Anemoside A3 Inhibits Macrophage M2-Like Polarization to Prevent Triple-Negative Breast Cancer Metastasis
Abstract
:1. Introduction
2. Results
2.1. A3 Suppresses Triple-Negative Breast Cancer Metastasis In Vivo by Inhibiting TAMs Polarization to M2-Type
2.2. A3 Attenuates IL-4-Induced M2-Type Polarization of BMDM Cells
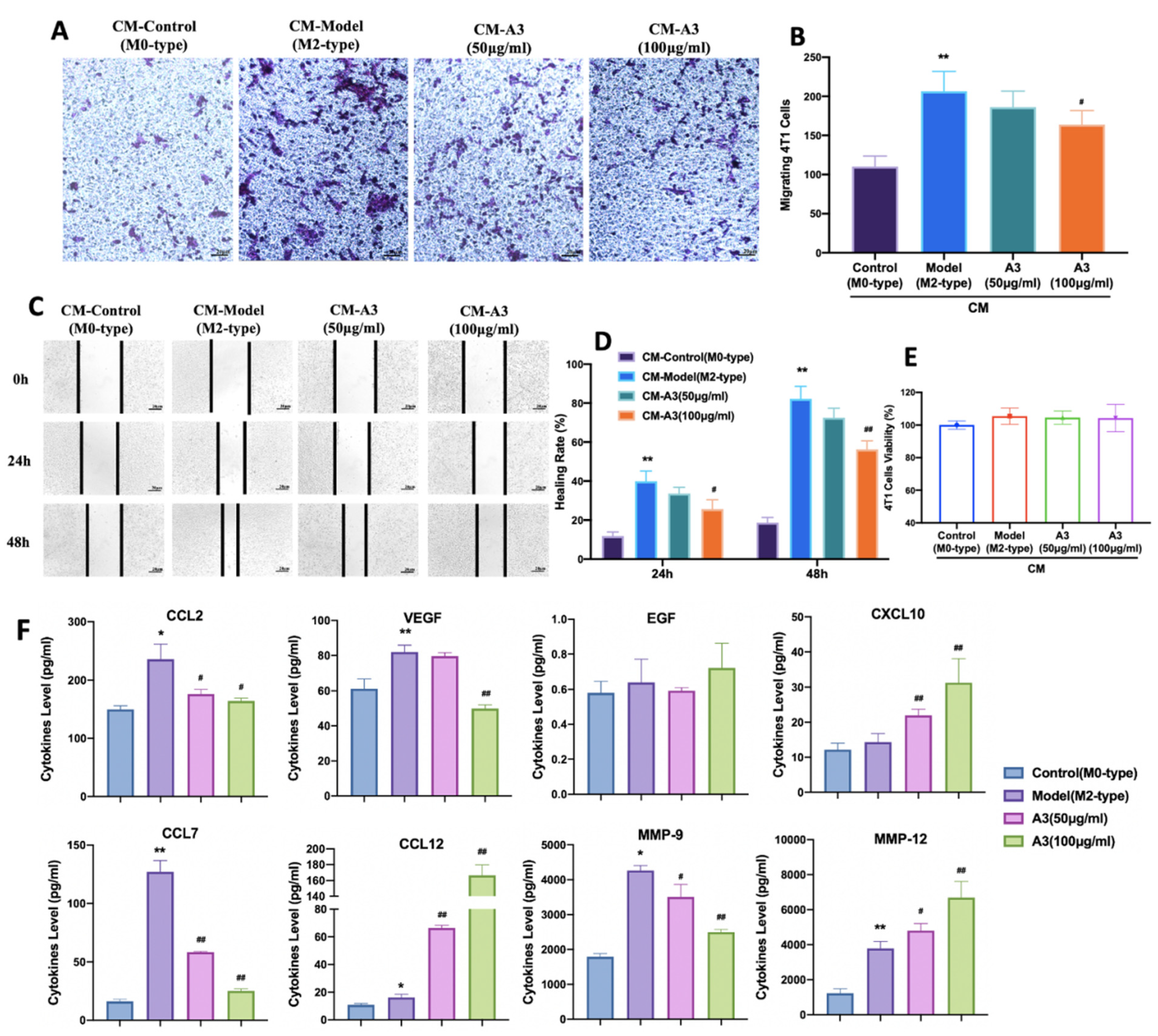
2.3. Inhibition of 4 T1 Cell Migration Is Macrophage-Dependent
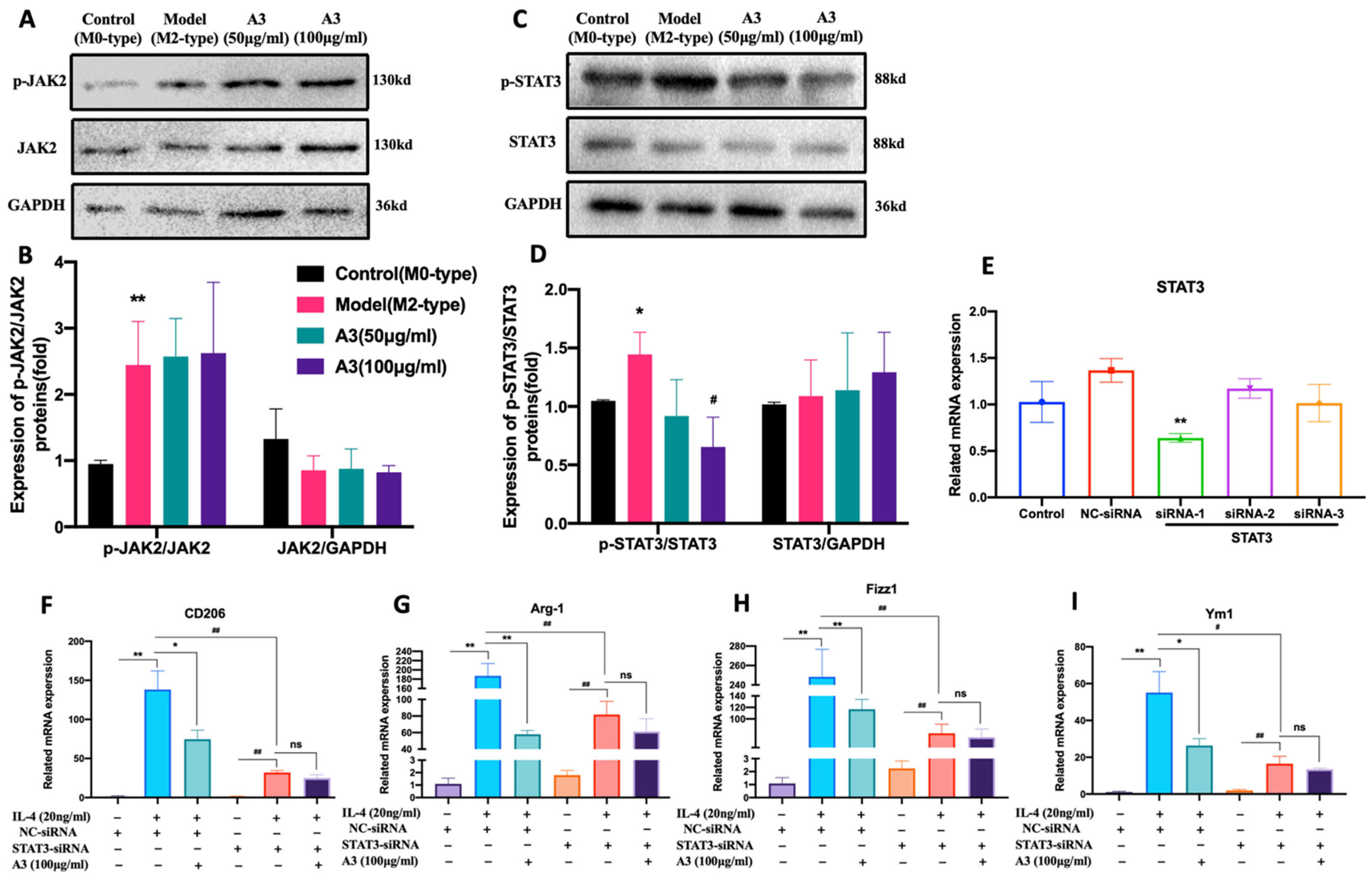
2.4. A3 Inhibits M2-Type Polarization of Macrophages via STAT3 Dependent Pathway in BMDM Cells
3. Discussion
4. Materials and Methods
4.1. Compounds and Drugs
4.2. Cell Lines and Cell Culture
4.3. Animal Care
4.4. In Vivo Tumor Models
4.5. In Vivo Bioluminescence Imaging
4.6. He Staining and Immunohistochemical Analysis
4.7. Production of L929 Cell-Conditioned Medium (LCCM) and BMDM Differentiation
4.8. Cell Viability
4.9. Flow Cytometry
4.10. Quantitative RT-PCR
4.11. Production of BMDM-Conditioned Medium
4.12. Transwell Matrix Penetration and Wound Healing Assay
4.13. Quantification of Cytokines and Chemokines
4.14. Western Blot Analysis
4.15. Small Interfering RNA (siRNA) Transfections
4.16. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Chang, C.-H.; Bijian, K.; Wernic, D.; Su, J.; da Silva, S.D.; Yu, H.; Qiu, D.; Asslan, M.; Alaoui-Jamali, M.A. A novel orally available seleno-purine molecule suppresses triple-negative breast cancer cell proliferation and progression to metastasis by inducing cytostatic autophagy. Autophagy 2019, 15, 1376–1390. [Google Scholar] [CrossRef] [PubMed]
- Lyons, T.G. Targeted Therapies for Triple-Negative Breast Cancer. Curr. Treat. Options Oncol. 2019, 20, 82. [Google Scholar] [CrossRef]
- Harbeck, N.; Gnant, M. Breast cancer. Lancet 2017, 389, 1134–1150. [Google Scholar] [CrossRef]
- Marra, A.; Viale, G.; Curigliano, G. Recent advances in triple negative breast cancer: The immunotherapy era. BMC Med. 2019, 17, 90. [Google Scholar] [CrossRef]
- Choi, J.; Gyamfi, J.; Jang, H.; Koo, J.S. The role of tumor-associated macrophage in breast cancer biology. Histol. Histopathol. 2018, 33, 133–145. [Google Scholar] [PubMed]
