Phytochemicals, Antioxidant and Antimicrobial Potentials and LC-MS Analysis of Centaurea parviflora Desf. Extracts
Abstract
1. Introduction
2. Results and Discussion
2.1. Extraction Yield
2.2. Qualitative Analysis
2.2.1. Thin-Layer Chromatography Analysis (TLC)
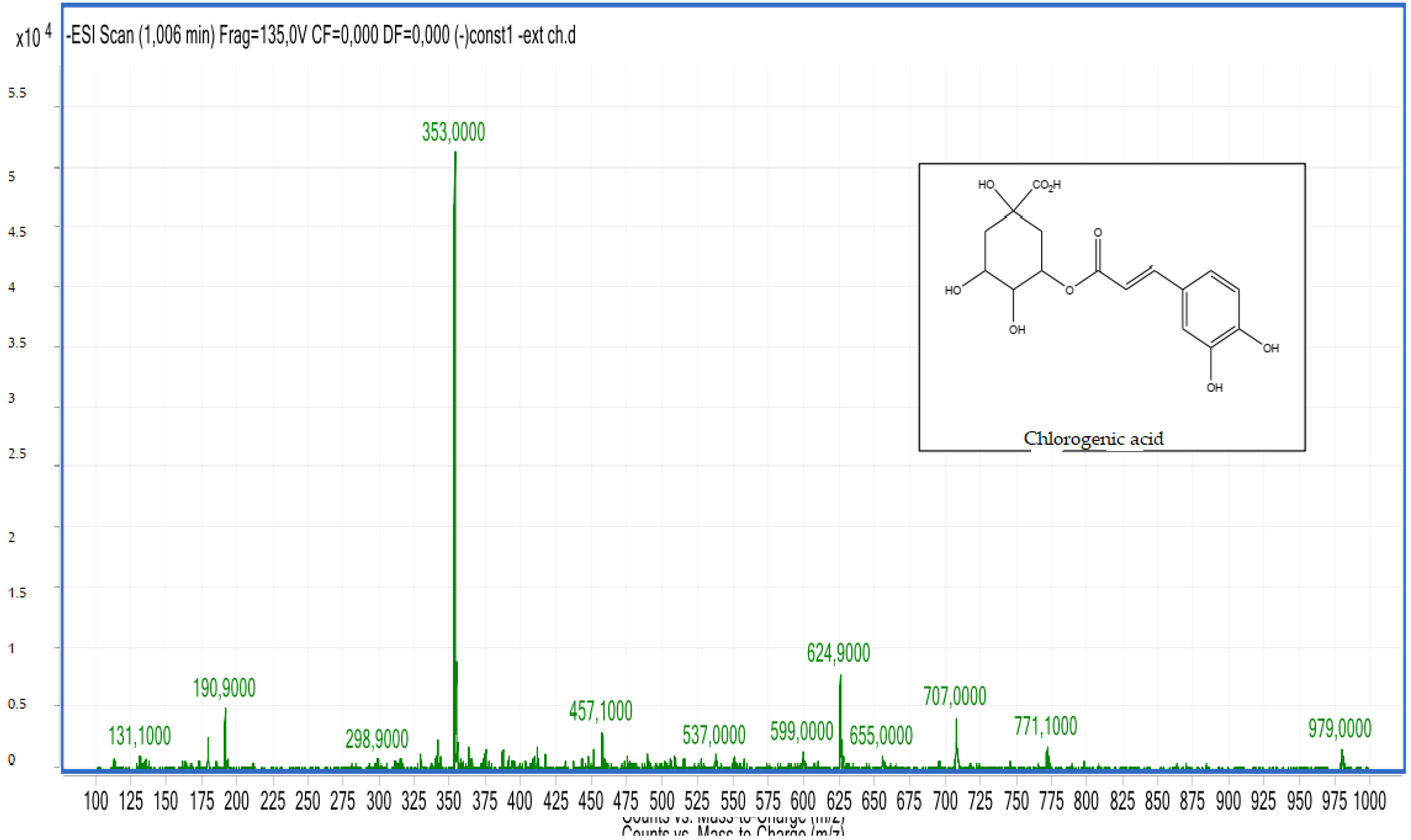
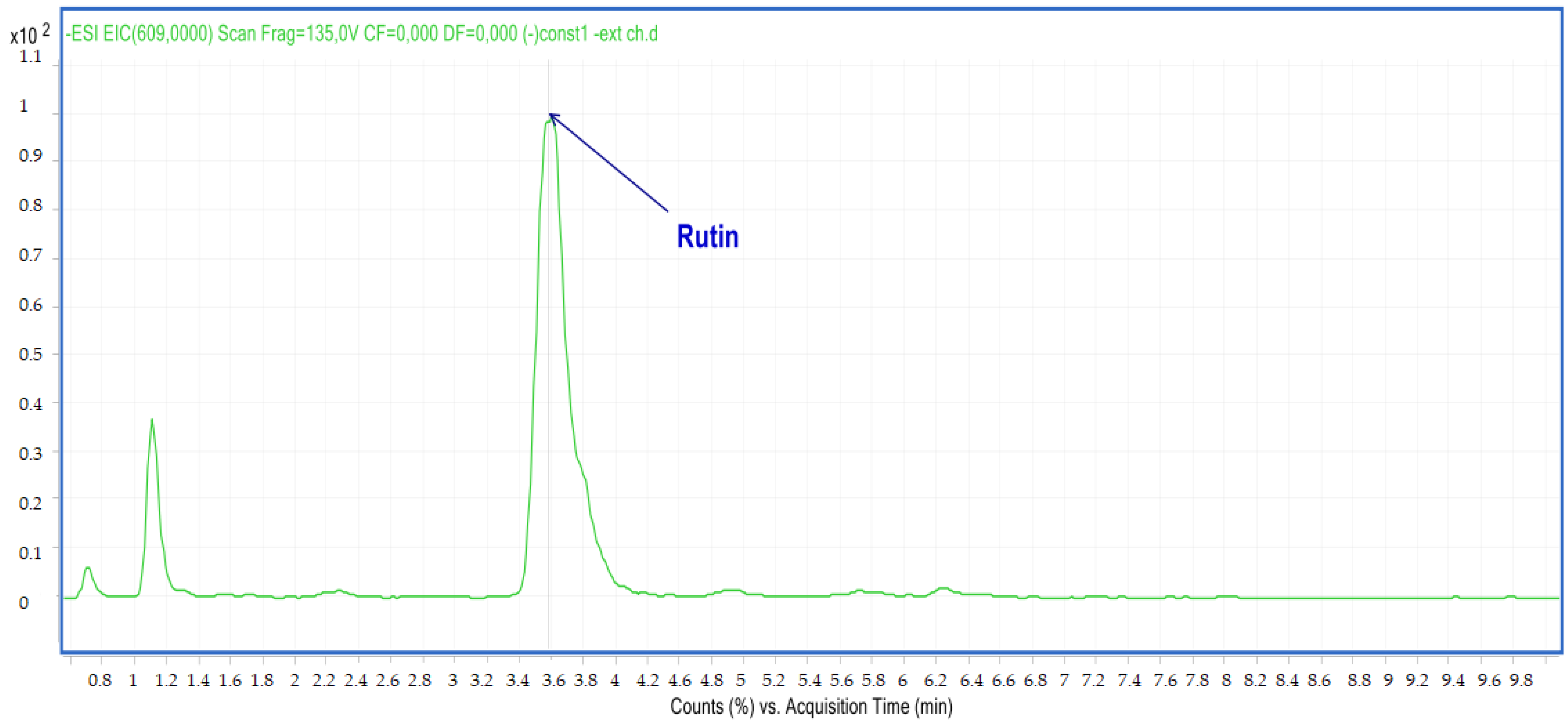
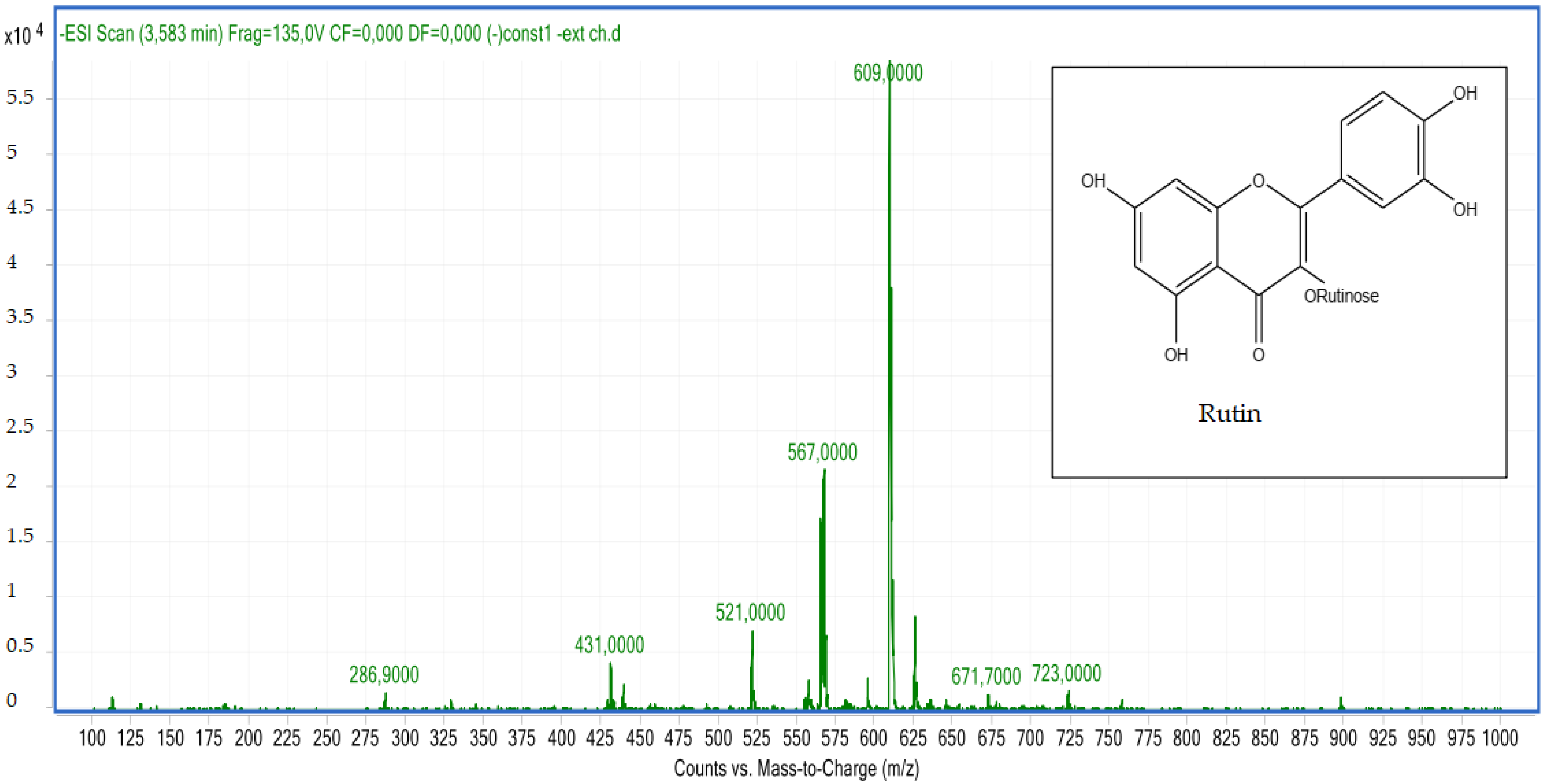
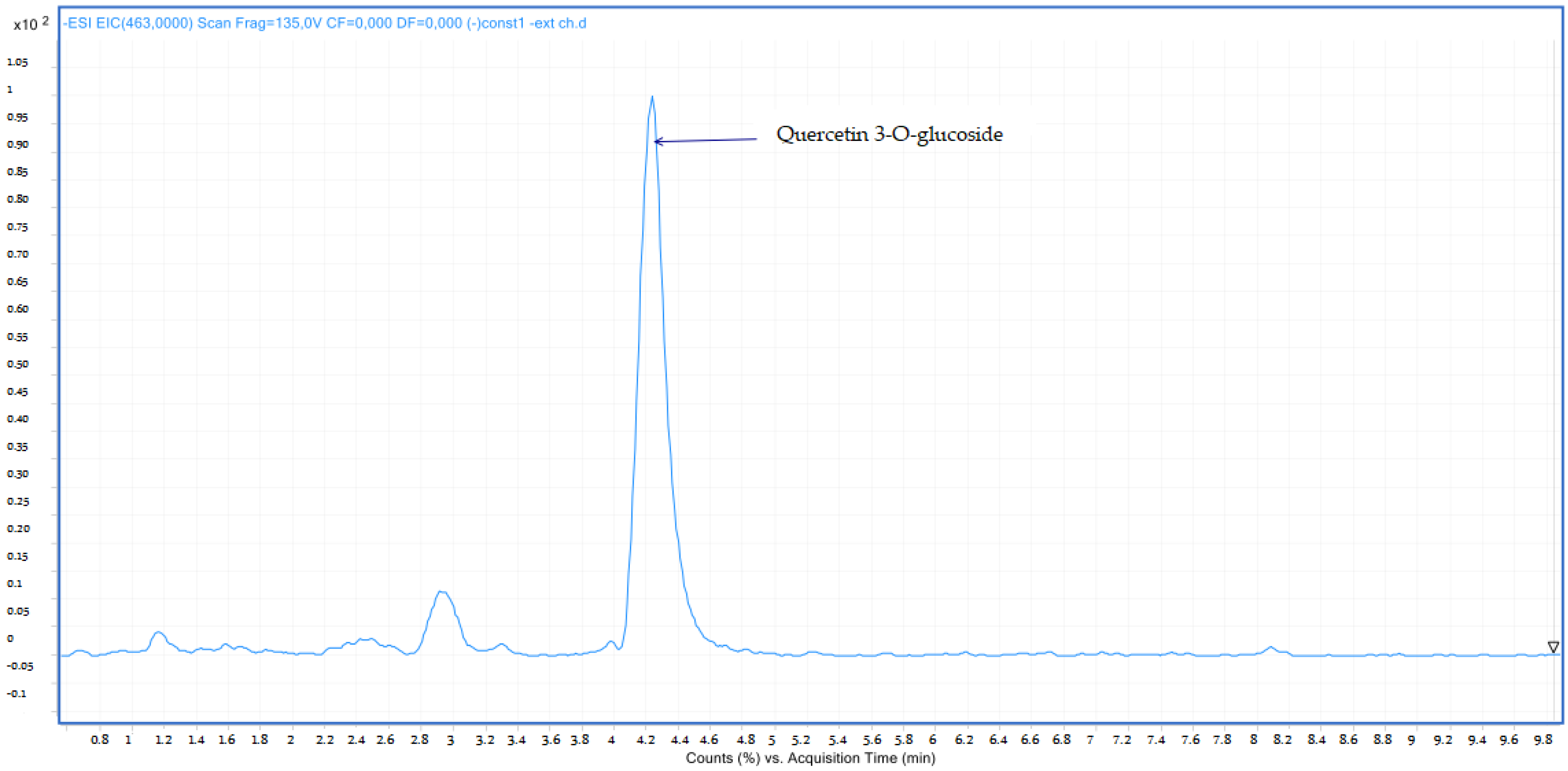
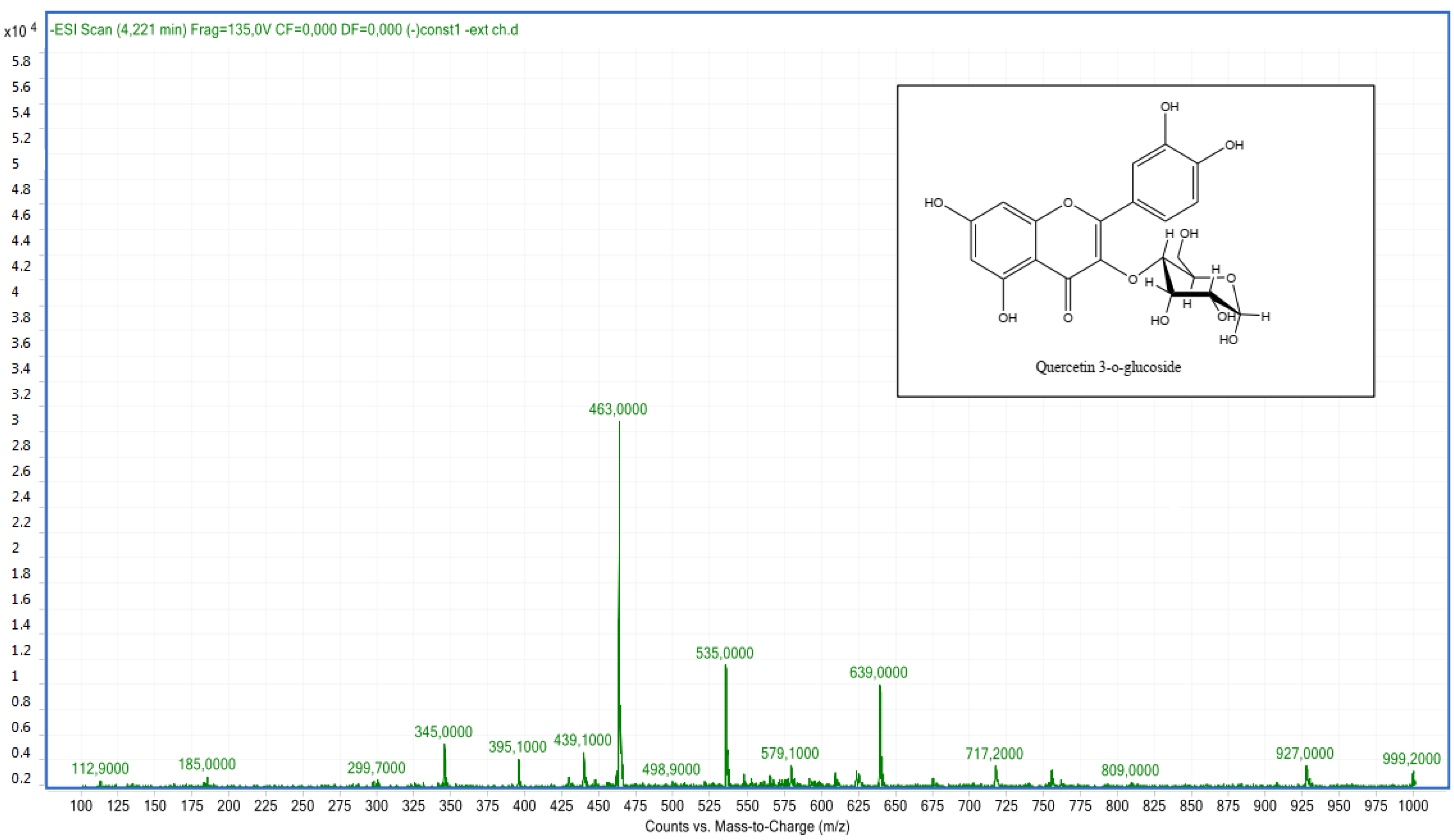
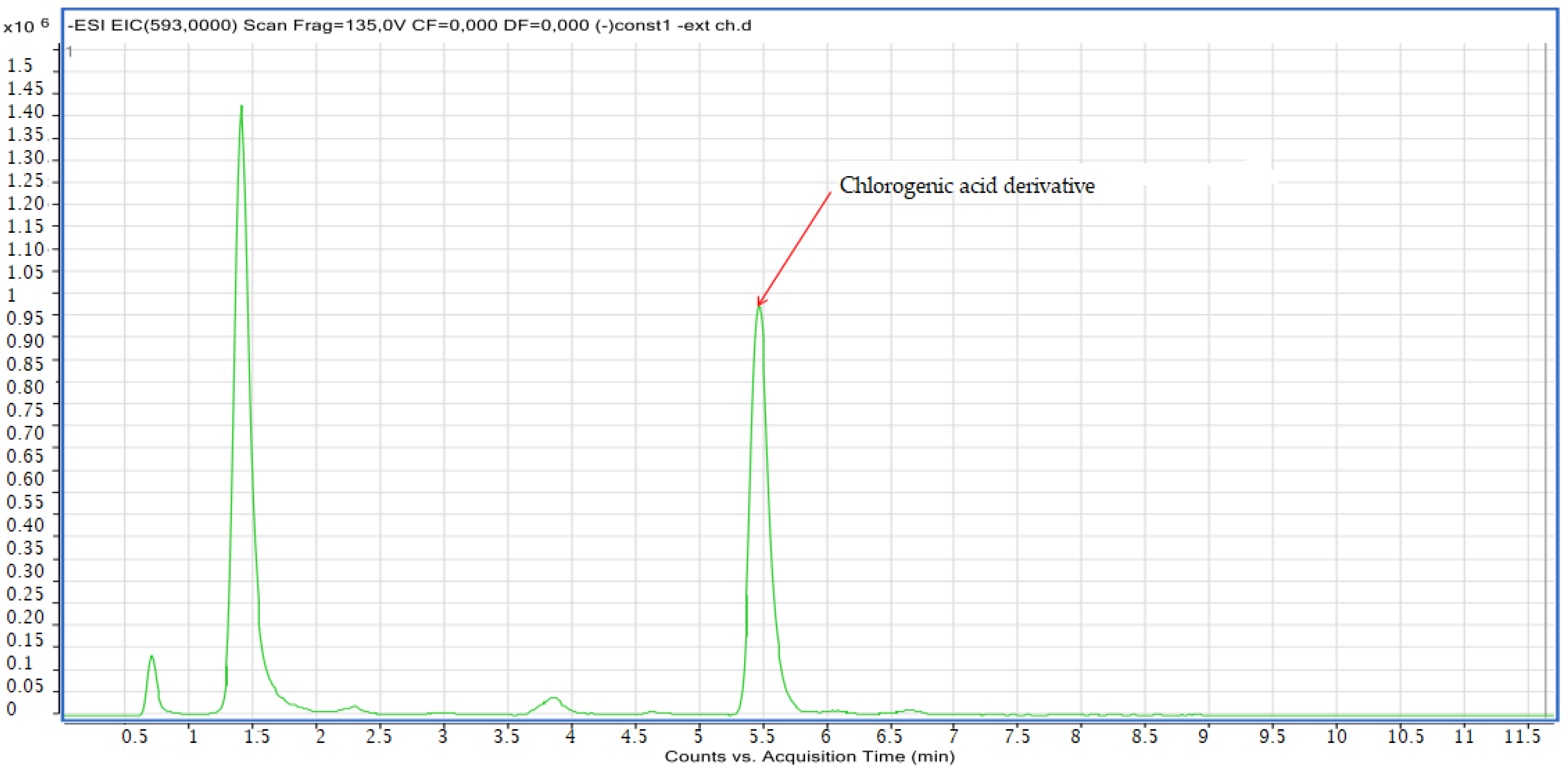
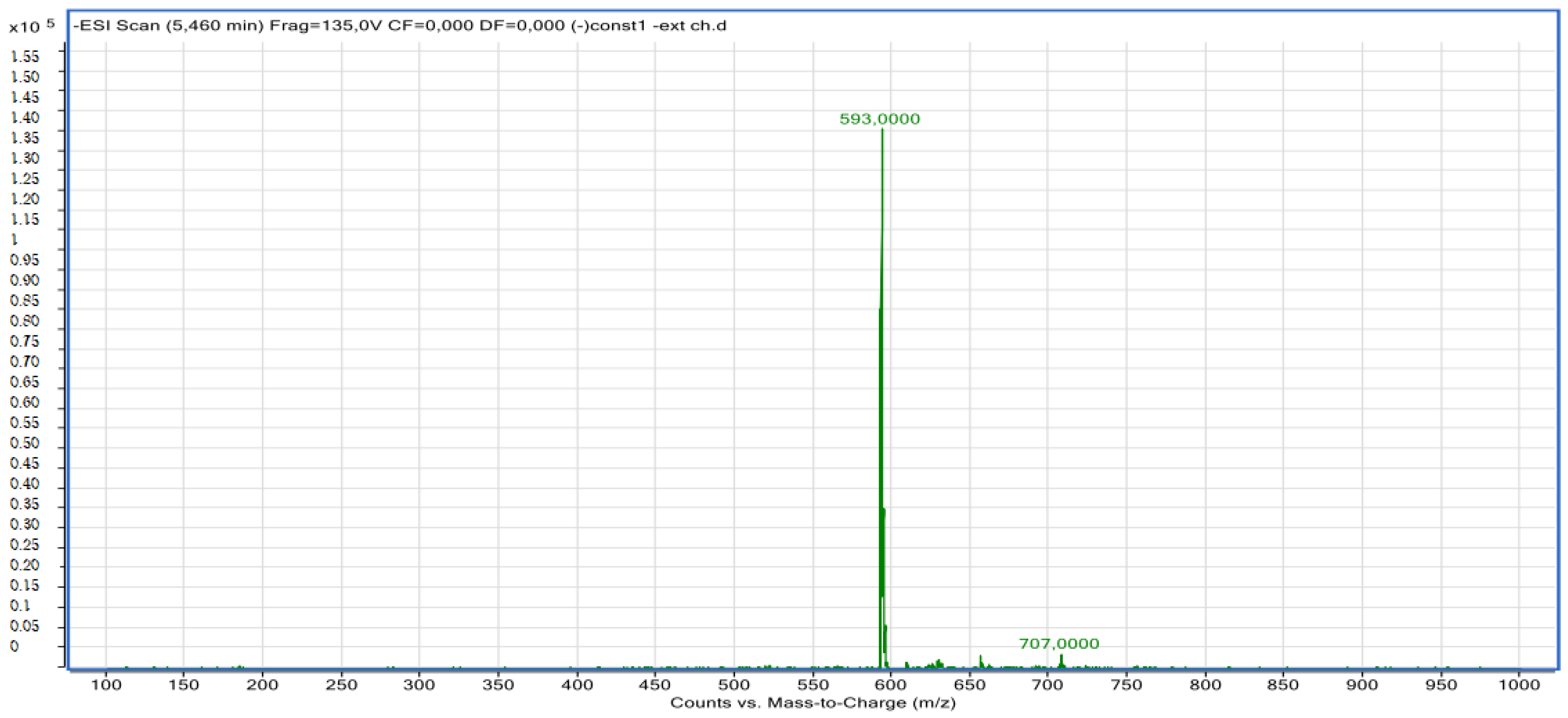
2.2.2. LC-MS Analyses
2.3. Antioxidant Activity
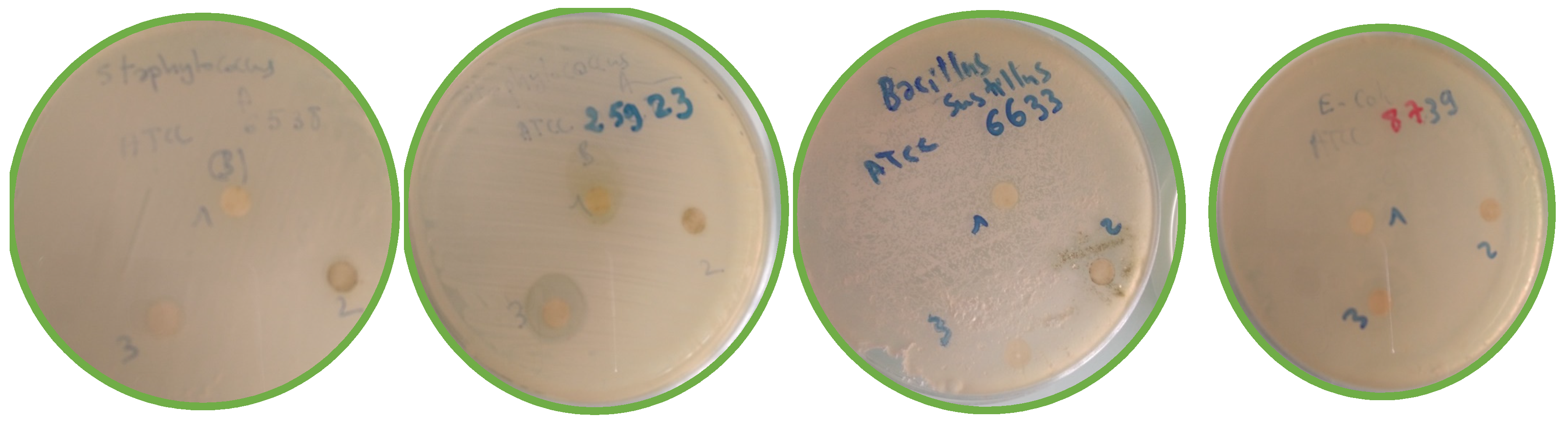
Antimicrobial Activity
3. Material and Methods
3.1. Plant Materials
3.2. Extraction and Fractionation
Chemicals
3.3. Chromatographic Analysis
3.3.1. Qualitative Analysis with Thin-Layer Chromatography (TLC)
3.3.2. HPLC Analysis
3.4. Antioxidant Activities
3.4.1. Total Phenolic Content (TPC)
3.4.2. Total Flavonoid Content (TFC)
3.4.3. Total Flavonol (TFOL) Content
3.4.4. DPPH Free-Radical Scavenging
3.4.5. Galvinoxyl Free-Radical Scavenging
3.4.6. ABTS-Scavenging Assay
3.4.7. Superoxide-Radical-Scavenging Activity (O2•−)
3.4.8. CUPric Reducing Antioxidant Capacity (CUPRAC)
3.4.9. Ferric-Reducing Power
3.4.10. Phenanthroline Test
3.4.11. Microorganisms
3.5. Statistical Analyses
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Sánchez-Chávez, E.; Rodríguez, A.; Castro-Castro, A.; Angel Pérez- Farrera, M.; Sosa, V. Spatio-temporal evolution of climbing habit in the Dahlia-Hidalgoa group (Coreopsidae, Asteraceae). Mol. Phylogenet. Evol. 2019, 135, 166–176. [Google Scholar] [CrossRef]
- Lawrence, G.H.M. Taxonomy of Vascular Plants; Rakes Press; Oxford and IBM Publishing Co.: New Delhi, India, 1973. [Google Scholar]
- Matsuda, H.; Morikawa, T.; Toguchida, I.; Harima, S.; Yoshikawa, M. Medicinal Flowers. VI. Absolute Stereostructures of Two New Flavanone Glycosides and a Phenylbutanoid Glycoside from the Flowers of Chrysanthemum indicum L.: Their Inhibitory Activities for Rat Lens Aldose Reductase. Chem. Pharm. Bull. 2002, 50, 972. [Google Scholar] [CrossRef]
- Da Silva, A.C.N.; do Nascimento, R.M.C.; do Rodrigues, D.C.N.; Ferreira, P.M.P.; Pessoa, C.; Lima, D.J.B.; de Moraes Filho, M.O.; de Almeida, R.M.; Ferreira, S.R.; Fujiwara, R.T.; et al. In vitro activity evaluation of seven Brazilian Asteraceae against cancer cells and Leishmania amazonensis. S. Afr. J. Bot. 2019, 121, 267–273. [Google Scholar] [CrossRef]
- Sarembaud, A.; Poitevin, B. Médicament à usage homéopathique; Ed. Masson: Paris, France, 1996; p. 256. [Google Scholar]
