Quantitative Proteomic Analysis of Tibetan Pig Livers at Different Altitudes
Abstract
1. Introduction
2. Results
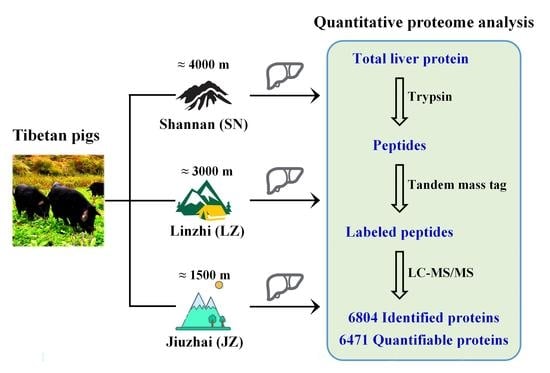
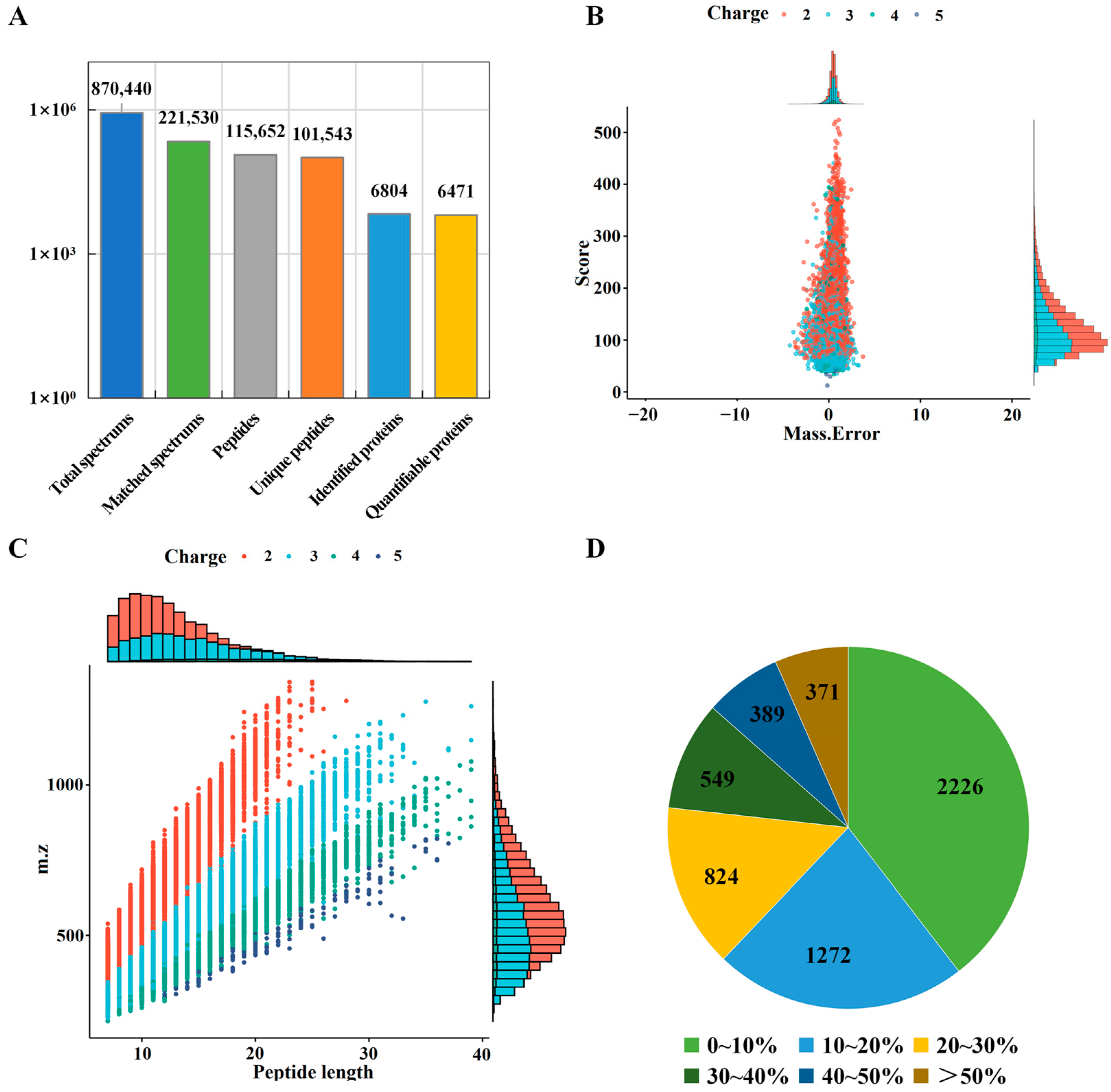
2.1. Proteome of Tibetan Pig Liver
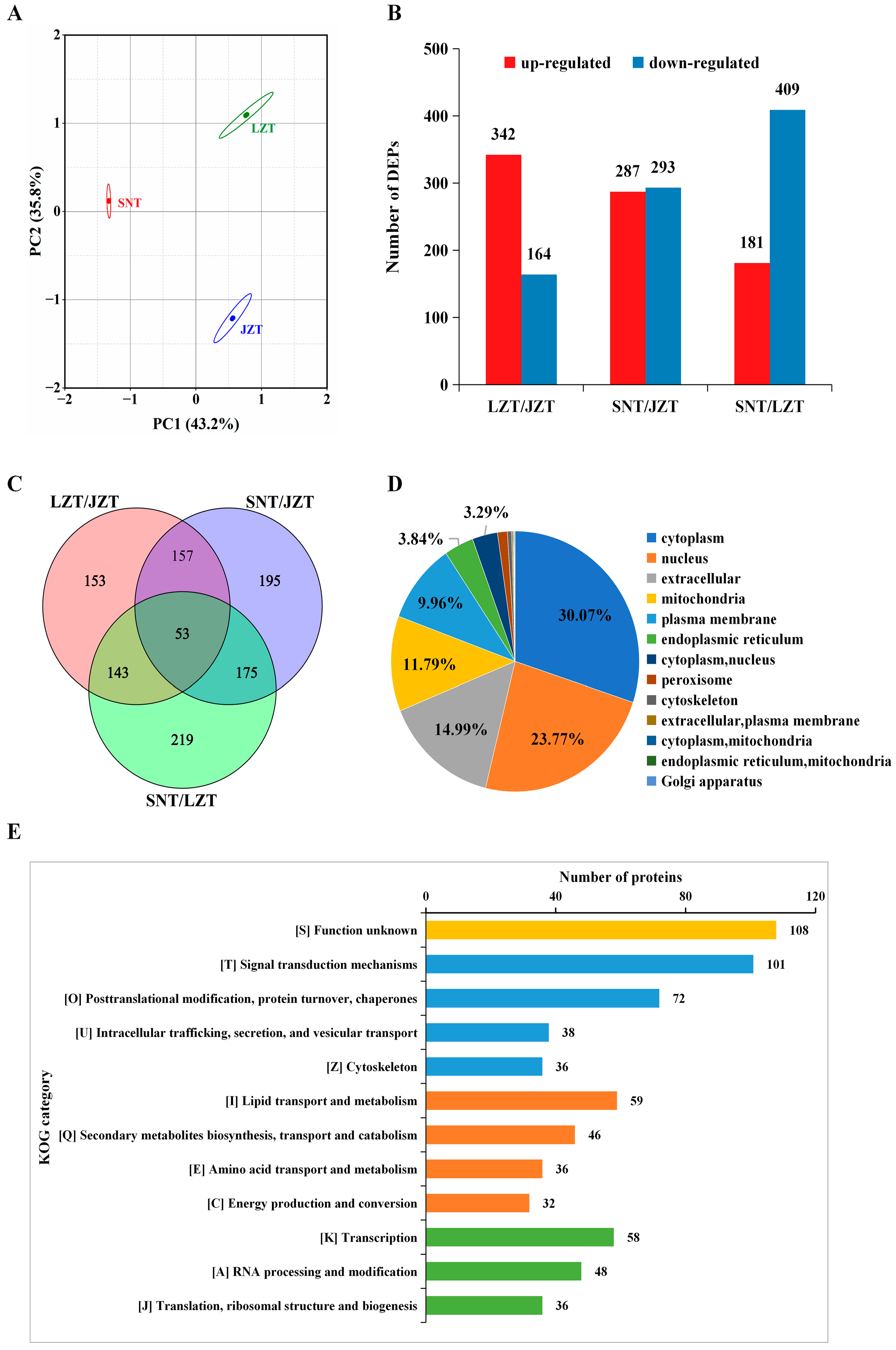
2.2. Differentially Expressed Proteins (DEPs)
2.3. Annotation Analysis of DEPs
2.3.1. Subcellular Localization
2.3.2. COG/KOG Annotations
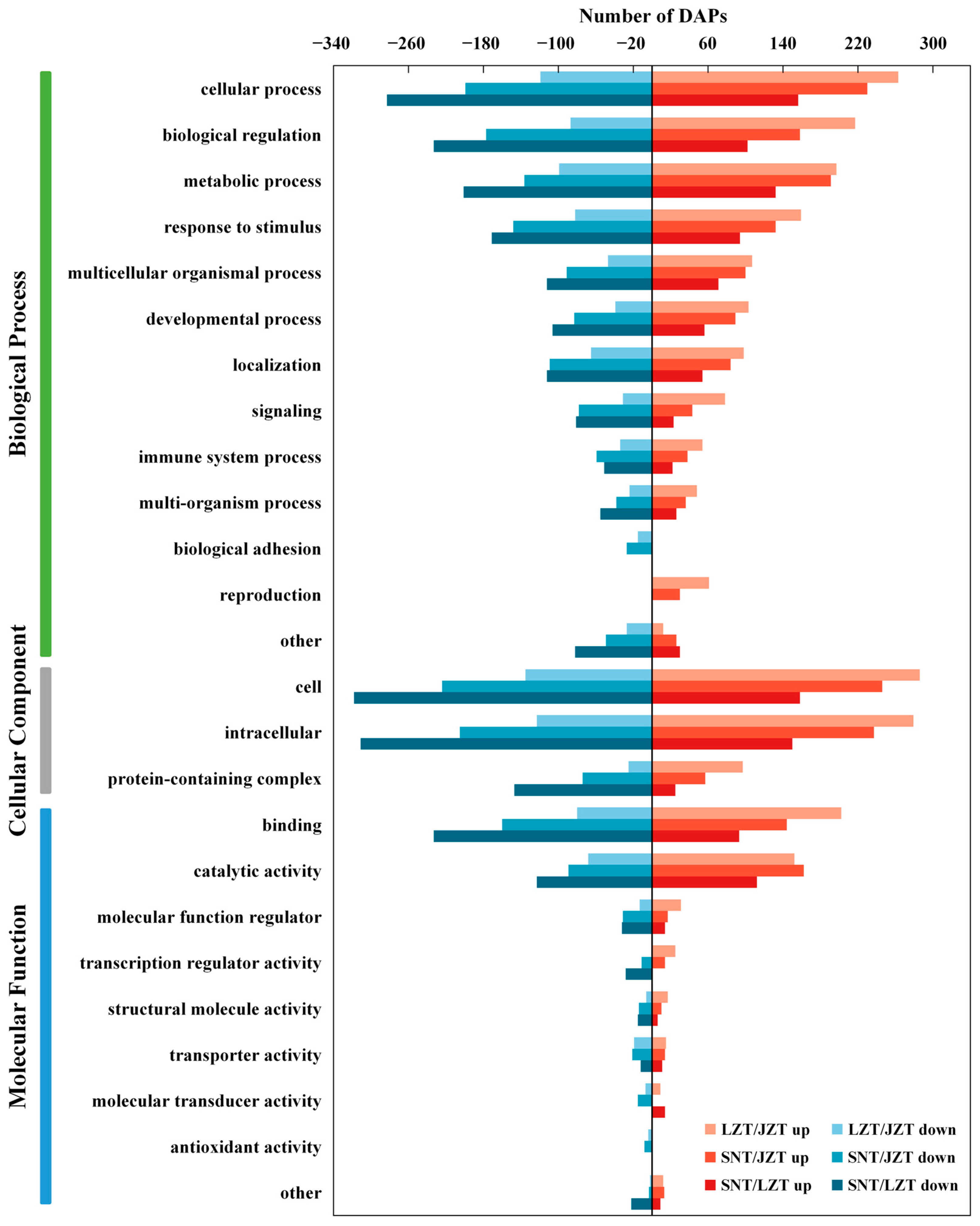
2.3.3. GO Annotations of DEPs
2.4. Enrichment Analysis of DEPs
2.4.1. GO Enrichment Analysis
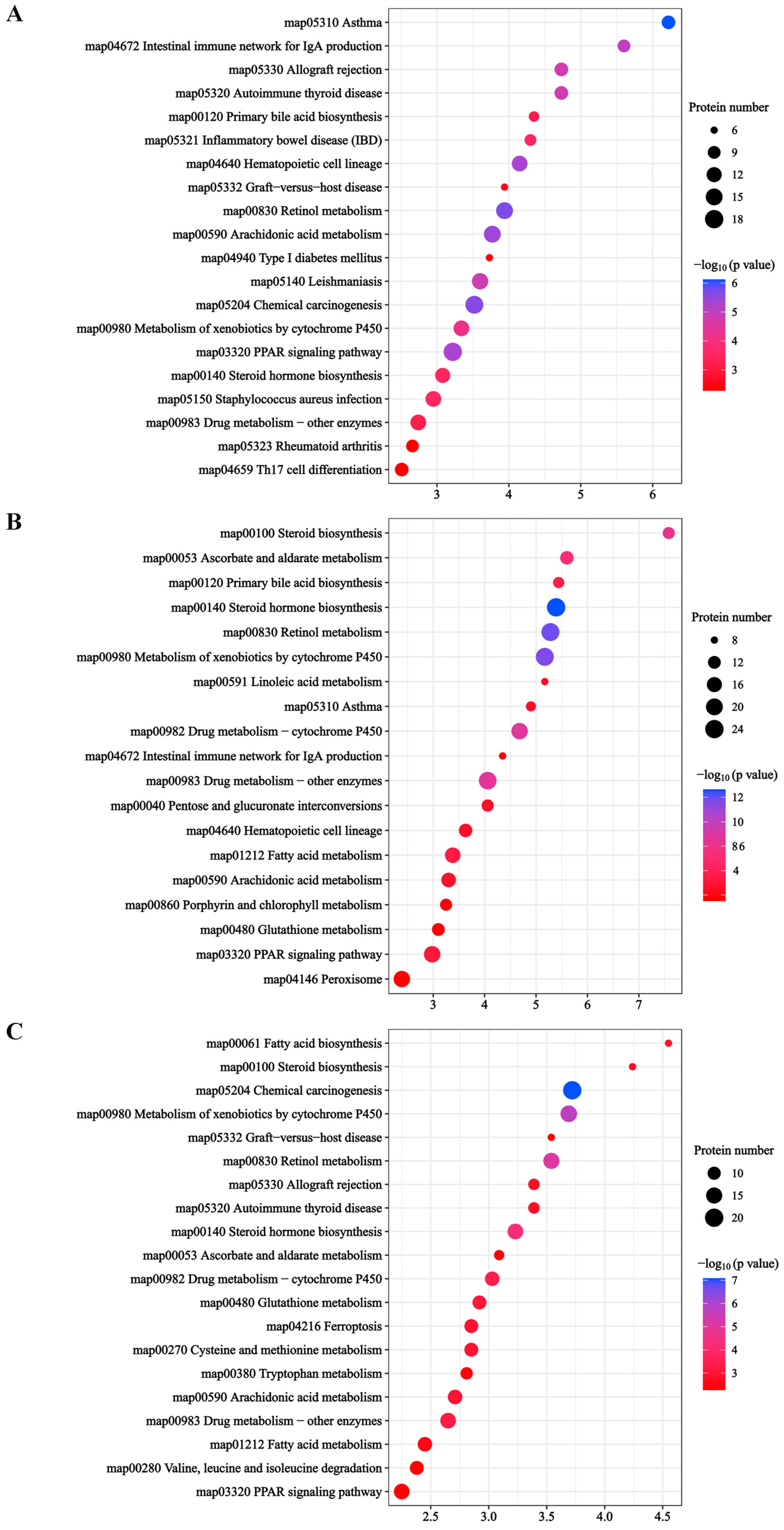
2.4.2. KEGG Enrichment Analysis
3. Discussion
3.1. Steroid Hormones Promote Liver Detoxification
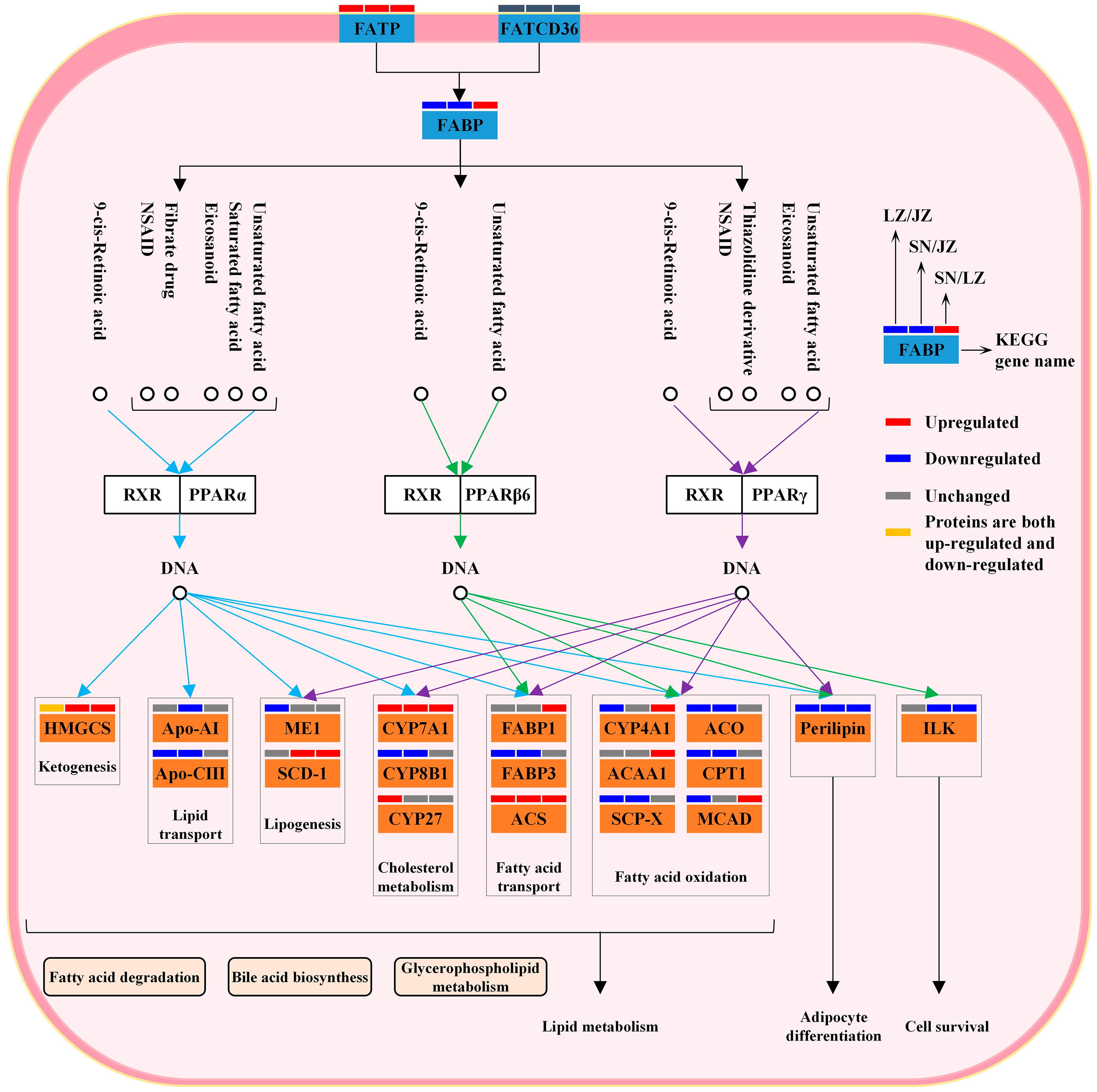
3.2. Lipid Metabolism Maintains Energy Homeostasis
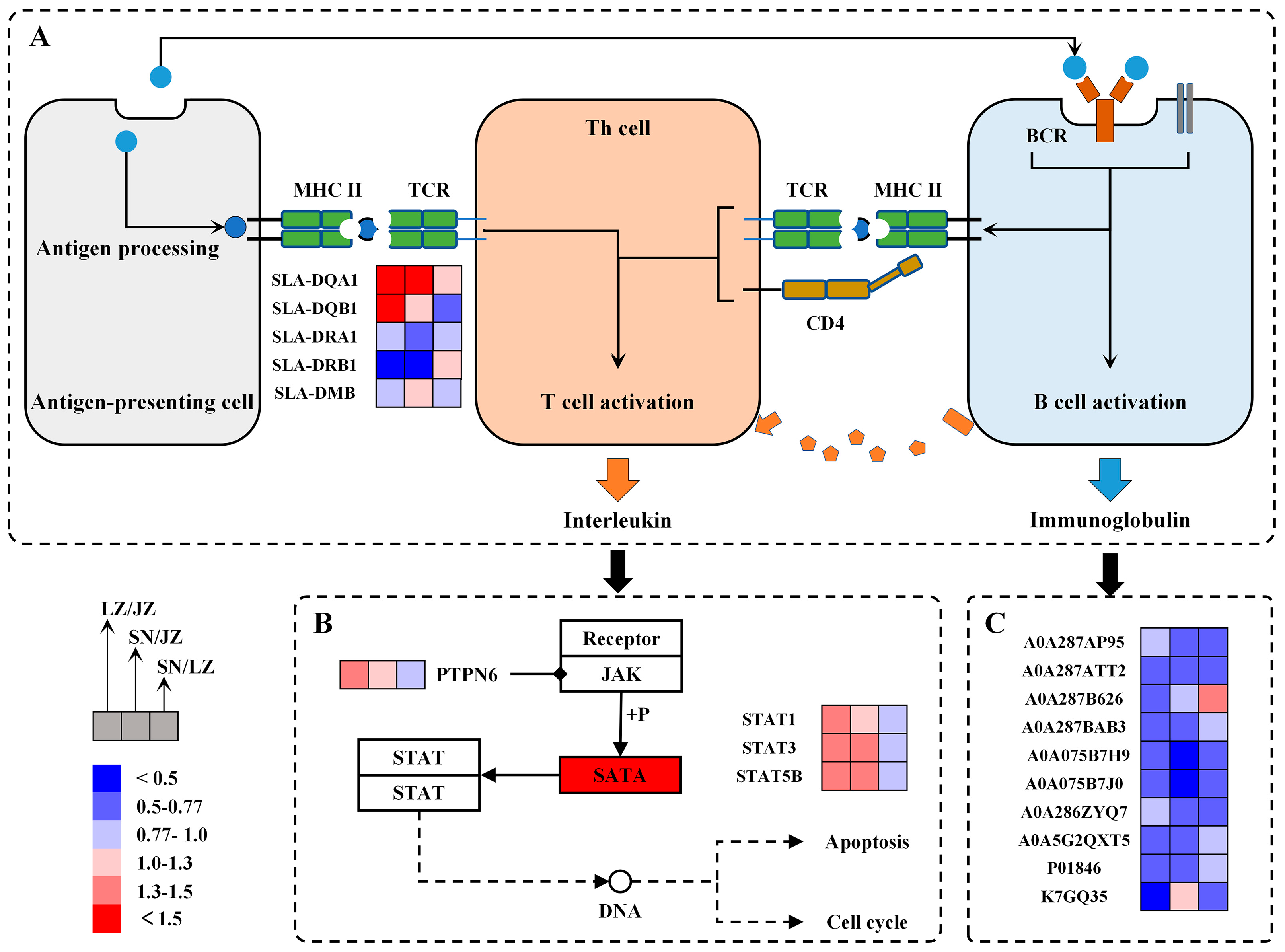
3.3. Immune Defense and Immune Regulation
4. Materials and Methods
4.1. Sample Collection
4.2. Protein Extraction and Digestion
4.3. TMT Labelling and Fractionation
4.4. LC-MS/MS
4.5. Bioinformatics Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, B.; Chamba, Y.; Shang, P.; Wang, Z.; Ma, J.; Wang, L.; Zhang, H. Comparative transcriptomic and proteomic analyses provide insights into the key genes involved in high-altitude adaptation in the Tibetan pig. Sci. Rep. 2017, 7, 3654. [Google Scholar] [CrossRef]
- Mi, S.; Shang, K.; Li, X.; Zhang, C.-H.; Liu, J.-Q.; Huang, D.-Q. Characterization and discrimination of selected China’s domestic pork using an LC-MS-based lipidomics approach. Food Control 2019, 100, 305–314. [Google Scholar] [CrossRef]
- Li, M.; Tian, S.; Jin, L.; Zhou, G.; Li, Y.; Zhang, Y.; Wang, T.; Yeung, C.K.L.; Chen, L.; Ma, J.; et al. Genomic analyses identify distinct patterns of selection in domesticated pigs and Tibetan wild boars. Nat. Genet. 2013, 45, 1431–1438. [Google Scholar] [CrossRef] [PubMed]
