Discovery of Anti-Inflammatory Triterpenoid Glucosides from the Heritiera littoralis Dryand
Abstract
:1. Introduction
2. Results and Discussion
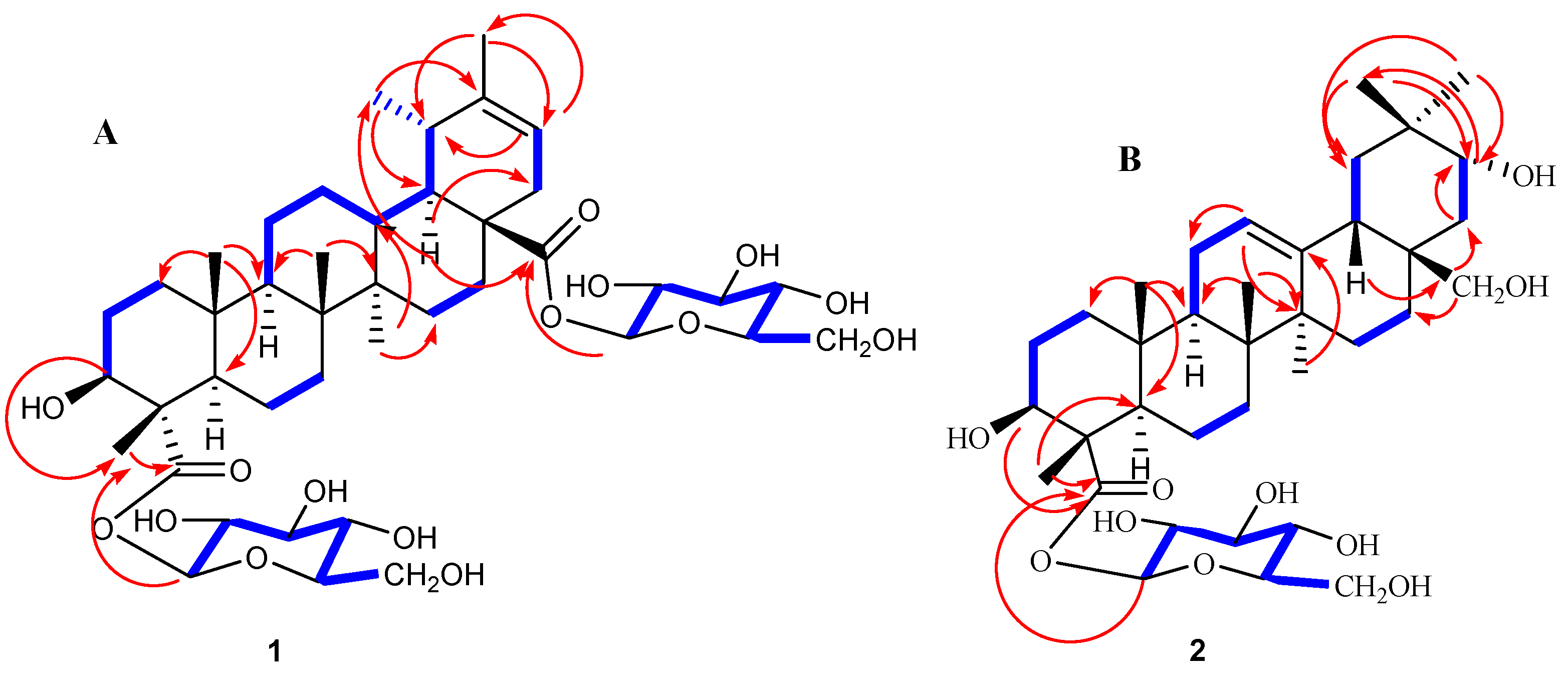
2.1. Elucidation of the Chemical Structure of Heritiera A (1) and Heritiera B (2)
2.2. Anti-Inflammatory Assay of the Isolates
2.3. Similarities and Differences of Some Triterpenoids and Their Anti-Inflammatory Activity from Malvaceae
3. Experimental Section
3.1. General Experimental Procedures
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Characterization of the Isolates
3.5. Enzymatic Hydrolysis of Compounds 1–2
3.6. Cell Viability and Anti-Inflammatory Activity Test [41,42]
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Medzhitov, R. Origin and physiological roles of inflammation. Nature 2008, 454, 428–435. [Google Scholar]
- Antonelli, M.; Kushner, I. It’s time to redefine inflammation. FASEB J. 2017, 31, 1787–1791. [Google Scholar]
- Gomes, A.; Fernandes, E.; Lima, J.L.; Mira, L.; Corvo, M.L. Molecular mechanisms of anti-inflammatory activity mediated by flavonoids. Curr. Med. Chem. 2008, 15, 1586–1605. [Google Scholar]
- Zamora, R.; Vodovotz, Y.; Billiar, T.R. Inducible nitric oxide synthase and inflammatory diseases. Mol. Med. 2000, 6, 347–373. [Google Scholar]
- Pereira-Leite, C.; Nunes, C.; Jamal, S.K.; Cuccovia, I.M.; Reis, S. Nonsteroidal anti-inflammatory therapy: A journey toward safety. Med. Res. Rev. 2017, 37, 802–859. [Google Scholar] [CrossRef]
- Prakash, V. Terpenoids as source of anti-inflammatory compounds. Asian J. Pharm. Clin. Res. 2017, 10, 68–76. [Google Scholar] [CrossRef]
- Kuo, P.C.; Tai, S.H.; Hung, C.C.; Hwang, T.L.; Kuo, L.M.; Lam, S.H.; Cheng, K.C.; Kuo, D.H.; Hung, H.Y.; Wu, T.S. Antiinflammatory triterpenoids from the fruiting bodies of Fomitopsis pinicola. Bioorg. Chem. 2021, 108, 104562. [Google Scholar]
- Kuang, Y.; Li, B.; Wang, Z.L.; Qiao, X.; Ye, M. Terpenoids from the medicinal mushroom Antrodia camphorata: Chemistry and medicinal potential. Nat. Prod. Rep. 2021, 38, 83–102. [Google Scholar]
- Lim, H.J.; Jang, H.J.; Kim, M.H.; Lee, S.; Lee, S.W.; Rho, M.C. Oleanolic acid acetate exerts anti-inflammatory activity via IKKα/β suppression in TLR3-mediated NF-κB activation. Molecules 2019, 24, 4002. [Google Scholar] [CrossRef]
- Honda, T.; Finlay, H.J.; Gribble, G.W.; Suh, N.; Sporn, M.B. New enone derivatives of oleanolic acid and ursolic acid as inhibitors of nitric oxide production in mouse macrophages. Bioorg. Med. Chem. Lett. 1997, 7, 1623–1628. [Google Scholar] [CrossRef]
