Molecular Simulation Study on the Interaction between Porcine CR1-like and C3b
Abstract
1. Introduction
2. Results
2.1. Sequence and Binding Interface Analyses
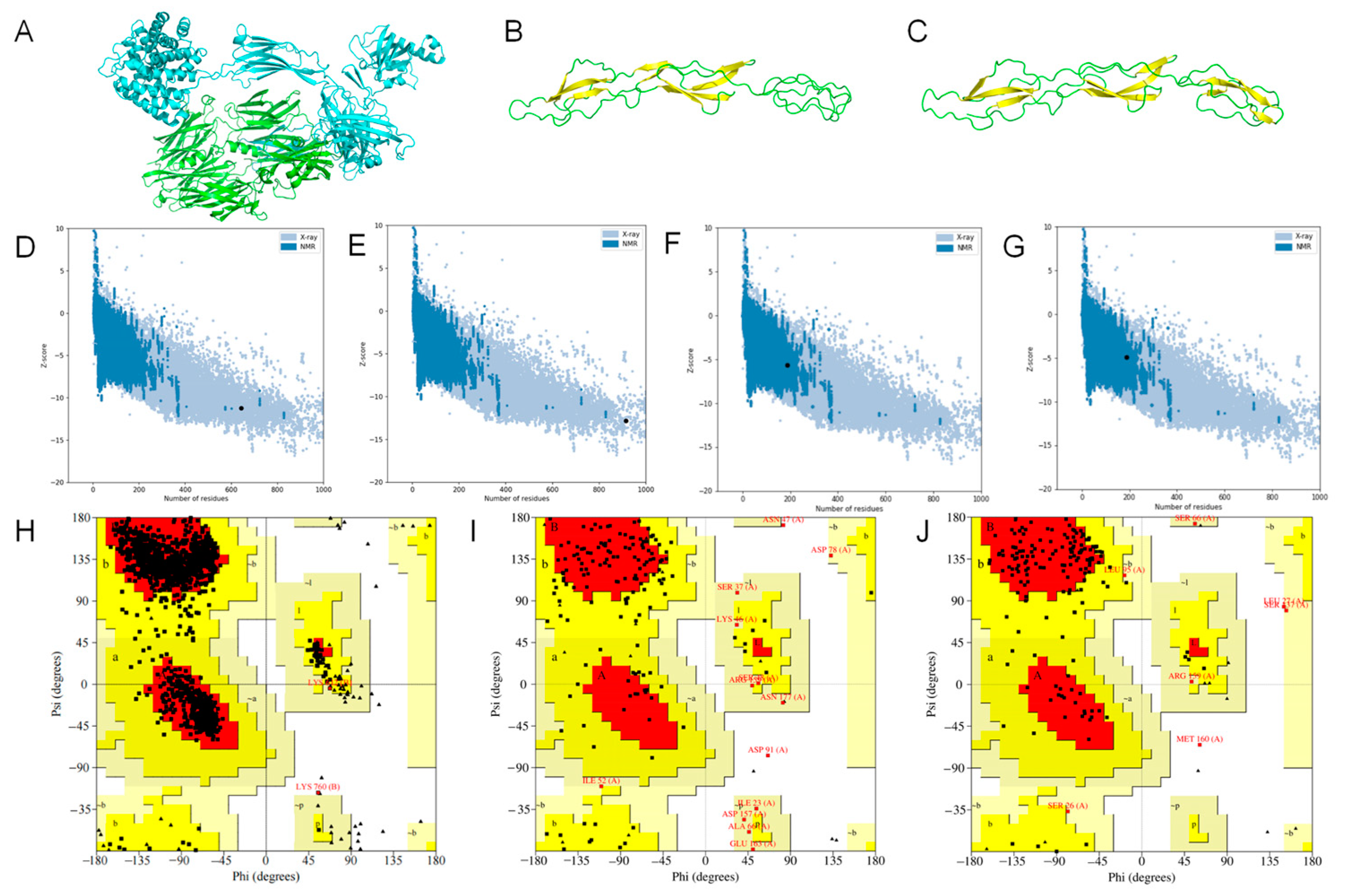
2.2. Homology Modeling and Evaluation
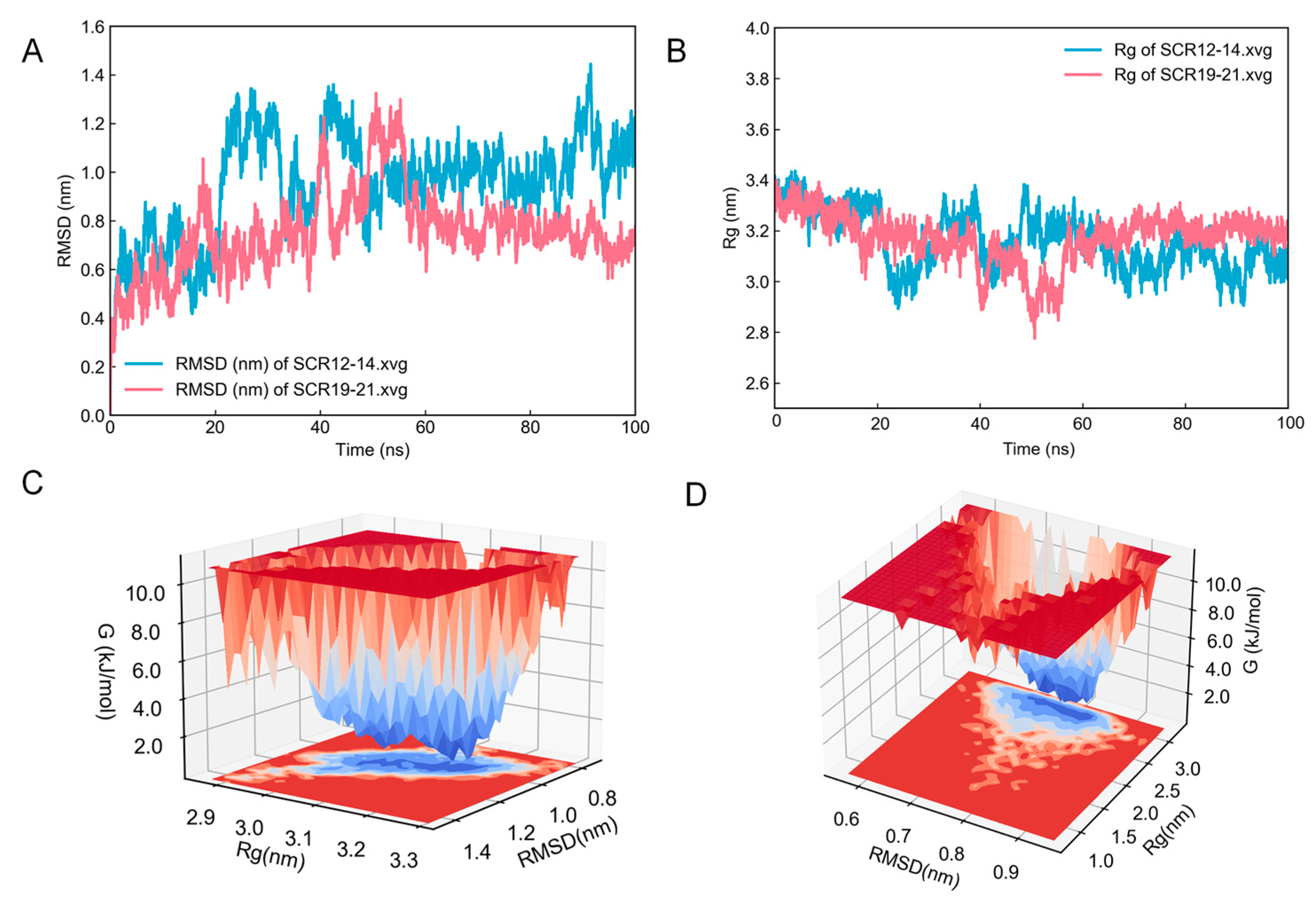
2.3. Model Optimization and Evaluation
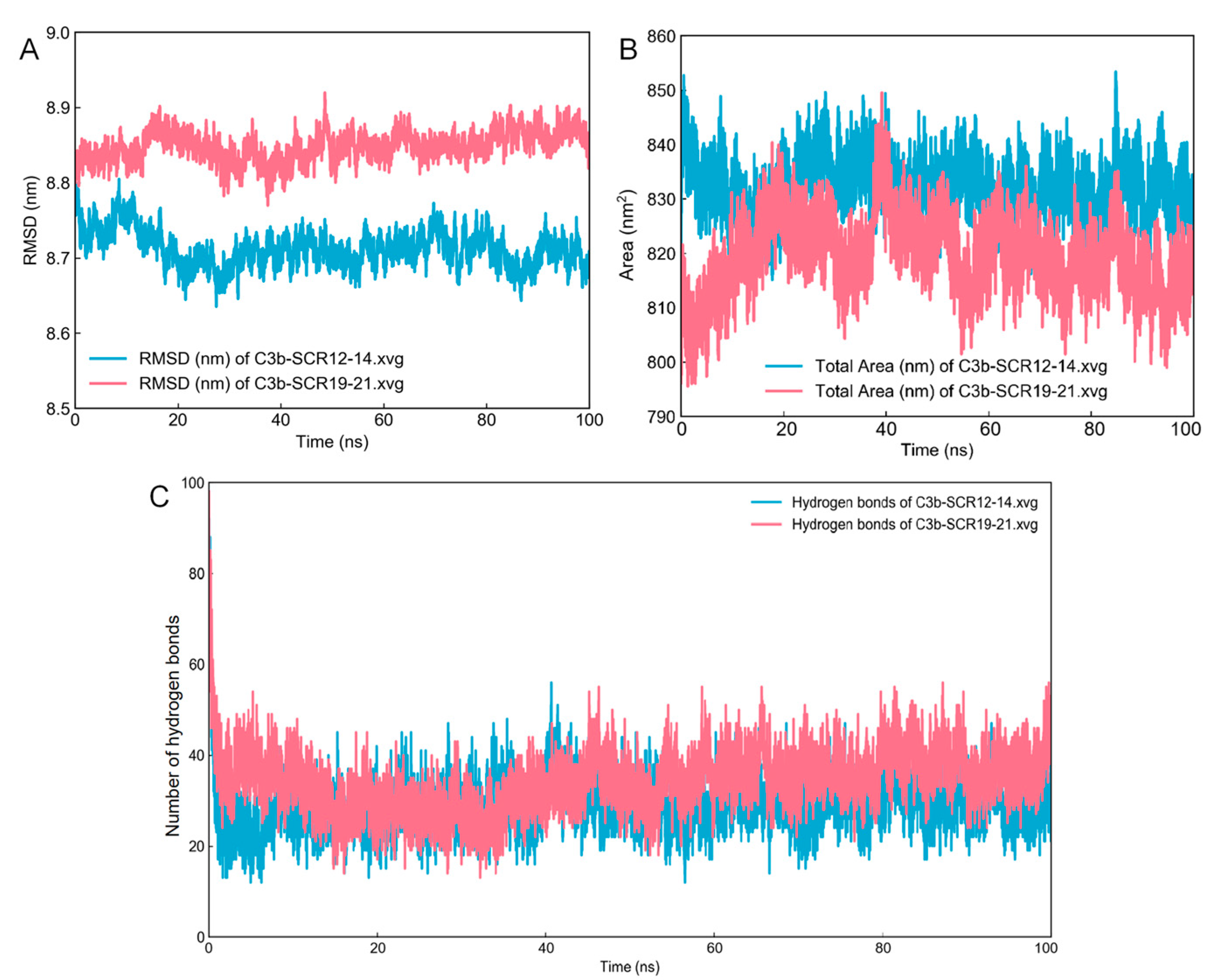
2.4. Molecular Docking and Dynamics Simulation
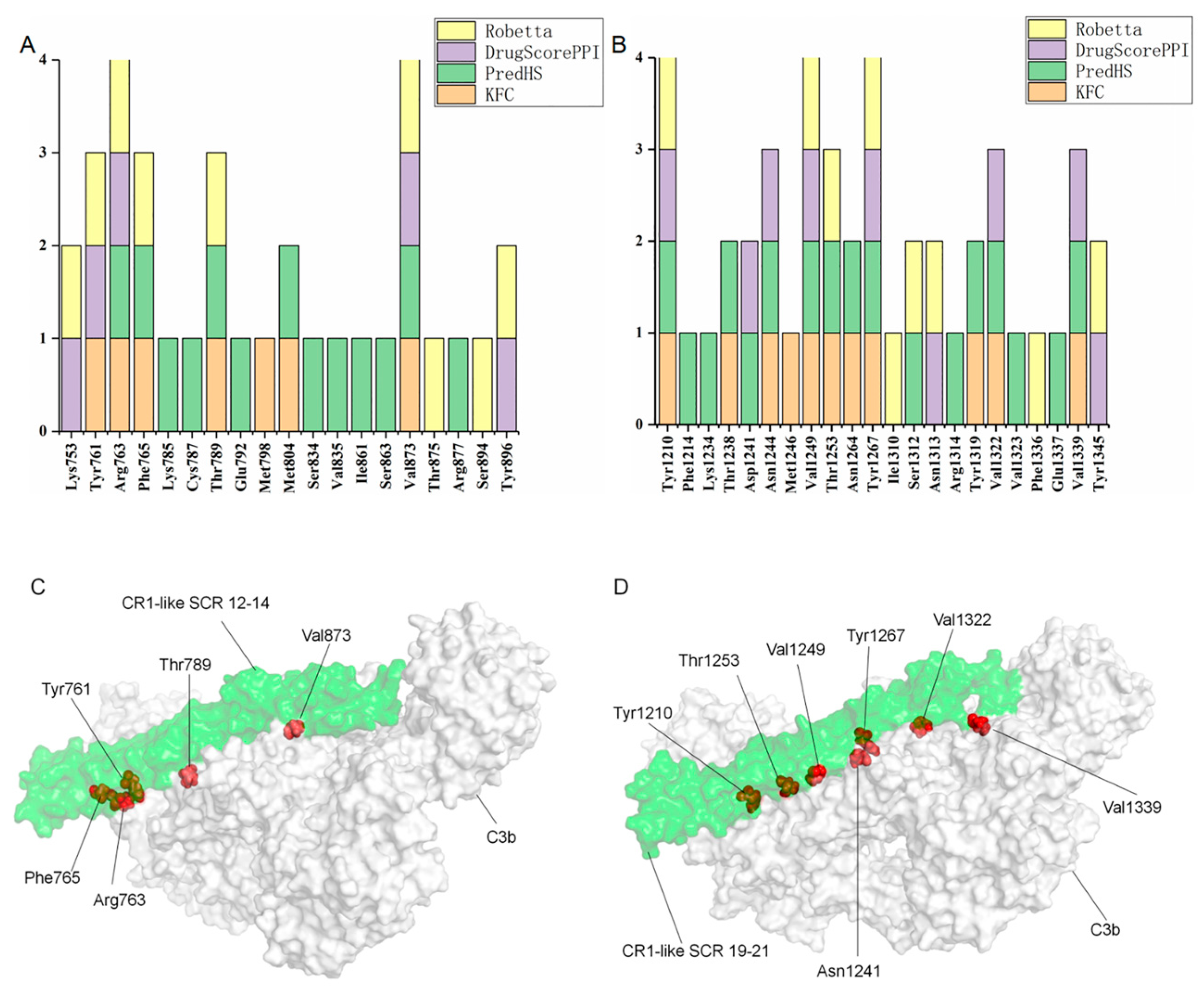
2.5. Protein Interaction Analysis
3. Discussion
4. Materials and Methods
4.1. Protein Binding Interface Analyses
4.2. Homology Modeling and Validation
4.3. Molecular Dynamics Simulation of Proteins
4.4. Molecular Docking and Affinity Calculations of CR1-like and C3b
4.5. Prediction of Key Amino Acids of the CR1-like-C3b Interaction
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Salam, K.A.; Wang, R.Y.; Grandinetti, T.; De Giorgi, V.; Alter, H.J.; Allison, R.D. Binding of Free and Immune Complex-Associated Hepatitis C Virus to Erythrocytes Is Mediated by the Complement System. Hepatology 2018, 68, 2118–2129. [Google Scholar] [CrossRef] [PubMed]
- Anand, D.; Kumar, U.; Kanjilal, M.; Kaur, S.; Das, N. Leucocyte complement receptor 1 (CR1/CD35) transcript and its correlation with the clinical disease activity in rheumatoid arthritis patients. Clin. Exp. Immunol. 2014, 176, 327–335. [Google Scholar] [CrossRef]
- Chen, C.H.; Tai, S.B.; Chen, H.C.; Yang, D.H.; Peng, M.Y.; Lin, Y.F. Analysis of Erythrocyte C4d to Complement Receptor 1 Ratio: Use in Distinguishing between Infection and Flare-Up in Febrile Patients with Systemic Lupus Erythematosus. Biomed. Res. Int. 2015, 2015, 939783. [Google Scholar] [CrossRef] [PubMed]
