Reliable N-Glycan Analysis–Removal of Frequently Occurring Oligosaccharide Impurities by Enzymatic Degradation
Abstract
1. Introduction
2. Results
2.1. Depletion of OSIs on the Glycoprotein Level
2.2. Enzymatic Degradation of APTS-Labeled Maltodextrins and Dextrans
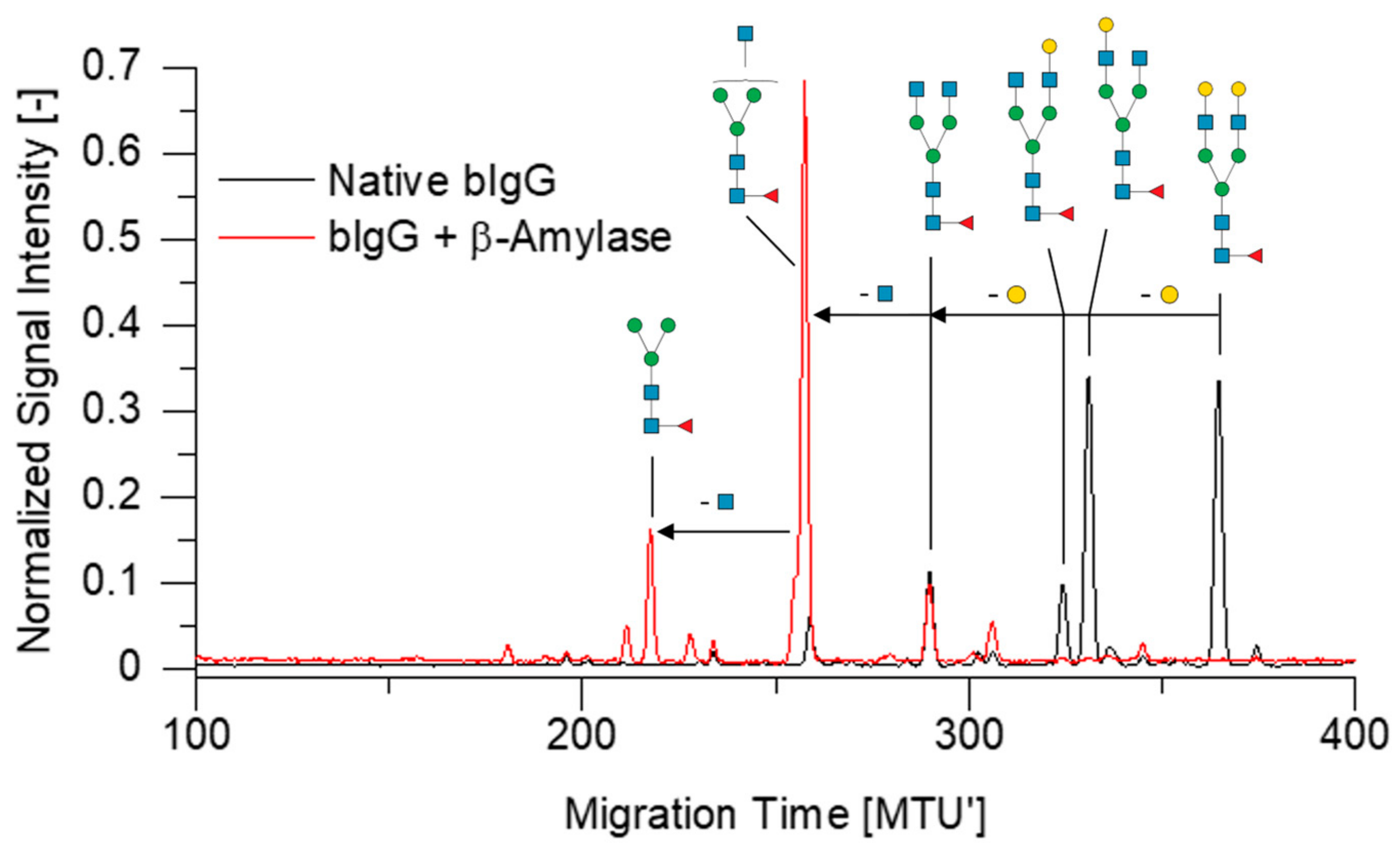
2.3. Assessment of Side Reactions with APTS-Labeled N-Glycans
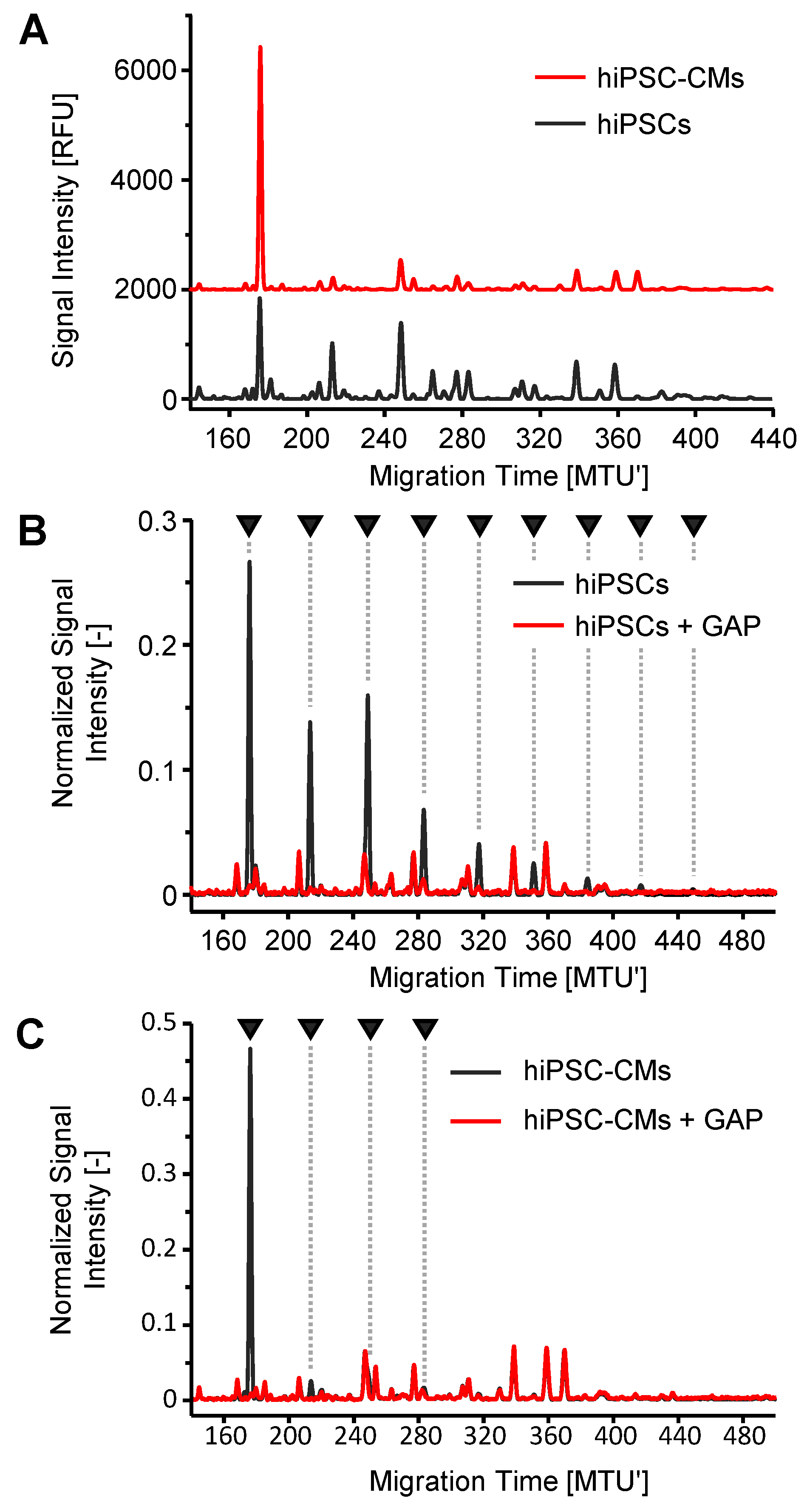
2.4. Enzymatic Degradation of OSIs in N-Glycan Samples
3. Discussion
4. Material and Methods
4.1. Materials
4.2. Non-Enzymatic Approaches for Depletion of OSIs on the Glycoprotein Level
4.3. xCGE-LIF Analysis of N-Glycans and Oligosaccharides
4.4. Enzymatic Degradation of APTS-Labeled Maltodextrins and Dextrans
4.5. Test for Side Reactions with APTS-Labeled N-Glycans
4.6. Enzymatic Degradation of OSIs in Complex N-Glycan Samples
5. Concluding Remarks
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACN | Acetonitrile |
| APTS | 8-aminopyrene-1,3,6-trisulfonic acid |
| CE | Capillary electrophoresis |
| DxChe | Dextranase from Chaetomium erraticum |
| DxPsp | Dextranase from Penicillium species |
| GAP | Glucoamylase P |
| hESCs | Human Embryonic Stem Cells |
| hiPSCs | Human-Induced Pluripotent Stem Cells |
| hiPSCs-CM | hiPSC-derived Cardiomyocytes |
| IgG | immunoglobulin G |
| mESCs | Murine Embryonic Stem Cells |
| MTU’ | Normalized Migration Time Units |
| OSIs | Oligosaccharide Impurities |
| PBS | Phosphate Buffered Saline |
| PVDF | polyvinylidene difluoride |
| RFU | Relative Fluorescence Units |
| SDS | Sodium Dodecyl Sulfate |
| SDS-PAGE | sodium dodecyl sulfate polyacrylamide gel electrophoresis |
| xCGE-LIF | multiplexed Capillary Gel Electrophoresis with Laser-Induced Fluorescence Detection |
References
- Beimdiek, J.; Hennig, R.; Burock, R.; Puk, O.; Biskup, S.; Rapp, E.; Lesinski-Schiedat, A.; Buettner, F.F.R.; Das, A.M. Serum N-glycomics of a novel CDG-IIb patient reveals aberrant IgG glycosylation. Glycobiology 2022, 32, 380–390. [Google Scholar] [CrossRef] [PubMed]
- Wittenbecher, C.; Štambuk, T.; Kuxhaus, O.; Rudman, N.; Vučković, F.; Štambuk, J.; Schiborn, C.; Rahelić, D.; Dietrich, S.; Gornik, O.; et al. Plasma N-Glycans as Emerging Biomarkers of Cardiometabolic Risk: A Prospective Investigation in the EPIC-Potsdam Cohort Study. Diabetes Care 2020, 43, 661–668. [Google Scholar] [CrossRef] [PubMed]
- Almeida, A.; Kolarich, D. The promise of protein glycosylation for personalised medicine. Biochim. Biophys. Acta 2016, 1860, 1583–1595. [Google Scholar] [CrossRef] [PubMed]
- Vanderschaeghe, D.; Laroy, W.; Sablon, E.; Halfon, P.; van Hecke, A.; Delanghe, J.; Callewaert, N. GlycoFibroTest is a highly performant liver fibrosis biomarker derived from DNA sequencer-based serum protein glycomics. Mol. Cell. Proteom. 2009, 8, 986–994. [Google Scholar] [CrossRef]