- Wanderley, C.W.; Colón, D.F.; Luiz, J.P.M.; Oliveira, F.F.; Viacava, P.R.; Leite, C.A.; Pereira, J.A.; Silva, C.M.; Silva, C.R.; Silva, R.L.; et al. Paclitaxel reduces tumor growth by reprogramming tumor-associated macrophages to an M1- profile in a TLR4-dependent manner. Cancer Res. 2018, 78, 5891–5900. [Google Scholar] [CrossRef]
- Zhang, S.Y.; Song, X.Y.; Li, Y.; Ye, L.L.; Zhou, Q.; Yang, W.B. Tumor-associated macrophages: A promising target for a cancer im-munotherapeutic strategy. Pharmacol. Res. 2020, 161, 105111. [Google Scholar] [CrossRef]
- Lewis, C.; Leek, R.; Harris, A.; McGee, J. Cytokine regulation of angiogenesis in breast cancer: The role of tumor-associated macrophages. J. Leukoc. Biol. 1995, 57, 747–751. [Google Scholar] [CrossRef] [PubMed]
- Qian, B.-Z.; Pollard, J.W. Macrophage Diversity Enhances Tumor Progression and Metastasis. Cell 2010, 141, 39–51. [Google Scholar] [CrossRef]
- Williams, C.B.; Yeh, E.S.; Soloff, A.C. Tumor-associated macrophages: Unwitting accomplices in breast cancer malignancy. NPJ Breast Cancer 2016, 2, 15025–15031. [Google Scholar] [CrossRef] [PubMed]
- Chistiakov, D.A.; Myasoedova, V.A.; Revin, V.V.; Orekhov, A.N.; Bobryshev, Y.V. The impact of interferon-regulatory factors to macrophage differentiation and polarization into M1 and M2. Immunobiology 2018, 223, 101–111. [Google Scholar] [CrossRef]
- Najafi, M.; Hashemi Goradel, N.; Farhood, B.; Salehi, E.; Nashtaei, M.S.; Khanlarkhani, N.; Khezri, Z.; Majidpoor, J.; Abouzaripour, M.; Habibi, M.; et al. Macrophage polarity in cancer: A review. J. Cell. Biochem. 2019, 120, 2756–2765. [Google Scholar] [CrossRef]
- Vitale, I.; Manic, G.; Coussens, L.M.; Kroemer, G.; Galluzzi, L. Macrophages and Metabolism in the Tumor Microenvironment. Cell Metab. 2019, 30, 36–50. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Zhao, X.; Gu, X.; Sun, H.; Cheng, R.; Zhong, Z.; Deng, C. CD44-Targeted Multifunctional Nanomedicines Based on a Sin-gle-Component Hyaluronic Acid Conjugate with All-Natural Precursors: Construction and Treatment of Metastatic Breast Tumors in Vivo. Biomacromolecules 2020, 21, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Fan, Z.; Liu, P.; Chen, L.; Guan, Z.; Liu, Y.; Luo, Y. Anemoside A3 activates TLR4-dependent M1-phenotype macrophage polarization to represses breast tumor growth and angiogenesis. Toxicol. Appl. Pharmacol. 