Removal of 241Am from Aqueous Solutions by Adsorption on Sponge Gourd Biochar
Abstract
1. Introduction
2. Results and Discussion
2.1. Effect of Contact Time on the 241Am Adsorption
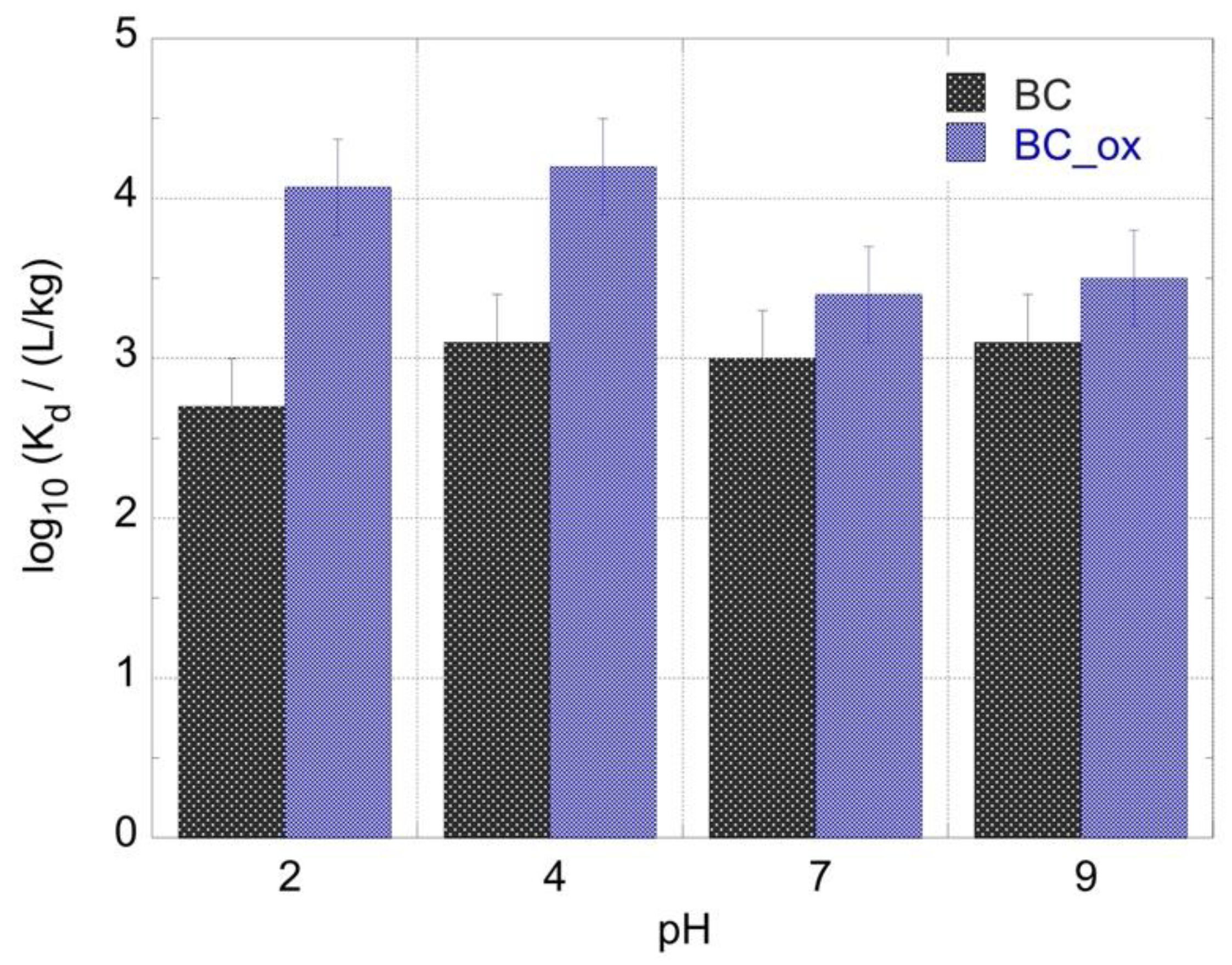
2.2. Effect of pH on 241Am Adsorption
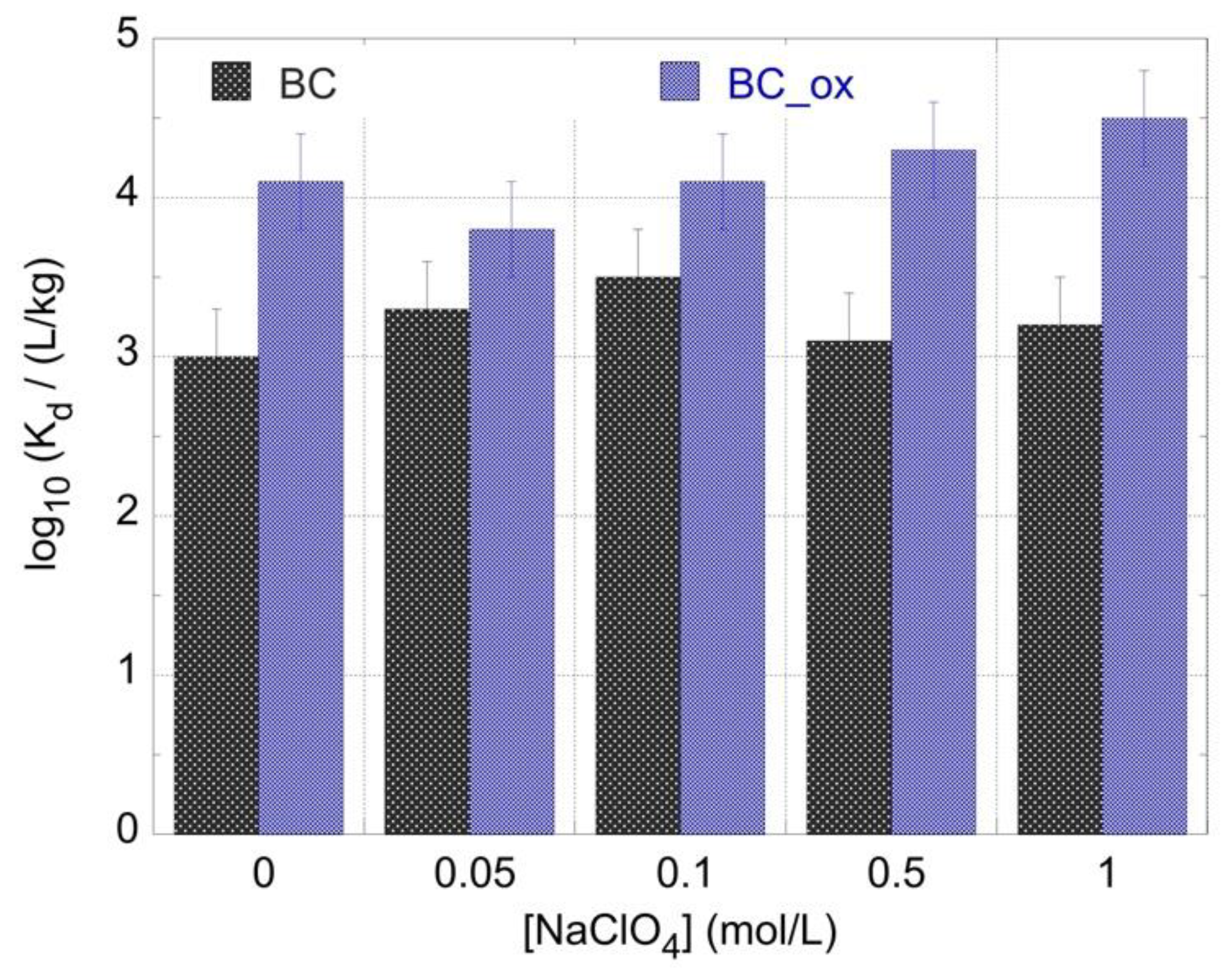
2.3. Effect of Ionic Strength on 241Am Adsorption
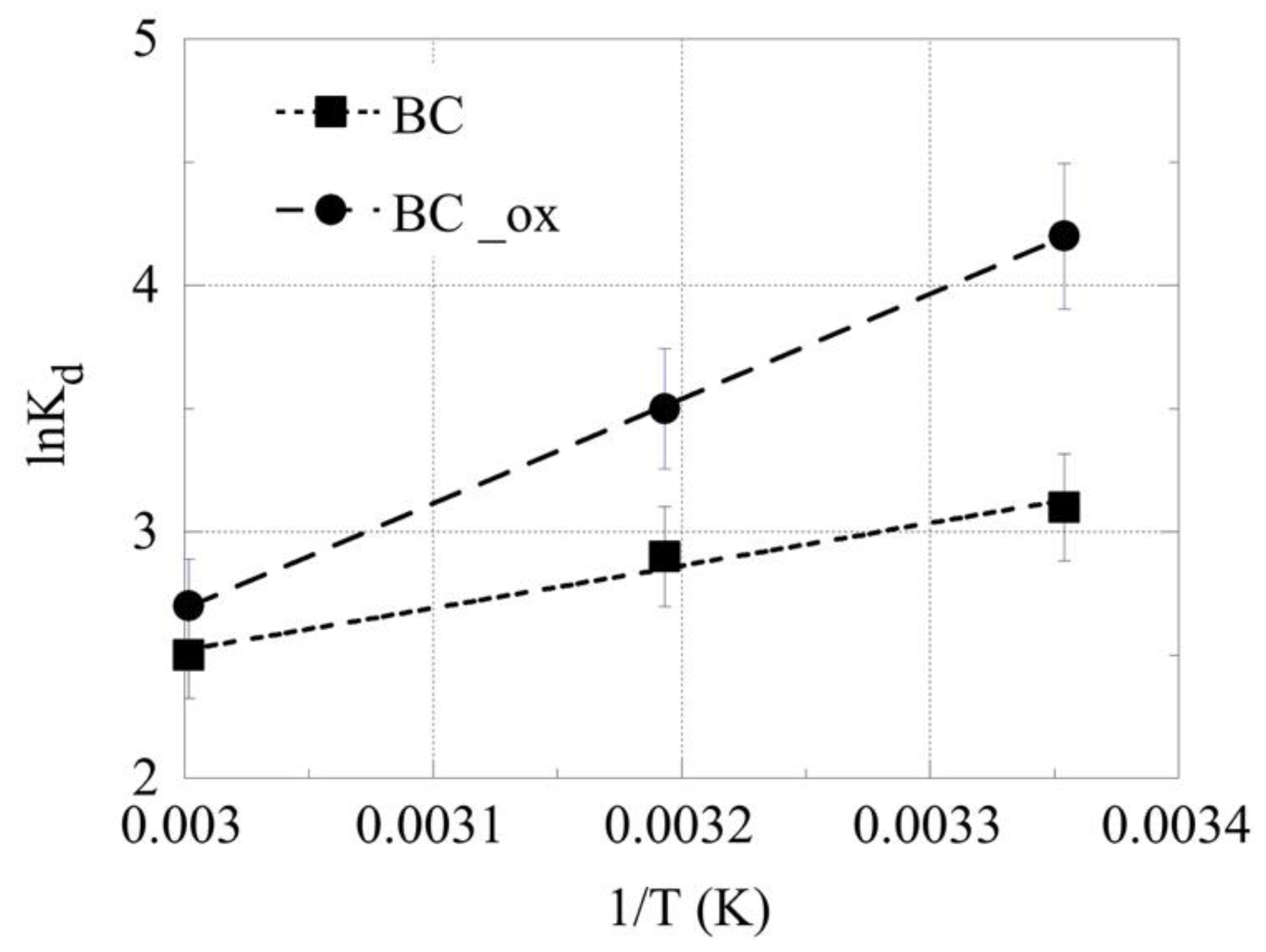
2.4. Effect of Temperature on 241Am Adsorption
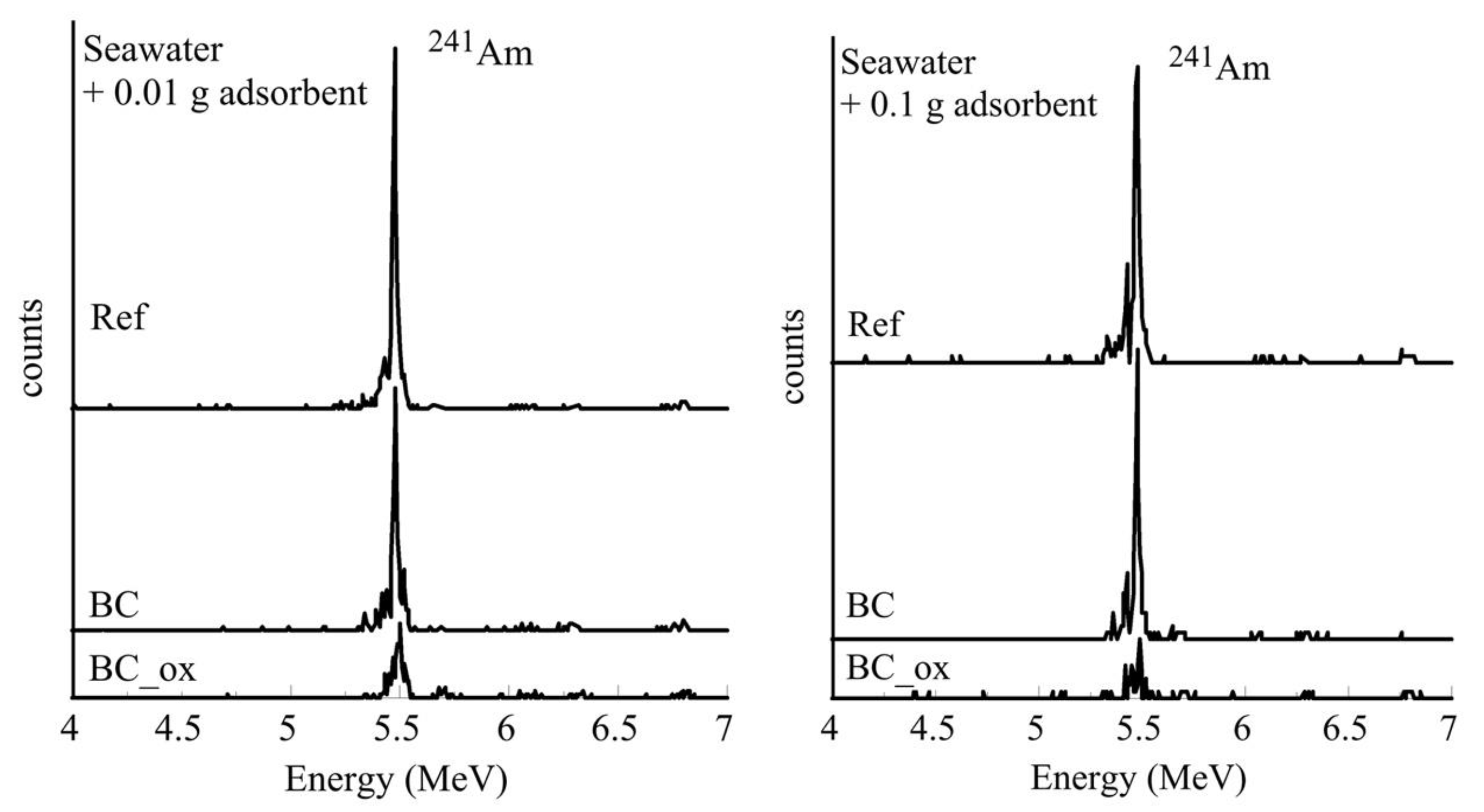
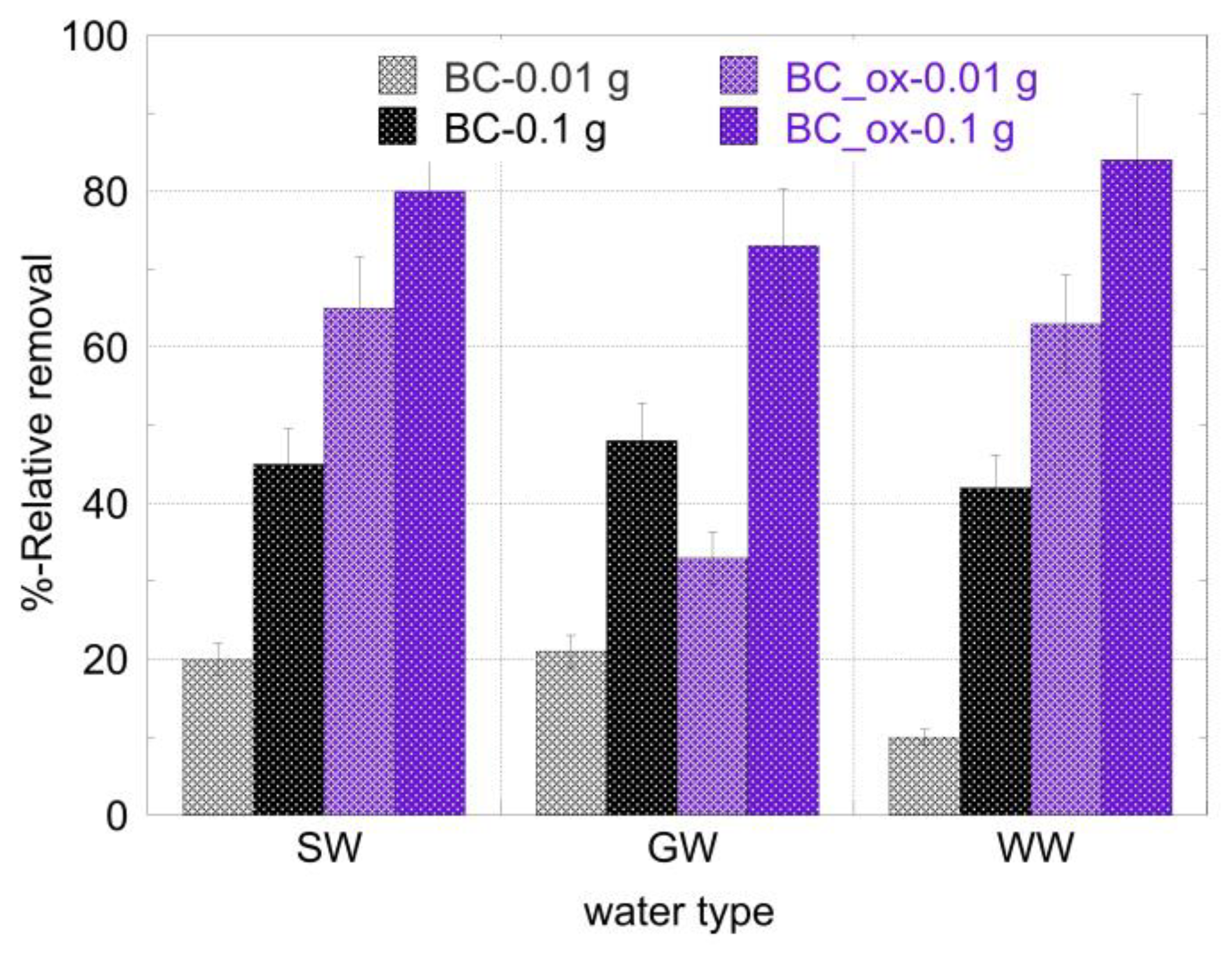
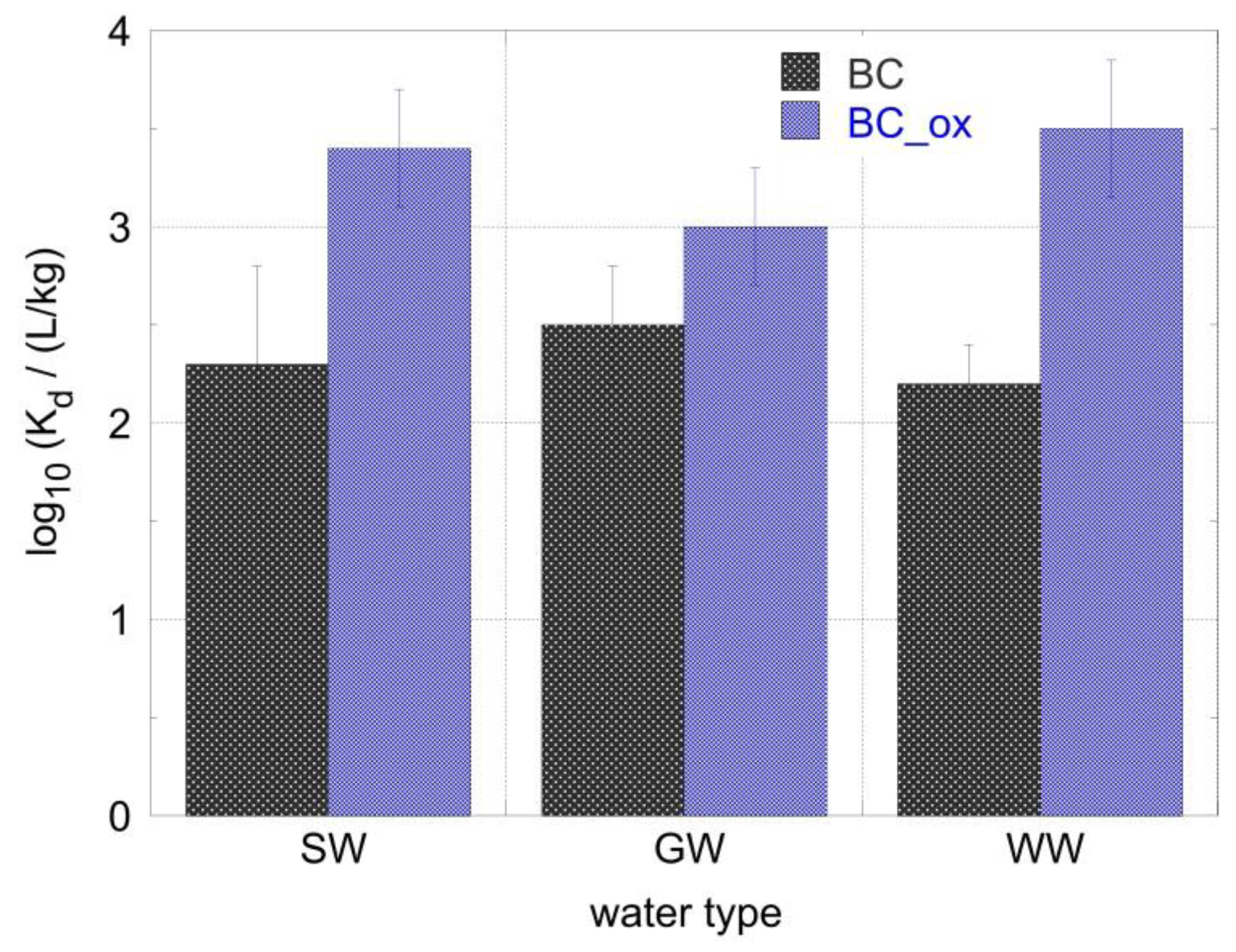
2.5. Removal of 241Am from Seawater, Groundwater, and Wastewater
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Warwick, P.E.; Croudace, I.W.; Carpenter, R. Review of analytical techniques for the determination of americium-241 in soils and sediments. Appl. Radiat. Isot. 1996, 47, 627–642. [Google Scholar] [CrossRef]
- Aggarwal, S.K. A review on the mass spectrometric studies of americium: Present status and future perspective. Mass Spectrom. Rev. 2018, 37, 43–56. [Google Scholar] [CrossRef]
- Nash, K.L. A review of the basic chemistry and recent developments in trivalent f-elements separations. Solvent Extr. Ion Exch. 1993, 11, 729–768. [Google Scholar] [CrossRef]
- Stadler, S.; Kim, J.I. Hydrolysis Reactions of Am (III) and Am (V). Radiochim. Acta 1988, 44, 39–44. [Google Scholar] [CrossRef]
- Meinrath, G.; Kim, J.I. The carbonate complexation of the Am (III) ion. Radiochim. Acta 1991, 52, 29–34. [Google Scholar] [CrossRef]
