1. Introduction
Electrocatalytic overall water splitting exhibits great potential in sustainable hydrogen production, which is composed of two half reactions, one being a hydrogen evolution reaction (HER) and the other being an oxygen evolution reaction (OER) [
1,
2]. However, the efficiency of water splitting has been strongly hindered by the sluggish kinetics process [
3]. Previous reports have proved that the traditional noble metal–based electrocatalysts have high catalytic performance on HER or OER, such as iridium and ruthenium oxides for OER and platinum for HER [
4,
5]. However, these catalysts might only be suitable for the half reaction, and commonly suffer from expensive costs and scarce reserves. In general, several requirements must be met for water splitting catalysts to achieve practical applications: (1) stable and high active sites at large current density [
6]; (2) fast electron transfer [
7]; and (3) low-cost and easy access [
8]. Recently, some progress was achieved in electrochemical water splitting; nevertheless, most of the reported electrodes were evaluated in terms of overpotentials and stability at low current densities, which are not practical for large-scale applications [
9]. It remains an urgent need to explore high-performance catalysts with large current density and robust durability for overall water splitting [
3,
10]. The development of non-noble metal bifunctional electrocatalysts with robust and stable catalytic performance has exhibited great potential in practical applications, and the exploration of bifunctional catalysts with both HER and OER will significantly simplify the water splitting devices.
Currently, tremendous efforts have been made in developing efficient and scalable overall water splitting electrocatalysts [
11,
12]. It has been established that the transition metal phosphides, such as Fe and Ni-based phosphides, possess superior electrical conductivity and high electron density near the Fermi level. They can effectively improve the intrinsic conductivity of materials to realize large charge carrier transfer efficiency and catalytic capability in the electrocatalytic process [
13,
14]. The positively charged metal cations in metal phosphides can be regarded as the hydroxyl receptors, and negatively charged phosphorous active sites can accelerate the dissociation of H
2 to boost HER activity. Furthermore, the introduction of secondary metal into the metal center can increase the active sites, optimize the
eg orbitals, change the charge transfer path, and modulate the electronic structure for better electrocatalytic capabilities in overall water splitting [
15]. To date, however, the uncontrolled generation of multiple segregated phases due to the reactive difference of metal centers still makes it challenging to synthesize homogeneous bimetallic phosphides.
As a new class of crystalline materials, metal-organic frameworks (MOFs) are constructed with metal nodes and organic ligands [
16,
17,
18]. Their compositions and chemical environments can be easily modulated and systematically designed via changes of metal ions in the MOF precursor, or by annealing processes in various environments [
19]. In addition, the structure and morphology of the obtained products can be well preserved [
20,
21]. For now, numerous different functional MOFs have been created by changing the metal ions/clusters and organic linkers which have been regarded as promising candidates for carbon-based materials [
22,
23].
In this work, we report the designing and fabricating of Ni2P/FeP heterojunction encapsulated in N, P-doped carbon frameworks (donated as Ni2P/FeP@NPC) through a bimetallic MOF precursor topochemical conversion, aiming at boosting both the activity and durability of OER and HER electrocatalysts. First, we synthesized the Ni/Fe MOF, which was then followed by pyrolysis, and the phosphating of the Ni2P/FeP@NPC was conducted. Because of the remarkable HER and OER activity, this bifunctional Ni2P/FeP@NPC was integrated in an alkaline electrolyzer as both the anode and cathode electrodes. The experimental results demonstrated that only a cell voltage of 1.64 V could deliver 10 mA cm−2, and a cell voltage of 2.37 V was needed to deliver 500 mA cm−2 with 200 h durability, outperforming most catalysts with similar functions and those of precious metal catalysts (Pt/C//RuO2, 2.41 V for 500 mA cm−2).
