The Gemstone Cyborg: How Diamond Films Are Creating New Platforms for Cell Regeneration and Biointerfacing
Abstract
1. Introduction
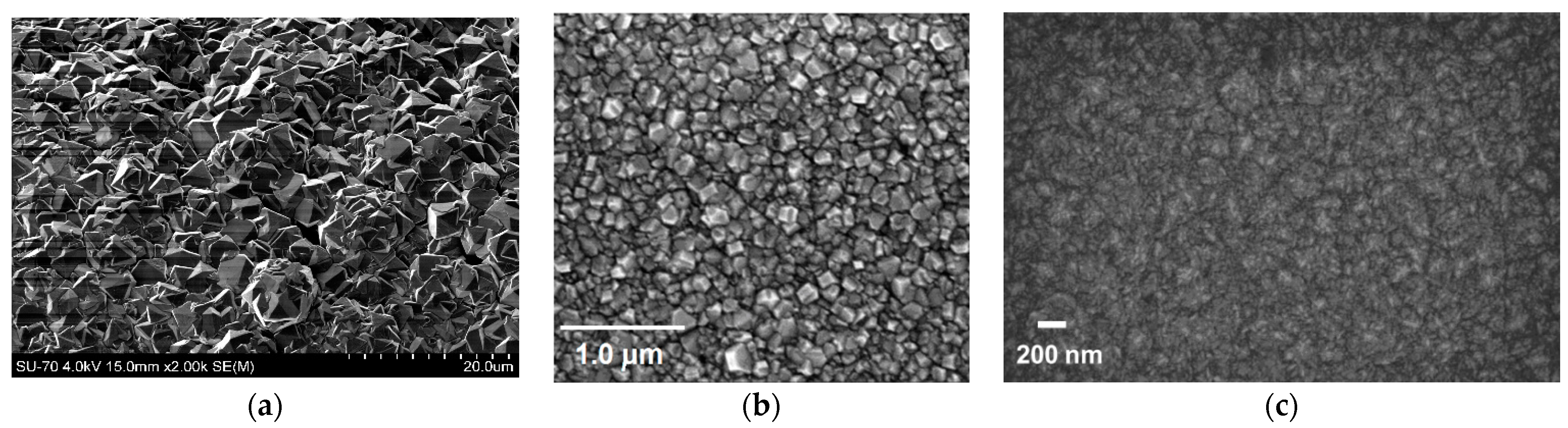
1.1. Growth of Diamond by Chemical Vapour Deposition
2. Diamond as a Substrate for Cell Culture
2.1. Fibroblasts
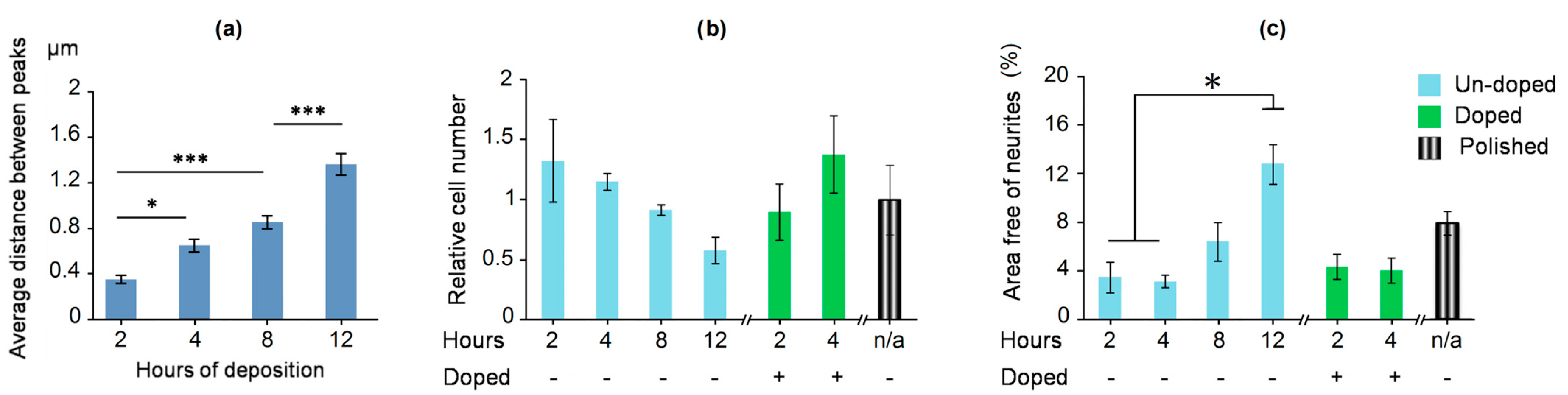
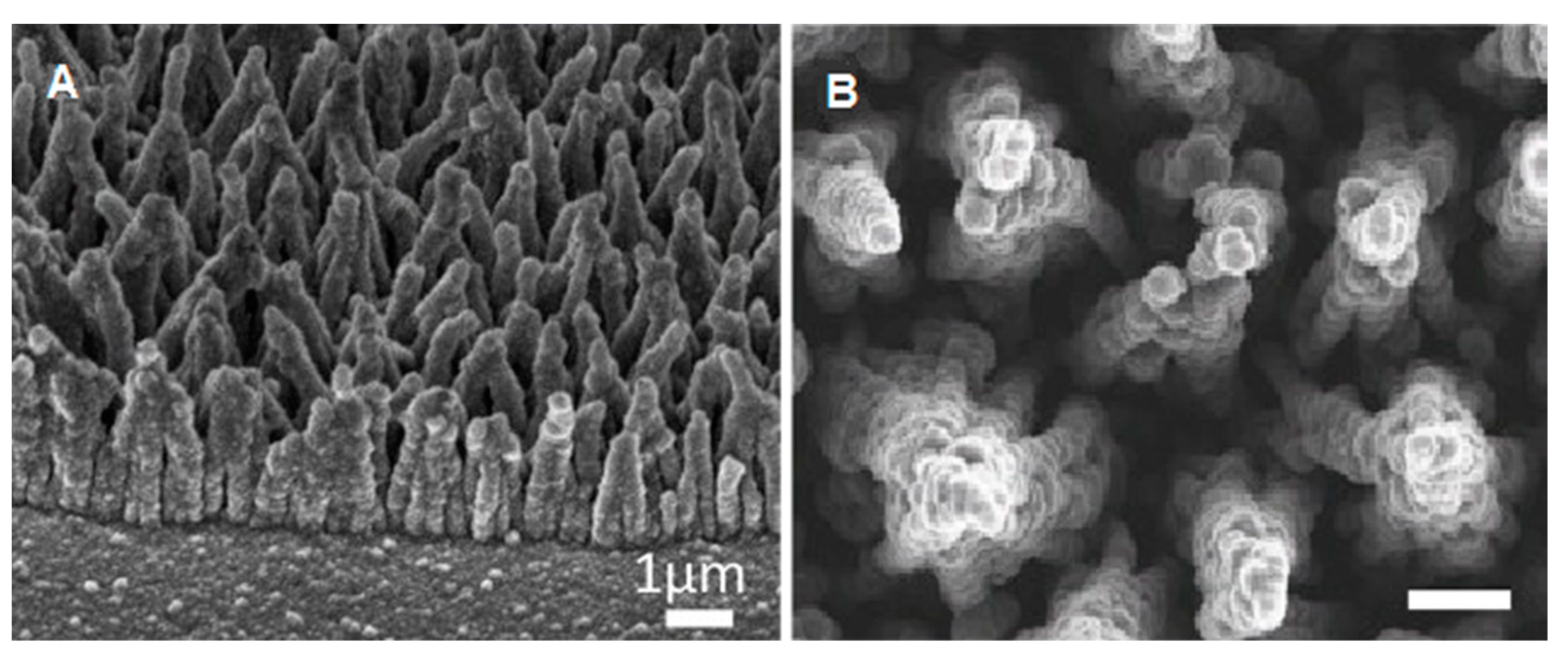
2.1.1. Growth of Fibroblasts on UNCD Substrates
2.1.2. Growth of Fibroblasts on Large-Grain NCD Substrates
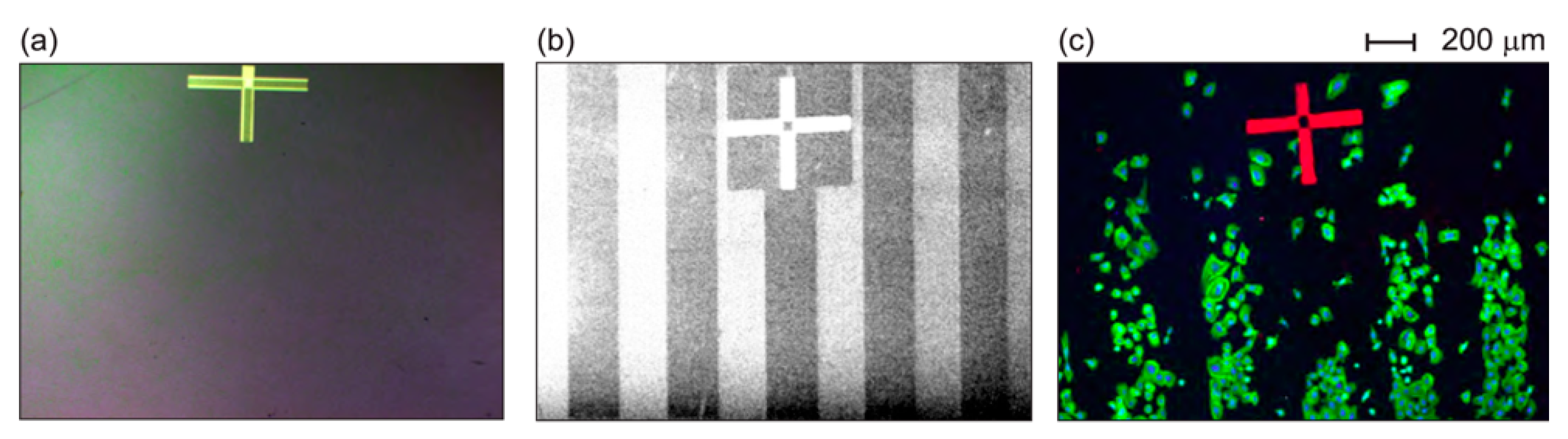
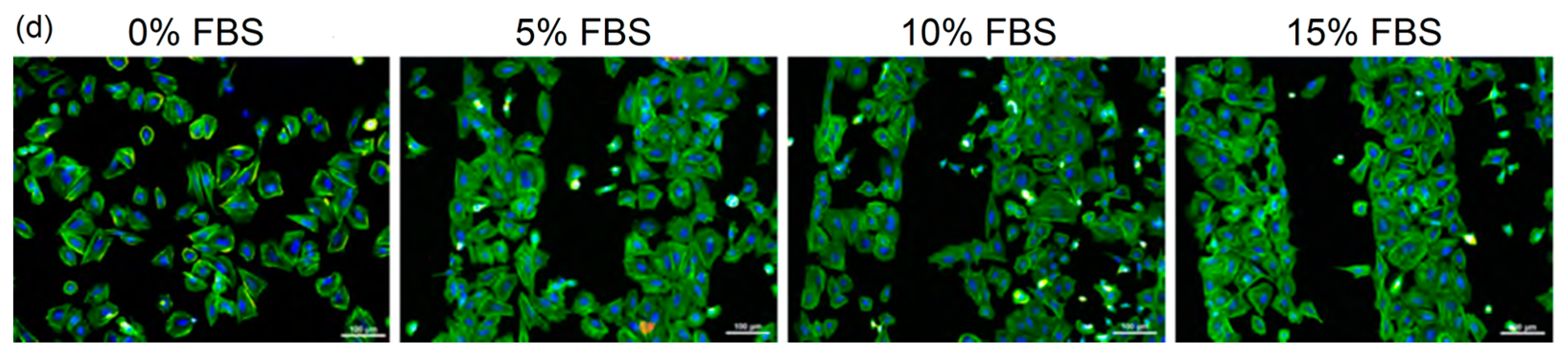
2.2. Kidney Epithelial Cells
2.3. Osteoblasts and Osteoblast-like Cells
NCD as Coating for Prosthetic Implants
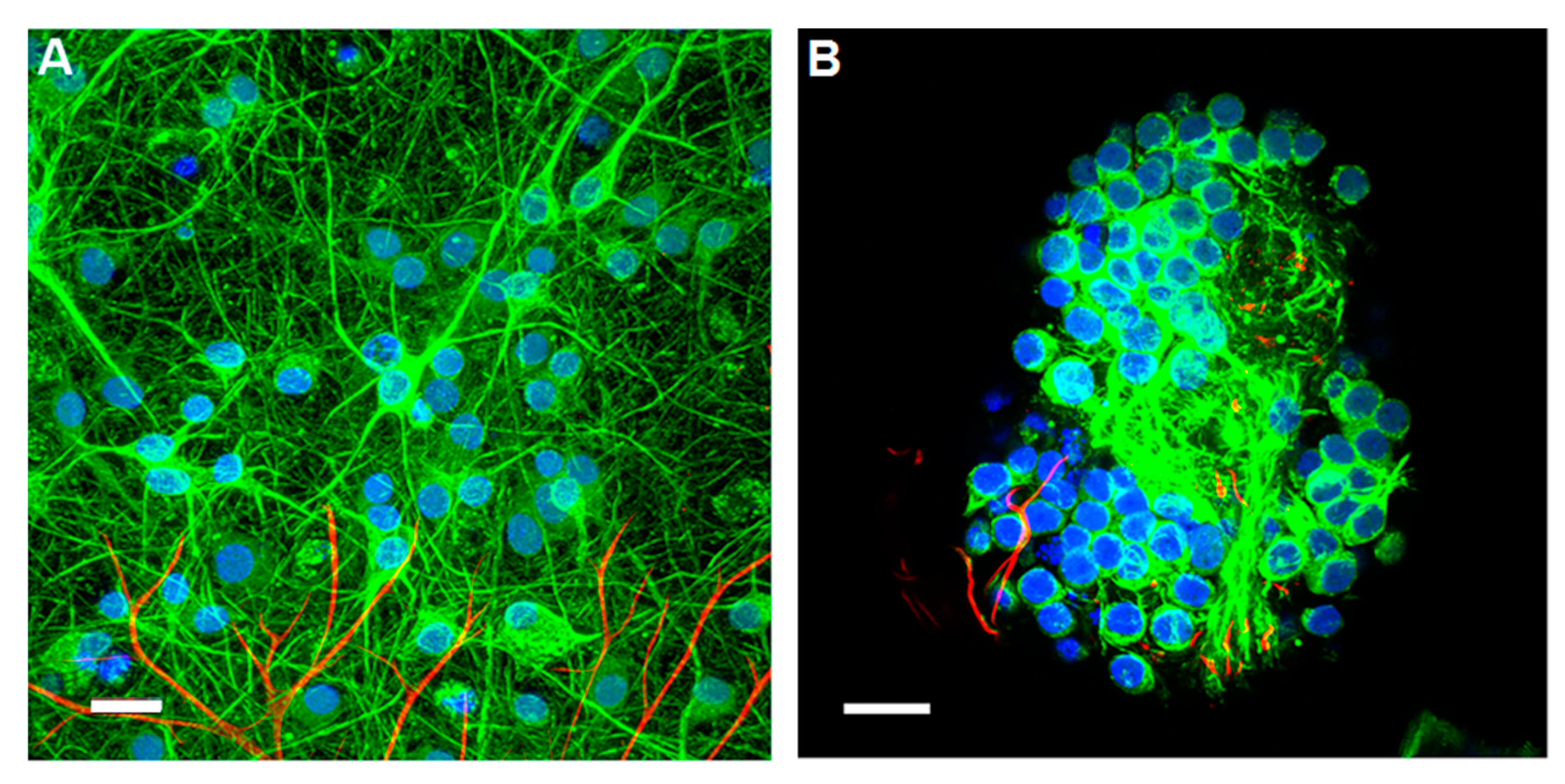
2.4. Neural Stem Cells and Neurons
2.4.1. Stem Cells Differentiation with UNCD Films
2.4.2. Culture of Differentiated Neural Cells on Diamond Surfaces
3. Diamond as a Biomedical Tool for Biointerfacing
3.1. “Hello, Is There Anybody out There?”—Cell Signal Reception with Diamond-Based Devices
3.2. Diamond-Based Retinal Prostesis for Artificial Vision
4. Discussion
5. Conclusions and Future Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nistor, P.A.; May, P.W. Diamond thin films: Giving biomedical applications a new shine. J. R. Soc. Interface 2017, 14, 20170382. [Google Scholar] [CrossRef]
- Raymakers, J.; Haenen, K.; Maes, W. Diamond surface functionalization: From gemstone to photoelectrochemical applications. J. Mater. Chem. C 2019, 7, 10134–10165. [Google Scholar] [CrossRef]
- Kawarada, H. Hydrogen-terminated diamond surfaces and interfaces. Surf. Sci. Rep. 1996, 26, 205–259. [Google Scholar] [CrossRef]
- Santos, N.E.; Figueira, F.; Neto, M.; Paz, F.A.A.; Braga, S.S.; Mendes, J.C. Diamonds for life: Developments in sensors for biomolecules. Appl. Sci. 2022, 12, 3000. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Lee, D.-C.; Hsiao, C.-Y.; Chung, Y.-F.; Chen, H.-C.; Thomas, J.P.; Pong, W.