- Runde, W.; Meinrath, G.; Kim, J.I. A study of solid-liquid phase equilibria of trivalent lanthanide and actinide ions in carbonate systems. Radiochim. Acta 1992, 58, 93–100. [Google Scholar] [CrossRef]
- Ioannidis, I.; Xenofontos, A.; Anastopoulos, I.; Pashalidis, I. Americium Sorption by Microplastics in Aqueous Solutions. Coatings 2022, 12, 1452. [Google Scholar] [CrossRef]
- Hossain, F. Natural and anthropogenic radionuclides in water and wastewater: Sources, treatments and recoveries. J. Environ. Radioact. 2020, 225, 106423. [Google Scholar] [CrossRef] [PubMed]
- Panagiotou, N.; Liatsou, I.; Pournara, A.; Angeli, G.K.; Giappa, R.M.; Tylianakis, E.; Manos, M.J.; Froudakis, G.E.; Trikalitis, P.N.; Pashalidis, I.; et al. Water-stable 2-D Zr MOFs with exceptional UO22+ sorption capability. J. Mater. Chem. A 2020, 8, 1849–1857. [Google Scholar] [CrossRef]
- Georgiou, E.; Pashalidis, I.; Raptopoulos, G.; Paraskevopoulou, P. Efficient Removal of Polyvalent Metal Ions (Eu (III) and Th (IV)) from Aqueous Solutions by Polyurea-Crosslinked Alginate Aerogels. Gels 2022, 8, 478. [Google Scholar] [CrossRef]
- Georgiou, E.; Raptopoulos, G.; Papastergiou, M.; Paraskevopoulou, P.; Pashalidis, I. Extremely Efficient Uranium Removal from Aqueous Environments with Polyurea-Cross-Linked Alginate Aerogel Beads. ACS Appl. Polym. Mater. 2022, 4, 920–928. [Google Scholar] [CrossRef]
- Georgiou, E.; Raptopoulos, G.; Anastopoulos, I.; Giannakoudakis, D.A.; Arkas, M.; Paraskevopoulou, P.; Pashalidis, I. Uranium Removal from Aqueous Solutions by Aerogel-Based Adsorbents—A Critical Review. Nanomaterials 2023, 13, 363. [Google Scholar] [CrossRef]