- Wang, J.-R.; Gao, W.-N.; Grimm, R.; Jiang, S.; Liang, Y.; Ye, H.; Li, Z.-G.; Yau, L.-F.; Huang, H.; Liu, J.; et al. A method to identify trace sulfated IgG N-glycans as biomarkers for rheumatoid arthritis. Nat. Commun. 2017, 8, 631. [Google Scholar] [CrossRef]
- Everest-Dass, A.V.; Moh, E.S.X.; Ashwood, C.; Shathili, A.M.M.; Packer, N.H. Human disease glycomics: Technology advances enabling protein glycosylation analysis—Part 2. Expert Rev. Proteom. 2018, 15, 341–352. [Google Scholar] [CrossRef]
- Satomaa, T.; Heiskanen, A.; Mikkola, M.; Olsson, C.; Blomqvist, M.; Tiittanen, M.; Jaatinen, T.; Aitio, O.; Olonen, A.; Helin, J.; et al. The N-glycome of human embryonic stem cells. BMC Cell Biol. 2009, 10, 42. [Google Scholar] [CrossRef]
- Hasehira, K.; Tateno, H.; Onuma, Y.; Ito, Y.; Asashima, M.; Hirabayashi, J. Structural and quantitative evidence for dynamic glycome shift on production of induced pluripotent stem cells. Mol. Cell. Proteom. 2012, 11, 1913–1923. [Google Scholar] [CrossRef]
- Hamouda, H.; Ullah, M.; Berger, M.; Sittinger, M.; Tauber, R.; Ringe, J.; Blanchard, V. N-glycosylation profile of undifferentiated and adipogenically differentiated human bone marrow mesenchymal stem cells: Towards a next generation of stem cell markers. Stem Cells Dev. 2013, 22, 3100–3113. [Google Scholar] [CrossRef]
- Konze, S.A.; Cajic, S.; Oberbeck, A.; Hennig, R.; Pich, A.; Rapp, E.; Buettner, F.F.R. Quantitative Assessment of Sialo-Glycoproteins and N-Glycans during Cardiomyogenic Differentiation of Human Induced Pluripotent Stem Cells. Chembiochem 2017, 18, 1317–1331. [Google Scholar] [CrossRef]
- Reusch, D.; Tejada, M.L. Fc glycans of therapeutic antibodies as critical quality attributes. Glycobiology 2015, 25, 1325–1334. [Google Scholar] [CrossRef]
- Zhang, P.; Woen, S.; Wang, T.; Liau, B.; Zhao, S.; Chen, C.; Yang, Y.; Song, Z.; Wormald, M.R.; Yu, C.; et al. Challenges of glycosylation analysis and control: An integrated approach to producing optimal and consistent therapeutic drugs. Drug Discov. Today 2016, 21, 740–765. [Google Scholar] [CrossRef] [PubMed]
- Szekrenyes, A.; Szigeti, M.; Dvorakova, V.; Jarvas, G.; Guttman, A. Quantitative comparison of the N-glycosylation of therapeutic glycoproteins using the Glycosimilarity Index. A tutorial. TrAC Trends Anal. Chem. 2020, 122, 115728. [Google Scholar] [CrossRef]
- Schiestl, M.; Li, J.; Abas, A.; Vallin, A.; Millband, J.; Gao, K.; Joung, J.; Pluschkell, S.; Go, T.; Kang, H.-N. The role of the quality assessment in the determination of overall biosimilarity: A simulated case study exercise. Biologicals 2014, 42, 128–132. [Google Scholar] [CrossRef]
- Blundell, P.A.; Lu, D.; Dell, A.; Haslam, S.; Pleass, R.J. Choice of Host Cell Line Is Essential for the Functional Glycosylation of the Fc Region of Human IgG1 Inhibitors of Influenza B Viruses. J. Immunol. 2020, 204, 1022–1034. [Google Scholar] [CrossRef]
