Control of Explosive Chemical Reactions by Optical Excitations: Defect-Induced Decomposition of Trinitrotoluene at Metal Oxide Surfaces
Abstract
1. Introduction
2. Details of Calculations
3. Electronic and Optical Properties of TNT, MgO, and TNT-MgO Interfaces
3.1. Molecular and Crystalline TNT
3.2. Pristine and Defect-Containing MgO Surface
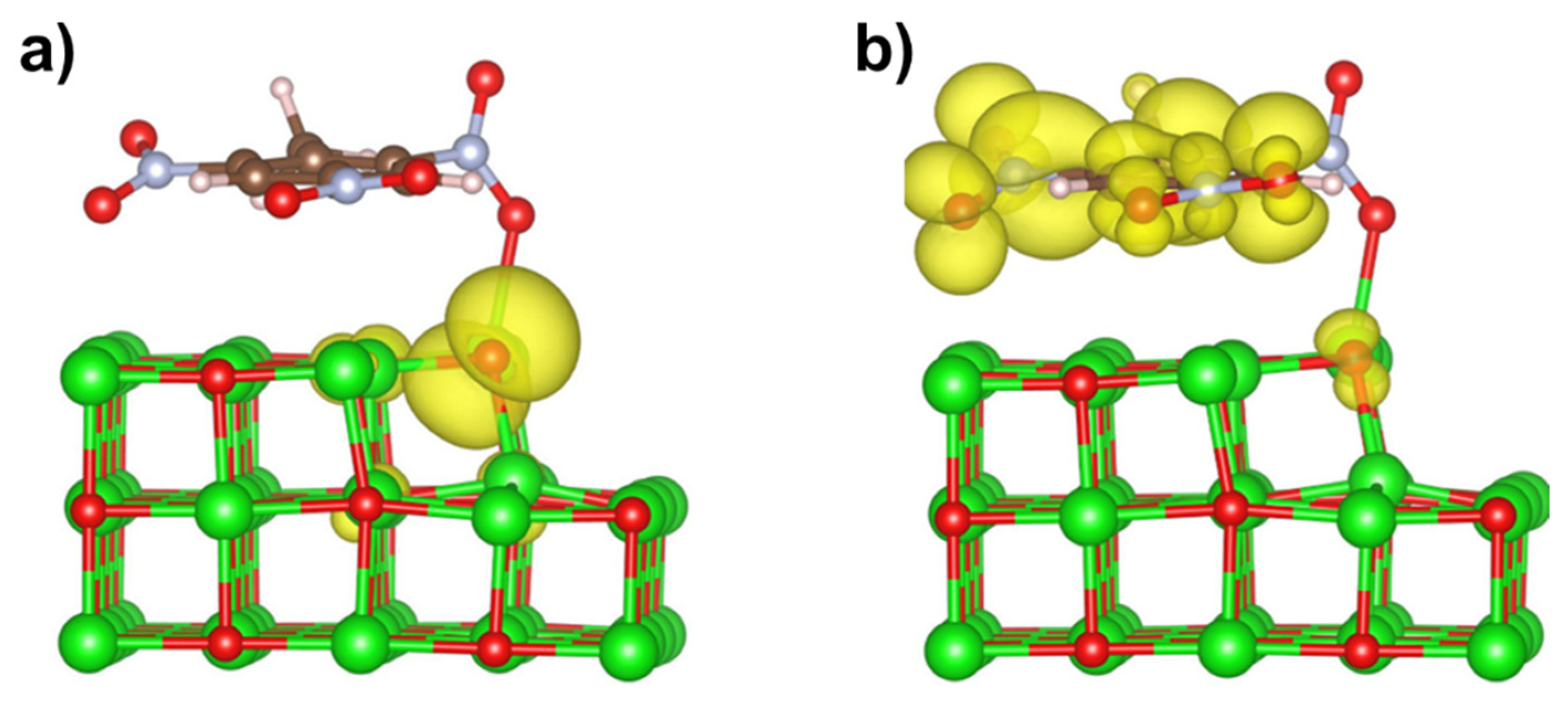
3.3. TNT at MgO (001) Surface
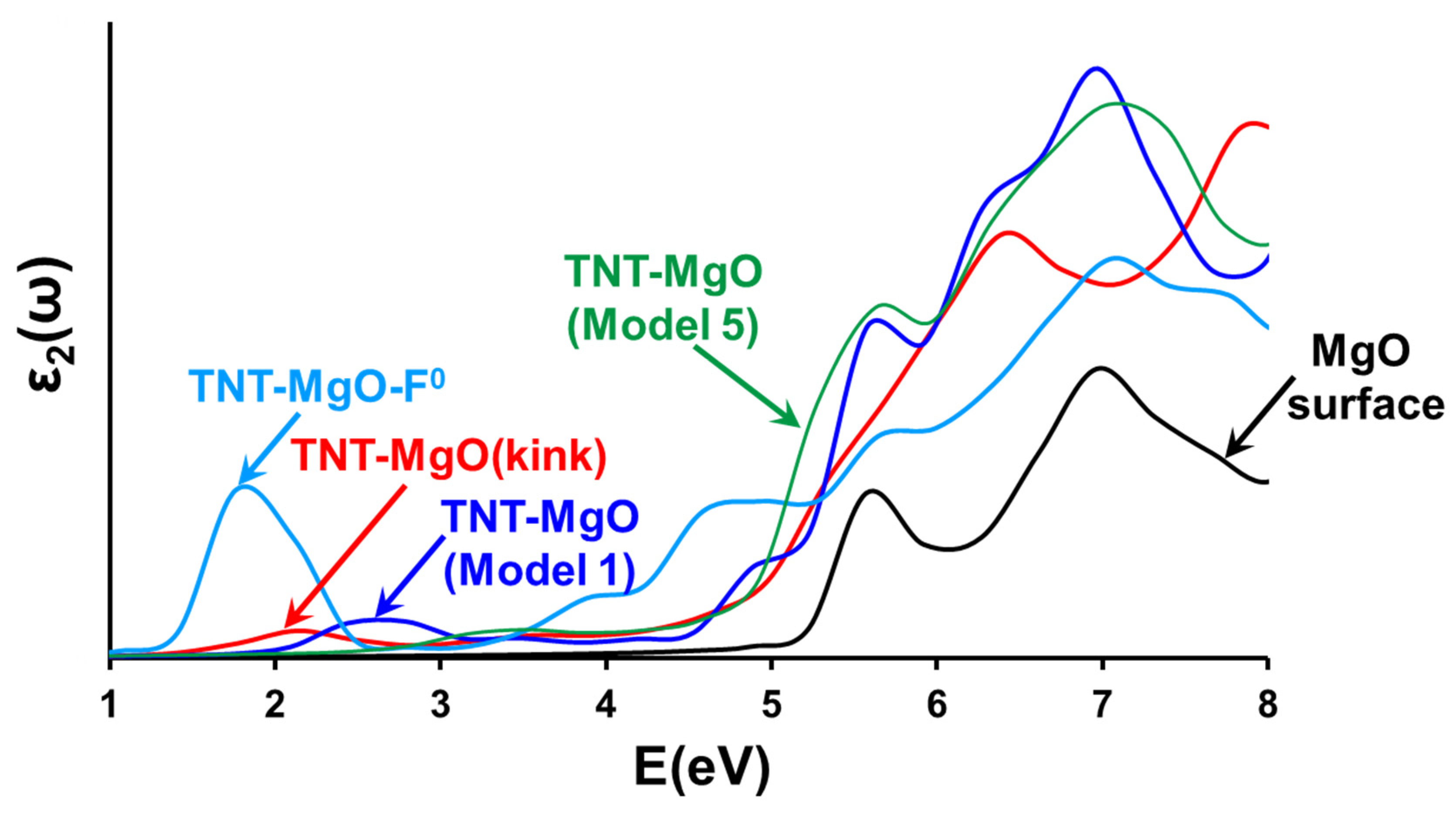
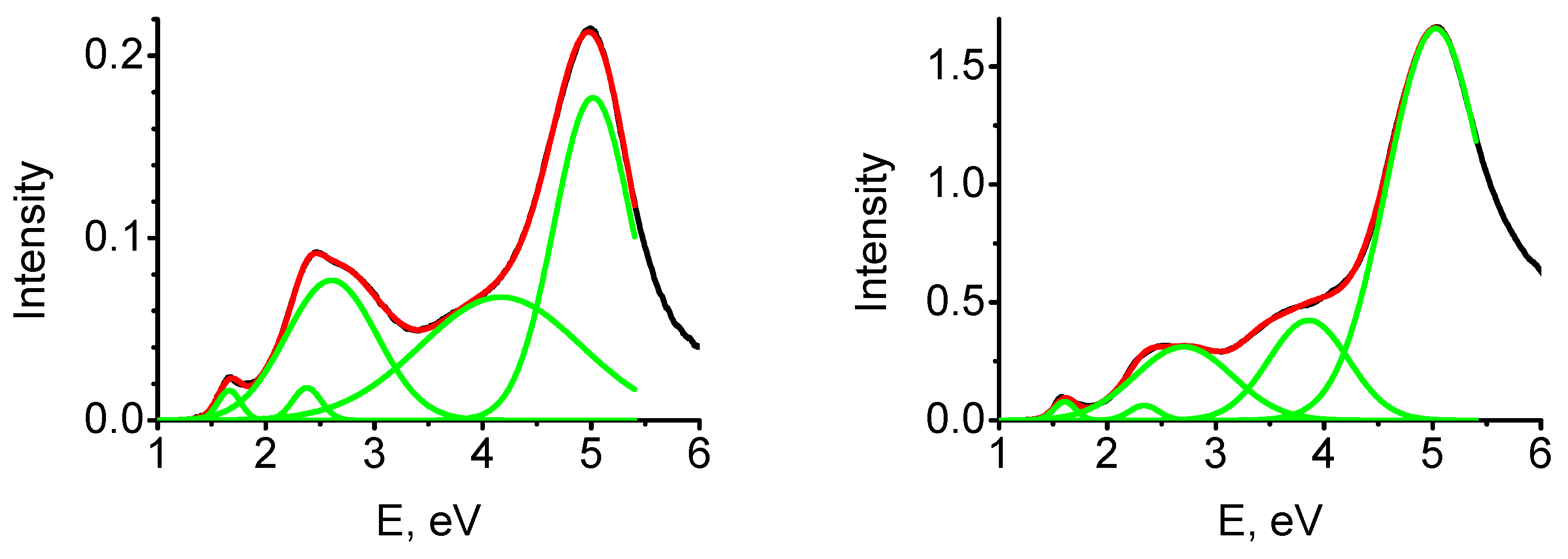
3.4. Optical Absorption at TNT-MgO Interfaces: Comparison to Available Experiments
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Seifert, G. The physics of explosive chemistry. Nat. Phys. 2008, 4, 12–13. [Google Scholar] [CrossRef]
- Grirrane, A.; Corma, A.; García, H. Gold-catalyzed synthesis of aromatic azo compounds from anilines and nitroaromatics. Science 2008, 322, 1661–1664. [Google Scholar] [CrossRef] [PubMed]
- DeCarli, P.S.; Jamieson, J.C. Formation of diamond by explosive shock. Science 1961, 133, 1821–1822. [Google Scholar] [CrossRef] [PubMed]
- Greiner, N.R.; Phillips, D.S.; Johnson, J.D.; Volk, F. Diamonds in detonation soot. Nature 1988, 333, 440–442. [Google Scholar] [CrossRef]
- Koppaka, V.; Thompson, D.C.; Chen, Y.; Ellermann, M.; Nicolaou, K.C.; Juvonen, R.O.; Petersen, D.; Deitrich, R.A.; Hurley, T.D.; Vasiliou, V. Aldehyde Dehydrogenase Inhibitors: A Comprehensive Review of the Pharmacology, Mechanism of Action, Substrate Specificity, and Clinical Application. Pharmacol. Rev. 2012, 64, 520–539. [Google Scholar] [CrossRef] [PubMed]
- Fabbiani, F.P.A.; Pulham, C.R. High-pressure studies of pharmaceutical compounds and energetic materials. Chem. Soc. Rev. 2006, 35, 932–942. [Google Scholar] [CrossRef]
