Bench-to-Bedside Studies of Arginine Deprivation in Cancer
Abstract
1. Introduction
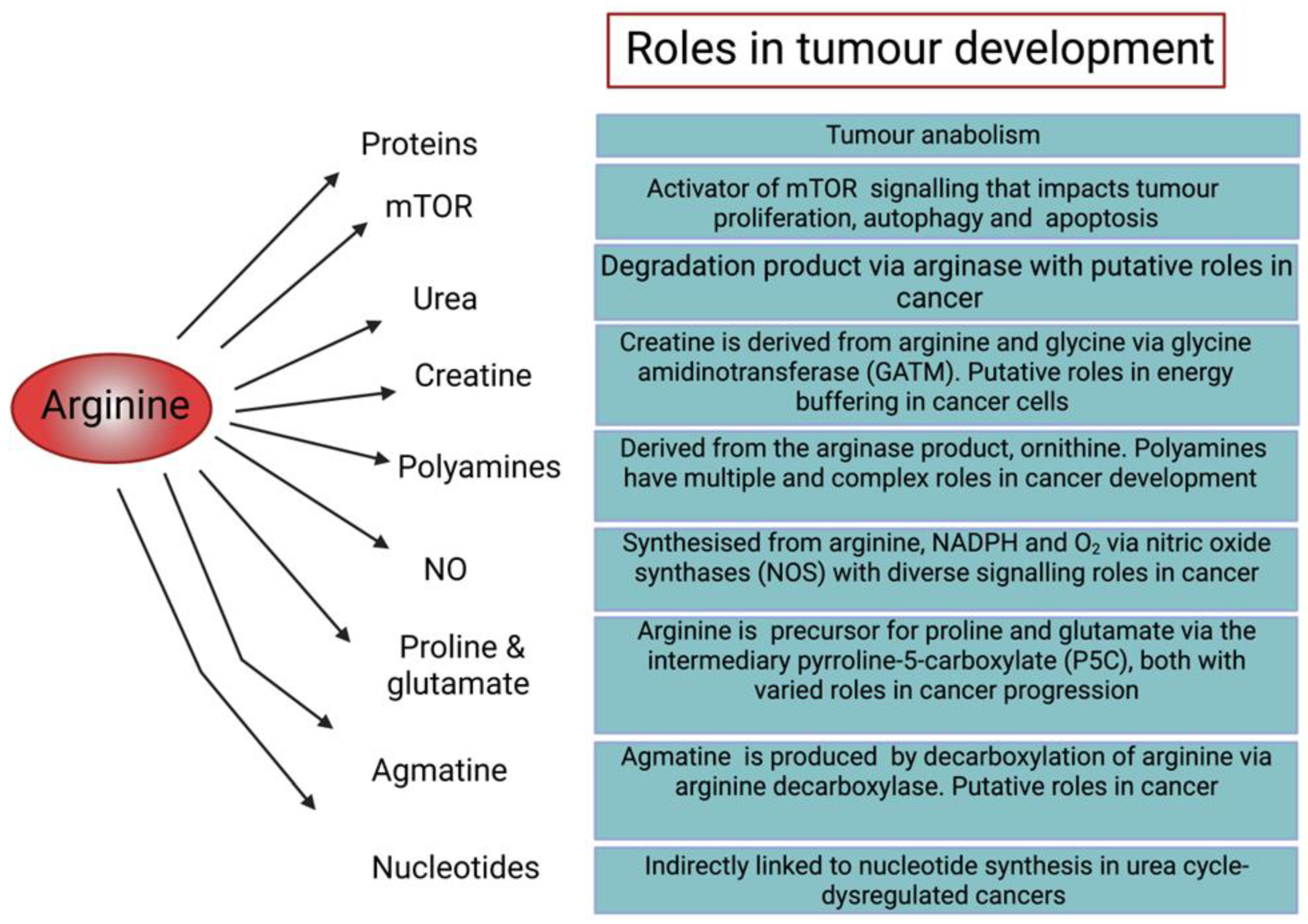
1.1. Role of Arginine in Cellular Function
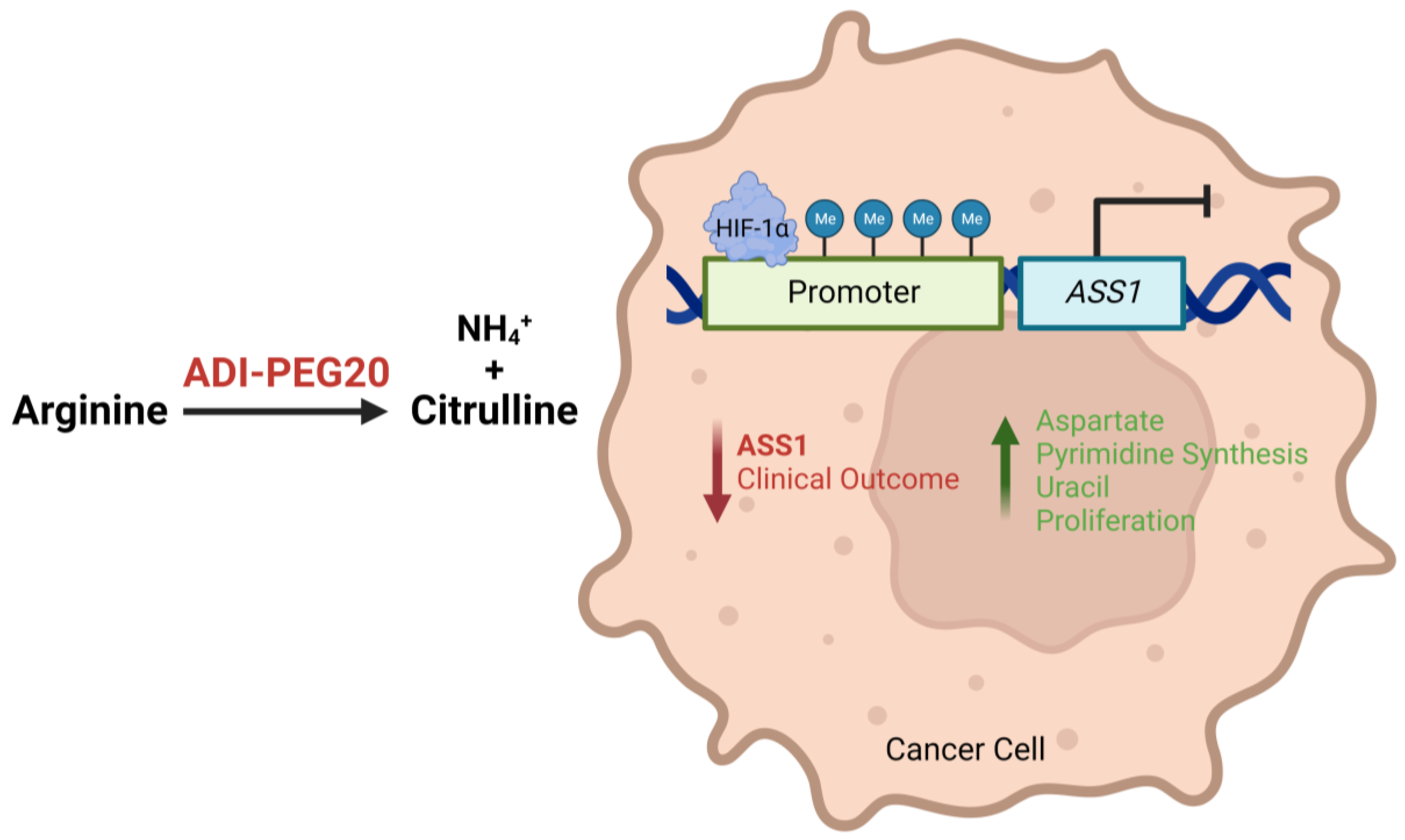
1.2. Tumoral ASS1 Deficiency and Arginine Dependency
1.3. Arginine Deprivation: Preclinical Data
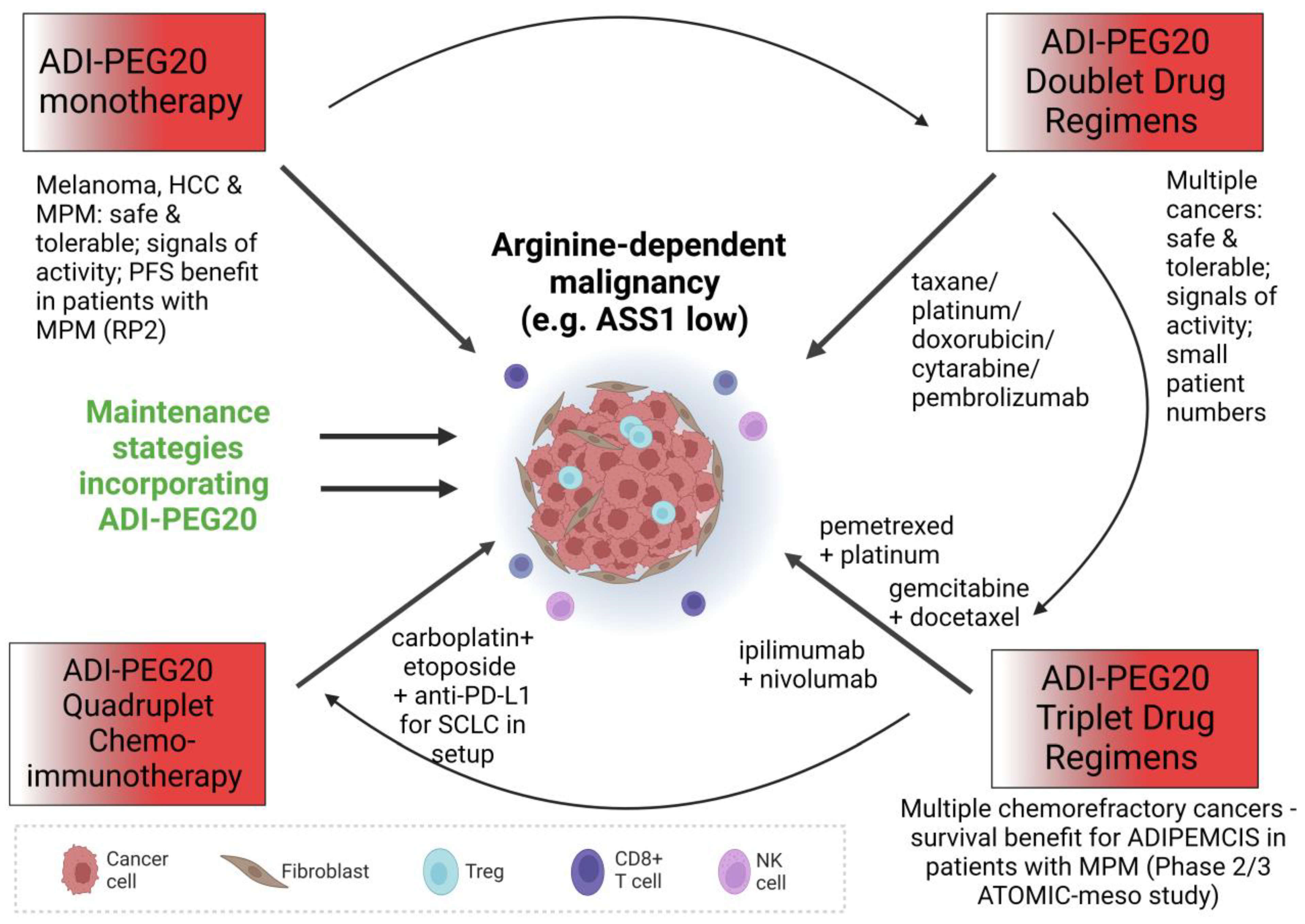
2. Monotherapy
3. Combination Therapy
3.1. ADI-PEG20 and Antifolates
3.2. ADI-PEG20 and Gemcitabine
3.3. ADI-PEG20 and Taxanes
3.4. ADI-PEG20 and Platinum
3.5. ADI-PEG20 and Doxorubicin
3.6. ADI-PEG20 and Cytarabine
3.7. ADI-PEG20 and Immunotherapy
4. ADI-PEG20 Resistance
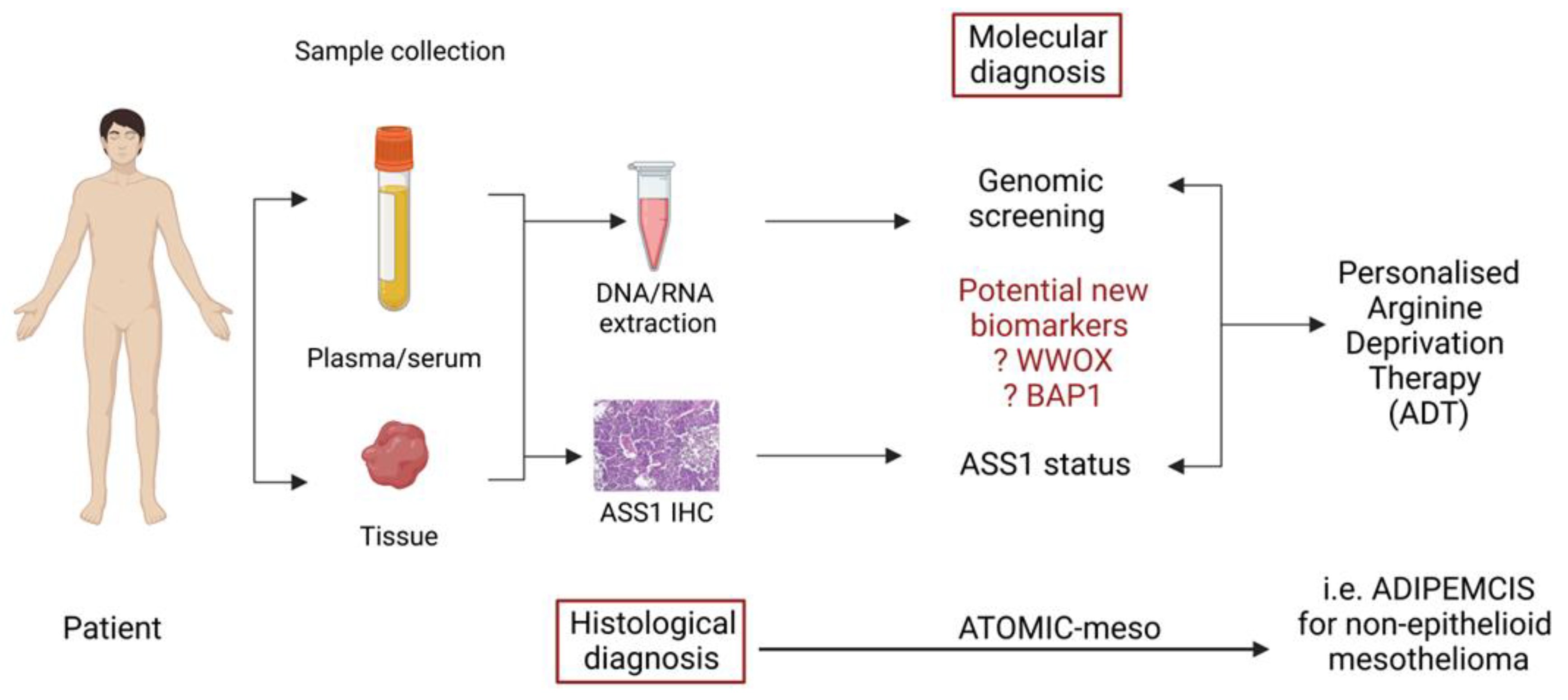
5. Biomarkers and Arginine Deprivation Treatment
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Patil, M.D.; Bhaumik, J.; Babykutty, S.; Banerjee, U.C.; Fukumura, D. Arginine dependence of tumor cells: Targeting a chink in cancer’s armor. Oncogene 2016, 35, 4957–4972. [Google Scholar] [CrossRef]
- Wu, G.; Morris, S.M. Arginine metabolism: Nitric oxide and beyond. Biochem. J. 1998, 336, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Husson, A.; Brasse-Lagnel, C.; Fairand, A.; Renouf, S.; Lavoinne, A. Argininosuccinate synthetase from the urea cycle to the citrulline-NO cycle. Eur. J. Biochem. 2003, 270, 1887–1899. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.L.; Hsu, S.C.; Ann, D.K.; Yen, Y.; Kung, H.J. Arginine Signaling and Cancer Metabolism. Cancers 2021, 13, 3541. [Google Scholar] [CrossRef] [PubMed]
- Martí i Líndez, A.A.; Reith, W. Arginine-dependent immune responses. Cell. Mol. Life Sci. 2021, 78, 5303–5324. [Google Scholar] [CrossRef]
- Kazak, L.; Cohen, P. Creatine metabolism: Energy homeostasis, immunity and cancer biology. Nat. Rev. Endocrinol. 2020, 16, 421–436. [Google Scholar] [CrossRef] [PubMed]
- Barua, S.; Sim, A.Y.; Kim, J.Y.; Shin, I.; Lee, J.E. Maintenance of the Neuroprotective Function of the Amino Group Blocked Fluorescence-Agmatine. Neurochem. Res. 2021, 46, 1933–1940. [Google Scholar] [CrossRef] [PubMed]
- Delage, B.; Fennell, D.A.; Nicholson, L.; McNeish, I.; Lemoine, N.R.; Crook, T.; Szlosarek, P.W. Arginine deprivation and argininosuccinate synthetase expression in the treatment of cancer. Int. J. Cancer 2010, 126, 2762–2772. [Google Scholar] [CrossRef]
- Szlosarek, P.W.; Klabatsa, A.; Pallaska, A.; Sheaff, M.; Smith, P.; Crook, T.; Grimshaw, M.J.; Steele, J.P.; Rudd, R.M.; Balkwill, F.R.; et al. In vivo Loss of Expression of Argininosuccinate Synthetase in Malignant Pleural Mesothelioma Is a Biomarker for Susceptibility to Arginine Depletion. Clin. Cancer Res. 2006, 12, 7126–7131. [Google Scholar] [CrossRef]
- Rabinovich, S.; Adler, L.; Yizhak, K.; Sarver, A.; Silberman, A.; Agron, S.; Stettner, N.; Sun, Q.; Brandis., A.; Helbling, D.; et al. Diversion of aspartate in ASS1-deficient tumours fosters de novo pyrimidine synthesis. Nature 2015, 527, 379–383. [Google Scholar] [CrossRef]