- Guérardel, Y.; Chang, L.-Y.; Maes, E.; Huang, C.-J.; Khoo, K.-H. Glycomic survey mapping of zebrafish identifies unique sialylation pattern. Glycobiology 2006, 16, 244–257. [Google Scholar] [CrossRef]
- Kawasaki, N.; Lin, C.-W.; Inoue, R.; Khoo, K.-H.; Kawasaki, N.; Ma, B.Y.; Oka, S.; Ishiguro, M.; Sawada, T.; Ishida, H.; et al. Highly fucosylated N-glycan ligands for mannan-binding protein expressed specifically on CD26 (DPPVI) isolated from a human colorectal carcinoma cell line, SW1116. Glycobiology 2009, 19, 437–450. [Google Scholar] [CrossRef] [PubMed]
- Dumych, T.; Yamakawa, N.; Sivignon, A.; Garenaux, E.; Robakiewicz, S.; Coddeville, B.; Bongiovanni, A.; Bray, F.; Barnich, N.; Szunerits, S.; et al. Oligomannose-Rich Membranes of Dying Intestinal Epithelial Cells Promote Host Colonization by Adherent-Invasive E. coli. Front. Microbiol. 2018, 9, 742. [Google Scholar] [CrossRef]
- Rosenlöcher, J.; Böhrsch, V.; Sacharjat, M.; Blanchard, V.; Giese, C.; Sandig, V.; Hackenberger, C.P.R.; Hinderlich, S. Applying Acylated Fucose Analogues to Metabolic Glycoengineering. Bioengineering 2015, 2, 213–234. [Google Scholar] [CrossRef]
- Xie, B.; Luo, X.; Zhao, C.; Priest, C.M.; Chan, S.-Y.; O’ Connor, P.B.; Kirschner, D.A.; Costello, C.E. Molecular Characterization of Myelin Protein Zero in Xenopus laevis Peripheral Nerve: Equilibrium between Non-covalently Associated Dimer and Monomer. Int. J. Mass Spectrom. 2007, 268, 304–315. [Google Scholar] [CrossRef]
- Xie, B.; Zhou, G.; Chan, S.-Y.; Shapiro, E.; Kong, X.-P.; Wu, X.-R.; Sun, T.-T.; Costello, C.E. Distinct glycan structures of uroplakins Ia and Ib: Structural basis for the selective binding of FimH adhesin to uroplakin Ia. J. Biol. Chem. 2006, 281, 14644–14653. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Nie, H.; Zhang, Y.; Yao, Y.; Maitikabili, A.; Qu, Y.; Shi, S.; Chen, C.; Li, Y. Cell surface-specific N-glycan profiling in breast cancer. PLoS ONE 2013, 8, e72704. [Google Scholar] [CrossRef] [PubMed]
- Harvey, D.J. Analysis of carbohydrates and glycoconjugates by matrix-assisted laser desorption/ionization mass spectrometry: An update for 2013–2014. Mass Spectrom. Rev. 2018, 37, 353–491. [Google Scholar] [CrossRef] [PubMed]
- Mehta, N.; Porterfield, M.; Struwe, W.B.; Heiss, C.; Azadi, P.; Rudd, P.M.; Tiemeyer, M.; Aoki, K. Mass Spectrometric Quantification of N-Linked Glycans by Reference to Exogenous Standards. J. Proteome Res. 2016, 15, 2969–2980. [Google Scholar] [CrossRef]
- Hanzawa, K.; Suzuki, N.; Natsuka, S. Structures and developmental alterations of N-glycans of zebrafish embryos. Glycobiology 2017, 27, 228–245. [Google Scholar] [CrossRef] [PubMed]
- Barker, C.; Dell, A.; Rogers, M.; Winchester, B. Comparison of the fucose-containing storage products in different tissues of a dog with fucosidosis. Biochem. Soc. Trans. 1986, 14, 709–710. [Google Scholar] [CrossRef]
