Recovery of Bioactive Compounds from Marine Organisms: Focus on the Future Perspectives for Pharmacological, Biomedical and Regenerative Medicine Applications of Marine Collagen
Abstract
1. Introduction
2. Food and Human Health
3. Sources of Marine Bioactive Compounds
4. Collagen Characterization
5. Use of Marine Collagen in Blends
6. Marine Collagen and Pharmacological Applications
7. Marine Collagen and Biomedical Applications
8. Use of Marine Collagen for Drug Delivery
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kienberger, K.; Riera-Buch, M.; Schönemann, A.M.; Bartsch, V.; Halbauer, R.; Prieto, L. First Description of the Life Cycle of the Jellyfish Rhizostoma luteum (Scyphozoa: Rhizostomeae). PLoS ONE 2018, 13, e0202093. [Google Scholar] [CrossRef] [PubMed]
- Falkenhaug, T. Review of Jellyfish Blooms in the Mediterranean and Black Sea. Mar. Biol. Res. 2014, 10, 1038–1039. [Google Scholar] [CrossRef]
- Coll, M.; Piroddi, C.; Albouy, C.; ben Rais Lasram, F.; Cheung, W.W.L.; Christensen, V.; Karpouzi, V.S.; Guilhaumon, F.; Mouillot, D.; Paleczny, M.; et al. The Mediterranean Sea under Siege: Spatial Overlap be-tween Marine Biodiversity, Cumulative Threats and Marine Reserves. Glob. Ecol. Biogeogr. 2012, 21, 465–480. [Google Scholar] [CrossRef]
- Goldstein, J.; Steiner, U.K. Ecological Drivers of Jellyfish Blooms–The Complex Life History of a ‘Well-Known’ Medusa (Aurelia Aurita). J. Anim. Ecol. 2020, 89, 910–920. [Google Scholar] [CrossRef]
- Xu, N.; Peng, X.L.; Li, H.R.; Liu, J.X.; Cheng, J.S.Y.; Qi, X.Y.; Ye, S.J.; Gong, H.L.; Zhao, X.H.; Yu, J.; et al. Marine-Derived Collagen as Biomaterials for Human Health. Front. Nutr. 2021, 8, 493. [Google Scholar] [CrossRef]
- Hamed, I.; Özogul, F.; Özogul, Y.; Regenstein, J.M. Marine Bioactive Compounds and Their Health Benefits: A Review. Compr. Rev. Food Sci. Food Saf. 2015, 14, 446–465. [Google Scholar] [CrossRef]
- Lordan, S.; Ross, R.P.; Stanton, C. Marine Bioactives as Functional Food Ingredients: Potential to Reduce the Incidence of Chronic Diseases. Mar. Drugs 2011, 9, 1056. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, R.S.; Morrissey, M.T. Marine Biotechnology for Production of Food Ingredients. Adv. Food Nutr. Res. 2007, 52, 237–292. [Google Scholar]
- Batista, M.P.; Fernández, N.; Gaspar, F.B.; Bronze, M.d.R.; Duarte, A.R.C. Extraction of Biocompatible Collagen From Blue Shark Skins Through the Conventional Extraction Process Intensification Using Natural Deep Eutectic Solvents. Front. Chem. 2022, 10, 711. [Google Scholar] [CrossRef]
- Furtado, M.; Chen, L.; Chen, Z.; Chen, A.; Cui, W. Development of Fish Collagen in Tissue Regeneration and Drug Delivery. Eng. Reg. 2022, 3, 217–231. [Google Scholar] [CrossRef]
- Siró, I.; Kápolna, E.; Kápolna, B.; Lugasi, A. Functional Food. Product Development, Marketing and Consumer Acceptance—A Review. Appetite 2008, 51, 456–467. [Google Scholar]
- Tortosa, A.; Bes-Rastrollo, M.; Sanchez-Villegas, A.; Basterra-Gortari, F.J.; Nũnez-Cordoba, J.M.; Martinez-Gonzalez, M.A. Mediterranean Diet Inversely Associated with the Incidence of Metabolic Syndrome: The SUN Prospective Cohort. Diabetes Care. 2007, 30, 2957–2959. [Google Scholar] [CrossRef] [PubMed]