- Brubaker, W.D.; Crane, A.; Johansson, J.U. Peripheral complement interactions with amyloid β peptide: Erythrocyte clearance mechanisms. Alzheimers Dement. 2017, 13, 1397–1409. [Google Scholar] [CrossRef] [PubMed]
- Khera, R.; Das, N. Complement Receptor 1: Disease associations and therapeutic implications. Mol. Immunol. 2009, 46, 761–772. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.Z.; Zhao, H.S.; Li, X.W.; Zhang, L.C.; Hu, C.W.; Shao, B.; Sun, H.; Bah, A.A.; Li, Y.F.; Zhang, Z.G. Effects of Subchronic Aluminum Exposure on the Immune Function of Erythrocytes in Rats. Biol. Trace Elem. Res. 2011, 143, 1576–1580. [Google Scholar] [CrossRef]
- Zheng, S.; Yiyang, G.E.; Hongwei, M.A.; Gao, X.; Liu, C. Effect of goose source H5N1 avian influenza virus infection to erythrocyte immune function of duckling. J. Northeast Agric. Univ. 2019, 50, 45–51. [Google Scholar] [CrossRef]
- Jiang, J.B.; Wu, C.H.; Gao, H.; Song, J.D.; Li, H.Q. Effects of astragalus polysaccharides on immunologic function of erythrocyte in chickens infected with infectious bursa disease virus. Vaccine 2010, 28, 5614–5616. [Google Scholar] [CrossRef]
- Bao, E.; Su, J. Studies on the Red Blood Cell immune function of periphery blood of chickens which embryonally vaccinated with Marek’s disease vaccine. Chin. J. Anim. Vet. Sci. 2000, 5, 436–440. [Google Scholar] [CrossRef]
- Yin, W.; Xue, Y.P.; Jiang, J.B.; Fan, K.H.; Sun, N.; Sun, Y.G.; Hai-Li, M.A.; Bu-Gao, L.I.; Wang, Z.W.; Hong, H.L. Initial study of CR1-like expressed on porcine erythrocytes surface. Chin. Vet. Sci. 2015, 45, 985–990. [Google Scholar] [CrossRef]