- Donczo, B.; Kiraly, G.; Guttman, A. Effect of the elapsed time between sampling and formalin fixation on the N-glycosylation profile of mouse tissue specimens. Electrophoresis 2019, 40, 3057–3061. [Google Scholar] [CrossRef]
- Geyer, H.; Schmidt, M.; Müller, M.; Schnabel, R.; Geyer, R. Mass spectrometric comparison of N-glycan profiles from Caenorhabditis elegans mutant embryos. Glycoconj. J. 2012, 29, 135–145. [Google Scholar] [CrossRef]
- Jeong, H.-J.; Park, H.-M.; Kim, K.-J.; Kim, Y.-W.; Bhatia, S.K.; Lee, Y.K.; Yang, Y.-H.; Kim, B.-G.; Kim, Y.-G. Highly sensitive glycosylation analysis of membrane glycoproteins avoiding polymeric contaminants. Biotechnol. Bioprocess Eng. 2014, 19, 545–550. [Google Scholar] [CrossRef]
- Gao, W.; Jiang, Y.; Zhang, Z.; Zhang, Y.; Liu, Y.; Zhou, Y.; Liu, X. A facile method for cellular N-glycomic profiling by matrix-assisted laser desorption/ionization mass spectrometry. RSC Adv. 2017, 7, 35687–35693. [Google Scholar] [CrossRef]
- Herrera, H.; Dilday, T.; Uber, A.; Scott, D.; Zambrano, J.N.; Wang, M.; Angel, P.M.; Mehta, A.S.; Drake, R.R.; Hill, E.G.; et al. Core-Fucosylated Tetra-Antennary N-Glycan Containing A Single N-Acetyllactosamine Branch Is Associated with Poor Survival Outcome in Breast Cancer. Int. J. Mol. Sci. 2019, 20, 2528. [Google Scholar] [CrossRef] [PubMed]
- Montacir, H. Glycan-Based Biomarkers for the Quality Assurance in Stem Cell Therapy; Freie Universität Berlin: Berlin, Germany, 2014. [Google Scholar]
- Ruhaak, L.R.; Zauner, G.; Huhn, C.; Bruggink, C.; Deelder, A.M.; Wuhrer, M. Glycan labeling strategies and their use in identification and quantification. Anal. Bioanal. Chem. 2010, 397, 3457–3481. [Google Scholar] [CrossRef] [PubMed]
- Alagesan, K.; Everest-Dass, A.; Kolarich, D. Isomeric Separation and Characterisation of Glycoconjugates. Adv. Exp. Med. Biol. 2018, 1104, 77–99. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, M.; Leventis, P.A.; Silvescu, C.I.; Reinhold, V.N.; Schachter, H.; Boulianne, G.L. Null mutations in Drosophila N-acetylglucosaminyltransferase I produce defects in locomotion and a reduced life span. J. Biol. Chem. 2006, 281, 12776–12785. [Google Scholar] [CrossRef] [PubMed]
- Aoki, K.; Porterfield, M.; Lee, S.S.; Dong, B.; Nguyen, K.; McGlamry, K.H.; Tiemeyer, M. The diversity of O-linked glycans expressed during Drosophila melanogaster development reflects stage- and tissue-specific requirements for cell signaling. J. Biol. Chem. 2008, 283, 30385–30400. [Google Scholar] [CrossRef]
- Aoki, K.; Perlman, M.; Lim, J.-M.; Cantu, R.; Wells, L.; Tiemeyer, M. Dynamic developmental elaboration of N-linked glycan complexity in the Drosophila melanogaster embryo. J. Biol. Chem. 2007, 282, 9127–9142. [Google Scholar] [CrossRef]