- Labed, F.; Masullo, M.; Mirra, V.; Nazzaro, F.; Benayache, F.; Benayache, S.; Piacente, S. Amino acid-sesquiterpene lactone conjugates from the aerial parts of Centaurea pungens and evaluation of their antimicrobial activity. Fitoterapia 2019, 133, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Hellwig, F.H. Centaureinae (Asteraceae) in the Mediterranean history of ecogeographical radiation. Plant. Syst. Evol. 2004, 246, 137–162. [Google Scholar] [CrossRef]
- Bellakhdar, J. La pharmacopée marocaine traditionnelle. In Médecine Arabe Ancienne et Savoirs Populaires; Etienne, S., Ed.; Ibis Press: Paris, France, 1997; p. 764. [Google Scholar]
- Adekenov, S.M. Sesquiterpene lactones with unusual structure. Their biogenesis and biological activity. Fitoterapia 2017, 121, 16–30. [Google Scholar] [CrossRef] [PubMed]
- Formisano, C.; Formisano, C.; Sirignano, C.; Rigano, D.; Chianese, G.; Zengin, G.; Seo, E.J.; Efferth, T.; Taglialatela-Scafati, O. Antiproliferative activity against leukemia cells of sesquiterpene lactones from the Turkish endemic plant Centaurea drabifolia subsp detonsa. Fitoterapia 2017, 120, 98–102. [Google Scholar] [CrossRef]
- Shakeri, A.; Amini, E.; Asili, J.; Masullo, M.; Piacente, S.; Iranshahi, M. Screening of several biological activities induced by different sesquiterpene lactones isolated from Centaurea behen L. and Rhaponticum repens (L.) Hidalgo. Nat. Prod. Res. 2017, 32, 1436–1440. [Google Scholar] [CrossRef]
- Ayaz, F.A.; Ozcan, M.; Kurt, A.; Karayigit, B.; Ozogul, Y.; Glew, R.; Ozogul, F. Fatty acid composition and antioxidant capacity of cypselas in Centaurea s.l. taxa (Asteraceae, Cardueae) from NE Anatolia. S. Afr. J. Bot. 2017, 112, 474–482. [Google Scholar] [CrossRef]
- Quezel, P.; Santa, S. Nouvelle flore de l’Algérie et des régions désertiques méridionales; Tome, I., Ed.; C.N.R.S. Paris: Paris, France, 1963; p. 1023. [Google Scholar]
- Baharfar, R.; Khalilzadeh, M.A.; Gheibi, S.; Jazayeri, O.; Azimi, R.; Tajbakhsh, M. Antioxidant and antibacterial activities of the methanolic extract of Centaurea zuvandica Sosn. JICS 2009, 3, 172–177. [Google Scholar]
- Koca, U.; Süntar, I.P.; Keles, H.; Yesilada, E.; Akkol, E.K. In vivo antiinflammatory and wound healing activities of Centaurea iberica Trev. ex Spreng. J. Ethnopharmacol. 2009, 126, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Leonardi, M.C.; Ivaldi, G.B.; Santoro, L.; Lazzari, R.; Ferrari, A.; Morra, A.; Caldarella, P.; Burgoa, L.; Bassi, F.D.; Sangalli, C. Long-term side effects and cosmetic outcome in a pool of breast cancer patients treated with intraoperative radiotherapy with electrons as sole treatmen. Tumori. J. 2011, 98, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Kilic, O. Essential oil compounds of three Centaurea, L. taxa from Turkey and their chemotaxonomy. J. Med. Plants. Res. 2013, 7, 1344–1350. [Google Scholar]