- Giannakoudakis, D.A.; Anastopoulos, I.; Barczak, M.; Antoniou, Ε.; Terpiłowski, K.; Mohammadi, E.; Shams, M.; Coy, E.; Bakandritsos, A.; Katsoyannis, I.A.; et al. Enhanced uranium removal from acidic wastewater by phosphonate-functionalized ordered mesoporous silica: Surface chemistry matters the most. J. Hazard. Mater. 2021, 413, 125279. [Google Scholar] [CrossRef]
- Christou, C.; Philippou, K.; Krasia-Christoforou, T.; Pashalidis, I. Uranium adsorption by polyvinylpyrrolidone/chitosan blended nanofibers. Carbohydr. Polym. 2019, 219, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Anastopoulos, I.; Milojković, J.V.; Tsigkou, K.; Zafiri, C.; Lopičić, Z.R.; Kornaros, M.; Pashalidis, I. A nappies management by-product for the treatment of uranium-contaminated waters. J. Hazard. Mater. 2021, 404, 124147. [Google Scholar] [CrossRef] [PubMed]
- Prodromou, M.; Pashalidis, I. Uranium adsorption by non-treated and chemically modified cactus fibres in aqueous solutions. J. Radioanal. Nucl. Chem. 2013, 298, 1587–1595. [Google Scholar] [CrossRef]
- Anastopoulos, I.; Pashalidis, I.; Hosseini-Bandegharaei, A.; Giannakoudakis, D.A.; Robalds, A.; Usman, M.; Lima, É.C. Agricultural biomass/waste as adsorbents for toxic metal decontamination of aqueous solutions. J. Mol. Liq. 2019, 295, 111684. [Google Scholar] [CrossRef]
- Konstantinou, M.; Pashalidis, I. Adsorption of hexavalent uranium on biomass by-product. J. Radioanal. Nucl. Chem. 2007, 273, 549–552. [Google Scholar] [CrossRef]