- Huang, H.Y.; Wu, W.R.; Wang, Y.H.; Wang, J.W.; Fang, F.M.; Tsai, J.W.; Li, S.H.; Hung, H.C.; Yu, S.C.; Lan, J.; et al. ASS1 as a Novel Tumor Suppressor Gene in Myxofibrosarcomas: Aberrant Loss via Epigenetic DNA Methylation Confers Aggressive Phenotypes, Negative Prognostic Impact, and Therapeutic Relevance. Clin. Cancer Res. 2013, 19, 2861–2872. [Google Scholar] [CrossRef] [PubMed]
- Allen, M.D.; Luong, P.; Hudson, C.; Leyton, J.; Delage, B.; Ghazaly, E.; Cutts, R.; Yuan, M.; Syed, N.; Lo Nigro, C.; et al. Prognostic and Therapeutic Impact of Argininosuccinate Synthetase 1 Control in Bladder Cancer as Monitored Longitudinally by PET Imaging. Cancer Res. 2014, 74, 896–907. [Google Scholar] [CrossRef] [PubMed]
- Giatromanolaki, A.; Harris, A.L.; Koukourakis, M.I. The prognostic and therapeutic implications of distinct patterns of argininosuccinate synthase 1 (ASS1) and arginase-2 (ARG2) expression by cancer cells and tumor stroma in non-small-cell lung cancer. Cancer Metab. 2021, 9, 28. [Google Scholar] [CrossRef] [PubMed]
- Delage, B.; Luong, P.; Maharaj, L.; O’Riain, C.; Syed, N.; Crook, T.; Hatzimichael, E.; Papoudou-Bai, A.; Mitchell, T.J.; Whittaker, S.J.; et al. Promoter methylation of argininosuccinate synthetase-1 sensitises lymphomas to arginine deiminase treatment, autophagy and caspase-dependent apoptosis. Cell Death Dis. 2012, 3, e342. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, L.J.; Smith, P.R.; Hiller, L.; Szlosarek, P.W.; Kimberley, C.; Sehouli, J.; Koensgen, D.; Mustea, A.; Schmid, P.; Crook, T. Epigenetic silencing of argininosuccinate synthetase confers resistance to platinum-induced cell death but collateral sensitivity to arginine auxotrophy in ovarian cancer. Int. J. Cancer 2009, 125, 1454–1463. [Google Scholar] [CrossRef]
- Syed, N.; Langer, J.; Janczar, K.; Singh, P.; lo Nigro, C.; Lattanzio, L.; Coley, H.M.; Hatzimichael, E.; Bromalaski, J.; Szlosarek, P.W.; et al. Epigenetic status of argininosuccinate synthetase and argininosuccinate lyase modulates autophagy and cell death in glioblastoma. Cell Death Dis. 2013, 4, e458. [Google Scholar] [CrossRef]
- Tsai, W.B.; Aiba, I.; Lee, S.Y.; Feun, L.; Savaraj, N.; Kuo, M.T. Resistance to arginine deiminase treatment in melanoma cells is associated with induced argininosuccinate synthetase expression involving c-Myc/HIF-1α/Sp4. Mol. Cancer Ther. 2009, 8, 3223–3233. [Google Scholar] [CrossRef]
- Hajaj, E.; Sciacovelli, M.; Frezza, C.; Erez, A. The context-specific roles of urea cycle enzymes in tumorigenesis. Mol. Cell 2021, 81, 3749–3759. [Google Scholar] [CrossRef]
- Zou, Z.; Tao, T.; Li, H.; Zhu, X. mTOR signaling pathway and mTOR inhibitors in cancer: Progress and challenges. Cell Biosci. 2020, 10, 31. [Google Scholar] [CrossRef]