2. Results and Discussion
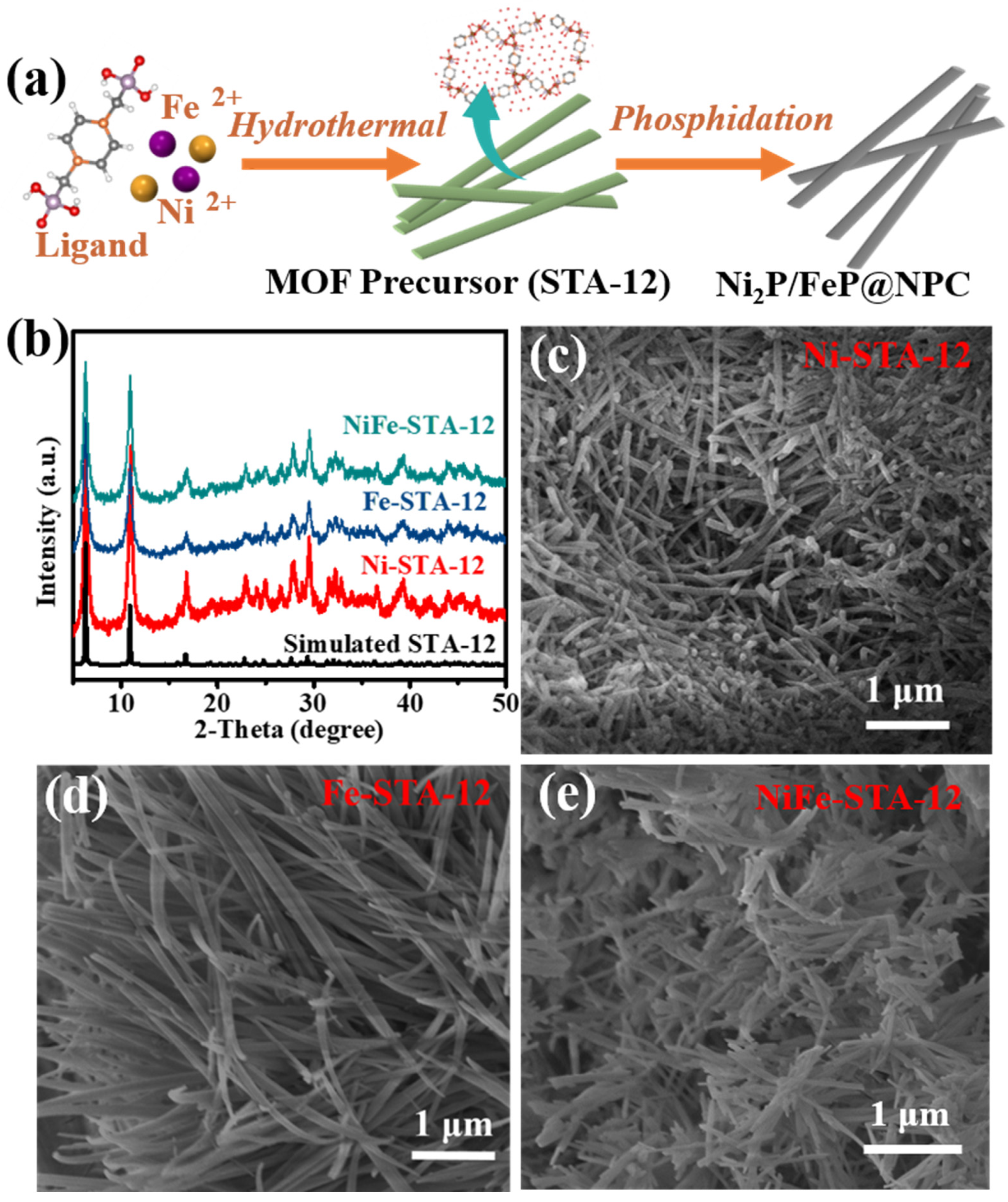
The synthetic step of the Ni
2P/FeP@NPC catalysts is illustrated in
Figure 1a. The MOFs precursors (denoted as STA-12) were prepared with a hydrothermal method according to previous work with small modifications (all details can be obtained from the
Supporting information) [
24]. The metal nodes in STA-12 were tailored by adjusting the species of metal salts in the preparation process. As shown in
Figure 1b, the powder X-ray diffraction (PXRD) patterns demonstrated that all STA-12 crystals possessed the same PXRD pattern and exhibited high agreement with the reference [
24], which confirmed that the bimetallic STA-12 crystals possessed the same crystal structure with single-metal-center STA-12. The scanning electron microscopy images (SEM) in
Figure 1c–e show that all the STA-12 exhibited similar regular nanorod morphologies. Interestingly, as shown in
Figure 1e, compared with single-metal-center STA-12, the bimetallic STA-12-FeNi exhibited much slender morphologies, which might be caused by the presence of Fe that caused the MOF crystal growing along specific lattice plane, limiting the growth in other lattice planes.
