Phenolic Composition, Wound Healing, Antinociceptive, and Anticancer Effects of Caralluma europaea Extracts
Abstract
1. Introduction
2. Results
2.1. Chemical Analysis of C. europaea Polyphenolic Extract
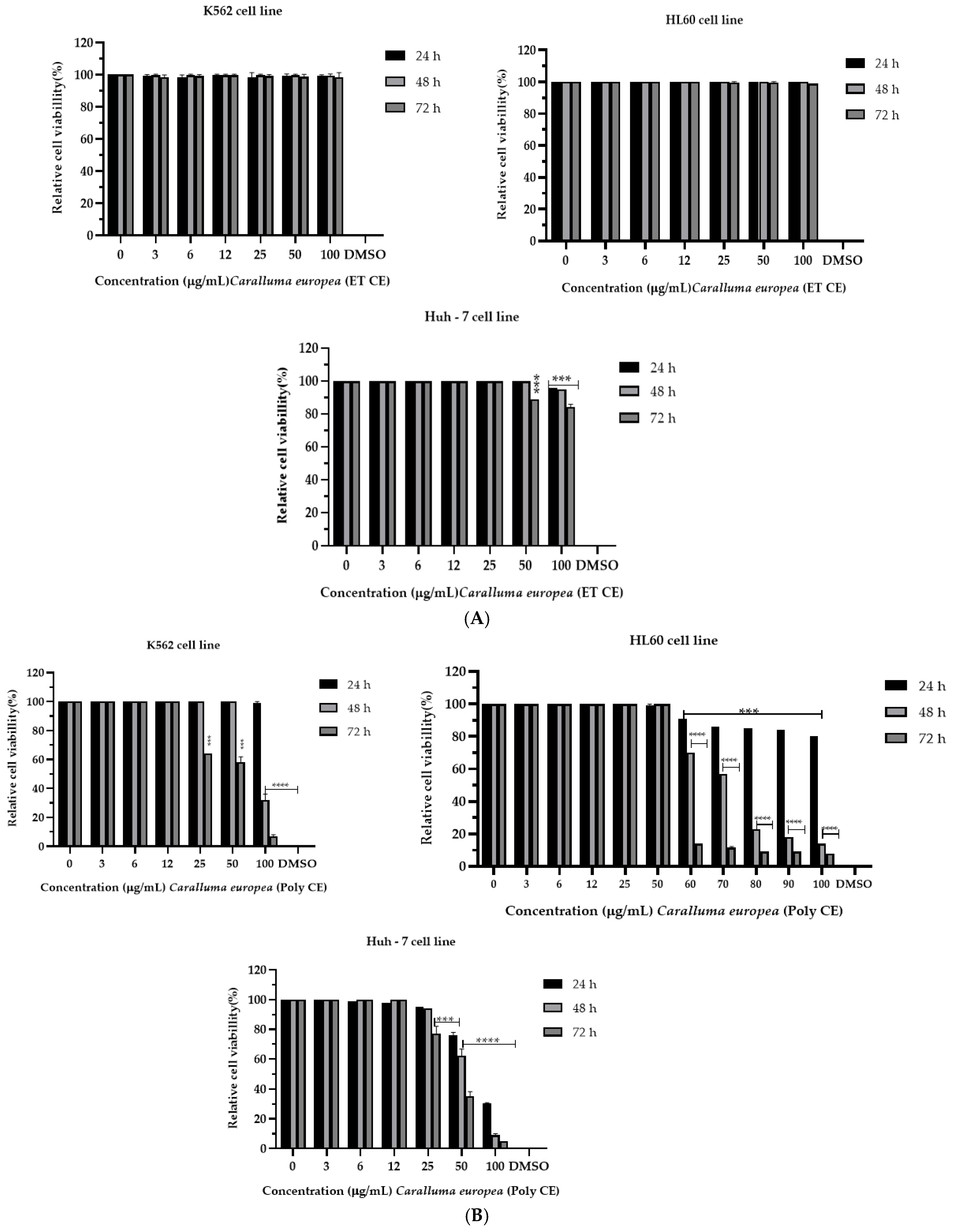
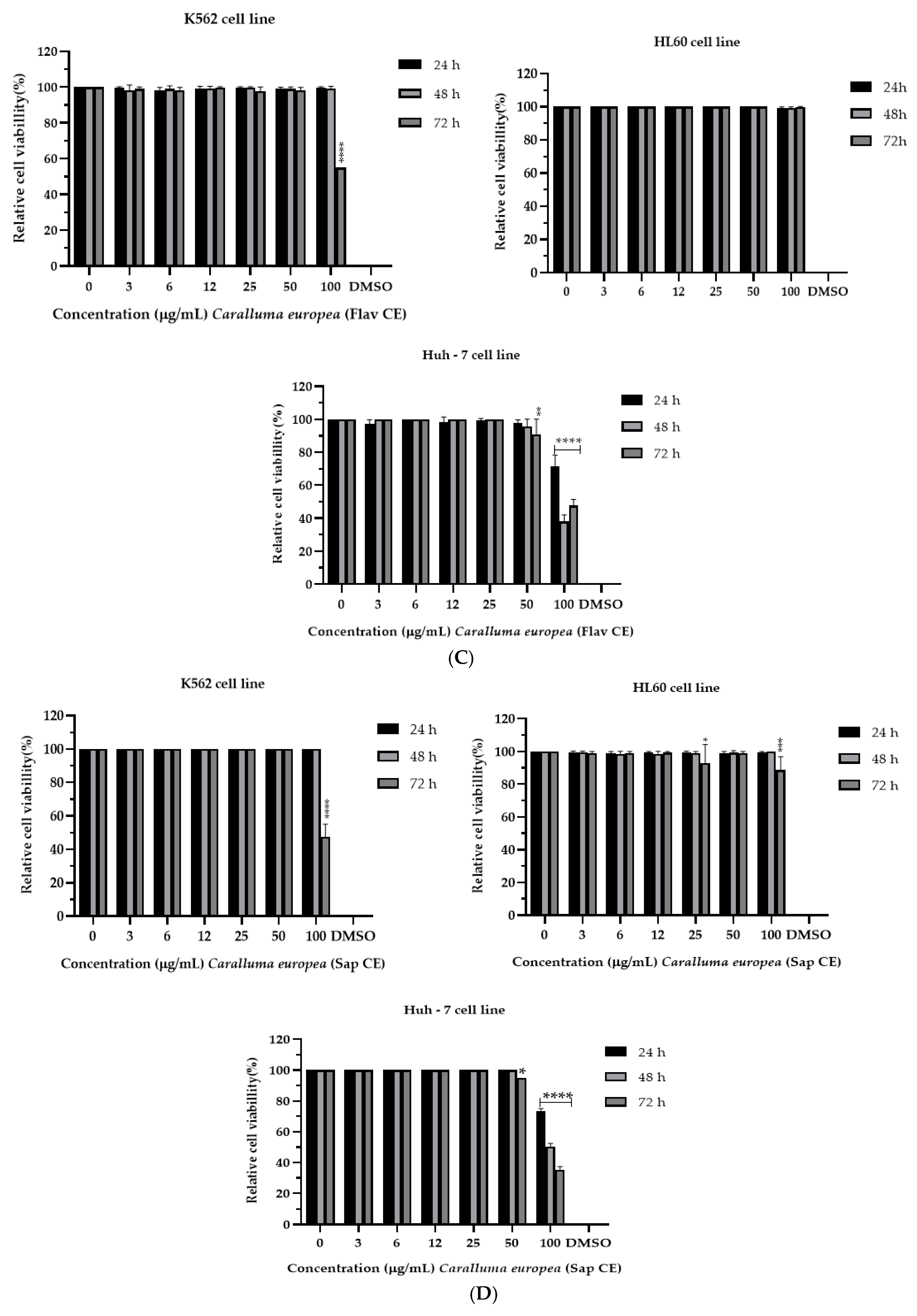
2.2. Cytotoxicity of C. europaea on Human Leukemia and Hepatocellular Carcinoma
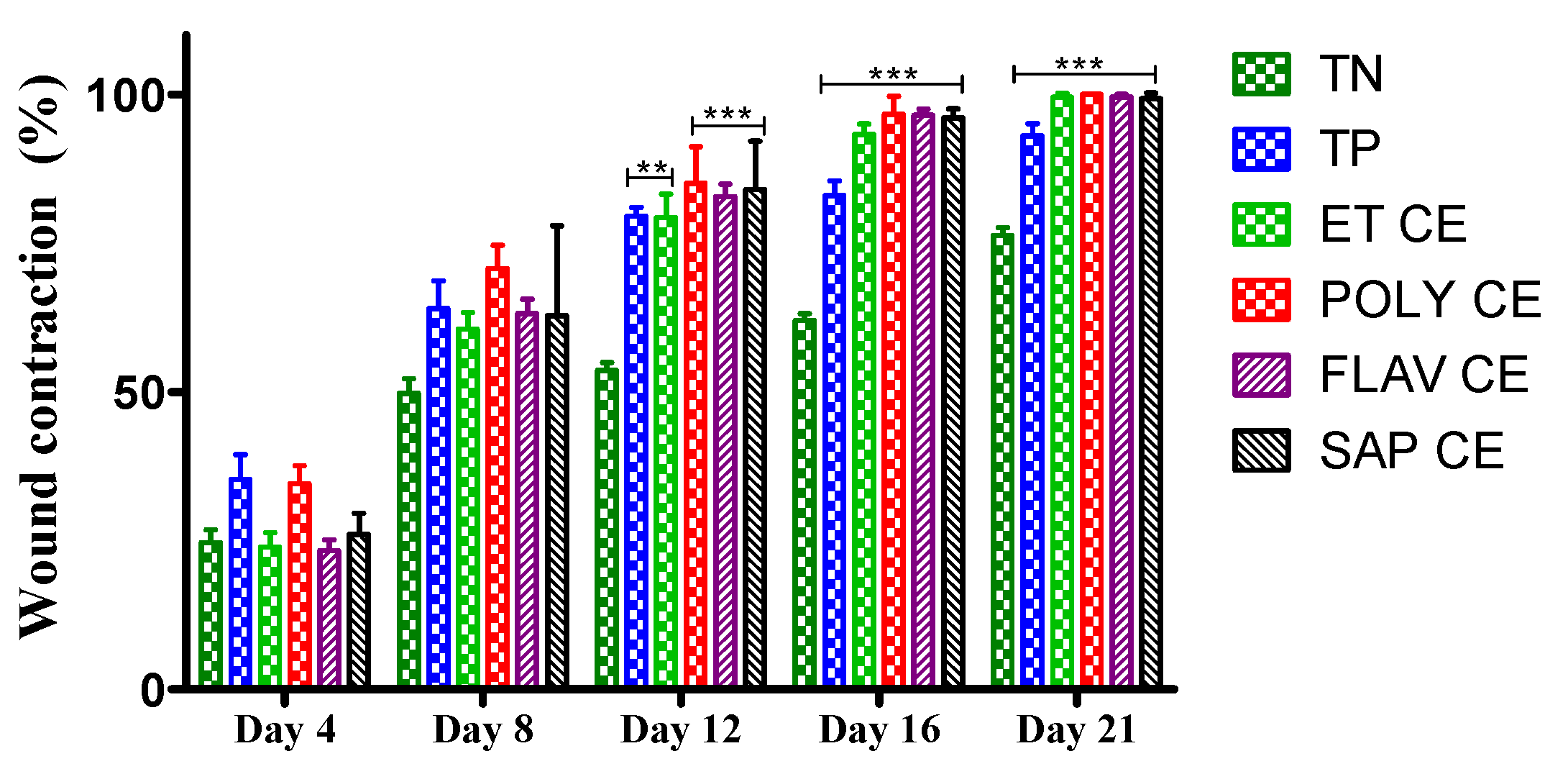