- Federoff, B.T.; Aaronson, H.A.; Reese, E.F.; Sheffield, O.E.; Clift, G.D. Encyclopedia of Explosives and Related Items; Picatinny Arsenal: Dover, DE, USA, 1960. [Google Scholar]
- Bhattacharjee, Y. New Efforts to Detect Explosives Require Advances on Many Fronts. Science 2008, 320, 1416–1417. [Google Scholar] [CrossRef]
- Dasary, S.S.R.; Senapati, D.; Singh, A.K.; Anjaneyulu, Y.; Yu, H.; Ray, P.C. A Highly Sensitive and Selective Dynamic Light Scattering Assay for TNT Detection Using p-ATP Attached Gold Nanoparticle. ACS Appl. Mater. Interfaces 2010, 2, 3455–3460. [Google Scholar] [CrossRef]
- Rylott, E.L.; Jackson, R.G.; Edwards, J.; Womack, G.L.; Seth-Smith, H.M.B.; Rathbone, D.A.; Strand, S.E.; Bruce, N.C. An explosive-degrading cytochrome P450 activity and its targeted application for the phytoremediation of RDX. Nat. Biotechnol. 2006, 24, 216–219. [Google Scholar] [CrossRef]
- Aluker, E.D.; Krechetov, A.G.; Mitrofanov, A.Y.; Zverev, A.S.; Kuklja, M.M. Topography of photochemical initiation in molecular materials. Molecules 2013, 18, 14148–14160. [Google Scholar] [CrossRef]
- Tsyshevsky, R.V.; Rashkeev, S.N.; Kuklja, M.M. Defect states at organic–inorganic interfaces: Insight from first principles calculations for pentaerythritol tetranitrate on MgO surface. Surf. Sci. 2015, 637–638, 19–28. [Google Scholar] [CrossRef]
- Wang, F.; Tsyshevsky, R.; Zverev, A.; Mitrofanov, A.; Kuklja, M.M. Can a Photosensitive Oxide Catalyze Decomposition of Energetic Materials? J. Phys. Chem. C 2017, 121, 1153–1161. [Google Scholar] [CrossRef]