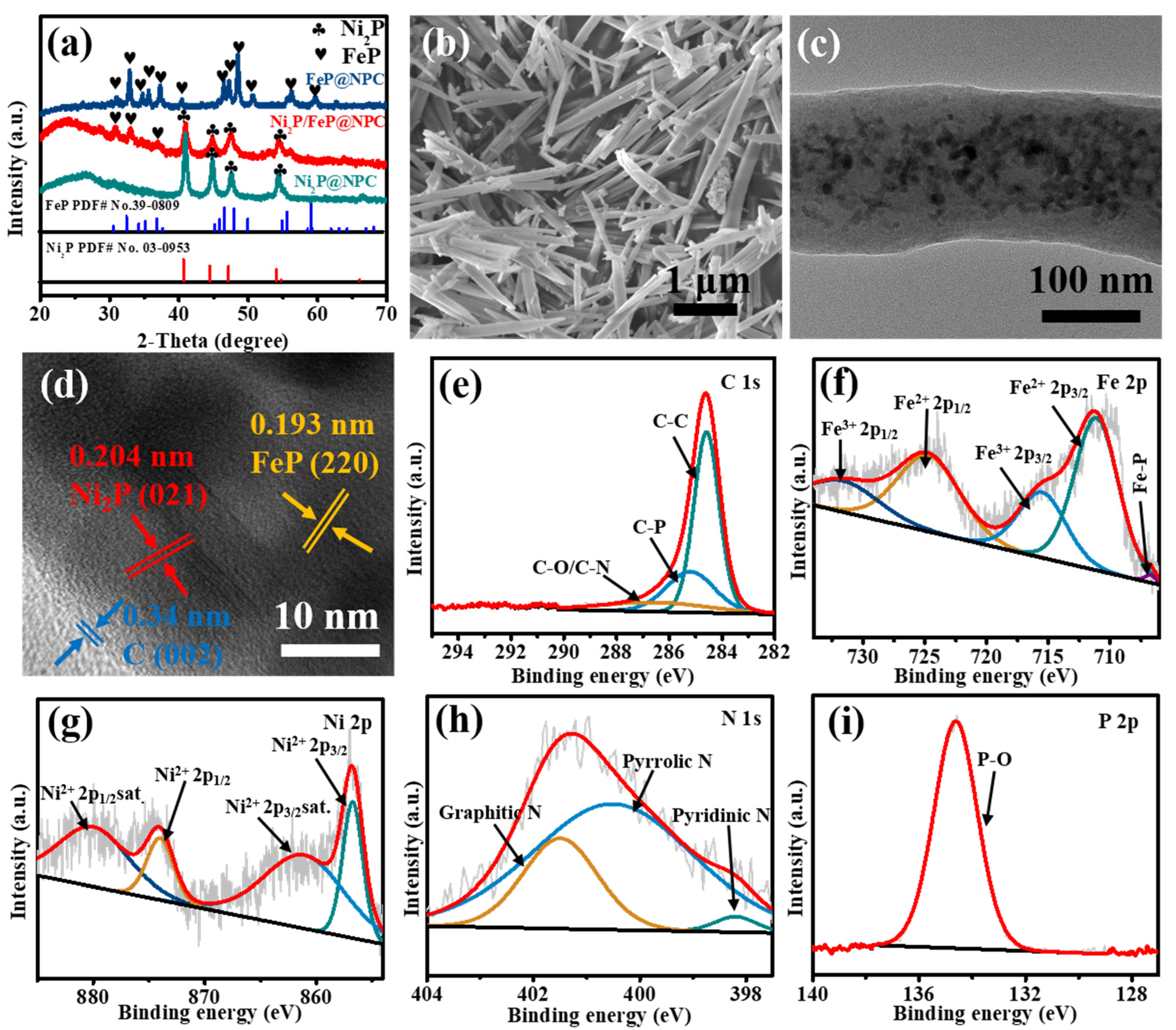
After carbonization and phosphorization of the MOF precursor, the XRD patterns of Ni
2P@NPC, FeP@NPC, and Ni
2P/FeP@NPC are shown in
Figure 2a. The peaks at 2θ of 31.83°, 35.33°, 40.75°, 44.64°, 54.15° and 54.37° are matched well with hexagonal Ni
2P crystal planes (JCPDS No. 65-3544) of (011), (111), (021), (210), (300) and (002), respectively. Meanwhile, the peaks of FeP at 2θ of 32.78°, 37.18°, 46.32°, 46.98°, 48.36°, 56.09° are also illustrated, which belong to the (011), (111), (112), (202), (211) and (212) crystal planes respectively, and exhibited high agreement with orthorhombic FeP (JCPDS No. 65-2595). As shown in
Figure S1, the obtained Raman spectrums of the all catalysts exhibited two broad and obvious peaks positioned at 1355 and 1603 cm
−1 corresponding to the typical D and G bands of graphene, respectively. The detection of these two peaks demonstrated the existence of substantial defects or disordered sites, which might be due to the concurrent doping and absence of C atoms. The SEM images were then collected to explore the morphology of the materials after the carbonization of MOF precursors (
Figure 2b and S2). It can be seen that the morphology of Ni
2P@NPC, Ni
2P/FeP@NPC and FeP@NPC are similar to the MOF precursors, indicating that the morphology can be well preserved during the phase transformation process. As shown in
Figure 2c, the transmission electron microscopy (TEM) image exhibited that Ni
2P/FeP@NPC possessed a typical rod-shaped morphology with a diameter of approximately 120 nm. From high-resolution TEM (HRTEM) images (
Figure 2d), lattice fringes could be observed in the Ni
2P/FeP@NPC, in which the spacing of 0.204 nm was attributed to the (021) planes of Ni
2P. In contrast, the lattice fringe with an inter-planar distance of 0.193 nm was corresponding to the (220) crystal planes of FeP. All of these results exhibited high consistency with the XRD analysis, proving the successful preparation of Ni
2P/FeP@NPC. As shown in
Figure 2d, the obvious phase boundary between the marked Ni
2P and FeP proved the production of a heterostructure, which could generate an interfacial bonding effect that facilitates the exposure of more active sites. Furthermore, the lattice fringe with a spacing distance of 0.34 nm could be ascribed to the (002) plane of graphitic carbon, confirming the existence of carbon substrate through the pyrolysis process. The energy-dispersive X-ray spectrometry (EDX) mapping was applied to reveal the element distribution of Ni
2P/FeP. As shown in
Figure S3, the elements of C, Ni, Fe, P, and N are homogeneously dispersed in the structures, demonstrating the successful preparation of Ni
2P/FeP@NPC compounds.