2.3. Wound Healing Activity of C. europaea Extracts
2.4. C. europaea Extracts May Have a Significant Antinociceptive Effect
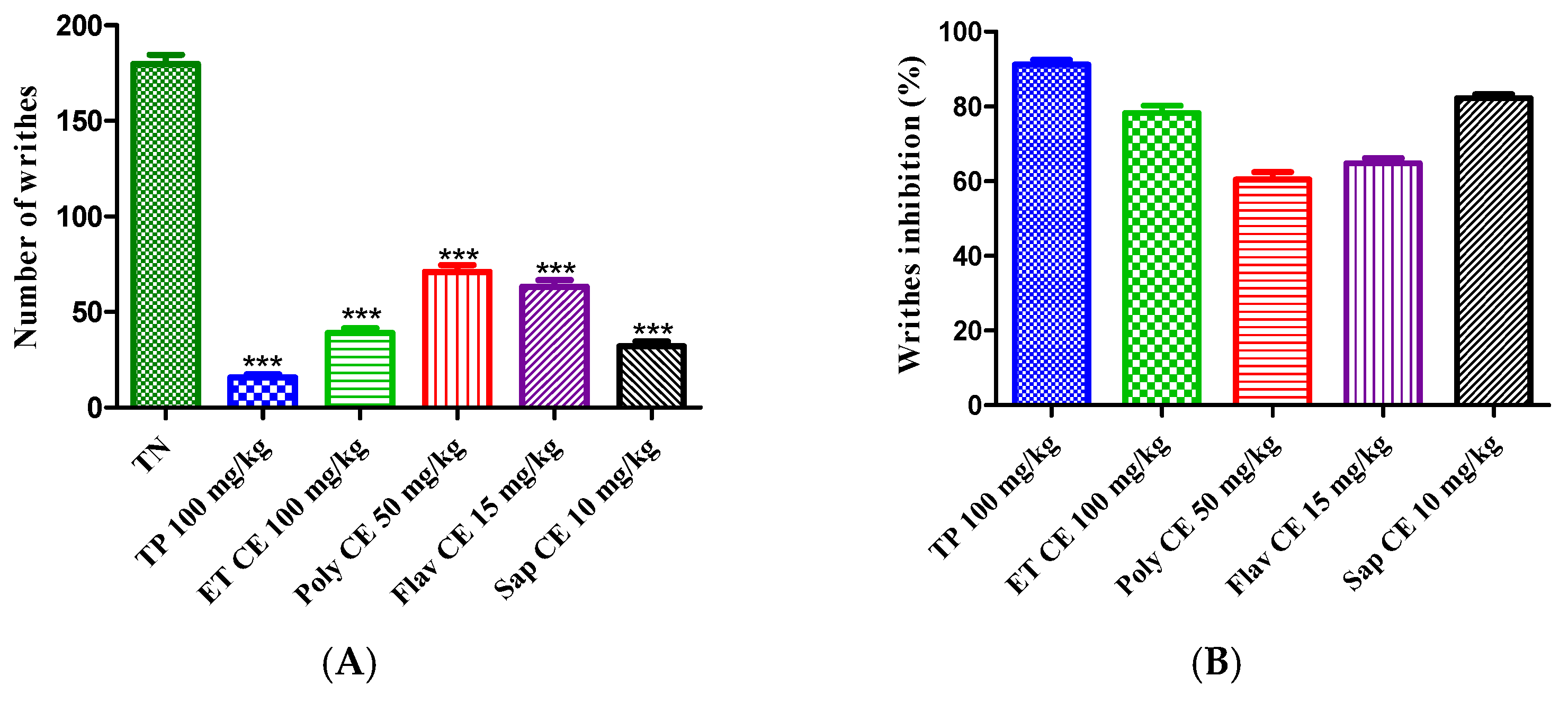
2.4.1. Acetic Acid-Induced Writhing
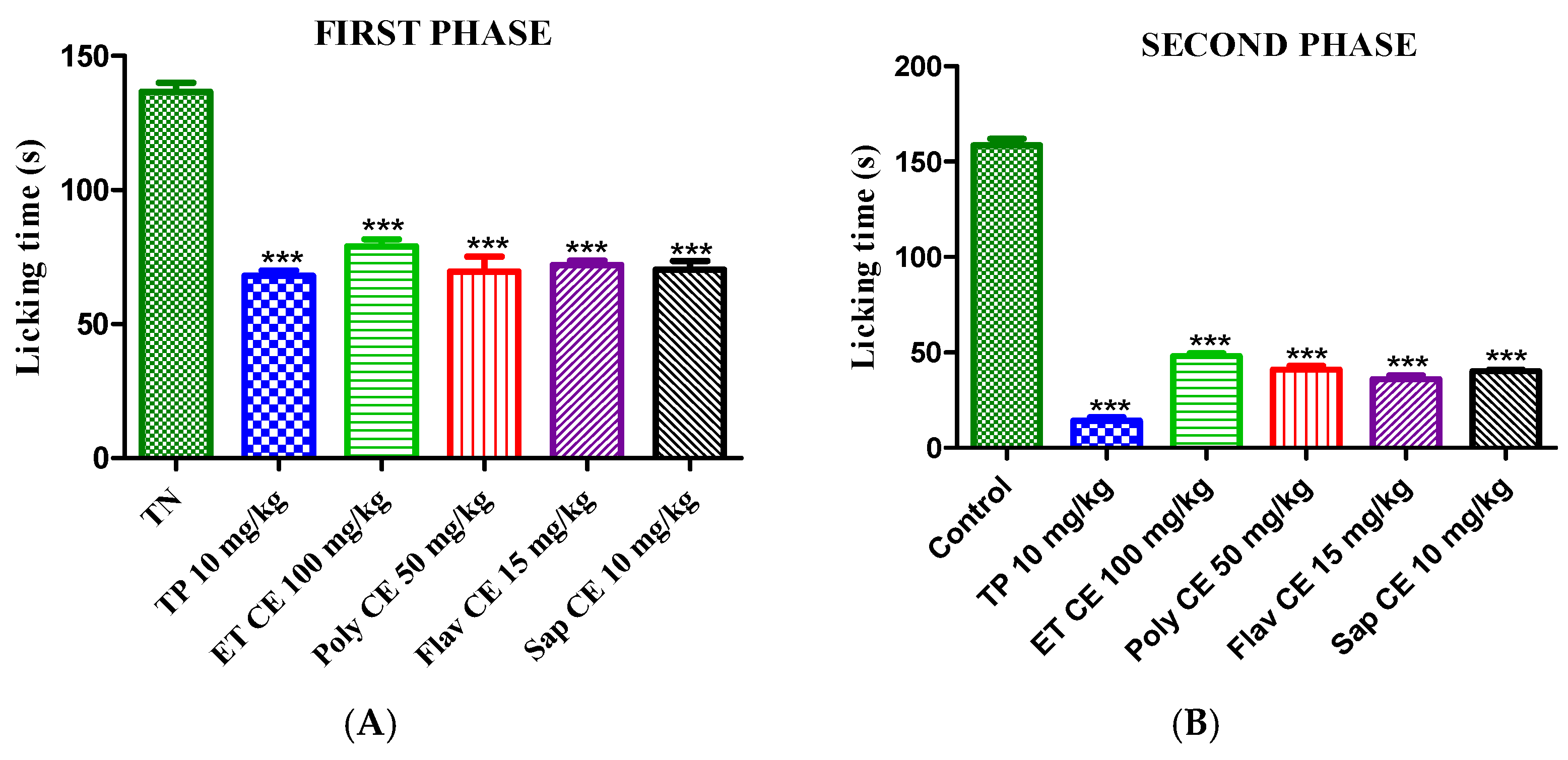
2.4.2. Formalin-Induced Paw Licking Test
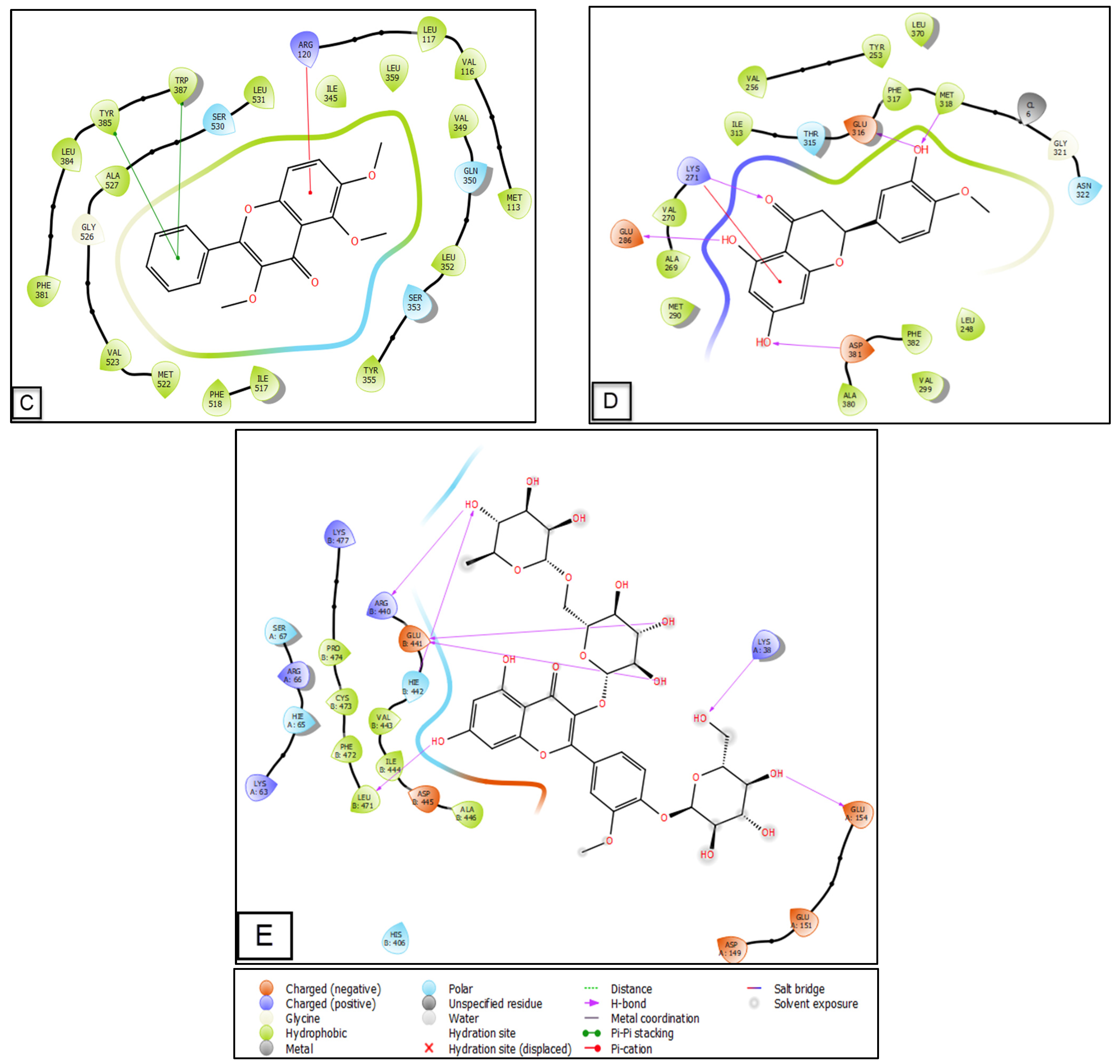
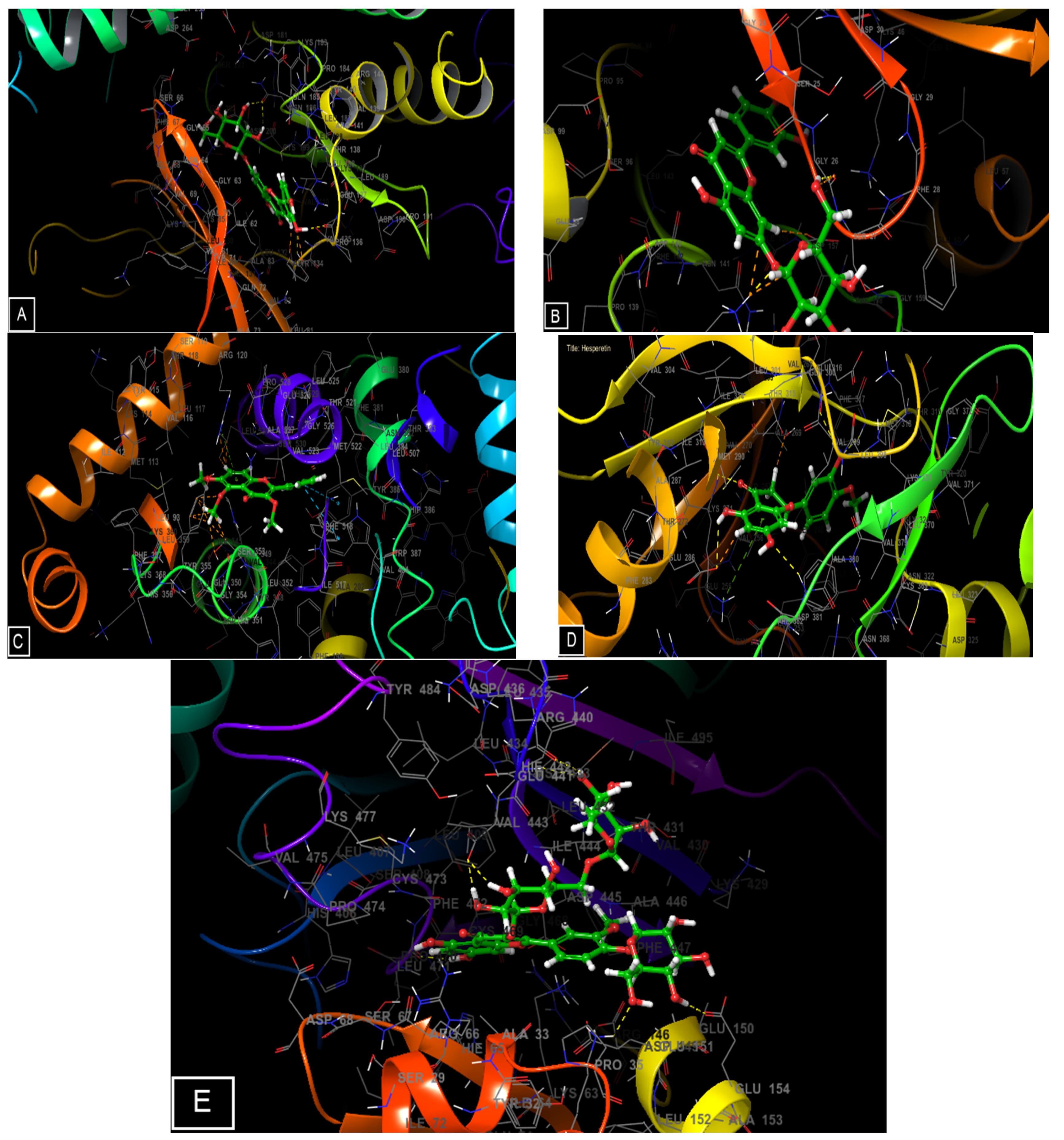
2.5. Molecular Docking of the Main Phenolic Compounds of C. europaea Polyphenolic Extract
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Animal Material
4.3. Preparation of C. europaea Extracts
4.3.1. Hydroethanolic Extract
4.3.2. Polyphenolic Extract
4.3.3. Flavonoids Extract
4.3.4. Saponins Extract
4.4. Phytochemical Composition of C. europaea Polyphenolic Extract
4.4.1. Solvents and Reagents
4.4.2. Analytical Equipment
4.5. Cytotoxicity Assay of C. europaea Extracts
4.6. Wound Healing Activity of C. europaea Extracts
4.6.1. Ointment Preparation
4.6.2. Burn Wound Induction
4.7. Antinociceptive Tests of C. europaea Extracts
4.7.1. Acetic Acid Test
- Group 1: Negative control (0.9% NaCl);
- Group 2: Paracetamol (100 mg/kg);
- Group 3: Hydroethanolic fraction (100 mg/kg);
- Group 4: Polyphenols fraction (50 mg/kg);
- Group 5: Flavonoids fraction (15 mg/kg);
- Group 6: Saponins fraction (10 mg/kg).