- Shang, P.; Li, W.; Tan, Z.; Zhang, J.; Dong, S.; Wang, K.; Chamba, Y. Population Genetic Analysis of Ten Geographically Isolated Tibetan Pig Populations. Animals 2020, 10, 1297. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Li, Y.; Yang, Y.; Xu, K.; Yang, L.; Qiao, S.; Pan, H. Effect of a Plateau Environment on the Oxidation State of the Heart and Liver through AMPK/p38 MAPK/Nrf2-ARE Signaling Pathways in Tibetan and DLY Pigs. Animals 2022, 12, 1219. [Google Scholar] [CrossRef]
- Mi, S.; Shang, K.; Jia, W.; Zhang, C.-H.; Liu, J.-Q.; Huang, D.-Q. Composition of chemical elements in the edible viscera of Tibetan pigs and its correlation with environment and feed. Food Res. Int. 2020, 129, 108832. [Google Scholar] [CrossRef]
- Shen, L.; Lei, H.; Zhang, S.; Li, X.; Li, M.; Jiang, X.; Zhu, K.; Zhu, L. The comparison of energy metabolism and meat quality among three pig breeds. Anim. Sci. J. 2014, 85, 770–779. [Google Scholar] [CrossRef]
- Gan, M.; Shen, L.; Fan, Y.; Guo, Z.; Liu, B.; Chen, L.; Tang, G.; Jiang, Y.; Li, X.; Zhang, S.; et al. High Altitude Adaptability and Meat Quality in Tibetan Pigs: A Reference for Local Pork Processing and Genetic Improvement. Animals 2019, 9, 1080. [Google Scholar] [CrossRef]
- Naya, D.E.; Veloso, C.; Sabat, P.; Bozinovic, F. Seasonal flexibility of organ mass and intestinal function for the Andean lizard Liolaemus nigroviridis. J. Exp. Zool. Part A Ecol. Genet. Physiol. 2009, 311A, 270–277. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, J.; Li, C.; Xu, H.; Chen, W.; Zeng, Y.; Wang, H. Molecular cloning, sequence characteristics, and tissue expression analysis of ECE1 gene in Tibetan pig. Gene 2015, 571, 237–244. [Google Scholar] [CrossRef]