- Belkacem, S.; Belbache, H.; Boubekril, C.; Mosset, P.; RachedMosbah, O.; Marchioni, E.; Benayache1, S.; Benayache, F. Biological and Chemical Sciences Chemical Constituents from C. parviflora Desf. Res. J. Pharm. 2014, 5, 1275–1279. [Google Scholar]
- Grafakou, M.E.; Djeddi, S.; Tarek, H.; Skaltsa, H. Secondary metabolites from the aerial parts of Centaurea papposa (Coss.) Greuter. Biochem. Syst. Ecol. 2018, 76, 15–22. [Google Scholar] [CrossRef]
- Arfaoui, L. Dietary plant polyphenols: Effects of food processing on their content and bioavailability. Molecules 2021, 26, 2959. [Google Scholar] [CrossRef]
- Altemimi, A.; Lakhssassi, N.; Baharlouei, A.; Watson, D.G.; Lightfoot, D.A. Phytochemicals: Extraction, isolation, and identification of bioactive compounds from plant extracts. Plants 2017, 6, 42. [Google Scholar] [CrossRef]
- Do, Q.D.; Angkawijaya, A.E.; Tran-Nguyen, P.L.; Huynh, L.H.; Soetaredjo, F.E.; Ismadji, S.; Ju, Y.H. Effect of extraction solvent on total phenol content, total flavonoid content, and antioxidant activity of Limnophila aromatica. J. Food Drug Anal. 2014, 22, 296–302. [Google Scholar] [CrossRef]
- Bui, N.T.; Pham, T.L.T.; Nguyen, K.T.; Le, P.H.; Kim, K.H. Effect of extraction solvent on total phenol, flavonoid content, and antioxidant activity of Avicennia officinalis. Res. Appl. Chem. 2021, 12, 2678–2690. [Google Scholar]
- Bougandoura, N. Pouvoir antioxydant et antimicrobien des extraits d’espèces végétales Satureja Calamintha Ssp Nepeta (nabta) et Ajuga iva L.(chendgoura) de l’ouest d’Algérie; Mémoire du Master en biologie, Université Abou Bekr Belkaid-Tlemcen: Tlemcen, Algeria, 2011; p. 52. [Google Scholar]
- Markham, K.R. Techniques of Flavonoid Identification; Academic Press: New York, NY, USA, 1982. [Google Scholar]
- Aslam, M.S.; Ahmad, M.S.; Mamat, A.S. Phytochemical evaluation of polyherbal formulation of Clinacanthus nutans and Elephantopus scaber to identify flavonoids. Pharmacogn. Rev. 2016, 8, 534–541. [Google Scholar] [CrossRef]
- Wang, H.; Nair, M.G.; Strasburg, G.M.; Booren, A.M.; Gray, J.I. Antioxidant polyphenols from tart cherries (Prunus cerasus). J. Agric. Food Chem. 1999, 47, 840–844. [Google Scholar] [CrossRef]
- Mbaïhougadobé, S.; Ngakegni-Limbili, A.C.; Gouollaly, T.; Koane, J.N.; Ngaïssona, P.; Loumpangou, C.N.; Mahmout, Y.; Ouamba, J.M. Evaluation de l’activité anti-oxydante de trois espèces de plantes utilisées dans le traitement de la goutte au Tchad. Méd. Tradit. Pharmacop. 2017, 18, 28–35. [Google Scholar]