- Prodromou, M.; Pashalidis, I. Chapter 1—(Radio)toxic metal ion adsorption by plant fibers. In Biomass-Derived Materials for Environmental Applications; Anastopoulos, I., Meili, L., Giannakoudakis, D., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 1–12. [Google Scholar] [CrossRef]
- Hadjittofi, L.; Prodromou, Μ.; Pashalidis, Ι. Activated biochar derived from cactus fibres—Preparation, characterization and application on Cu(II) removal from aqueous solutions. Bioresour. Technol. 2014, 159, 460–464. [Google Scholar] [CrossRef]
- Liatsou, I.; Michail, G.; Demetriou, M.; Pashalidis, I. Uranium binding by biochar fibres derived from Luffa cylindrica after controlled surface oxidation. J. Radioanal. Nucl. Chem. 2017, 311, 871–875. [Google Scholar] [CrossRef]
- Philippou, K.; Savva, I.; Pashalidis, I. Uranium (VI) binding by pine needles prior and after chemical modification. J. Radioanal. Nucl. Chem. 2018, 318, 2205–2211. [Google Scholar] [CrossRef]
- Philippou, M.; Pashalidis, I.; Theocharis, C.R. Uranium Isotope (U-232) Removal from Waters by Biochar Fibers: An Adsorption Study in the Sub-Picomolar Concentration Range. Molecules 2022, 27, 6765. [Google Scholar] [CrossRef] [PubMed]
- Philippou, K.; Pashalidis, I. Polyvalent metal ion adsorption by chemically modified biochar fibers. In Biomass-Derived Materials for Environmental Applications; Elsevier: Amsterdam, The Netherlands, 2022; pp. 267–286. [Google Scholar] [CrossRef]