- Thiesler, C.T.; Cajic, S.; Hoffmann, D.; Thiel, C.; van Diepen, L.; Hennig, R.; Sgodda, M.; Weiβmann, R.; Reichl, U.; Steinemann, D.; et al. Glycomic Characterization of Induced Pluripotent Stem Cells Derived from a Patient Suffering from Phosphomannomutase 2 Congenital Disorder of Glycosylation (PMM2-CDG). Mol. Cell. Proteom. 2016, 15, 1435–1452. [Google Scholar] [CrossRef]
- Breloy, I.; Pacharra, S.; Aust, C.; Hanisch, F.-G. A sensitive gel-based global O-glycomics approach reveals high levels of mannosyl glycans in the high mass region of the mouse brain proteome. Biol. Chem. 2012, 393, 709–717. [Google Scholar] [CrossRef]
- Hamouda, H.; Kaup, M.; Ullah, M.; Berger, M.; Sandig, V.; Tauber, R.; Blanchard, V. Rapid analysis of cell surface N-glycosylation from living cells using mass spectrometry. J. Proteome Res. 2014, 13, 6144–6151. [Google Scholar] [CrossRef]
- Hemmoranta, H.; Satomaa, T.; Blomqvist, M.; Heiskanen, A.; Aitio, O.; Saarinen, J.; Natunen, J.; Partanen, J.; Laine, J.; Jaatinen, T. N-glycan structures and associated gene expression reflect the characteristic N-glycosylation pattern of human hematopoietic stem and progenitor cells. Exp. Hematol. 2007, 35, 1279–1292. [Google Scholar] [CrossRef]
- Heiskanen, A.; Hirvonen, T.; Salo, H.; Impola, U.; Olonen, A.; Laitinen, A.; Tiitinen, S.; Natunen, S.; Aitio, O.; Miller-Podraza, H.; et al. Glycomics of bone marrow-derived mesenchymal stem cells can be used to evaluate their cellular differentiation stage. Glycoconj. J. 2009, 26, 367–384. [Google Scholar] [CrossRef] [PubMed]
- Stanley, P.; Taniguchi, N.; Aebi, M. Essentials of Glycobiology: N-Glycans. In Essentials of glycobiology, 3rd ed.; Varki, A., Cummings, R.D., Esko, J.D., Stanley, P., Hart, G.W., Aebi, M., Darvill, A.G., Kinoshita, T., Packer, N.H., Prestegard, J.H., et al., Eds.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2017; pp. 99–112. ISBN 9781621821328. [Google Scholar]
- Reiding, K.R.; Bondt, A.; Hennig, R.; Gardner, R.A.; O’Flaherty, R.; Trbojević-Akmačić, I.; Shubhakar, A.; Hazes, J.M.W.; Reichl, U.; Fernandes, D.L.; et al. High-throughput Serum N-Glycomics: Method Comparison and Application to Study Rheumatoid Arthritis and Pregnancy-associated Changes. Mol. Cell. Proteom. 2019, 18, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Cajic, S.; Hennig, R.; Burock, R.; Rapp, E. Capillary (Gel) Electrophoresis-Based Methods for Immunoglobulin (G) Glycosylation Analysis. Exp. Suppl. 2021, 112, 137–172. [Google Scholar] [CrossRef] [PubMed]
- Chuzel, L.; Fossa, S.L.; Boisvert, M.L.; Cajic, S.; Hennig, R.; Ganatra, M.B.; Reichl, U.; Rapp, E.; Taron, C.H. Combining functional metagenomics and glycoanalytics to identify enzymes that facilitate structural characterization of sulfated N-glycans. Microb. Cell Fact. 2021, 20, 162. [Google Scholar] [CrossRef] [PubMed]