- Fernando, L. Purification of fresh cassava root polyphenols by solidphase extraction with Amberlite XAD-8 resin. J. Chromatogr. A 2009, 657, 445–449. [Google Scholar]
- Dai, J.; Mumper, R.J. Plant phenolics: Extraction, analysis and their antioxidant and anticancer properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Liu, C.Z. Comparison of techniques for the extraction of flavonoids from cultured cells of Saussurea medusa Maxim. World. J. Microbiol. Biotechnol. 2005, 21, 1461–1463. [Google Scholar] [CrossRef]
- Uttara, B.; Singh, A.V.; Zamboni, P.; Mahajan, R. Oxidative stress and neurodegenerative diseases: A review of upstream and downstream antioxidant therapeutic options. Curr. Neuropharmacol. 2009, 7, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Lima, G.P.P.; Vianello, F.; Corrêa, C.R.; Campos, R.A.D.S.; Borguini, M.G. Polyphenols in fruits and vegetables and its effect on human health. Food. Sci. Nutr. 2014, 1065–1082. [Google Scholar] [CrossRef]
- Grgić, J.; Šelo, G.; Planinić, M.; Tišma, M.; Bucić-Kojić, A. Role of the encapsulation in bioavailability of phenolic compounds. J. Antioxid. 2020, 9, 923. [Google Scholar] [CrossRef]
- Tomás-Barberán, F.A.; Espín, J.C. Phenolic compounds and related enzymes as determinants of quality in fruits and vegetables. J. Sci. Food. Agric. 2001, 81, 853–876. [Google Scholar] [CrossRef]
- Pietta, P.G. Flavonoids as antioxidants. J. Nat. Prod. 2000, 63, 1035–1042. [Google Scholar] [CrossRef]
- Escarpa, A.; González, M.C. High-performance liquid chromatography with diode-array detection for the determination of phenolic compounds in peel and pulp from different apple varieties. J. Chromatogr. A 1998, 823, 331–337. [Google Scholar] [CrossRef]
- Zhang, L.Y.; Cosma, G.; Gardner, H.; Vallyathan, V.; Castranova, V. Effect of chlorogenic acid on hydroxyl radical. Mol. Cell. Biochem. 2003, 247, 205–210. [Google Scholar] [CrossRef]
- Mariadoss, A.V.A.; Park, S.; Saravanakumar, K.; Sathiyaseelan, A.; Wang, M.H. Ethyl acetate fraction of Helianthus tuberosus L. induces anti-diabetic, and wound-healing activities in insulin-resistant human liver cancer and mouse fibroblast cells. J. Antioxid. 2021, 10, 99. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, R.; Mohan Rao, L.J. An outlook on chlorogenic acids-occurrence, chemistry, technology, and biological activities. Crit. Rev. Food. Sci. Nutr. 2013, 53, 968–984. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Itagaki, S.; Kurokawa, T.; Ogura, J.; Kobayashi, M.; Hirano, T.; .Sugawara, M.; Iseki, K. In vitro and in vivo antioxidant properties of chlorogenic acid and caffeic acid. Int. J. Pharm. 2011, 403, 136–138. [Google Scholar] [CrossRef] [PubMed]