-F.; Tai, N.-H.; Lin, I.-N.; Chiu, I.-M. The effect of ultra-nanocrystalline diamond films on the proliferation and differentiation of neural stem cells. Biomaterials 2009, 30, 3428–3435. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Lee, D.-C.; Tsai, T.-Y.; Hsiao, C.-Y.; Liu, J.-W.; Kao, C.-Y.; Lin, H.-K.; Chen, H.-C.; Palathinkal, T.J.; Pong, W.-F.; et al. Induction and regulation of differentiation in neural stem cells on ultra-nanocrystalline diamond films. Biomaterials 2010, 31, 5575–5587. [Google Scholar] [CrossRef]
- Vickers, N.J. Animal communication: When I’m calling you, will you answer too? Curr. Biol. 2017, 27, R713–R715. [Google Scholar] [CrossRef]
- May, P.W.; Regan, E.M.; Taylor, A.; Uney, J.; Dick, A.D.; McGeehan, J. Spatially controlling neuronal adhesion on CVD diamond. Diam. Relat. Mater. 2012, 23, 100–104. [Google Scholar] [CrossRef]
- Specht, C.G.; Williams, O.A.; Jackman, R.B.; Schoepfer, R. Ordered growth of neurons on diamond. Biomaterials 2004, 25, 4073–4078. [Google Scholar] [CrossRef]
- Ariano, P.; Budnyk, O.; Dalmazzo, S.; Lovisolo, D.; Manfredotti, C.; Rivolo, P.; Vittone, E. On diamond surface properties and interactions with neurons. Eur. Phys. J. E 2009, 30, 149–156. [Google Scholar] [CrossRef]
- Shi, B.; Jin, Q.; Chen, L.; Woods, A.S.; Schultz, A.J.; Auciello, O. Cell growth on different types of ultrananocrystalline diamond thin films. J. Funct. Biomater. 2012, 3, 588–600. [Google Scholar] [CrossRef]
- Chong, K.F.; Loh, K.P.; Vedula, S.R.K.; Lim, C.T.; Sternschulte, H.; Steinmüller, D.; Sheu, F.-S.; Zhong, Y.L. Cell adhesion properties on photochemically functionalized diamond. Langmuir 2007, 23, 5615–5621. [Google Scholar] [CrossRef]
- Tong, W.; Tran, P.A.; Turnley, A.M.; Aramesh, M.; Prawer, S.; Brandt, M.; Fox, K. The influence of sterilization on nitrogen-included ultrananocrystalline diamond for biomedical applications. Mater. Sci. Eng. C 2016, 61, 324–332. [Google Scholar] [CrossRef]