- Vanderschaeghe, D.; Guttman, A.; Callewaert, N. High-throughput profiling of the serum N-glycome on capillary electrophoresis microfluidics systems. Methods Mol. Biol. 2013, 919, 87–96. [Google Scholar] [CrossRef]
- Mittermayr, S.; Bones, J.; Doherty, M.; Guttman, A.; Rudd, P.M. Multiplexed analytical glycomics: Rapid and confident IgG N-glycan structural elucidation. J. Proteome Res. 2011, 10, 3820–3829. [Google Scholar] [CrossRef]
- Reusch, D.; Haberger, M.; Kailich, T.; Heidenreich, A.-K.; Kampe, M.; Bulau, P.; Wuhrer, M. High-throughput glycosylation analysis of therapeutic immunoglobulin G by capillary gel electrophoresis using a DNA analyzer. MAbs 2014, 6, 185–196. [Google Scholar] [CrossRef]
- Schwarzer, J.; Rapp, E.; Reichl, U. N-glycan analysis by CGE-LIF: Profiling influenza A virus hemagglutinin N-glycosylation during vaccine production. Electrophoresis 2008, 29, 4203–4214. [Google Scholar] [CrossRef]
- Abeln, M.; Borst, K.M.; Cajic, S.; Thiesler, H.; Kats, E.; Albers, I.; Kuhn, M.; Kaever, V.; Rapp, E.; Münster-Kühnel, A.; et al. Sialylation Is Dispensable for Early Murine Embryonic Development in Vitro. Chembiochem 2017, 18, 1305–1316. [Google Scholar] [CrossRef]
- Hennig, R.; Cajic, S.; Borowiak, M.; Hoffmann, M.; Kottler, R.; Reichl, U.; Rapp, E. Towards personalized diagnostics via longitudinal study of the human plasma N-glycome. Biochim. Biophys. Acta 2016, 1860, 1728–1738. [Google Scholar] [CrossRef]
- Hennig, R.; Rapp, E.; Kottler, R.; Cajic, S.; Borowiak, M.; Reichl, U. N-Glycosylation Fingerprinting of Viral Glycoproteins by xCGE-LIF. Methods Mol. Biol. 2015, 1331, 123–143. [Google Scholar] [CrossRef] [PubMed]
- Varki, A.; Cummings, R.D.; Aebi, M.; Packer, N.H.; Seeberger, P.H.; Esko, J.D.; Stanley, P.; Hart, G.; Darvill, A.; Kinoshita, T.; et al. Symbol Nomenclature for Graphical Representations of Glycans. Glycobiology 2015, 25, 1323–1324. [Google Scholar] [CrossRef] [PubMed]
- Meininger, M.; Stepath, M.; Hennig, R.; Cajic, S.; Rapp, E.; Rotering, H.; Wolff, M.W.; Reichl, U. Sialic acid-specific affinity chromatography for the separation of erythropoietin glycoforms using serotonin as a ligand. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2016, 1012–1013, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Puan, K.J.; San Luis, B.; Yusof, N.; Kumar, D.; Andiappan, A.K.; Lee, W.; Cajic, S.; Vuckovic, D.; de Chan, J.; Döllner, T.; et al. FUT6 deficiency compromises basophil function by selectively abrogating their sialyl-Lewis x expression. Commun. Biol. 2021, 4, 832. [Google Scholar] [CrossRef]
- Séveno, M.; Cabrera, G.; Triguero, A.; Burel, C.; Leprince, J.; Rihouey, C.; Vézina, L.-P.; D’Aoust, M.-A.; Rudd, P.M.; Royle, L.; et al. Plant N-glycan profiling of minute amounts of material. Anal. Biochem. 2008, 379, 66–72. [Google Scholar] [CrossRef]