- Xi, Y.; Jiao, W.; Cao, J.; Jiang, W. Effects of chlorogenic acid on capacity of free radicals scavenging and proteomic changes in postharvest fruit of nectarine. PLoS ONE 2017, 2, e0182494. [Google Scholar] [CrossRef]
- Wang, Z.; Ding, Z.; Li, Z.; Ding, Y.; Jiang, F.; Liu, J. Antioxidant and antibacterial study of 10 flavonoids revealed rutin as a potential antibiofilm agent in Klebsiella pneumoniae strains isolated from hospitalized patients. Microb. Pathog. 2021, 159, 105121. [Google Scholar] [CrossRef]
- Babiaka, S.B.; Nia, R.; Abuga, K.O.; Mbah, J.A.; Vincent de Paul, N.N.; Paper, D.H.; Ntie-Kang, F. Antioxidant potential of flavonoid glycosides from Manniophyton fulvum Müll.(Euphorbiaceae): Identification and molecular modeling. Sci. Afr. 2020, 8, e00423. [Google Scholar] [CrossRef]
- Razavi, S.M.; Zahri, S.; Zarrini, G.; Nazemiyeh, H.; Mohammadi, S. Biological activity of quercetin-3-O-glucoside, a known plant flavonoid. Russ. J. Bioorganic Chem. 2009, 35, 376–378. [Google Scholar] [CrossRef]
- Naeim, H.; El-Hawiet, A.; Abdel Rahman, R.A.; Hussein, A.; El Demellawy, M.A.; Embaby, A.M. Antibacterial activity of Centaurea pumilio L. root and aerial part extracts against some multidrug resistant bacteria. BMC Complement. Med. Ther. 2020, 20, 79. [Google Scholar] [CrossRef]
- Karamenderes, C.; Khan, S.; Tekwani, B.L.; Jacob, M.R.; Khan, I.A. Antiprotozoal and Antimicrobial Activities of Centaurea. Species Growing in Turkey. Pharm. Biol. 2006, 44, 534–539. [Google Scholar] [CrossRef]
- Jafari-Sales, A.; Mobaiyen, H.; Jafari, B.; Sayyahi, J. Assessment of Antibacterial Effect of Alcoholic Extract of Centaurea depressa M.B., Reseda lutea L. and Fumaria asepala on Selected Standard Strains in vitro. Sci. J. Nurs. Midwifery 2019, 5, 63–73. [Google Scholar]
- Zink, D.L. The impact of consumer demands and trends on food processing. Emerging. Infect Dis. 1997, 3, 467–469. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, M.; Epifano, F.; Fiorito, S.; Álvarez-Suarez, J.M. Phytochemical analysis and biological investigation of Nepeta juncea Benth. different extracts. Plants 2020, 9, 646. [Google Scholar] [CrossRef] [PubMed]
- Valanciene, E.; Malys, N. Advances in Production of Hydroxycinnamoyl-Quinic Acids: From Natural Sources to Biotechnology. Antioxidants 2022, 11, 2427. [Google Scholar] [CrossRef] [PubMed]
- Zaak, H.; Bendif, H.; Rebbas, K.; Aouati, L.; Abdennour, A.; Hamza, A.; Wandjou, J.G.N.; Maggi, F. Essential oil composition and biological activities of Ononis alba Poir (Fabaceae). Nat. Prod. Res. 2022, 36, 2418–2423. [Google Scholar] [CrossRef]