- Amaral, M.; Gomes, P.S.; Lopes, M.A.; Santos, J.D.; Silva, R.F.; Fernandes, M.H. Cytotoxicity evaluation of nanocrystalline diamond coatings by fibroblast cell cultures. Acta Biomater. 2009, 5, 755–763. [Google Scholar] [CrossRef]
- Rezek, B.; Michalíková, L.; Ukraintsev, E.; Kromka, A.; Kalbacova, M. Micro-pattern guided adhesion of osteoblasts on diamond surfaces. Sensors 2009, 9, 3549–3562. [Google Scholar] [CrossRef]
- Grausova, L.; Kromka, A.; Burdikova, Z.; Eckhardt, A.; Rezek, B.; Vacik, J.; Haenen, K.; Lisa, V.; Bacakova, L. Enhanced growth and osteogenic differentiation of human osteoblast-like cells on boron-doped nanocrystalline diamond thin films. PLoS ONE 2011, 6, e20943. [Google Scholar] [CrossRef]
- Amaral, M.; Dias, A.G.; Gomes, P.S.; Lopes, M.A.; Silva, R.F.; Santos, J.D.; Fernandes, M.H. Nanocrystalline diamond: In vitro biocompatibility assessment by MG63 and human bone marrow cells cultures. J. Biomed. Mater. Res. A 2008, 87A, 91–99. [Google Scholar] [CrossRef]
- Lechleitner, T.; Klauser, F.; Seppi, T.; Lechner, J.; Jennings, P.; Perco, P.; Mayer, B.; Steinmüller-Nethl, D.; Preiner, J.; Hinterdorfer, P.; et al. The surface properties of nanocrystalline diamond and nanoparticulate diamond powder and their suitability as cell growth support surfaces. Biomaterials 2008, 29, 4275–4284. [Google Scholar] [CrossRef]
- Yang, K.-H.; Narayan, R.J. Biocompatibility and functionalization of diamond for neural applications. Curr. Opin. Biomed. Eng. 2019, 10, 60–68. [Google Scholar] [CrossRef]
- Van der Drift, A. Evolutionary selection, a principle governing growth orientation in vapour-deposited layers. Philips Res. Rep. 1967, 22, 267–288. [Google Scholar]
- Gracio, J.J.; Fan, Q.H.; Madaleno, J.C. Diamond growth by chemical vapour deposition. J. Phys. D Appl. Phys. 2010, 43, 374017. [Google Scholar] [CrossRef]
