. [Google Scholar]
- Mann, F.G.; Stewart, F.H. The Action of Magnesium and of Grignard Reagents on Certain Benzyl Ethers. Part I. The Action of Magnesium on o-, m-, and p-Alkoxy-and-phenoxy-methylbenzyl Chlorides. J. Chem. Soc. 1954, 2826–2832. [Google Scholar] [CrossRef]
- Pidathala, C.; Amewu, R.; Pacorel, B.; Nixon, G.L.; Gibbons, P.; Hong, W.D.; Leung, S.C.; Berry, N.G.; Sharma, R.; Stocks, P.A.; et al. Identification, design and biological evaluation of bisaryl quinolones targeting Plasmodium falciparum type II NADH: Quinone oxidoreductase (PfNDH2). J. Med. Chem. 2012, 55, 1831–1843. [Google Scholar] [CrossRef]
- Amporndanai, K.; Pinthong, N.; O’Neill, P.M.; Hong, W.D.; Amewu, R.K.; Pidathala, C.; Berry, N.G.; Leung, S.C.; Ward, S.A.; Biagini, G.A.; et al. Targeting the Ubiquinol-Reduction (Qi) Site of the Mitochondrial Cytochrome bc1 Complex for the Development of Next Generation Quinolone Antimalarials. Biology 2022, 11, 1109. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Zhou, Z.; Kwon, D.H.; Coombs, J.; Jones, S.; Behnke, N.E.; Ess, D.H.; Kürti, L. Rapid heteroatom transfer to arylmetals utilizing multifunctional reagent scaffolds. Nat. Chem. 2017, 9, 681–688. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, T.; Imoto, M.; Takeda, M.; Nakai, T.; Mihara, M.; Iwai, T.; Ito, T.; Mizuno, T.; Nomoto, A.; Ogawa, A. Hydrolysis of Diazonium Salts Using a Two-Phase System (CPME and Water). Heteroat. Chem. 2015, 26, 411–416. [Google Scholar] [CrossRef]