- Yin, W.; Cui, J.Y.; Jiang, J.B.; Zhao, J.X.; Fan, K.H.; Sun, N.; Wang, Z.W.; Sun, Y.G.; Ma, H.L.; Li, H.Q. The immune adherence receptor CR1-like existed on porcine erythrocytes membrane. Sci. Rep. 2015, 5, 13290. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.; Wang, C.; Fan, K.H.; Sun, N.; Sun, Y.G.; Li, H.Q. In vitro observation: The GFP-E-coli adhering to porcine erythrocytes can be removed by porcine alveolar macrophages. PeerJ 2019, 7, e6439. [Google Scholar] [CrossRef]
- Lim, N.T.Y.; Harder, M.J.; Kennedy, A.T.; Lin, C.S.; Weir, C.; Cowman, A.F.; Call, M.J.; Schmidt, C.Q.; Tham, W.H. Characterization of Inhibitors and Monoclonal Antibodies That Modulate the Interaction between Plasmodium falciparum Adhesin PfRh4 with Its Erythrocyte Receptor Complement Receptor 1. J. Biol. Chem. 2015, 290, 25307–25321. [Google Scholar] [CrossRef] [PubMed]
- Ng, Y.C.; Peters, D.K.; Cederholm-Williams, S.A.; Walport, M.J. A lysine-binding protein in SLE sera inhibits the binding of immune complexes to normal erythrocyte CR1 (complement receptor type 1). Clin. Exp. Immunol. 1987, 69, 89–97. [Google Scholar]
- Furtado, P.B.; Huang, C.Y.; Ihyembe, D.; Hammond, R.A.; Marsh, H.C.; Perkins, S.J. The Partly Folded Back Solution Structure Arrangement of the 30 SCR Domains in Human Complement Receptor Type 1 (CR1) Permits Access to Its C3b and C4b Ligands. J. Mol. Biol. 2008, 375, 102–118. [Google Scholar] [CrossRef]
- Liu, D.; Niu, Z.-X. The Structure, Genetic Polymorphisms, Expression and Biological Functions of Complement Receptor Type 1 (CR1/CD35). Immunopharmacol. Immunotoxicol. 2009, 31, 524–535. [Google Scholar] [CrossRef]
- Soares, D.C.; Gerloff, D.L.; Syme, N.R.; Coulson, A.F.W.; Parkinson, J.; Barlow, P.N. Large-Scale Modelling as a Route to Multiple Surface Comparisons of the CCP Module Family. Protein Eng. Des. Sel. 2005, 18, 379–388. [Google Scholar] [CrossRef]
- Geisbrecht, B.V.; Lambris, J.D.; Gros, P. Complement Component C3: A Structural Perspective and Potential Therapeutic Implications. Semin. Immunol. 2022, 59, 101627. [Google Scholar] [CrossRef]