- Gruen, D.M. Nanocrystalline diamond films. Annu. Rev. Mater. Res. 1999, 29, 211. [Google Scholar] [CrossRef]
- Williams, O.A.; Daenen, M.; D’Haen, J.; Haenen, K.; Maes, J.; Moshchalkov, V.; Nesládek, M.; Gruen, D.J. Comparison of the growth and properties of ultrananocrystalline diamond and nanocrystalline diamond. Diam. Relat. Mater. 2006, 15, 654–658. [Google Scholar] [CrossRef]
- Sumant, A.V.; Grierson, D.S.; Gerbi, J.E.; Carlisle, J.A.; Auciello, O.; Carpick, R.W. Surface chemistry and bonding configuration of ultrananocrystalline diamond surfaces and their effects on nanotribological properties. Phys. Rev. B 2007, 76, 235429. [Google Scholar] [CrossRef]
- Armstrong, R.J.E.; Svendsen, C.N. Neural stem cells: From cell biology to cell replacement. Cell Transplant. 2000, 9, 139–152. [Google Scholar] [CrossRef]
- Bithell, A.; Williams, B.P. Neural stem cells and cell replacement therapy: Making the right cells. Clin. Sci. 2004, 108, 13–22. [Google Scholar] [CrossRef]
- Stockton, R.A.; Jacobson, B.S. Modulation of cell-substrate adhesion by arachidonic acid: Lipoxygenase regulates cell spreading and ERK1/2-inducible cyclooxygenase regulates cell migration in NIH-3T3 fibroblasts. Mol. Biol. Cell 2001, 12, 1937–1956. [Google Scholar] [CrossRef]
- Hong, S.; Ergezen, E.; Lec, R.; Barbee, K.A. Real-time analysis of cell–surface adhesive interactions using thickness shear mode resonator. Biomaterials 2006, 27, 5813–5820. [Google Scholar] [CrossRef]
- McEver, R.P.; Luscinskas, F.W. Cell adhesion. In Hematology, 7th ed.; Hoffman, R., Benz, E.J., Silberstein, L.E., Heslop, H.E., Weitz, J.I., Anastasi, J., Salama, M.E., Abutalib, S.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; Chapter 12; pp. 127–134. [Google Scholar]
- Butler, J.E.; Sumant, A.V. The CVD of Nanodiamond Materials. Chem. Vap. Depos. 2008, 14, 145–160. [Google Scholar] [CrossRef]