2021, 432, 115755. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, L.; Han, X.; Wang, Y.; Mi, J.; Wang, C.; Sun, D.; Fu, Y.; Zhao, X.; Guo, H.; et al. Saikosaponin A Inhibits Triple-Negative Breast Cancer Growth and Metastasis Through Downregulation of CXCR4. Front. Oncol. 2020, 9, 1487. [Google Scholar] [CrossRef]
- Pulaski, B.A.; Ostrand-Rosenberg, S. Mouse 4T1 breast tumor model. Curr. Protoc. Immunol. 2000, 39, 20–22. [Google Scholar] [CrossRef]
- Tariq, M.; Zhang, J.-Q.; Liang, G.-K.; He, Q.-J.; Ding, L.; Yang, B. Gefitinib inhibits M2-like polarization of tumor-associated macrophages in Lewis lung cancer by targeting the STAT6 signaling pathway. Acta Pharmacol. Sin. 2017, 38, 1501–1511. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.; Zhang, J.; Zhang, B.; Liang, G.; Chen, X.; Yao, F.; Xu, X.; Wu, H.; He, Q.; Ding, L.; et al. Imatinib prevents lung cancer metastasis by inhibiting M2-like polarization of macrophages. Pharmacol. Res. 2018, 133, 121–131. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef]
- Karnevi, E.; Andersson, R.; Rosendahl, A.H. Tumour-educated macrophages display a mixed polarisation and enhance pancreatic cancer cell invasion. Immunol. Cell Biol. 2014, 92, 543–552. [Google Scholar] [CrossRef] [PubMed]
- Condeelis, J.; Pollard, J.W. Macrophages: Obligate Partners for Tumor Cell Migration, Invasion, and Metastasis. Cell 2006, 124, 263–266. [Google Scholar] [CrossRef] [PubMed]
- Qian, B.-Z.; Li, J.; Zhang, H.; Kitamura, T.; Zhang, J.; Campion, L.R.; Kaiser, E.A.; Snyder, L.A.; Pollard, J.W. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature 2011, 475, 222–225. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, S.; Wang, Q.; Zhang, X. Tumor-recruited M2 macrophages promote gastric and breast cancer metastasis via M2 macrophage-secreted CHI3L1 protein. J. Hematol. Oncol. 2017, 10, 36. [Google Scholar] [CrossRef] [PubMed]