- Sun, Y.C.; Jia, R.P.; Fan, K.H.; Sun, N.; Sun, Y.G.; Sun, P.P.; Li, H.Q.; Yin, W. Detection of Interaction Between Porcine Type I Complement Receptor and C3b Active Fragment in Vitro. China Agric. Sci. 2021, 54, 4243–4254. [Google Scholar] [CrossRef]
- Forneris, F.; Wu, J.; Xue, X.; Ricklin, D.; Lin, Z.; Sfyroera, G.; Tzekou, A.; Volokhina, E.; Granneman, J.C.M.; Hauhart, R.; et al. Regulators of complement activity mediate inhibitory mechanisms through a common C3b-binding mode. EMBO J. 2016, 35, 1133–1149. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Lin, K.; Zhang, X.; Zou, J.; Zhang, L.; Gong, P.; Zhao, J.; Han, C.; Liu, Y.; Yi, H.; et al. Insight into the Molecular-Level Details of As1 Casein Interactions with IgG: Combining with LC-MS/MS and Molecular Modelling Techniques. Food Chem. 2023, 399, 133987. [Google Scholar] [CrossRef] [PubMed]
- Rosell, M.; Fernández-Recio, J. Docking Approaches for Modeling Multi-Molecular Assemblies. Curr. Opin. Struct. Biol. 2020, 64, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Porter, K.A.; Desta, I.; Kozakov, D.; Vajda, S. What Method to Use for Protein–Protein Docking? Curr. Opin. Struct. Biol. 2019, 55, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.O.; Mallin, R.L.; Krych-Goldberg, M.; Wang, X.; Hauhart, R.E.; Bromek, K.; Uhrin, D.; Atkinson, J.P.; Barlow, P.N. Structure of the C3b Binding Site of CR1 (CD35), the Immune Adherence Receptor. Cell 2002, 108, 769–780. [Google Scholar] [CrossRef] [PubMed]
- Krych-Goldberg, M.; Hauhart, R.E.; Subramanian, V.B.; Yurcisin, B.M.; Crimmins, D.L.; Hourcade, D.E.; Atkinson, J.P. Decay Accelerating Activity of Complement Receptor Type 1 (CD35). J. Biol. Chem. 1999, 274, 31160–31168. [Google Scholar] [CrossRef]