- Aspenberg, P.; Anttila, A.; Konttinen, Y.T.; Lappalainen, R.; Goodman, S.B.; Nordsletten, L.; Santavirta, S. Benign response to particles of diamond and SiC: Bone chamber studies of new joint replacement coating materials in rabbits. Biomaterials 1996, 17, 807–812. [Google Scholar] [CrossRef]
- Popov, C.; Kulisch, W.; Jelinek, M.; Bock, A.; Strnad, J. Nanocrystalline diamond/amorphous carbon composite films for applications in tribology, optics and biomedicine. Thin Solid Films 2006, 494, 92–97. [Google Scholar] [CrossRef]
- Steinmüller-Nethl, D.; Kloss, F.R.; Najam-Ul-Haq, M.; Rainer, M.; Larsson, K.; Linsmeier, C.; Köhler, G.; Fehrer, C.; Lepperdinger, G.; Liu, X.; et al. Strong binding of bioactive BMP-2 to nanocrystalline diamond by physisorption. Biomaterials 2006, 27, 4547–4556. [Google Scholar] [CrossRef]
- Hsu, Y.-C.; Lee, D.-C.; Chen, S.-L.; Liao, W.-C.; Lin, J.-W.; Chiu, W.-T.; Chiu, I.-M. Brain-specific 1B promoter of FGF1 gene facilitates the isolation of neural stem/progenitor cells with self-renewal and multipotent capacities. Dev. Dyn. 2009, 238, 302–314. [Google Scholar] [CrossRef]
- Reynolds, B.A.; Rietze, R.L. Neural stem cells and neurospheres—Re-evaluating the relationship. Nat. Methods 2005, 2, 333–336. [Google Scholar] [CrossRef]
- Edgington, R.J.; Thalhammer, A.; Welch, J.O.; Bongrain, A.; Bergonzo, P.; Scorsone, E.; Jackman, R.B.; Schoepfer, R. Patterned neuronal networks using nanodiamonds and the effect of varying nanodiamond properties on neuronal adhesion and outgrowth. J. Neural Eng. 2013, 10, 056022. [Google Scholar] [CrossRef]
- Gordon, J.; Amini, S. General overview of neuronal cell culture. In Neuronal Cell Culture: Methods and Protocols; Amini, S., White, M.K., Eds.; Springer: New York, NY, USA, 2021; pp. 1–8. [Google Scholar]
- Ariano, P.; Baldelli, P.; Carbone, E.; Gilardino, A.; Lo Giudice, A.; Lovisolo, D.; Manfredotti, C.; Novara, M.; Sternschulte, H.; Vittone, E. Cellular adhesion and neuronal excitability on functionalised diamond surfaces. Diam. Relat. Mater. 2005, 14, 669–674. [Google Scholar] [CrossRef]