- Aguiar, T.Q.; Maaheimo, H.; Heiskanen, A.; Wiebe, M.G.; Penttilä, M.; Domingues, L. Characterization of the Ashbya gossypii secreted N-glycome and genomic insights into its N-glycosylation pathway. Carbohydr. Res. 2013, 381, 19–27. [Google Scholar] [CrossRef]
- Royle, L.; Radcliffe, C.M.; Dwek, R.A.; Rudd, P.M. Detailed structural analysis of N-glycans released from glycoproteins in SDS-PAGE gel bands using HPLC combined with exoglycosidase array digestions. In Glycobiology Protocols; Inka, B., Ed.; Humana Press: Totowa, NJ, USA, 2006; pp. 125–143. ISBN 1-59745-167-3. [Google Scholar]
- Batista, F.R.X.; Pereira, C.A.; Mendonça, R.Z.; Moraes, A.M. Enhancement of Sf9 Cells and Baculovirus Production Employing Grace’s Medium Supplemented with Milk Whey Ultrafiltrate. Cytotechnology 2005, 49, 1–9. [Google Scholar] [CrossRef]
- Jensen, P.H.; Karlsson, N.G.; Kolarich, D.; Packer, N.H. Structural analysis of N- and O-glycans released from glycoproteins. Nat. Protoc. 2012, 7, 1299–1310. [Google Scholar] [CrossRef]
- Bairoch, A. The ENZYME database in 2000. Nucleic Acids Res. 2000, 28, 304–305. [Google Scholar] [CrossRef]
- Fagerström, R. Purification and specificity of recombinant Hormoconis resinae glucoamylase P and endogenous glucoamylase from Trichoderma reesei. Enzyme Microb. Technol. 1994, 16, 36–42. [Google Scholar] [CrossRef]
- Davies, G.; Henrissat, B. Structures and mechanisms of glycosyl hydrolases. Structure 1995, 3, 853–859. [Google Scholar] [CrossRef] [PubMed]
- Morell, M.K.; Samuel, M.S.; O’Shea, M.G. Analysis of starch structure using fluorophore-assisted carbohydrate electrophoresis. Electrophoresis 1998, 19, 2603–2611. [Google Scholar] [CrossRef] [PubMed]
- Yoshigi, N.; Okada, Y.; Sahara, H.; Koshino, S. Expression in Escherichia coli of cDNA encoding barley beta-amylase and properties of recombinant beta-amylase. Biosci. Biotechnol. Biochem. 1994, 58, 1080–1086. [Google Scholar] [CrossRef]
- Lebrilla, C.B.; Liu, J.; Widmalm, G.; Prestegard, J.H. Essentials of Glycobiology: Oligosaccharides and Polysaccharides, 4th ed.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2022; ISBN 9781621824213. [Google Scholar]
- Vettori, M.H.P.B.; Franchetti, S.M.M.; Contiero, J. Structural characterization of a new dextran with a low degree of branching produced by Leuconostoc mesenteroides FT045B dextransucrase. Carbohydr. Polym. 2012, 88, 1440–1444. [Google Scholar] [CrossRef]
- Monsan, P.; Lopez, A. On the production of dextran by free and immobilized dextransucrase. Biotechnol. Bioeng. 1981, 23, 2027–2037. [Google Scholar] [CrossRef]
- Wang, Y.-C.; Lin, V.; Loring, J.F.; Peterson, S.E. The ‘sweet’ spot of cellular pluripotency: Protein glycosylation in human pluripotent stem cells and its applications in regenerative medicine. Expert Opin. Biol. Ther. 2015, 15, 679–687. [Google Scholar] [CrossRef]