- Tsyshevsky, R.V.; Zverev, A.; Mitrofanov, A.; Sergey, N.; Rashkeev, S.N.; Kuklja, M.M. Photochemistry of the α-Al2O3-PETN Interface. Molecules 2016, 21, 289. [Google Scholar] [CrossRef] [PubMed]
- Kuklja, M.M.; Tsyshevskiy, R.; Zverev, A.S.; Mitrofanov, A.; Ilyakova, N.; Nurmukhametov, D.R.; Rashkeev, S.N. Achieving Tunable Chemical Reactivity through Photo Initiation of Energetic Materials at Metal Oxide Surfaces. Phys. Chem. Chem. Phys. 2020, 22, 25284–25296. [Google Scholar] [CrossRef] [PubMed]
- Mastrikov, Y.; Tsyshevsky, R.; Wang, F.; Kuklja, M.M. Recruiting Perovskites to Degrade Toxic Trinitrotoluene. Materials 2021, 14, 7387. [Google Scholar] [CrossRef]
- Kuklja, M.; Kotomin, E.; Merkle, R.; Mastrikov, Y.; Maier, J. Combined theoretical and experimental analysis of processes determining cathode performance in solid oxide fuel cells. Phys. Chem. Chem. Phys. 2013, 15, 5443–5471. [Google Scholar] [CrossRef]
- Tsyshevsky, R.; Sharia, O.; Kuklja, M. Molecular theory of detonation initiation: Insight from first principles modeling of the decomposition mechanisms of organic nitro energetic materials. Molecules 2016, 21, 236. [Google Scholar] [CrossRef]
- Kuklja, M. Quantum-chemical modeling of energetic materials: Chemical reactions triggered by defects, deformations, and electronic excitations. Adv. Quantum Chem. 2014, 69, 71–145. [Google Scholar]
- Sultana, S.; Rafiuddin; Khan, M.Z.; Umar, K.; Ahmed, A.S.; Shahadat, M. SnO2-SrO based nanocomposites and their photocatalytic activity for the treatment of organic pollutants. J. Mol. Struct. 2015, 1098, 393–399. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Noor, N.H.M.; Umar, K.; Adnan, R.; Ibrahim, M.N.M.; Rashid, M. Graphene oxide–ZnO nanocomposite: An efficient visible light photocatalyst for degradation of rhodamine B. Appl. Nanosci. 2021, 11, 1291–1302. [Google Scholar] [CrossRef]