- Krych, M.; Hauhart, R.; Atkinson, J.P. Structure-Function Analysis of the Active Sites of Complement Receptor Type 1. J. Biol. Chem. 1998, 273, 8623–8629. [Google Scholar] [CrossRef]
- Fábio, M.; Mi, P.; Joon, L.; Nicola, B.; Tamer, G.; Nandana, M. The EMBL-EBI search and sequence analysis tools APIs in 2019. Nucleic Acids Res. 2019, 47, 636–641. [Google Scholar] [CrossRef]
- Sarkar, S.; Gupta, S.; Chakraborty, W.; Senapati, S.; Gachhui, R. Homology modeling, molecular docking and molecular dynamics studies of the catalytic domain of chitin deacetylase from Cryptococcus laurentii strain RY1. Int. J. Biol. Macromol. 2017, 104, 1682–1691. [Google Scholar] [CrossRef] [PubMed]
- Letunic, I.; Khedkar, S.; Bork, P. SMART: Recent Updates, New Developments and Status in 2020. Nucleic Acids Res. 2020, 49, D458–D460. [Google Scholar] [CrossRef] [PubMed]
- Moller, S.; Croning, M.D.R.; Apweiler, R. Evaluation of methods for the prediction of membrane spanning regions. Bioinformatics 2001, 17, 646–653. [Google Scholar] [CrossRef]
- Teufel, F.; Almagro Armenteros, J.J.; Johansen, A.R.; Gíslason, M.H.; Pihl, S.I.; Tsirigos, K.D.; Winther, O.; Brunak, S.; von Heijne, G.; Nielsen, H. SignalP 6.0 Predicts All Five Types of Signal Peptides Using Protein Language Models. Nat. Biotechnol. 2022, 40, 1023–1025. [Google Scholar] [CrossRef]
- Wang, H.; Liu, C.; Deng, L. Enhanced Prediction of Hot Spots at Protein-Protein Interfaces Using Extreme Gradient Boosting. Sci. Rep. 2018, 8, 14285. [Google Scholar] [CrossRef]
- Zhu, X.; Mitchell, J.C. KFC2: A Knowledge-Based Hot Spot Prediction Method Based on Interface Solvation, Atomic Density, and Plasticity Features. Proteins 2011, 79, 2671–2683. [Google Scholar] [CrossRef] [PubMed]