- Deji, B.Z.; Shang, P.; Danzeng, W.J.; Zhang, H.; Qiangba, Y.Z. Expression and hypoxia adaptation analysis of the EPO gene in different tissues of plateau Tibetan pigs. Genet. Mol. Res. 2015, 14, 1700–1706. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, X.; Sun, W.; Cheng, C.; Chen, Y.; Zeng, K.; Chen, X.; Gu, Y.; Gao, R.; Liu, R.; et al. Comparison of liver microRNA transcriptomes of Tibetan and Yorkshire pigs by deep sequencing. Gene 2016, 577, 244–250. [Google Scholar] [CrossRef]
- Duan, M.; Wang, Z.; Guo, X.; Wang, K.; Liu, S.; Zhang, B.; Shang, P. Integrated analysis of transcriptomic and proteomic analyses reveals different metabolic patterns in the livers of Tibetan and Yorkshire pigs. Anim. Biosci. 2020, 34, 922–930. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Gao, Y.; Luo, Z.; Yang, L.; Chi, F.; Xiao, J.; Wang, W.; Geng, F. In-depth mapping of the proteome of Tibetan pig tenderloin (longissimus dorsi) using offline high-pH reversed-phase fractionation and LC-MS/MS. J. Food Biochem. 2019, 43, e13015. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xiao, J.; Liu, X.; Gao, Y.; Luo, Z.; Gu, X.; Zhang, J.; Wu, D.; Geng, F. Tandem mass tag-labeled quantitative proteomic analysis of tenderloins between Tibetan and Yorkshire pigs. Meat Sci. 2021, 172, 108343. [Google Scholar] [CrossRef]
- Ge, Q.-Y.; Cai, Y.; Chen, G.-S.; Jiang, T.-T.; Yang, Q.-L.; Huang, X.-Y.; Zhao, S.-G. The complete mitochondrial genome of the Qinghai Tibetan pig. Mitochondrial DNA Part B 2018, 3, 864–865. [Google Scholar] [CrossRef]
- Zhang, B.; Ban, D.; Gou, X.; Zhang, Y.; Yang, L.; Chamba, Y.; Zhang, H. Genome-wide DNA methylation profiles in Tibetan and Yorkshire pigs under high-altitude hypoxia. J. Anim. Sci. Biotechnol. 2019, 10, 25. [Google Scholar] [CrossRef] [PubMed]