- Mukhtar, R.A.; Nseyo, O.; Campbell, M.J.; Esserman, L.J. Tumor-associated macrophages in breast cancer as potential biomarkers for new treatments and diagnostics. Expert Rev. Mol. Diagn. 2011, 11, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Allavena, P. The interaction of anticancer therapies with tumor-associated macrophages. J. Exp. Med. 2015, 212, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Franklin, R.A.; Liao, W.; Sarkar, A.; Kim, M.V.; Bivona, M.R.; Liu, K.; Pamer, E.G.; Li, M.O. The cellular and molecular origin of tu-mor-associated macrophages. Science 2014, 344, 921–925. [Google Scholar] [CrossRef]
- Smith, H.A.; Kang, Y. The metastasis-promoting roles of tumor-associated immune cells. J. Mol. Med. 2013, 91, 411–429. [Google Scholar] [CrossRef]
- Li, H.; Huang, N.; Zhu, W.; Wu, J.; Yang, X.; Teng, W.; Tian, J.; Fang, Z.; Luo, Y.; Chen, M.; et al. Modulation the crosstalk between tu-mor-associated macrophages and non-small cell lung cancer to inhibit tumor migration and invasion by ginsenoside Rh2. BMC Cancer 2018, 18, 579. [Google Scholar]
- Agraval, H.; Yadav, U.C. MMP-2 and MMP-9 mediate cigarette smoke extract-induced epithelial-mesenchymal transition in airway epithelial cells via EGFR/Akt/GSK3β/β-catenin pathway: Amelioration by fisetin. Chem. Interact. 2019, 314, 108846. [Google Scholar] [CrossRef]
- Javadian, M.; Gharibi, T.; Shekari, N.; Abdollahpour-Alitappeh, M.; Mohammadi, A.; Hossieni, A.; Mohammadi, H.; Kazemi, T. The role of microRNAs regulating the expression of matrix metalloproteinases (MMPs) in breast cancer development, progression, and metastasis. J. Cell. Physiol. 2019, 234, 5399–5412. [Google Scholar] [CrossRef]
- Yoshimura, T. The production of monocyte chemoattractant protein-1 (MCP-1)/CCL2 in tumor microenvironments. Cytokine 2017, 98, 71–78. [Google Scholar] [CrossRef]
- Dutta, P.; Sarkissyan, M.; Paico, K.; Wu, Y.; Vadgama, J.V. MCP-1 is overexpressed in triple-negative breast cancers and drives cancer invasiveness and metastasis. Breast Cancer Res. Treat. 2018, 170, 477–486. [Google Scholar] [CrossRef]
- Lawrence, T.; Natoli, G. Transcriptional regulation of macrophage polarization: Enabling diversity with identity. Nat. Rev. Immunol. 2011, 11, 750–761. [Google Scholar] [CrossRef]