- Kortemme, T.; Kim, D.E.; Baker, D. Computational Alanine Scanning of Protein-Protein Interfaces. Sci. STKE 2004, 2004, pl2. [Google Scholar] [CrossRef]
- Kruger, D.M.; Gohlke, H. DrugScore (PPI) webserver: Fast and accurate in silico alanine scanning for scoring protein-protein interactions. Nucleic Acids Res. 2010, 38, 480–486. [Google Scholar] [CrossRef]
- Evans, R.; O’Neill, M.; Pritzel, A.; Antropova, N.; Senior, A.; Green, T.; Žídek, A.; Bates, R.; Blackwell, S.; Yim, J.; et al. Protein Complex Prediction with AlphaFold-Multimer. BioRxiv 2021, 1–25. [Google Scholar] [CrossRef]
- Du, Z.; Su, H.; Wang, W.; Ye, L.; Wei, H.; Peng, Z.; Anishchenko, I.; Baker, D.; Yang, J. The TrRosetta Server for Fast and Accurate Protein Structure Prediction. Nat. Protoc. 2021, 16, 5634–5651. [Google Scholar] [CrossRef] [PubMed]
- Baek, M.; DiMaio, F.; Anishchenko, I.; Dauparas, J.; Ovchinnikov, S.; Lee, G.R.; Wang, J.; Cong, Q.; Kinch, L.N.; Schaeffer, R.D.; et al. Accurate Prediction of Protein Structures and Interactions Using a Three-Track Neural Network. Science 2021, 373, 871–876. [Google Scholar] [CrossRef] [PubMed]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly Accurate Protein Structure Prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef]
- Yang, J.Y.; Zhang, Y. I-TASSER server: New development for protein structure and function predictions. Nucleic Acids Res. 2015, 43, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Laskowski, R.A.; MacArthur, M.W.; Moss, D.S.; Thornton, J.M. PROCHECK: A Program to Check the Stereochemical Quality of Protein Structures. J. Appl. Crystallogr. 1993, 26, 283–291. [Google Scholar] [CrossRef]