- Schiffer, L.; Barnard, L.; Baranowski, E.S.; Gilligan, L.C.; Taylor, A.E.; Arlt, W.; Shackleton, C.H.L.; Storbeck, K.-H. Human steroid biosynthesis, metabolism and excretion are differentially reflected by serum and urine steroid metabolomes: A comprehensive review. J. Steroid Biochem. Mol. Biol. 2019, 194, 105439. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Papadopoulos, V.; Vihma, V. Steroid biosynthesis in adipose tissue. Steroids 2015, 103, 89–104. [Google Scholar] [CrossRef]
- Saloniemi, T.; Jokela, H.; Strauss, L.; Pakarinen, P.; Poutanen, M. The diversity of sex steroid action: Novel functions of hydroxysteroid (17β) dehydrogenases as revealed by genetically modified mouse models. J. Endocrinol. 2012, 212, 27–40. [Google Scholar] [CrossRef]
- Su, W.; Mao, Z.; Liu, Y.; Zhang, X.; Zhang, W.; Gustafsson, J.-A.; Guan, Y. Role of HSD17B13 in the liver physiology and pathophysiology. Mol. Cell. Endocrinol. 2019, 489, 119–125. [Google Scholar] [CrossRef]
- Nikolaou, N.; Gathercole, L.L.; Marchand, L.; Althari, S.; Dempster, N.J.; Green, C.J.; van de Bunt, M.; McNeil, C.; Arvaniti, A.; Hughes, B.A.; et al. AKR1D1 is a novel regulator of metabolic phenotype in human hepatocytes and is dysregulated in non-alcoholic fatty liver disease. Metabolism. 2019, 99, 67–80. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Hou, Y.; Liu, F.; Liu, A.; Jing, L.; Zhao, C.; Luan, Y.; Miao, Y.; Zhao, S.; Li, X. Transcriptome Analysis Reveals that Vitamin A Metabolism in the Liver Affects Feed Efficiency in Pigs. G3 Genes Genomes Genet. 2016, 6, 3615–3624. [Google Scholar] [CrossRef] [PubMed]
- Skupinska, K.; Misiewicz-Krzeminska, I.; Lubelska, K.; Kasprzycka-Guttman, T. The effect of isothiocyanates on CYP1A1 and CYP1A2 activities induced by polycyclic aromatic hydrocarbons in Mcf7 cells. Toxicol. Vitr. 2009, 23, 763–771. [Google Scholar] [CrossRef] [PubMed]
- Yao, M.; Dai, M.; Liu, Z.; Cui, W.; Li, D.; Zhang, H.; Li, J.; Liu, Y.; Yuan, Z. mRNA expression profiles of P450 3A enzymes in the liver and small intestine of the domestic pig. Res. Vet. Sci. 2012, 93, 360–365. [Google Scholar] [CrossRef]