- Sun, Y.; Li, J.; Qu, Z.; Yang, Z.; Jia, X.; Lin, Y.; He, Q.; Zhang, L.; Luo, Y. Causal Associations between Serum Urea and Cancer: A Mendelian Randomization Study. Genes 2021, 12, 498. [Google Scholar] [CrossRef]
- Holbert, C.E.; Cullen, M.T.; Casero, R.A.; Stewart, T.M. Polyamines in cancer: Integrating organismal metabolism and antitumour immunity. Nat. Rev. Cancer 2022, 22, 467–480. [Google Scholar] [CrossRef]
- Reddy, T.P.; Glynn, S.A.; Billiar, T.R.; Wink, D.A.; Chang, J.C. Targeting Nitric Oxide: Say NO to Metastasis. Clin. Cancer Res. 2022, 22, 2791. [Google Scholar] [CrossRef] [PubMed]
- D’Aniello, C.; Patriarca, E.J.; Phang, J.M.; Minchiotti, G. Proline Metabolism in Tumor Growth and Metastatic Progression. Front Oncol. 2020, 10, 776. [Google Scholar] [CrossRef] [PubMed]
- García-Gaytán, A.C.; Hernández-Abrego, A.; Díaz-Muñoz, M.; Méndez, I. Glutamatergic system components as potential biomarkers and therapeutic targets in cancer in non-neural organs. Front Endocrinol. 2022, 13, 1029210. [Google Scholar] [CrossRef] [PubMed]
- Piletz, J.E.; Aricioglu, F.; Cheng, J.T.; Fairbanks, C.A.; Gilad, V.H.; Haenisch, B.; Halaris, A.; Hong, S.; Lee, J.E.; Li, J.; et al. Agmatine: Clinical applications after 100 years in translation. Drug Discov. Today 2013, 18, 880–893. [Google Scholar] [CrossRef] [PubMed]
- Keshet, R.; Szlosarek, P.; Carracedo, A.; Erez, A. Rewiring urea cycle metabolism in cancer to support anabolism. Nat. Rev. Cancer 2018, 18, 634–645. [Google Scholar] [CrossRef]
- Noens, E.E.E.; Lolkema, J.S. Convergent evolution of the arginine deiminase pathway: The ArcD and ArcE arginine/ornithine exchangers. Microbiologyopen 2017, 6, e00412. [Google Scholar] [CrossRef]
- Novák, L.; Zubáčová, Z.; Karnkowska, A.; Kolisko, M.; Hroudová, M.; Stairs, C.W.; Simpson, A.G.B.; Keeling, P.J.; Roger, A.J.; Čepička, I.; et al. Arginine deiminase pathway enzymes: Evolutionary history in metamonads and other eukaryotes. BMC Evol. Biol. 2016, 16, 197. [Google Scholar] [CrossRef]
- Ensor, C.M.; Holtsberg, F.W.; Bomalaski, J.S.; Clark, M.A. Pegylated arginine deiminase (ADI-SS PEG20,000 mw) inhibits human melanomas and hepatocellular carcinomas in vitro and in vivo. Cancer Res. 2002, 62, 5443–5450. [Google Scholar]
- Bowles, T.L.; Kim, R.; Galante, J.; Parsons, C.M.; Virudachalam, S.; Kung, H.J.; Bold, R.J. Pancreatic cancer cell lines deficient in argininosuccinate synthetase are sensitive to arginine deprivation by arginine deiminase. Int. J. Cancer 2008, 123, 1950–1955. [Google Scholar] [CrossRef] [PubMed]