- Mastrikov, Y.A.; Merkle, R.; Kotomin, E.A.; Kuklja, M.M.; Maier, J. Surface termination effects on the oxygen reduction reaction rate at fuel cell cathodes. J. Mater. Chem. A 2018, 6, 11929–11940. [Google Scholar] [CrossRef]
- Kuklja, M.M.; Tsyshevsky, R.V.; Sharia, O. Effect of Polar Surfaces on Decomposition of Molecular Materials. J. Am. Chem. Soc. 2014, 136, 13289–13302. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Wright, D.; Cliffel, D.; Haglund, R.; Pantelides, S.T. Ionization-Enhanced Decomposition of 2,4,6-Trinitrotoluene (TNT) Molecules. J. Phys. Chem. A 2011, 115, 8142–8146. [Google Scholar] [CrossRef]
- Aluker, E.D.; Krechetov, A.G.; Mitrofanov, A.Y.; Nurmukhametov, D.R.; Kuklja, M.M. Laser Initiation of Energetic Materials: Selective Photoinitiation Regime in Pentaerythritol Tetranitrate. J. Phys. Chem. C 2011, 115, 6893–6901. [Google Scholar] [CrossRef]
- Kimmel, A.V.; Sushko, P.V.; Shluger, A.L.; Kuklja, M.M. Effect of charged and excited states on the decomposition of 1,1-diamino-2,2-dinitroethylene molecules. J. Chem. Phys. 2007, 126, 234711. [Google Scholar] [CrossRef]
- Tsyshevskiy, R.; Rashkeev, S.N.; Kukla, M.M. Defect-Induced Decomposition of Energetic Nitro Compounds at MgO Surface. Surf. Sci. 2022, 722, 122085. [Google Scholar] [CrossRef]
- Klapötke, T.M. Energetic Materials Encyclopedia; de Gruyter: Berlin, Germany, 2018. [Google Scholar]
- Hohenberg, P.; Kohn, W. Inhomogeneous electron gas. Phys. Rev. 1964, 136, B864–B871. [Google Scholar] [CrossRef]