4.7.2. Formalin Test
- Group 1: Negative control (0.9% NaCl);
- Group 2: Positive control (Tramadol);
- Group 3: Hydroethanolic extract (100 mg/kg);
- Group 4: Polyphenols extract (50 mg/kg);
- Group 5: Flavonoids extract (15 mg/kg);
- Group 6: Saponins extract (10 mg/kg)
4.8. Molecular Docking
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Disclaimer
References
- Moni, J.N.R.; Adnan, M.; Tareq, A.M.; Kabir, M.I.; Reza, A.A.; Nasrin, M.S.; Chowdhury, K.H.; Sayem, S.A.J.; Rahman, M.A.; Alam, A.K. Therapeutic potentials of Syzygium fruticosum fruit (seed) reflected into an array of pharmacological assays and prospective receptors-mediated pathways. Life 2021, 11, 155. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Jiang, L.; Guan, X.-Y. The genetic and epigenetic alterations in human hepatocellular carcinoma: A recent update. Protein Cell. 2014, 5, 673–691. [Google Scholar] [CrossRef] [PubMed]
- Lejman, M.; Chałupnik, A.; Chilimoniuk, Z.; Dobosz, M. Genetic biomarkers and their clinical implications in B-cell acute lymphoblastic leukemia in children. Int. J. Mol. Sci. 2022, 23, 2755. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed]
- Mulcahy, A.; Mulligan, S.P.; Shumack, S.P. Recommendations for skin cancer monitoring for patients with chronic lymphocytic leukemia. Leuk. Lymphoma 2018, 59, 578–582. [Google Scholar] [CrossRef] [PubMed]
- Olson, K.; Amari, A. Self-reported pain in adolescents with leukemia or a brain tumor: A systematic review. Cancer Nurs. 2015, 38, E43–E53. [Google Scholar] [CrossRef]
- Issiki, Z.; Moundir, C.; Marnissi, F.; Seddik, N.; Benjelloun, N.; Zaid, Y.; Oudghiri, M. Toxicological evaluation of the aqueous extract of Caralluma europaea and its immunomodulatory and inflammatory activities. Pharmacognosy Res. 2017, 9, 390. [Google Scholar]
- Amrati, F.E.-Z.; Bourhia, M.; Slighoua, M.; Boukhira, S.; Ullah, R.; Ezzeldin, E.; Mostafa, G.A.E.; Grafov, A.; Bousta, D. Protective Effect of Chemically Characterized Polyphenol-Rich Fraction from Apteranthes europaea (Guss.) Murb. subsp. maroccana (Hook.f.) Plowes on Carbon Tetrachloride-Induced Liver Injury in Mice. Appl. Sci. 2021, 11, 554. [Google Scholar] [CrossRef]
- Amrati, F.E.-Z.; Bourhia, M.; Saghrouchni, H.; Slighoua, M.; Grafov, A.; Ullah, R.; Ezzeldin, E.; Mostafa, G.A.; Bari, A.; Ibenmoussa, S. Caralluma europaea (Guss.) NE Br.: Anti-inflammatory, antifungal, and antibacterial activities against nosocomial antibiotic-resistant microbes of chemically characterized fractions. Molecules 2021, 26, 636. [Google Scholar] [CrossRef]
- Amrati, F.E.-Z.; Bourhia, M.; Slighoua, M.; Ibnemoussa, S.; Bari, A.; Ullah, R.; Amaghnouje, A.; Di Cristo, F.; El Mzibri, M.; Calarco, A.; et al. Phytochemical study on antioxidant and antiproliferative activities of Moroccan Caralluma europaea extract and its bioactive compound classes. Evid. Based Complement. Altern. Med. 2020, 2020, 8409718. [Google Scholar] [CrossRef]
- Li, Z.H.; Guo, H.; Xu, W.B.; Ge, J.; Li, X.; Alimu, M.; He, D.-J. Rapid Identification of Flavonoid Constituents Directly from PTP1B Inhibitive Extract of Raspberry (Rubus idaeus L.) Leaves by HPLC-ESI-QTOF-MS-MS. J. Chromatogr. Sci. 2016, 54, 805–810. [Google Scholar] [CrossRef]
- Seraglio, S.K.T.; Valese, A.C.; Daguer, H.; Bergamo, G.; Azevedo, M.S.; Gonzaga, L.V.; Fett, R.; Costa, A.C.O. Development and validation of a LC-ESI-MS/MS method for the determination of phenolic compounds in honeydew honeys with the diluted-and-shoot approach. Food Res. Int. 2016, 87, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Nichitoi, M.M.; Costache, T.; Josceanu, A.M.; Isopescu, R.; Isopencu, G.; Lavric, V. Development and Application of an LC-MS/MS Method for Identification of Polyphenols in Propolis Extract. Proceedings 2020, 55, 10. [Google Scholar]
- Cozza, G.; Gianoncelli, A.; Montopoli, M.; Caparrotta, L.; Venerando, A.; Meggio, F.; Pinna, L.A.; Zagotto, G.; Moro, S. Identification of novel protein kinase CK1 delta (CK1δ) inhibitors through structure-based virtual screening. Bioorganic Med. Chem. Lett. 2008, 18, 5672–5675. [Google Scholar] [CrossRef] [PubMed]
- Kruggel, S.; Lemcke, T. Comparative Investigation of the ATP-Binding Site of Human and Plasmodial Glycogen Synthase Kinase-3. QSAR Comb. Sci. 2009, 28, 885–890. [Google Scholar] [CrossRef]
- Raichurkar, A.K.; Kulkarni, V. 3D-QSAR of Cyclooxygenase-2 Inhibitors by Genetic Function Approximation. Internet Electron. J. Mol. Des. 2003, 2, 242–261. [Google Scholar]
- Lamers, M.B.A.C.; Antson, A.A.; Hubbard, R.E.; Scott, R.K.; Williams, D.H. Structure of the protein tyrosine kinase domain of C-terminal Src kinase (CSK) in complex with staurosporine. J. Mol. Biol. 1999, 285, 713–725. [Google Scholar] [CrossRef]