- Shabanipour, S.; Dalvand, A.; Jiao, X.; Balaei, M.R.; Chung, S.H.; Kong, J.; Del Bigio, M.R.; Marzban, H. Primary culture of neurons isolated from embryonic mouse cerebellum. J. Vis. Exp. 2019, 152, e60168. [Google Scholar] [CrossRef]
- Nistor, P.A.; May, P.W.; Tamagnini, F.; Randall, A.D.; Caldwell, M.A. Long-term culture of pluripotent stem-cell-derived human neurons on diamond—A substrate for neurodegeneration research and therapy. Biomaterials 2015, 61, 139–149. [Google Scholar] [CrossRef]
- Ojovan, S.M.; McDonald, M.; Rabieh, N.; Shmuel, N.; Erez, H.; Nesladek, M.; Spira, M.E. Nanocrystalline diamond surfaces for adhesion and growth of primary neurons, conflicting results and rational explanation. Front. Neuroeng. 2014, 7, 17. [Google Scholar] [CrossRef]
- Majchrowicz, D.; Ficek, M.; Baran, T.; Wąsowicz, M.; Struk, P.; Jędrzejewska-Szczerska, M. Diamond-based protective layer for optical biosensors. In Proceedings of the Nanostructured Thin Films IX, San Diego, CA, USA, 30 August–1 September 2016; Volume 9929, p. 99291J. [Google Scholar]
- Nebel, C.E.; Rezek, B.; Shin, D.; Uetsuka, H.; Yang, N. Diamond for bio-sensor applications. J. Phys. D Appl. Phys. 2007, 40, 6443–6466. [Google Scholar] [CrossRef]
- McDonald, M.; Monaco, A.; Vahidpour, F.; Haenen, K.; Giugliano, M.; Nesladek, M. Diamond microelectrode arrays for in vitro neuronal recordings. MRS Commun. 2017, 7, 683–690. [Google Scholar] [CrossRef]
- Hébert, C.; Warnking, J.; Depaulis, A.; Garçon, L.A.; Mermoux, M.; Eon, D.; Mailley, P.; Omnès, F. Microfabrication, characterization and in vivo MRI compatibility of diamond microelectrodes array for neural interfacing. Mater. Sci. Eng. C 2015, 46, 25–31. [Google Scholar] [CrossRef]