- Juhas, U.; Ryba-Stanisławowska, M.; Szargiej, P.; Myśliwska, J. Different pathways of macrophage activation and polarization. Adv. Hyg. Exp. Med. 2015, 69, 496–502. [Google Scholar] [CrossRef]
- Zhou, D.; Huang, C.; Lin, Z.; Zhan, S.; Kong, L.; Fang, C.; Li, J. Macrophage polarization and function with emphasis on the evolving roles of coordinated regulation of cellular signaling pathways. Cell. Signal. 2014, 26, 192–197. [Google Scholar] [CrossRef]
- Yu, H.; Pardoll, D.; Jove, R. STATs in cancer inflammation and immunity: A leading role for STAT3. Nat. Rev. Cancer 2009, 9, 798–809. [Google Scholar] [CrossRef]
- Wills-Karp, M.; Finkelman, F.D. Untangling the complex web of IL-4- and IL-13-mediated signaling pathways. Sci. Signal. 2008, 1, pe55. [Google Scholar] [CrossRef]
- Bhattacharjee, A.; Shukla, M.; Yakubenko, V.P.; Mulya, A.; Kundu, S.; Cathcart, M.K. IL-4 and IL-13 employ discrete signaling pathways for target gene expression in alternatively activated monocytes/macrophages. Free. Radic. Biol. Med. 2013, 54, 1–16. [Google Scholar] [CrossRef]
- Fu, X.L.; Duan, W.; Su, C.Y.; Mao, F.Y.; Lv, Y.P.; Teng, Y.S.; Yu, P.W.; Zhuang, Y.; Zhao, Y.L. Interleukin 6 induces M2 macrophage differ-entiation by STAT3 activation that correlates with gastric cancer progression. Cancer Immunol. Immunother. 2017, 66, 1597–1608. [Google Scholar] [CrossRef]
- Dallagi, A.; Girouard, J.; Hamelin-Morrissette, J.; Dadzie, R.; Laurent, L.; Vaillancourt, C.; Lafond, J.; Carrier, C.; Reyes-Moreno, C. The activating effect of IFN-γ on monocytes/macrophages is regulated by the LIF–trophoblast–IL-10 axis via Stat1 inhibition and Stat3 activation. Cell. Mol. Immunol. 2015, 12, 326–341. [Google Scholar] [CrossRef] [PubMed]
- Khatami, M. Chronic Inflammation: Synergistic Interactions of Recruiting Macrophages (TAMs) and Eosinophils (Eos) with Host Mast Cells (MCs) and Tumorigenesis in CALTs. M-CSF, Suitable Biomarker for Cancer Diagnosis! Cancers 2014, 6, 297–322. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, N.; Zhang, X.; Horng, T. Mitochondrial metabolism regulates macrophage biology. J. Biol. Chem. 2021, 297, 100904. [Google Scholar] [CrossRef]



| Groups | Body Weight (g) | ||||||