- Nakamura, N. The Role of the Transmembrane RING Finger Proteins in Cellular and Organelle Function. Membranes 2011, 1, 354–393. [Google Scholar] [CrossRef]
- Al-Nemi, R.; Makki, A.A.; Sawalha, K.; Hajjar, D.; Jaremko, M. Untargeted Metabolomic Profiling and Antioxidant Capacities of Different Solvent Crude Extracts of Ephedra foeminea. Metabolites 2022, 12, 451. [Google Scholar] [CrossRef]
- Jemima, L.W.; Andrew, A.S.; Jill, A.H. Wnt Signaling and Injury Repair. Cold Spring Harb. Perspect. Biol. 2012, 4, a008078. [Google Scholar]
- Özay, Y.; Güzel, S.; Yumrutaş, Ö.; Pehlivanoğlu, B.; Erdoğdu, İ.H.; Yildirim, Z.; Türk, B.A.; Darcan, S. Wound healing effect of kaempferol in diabetic and nondiabetic rats. J. Surg. Res. 2019, 233, 284–296. [Google Scholar] [CrossRef] [PubMed]
- Gendrisch, F.; Esser, P.R.; Schempp, C.M.; Wölfle, U. Luteolin as a modulator of skin aging and inflammation. Biofactors 2021, 47, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Ghaisas, M.M.; Kshirsagar, S.B.; Sahane, R.S. Evaluation of wound healing activity of ferulic acid in diabetic rats. Int. Wound J. 2014, 11, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Dorjsembe, B.; Lee, H.J.; Kim, M.; Dulamjav, B.; Jigjid, T.; Nho, C.W. Achillea asiatica extract and its active compounds induce cutaneous wound healing. J. Ethnopharmacol. 2017, 206, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.-H.; Cao, G.; Wu, X.-Q.; Vaziri, N.D.; Zhao, Y.-Y. Wnt signaling pathway in aging-related tissue fibrosis and therapies. Ageing Res. Rev. 2020, 60, 101063. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Yan, R.; Zhang, X.; Wang, L.; Ke, X.; Qu, Y. Activating Wnt/β-catenin signaling pathway for disease therapy: Challenges and opportunities. Pharmacol. Ther. 2019, 196, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Reshad, R.A.I.; Alam, S.; Raihan, H.B.; Meem, K.N.; Rahman, F.; Zahid, F.; Rafid, M.; Rahman, S.; Omit, S.; Ali, M. In silico investigations on curcuminoids from Curcuma longa as positive regulators of the Wnt/β-catenin signaling pathway in wound healing. Egypt. J. Med. Hum. Genet. 2021, 22, 65. [Google Scholar] [CrossRef]
- Mi, Y.; Zhong, L.; Lu, S.; Hu, P.; Pan, Y.; Ma, X.; Yan, B.; Wei, Z.; Yang, G. Quercetin promotes cutaneous wound healing in mice through Wnt/β-catenin signaling pathway. J. Ethnopharmacol. 2022, 290, 115066. [Google Scholar] [CrossRef]
- Slighoua, M.; Chebaibi, M.; Mahdi, I.; Amrati, F.E.-Z.; Conte, R.; Cordero, M.A.W.; Alotaibi, A.; Saghrouchni, H.; Agour, A.; Zair, T.; et al. The LC-MS/MS Identification and Analgesic and Wound Healing Activities of Lavandula officinalis Chaix: In Vivo and In Silico Approaches. Plants 2022, 11, 3222. [Google Scholar] [CrossRef]
- Shafie, A.; Khan, S.; Zehra; Mohammad, T.; Anjum, F.; Hasan, G.M.; Yadav, D.K.; Hassan, M.I. Identification of Phytoconstituents as Potent Inhibitors of Casein Kinase-1 Alpha Using Virtual Screening and Molecular Dynamics Simulations. Pharmaceutics 2021, 13, 2157. [Google Scholar] [CrossRef]
- Kebbou, A.; Laaradia, M.A.; Oufquir, S.; Aarab, A.; Gabbas, Z.E.; Rais, H.; Zyad, A.; Chait, A. Antioxidant Activity, Anti-Inflammatory and Analgesic Effects of Caralluma europaea (Eddaghmouss) in Mice. Online J. Biol. Sci. 2019, 19, 272–285. [Google Scholar] [CrossRef]
- Zhao, J.; Xu, F.; Huang, H.; Gu, Z.; Wang, L.; Tan, W.; He, J.; Chen, Y.; Li, C. Evaluation on anti-inflammatory, analgesic, antitumor, and antioxidant potential of total saponins from Nigella glandulifera seeds. Evid. Based Complement. Altern. Med. 2013, 2013, 827230. [Google Scholar] [CrossRef] [PubMed]
- Shi, G.-N.; Liu, Y.-L.; Lin, H.-M.; Yang, S.-L.; Feng, Y.-L.; Reid, P.F.; Qin, Z.-H. Involvement of cholinergic system in suppression of formalin-induced inflammatory pain by cobratoxin. Acta Pharmacol. Sin. 2011, 32, 1233–1238. [Google Scholar] [CrossRef] [PubMed]
- Mountassir, M.; Chaib, S.; Selami, Y.; Khalki, H.; Ouachrif, A.; Moubtakir, S.; Aboufatima, R.; Zyad, A.; Benharref, A.; Chait, A. Antinociceptive activity and acute toxicity of Moroccan black propolis. Int. J. Eng. Sci. 2014, 3, 2393–2397. [Google Scholar]
- Calixto-Campos, C.S.; Carvalho, T.T.; Hohmann, M.S.; Pinho-Ribeiro, F.A.; Fattori, V.; Manchope, M.F.; Zarpelon, A.C.; Baracat, M.M.; Georgetti, S.R.; Casagrande, R. Vanillic acid inhibits inflammatory pain by inhibiting neutrophil recruitment, oxidative stress, cytokine production, and NFκB activation in mice. J. Nat. Prod. 2015, 78, 1799–1808. [Google Scholar] [CrossRef]