- Piret, G.; Hébert, C.; Mazellier, J.-P.; Rousseau, L.; Scorsone, E.; Cottance, M.; Lissorgues, G.; Heuschkel, M.O.; Picaud, S.; Bergonzo, P.; et al. 3D-nanostructured boron-doped diamond for microelectrode array neural interfacing. Biomaterials 2015, 53, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Seyock, S.; Maybeck, V.; Scorsone, E.; Rousseau, L.; Hébert, C.; Lissorgues, G.; Bergonzo, P.; Offenhäusser, A. Interfacing neurons on carbon nanotubes covered with diamond. RSC Adv. 2017, 7, 153–160. [Google Scholar] [CrossRef]
- Hadjinicolaou, A.E.; Leung, R.T.; Garrett, D.J.; Ganesan, K.; Fox, K.; Nayagam, D.A.; Shivdasani, M.N.; Meffin, H.; Ibbotson, M.; Prawer, S.; et al. Electrical stimulation of retinal ganglion cells with diamond and the development of an all diamond retinal prosthesis. Biomaterials 2012, 33, 5812–5820. [Google Scholar] [CrossRef]
- Bendali, A.; Agnès, C.; Meffert, S.; Forster, V.; Bongrain, A.; Arnault, J.-C.; Sahel, J.-A.; Offenhäusser, A.; Bergonzo, P.; Picaud, S. Distinctive glial and neuronal interfacing on nanocrystalline diamond. PLoS ONE 2014, 9, e92562. [Google Scholar] [CrossRef]
- Rousseau, L.; Scorsone, E.; Bendali, A.; Djilas, M.; Girard, H.; Cottance, M.; Joucla, S.; Dubus, E.; Degardin, J.; Yvert, B.; et al. Soft 3D retinal implants with diamond electrode a way for focal stimulation. In Proceedings of the 2013 Transducers & Eurosensors XXVII: The 17th International Conference on Solid-State Sensors, Actuators and Microsystems (Transducers & Eurosensors XXVII), Barcelona, Spain, 16–20 June 2013; pp. 1227–1230. [Google Scholar]
- Ganesan, K.; Garrett, D.J.; Ahnood, A.; Shivdasani, M.N.; Tong, W.; Turnley, A.M.; Fox, K.; Meffin, H.; Prawer, S. An all-diamond, hermetic electrical feedthrough array for a retinal prosthesis. Biomaterials 2014, 35, 908–915. [Google Scholar] [CrossRef]
- Ahnood, A.; Escudie, M.C.; Cicione, R.; Abeyrathne, C.D.; Ganesan, K.; Fox, K.E.; Garrett, D.J.; Stacey, A.; Apollo, N.V.; Lichter, S.G.; et al. Ultrananocrystalline diamond-CMOS device integration route for high acuity retinal prostheses. Biomed. Microdevices 2015, 17, 50. [Google Scholar] [CrossRef]