- Wasilewska, M.; Derylo-Marczewska, A. Adsorption of Non-Steroidal Anti-Inflammatory Drugs on Alginate-Carbon Composites—Equilibrium and Kinetics. Materials 2022, 15, 6049. [Google Scholar] [CrossRef]
- Ioannou, K.; Hadjiyiannis, P.; Liatsou, I.; Pashalidis, I. U (VI) adsorption by biochar fiber–MnO2 composites. J. Radioanal. Nucl. Chem. 2022, 320, 425–432. [Google Scholar] [CrossRef]
- Philippou, K.; Anastopoulos, I.; Dosche, C.; Pashalidis, I. Synthesis and characterization of a novel Fe3O4-loaded oxidized biochar from pine needles and its application for uranium removal. Kinetic, thermodynamic, and mechanistic analysis. J. Environ. Manag. 2019, 252, 109677. [Google Scholar] [CrossRef]
- Liatsou, I.; Pashalidis, I.; Nicolaides, A. Triggering selective uranium separation from aqueous solutions by using salophen-modified biochar fibers. J. Radioanal. Nucl. Chem. 2018, 318, 2199–2203. [Google Scholar] [CrossRef]
- Palansooriya, K.N.; Yoon, I.-N.; Kim, S.-M.; Wang, C.-H.; Kwon, H.; Lee, S.-H.; Igalavithana, A.D.; Mukhopadhyay, R.; Sarkar, B.; Ok, Y.S. Designer biochar with enhanced functionality for efficient removal of radioactive cesium and strontium from water. Environ. Res. 2022, 214, 114072. [Google Scholar] [CrossRef]
- Sumalatha, B.; Narayana, A.V.; Khan, A.A.; Venkateswarulu, T.C.; Reddy, G.S.; Reddy, P.R.; Babu, D.J. A Sustainable Green Approach for Efficient Capture of Strontium from Simulated Radioactive Wastewater Using Modified Biochar. Int. J. Environ. Res. 2022, 16, 75. [Google Scholar] [CrossRef]