- Mirmiran, P.; Moslehi, N.; Mahmoudof, H.; Sadeghi, M.; Azizi, F. A Longitudinal Study of Adherence to the Mediterranean Dietary Pattern and Metabolic Syndrome in a Non-Mediterranean Population. Int. J. Endocrinol. Metab. 2015, 13, 26128. [Google Scholar] [CrossRef]
- Mollet, B.; Rowland, I. Functional Foods: At the Frontier between Food and Pharma. Curr. Opin. Biotechnol. 2002, 13, 483–485. [Google Scholar] [CrossRef]
- Burdge, G.C.; Calder, P.C. Conversion of Alpha-Linolenic Acid to Longer-Chain Polyunsaturated Fatty Acids in Human Adults. Reprod. Nutr. Dev. 2005, 45, 581–597. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P. Human Requirement for N-3 Polyunsaturated Fatty Acids. Poult. Sci. 2000, 79, 961–970. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Wu, J.H.Y. Omega-3 Fatty Acids and Cardiovascular Disease: Effects on Risk Factors, Molecular Pathways, and Clinical Events. J. Am. Coll. Cardiol. 2011, 58, 2047–2067. [Google Scholar] [CrossRef]
- Cleland, L.G.; James, M.J.; Proudman, S.M. The Role of Fish Oils in the Treatment of Rheumatoid Arthritis. Drugs 2003, 63, 845–853. [Google Scholar] [CrossRef]
- Gómez Candela, C.; Bermejo López, L.M.; Loria Kohen, V. Importance of a Balanced Omega 6/Omega 3 Ratio for the Maintenance of Health: Nutritional Recommendations. Nutr. Hosp. 2011, 26, 323–329. [Google Scholar] [PubMed]
- Yashodhara, B.M.; Umakanth, S.; Pappachan, J.M.; Bhat, S.K.; Kamath, R.; Choo, B.H. Omega-3 Fatty Acids: A Comprehensive Review of Their Role in Health and Disease. Postgrad. Med. J. 2009, 85, 84–90. [Google Scholar]
- Rocha, J.; Peixe, L.; Gomes, N.C.M.; Calado, R. Cnidarians as a Source of New Marine Bioactive Compounds--an Overview of the Last Decade and Future Steps for Bioprospecting. Mar. Drugs 2011, 9, 1860–1886. [Google Scholar] [CrossRef] [PubMed]
- Green, D.W.; Padula, M.P.; Santos, J.; Chou, J.; Milthorpe, B.; Ben-Nissan, B. A Therapeutic Potential for Marine Skeletal Proteins in Bone Regeneration. Mar. Drugs 2013, 11, 1203–1220. [Google Scholar] [CrossRef]
- Tziveleka, L.A.; Ioannou, E.; Tsiourvas, D.; Berillis, P.; Foufa, E.; Roussis, V. Collagen from the Marine Sponges Axinella Cannabina and Suberites Carnosus: Isolation and Morphological, Biochemical, and Biophysical Characterization. Mar. Drugs 2017, 15, 152. [Google Scholar] [CrossRef]
- Jankangram, W.; Chooluck, S.; Pomthong, B. Comparison of the Properties of Collagen Extracted from Dried Jellyfish and Dried Squid. Afr. J. Biotechnol. 2016, 15, 642–648. [Google Scholar]
- Langasco, R.; Cadeddu, B.; Formato, M.; Lepedda, A.J.; Cossu, M.; Giunchedi, P.; Pronzato, R.; Rassu, G.; Manconi, R.; Gavini, E. Natural Collagenic Skeleton of Marine Sponges in Pharmaceutics: Innovative Bio-material for Topical Drug Delivery. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 70, 710–720. [Google Scholar] [CrossRef]