- Ahnood, A.; Meffin, H.; Garrett, D.J.; Fox, K.; Ganesan, K.; Stacey, A.; Apollo, N.V.; Wong, Y.T.; Lichter, S.G.; Kentler, W.; et al. Diamond devices for high acuity prosthetic vision. Adv. Biosyst. 2017, 1, 1600003. [Google Scholar] [CrossRef]
- Ahnood, A.; Cheriton, R.; Bruneau, A.; Belcourt, J.A.; Ndabakuranye, J.P.; Lemaire, W.; Hilkes, R.; Fontaine, R.; Cook, J.P.D.; Hinzer, K.; et al. Laser driven miniature diamond implant for wireless retinal prostheses. Adv. Biosyst. 2020, 4, 2000055. [Google Scholar] [CrossRef]
- Longitudinal Study of a Bionic Eye. US National Institute of Health Clinical Trial, Registry NCT05158049. Available online: https://clinicaltrials.gov/ct2/show/NCT05158049 (accessed on 18 January 2023).
- iBionics Clinical Timeline. Available online: http://ibionics.ca/timeline/ (accessed on 18 January 2023).




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, N.E.; Mendes, J.C.; Braga, S.S. The Gemstone Cyborg: How Diamond Films Are Creating New Platforms for Cell Regeneration and Biointerfacing. Molecules 2023, 28, 1626. https://doi.org/10.3390/molecules28041626
Santos NE, Mendes JC, Braga SS. The Gemstone Cyborg: How Diamond Films Are Creating New Platforms for Cell Regeneration and Biointerfacing. Molecules. 2023; 28(4):1626. https://doi.org/10.3390/molecules28041626
Chicago/Turabian StyleSantos, Nádia E., Joana C. Mendes, and Susana Santos Braga. 2023. "The Gemstone Cyborg: How Diamond Films Are Creating New Platforms for Cell Regeneration and Biointerfacing" Molecules 28, no. 4: 1626. https://doi.org/10.3390/molecules28041626
APA StyleSantos, N. E., Mendes, J. C., & Braga, S. S. (2023). The Gemstone Cyborg: How Diamond Films Are Creating New Platforms for Cell Regeneration and Biointerfacing. Molecules, 28(4), 1626. https://doi.org/10.3390/molecules28041626