- Silva, M.L.; David, J.P.; Silva, L.C.R.C.; Santos, R.A.F.; David, J.M.; Lima, L.; Reis, P.S.; Fontana, R. Bioactive oleanane, lupane and ursane triterpene acid derivatives. Molecules 2012, 17, 12197–12205. [Google Scholar] [CrossRef]
- Editorial Committee of Flora of China. Flora of China; Science Press: Beijing, China, 1984; Volume 49, pp. 39–40. [Google Scholar]
- Lin, P. Medicinal plants of mangrove in China. J. Mar. Drugs 1984, 12, 45–51. [Google Scholar]
- Shao, C.L.; Fu, X.M.; Wang, C.Y.; Han, L.; Fang, Y.C.; Li, G.Q.; Zeng, X.Q.; Liu, G.X.; Guan, H.S. Investigation on the status of mangrove resources and medicinal research in China III;. Status of folk medicinal usage and medicinal research. J. Ocean Univ. Chin. 2009, 39, 712–718. [Google Scholar]
- Du, Q.; Wei, W.M.; Mi, D.Q. Knowledge and existing status of medicinal ethnobotany of mangrove among Jing People in Guangxi. Guihaia 2016, 36, 405–412. [Google Scholar]
- Ning, X.Q.; Li, J.F.; Huang, Y.; Tan, Y.F.; Deng, J.G. Study on the species of medicinal mangroves and their folk medicinal efficacy in Guangxi. Guide Chin. Med. 2013, 11, 73–75. [Google Scholar]
- Fan, H.Q.; Liang, S.C. (Eds.) Research and Management on China Mangroves; Science Press: Beijing, China, 1995; pp. 164–172. [Google Scholar]
- Cui, J.G.; Lu, Y.; Huang, Y.M. Review on bioactive substances from mangrove. Nat. Prod. Res. Dev. 2017, 29, 1626–1633. [Google Scholar]
- Ge, L.; Li, Y.J.; Yang, K.D. Chemical constituents of the leaves of Heritiera littoralis. Chem. Nat. Compd. 2016, 52, 603–604. [Google Scholar] [CrossRef]
- Miles, D.H.; Vallapa, C.W. Toxicants from mangrove plants, VII. Vallapin and vallapianin, novel sesquiterpene lactones from the mangrove plant Heritiera littoralis. J. Nat. Prod. 1991, 54, 286–289. [Google Scholar]
- Takeda, Y.; Miyazaki, K.; Shimizu, H.; Masuda, T.; Otsuka, H. A new phenylpropanoid-glycerol conjugate from Heritiera littoralis Dryand. Nat. Med. 2000, 54, 22–25. [Google Scholar]
- Tian, Y.; Wu, J.; Xi, S.H.; Zhang, D.J.; Xu, L.R.; Zhang, S. Studies on the triterpenoid components of Heritiera littoralis. Chin. Trad. Herb. Drugs 2007, 3, 35–36. [Google Scholar]
- Tian, Y.; Wu, J.; Zhang, S. Flavonoids from leaves of Heritiera littoralis. J. Chin. Pharm. Sci. 2004, 13, 214–216. [Google Scholar]