- W Kohn, W.; Sham, L.J. Self-consistent equations including exchange and correlation effects. Phys. Rev. A 1965, 140, A1133–A1138. [Google Scholar] [CrossRef]
- Dion, M.; Rydberg, H.; Schroder, E.; Langreth, D.C.; Lundqvist, B.I. Van der Waals Density Functional for General Geometries. Phys. Rev. Lett. 2004, 92, 246401. [Google Scholar] [CrossRef]
- Thonhauser, T.; Cooper, V.R.; Li, S.; Puzder, A.; Hyldgaard, P.; Langreth, D.C. Van der Waals density functional: Self-consistent potential and the nature of the van der Waals bond. Phys. Rev. B 2007, 76, 125112. [Google Scholar] [CrossRef]
- Roman-Perez, G.; Soler, J.M. Efficient Implementation of a van der Waals Density Functional: Application to Double-Wall Carbon Nanotubes. Phys. Rev. Lett. 2009, 103, 096102. [Google Scholar] [CrossRef]
- Kresse, G.; Futhmuller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmuller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Hafner, J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B 1993, 47, 558–561. [Google Scholar] [CrossRef] [PubMed]
- Adamo, C.; Barone, V. Toward reliable density functional methods without adjustable parameters: The PBE0 model. J. Chem. Phys. 1999, 110, 6158–6169. [Google Scholar] [CrossRef]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef]
- Whited, R.C.; Walker, W.C. Exciton Spectra of CaO and MgO. Phys. Rev. Lett. 1969, 22, 1428–1430. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision B.01; Gaussian, Inc.: Wallingford, UK, 2010. [Google Scholar]
- Bauernschmitt, R.; Ahlrichs, R. Treatment of Electronic Excitations within the Adiabatic Approximation of Time Dependent Density Functional Theory. Chem. Phys. Lett. 1996, 256, 454–464. [Google Scholar] [CrossRef]