- Ruzin, S.E. Plant Microtechnique and Microscopy; Oxford University Press: New York, NY, USA, 1999; ISBN 0195089561. [Google Scholar]
- Royle, L.; Campbell, M.P.; Radcliffe, C.M.; White, D.M.; Harvey, D.J.; Abrahams, J.L.; Kim, Y.-G.; Henry, G.W.; Shadick, N.A.; Weinblatt, M.E.; et al. HPLC-based analysis of serum N-glycans on a 96-well plate platform with dedicated database software. Anal. Biochem. 2008, 376, 1–12. [Google Scholar] [CrossRef]
- Hennig, R.; Reichl, U.; Rapp, E. A Software Tool for Automated High-Throughput Processing of CGE-LIF Based Glycoanalysis Data, Generated by a Multiplexing Capillary DNA Sequencer. Glycoconj. J. 2011, 28, 331–332. [Google Scholar]



| Substrate | Maltodextrin | Dextran | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Peak of | DP1 [%] | DP2 [%] | DP3 [%] | ≥DP4 [%] | DP1 [%] | DP2 [%] | DP3 [%] | ≥DP4 [%] | |
| Enzyme | |||||||||
| none | 1.9 | 5.9 | 13.7 | 78.5 | 0.0 | 0.0 | 1.2 | 98.8 | |
| ß-amylase | 2.9 | 57.4 | 33.2 | 6.5 | 0.0 | 0.0 | 1.4 | 98.6 | |
| GAP * | 0.0 | 85.3 | 14.7 | 0.0 | 1.3 | 62.2 | 0.0 | 36.5 | |
| Oligo-1,6-glucosidase | 5.4 | 6.6 | 13.4 | 74.6 | 47.3 | 4.7 | 0.0 | 48.0 | |
| DxChe | 0.9 | 10.8 | 73.2 | 15.1 | 0.2 | 60.3 | 29.8 | 9.7 | |
| DxPsp | 0.0 | 81.4 | 5.7 | 12.9 | 0.0 | 43.9 | 50.0 | 6.1 | |
| α-amylase II-A | 2.0 | 6.7 | 40.7 | 50.6 | 0.0 | 0.0 | 1.1 | 98.9 | |
| α-amylase IX-A | 2.1 | 31.7 | 56.4 | 9.8 | 0.0 | 0.0 | 1.2 | 98.8 | |
| α-amylase XIII-A | 2.4 | 29.4 | 60.9 | 7.3 | 0.0 | 0.0 | 1.3 | 98.7 | |
| α-glucosidase | 46.0 | 0.0 | 0.0 | 54.0 | 0.0 | 0.0 | 1.1 | 98.9 | |
| Oligo-α-(1,4-1,6)-glucosidase | 1.7 | 5.4 | 13.0 | 79.9 | 0.0 | 0.0 | 1.0 | 99.0 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burock, R.; Cajic, S.; Hennig, R.; Buettner, F.F.R.; Reichl, U.; Rapp, E. Reliable N-Glycan Analysis–Removal of Frequently Occurring Oligosaccharide Impurities by Enzymatic Degradation. Molecules 2023, 28, 1843. https://doi.org/10.3390/molecules28041843
Burock R, Cajic S, Hennig R, Buettner FFR, Reichl U, Rapp E. Reliable N-Glycan Analysis–Removal of Frequently Occurring Oligosaccharide Impurities by Enzymatic Degradation. Molecules. 2023; 28(4):1843. https://doi.org/10.3390/molecules28041843
Chicago/Turabian StyleBurock, Robert, Samanta Cajic, René Hennig, Falk F. R. Buettner, Udo Reichl, and Erdmann Rapp. 2023. "Reliable N-Glycan Analysis–Removal of Frequently Occurring Oligosaccharide Impurities by Enzymatic Degradation" Molecules 28, no. 4: 1843. https://doi.org/10.3390/molecules28041843
APA StyleBurock, R., Cajic, S., Hennig, R., Buettner, F. F. R., Reichl, U., & Rapp, E. (2023). Reliable N-Glycan Analysis–Removal of Frequently Occurring Oligosaccharide Impurities by Enzymatic Degradation. Molecules, 28(4), 1843. https://doi.org/10.3390/molecules28041843