- Burkina, V.; Rasmussen, M.K.; Oliinychenko, Y.; Zamaratskaia, G. Porcine cytochrome 2A19 and 2E1. Basic Clin. Pharmacol. Toxicol. 2019, 124, 32–39. [Google Scholar] [CrossRef]
- Chen, J.; Jiang, S.; Wang, J.; Renukuntla, J.; Sirimulla, S.; Chen, J. A comprehensive review of cytochrome P450 2E1 for xenobiotic metabolism. Drug Metab. Rev. 2019, 51, 178–195. [Google Scholar] [CrossRef]
- Hu, D.G.; Meech, R.; McKinnon, R.A.; Mackenzie, P.I. Transcriptional regulation of human UDP-glucuronosyltransferase genes. Drug Metab. Rev. 2014, 46, 421–458. [Google Scholar] [CrossRef]
- Prysyazhnyuk, P.; Dzuryak, V.; Buzdugan, I.; Bobkovych, K.; Prysiazhniuk, I.; Sydorchuk, R.; Sydorchuk, L.; Prysyazhnyuk, V. Glutathione-S-transferases genes-promising predictors of hepatic dysfunction. World J. Hepatol. 2021, 13, 620–633. [Google Scholar] [CrossRef]
- Pajaud, J.; Ribault, C.; Ben Mosbah, I.; Rauch, C.; Henderson, C.; Bellaud, P.; Aninat, C.; Loyer, P.; Morel, F.; Corlu, A. Glutathione transferases P1/P2 regulate the timing of signaling pathway activations and cell cycle progression during mouse liver regeneration. Cell Death Dis. 2015, 6, e1598. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Wang, X.; Liu, Y.; Fang, S.; Wu, Z.; Han, C.; Shi, W.; Bao, Y. Effects of early florfenicol exposure on glutathione signaling pathway and PPAR signaling pathway in chick liver. Ecotoxicol. Environ. Saf. 2022, 237, 113529. [Google Scholar] [CrossRef]
- Jing, X.P.; Wang, W.J.; Degen, A.A.; Guo, Y.M.; Kang, J.P.; Liu, P.P.; Ding, L.M.; Shang, Z.H.; Zhou, J.W.; Long, R.J. Energy substrate metabolism in skeletal muscle and liver when consuming diets of different energy levels: Comparison between Tibetan and Small-tailed Han sheep. Animal 2021, 15, 100162. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Du, M.; Zhang, J.; Liang, Z.; Ahmad, A.A.; Shen, J.; Salekdeh, G.H.; Ding, X. Transcriptomic and Metabolomic Analyses Reveal Inhibition of Hepatic Adipogenesis and Fat Catabolism in Yak for Adaptation to Forage Shortage During Cold Season. Front. Cell Dev. Biol. 2022, 9, 759521. [Google Scholar] [CrossRef] [PubMed]
- Peng, M.; Han, J.; Li, L.; Ma, H. Suppression of fat deposition in broiler chickens by (-)-hydroxycitric acid supplementation: A proteomics perspective. Sci. Rep. 2016, 6, 32580. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.H.; Kim, S.G. AMPK-Dependent Metabolic Regulation by PPAR Agonists. PPAR Res. 2010, 2010, e549101. [Google Scholar] [CrossRef] [PubMed]
- Falomir Lockhart, L.J.; Burgardt, N.I.; Ferreyra, R.G.; Ceolin, M.; Ermácora, M.R.; Córsico, B. Fatty Acid Transfer from Yarrowia lipolytica Sterol Carrier Protein 2 to Phospholipid Membranes. Biophys. J. 2009, 97, 248–256. [Google Scholar] [CrossRef]
- Jin, Y.; Tan, Y.; Chen, L.; Liu, Y.; Ren, Z. Reactive Oxygen Species Induces Lipid Droplet Accumulation in HepG2 Cells by Increasing Perilipin 2 Expression. Int. J. Mol. Sci. 2018, 19, 3445. [Google Scholar] [CrossRef]
- Li, H.; Song, Y.; Zhang, L.-J.; Gu, Y.; Li, F.-F.; Pan, S.-Y.; Jiang, L.-N.; Liu, F.; Ye, J.; Li, Q. LSDP5 Enhances Triglyceride Storage in Hepatocytes by Influencing Lipolysis and Fatty Acid β-Oxidation of Lipid Droplets. PLoS ONE 2012, 7, e36712. [Google Scholar] [CrossRef]
- Kong, Z.; Zhou, C.; Li, B.; Jiao, J.; Chen, L.; Ren, A.; Jie, H.; Tan, Z. Integrative plasma proteomic and microRNA analysis of Jersey cattle in response to high-altitude hypoxia. J. Dairy Sci. 2019, 102, 4606–4618. [Google Scholar] [CrossRef]
- Mishra, K.P.; Ganju, L. Influence of high altitude exposure on the immune system: A review. Immunol. Invest. 2010, 39, 219–234. [Google Scholar] [CrossRef]