- Casida, M.E.; Jamorski, C.; Casida, K.C.; Salahub, D.R. Molecular Excitation Energies to High-lying Bound States from Time-dependent Density-functional Response Theory: Characterization and Correction of the Time-dependent Local Density Approximation Ionization Threshold. J. Chem. Phys. 1998, 108, 4439–4449. [Google Scholar] [CrossRef]
- Roessler, M.; Walker, W.C. Electronic Spectrum and Ultraviolet Optical Properties of Crystalline MgO. Phys. Rev. 1967, 159, 733–738. [Google Scholar] [CrossRef]
- Cox, P.A.; Williams, A.A. Surface excitons on ionic crystals. Surf. Sci. Lett. 1986, 175, L782–L786. [Google Scholar]
- Hess, W.P.; Joly, A.G.; Beck, K.M.; Henyk, M.; Sushko, P.V.; Trevisanutto, P.E.; Shluger, A.L. Laser Control of Desorption through Selective Surface Excitation. J. Phys. Chem. B 2005, 109, 19563–19578. [Google Scholar] [CrossRef] [PubMed]
- Sterrer, M.; Diwald, O.; Knözinger, E.; Sushko, P.V.; Shluger, A.L. Energies and Dynamics of Photoinduced Electron and Hole Processes on MgO Powders. J. Phys. Chem. B 2002, 106, 12478–12482. [Google Scholar] [CrossRef]
- Zecchina, A.; Lofthouse, M.G.; Stone, F.S. Reflectance Spectra of Surface States in Magnesium Oxide and Calcium Oxide. J. Chem. Soc. Faraday Trans. I 1975, 71, 1476–1490. [Google Scholar] [CrossRef]
- Coluccia, S.; Deane, A.M.; Tench, A.J. Photoluminescent Spectra of Surface States in Alkaline Earth Oxides. J. Chem. Soc. Faraday Trans. I 1978, 74, 2913–2922. [Google Scholar] [CrossRef]