- Eisenberg, D.; Lüthy, R.; Bowie, J.U. VERIFY3D: Assessment of Protein Models with Three-Dimensional Profiles. Methods Enzymol. 1997, 277, 396–404. [Google Scholar] [CrossRef]
- Wiederstein, M.; Sippl, M.J. ProSA-web: Interactive web service for the recognition of errors in three-dimensional structures of proteins. Nucleic Acids Res. 2007, 35, 407–410. [Google Scholar] [CrossRef]
- Huang, J.; MacKerell, A.D. CHARMM36 all-atom additive protein force field: Validation based on comparison to NMR data. J. Comput. Chem. 2013, 34, 2135–2145. [Google Scholar] [CrossRef]
- Srikumar, P.S.; Rohini, K.; Rajesh, P.K. Molecular Dynamics Simulations and Principal Component Analysis on Human Laforin Mutation W32G and W32G/K87A. Protein J. 2014, 33, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Papaleo, E.; Mereghetti, P.; Fantucci, P.; Grandori, R.; De Gioia, L. Free-Energy Landscape, Principal Component Analysis, and Structural Clustering to Identify Representative Conformations from Molecular Dynamics Simulations: The Myoglobin Case. J. Mol. Graph. Model. 2009, 27, 889–899. [Google Scholar] [CrossRef]
- Desta, I.T.; Porter, K.A.; Xia, B.; Kozakov, D.; Vajda, S. Performance and Its Limits in Rigid Body Protein-Protein Docking. Structure 2020, 28, 1071–1081. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Li, L.; Weng, Z.P. ZDOCK: An initial-stage protein-docking algorithm. Proteins 2003, 52, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Aportela, E.; Lopez-Blanco, J.R.; Chacon, P. FRODOCK 2.0: Fast protein-protein docking server. Bioinformatics 2016, 32, 2386–2388. [Google Scholar] [CrossRef]
- Tovchigrechko, A.; Vakser, I.A. GRAMM-X public web server for protein-protein docking. Nucleic Acids Res. 2006, 34, 310–314. [Google Scholar] [CrossRef]