X-ray photoelectron spectroscopy (XPS) was employed to evaluate the valence state and composition of the prepared samples more accurately. The XPS survey scan spectrum in
Figure S4 indicated the existence of C, Fe, Ni, N, and P in the architecture. The high-resolution spectra of these elements further confirmed the formation of Ni
2P/FeP@NPC. The C 1s XPS spectrum in
Figure 2e could be deconvoluted into three peaks positioned at 286.5, 285.2, and 284.6 eV, which were ascribed to the C-O/C-N, C-P, and C-C/C = C groups, respectively [
25]. The existence of C-O, C-P, and C-N offered numerous anchoring sites for electrochemically active materials to inhibit agglomeration or disengagement of particles from electrodes. As shown in
Figure 2f, two main spin-orbit doublets in the Fe 2p spectrum could be divided into four components: the first doublet located at 711.1 eV and 724.6 eV belonged to Fe
2+ 2p
3/2 and Fe
2+ 2p
1/2, and the second doublet located at 715.5 eV and 731.6 eV was ascribed to the splitting peaks of Fe
3+ 2p
3/2 and Fe
3+ 2p
1/2, respectively. In addition, the small peak positioned at 706.7 eV indicated the existence of metallic Fe [
26]. As shown in
Figure 2g, the two spin-orbit doublet peaks at 856.7 and 874.0 eV with two shake-up satellites at 861.1 and 880.0 eV indicated the existence of oxidized Ni species in Ni
2P/FeP@NPC, ascribed to the surface oxidation in air [
27]. As shown in
Figure 2h, the peak located at 133.7 eV in the P 2p region was attributed to the oxidized phosphide (P-O) [
26]. Furthermore, the N 1s spectrum in
Figure 2i could be deconvoluted into pyridinic-N (398.2 eV), pyrrolic-N (400.5 eV), and quaternary-N (401.5 eV), confirming the incorporation of N into Ni
2P/FeP@NPC. Previous reports have proved that the incorporation of the N species in the catalysts could efficiently improve the intrinsic electrocatalytic activities, which could effectively improve the catalytic performance [
28]. All of these results demonstrated that Ni
2P/FeP@NPC catalysts with the combination of conductive N, P-doped carbon and highly active Ni
2P/FeP heterostructures have been established.
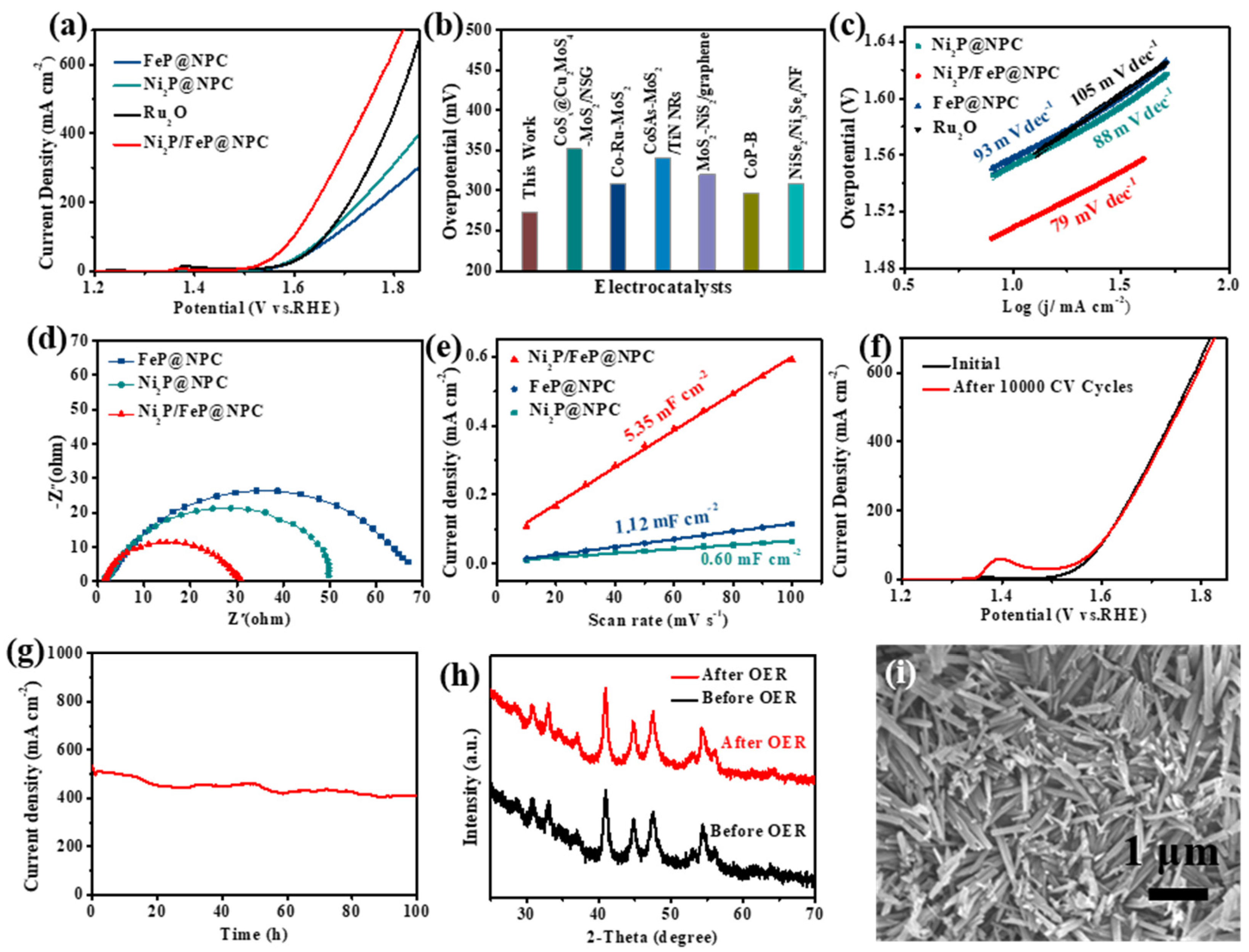
The catalytic performance of the Ni
2P/FeP@NPC hybrid catalyst was then investigated. Firstly, the OER activity of the Ni
2P/FeP@NPC catalysts in 1.0 M KOH electrolyte was investigated. As shown in
Figure 3a, the representative polarization curves exhibited the geometric current density plotted against applied potential vs the reversible hydrogen electrode (RHE) of this Ni