- Kelly, M.P.; Jungbluth, A.A.; Wu, B.W.; Bomalaski, J.; Old, L.J.; Ritter, G. Arginine deiminase PEG20 inhibits growth of small cell lung cancers lacking expression of argininosuccinate synthetase. Br. J. Cancer 2012, 106, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Bean, G.R.; Kremer, J.C.; Prudner, B.C.; Schenone, A.D.; Yao, J.C.; Schultze, M.B.; Chen, D.Y.; Tanas, M.R.; Adkins, D.R.; Bomalaski, J.; et al. A metabolic synthetic lethal strategy with arginine deprivation and chloroquine leads to cell death in ASS1-deficient sarcomas. Cell Death Dis. 2016, 7, e2406. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.X.; Cochrane, D.R.; Tessier-Cloutier, B.; Chen, S.Y.; Ho, G.; Pathak, K.V.; Alcazar, I.N.; Farnell, D.; Leung, S.; Cheng, A.; et al. Arginine Depletion Therapy with ADI-PEG20 Limits Tumor Growth in Argininosuccinate Synthase–Deficient Ovarian Cancer, Including Small-Cell Carcinoma of the Ovary, Hypercalcemic Type. Clin. Cancer Res. 2020, 26, 4402–4413. [Google Scholar] [CrossRef] [PubMed]
- Savaraj, N.; Wu, C.; Kuo, M.T.; You, M.; Wangpaichitr, M.; Robles, C.; Spector, S.; Feun, L. The relationship of arginine deprivation, argininosuccinate synthetase and cell death in melanoma. Drug Target Insights 2007, 2, 119–128. [Google Scholar] [CrossRef]
- Kim, R.H.; Coates, J.M.; Bowles, T.L.; McNerney, G.P.; Sutcliffe, J.; Jung, J.U.; Gandour-Edwards, R.; Chuang, F.Y.S.; Bold, R.J.; Kung, H.J. Arginine Deiminase as a Novel Therapy for Prostate Cancer Induces Autophagy and Caspase-Independent Apoptosis. Cancer Res. 2009, 69, 700–708. [Google Scholar] [CrossRef]
- Cheng, C.T.; Qi, Y.; Wang, Y.C.; Chi, K.K.; Chung, Y.; Ouyang, C.; Chen, Y.R.; Oh, M.E.; Sheng, X.; Tang, Y.; et al. Arginine starvation kills tumor cells through aspartate exhaustion and mitochondrial dysfunction. Commun. Biol. 2018, 1, 178. [Google Scholar] [CrossRef]
- Storr, J.M.; Burton, A.F. The Effects of Arginine Deficiency on Lymphoma Cells. Br. J. Cancer 1974, 30, 50–59. [Google Scholar] [CrossRef]
- Savoca, K.V.; Davis, F.F.; van Es, T.; McCoy, J.R.; Palczuk, N.C. Cancer therapy with chemically modified enzymes. II. The therapeutic effectiveness of arginase, and arginase modified by the covalent attachment of polyethylene glycol, on the taper liver tumor and the L5178Y murine leukemia. Cancer Biochem. Biophys. 1984, 7, 261–268. [Google Scholar]
- Cheng, P.N.M.; Lam, T.L.; Lam, W.M.; Tsui, S.M.; Cheng, A.W.M.; Lo, W.H.; Leung, Y.C. Pegylated Recombinant Human Arginase (rhArg-peg5,000mw) Inhibits the In vitro and In vivo Proliferation of Human Hepatocellular Carcinoma through Arginine Depletion. Cancer Res. 2007, 67, 309–317. [Google Scholar] [CrossRef]
- Agnello, G.; Alters, S.E.; Rowlinson, S.W. Preclinical safety and antitumor activity of the arginine-degrading therapeutic enzyme pegzilarginase, a PEGylated, cobalt-substituted recombinant human arginase 1. Transl. Res. 2020, 217, 11–22. [Google Scholar] [CrossRef]
- Yu, K.M.; Pang, T.P.S.; Cutler, M.; Tian, M.; Huang, L.; Lau, J.Y.N.; Chung, S.F.; Lo, T.W.H.; Leung, T.Y.C. Rational design, engineer, and characterization of a novel pegylated single isomer human arginase for arginine depriving anti-cancer treatment. Life Sci. 2021, 264, 118674. [Google Scholar] [CrossRef] [PubMed]