- Lim, Y.S.; Ok, Y.J.; Hwang, S.Y.; Kwak, J.Y.; Yoon, S. Marine Collagen as A Promising Biomaterial for Biomedical Applications. Mar. Drugs 2019, 17, 467. [Google Scholar] [CrossRef] [PubMed]
- Merquiol, L.; Romano, G.; Ianora, A.; D’Ambra, I. Biotechnological Applications of Scyphomedusae. Mar. Drugs 2019, 17, 604. [Google Scholar] [CrossRef]
- Steinberger, L.R.; Gulakhmedova, T.; Barkay, Z.; Gozin, M.; Richter, S. Jellyfish-Based Plastic. Adv. Sustain. Syst. 2019, 3, 1900016. [Google Scholar] [CrossRef]
- O’Gorman, D.M.; O’Carroll, C.; Carmody, R.J. Evidence That Marine-Derived, Multi-Mineral, Aquamin Inhibits the NF-ΚB Signaling Pathway in Vitro. Phytother. Res. 2012, 26, 630–632. [Google Scholar] [CrossRef]
- Carson, M.A.; Clarke, S.A. Bioactive Compounds from Marine Organisms: Potential for Bone Growth and Healing. Mar. Drugs 2018, 16, 340. [Google Scholar] [CrossRef]
- Fitton, J.H. Therapies from Fucoidan; Multifunctional Marine Polymers. Mar. Drugs 2011, 9, 1731. [Google Scholar] [CrossRef] [PubMed]
- Liu, C. Application of Marine Collagen for Stem cell based Therapy and Tissue Regeneration (Review). Med. Int. 2021, 1, 1–10. [Google Scholar] [CrossRef]
- Lee, C.H.; Singla, A.; Lee, Y. Biomedical Applications of Collagen. Int. J. Pharm. 2001, 221, 1–22. [Google Scholar] [CrossRef]
- Carfì Pavia, F.; Ciappa, M.; Lepedda, A.; Fiorentino, S.M.; Rigogliuso, S.; Brucato, V.; Formato, M.; Ghersi, G.; la Carrubba, V.; di Maio, E. A Poly-L-Lactic Acid/Collagen/Glycosaminoglycan Matrix for Tissue Engineering Applications. J. Cell. Plast. 2017, 53, 537–549. [Google Scholar] [CrossRef]
- Rodríguez, M.I.A.; Barroso, L.G.R.; Sánchez, M.L. Collagen: A Review on Its Sources and Potential Cosmetic Applications. J. Cosmet. Dermatol. 2018, 17, 20–26. [Google Scholar] [CrossRef]
- Gordon, M.K.; Hahn, R.A. Collagens. Cell Tissue Res. 2010, 339, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Campora, S.; Alberte, P.S.; Bruno, C.; Ghersi, G. Isolation of Adult Rat Cardiomyocytes Using Recombinant Collagenases. Chem. Eng. Trans. 2018, 64, 25–30. [Google Scholar]
- Oliveira, V.d.M.; Assis, C.R.D.; Costa, B.d.A.M.; Neri, R.C.d.A.; Monte, F.T.; Freitas, H.M.S.d.C.V.; França, R.C.P.; Santos, J.; Bezerra, R.d.S.; Porto, A.L.F. Physical, Biochemical, Densitometric and Spectroscopic Techniques for Characterization Collagen from Alternative Sources: A Review Based on the Sustainable Valorization of Aquatic by-Products. J. Mol. Struct. 2021, 1224, 129023. [Google Scholar] [CrossRef]
- Shoulders, M.D.; Raines, R.T. Collagen Structure and Stability. Annu. Rev. Biochem. 2009, 78, 929–958. [Google Scholar] [CrossRef]
- Ricard-Blum, S. The Collagen Family. Cold Spring Harb. Perspect. Biol. 2011, 3, 1–19. [Google Scholar] [CrossRef]
- Davison-Kotler, E.; Marshall, W.S.; García-Gareta, E. Sources of Collagen for Biomaterials in Skin Wound Healing. Bioengineering 2019, 6, 56. [Google Scholar] [CrossRef]
- Lin, K.; Zhang, D.; Macedo, M.H.; Cui, W.; Sarmento, B.; Shen, G. Advanced Collagen-Based Biomaterials for Regenerative Biomedicine. Adv. Funct. Mater. 2019, 29, 1804943. [Google Scholar] [CrossRef]