- Černe, M.; Palčić, I.; Major, N.; Pasković, I.; Perković, J.; Užila, Z.; Filipović, V.; Romić, M.; Goreta Ban, S.; Jaćimović, R.; et al. Effect of sewage sludge derived compost or biochar amendment on the phytoaccumulation of potentially toxic elements and radionuclides by Chinese cabbage. J. Environ. Manag. 2021, 293, 112955. [Google Scholar] [CrossRef]
- Szewczak, K.; Jednoróg, S.; Wołoszczuk, K.; Szlązak, R.; Podgórska, Z.; Rafalska-Przysucha, A.; Gluba, Ł.; Łukowski, M. Impact of soil incorporation of biochar on environmental radioactivity. J. Environ. Qual. 2020, 49, 428–439. [Google Scholar] [CrossRef]
- Zhang, W.; He, X.; Ye, G.; Yi, R.; Chen, J. Americium (III) capture using phosphonic acid-functionalized silicas with different mesoporous morphologies: Adsorption behavior study and mechanism investigation by EXAFS/XPS. Environ. Sci. Technol. 2014, 48, 6874–6881. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Kim, S.-Y.; Ito, T.; Hitomi, K.; Kuraoka, E.; Usuda, S.; Ishii, K. Adsorption behavior of trivalent americium and rare earth ions onto a microporous silica-based isobutyl-BTP/SiO2 adsorbent in nitric acid solution. J. Radioanal. Nucl. Chem. 2014, 299, 149–155. [Google Scholar] [CrossRef]
- Shu, Q.; Khayambashi, A.; Zhou, Q.; Wang, X.; Wei, Y.; He, L.; Tang, F. Studies on adsorption and separation characteristics of americium and lanthanides using a silica-based microporous bi(2-ethylhexyl)phosphoric acid (HDEHP) adsorbent. J. Radioanal. Nucl. Chem. 2017, 313, 29–37. [Google Scholar] [CrossRef]