- Enam, T.; Agarwala, R.; Chowdhury, M.d.H.U.; Chy, M.N.U.; Adnan, M. An in silico ADME/T and molecular docking studies of phytochemicals derived from Holigarna caustica (Dennst.) for the management of pain. J. Phytomol. Pharmacol. 2022, 1, 37–46. [Google Scholar] [CrossRef]
- Rostaing, R.S.; Guerin, J. Cancer pain management: Good clinical practices, use of strong opioids. Presse Med. 2014, 43, 252–262. [Google Scholar]
- O’Neil, C.K.; Hanlon, J.T.; Marcum, Z.A. Adverse effects of analgesics commonly used by older adults with osteoarthritis: Focus on non-opioid and opioid analgesics. Am. J. Geriatr. Pharmac. 2012, 10, 331–342. [Google Scholar] [CrossRef]
- Sakthivel, K.; Guruvayoorappan, C. Amentoflavone inhibits iNOS, COX-2 expression and modulates cytokine profile, NF-κB signal transduction pathways in rats with ulcerative colitis. Int. Immunopharmacol. 2013, 17, 907–916. [Google Scholar] [CrossRef]
- Moslehi, J.J.; Deininger, M. Tyrosine kinase inhibitor–associated cardiovascular toxicity in chronic myeloid leukemia. J. Clin. Oncol. 2015, 33, 4210. [Google Scholar] [CrossRef]
- Adan, A.; Baran, Y. Fisetin and hesperetin induced apoptosis and cell cycle arrest in chronic myeloid leukemia cells accompanied by modulation of cellular signaling. Tumor Biol. 2016, 37, 5781–5795. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, H.A.; Almahmoud, S.A.; El-Ghaly, E.S.M.; Khan, F.A.; Emwas, A.H.; Jaremko, M.; Almulhim, F.; Khan, R.A.; Ragab, E.A. Comparative Anticancer Potentials of Taxifolin and Quercetin Methylated Derivatives against HCT-116 Cell Lines: Effects of O-Methylation on Taxifolin and Quercetin as Preliminary Natural Leads. ACS Omega 2022, 7, 46629–46639. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.-Y.; Lu, H.-F.; Chou, Y.-C.; Shih, Y.-L.; Bau, D.-T.; Chen, J.-C.; Hsu, S.-C.; Chung, J.-G. Kaempferol induces DNA damage and inhibits DNA repair associated protein expressions in human promyelocytic leukemia HL-60 cells. Am. J. Chin. Med. 2015, 43, 365–382. [Google Scholar] [CrossRef] [PubMed]
- Hassanzadeh, A.; Hosseinzadeh, E.; Rezapour, S.; Vahedi, G.; Haghnavaz, N.; Marofi, F. Quercetin promotes cell cycle arrest and apoptosis and attenuates the proliferation of human chronic myeloid leukemia cell line-K562 through interaction with HSPs (70 and 90), MAT2A and FOXM1. Anticancer Agents Med. Chem. 2019, 19, 1523–1534. [Google Scholar] [CrossRef]
- Cheng, A.-C.; Huang, T.-C.; Lai, C.-S.; Pan, M.-H. Induction of apoptosis by luteolin through cleavage of Bcl-2 family in human leukemia HL-60 cells. Eur. J. Pharmacol. 2005, 509, 1–10. [Google Scholar] [CrossRef]
- Ntie-Kang, F.; Nwodo, J.N.; Ibezim, A.; Simoben, C.V.; Karaman, B.; Ngwa, V.F.; Sippl, W.; Adikwu, M.U.; Mbaze, L.M. Molecular Modeling of Potential Anticancer Agents from African Medicinal Plants. J. Chem. Inf. Model. 2014, 54, 2433–2450. [Google Scholar] [CrossRef]
- Ciftci, H.I.; Ozturk, S.E.; Ali, T.F.S.; Radwan, M.O.; Tateishi, H.; Koga, R.; Ocak, Z.; Can, M.; Otsuka, M.; Fujita, M. The First Pentacyclic Triterpenoid Gypsogenin Derivative Exhibiting Anti-ABL1 Kinase and Anti-chronic Myelogenous Leukemia Activities. Biol. Pharm. Bull. 2018, 41, 570–574. [Google Scholar] [CrossRef]
- Sheikh, M.S.; Huang, Y. Death Receptor Activation Complexes: It Takes Two to Activate TNF Receptor 1. Cell Cycle. 2003, 2, 549–551. [Google Scholar] [CrossRef]
- Spence, T.; Allsopp, P.J.; Yeates, A.J.; Mulhern, M.S.; Strain, J.; McSorley, E.M. Maternal serum cytokine concentrations in healthy pregnancy and preeclampsia. J. Pregnancy 2021, 2021, 6649608. [Google Scholar] [CrossRef]
- Bellail, A.C.; Tse, M.C.L.; Song, J.H.; Phuphanich, S.; Olson, J.J.; Sun, S.Y.; Hao, C. DR5-mediated DISC controls caspase-8 cleavage and initiation of apoptosis in human glioblastomas. J. Cell. Mol. Med. 2010, 14, 1303–1317. [Google Scholar] [CrossRef]
- Wu, H. Assembly of Post-Receptor Signaling Complexes for the Tumor Necrosis Factor Receptor Superfamily. Adv. Protein Chem. 2004, 68, 225–279. [Google Scholar]
- Amrati, F.E.-Z.; Bourhia, M.; Slighoua, M.; Salamatullah, A.M.; Alzahrani, A.; Ullah, R.; Bari, A.; Bousta, D. Traditional medicinal knowledge of plants used for cancer treatment by communities of mountainous areas of Fez-Meknes-Morocco. Saudi Pharm. J. 2021, 29, 1185–1204. [Google Scholar] [CrossRef] [PubMed]
- National Research Council. Guide for the Care and Use of Laboratory Animals, The National Academies Collection: Reports Funded by National Institutes of Health, 8th ed.; National Academies Press (US): Washington, DC, USA, 2011. [Google Scholar]