- Xu, J.; Cai, Z.; Liu, Y.; Wang, C.; Fu, W.; Wang, H.; Wang, W.; Zhang, Q. Expression Profile and Porcin DQB1 Gene Association Analysis of the with Peripheral Blood T Lymphocyte Subsets. Ital. J. Anim. Sci. 2015, 14, 3864. [Google Scholar] [CrossRef]
- Bao, W.; Ye, L.; Zi, C.; Liu, L.; Zhu, J.; Pan, Z.; Zhu, G.; Huang, X.; Wu, S. Relationship between the expression level of SLA-DQA and Escherichia coli F18 infection in piglets. Gene 2012, 494, 140–144. [Google Scholar] [CrossRef] [PubMed]
- Zhai, T.; Wu, C.; Wang, N.; Shi, B.; Li, J.; Chen, R.; Dong, J.; Zhang, Y.; Zhou, E.-M.; Nan, Y. Development of a monoclonal antibody against swine leukocyte antigen (SLA)-DR α chain and evaluation of SLA-DR expression in bone marrow-derived dendritic cells after PRRSV infection. Vet. Immunol. Immunopathol. 2019, 211, 19–24. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Y.; Liu, B.; He, Y.; Li, Z.; Zhao, Q.; Nan, Y.; Wu, C. Porcine Epidemic Diarrhea Virus Envelope Protein Blocks SLA-DR Expression in Barrow-Derived Dendritic Cells by Inhibiting Promoters Activation. Front. Immunol. 2021, 12, 741425. [Google Scholar] [CrossRef] [PubMed]
- Qin, M.; Chen, W.; Li, Z.; Wang, L.; Ma, L.; Geng, J.; Zhang, Y.; Zhao, J.; Zeng, Y. Role of IFNLR1 gene in PRRSV infection of PAM cells. J. Vet. Sci. 2021, 22, e39. [Google Scholar] [CrossRef]
- Aguilar-Melero, P.; Luque, A.; Machuca, M.M.; Pérez de Obanos, M.P.; Navarrete, R.; Rodríguez-García, I.C.; Briceño, J.; Iñiguez, M.; Ruiz, J.; Prieto, J.; et al. Cardiotrophin-1 reduces ischemia/reperfusion injury during liver transplant. J. Surg. Res. 2013, 181, e83–e91. [Google Scholar] [CrossRef]
- Li, J.; Andreyev, O.; Chen, M.; Marco, M.; Iwase, H.; Long, C.; Ayares, D.; Shen, Z.; Cooper, D.K.C.; Ezzelarab, M.B. Human T cells upregulate CD69 after coculture with xenogeneic genetically-modified pig mesenchymal stromal cells. Cell. Immunol. 2013, 285, 23–30. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J.Q.; Shi, Y.Q.; Ye, H.L.; Luo, W.; Geng, F. Quantitative proteomic analyses during formation of chicken egg yolk. Food Chem. 2022, 374, 131828. [Google Scholar] [CrossRef]