- Yau, T.; Cheng, P.N.M.; Chiu, J.; Kwok, G.G.W.; Leung, R.; Liu, A.M.; Cheung, T.T.; Ng, C.T. A phase 1 study of pegylated recombinant arginase (PEG-BCT-100) in combination with systemic chemotherapy (capecitabine and oxaliplatin)[PACOX] in advanced hepatocellular carcinoma patients. Investig. New Drugs 2022, 40, 314–321. [Google Scholar] [CrossRef] [PubMed]
- de Santo, C.; Cheng, P.; Beggs, A.; Egan, S.; Bessudo, A.; Mussai, F. Metabolic therapy with PEG-arginase induces a sustained complete remission in immunotherapy-resistant melanoma. J. Hematol. Oncol. 2018, 11, 68. [Google Scholar] [CrossRef]
- Chan, S.L.; Cheng, P.N.M.; Liu, A.M.; Chan, L.L.; Li, L.; Chu, C.M.; Chong, C.C.N.; Lau, Y.M.; Yeo, W.; Ng, K.K.C.; et al. A phase II clinical study on the efficacy and predictive biomarker of pegylated recombinant arginase on hepatocellular carcinoma. Investig. New Drugs 2021, 39, 1375–1382. [Google Scholar] [CrossRef]
- Izzo, F.; Marra, P.; Beneduce, G.; Castello, G.; Vallone, P.; de Rosa, V.; Cremona, F.; Ensor, C.M.; Holtsberg, F.W.; Bomalaski, J.S.; et al. Pegylated Arginine Deiminase Treatment of Patients With Unresectable Hepatocellular Carcinoma: Results From Phase I/II Studies. J. Clin. Oncol. 2004, 22, 1815–1822. [Google Scholar] [CrossRef] [PubMed]
- Glazer, E.S.; Piccirillo, M.; Albino, V.; di Giacomo, R.; Palaia, R.; Mastro, A.A.; Beneduce, G.; Castello, G.; De Rosa, V.; Petrillo, A.; et al. Phase II Study of Pegylated Arginine Deiminase for Nonresectable and Metastatic Hepatocellular Carcinoma. J. Clin. Oncol. 2010, 28, 2220–2226. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.S.; Lu, S.N.; Chao, Y.; Sheen, I.S.; Lin, C.C.; Wang, T.E.; Chen, S.C.; Wang, J.H.; Liao, L.Y.; Thomson, J.A.; et al. A randomised phase II study of pegylated arginine deiminase (ADI-PEG 20) in Asian advanced hepatocellular carcinoma patients. Br. J. Cancer 2010, 103, 954–960. [Google Scholar] [CrossRef]
- Abou-Alfa, G.K.; Qin, S.; Ryoo, B.Y.; Lu, S.N.; Yen, C.J.; Feng, Y.H.; Lim, H.Y.; Izzo, F.; Colombo, M.; Sarker, D.; et al. Phase III randomized study of second line ADI-PEG 20 plus best supportive care versus placebo plus best supportive care in patients with advanced hepatocellular carcinoma. Ann. Oncol. 2018, 29, 1402–1408. [Google Scholar] [CrossRef]
- Feun, L.G.; Marini, A.; Walker, G.; Elgart, G.; Moffat, F.; Rodgers, S.E.; Wu, C.J.; You, M.; Wangpaichitr, M.; Juo, M.T.; et al. Negative argininosuccinate synthetase expression in melanoma tumours may predict clinical benefit from arginine-depleting therapy with pegylated arginine deiminase. Br. J. Cancer 2012, 106, 1481–1485. [Google Scholar] [CrossRef]
- Szlosarek, P.W.; Phillips, M.M.; Pavlyk, I.; Steele, J.; Shamash, J.; Spicer, J.; Kumar, S.; Pacey, S.; Feng, X.; Johnston, A.; et al. Expansion Phase 1 Study of Pegargiminase Plus Pemetrexed and Cisplatin in Patients With Argininosuccinate Synthetase 1–Deficient Mesothelioma: Safety, Efficacy, and Resistance Mechanisms. JTO Clin. Res. Rep. 2020, 1, 100093. [Google Scholar] [CrossRef]