- Coppola, D.; Oliviero, M.; Vitale, G.A.; Lauritano, C.; D’Ambra, I.; Iannace, S.; de Pascale, D. Marine Collagen from Alternative and Sustainable Sources: Extraction, Processing and Applications. Mar. Drugs 2020, 18, 214. [Google Scholar] [CrossRef] [PubMed]
- Subhan, F.; Hussain, Z.; Tauseef, I.; Shehzad, A.; Wahid, F. A Review on Recent Advances and Applications of Fish Collagen. Crit. Rev. Food Sci. Nutr. 2020, 61, 1027–1037. [Google Scholar] [CrossRef]
- Salamone, M.; Saladino, S.; Pampalone, M.; Campora, S.; Ghersi, G. Tissue Dissociation and Primary Cells Isolation Using Recombinant Collagenases Class I and II. Chem. Eng. Trans. 2014, 38, 247. [Google Scholar] [CrossRef]
- Salamone, M.; Rigogliuso, S.; Nicosia, A.; Campora, S.; Bruno, C.M.; Ghersi, G. 3D Collagen Hydrogel Pro-motes In Vitro Langerhans Islets Vascularization through Ad-MVFs Angiogenic Activity. Biomedicines 2021, 9, 739. [Google Scholar] [CrossRef]
- Salamone, M.; Rigogliuso, S.; Nicosia, A.; Tagliavia, M.; Campora, S.; Cinà, P.; Bruno, C.; Ghersi, G. Neural Crest-Derived Chondrocytes Isolation for Tissue Engineering in Regenerative Medicine. Cells 2020, 14, 962. [Google Scholar] [CrossRef]
- Randelli, F.; Sartori, P.; Carlomagno, C.; Bedoni, M.; Menon, A.; Vezzoli, E.; Sommariva, M.; Gagliano, N. The Collagen-Based Medical Device MD-Tissue Acts as a Mechanical Scaffold Influencing Morpho-Functional Properties of Cultured Human Tenocytes. Cells 2020, 9, 2401. [Google Scholar] [CrossRef] [PubMed]
- Rezvani Ghomi, E.; Nourbakhsh, N.; Akbari Kenari, M.; Zare, M.; Ramakrishna, S. Collagen-Based Bio-materials for Biomedical Applications. J. Biomed. Mater. Res. B Appl. Biomater. 2021, 109, 1986–1999. [Google Scholar] [CrossRef] [PubMed]
- Gorlov, I.F.; Titov, E.I.; Semenov, G.V.; Slozhenkina, M.I.; Sokolov, A.Y.; Omarov, R.S.; Goncharov, A.I.; Zlo-bina, E.Y.; Litvinova, E.V.; Karpenko, E.V. Collagen from Porcine Skin: A Method of Extraction and Structural Properties. Int. J. Food Prop. 2018, 21, 1031–1042. [Google Scholar] [CrossRef]
- Noorzai, S.; Verbeek, C.J.R.; Lay, M.C.; Swan, J. Collagen Extraction from Various Waste Bovine Hide Sources. Waste Biomass Valoriz. 2020, 11, 5687–5698. [Google Scholar] [CrossRef]
- Muthukumar, T.; Sreekumar, G.; Sastry, T.P.; Chamundeeswari, M. Collagen as a Potential Biomaterial in Biomedical Applications. Adv. Mater. Sci. 2018, 53, 29–39. [Google Scholar] [CrossRef]
- Silvipriya, K.S.; Krishna Kumar, K.; Bhat, A.R.; Dinesh Kumar, B.; John, A.; Lakshmanan, P. Collagen: Animal Sources and Biomedical Application. J. Appl. Pharm. Sci. 2015, 5, 123–127. [Google Scholar] [CrossRef]
- Santos, J.A.N.; Gouvea, I.E.; Júdice, W.A.S.; Izidoro, M.A.; Alves, F.M.; Melo, R.L.; Juliano, M.A.; Skern, T.; Juliano, L. Hydrolytic Properties and Substrate Specificity of the Foot-and-Mouth Disease Leader Protease. Biochemistry 2009, 48, 7948–7958. [Google Scholar] [CrossRef]
- Kuehnel, E.; Cencic, R.; Foeger, N.; Skern, T. Foot-and-Mouth Disease Virus Leader Proteinase: Specificity at the P2 and P3 Positions and Comparison with Other Papain-like Enzymes. Biochemistry 2004, 43, 11482–11490. [Google Scholar] [CrossRef]