- Christopher, R.; Nyandoro, S.S.; Chacha, M.; De Koning, C.B. A new cinnamoylglycoflavonid antimycobacterial and antioxidant constituents from Heritiera littoralis leaf extracts. Nat. Prod. Res. 2014, 28, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.J.; Wang, K.; Chen, H.C.; He, R.J.; Cai, R.L.; Li, J.; Zhou, D.X.; Liu, W.; Huang, X.S.; Yang, R.Y.; et al. Anti-inflammatory lignans and phenylethanoid glycosides from the root of Isodon ternifolius (D.Don) Kudô. Phytochemistry 2018, 153, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Ansari, A.A.; Kenne, L.; Atta-ur-Rahman. Isolation and characterization of two saponins from Fagonia indica. Phytochemistry 1987, 26, 1487–1490. [Google Scholar] [CrossRef]
- Kanwal, N.; Adhikari, A.; Hameed, A.; Hafizur, R.M.; Musharraf, S.G. Isolation and characterization of non-sulfated and sulfated triterpenoid saponins from Fagonia indica. Phytochemistry 2017, 143, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Khalik, S.A.; Miyase, T.; El-Ashaal, H.A.; Melek, F. Triterpenoid saponins from Fagonia cretica. Phytochemistry 2000, 54, 853–859. [Google Scholar] [PubMed]
- Xue, H.Z.; Lu, Z.Z.; Konno, C.; Soejarto, D.D.; Cordell, G.A.; Fong, H.H.G.; Hodgson, W. β-(3,4-dihydroxycinnamoyl)-erythrodiol and 3β-(4-hydroxycinnamoyl)-erythrodiol from Larrea tridentate. Phytochemistry 1988, 27, 233–235. [Google Scholar]
- Tan, J.Y.; Cheng, Y.G.; Li, J.L.; Ren, H.Q.; Huang, Y.R.; Qiao, Y.B.; Li, Q.S.; Wang, Y.L. New taraxasterane-type triterpenes from Diaphragma juglandis Fructus. Tetrahedron Lett. 2022, 100, 153868. [Google Scholar]
- Adnyana, I.K.; Tezuka, Y.; Banskota, A.H.; Xiong, Q.B.; Tran, K.Q.; Kadota, S. Quadranosides I-V, new triterpenoid glucosides from the seeds of Combretum quadrangulare. J. Nat. Prod. 2000, 63, 496–500. [Google Scholar]
- Yang, H.M.; Yin, Z.Q.; Zha, M.G.; Jiang, C.H.; Pan, K. Pentacyclic triterpenoids from Cyclocarya paliurus and their antioxidant activities in FFA-induced HepG2 steatosis cells. Phytochemistry 2018, 151, 119–127. [Google Scholar] [CrossRef]
- Bisoli, E.; Garcez, W.S.; Hamerski, L.; Tieppo, G.; Garcez, F.R. Bioactive pentacyclic triterpenoids from the stems of Combretum laxum. Molecules 2008, 13, 2717–2728. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.J.; Jeong, E.J.; Kim, J.W.; Sung, S.H.; Kim, Y.C. Antiproliferative triterpenes from the leaves and twigs of Juglans sinensis on HSC-T6 cells. J. Nat. Prod. 2011, 74, 751–756. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Kong, L.Y.; Zhang, H.Q.; He, S.A. Triterpenes from Angelica cartilagino–marginata var. foliata Yuan et Shan and their significance as chemosystematic characters. Biochem. Syst. Ecol. 2005, 33, 1121–1129. [Google Scholar]