|---|---|---|---|---|---|---|---|
| 0 d | 2 d | 4 d | 6 d | 8 d | 10 d | 12 d | |
| Control | 19.33 ± 0.94 | 19.58 ± 0.92 | 19.84 ± 0.55 | 20.33 ± 0.73 | 20.35 ± 0.48 | 20.60 ± 0.52 | 20.69 ± 0.62 |
| Model | 19.41 ± 0.39 | 19.86 ± 0.43 | 19.93 ± 0.81 | 18.21 ± 1.14 ** | 16.88 ± 0.79 ** | 15.53 ± 0.80 ** | 15.27 ± 0.77 ** |
| A3–5 mg/kg | 19.71 ± 0.56 | 20.14 ± 0.72 | 20.43 ± 0.69 | 18.41 ± 1.19 | 16.94 ± 0.98 | 15.80 ± 0.53 | 15.43 ± 0.88 |
| A3–10 mg/kg | 19.60 ± 0.47 | 19.99 ± 0.83 | 20.24 ± 0.40 | 19.10 ± 0.75 | 17.14 ± 0.94 | 16.41 ± 0.83 | 15.87 ± 1.14 |
| A3–20 mg/kg | 19.28 ± 0.73 | 19.54 ± 0.45 | 19.99 ± 0.51 | 19.30 ± 0.69 | 17.76 ± 0.58 | 16.79 ± 0.92 # | 16.64 ± 0.72 # |
| PCX-15 mg/kg | 19.43 ± 0.93 | 19.22 ± 0.30 | 19.67 ± 0.95 | 18.30 ± 1.08 | 15.93 ± 0.82 | 15.20 ± 0.55 | 14.77 ± 0.54 |
| Gene | Sense Strand (5′-3′) | Antisense Strand (3′-5′) |
|---|---|---|
| CD206 | AGGGACCTGGATGGATGACA | TGTACCGCACCCTCCATCTA |
| Arg-1 | AACACGGCAGTGGCTTTAAC | GTCAGTCCCTGGCTTATGGTT |
| Fizz1 | CCCTGCTGGGATGACTGCTA | TGCAAGTATCTCCACTCTGGATCT |
| Ym1 | GGCGCTGTCATCGATTTCTT | ATAGAGTCGCCACCCTGATG |
| β-actin | GGTCATCACTATGGCAACG | ACGGATGTCAACGTCACACT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, P.; Liu, Y.; Chen, L.; Fan, Z.; Luo, Y.; Cui, Y. Anemoside A3 Inhibits Macrophage M2-Like Polarization to Prevent Triple-Negative Breast Cancer Metastasis. Molecules 2023, 28, 1611. https://doi.org/10.3390/molecules28041611
Liu P, Liu Y, Chen L, Fan Z, Luo Y, Cui Y. Anemoside A3 Inhibits Macrophage M2-Like Polarization to Prevent Triple-Negative Breast Cancer Metastasis. Molecules. 2023; 28(4):1611. https://doi.org/10.3390/molecules28041611
Chicago/Turabian StyleLiu, Peng, Yahui Liu, Lanying Chen, Zeping Fan, Yingying Luo, and Yaru Cui. 2023. "Anemoside A3 Inhibits Macrophage M2-Like Polarization to Prevent Triple-Negative Breast Cancer Metastasis" Molecules 28, no. 4: 1611. https://doi.org/10.3390/molecules28041611
APA StyleLiu, P., Liu, Y., Chen, L., Fan, Z., Luo, Y., & Cui, Y. (2023). Anemoside A3 Inhibits Macrophage M2-Like Polarization to Prevent Triple-Negative Breast Cancer Metastasis. Molecules, 28(4), 1611. https://doi.org/10.3390/molecules28041611