- Szlosarek, P.W.; Wimalasingham, A.G.; Phillips, M.M.; Hall, P.E.; Chan, P.Y.; Conibear, J.; Lim, L.; Rashid, S.; Steele, J.; Wells, P.; et al. Phase 1, pharmacogenomic, dose-expansion study of pegargiminase plus pemetrexed and cisplatin in patients with ASS1-deficient non-squamous non-small cell lung cancer. Cancer Med. 2021, 10, 6642–6652. [Google Scholar] [CrossRef]
- Ascierto, P.A.; Scala, S.; Castello, G.; Daponte, A.; Simeone, E.; Ottaiano, A.; Geneduce, G.; De Rosa, V.; Izzo, F.; Melucci, M.T.; et al. Pegylated Arginine Deiminase Treatment of Patients With Metastatic Melanoma: Results From Phase I and II Studies. J. Clin. Oncol. 2005, 23, 7660–7668. [Google Scholar] [CrossRef] [PubMed]
- Ott, P.A.; Carvajal, R.D.; Pandit-Taskar, N.; Jungbluth, A.A.; Hoffman, E.W.; Wu, B.W.; Bomalaski, J.S.; Venhaus, R.; Pan, L.; Old, L.J.; et al. Phase I/II study of pegylated arginine deiminase (ADI-PEG 20) in patients with advanced melanoma. Investig. New Drugs 2013, 31, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Szlosarek, P.W.; Steele, J.P.; Nolan, L.; Gilligan, D.; Taylor, P.; Spicer, J.; Lind, M.; Mitra, S.; Shamash, J.; Phillips, M.M.; et al. Arginine Deprivation With Pegylated Arginine Deiminase in Patients With Argininosuccinate Synthetase 1–Deficient Malignant Pleural Mesothelioma. JAMA Oncol. 2017, 3, 58. [Google Scholar] [CrossRef] [PubMed]
- Beddowes, E.; Spicer, J.; Chan, P.Y.; Khadeir, R.; Corbacho, J.G.; Repana, D.; Steele, J.P.; Schmid, P.; Szyszko, T.; Cook, T.; et al. Phase 1 Dose-Escalation Study of Pegylated Arginine Deiminase, Cisplatin, and Pemetrexed in Patients With Argininosuccinate Synthetase 1–Deficient Thoracic Cancers. J. Clin. Oncol. 2017, 35, 1778–1785. [Google Scholar] [CrossRef]
- Hall, P.E.; Lewis, R.; Syed, N.; Shaffer, R.; Evanson, J.; Ellis, S.; Williams, M.; Feng, X.; Johnston, A.; Thomson, J.A.; et al. A Phase I Study of Pegylated Arginine Deiminase (Pegargiminase), Cisplatin, and Pemetrexed in Argininosuccinate Synthetase 1-Deficient Recurrent High-grade Glioma. Clin. Cancer Res. 2019, 25, 2708–2716. [Google Scholar] [CrossRef]
- Chan, P.Y.; Phillips, M.M.; Ellis, S.; Johnston, A.; Feng, X.; Arora, A.; Hay, G.; Cohen, V.M.; Sagoo, M.S.; Bomalaski, J.S.; et al. A Phase 1 study of <scp>ADI-PEG20</scp> (pegargiminase) combined with cisplatin and pemetrexed in ASS1-negative metastatic uveal melanoma. Pigment. Cell Melanoma Res. 2022, 35, 461–470. [Google Scholar]
- Polaris Group. Polaris Group Announces Positive Top-Line ResultsFrom Phase 2/3 ATOMIC Study In Patients With Malignant Pleural Mesothelioma To Assess ADI-PEG 20 With Pemetrexed and Cisplatin. Available online: https://polarispharma.com/2022/09/21/20220921001/?lang=en (accessed on 20 December 2022).