2P/FeP@NPC catalyst. The OER activities of Ni
2P@NPC, FeP@NPC, and RuO
2 were also evaluated by the same procedure. As shown in
Figure 3a, Ni
2P@NPC and FeP@NPC show larger overpotential of 621 and 699 mV at 400 mA cm
−2, respectively, while Ni
2P/FeP@NPC exhibited a much higher OER catalytic activity possessing the overpotential of 487 mV under the same condition. Specifically, the OER activities of other available bifunctional catalysts were compared with Ni
2P/FeP@NPC in
Figure 3b and
Table S1. Experimental results exhibited that the catalyst in this work required the lowest overpotential of 273 mV to achieve 10 mA cm
−2, indicating the potential application in overall water splitting at the small cell voltage. The OER kinetics of Ni
2P/FeP@NPC was further investigated by the Tafel slope to disclose the inherent property of catalysts. As shown in
Figure 3c, the measured Tafel slopes of Ni
2P/FeP@NPC was 79 mV dec
−1, was considerably lower than the counterparts of FeP (93 mV dec
−1), RuO
2 (105 mV dec
−1), and Ni
2P (88 mV dec
−1). The much smaller Tafel slopes of Ni
2P/FeP@NPC revealed the favorable OER kinetics of Ni
2P/FeP@NPC. Electrochemical impedance spectroscopy (EIS) measurements of Ni
2P@NPC, Ni
2P/FeP@NPC and FeP@NPC in
Figure 3d were conducted at OER conditions to reveal the charge transfer kinetics. The semicircle of Ni
2P/FeP@NPC is much smaller than that of Ni
2P@NPC and FeP@NPC, demonstrating that the bimetallic phosphide Ni
2P/FeP@NPC possessed smaller charge transfer resistance. The electrochemically active surface area values of catalysts were further investigated by double-layer capacitances (Cdl), which were obtained by calculating the CV curves at different scan rates (
Figures S5–S7). As shown in
Figure 3e, the Cdl values of Ni
2P/FeP@NPC (5.35 mF cm
−2) are much higher than those of Ni
2P (0.60 mF cm
−2) and FeP (1.12 mF cm
−2), demonstrating the higher exposed catalytic sites of Ni
2P/FeP@NPC. Stability is another important indicator of electrocatalysts for practical applications. We further explored the stability of Ni
2P/FeP@NPC by the long-term cycling test and amperometric i–t measurement. As illustrated in
Figure 3f, the polarization curve of Ni
2P/FeP@NPC after 10,000 CV cycles almost overlaps with the initial curve. Furthermore, the Ni
2P/FeP@NPC maintained good stability for 100 h at 500 mA cm
−2 (
Figure 3g), which proves the long-term durability of Ni
2P/FeP@NPC under large current density.