- Sionkowska, A.; Adamiak, K.; Musial, K.; Gadomska, M. Collagen Based Materials in Cosmetic Applications: A Review. Materials 2020, 13, 4217. [Google Scholar] [CrossRef] [PubMed]
- Easterbrook, C.; Maddern, G. Porcine and Bovine Surgical Products: Jewish, Muslim, and Hindu Perspectives. Arch. Surg. 2008, 143, 366. [Google Scholar] [CrossRef]
- Báez, J.; Olsen, D.; Polarek, J.W. Recombinant Microbial Systems for the Production of Human Collagen and Gelatin. Appl. Microbiol. Biotechnol. 2005, 69, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Felician, F.F.; Xia, C.; Qi, W.; Xu, H. Collagen from Marine Biological Sources and Medical Applications. Chem. Biodivers. 2018, 15, e1700557. [Google Scholar] [CrossRef]
- Seror, J.; Stern, M.; Zarka, R.; Orr, N. The Potential Use of Novel Plant-Derived Recombinant Human Collagen in Aesthetic Medicine. Plast. Reconstr. Surg. 2021, 148, 32S–38S. [Google Scholar] [CrossRef]
- Fertala, A. Three Decades of Research on Recombinant Collagens: Reinventing the Wheel or Developing New Biomedical Products? Bioengineering 2020, 7, 155. [Google Scholar] [CrossRef]
- Rahman, A.; Silva, T.H. Collagens from Marine Organisms towards Biomedical Applications. Mar. Drugs 2022, 20, 170. [Google Scholar] [CrossRef]
- Khong, N.M.H.; Yusoff, F.M.; Jamilah, B.; Basri, M.; Maznah, I.; Chan, K.W.; Armania, N.; Nishikawa, J. Im-proved Collagen Extraction from Jellyfish (Acromitus Hardenbergi) with Increased Physical-Induced Solubilization Processes. Food Chem. 2018, 251, 41–50. [Google Scholar] [CrossRef]
- Khong, N.M.H.; Yusoff, F.M.; Jamilah, B.; Basri, M.; Maznah, I.; Chan, K.W.; Nishikawa, J. Nutritional Com-position and Total Collagen Content of Three Commercially Important Edible Jellyfish. Food Chem. 2016, 196, 953–960. [Google Scholar] [CrossRef] [PubMed]
- Addad, S.; Exposito, J.Y.; Faye, C.; Ricard-Blum, S.; Lethias, C. Isolation, Characterization and Biological Evaluation of Jellyfish Collagen for Use in Biomedical Applications. Mar. Drugs 2011, 9, 967–983. [Google Scholar] [CrossRef] [PubMed]
- Rigogliuso, S.; Salamone, M.; Barbarino, E.; Barbarino, M.; Nicosia, A.; Ghersi, G. Production of Injectable Marine Collagen-Based Hydrogel for the Maintenance of Differentiated Chondrocytes in Tissue Engineering Applications. Int. J. Mol. Sci. 2020, 21, 5798. [Google Scholar] [CrossRef] [PubMed]
- Raghavendran, H.R.B.; Mohan, S.; Genasan, K.; Murali, M.R.; Naveen, S.V.; Talebian, S.; McKean, R.; Kamarul, T. Synergistic Interaction of Platelet Derived Growth Factor (PDGF) with the Surface of PLLA/Col/HA and PLLA/HA Scaffolds Produces Rapid Osteogenic Differentiation. Colloids Surf. B Biointerfaces 2016, 139, 68–78. [Google Scholar] [CrossRef]
- Stoica, A.E.; Chircov, C.; Grumezescu, A.M. Hydrogel Dressings for the Treatment of Burn Wounds: An Up-To-Date Overview. Materials 2020, 13, 2853. [Google Scholar] [CrossRef]
- Shalaby, M.; Agwa, M.; Saeed, H.; Khedr, S.M.; Morsy, O.; El-Demellawy, M.A. Fish Scale Collagen Preparation, Characterization and Its Application in Wound Healing. J. Polym. Environ. 2020, 28, 166–178. [Google Scholar] [CrossRef]