- Ciccolini, J.; Serdjebi, C.; Peters, G.J.; Giovannetti, E. Pharmacokinetics and pharmacogenetics of Gemcitabine as a mainstay in adult and pediatric oncology: An EORTC-PAMM perspective. Cancer Chemother Pharm. 2016, 78, 1–12. [Google Scholar] [CrossRef]
- Prudner, B.C.; Rathore, R.; Robinson, A.M.; Godec, A.; Chang, S.F.; Hawkins, W.G.; Hirbe, A.C.; Van Tine, B.A. Arginine Starvation and Docetaxel Induce c-Myc–Driven hENT1 Surface Expression to Overcome Gemcitabine Resistance in ASS1-Negative Tumors. Clin. Cancer Res. 2019, 25, 5122–5134. [Google Scholar] [CrossRef]
- Daylami, R.; Muilenburg, D.J.; Virudachalam, S.; Bold, R.J. Pegylated arginine deiminase synergistically increases the cytotoxicity of gemcitabine in human pancreatic cancer. J. Exp. Clin. Cancer Res. 2014, 33, 102. [Google Scholar]
- Maki, R.G.; Wathen, J.K.; Patel, S.R.; Priebat, D.A.; Okuno, S.H.; Samuels, B.; Fanucchi, M.; Harmon, D.C.; Schuetze, S.M.; Reinke, D.; et al. Randomized Phase II Study of Gemcitabine and Docetaxel Compared With Gemcitabine Alone in Patients With Metastatic Soft Tissue Sarcomas: Results of Sarcoma Alliance for Research Through Collaboration Study 002. J. Clin. Oncol. 2007, 25, 2755–2763. [Google Scholar] [CrossRef]
- Van Tine, B.A.; Luo, J.; Oppelt, P.J.; Weiss, M.C.; Eulo, V.A.; Toeniskoetter, J.; Haarberg, S.; Abaricia, S.; Ruff, T.; Somalaski, J.S.; et al. Phase II trial of pegylated arginine deiminase in combination with gemcitabine and docetaxel for the treatment of soft tissue sarcoma. J. Clin. Oncol. 2021, 39, 11508. [Google Scholar] [CrossRef]
- Lowery, M.A.; Yu, K.H.; Kelsen, D.P.; Harding, J.J.; Bomalaski, J.S.; Glassman, D.C.; Covington, C.M.; Brenner, R.; Hollywood, E.; Barba, A.; et al. A phase 1/1B trial of ADI-PEG 20 plus nab-paclitaxel and gemcitabine in patients with advanced pancreatic adenocarcinoma. Cancer 2017, 123, 4556–4565. [Google Scholar]
- Fitzpatrick, J.M.; de Wit, R. Taxane Mechanisms of Action: Potential Implications for Treatment Sequencing in Metastatic Castration-resistant Prostate Cancer. Eur. Urol. 2014, 65, 1198–1204. [Google Scholar] [PubMed]
- Dumontet, C.; Jordan, M.A. Microtubule-binding agents: A dynamic field of cancer therapeutics. Nat. Rev. Drug Discov. 2010, 9, 790–803. [Google Scholar] [CrossRef] [PubMed]
- Montero, A.; Fossella, F.; Hortobagyi, G.; Valero, V. Docetaxel for treatment of solid tumours: A systematic review of clinical data. Lancet Oncol. 2005, 6, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Tomlinson, B.K.; Thomson, J.A.; Bomalaski, J.S.; Diaz, M.; Akande, T.; Mahaffey, N.; Li, T.; Dutia, M.P.; Kelly, K.; Gong, I.Y.; et al. Phase I Trial of Arginine Deprivation Therapy with ADI-PEG 20 Plus Docetaxel in Patients with Advanced Malignant Solid Tumors. Clin. Cancer Res. 2015, 21, 2480–2486. [Google Scholar]
- Von Hoff, D.D.; Ervin, T.; Arena, F.P.; Chiorean, E.G.; Infante, J.; Moore, M.; Seay, T.; Tjulandin, S.A.; Ma, W.W.; Saleh, M.N.; et al. Increased Survival in Pancreatic Cancer with nab-Paclitaxel plus Gemcitabine. N. Engl. J. Med. 2013, 369, 1691–1703. [Google Scholar]
- 70. Savaraj, N.; Wu, C.; Li, Y.Y.; Wangpaichitr, M.; You, M.; Bomalaski, J.; Wei, H.; Kuo, M.T.; Feun, L.G. Targeting argininosuccinate synthetase negative melanomas using combination of arginine degrading enzyme and cisplatin. Oncotarget 2015, 6, 6295–6309. [Google Scholar]
- Long, Y.; Tsai, W.B.; Chang, J.T.; Estecio, M.; Wangpaichitr, M.; Savaraj, N.; Feun, L.G.; Chen, H.H.W.; Kuo, M.T. Cisplatin-induced synthetic lethality to arginine-starvation therapy by transcriptional suppression of ASS1 is regulated by DEC1, HIF-1α, and c-Myc transcription network and is independent of ASS1 promoter DNA methylation. Oncotarget 2016, 7, 82658–82670. [Google Scholar]
- Yao, S.; Janku, F.; Subbiah, V.; Stewart, J.; Patel, S.P.; Kaseb, A.; Westin, S.N.; Naing, A.; Tsimberidou, A.M.; Hong, D.; et al. Phase 1 trial of ADI-PEG20 plus cisplatin in patients with pretreated metastatic melanoma or other advanced solid malignancies. Br. J. Cancer 2021, 124, 1533–1539. [Google Scholar] [CrossRef]
- Rollins, K.D.; Lindley, C. Pemetrexed: A multitargeted antifolate. Clin. Ther. 2005, 27, 1343–1382. [Google Scholar] [CrossRef] [PubMed]
- Harding, J.J.; Do, R.K.; Dika, I.E.; Hollywood, E.; Uhlitskykh, K.; Valentino, E.; Wan, P.; Hamilton, C.; Feng, X.; Johnston, A.; et al. A phase 1 study of ADI-PEG 20 and modified FOLFOX6 in patients with advanced hepatocellular carcinoma and other gastrointestinal malignancies. Cancer Chemother Pharm. 2018, 82, 429–440. [Google Scholar] [CrossRef] [PubMed]
- Taymaz-Nikerel, H.; Karabekmez, M.E.; Eraslan, S.; Kırdar, B. Doxorubicin induces an extensive transcriptional and metabolic rewiring in yeast cells. Sci. Rep. 2018, 8, 13672. [Google Scholar] [CrossRef] [PubMed]
- Qiu, F.; Chen, Y.R.; Liu, X.; Chu, C.Y.; Shen, L.J.; Xu, J.; Gaur, S.; Forman, H.J.; Zhang, H.; Zheng, S.; et al. Arginine Starvation Impairs Mitochondrial Respiratory Function in ASS1-Deficient Breast Cancer Cells. Sci. Signal. 2014, 7, ra31. [Google Scholar] [CrossRef]