- Saipriya, G.; Kumaresan, R.; Nayak, P.K.; Venkatesan, K.A.; Antony, M.P.; Kumar, T. Studies on the adsorption behavior of americium and europium on radiolytically degraded solvent impregnated resin containing neutral and acidic extractants. J. Radioanal. Nucl. Chem. 2017, 314, 2557–2568. [Google Scholar] [CrossRef]
- Khadir, A.; Motamedi, M.; Pakzad, E.; Silanpää, M.; Mahajan, S. The prospective utilization of Luffa fibres as a lignocellulosic bio-material for environmental remediation of aqueous media: A review. J. Environ. Chem. Eng. 2021, 9, 104691. [Google Scholar] [CrossRef]
- Panteli, S.; Savva, I.; Efstathiou, M.; Vekas, L.; Marinica, O.M.; Krasia-Christoforou, T.; Pashalidis, I. β-ketoester-functionalized magnetoactive electrospun polymer fibers as Eu (III) adsorbents. SN Appl. Sci. 2019, 1, 30. [Google Scholar] [CrossRef]
- Kiliari, T.; Pashalidis, I. Simplified alpha-spectroscopic analysis of uranium in natural waters after its separation by cation-exchange. Radiat. Meas. 2010, 45, 966–968. [Google Scholar] [CrossRef]
- Li, W.; Ding, Y.; Tao, Z. Americium (III) adsorption on polyethylene from very dilute aqueous solutions. J. Radioanal. Nucl. Chem. 2001, 250, 497–500. [Google Scholar] [CrossRef]
- Hadjittofi, L.; Charalambous, S.; Pashalidis, I. Removal of trivalent samarium from aqueous solutions by activated biochar derived from cactus fibres. J. Rare Earths 2016, 34, 99–104. [Google Scholar] [CrossRef]
- Liatsou, I.; Pashalidis, I.; Oezaslan, M.; Dosche, C. Surface characterization of oxidized biochar fibers derived from Luffa Cylindrica and lanthanide binding. J. Environ. Chem. Eng. 2017, 5, 4069–4074. [Google Scholar] [CrossRef]