- Mammeri, A.; Bendif, H.; Bensouici, C.; Benslama, A.; Rebas, K.; Bouasla, A.; Rebaia, I.; Souilah, N.; Miara, M.D. Total phenolic contents, in vitro antioxidant activity, enzymes inhibition and antiinflammatory effect of the selective extracts from the Algerian Lavandula multifida. ACTA Pharm. Sci. 2022, 60, 1. [Google Scholar] [CrossRef]
- Müller, L.; Gnoyke, S.; Popken, A.M.; Böhm, V. Antioxidant capacity and related parameters of different fruit formulations. LWT—Food Sci. Technol. 2010, 43, 992–999. [Google Scholar] [CrossRef]
- Topçu, G.; Ay, A.; Bilici, A.; Sarıkürkcü, C.; Öztürk, M.; Ulubelen, A. A new flavone from antioxidant extracts of Pistacia terebinthus. Food.Chem. 2007, 103, 816–822. [Google Scholar] [CrossRef]
- Kumaran, A.; Karunakaran, R.J. In vitro antioxidant activities of methanol extracts of five Phyllanthus speciesfromIndia. LWT 2007, 40, 344–352. [Google Scholar] [CrossRef]
- Blois, M.S. Antioxidant determinations by the use of a stable Free Radical. Nature 1958, 4617, 1119–1200. [Google Scholar] [CrossRef]
- Shi, H.; Noguchi, N.; Niki, E. Galvinoxyl method for standardizing electron and proton donation activity. Meth. Enzymol. 2001, 335, 157–166. [Google Scholar]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation de colorization assay. Free. Radical. Bio. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Elizabeth, K.; Rao, M.N.A. Effect of curcumin on hydroxyl radical generation through fenton reaction. Int. J. Pharm. 1989, 57, 173–176. [Google Scholar]
- Apak, R.; Guclu, K.; Ozyurek, M.; Karademir, S.E. Novel total antioxidant capacity index for dietary polyphenols and vitamins C and E, using their cupric ion reducing capability in the presence of neocuproine: CUPRAC method. J. Agric. Food. Chem. 2004, 52, 7970–7981. [Google Scholar] [CrossRef] [PubMed]
- Bouratoua, A.; Khalfallah, A.; Bensouici, C.; Kabouche, Z.; Alabdul Magid, A.; Harakat, D.; Voutquenne-Nazabadioko, L.; Kabouche, A. Chemical composition and antioxidant activity of aerial parts of Ferula longipes Coss. ex Bonnier and Maury. Nat. Prod. Res. 2017, 32, 1873–1880. [Google Scholar] [CrossRef] [PubMed]
- Szydlowskaczerniak, A.; Dianoczki, C.; Recseg, K.; Karlovits, G.; Szlyk, E. Determination of antioxidant capacities of vegetable oils by ferric-ion spectrophotometric methods. Talanta 2008, 76, 899–905. [Google Scholar]
- Aissani, F.; Grara, N.; Bensouici, C.; Bousbia, A.; Ayed, H.; Idris, M.H.M.; Teh, L.K. Algerian Sonchus oleraceus L.: A comparison of different extraction solvent on phytochemical composition, antioxidant properties and anti-cholinesterase activity. Adv. Tradit. Med. 2021, 22, 383–394. [Google Scholar] [CrossRef]

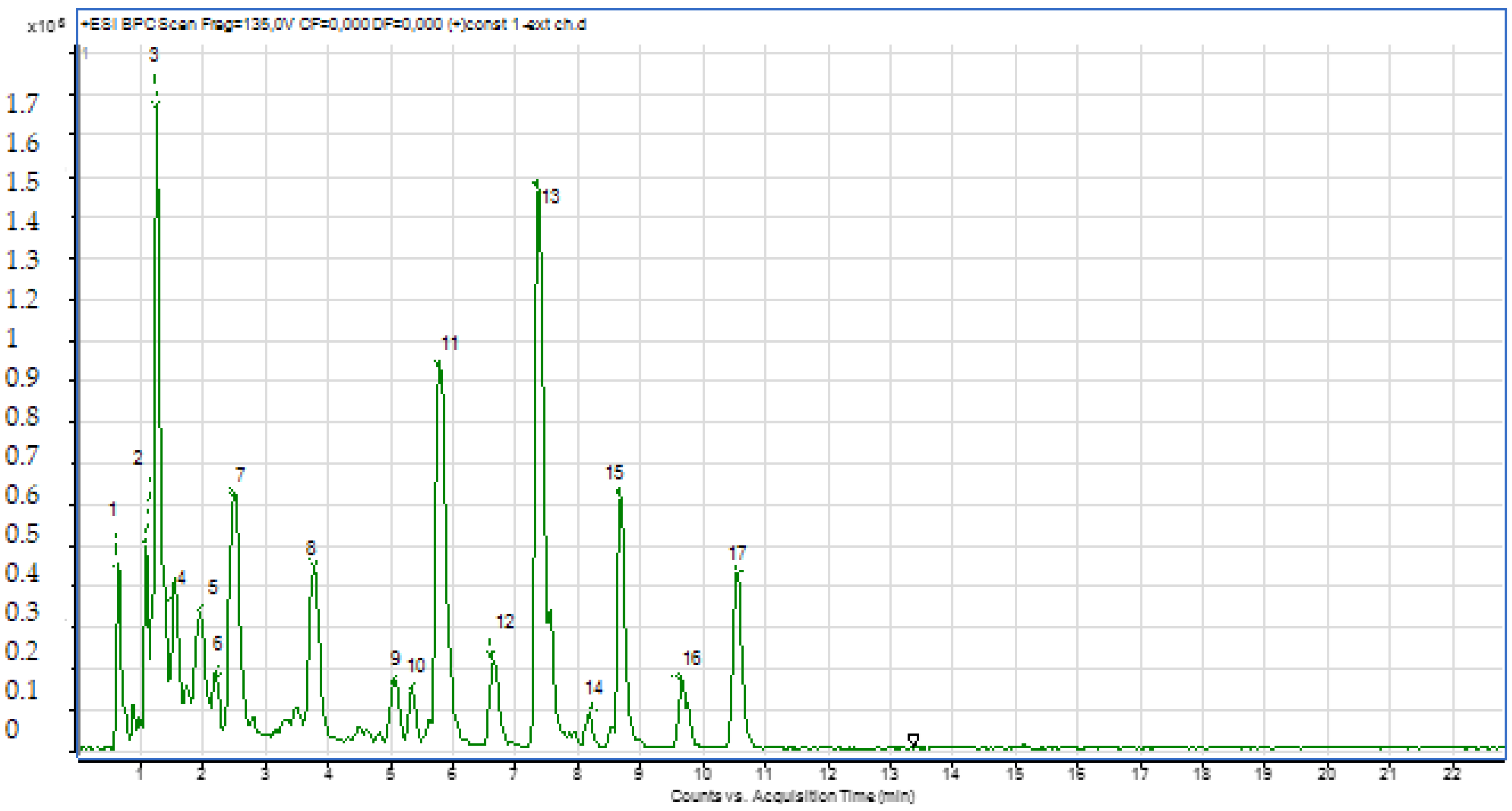
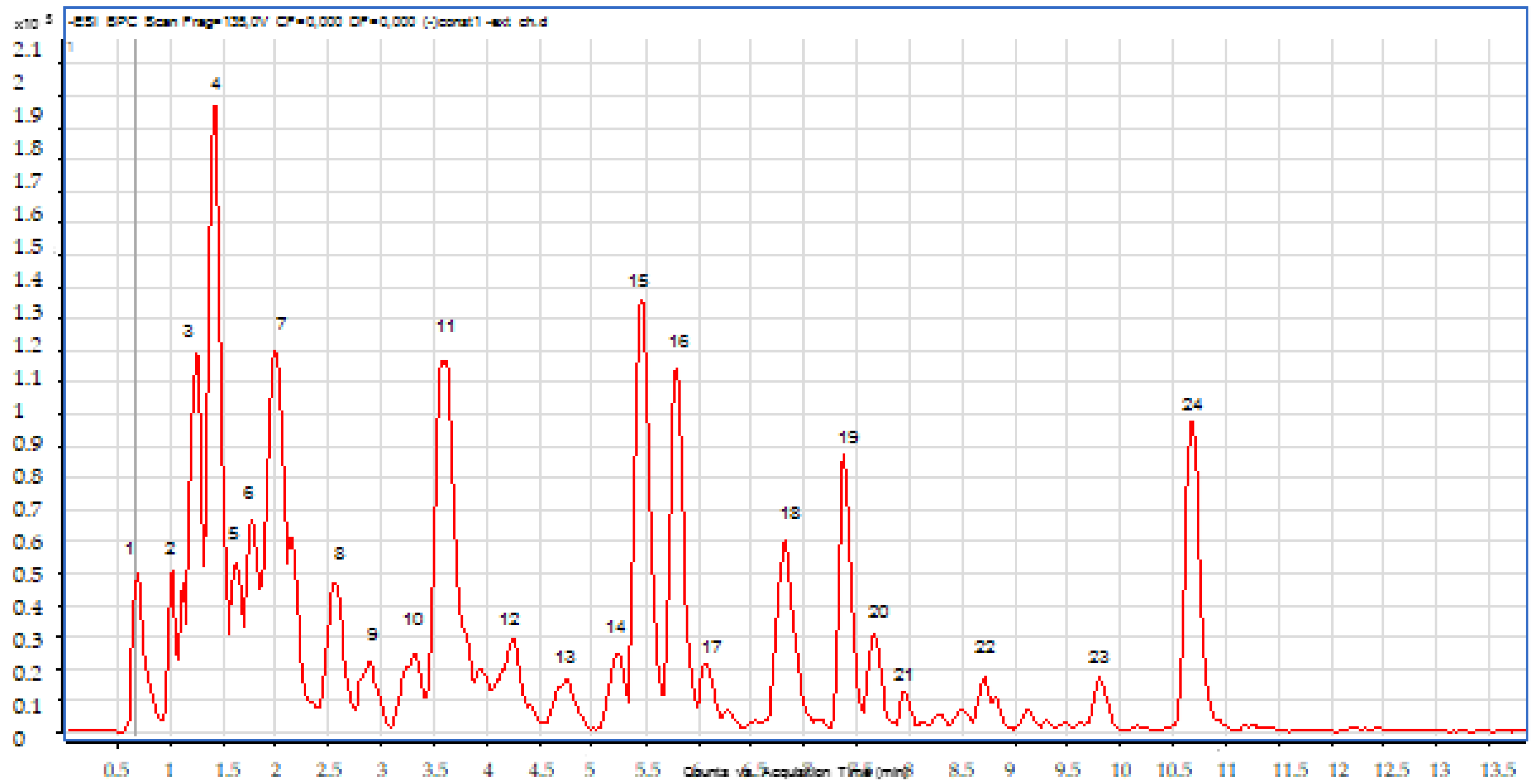
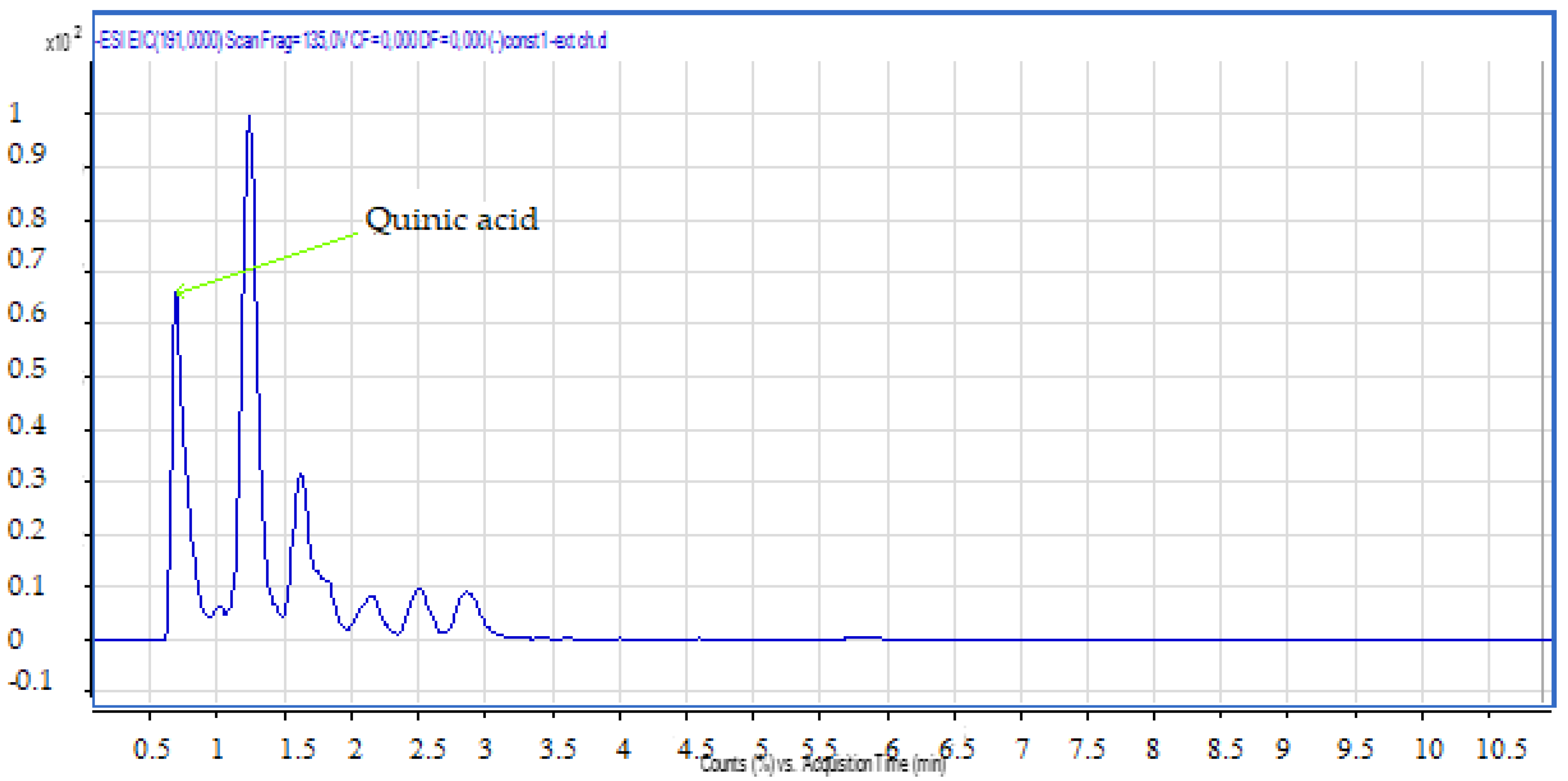