Because of the fantastic OER durability of Ni
2P/FeP@NPC, we further tried to investigate the origin of OER performance. XRD, SEM and XPS were used to study the characteristics of Ni
2P/FeP@NPC after OER stability testing (at the current density of 500 mA cm
−2 for 100 h). As shown in
Figure 3h, the XRD pattern of Ni
2P/FeP@NPC did not change obviously after OER, implying that the crystal structure remained unchanged. Meanwhile, the morphology of the sample after OER was tested and presented in
Figure 3i. It can be concluded that the Ni
2P/FeP@NPC maintained its morphology and dimension after the stability testing, demonstrating the excellent corrosion resistance and chemical stability of Ni
2P/FeP@NPC. XPS was further utilized to evaluate the change in the surface chemical composition and the electronic state after the catalytic process. The XPS spectrum after initial OER in
Figure S8 exhibited the presence of Ni, Fe, C, P and N elements in the catalysts. The corresponding peaks of C, Fe, Ni, N and P in the high-resolution XPS spectrum exhibited little change with the appearance of the peak attributed to O = C-O (292.1 eV) (
Figure S8b–f), suggesting the slight surface oxidation of Ni
2P/FeP@NPC. The result agreed with XRD analysis, which proved that the N, P-doped carbon could effectively prevent the Ni
2P/FeP from degradation, oxidation and corrosion during the OER. Therefore, the real active sites of Ni
2P/FeP@NPC electrocatalyst for OER were the combination of N, P-doped carbon, and the Ni
2P/FeP heterostructures.
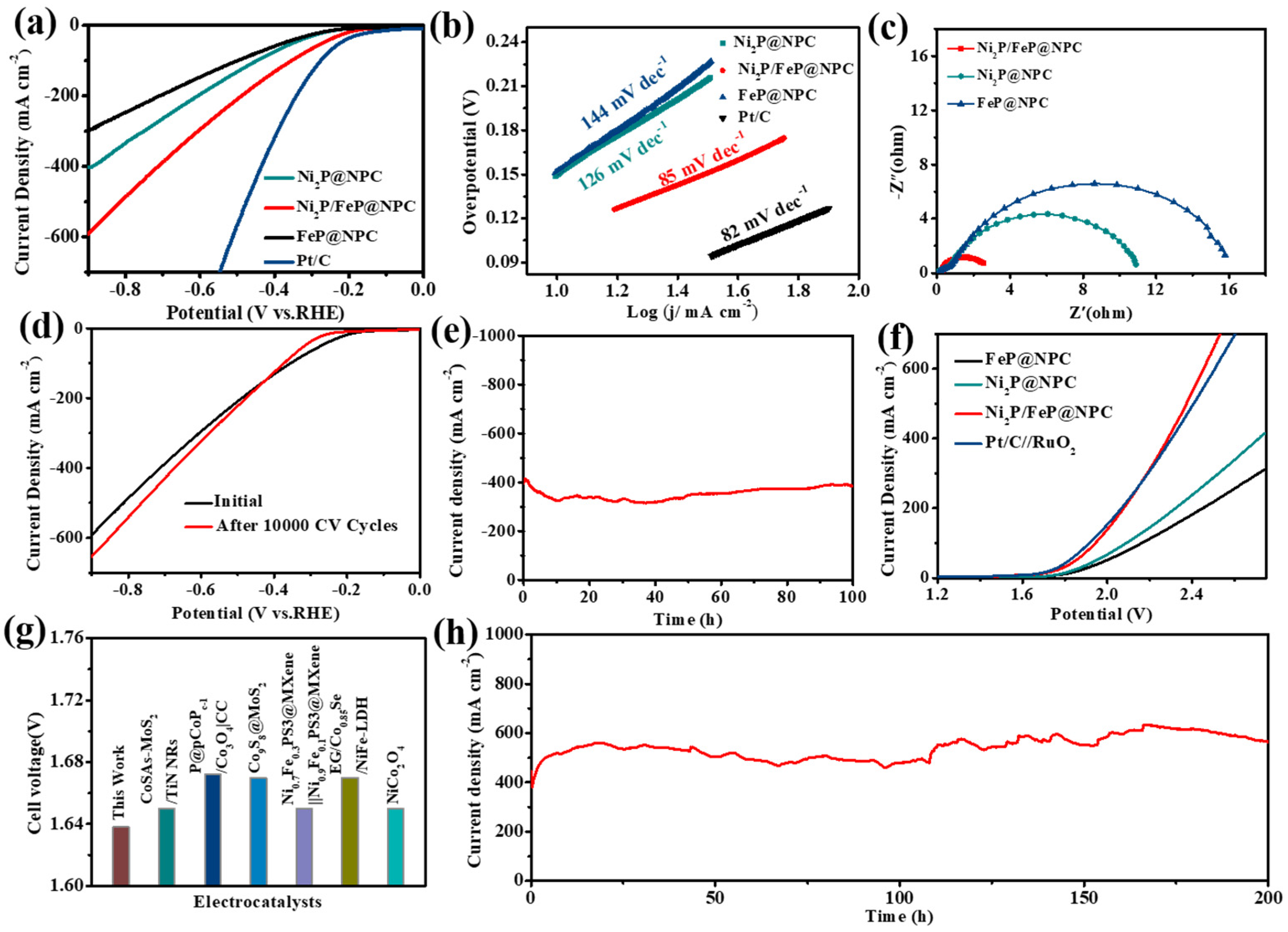
Next, we further explored the possibility of Ni
2P/FeP@NPC as a HER catalyst. As shown in
Figure 4a, Ni
2P/FeP@NPC exhibited considerable HER catalytic activity with low overpotentials of 182 mV to obtain the current density of 10 mA cm
−2, while the much higher overpotentials of 217 and 214 mV were needed to achieve the current density of 10 mA cm
−2 for FeP@NPC and Ni
2P@NPC, respectively. Moreover, the calculated Tafel slope of Ni
2P/FeP@NPC in
Figure 4b was 85 mV dec
−1, which was smaller than those of Ni
2P (126 mV dec
−1) and FeP (144 mV dec
−1), indicating that Ni
2P/FeP@NPC possessed the fastest HER kinetic process. Moreover, EIS was also conducted to evaluate the intrinsic HER electrocatalytic kinetics. The corresponding Rct of Ni
2P/FeP@NPC was smaller than those of Ni