- Sugahara, T.; Ueno, M.; Goto, Y.; Shiraishi, R.; Doi, M.; Akiyama, K.; Yamauchi, S. Immunostimulation Effect of Jellyfish Collagen. Biosci. Biotechnol. Biochem. 2006, 70, 2131–2137. [Google Scholar] [CrossRef]
- Barzideh, Z.; Latiff, A.A.; Gan, C.Y.; Abedin, M.Z.; Alias, A.K. ACE Inhibitory and Antioxidant Activities of Collagen Hydrolysates from the Ribbon Jellyfish ( Chrysaora Sp.). Food Technol. Biotechnol. 2014, 52, 495–504. [Google Scholar] [CrossRef]
- Ding, J.F.; Li, Y.Y.; Xu, J.J.; Su, X.R.; Gao, X.; Yue, F.P. Study on Effect of Jellyfish Collagen Hydrolysate on Anti-Fatigue and Anti-Oxidation. Food Hydrocoll. 2011, 25, 1350–1353. [Google Scholar] [CrossRef]
- Claverie, M.; McReynolds, C.; Petitpas, A.; Thomas, M.; Fernandes, S.C.M. Marine-Derived Polymeric Mate-rials and Biomimetics: An Overview. Polymers 2020, 12, 1002. [Google Scholar] [CrossRef]
- Cumming, M.H.; Hall, B.; Hofman, K. Isolation and Characterisation of Major and Minor Collagens from Hyaline Cartilage of Hoki ( Macruronus Novaezelandiae). Mar. Drugs 2019, 223. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, G.K.S.; Sharma, D.; Balakrishnan, R.M.; Ettiyappan, J.B.P. Extraction, Optimization and Characterization of Collagen from Sole Fish Skin. Sustain. Chem. Pharm. 2018, 9, 19–26. [Google Scholar] [CrossRef]
- Arnesen, J.A.; Gildberg, A. Preparation and Characterisation of Gelatine from the Skin of Harp Seal (Phoca Groendlandica). Bioresour. Technol. 2002, 82, 191–194. [Google Scholar] [CrossRef]
- Ward, A.G.; Courts, A. The Structure and Properties of Collagen. In The Science and Technology of Gelatine, 1st ed.; Academic Press: Cambridge, MA, USA, 1977; pp. 1–30. [Google Scholar]
- Shahidi, F.; Varatharajan, V.; Peng, H.; Senadheera, R. Utilization of Marine By-Products for the Recovery of Value-Added Products. J. Food Bioact. 2019, 6, 10–61. [Google Scholar] [CrossRef]
- Eastoe, J.E.; Leach, A.A. Chemical constitution of gelatine. In The Science and Technology of Gelatine, 1st ed.; Ward, A.G., Courts, A., Eds.; Academic Press: London, UK, 1977; pp. 73–107. [Google Scholar]
- Zhang, X.; Xu, S.; Shen, L.; Li, G. Factors Affecting Thermal Stability of Collagen from the Aspects of Extraction, Processing and Modification. J. Leather Sci. Eng. 2020, 2, 1–29. [Google Scholar] [CrossRef]
- Subhan, F.; Ikram, M.; Shehzad, A.; Ghafoor, A. Marine Collagen: An Emerging Player in Biomedical Applications. J. Food Sci. Technol. 2015, 52, 4703–4707. [Google Scholar] [CrossRef] [PubMed]
- Sionkowska, A. Collagen Blended with Natural Polymers: Recent Advances and Trends. Prog. Polym. Sci. 2021, 122, 101452. [Google Scholar] [CrossRef]
- Antezana, P.E.; Municoy, S.; Álvarez-Echazú, M.I.; Santo-Orihuela, P.L.; Catalano, P.N.; Al-Tel, T.H.; Kadumudi, F.B.; Dolatshahi-Pirouz, A.; Orive, G.; Desimone, M.F. The 3D Bioprinted Scaffolds for Wound Healing. Pharmaceutics 2022, 14, 464. [Google Scholar] [CrossRef]