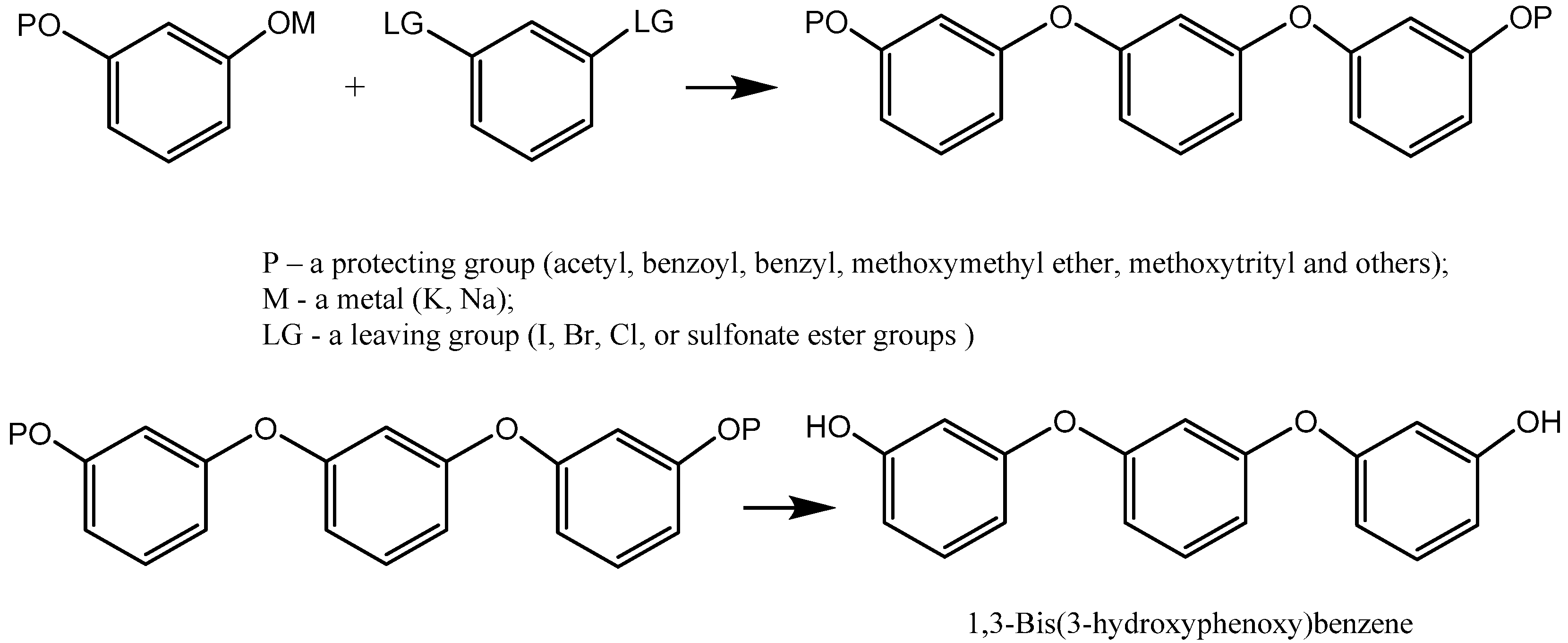
- Duvvuru, B.; Amankulova, D.; Gauden, S.; Haffemayer, T.; Clive, D.L. A mild alternative to the classical Ullmann coupling for preparation of 3-aryloxy phenols. Tetrahedron 2023, 133, 133264. [Google Scholar] [CrossRef]

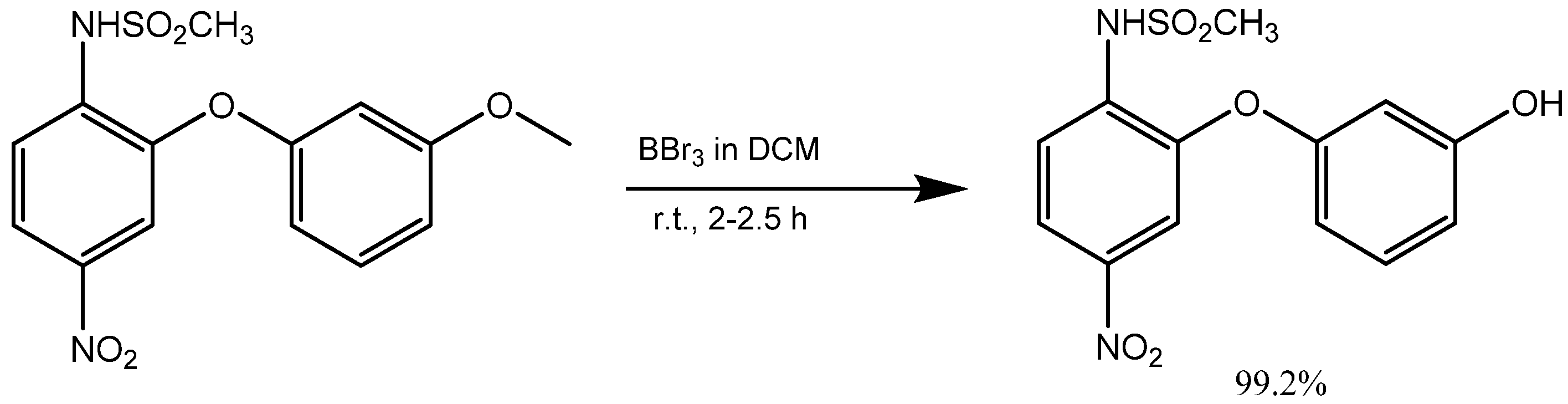












Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amankulova, D.; Berganayeva, G.; Kudaibergenova, B.; Zhetpisbay, D.; Sharipova, A.; Dyusebaeva, M. Recent Advances in the Synthesis and Applications of m-Aryloxy Phenols. Molecules 2023, 28, 2657. https://doi.org/10.3390/molecules28062657
Amankulova D, Berganayeva G, Kudaibergenova B, Zhetpisbay D, Sharipova A, Dyusebaeva M. Recent Advances in the Synthesis and Applications of m-Aryloxy Phenols. Molecules. 2023; 28(6):2657. https://doi.org/10.3390/molecules28062657
Chicago/Turabian StyleAmankulova, Dinara, Gulzat Berganayeva, Bates Kudaibergenova, Dinara Zhetpisbay, Ayshagul Sharipova, and Moldyr Dyusebaeva. 2023. "Recent Advances in the Synthesis and Applications of m-Aryloxy Phenols" Molecules 28, no. 6: 2657. https://doi.org/10.3390/molecules28062657
APA StyleAmankulova, D., Berganayeva, G., Kudaibergenova, B., Zhetpisbay, D., Sharipova, A., & Dyusebaeva, M. (2023). Recent Advances in the Synthesis and Applications of m-Aryloxy Phenols. Molecules, 28(6), 2657. https://doi.org/10.3390/molecules28062657