- Mongalo, N.I.; McGaw, L.J.; Finnie, J.F.; Van Staden, J. Isolation and characterization of antimicrobial and anti-inflflammatory triterpenoids from the acetone extract of Grewia flflava DC. (Malvaceae) Roots. S. Afr. J. Bot. 2022, 149, 87–95. [Google Scholar] [CrossRef]
- Araújo, C.R.R.; Silva, T.M.; Santos, M.G.; Ottoni, M.H.F.; Fagundes, E.M.S.; Fontoura, H.S.G.; Melo, E.B.A.; Alcântara, A.F.C. Anti-inflammatory and cytotoxic activities of the extracts, fractions, and chemical constituents isolated from Luehea ochrophylla Mart. BMC Complem. Altern. M. 2019, 19, 284. [Google Scholar]
- Benincá, J.P.; Dalmarco, J.B.; Pizzolatti, M.G.; Frode, T.S. Analysis of the antiinflammatory properties of Rosmarinus officinalis L. in mice. Food Chem. 2011, 124, 468–475. [Google Scholar] [CrossRef]
- Tsai, J.C.; Peng, W.H.; Chiu, T.H.; Lai, S.C.; Lee, C.Y. Anti-inflammatory effects of Scoparia dulcis L. and betulinic acid. Am. J. Chin. Med. 2011, 39, 943–956. [Google Scholar]
- Shakurova, É.R.; Parfenova, T.I.; Sufiyarova, R.S.; Khalilova, A.Z.; Akhmetova, V.R.; Bashkatov, A.S. Synthesis and anti-inflammatory activity of acyl derivatives of taraxasterol. Pharm. Chem. J. 2008, 42, 319–321. [Google Scholar] [CrossRef]
- Zhang, R.; Rupa, E.J.; Zheng, S.; Nahar, J.; Yang, D.C.; Kang, S.C.; Wang, Y. Panos-fermented extract-mediated nanoemulsion: Preparation, characterization, and in vitro anti-inflammatory effects on RAW 264.7 cells. Molecules 2022, 27, 218. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Huang, X.S.; Chen, H.C.; Zhou, D.X.; Yang, Z.M.; Wang, K.; Liu, W.; Deng, S.P.; Yang, R.Y.; Li, J.; et al. Discovery of anti-inflammatory terpenoids from Mallotus conspurcatus Croizat. J. Ethnopharmacol. 2019, 231, 170–178. [Google Scholar]


| 1 | 2 | |||
|---|---|---|---|---|
| Positition | δH (J = Hz) | δC (DEPT) | δH (J = Hz) | δC (DEPT) |
| 1 | 1.70, m; 1.20, m | 39.7, CH2 | 1.54, m | 38.5, CH2 |
| 2 | 1.96, m; 1.72, m | 28.2, CH2 | 1.89, m | 27.8, CH2 |
| 3 | 4.71, m | 75.7, CH | 4.64, dd, (10.6, 5.9) | 75.5, CH |
| 4 | 55.3, C | 55.5, C | ||
| 5 | 1.88, m | 52.8, CH | 1.90, m | 52.4, CH |
| 6 | 1.48, m | 21.9, CH2 | 1.48, m; 1.65, m | 18.3, CH2 |
| 7 | 1.52, m; 1.26 m | 22.3, CH2 | 1.72 m; 1.64, m | 22.1, CH2 |
| 8 | 42.6, C | 40.1, C | ||
| 9 | 1.46, m | 51.4, CH | 1.49, m | 49.8, CH |
| 10 | 37.3, C | 38.0, C | ||