2P@NPC and FeP@NPC, demonstrating the higher transfer coefficient and electronic conductivity (
Figure 4c). Furthermore, the Ni
2P/FeP@NPC catalyst possessed excellent stability during long-time HER operations. As shown in
Figure 4d, the catalytic performance exhibited no apparent deterioration after 10,000 cycles. As shown in
Figure 4e, Ni
2P/FeP@NPC maintained the current density of 400 mA cm
−2 for 100 h without obvious decline, demonstrating the fantastic durability of Ni
2P/FeP@NPC in the HER process. Such excellent HER durability of Ni
2P/FeP@NPC ranks at the top level of the reported HER electrocatalysts.
Because of the fantastic catalytic activity of Ni
2P/FeP@NPC for HER and OER, the two-electrode water splitting device was constructed using Ni
2P/FeP@NPC as the bifunctional catalyst for HER and OER. Remarkably, the cell voltage to afford the current density of 10 mA cm
−2 was low to 1.64 V (
Figure 4f), which was comparable to the coupled benchmark RuO
2//Pt/C catalysts (1.63 V), and better than many previously reported bifunctional electrocatalysts (
Figure 4g and
Table S2). In addition, as shown in
Figure 4h, the device exhibited outstanding stability for 200 h after the long-term test at the current density of 500 mA cm
−2.
To clarify the interfacial charge properties, theoretical models of Ni
2P@NPC, FeP@NPC, and Ni
2P/FeP@NPC heterostructures were established (
Figure S9) and DFT calculations were utilized to evaluate their surface energetics.
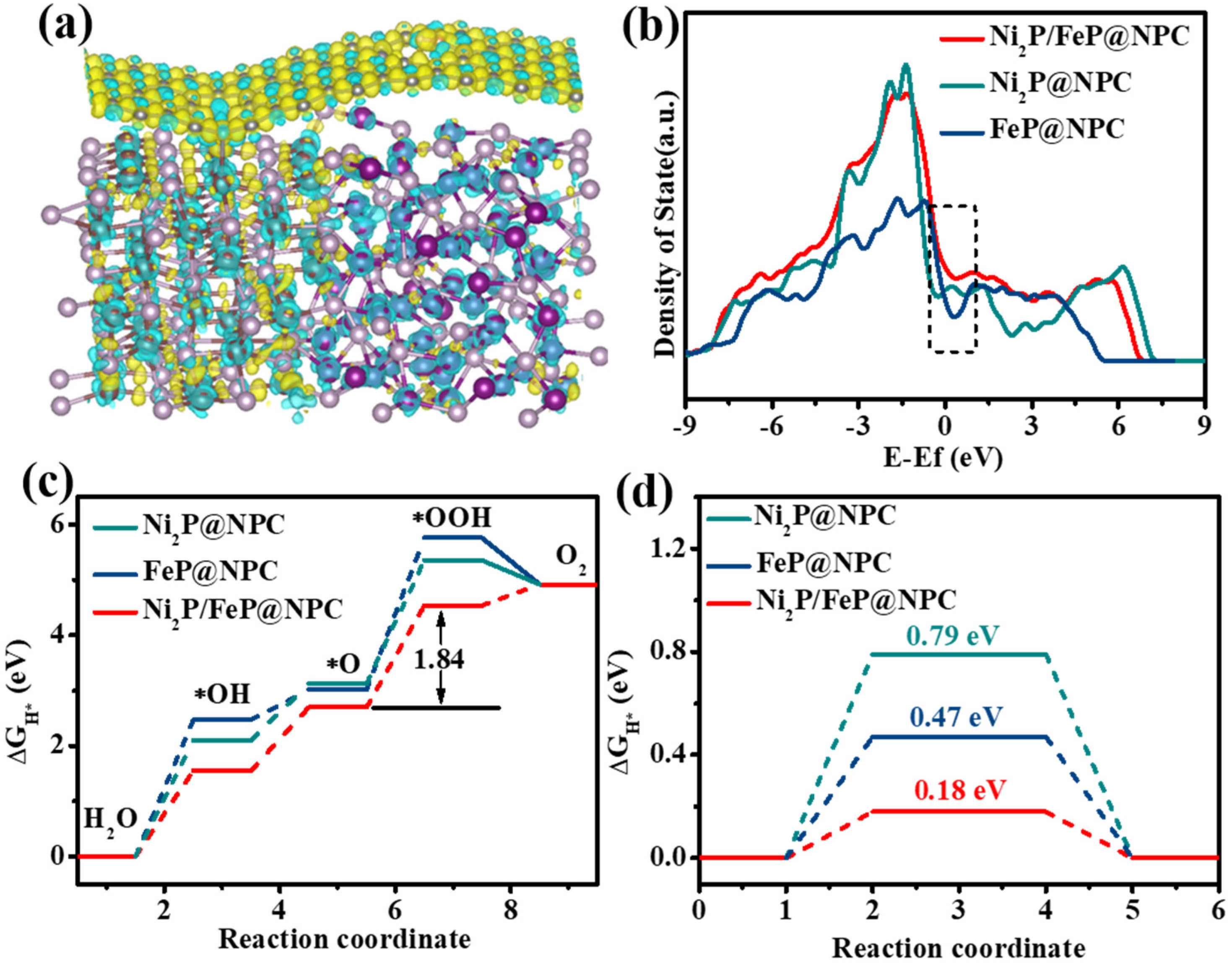
Figure 5a shows the differential charge density between N, P-doped carbon and Ni
2P/FeP in the heterostructure. Yellow and cyan refer to the positive and negative charges, respectively. As shown in
Figure 5a, the differential charge density at the Ni
2P/FeP@NPC interface indicated that the significant electron redistribution was achieved through the extensive electron transformation from the Ni
2P/FeP side to the NPC region. Thus, the NPC side was electron accumulating, whereas the Ni
2P/FeP domain was electron depleting, which indicated the strong electronic interaction between these two domains. This kind of interfacial electronic structure was beneficial for the surface adsorption of the intermediate. As shown in
Figure 5b, the density of states (DOS) was further conducted to evaluate their electrical conductivity. The results showed that the Ni
2P/FeP@NPC possessed higher carrier density around the Fermi level compared with Ni