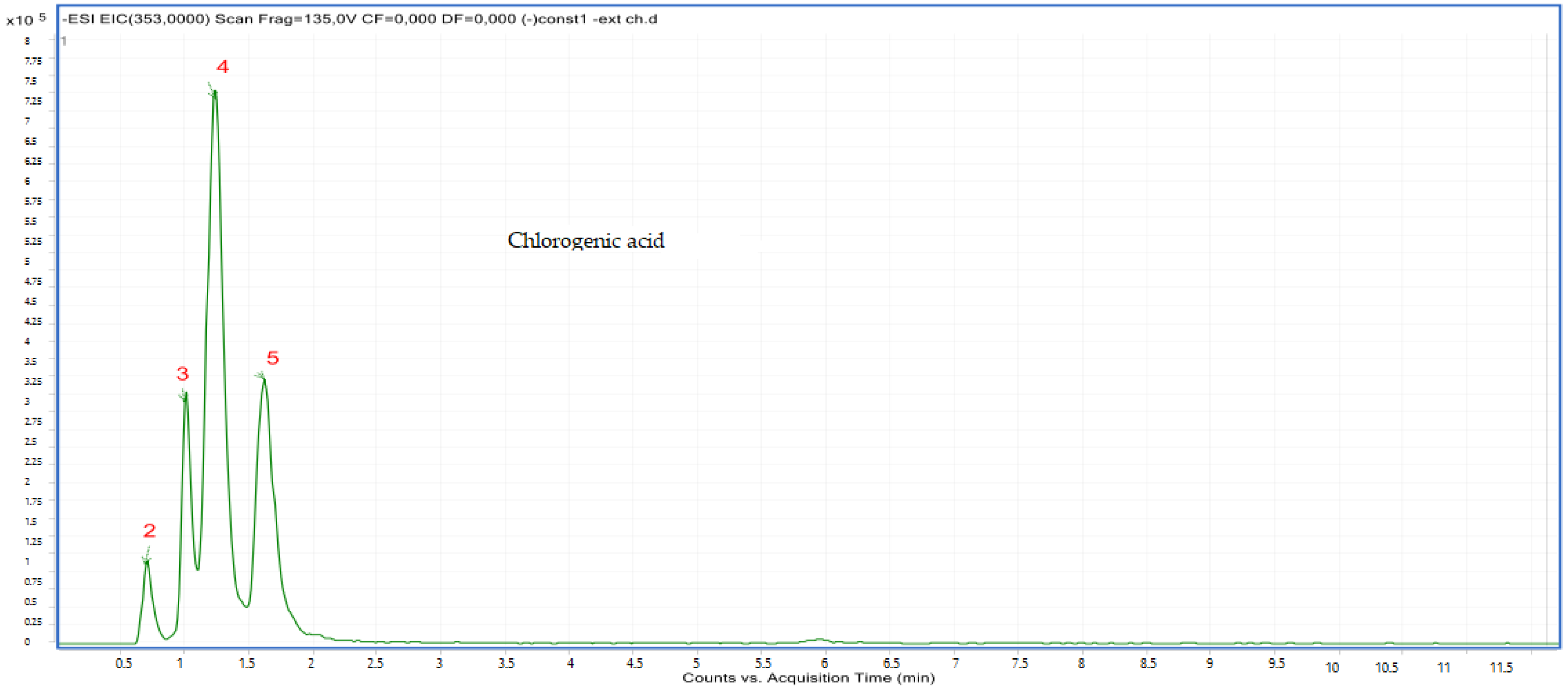

| N | Chemical Name | Solvent System | Rf Value | Spot Color | References |
|---|---|---|---|---|---|
| 1 | Flavonoids | Toluene/ | 0.12 | Pale pink | [26] |
| Chloroform/ | 0.18 | Light blue | |||
| Methanol (4/4/1) (v/v/v) | 0.56 | Pink | |||
| 0.6 | Pink | ||||
| 0.65 | Blue | ||||
| 0.78 | Purple | ||||
| 0.95 | Light blue | ||||
| 2 | Flavonoids | Chloroform/Methanol | 0.29 | Mauve | [27] |
| (10/1) (v/v) | 0.73 | Pale pink | |||
| 3 | Polyphenols/Flavonoids | Ethyl Acetate/Methanol | 0.12 | yellowish blue | [28] |
| /Water (8/1/1) (v/v/v) | 0.24 | Grey | |||
| 0.34 | Grey | ||||
| 0.39 | Grey | ||||
| 0.6 | Yellow | ||||
| 0.44 | Fluorescent White Blue Gray | ||||
| 0.74 | |||||
| 4 | Polyphenols | Methanol/Water (7/3) (v/v) | 0.88 | dark gray | [29] |
| 5 | Flavonoids | Butanol/Acetic acid/Water (40/10/50) (v/v/v) | 0.31 | Grey | - |
| 0.4 | dark gray | ||||
| 0.52 | Fluorescent white blue |
| Pic N | Compounds | Retention Time (min) | Molecular Formula | Experimental m/z | Calculated m/z | Ionization Mode |
|---|---|---|---|---|---|---|
| 1 | Quinic Acid | 0.68 | C7H12O6 | 191.00 | 191.05 | Neg |
| 2 | Chlorogenic acid derivative | 0.72 | C16H18O9 | 353.00 | 353.08 | Neg |
| 3 | Chlorogenic acid derivative | 1.03 | C16H18O9 | 353.00 | 353.08 | Neg |
| 4 | Chlorogenic acid derivative | 1.20 | C16H18O9 | 353.00 | 353.08 | Neg |
| 5 | Chlorogenic acid derivative | 1.60 | C16H18O9 | 353.00 | 353.08 | Neg |
| 6 | Rutin | 3.60 | C27H30O16 | 609.00 | 609.14 | Neg |
| 7 | Quercetin 3-O-glucoside | 4.26 | C21H20O12 | 463.00 | 463.08 | Neg |
| 8 | Chlorogenic acid derivative | 5.73 | C30H10O14 | 593.00 | 593.00 | Neg |
| DPPH (IC50 µg/mL) | Galvinoxyl (IC50 μg/mL) | ABTS (IC50 μg/mL) | CUPRAC (A0.5, μg/mL) | RP Phenanthroline (A0.5, μg/mL) | RP (A0.5, μg/mL) | Superoxide (IC50 μg/mL) | |
|---|---|---|---|---|---|---|---|
| CE | 160.92 ± 5.11 (a) | 88.87 ± 1.86 (a) | 162.39 ± 0.15 (a) | 128.44 ± 4.14 (a) | 46.56 ± 1.54 (a) | ˃200 | 21.91 ± 0.70 (a) |
| CHE | ˃200 | ˃200 | ˃200 | 188.33 ± 4.62 (b) | 75.67 ± 0.29 (a) | ˃200 | 64.77 ± 2.37 (b) |
| EAE | 144.75 ± 1.17 (a) | 97.72 ± 3.07 (a) | 124.22 ± 0.61 (a) | 92.00 ± 4.85 (a) | 56.56 ± 3.34 (a) | 193.67 ± 1.44 (a) | 14.36 ± 0.90 (a) |
| BUE | 59.38 ± 0.72 (b) | 36.25 ± 0.42 (b) | 49.52 ± 1.54 (b) | 71.80 ± 1.22 (a) | 20.29 ± 1.16 (b) | 119.17 ± 0.29 (b) | 13.61 ± 0.38 (a) |
| BHT | 22.32 ± 1.19 (b) | 3.32 ± 0.18 (c) | 1.29 ± 0.30 (c) | 9.62 ± 0.87 (c) | 2.24 ± 0.17 (c) | nd | nd |
| BHA | 5.73 ± 0.41 (c) | 5.38 ± 0.06 (c) | 1.81 ± 0.10 (c) | 3.64 ± 0.19 (c) | 0.93 ± 0.07 (c) | nd | nd |
| Tannic acid | nd | nd | nd | nd | nd | 5.39 ± 0.91 (c) | ˂3.125 |