- Diwald, O.; Sterrer, M.; Knözinger, E.; Sushko, P.V.; Shluger, A.L. Wavelength selective excitation of surface oxygen anions on highly dispersed MgO. J. Chem. Phys. 2002, 116, 1707–1712. [Google Scholar] [CrossRef]
- Stankic, S.; Müller, M.; Diwald, O.; Sterrer, M.; Knözinger, E.; Bernardi, J. Size-Dependent Optical Properties of MgO Nanocubes. Angew. Chem. Int. Ed. 2005, 44, 4917–4920. [Google Scholar] [CrossRef]
- Sushko, P.V.; Gavartin, J.L.; Shluger, A.L. Electronic properties of structural defects at the MgO (001) surface. J. Phys. Chem. B 2002, 106, 2269–2276. [Google Scholar] [CrossRef]
- Beck, K.M.; Joly, A.G.; Diwald, O.; Stankic, S.; Trevisanutto, P.E.; Sushko, P.V.; Shluger, A.L.; Hess, W.P. Energy and site selectivity in O-atom photodesorption from nanostructured MgO. Surf. Sci. 2008, 602, 1968–1973. [Google Scholar] [CrossRef]
- Underhill, P.R.; Gallon, T.E. The surface defect peak in the electron energy loss spectrum of MgO(100). Solid State Comm. 1982, 43, 9–11. [Google Scholar] [CrossRef]
- Chen, Y.; Williams, R.T.; Sibley, D.W. Defect Cluster Centers in MgO. Phys. Rev. 1969, 182, 960–964. [Google Scholar] [CrossRef]
- Wu, M.-C.; Truong, C.M.; Goodman, D.W. Electron-energy-loss-spectroscopy studies of thermally generated defects in pure and lithium-doped MgO(100) films on Mo(100). Phys. Rev. B 1992, 46, 12688–12694. [Google Scholar] [CrossRef] [PubMed]
- Zverev, A.; Mitrofanov, A. Private Communications; Kemerovo State University: Kemerovo, Russia, 2022. [Google Scholar]
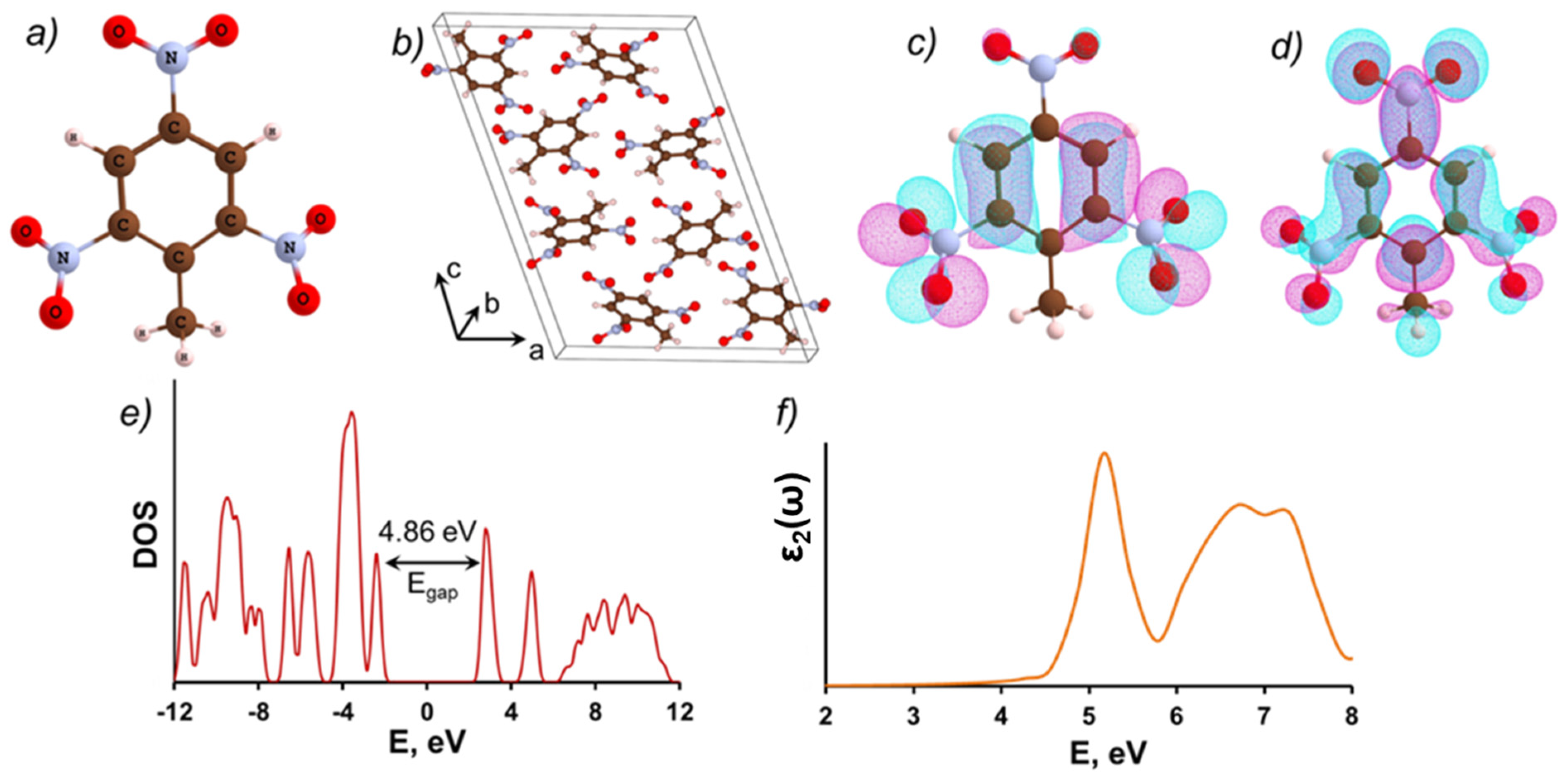
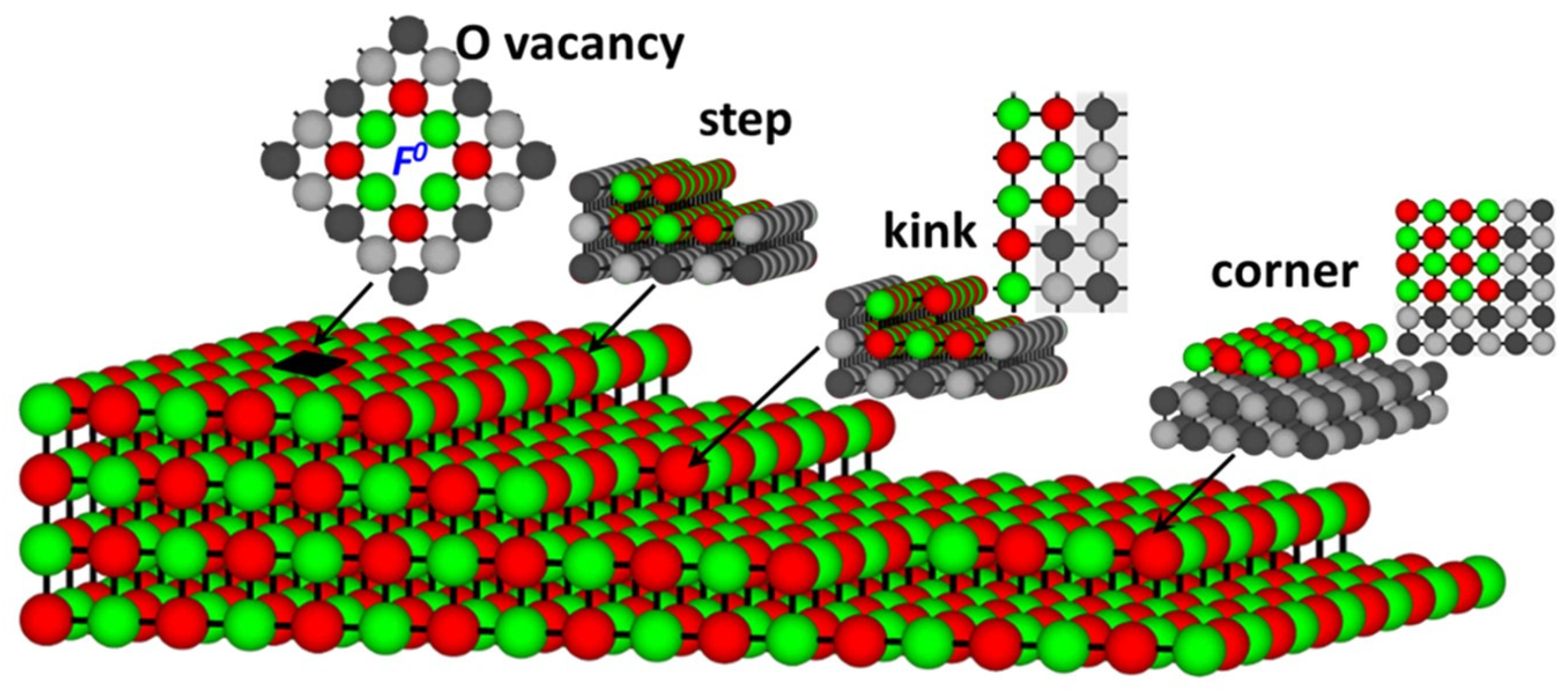
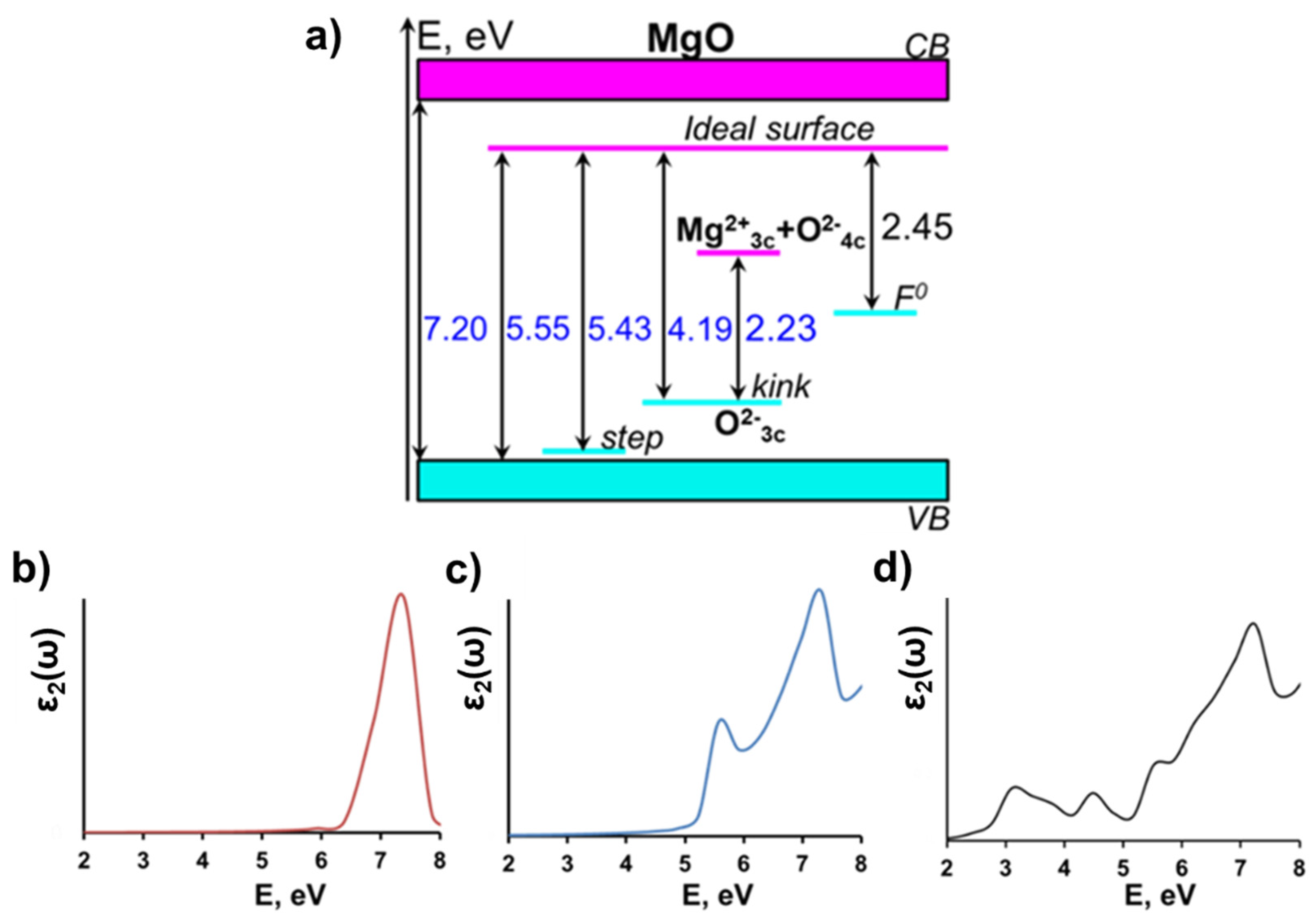

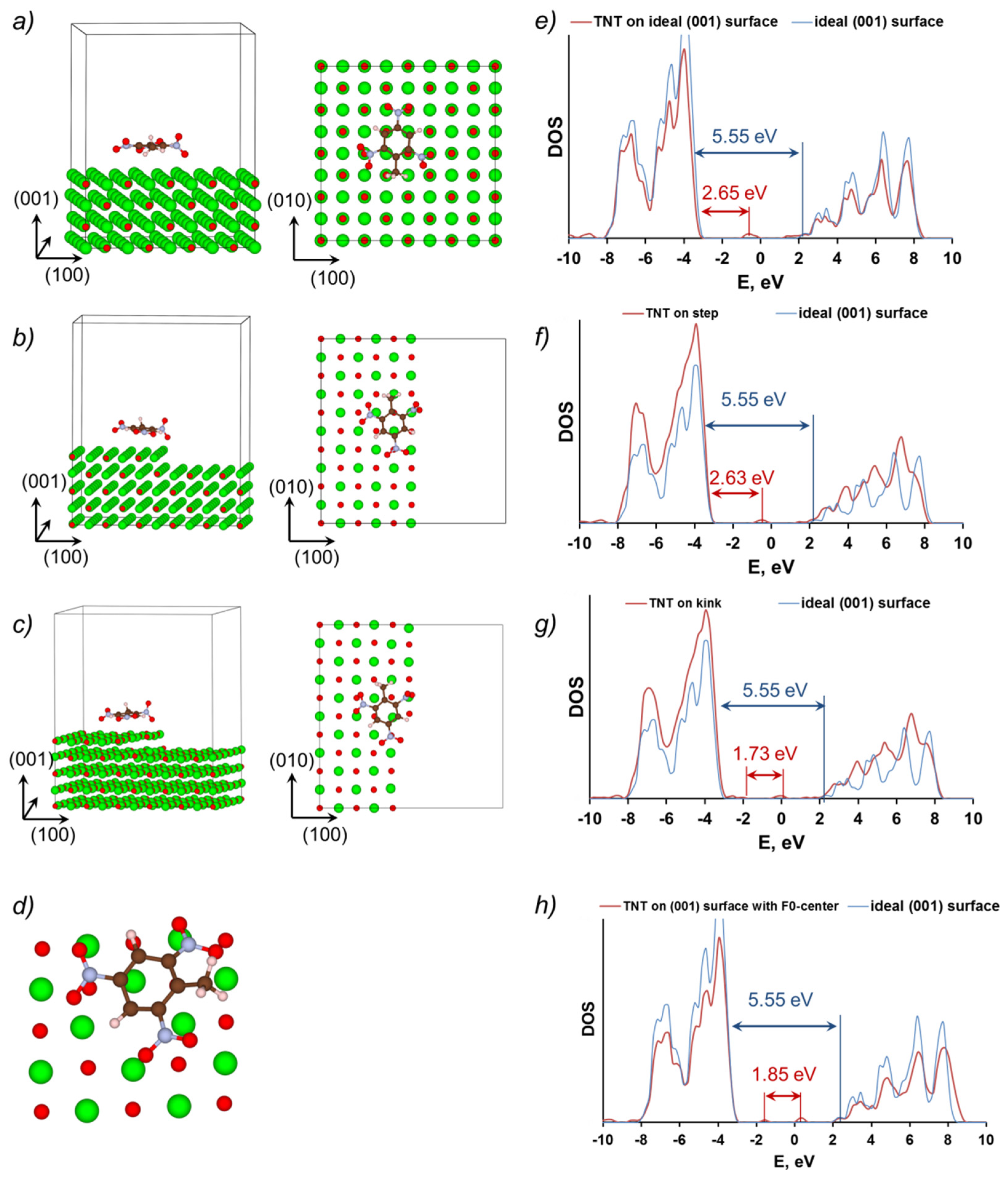

| Model | Egap(eV) |
|---|---|
| MgO | |
| Ideal Bulk Crystal | 7.20 |