2P@NPC and FeP@NPC, which demonstrated that better OER catalytic activity could be achieved through the improvement of the electrical conductivity. The intrinsic OER catalytic activities were then analyzed by calculating the surfaces Gibbs free energy (Δ
G) profiles of Ni
2P@NPC, FeP@NPC, and Ni
2P/FeP@NPC models through a four-step pathway at alkaline conditions (
Figure S10):
where * denotes the active sites on the catalyst surface. As shown in
Figure 5a, the Δ
G diagrams demonstrated that all electrocatalysts possessed the same rate-determining steps (RDS), which was the formation of the *OOH intermediate in the third step. The Ni
2P/FeP@NPC electrocatalyst displayed the smallest total energy barrier (Δ
G: 1.84 eV) compared with the Ni
2P@NPC (2.23 eV) and FeP@NPC (2.75 eV). These results indicated that the formation of the heterointerface could optimize the adsorption and desorption steps in Ni
2P/FeP@NPC to accelerate the whole OER process. As for the HER process, the intrinsic HER activities of the catalysts could be investigated through the Gibbs free energy of hydrogen (ΔG
H*) adsorption. The negative ΔG
H* is beneficial for the adsorption of H* but generates side effects on the desorption of products while too positive ΔG
H* does the opposite. When ΔG
H* is tending to zero, the H* and H
2 are more easily adsorbed and desorbed at the active center, which could be beneficial for the HER. The Ni
2P/FeP@NPC presented the positive ΔG
H* of 0.18 eV (
Figure 5d), which was much lower than Ni
2P@NPC (0.79 eV) and FeP@NPC (0.47 eV), confirming that the interaction between Ni
2P/FeP and N, P-doped carbon could adjust the ΔG
H* to zero to facilitate the reaction.
All above theoretical/experimental experiments demonstrated that the fantastic catalytic activities of Ni2P/FeP@NPC were ascribed to the three-phase heterojunction interface constructed by NPC, Ni2P and FeP, which could effectively optimize the desorption of intermediates and thus significantly boost the kinetics in OER, HER, and the overall water splitting process. The heterojunction interface optimized the adsorption/desorption ability of intermediates (O*, OH*, OOH* and H*), and the inner Ni2P/FeP possess superior electrical conductivity and high electron density near the Fermi level, which has better conductivity and a high transfer coefficient, could produce synergistic effects in promoting both HER and OER performance.