| 11 | 1.74, m | 34.7, CH2 | 2.61, dd, (14.8, 8.1); 1.88, m | 31.6, CH2 |
| 12 | 1.95, m; 1.16, m | 28.2, CH2 | 5.45, d, (8.0) | 117.6, CH |
| 13 | 2.67, m | 39.8, CH | 158.5, C | |
| 14 | 42.1, C | 38.1, C | ||
| 15 | 2.00, m; 1.04, m | 29.8, CH2 | 1.47, m; 1.58, m | 34.5, CH2 |
| 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 1′ 2′ 3′ 4′ 5′ 6′ 1″ 2″ 3″ 4″ 5″ 6′ | 2.44 m 1.29, m 2.55, d, (12.5) 5.35, d, (7.2) 2.40, m; 2.03, m 1.59, s 0.86, s 1.11, s 0.86, s 1.05, s 1.67, s 6.50, d, (8.2) 4.25, m 4.25, m 4.38, m 3.88, dd, (6.6, 3.2) 4.39, m 6.40, d, (8.2) 4.14, m 4.32, m 4.38, m 4.05, m 4.39, m | 33.7, CH2 49.8, C 49.7, CH 38.1, CH 143.6, C 117.8, CH 38.0, CH2 177.8, C 12.2, CH3 17.5, CH3 16.6, CH3 15.5, CH3 174.8, C 24.0, CH3 22.7, CH3 96.9, CH 74.9, CH 79.4, CH 71.5, CH 79.7, CH 62.6, CH2 95.7, CH 74.9, CH 79.3, CH 71.4, CH 80.0, CH 62.6, CH2 | 1.43, m; 1.80, m 0.75, dd, (13.4, 3.7) 1.81, m; 1.25, m 4.15, dd, (12.5, 3.5) 1.65, m; 2.51, m 1.60, s 0.92, s 1.00, s 0.96, s 3.63, m; 3.49, m 1.20, s 1.34, s 6.40, d, (8.0) 4.23, m 4.28, m 4.29, m 4.04, br s 4.40, d, (6.5); 4.32, dd, (11.8, 4.6) | 41.6, CH2 42.8, C 45.3, CH 38.7, CH2 35.1, C 73.1, CH 38.5, CH2 178.1, C 12.5, CH3 16.5, CH3 26.8, CH3 22.7, CH3 65.5, CH2 29.2, CH3 26.3, CH3 96.9, CH 74.7, CH 78.9, CH 71.5, CH 79.8, CH 62.5, CH2 |
| Compounds | IC50 (μM) |
|---|---|
| 1 | 32.11 ± 0.62 |
| 2 | 10.33 ± 0.43 |
| 3 | 39.32 ± 0.75 |
| 4 | 29.98 ± 0.42 |
| 5 | >50 |
| 6 | >50 |
| 7 | >50 |
| 8 | >50 |
| Dexamethasone b | 6.39 ± 0.64 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, X.; Niu, P.; Li, J.; Guan, X.; Zhang, Y.; Li, J. Discovery of Anti-Inflammatory Triterpenoid Glucosides from the Heritiera littoralis Dryand. Molecules 2023, 28, 1658. https://doi.org/10.3390/molecules28041658
Liang X, Niu P, Li J, Guan X, Zhang Y, Li J. Discovery of Anti-Inflammatory Triterpenoid Glucosides from the Heritiera littoralis Dryand. Molecules. 2023; 28(4):1658. https://doi.org/10.3390/molecules28041658
Chicago/Turabian StyleLiang, Xiaoqin, Peng Niu, Jun Li, Xinlan Guan, Yanjun Zhang, and Jian Li. 2023. "Discovery of Anti-Inflammatory Triterpenoid Glucosides from the Heritiera littoralis Dryand" Molecules 28, no. 4: 1658. https://doi.org/10.3390/molecules28041658
APA StyleLiang, X., Niu, P., Li, J., Guan, X., Zhang, Y., & Li, J. (2023). Discovery of Anti-Inflammatory Triterpenoid Glucosides from the Heritiera littoralis Dryand. Molecules, 28(4), 1658. https://doi.org/10.3390/molecules28041658