- Wang, J.; Xiao, J.; Liu, X.; Geng, F.; Huang, Q.; Zhao, J.; Xiang, D.; Zhao, G. Analysis of tartary buckwheat (Fagopyrum tataricum) seed proteome using offline two-dimensional liquid chromatography and tandem mass spectrometry. J. Food Biochem. 2019, 43, e12863. [Google Scholar] [CrossRef]
- Yang, R.; Geng, F.; Huang, X.; Qiu, N.; Li, S.; Teng, H.; Chen, L.; Song, H.; Huang, Q. Integrated proteomic, phosphoproteomic and N-glycoproteomic analyses of chicken eggshell matrix. Food Chem. 2020, 330, 127167. [Google Scholar] [CrossRef]
- Wang, J.; Xiao, J.; Geng, F.; Li, X.; Yu, J.; Zhang, Y.; Chen, Y.; Liu, D. Metabolic and proteomic analysis of morel fruiting body (Morchella importuna). J. Food Compos. Anal. 2019, 76, 51–57. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, X.; Chang, X.; Yang, L.; Chamba, Y.; Geng, F. Quantitative Proteomic Analysis of Tibetan Pig Livers at Different Altitudes. Molecules 2023, 28, 1694. https://doi.org/10.3390/molecules28041694
Gu X, Chang X, Yang L, Chamba Y, Geng F. Quantitative Proteomic Analysis of Tibetan Pig Livers at Different Altitudes. Molecules. 2023; 28(4):1694. https://doi.org/10.3390/molecules28041694
Chicago/Turabian StyleGu, Xuedong, Xinping Chang, Lin Yang, Yangzom Chamba, and Fang Geng. 2023. "Quantitative Proteomic Analysis of Tibetan Pig Livers at Different Altitudes" Molecules 28, no. 4: 1694. https://doi.org/10.3390/molecules28041694
APA StyleGu, X., Chang, X., Yang, L., Chamba, Y., & Geng, F. (2023). Quantitative Proteomic Analysis of Tibetan Pig Livers at Different Altitudes. Molecules, 28(4), 1694. https://doi.org/10.3390/molecules28041694