| Ascorbic acid | nd | nd | nd | nd | nd | 6.77 ± 1.15 | ˂3.125 |
| Microorganisms Tested | Extract Concentration | ||
|---|---|---|---|
| 20 mg/mL | 30 mg/mL | ||
| Inhibition Zone Diameter (IZD, mm) | |||
| Staphylococcus aureus ATCC6538 | Gram+ | 7.00 | 8.64 |
| Staphylococcus aureus ATCC25923 | Gram+ | 12.00 | 13.22 |
| Bacillus subtilis ATCC6633 | Gram+ | NA | NA |
| Escherichia coli ATCC8739 | Gram− | NA | NA |
| Salmonella sp. ATCC14028 | Gram− | 7.00 | 8.03 |
| Pseudomonas sp. ATCC27853 | Gram− | 12.12 | 15.23 |
| Candida albicans ATCC10234 | Fungus | NA | NA |
| Aspergillus niger ATCC16404 | Fungus | 7.00 | 8.22 |
| Extracts | Extraction Yield (%) | TPC (µg GAE/mgE) * | TFC (µg QE/mg E) * | TFOL (µg RE/mg E) * |
|---|---|---|---|---|
| Crude extract (CE) | 2.36 | 113.51 ± 2.95 (a) | 24.49 ± 0.49 (a) | 20.03 ± 0.63 (a) |
| Chloroform extract (CHE) | 1.45 | 105.47 ± 1.35 (a) | 17.78 ± 0.21 (a) | 22.89 ± 1.33 (a) |
| Ethyl Acetate Extract (EAE) | 0.95 | 136.94 ± 2.94 (b) | 20.83 ± 0.21 (a) | 27.86 ± 1.45 (a) |
| Butanol Extract (BUE) | 0.83 | 175.27 ± 2.79 (c) | 59.89 ± 0.91 (b) | 47.30 ± 0.51 (b) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hechaichi, F.Z.; Bendif, H.; Bensouici, C.; Alsalamah, S.A.; Zaidi, B.; Bouhenna, M.M.; Souilah, N.; Alghonaim, M.I.; Benslama, A.; Medjekal, S.; et al. Phytochemicals, Antioxidant and Antimicrobial Potentials and LC-MS Analysis of Centaurea parviflora Desf. Extracts. Molecules 2023, 28, 2263. https://doi.org/10.3390/molecules28052263
Hechaichi FZ, Bendif H, Bensouici C, Alsalamah SA, Zaidi B, Bouhenna MM, Souilah N, Alghonaim MI, Benslama A, Medjekal S, et al. Phytochemicals, Antioxidant and Antimicrobial Potentials and LC-MS Analysis of Centaurea parviflora Desf. Extracts. Molecules. 2023; 28(5):2263. https://doi.org/10.3390/molecules28052263
Chicago/Turabian StyleHechaichi, Fatima Zohra, Hamdi Bendif, Chawki Bensouici, Sulaiman A. Alsalamah, Boutheina Zaidi, Mustapha Mounir Bouhenna, Nabila Souilah, Mohammed I. Alghonaim, Abderrahim Benslama, Samir Medjekal, and et al. 2023. "Phytochemicals, Antioxidant and Antimicrobial Potentials and LC-MS Analysis of Centaurea parviflora Desf. Extracts" Molecules 28, no. 5: 2263. https://doi.org/10.3390/molecules28052263
APA StyleHechaichi, F. Z., Bendif, H., Bensouici, C., Alsalamah, S. A., Zaidi, B., Bouhenna, M. M., Souilah, N., Alghonaim, M. I., Benslama, A., Medjekal, S., Qurtam, A. A., Miara, M. D., & Boufahja, F. (2023). Phytochemicals, Antioxidant and Antimicrobial Potentials and LC-MS Analysis of Centaurea parviflora Desf. Extracts. Molecules, 28(5), 2263. https://doi.org/10.3390/molecules28052263