- Ramírez-Guinart, O.; Kaplan, D.; Rigol, A.; Vidal, M. Deriving Probabilistic Soil Distribution Coefficients (Kd). Part 3: Reducing Variability of Americium Kd Best Estimates Using Soil Properties and Chemical and Geological Material Analogues. J. Environ. Radioact. 2020, 223–224, 106378. [Google Scholar] [CrossRef] [PubMed]


| Parameter (mg/L) | Wastewater | Groundwater | Seawater |
|---|---|---|---|
| pH | 8.1 | 7.8 | 8.3 |
| K+ | 29 | <3 | 395 |
| Na+ | nd a | 40 | 10,680 |
| Ca2+ | 87 | 38 | 410 |
| Mg2+ | 55 | 70 | 1280 |
| Fe3+ | nd | <35 | 0.003 |
| Cu2+ | nd | <50 | 0.09 |
| Cl− | 298 | 54 | 19,200 |
| HCO3− | nd | 370 | 140 |
| SO42− | 111 | 95 | 2680 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Philippou, M.; Pashalidis, I.; Kalderis, D. Removal of 241Am from Aqueous Solutions by Adsorption on Sponge Gourd Biochar. Molecules 2023, 28, 2552. https://doi.org/10.3390/molecules28062552
Philippou M, Pashalidis I, Kalderis D. Removal of 241Am from Aqueous Solutions by Adsorption on Sponge Gourd Biochar. Molecules. 2023; 28(6):2552. https://doi.org/10.3390/molecules28062552
Chicago/Turabian StylePhilippou, Maria, Ioannis Pashalidis, and Dimitrios Kalderis. 2023. "Removal of 241Am from Aqueous Solutions by Adsorption on Sponge Gourd Biochar" Molecules 28, no. 6: 2552. https://doi.org/10.3390/molecules28062552
APA StylePhilippou, M., Pashalidis, I., & Kalderis, D. (2023). Removal of 241Am from Aqueous Solutions by Adsorption on Sponge Gourd Biochar. Molecules, 28(6), 2552. https://doi.org/10.3390/molecules28062552