- Honorato, R.V.; Koukos, P.I.; Jiménez-García, B.; Tsaregorodtsev, A.; Verlato, M.; Giachetti, A.; Rosato, A.; Bonvin, A.M. Structural Biology in the Clouds: The WeNMR-EOSC Ecosystem. Front. Mol. Biosci. 2021, 8, 729513. [Google Scholar] [CrossRef] [PubMed]
- Xue, L.C.; Rodrigues, J.; Kastritis, P.L.; Bonvin, A.; Vangone, A. PRODIGY: A web server for predicting the binding affinity of protein-protein complexes. Bioinformatics 2016, 32, 3676–3678. [Google Scholar] [CrossRef] [PubMed]


| Model | Ramachandran Plot (%) | Verify 3D (%) | |||
|---|---|---|---|---|---|
| Favoured | Allowed | General | Disallowed | Residues Score > 0.2% | |
| C3b | 92.2 | 7.7 | 0.1 | 0.1 | 87.2 |
| CR1-like(12–14) | 50.6 | 40.9 | 7.1 | 1.3 | 93.7 |
| CR1-like(19–21) | 72.2 | 23.4 | 3.2 | 1.3 | 95.2 |
| CR1-like(12–14) * | 85.7 | 12.3 | 1.9 | 0.0 | 81.5 |
| CR1-like(19–21) * | 88.0 | 10.8 | 1.3 | 0.0 | 89.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, Z.; Yin, W.; Hao, Z.; Fan, K.; Sun, N.; Sun, P.; Li, H. Molecular Simulation Study on the Interaction between Porcine CR1-like and C3b. Molecules 2023, 28, 2183. https://doi.org/10.3390/molecules28052183
Hou Z, Yin W, Hao Z, Fan K, Sun N, Sun P, Li H. Molecular Simulation Study on the Interaction between Porcine CR1-like and C3b. Molecules. 2023; 28(5):2183. https://doi.org/10.3390/molecules28052183
Chicago/Turabian StyleHou, Zhen, Wei Yin, Zhili Hao, Kuohai Fan, Na Sun, Panpan Sun, and Hongquan Li. 2023. "Molecular Simulation Study on the Interaction between Porcine CR1-like and C3b" Molecules 28, no. 5: 2183. https://doi.org/10.3390/molecules28052183
APA StyleHou, Z., Yin, W., Hao, Z., Fan, K., Sun, N., Sun, P., & Li, H. (2023). Molecular Simulation Study on the Interaction between Porcine CR1-like and C3b. Molecules, 28(5), 2183. https://doi.org/10.3390/molecules28052183