- Amrati, F.E.-Z.; Elmadbouh, O.H.M.; Chebaibi, M.; Soufi, B.; Conte, R.; Slighoua, M.; Saleh, A.; Al Kamaly, O.; Drioiche, A.; Zair, T. Evaluation of the toxicity of Caralluma europaea (CE) extracts and their effects on apoptosis and chemoresistance in pancreatic cancer cells. J. Biomol. Struct. Dyn. 2022, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Mssillou, I.; Agour, A.; Slighoua, M.; Chebaibi, M.; Amrati, F.E.-Z.; Alshawwa, S.Z.; Kamaly, O.A.; El Moussaoui, A.; Lyoussi, B.; Derwich, E. Ointment-based combination of Dittrichia viscosa L. and Marrubium vulgare L. accelerate burn wound healing. Pharmaceuticals 2022, 15, 289. [Google Scholar] [CrossRef]
- Brinkhaus, B.; Lindner, M.; Schuppan, D.; Hahn, E.G. Chemical, pharmacological and clinical profile of the East Asian medical plant Centella aslatica. Phytomedicine 2000, 7, 427–448. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Pérez, M.; Rabanal, R.M. Evaluation of the antinflammatory and analgesic activity of Sideritis canariensis var. pannosa in mice. J. Ethnopharmacol. 2002, 81, 43–47. [Google Scholar] [CrossRef]


| Polyphenolic Extract | ||
|---|---|---|
| Molecule | AUC | m/z |
| Gallic acid | 83,057 | 168.9 |
| Quercetin | 4,260,116 | 301 |
| Trans-ferulic acid | 478,543 | 193 |
| Hesperetin | 6,639,841 | 301.3 |
| Amentoflavone | 325,795 | 537.1 |
| Luteolin | 69,281,031 | 284.9 |
| Kaempferol-3-O-glucose | 1,458,547 | 609.1 |
| Quercetin-3-O-hexose deoxyhexose | 1,673,879 | 609.1 |
| Isorhamnetin-3-O-rutinoside | 1,432,451 | 623.1 |
| Isorhamnetin-7-O-pentose | 8,344,809 | 447.1 |
| Luteolin 7-O-glucoside | 8,266,394 | 447.1 |
| Kaempferol-3-O-glucuronic acid | 1,007,610 | 461.1 |
| Kaempferol-3-O-pentose | 1,665,472 | 417.1 |
| Kaempferol-3-O-hexose deoxyhexose | 19,807,364 | 593.1 |
| Tyrosol | 491,809 | 153.4 |
| Syringic acid | 365,324 | 198.9 |
| Ferulic acid | 214,942 | 193 |
| Kaempferol | 102,866,891 | 285 |
| C. europaea Extracts | IC50 (µM) | ||
|---|---|---|---|
| Human Chronic Myelogenous Leukemia (K562 Cell Line) | Human Acute Promyelocytic Leukemia (HL60 Cell Line) | Human Hepatocellular Carcinoma (Huh-7 Cell Line) | |
| Hydroethanolic | >100 | >100 | >100 |
| Polyphenols | 36.47 *** | 53.77 *** | 24.07 *** |
| Flavonoids | >100 | >100 | >100 |
| Saponins | >100 | >100 | 50.14 *** |
| Glide G-Score (Kcal/mol) | |||||
|---|---|---|---|---|---|
| Wound Healing Effect | Analgesic Activity (Cyclooxygenase-2) | Antileukemic Activity (Tyrosine Kinase) | Anti-Hepatocellular Carcinoma (TRADD) | ||
| Casein Kinase-1 | Glycogen Synthase Kinase-3β | ||||
| Luteolin 7-O-glucoside | −5.949 | −7.696 | - | - | −5.977 |
| Amentoflavone | −4.679 | −7.335 | - | - | - |
| Trimethoxyflavone | - | - | −8.572 | −8.304 | −3.975 |
| Luteolin | - | −7.258 | −8.431 | −8.464 | −6.885 |
| Quercetin | - | −7.236 | −7.749 | −8.687 | −6.312 |
| Hesperetin | - | −6.838 | −7.657 | −9.187 | −5.03 |
| Kaempferol | - | −6.716 | −7 | −7.964 | −6.569 |
| Ferulic Acid | - | −6.669 | −6.156 | −6.692 | - |
| Kaempferol-3-O-glucoside | −3.345 | −6.449 | - | - | - |
| Isorhamnetin-3-O-rutinoside | −5.171 | −6.196 | - | −7.47 | −7.416 |
| Tyrosol | - | −5.174 | −6.141 | −6.925 | −4.149 |
| Syringic acid | - | −5.047 | −6.404 | −6.075 | −4.794 |
| Gallic acid | - | −4.997 | −5.894 | −6.751 | −4.542 |
| Paracetamol | NA | NA | −5.644 | NA | NA |
| Tramadol | NA | NA | −6.710 | NA | NA |
| Obatoclax | NA | NA | NA | NA | −5.055 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amrati, F.E.-Z.; Chebaibi, M.; Galvão de Azevedo, R.; Conte, R.; Slighoua, M.; Mssillou, I.; Kiokias, S.; de Freitas Gomes, A.; Soares Pontes, G.; Bousta, D. Phenolic Composition, Wound Healing, Antinociceptive, and Anticancer Effects of Caralluma europaea Extracts. Molecules 2023, 28, 1780. https://doi.org/10.3390/molecules28041780
Amrati FE-Z, Chebaibi M, Galvão de Azevedo R, Conte R, Slighoua M, Mssillou I, Kiokias S, de Freitas Gomes A, Soares Pontes G, Bousta D. Phenolic Composition, Wound Healing, Antinociceptive, and Anticancer Effects of Caralluma europaea Extracts. Molecules. 2023; 28(4):1780. https://doi.org/10.3390/molecules28041780
Chicago/Turabian StyleAmrati, Fatima Ez-Zahra, Mohamed Chebaibi, Renata Galvão de Azevedo, Raffaele Conte, Meryem Slighoua, Ibrahim Mssillou, Sotirios Kiokias, Alice de Freitas Gomes, Gemilson Soares Pontes, and Dalila Bousta. 2023. "Phenolic Composition, Wound Healing, Antinociceptive, and Anticancer Effects of Caralluma europaea Extracts" Molecules 28, no. 4: 1780. https://doi.org/10.3390/molecules28041780
APA StyleAmrati, F. E.-Z., Chebaibi, M., Galvão de Azevedo, R., Conte, R., Slighoua, M., Mssillou, I., Kiokias, S., de Freitas Gomes, A., Soares Pontes, G., & Bousta, D. (2023). Phenolic Composition, Wound Healing, Antinociceptive, and Anticancer Effects of Caralluma europaea Extracts. Molecules, 28(4), 1780. https://doi.org/10.3390/molecules28041780