| Ideal (001) Surface | 5.55 |
| Step on (001) Surface | 5.43 |
| Kink on (001) Surface | 2.23 |
| F0- center on (001) Surface | 2.45 |
| TNT Molecule at MgO Surface | |
| Ideal (001) MgO Surface | 2.65 |
| (001) MgO Surface with Step | 2.63 |
| (001) MgO Surface with Kink | 1.73 |
| (001) MgO Surface with F0 Center | 1.85 |
| C (%)\Band | 1.6 eV | 2.3 eV | 2.6 eV | 3.9 eV | 5.0 eV |
|---|---|---|---|---|---|
| 0.27 | 0.00427 | 0.00585 | 0.08075 | 0.12555 | 0.15986 |
| 2.5 | 0.02209 | 0.01185 | 0.26136 | 0.60573 | 1.79894 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsyshevsky, R.V.; Rashkeev, S.N.; Kuklja, M.M. Control of Explosive Chemical Reactions by Optical Excitations: Defect-Induced Decomposition of Trinitrotoluene at Metal Oxide Surfaces. Molecules 2023, 28, 953. https://doi.org/10.3390/molecules28030953
Tsyshevsky RV, Rashkeev SN, Kuklja MM. Control of Explosive Chemical Reactions by Optical Excitations: Defect-Induced Decomposition of Trinitrotoluene at Metal Oxide Surfaces. Molecules. 2023; 28(3):953. https://doi.org/10.3390/molecules28030953
Chicago/Turabian StyleTsyshevsky, Roman V., Sergey N. Rashkeev, and Maija M. Kuklja. 2023. "Control of Explosive Chemical Reactions by Optical Excitations: Defect-Induced Decomposition of Trinitrotoluene at Metal Oxide Surfaces" Molecules 28, no. 3: 953. https://doi.org/10.3390/molecules28030953
APA StyleTsyshevsky, R. V., Rashkeev, S. N., & Kuklja, M. M. (2023). Control of Explosive Chemical Reactions by Optical Excitations: Defect-Induced Decomposition of Trinitrotoluene at Metal Oxide Surfaces. Molecules, 28(3), 953. https://doi.org/10.3390/molecules28030953