- Yao, S.; Janku, F.; Koenig, K.; Tsimberidou, A.M.; Piha-Paul, S.A.; Shi, N.; Stewart, J.; Johnston, A.; Bomalaski, J.S.; Meric-Bernstam, F.; et al. Phase 1 trial of ADI-PEG 20 and liposomal doxorubicin in patients with metastatic solid tumors. Cancer Med. 2022, 11, 340–347. [Google Scholar] [CrossRef]
- di Francia, R.; Crisci, S.; de Monaco, A.; Cafiero, C.; Re, A.; Iaccarino, G.; De Filippi, R.; Frigeri, F.; Corazzelli, G.; Micera, A.; et al. Response and Toxicity to Cytarabine Therapy in Leukemia and Lymphoma: From Dose Puzzle to Pharmacogenomic Biomarkers. Cancers 2021, 13, 966. [Google Scholar] [CrossRef]
- Miraki-Moud, F.; Ghazaly, E.; Ariza-McNaughton, L.; Hodby, K.A.; Clear, A.; Anjos-Afonso, F.; Liapis, K.; Grantham, M.; Sohrabi, F.; Cavenagh, J.; et al. Arginine deprivation using pegylated arginine deiminase has activity against primary acute myeloid leukemia cells in vivo. Blood 2015, 125, 4060–4068. [Google Scholar] [CrossRef]
- Tsai, H.; Hsiao, H.; Hsu, Y.; Liu, Y.; Kao, H.; Liu, T.; Cho, S.F.; Xiaoxing, F.; Johnston, A.; Bomalaski, J.S.; et al. Phase I study of ADI-PEG20 plus low-dose cytarabine for the treatment of acute myeloid leukemia. Cancer Med. 2021, 10, 2946–2955. [Google Scholar] [CrossRef]
- Brin, E.; Wu, K.; Lu, H.T.; He, Y.; Dai, Z.; He, W. PEGylated arginine deiminase can modulate tumor immune microenvironment by affecting immune checkpoint expression, decreasing regulatory T cell accumulation and inducing tumor T cell infiltration. Oncotarget 2017, 8, 58948–58963. [Google Scholar] [CrossRef]
- Chang, K.Y.; Chiang, N.J.; Wu, S.Y.; Yen, C.J.; Chen, S.H.; Yeh, Y.M.; Li, C.F.; Feng, X.; Wu, K.; Johnston, A.; et al. Phase 1b study of pegylated arginine deiminase (ADI-PEG 20) plus Pembrolizumab in advanced solid cancers. Oncoimmunology 2021, 10, 1943253. [Google Scholar] [CrossRef] [PubMed]
- Kraehenbuehl, L.; Holland, A.; Armstrong, E.; O’Shea, S.; Mangarin, L.; Chekalil, S.; Johnston, A.; Bomalaski, J.S.; Erinjeri, J.P.; Barker, C.A.; et al. Pilot Trial of Arginine Deprivation Plus Nivolumab and Ipilimumab in Patients with Metastatic Uveal Melanoma. Cancers 2022, 14, 2638. [Google Scholar] [CrossRef] [PubMed]
- Panetti, S.; McJannett, N.J.; Fultang, L.; Booth, S.; Gneo, L.; Scarpa, U.; Smith, C.; Vardon, A.; Vettore, L.; Whalley, C.; et al. Engineering amino acid uptake or catabolism promotes CAR-T cell adaption to the tumour environment. Blood Adv. 2022, 136, 1155–1160. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, T.; Lo, P.H.Y.; Saichi, N.; Ueda, K.; Hirata, M.; Tanikawa, C.; Matsuda, K. Argininosuccinate synthase 1 is an intrinsic Akt repressor transactivated by p53. Sci. Adv. 2017, 3, 1603204. [Google Scholar] [CrossRef]
- Chu, Y.D.; Liu, H.F.; Chen, Y.C.; Chou, C.H.; Yeh, C.T. WWOX-rs13338697 genotype predicts therapeutic efficacy of ADI-PEG 20 for patients with advanced hepatocellular carcinoma. Front. Oncol. 2022, 12. [Google Scholar] [CrossRef] [PubMed]
- de Mariano, M.; Gallesio, R.; Chierici, M.; Furlanello, C.; Conte, M.; Garaventa, A.; Croce, M.; Ferrini, S.; Tonini, G.P.; Longo, L. Identification of GALNT14 as a novel neuroblastoma predisposition gene. Oncotarget 2015, 6, 26335–26346. [Google Scholar] [CrossRef]
- Lin, W.R.; Hsu, C.W.; Yeh, C.S.H.; Chen, Y.C.; Chang, M.L.; Liang, K.H.; Lin, C.C.; Chu, Y.D.; Yeh, C.T. Combinations of single nucleotide polymorphisms WWOX -rs13338697, GALNT14 -rs9679162 and rs6025211 effectively stratify outcomes of chemotherapy in advanced hepatocellular carcinoma. Asia Pac. J. Clin. Oncol. 2018, 14, e54–e63. [Google Scholar] [CrossRef]
- Abu-Remaileh, M.; Khalaileh, A.; Pikarsky, E.; Aqeilan, R.I. WWOX controls hepatic HIF1α to suppress hepatocyte proliferation and neoplasia. Cell Death Dis. 2018, 9, 511. [Google Scholar] [CrossRef]
- Silberman, A.; Goldman, O.; Boukobza Assayag, O.; Jacob, A.; Rabinovich, S.; Adler, L.; Lee, J.S.; Keshet, R.; Sarver, A.; Frug, J.; et al. Acid-Induced Downregulation of ASS1 Contributes to the Maintenance of Intracellular pH in Cancer. Cancer Res. 2019, 79, 518–533. [Google Scholar] [CrossRef]
- Barnett, S.E.; Kenyani, J.; Tripari, M.; Butt, Z.; Grosman, R.; Querques, F.; Shaw, L.; Silva, L.C.; Goate, Z.; Marciniak, S.J.; et al. BAP1 Loss is associated with higher ASS1 expression in epithelioid mesothelioma: Implications for therapeutic stratification. Mol. Cancer Res. 2023, MCR-22-0635. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Field, G.C.; Pavlyk, I.; Szlosarek, P.W. Bench-to-Bedside Studies of Arginine Deprivation in Cancer. Molecules 2023, 28, 2150. https://doi.org/10.3390/molecules28052150
Field GC, Pavlyk I, Szlosarek PW. Bench-to-Bedside Studies of Arginine Deprivation in Cancer. Molecules. 2023; 28(5):2150. https://doi.org/10.3390/molecules28052150
Chicago/Turabian StyleField, George C., Iuliia Pavlyk, and Peter W. Szlosarek. 2023. "Bench-to-Bedside Studies of Arginine Deprivation in Cancer" Molecules 28, no. 5: 2150. https://doi.org/10.3390/molecules28052150
APA StyleField, G. C., Pavlyk, I., & Szlosarek, P. W. (2023). Bench-to-Bedside Studies of Arginine Deprivation in Cancer. Molecules, 28(5), 2150. https://doi.org/10.3390/molecules28052150