- Kim, S.-C.; Heo, S.-Y.; Oh, G.-W.; Yi, M.; Jung, W.-K.A.; Kim, S.-C.; Heo, S.-Y.; Oh, G.-W.; Yi, M.; Jung, W.-K. A 3D-Printed Polycaprolactone/Marine Collagen Scaffold Reinforced with Carbonated Hydroxyapatite from Fish Bones for Bone Regeneration. Mar. Drugs 2022, 20, 344. [Google Scholar] [CrossRef]
- Do Amaral, M.B.; Viana, R.B.; Viana, K.B.; Diagone, C.A.; Denis, A.B.; de Guzzi Plepis, A.M. In Vitro and in Vivo Response of Composites Based on Chitosan, Hydroxyapatite and Collagen. Acta Scientiarum. Technology 2019, 42, e41102. [Google Scholar]
- Perez-Puyana, V.; Jiménez-Rosado, M.; Romero, A.; Guerrero, A. Crosslinking of Hybrid Scaffolds Produced from Collagen and Chitosan. Int. J. Biol. Macromol. 2019, 139, 262–269. [Google Scholar] [CrossRef]
- Elango, J.; Lee, J.W.; Wang, S.; Henrotin, Y.; de Val, J.E.M.S.; Regenstein, J.M.; Lim, S.Y.; Bao, B.; Wu, W. Evaluation of Differentiated Bone Cells Proliferation by Blue Shark Skin Collagen via Biochemical for Bone Tissue Engineering. Mar. Drugs 2018, 16, 350. [Google Scholar] [CrossRef] [PubMed]
- Diogo, G.S.; López-Senra, E.; Pirraco, R.P.; Canadas, R.F.; Fernandes, E.M.; Serra, J.; Pérez-Martín, R.I.; Sotelo, C.G.; Marques, A.P.; González, P.; et al. Marine Collagen/Apatite Composite Scaffolds Envisaging Hard Tis-sue Applications. Mar. Drugs 2018, 16, 269. [Google Scholar] [CrossRef]
- Ennaas, N.; Hammami, R.; Gomaa, A.; Bédard, F.; Biron, É.; Subirade, M.; Beaulieu, L.; Fliss, I. Collagencin, an Antibacterial Peptide from Fish Collagen: Activity, Structure and Interaction Dynamics with Membrane. Biochem. Biophys. Res. Commun. 2016, 473, 642–647. [Google Scholar] [CrossRef] [PubMed]
- Chi, C.F.; Cao, Z.H.; Wang, B.; Hu, F.Y.; Li, Z.R.; Zhang, B. Antioxidant and Functional Properties of Collagen Hydrolysates from Spanish Mackerel Skin as Influenced by Average Molecular Weight. Molecules 2014, 19, 11211–11230. [Google Scholar] [CrossRef]
- Fan, J.; Zhuang, Y.; Li, B. Effects of Collagen and Collagen Hydrolysate from Jellyfish Umbrella on Histological and Immunity Changes of Mice Photoaging. Nutrients 2013, 5, 223–233. [Google Scholar] [CrossRef]
- Alemán, A.; Gómez-Guillén, M.C.; Montero, P. Identification of Ace-Inhibitory Peptides from Squid Skin Collagen after in Vitro Gastrointestinal Digestion. Food Res. Int. 2013, 54, 790–795. [Google Scholar] [CrossRef]
- Zhuang, Y.; Sun, L.; Zhang, Y.; Liu, G. Antihypertensive Effect of Long-Term Oral Administration of Jellyfish (Rhopilema esculentum) Collagen Peptides on Renovascular Hypertension. Mar. Drugs 2012, 10, 417–426. [Google Scholar] [CrossRef]
- Woo, M.; Song, Y.O.; Kang, K.H.; Noh, J.S. Anti-Obesity Effects of Collagen Peptide Derived from Skate (Raja Kenojei) Skin Through Regulation of Lipid Metabolism. Mar. Drugs 2018, 16, 306. [Google Scholar] [CrossRef] [PubMed]
- Ge, B.; Wang, H.; Li, J.; Liu, H.; Yin, Y.; Zhang, N.; Qin, S. Comprehensive Assessment of Nile Tilapia Skin ( Oreochromis Niloticus) Collagen Hydrogels for Wound Dressings. Mar. Drugs 2020, 18, 178. [Google Scholar] [CrossRef] [PubMed]
- Morishige, H.; Sugahara, T.; Nishimoto, S.; Muranaka, A.; Ohno, F.; Shiraishi, R.; Doi, M. Immunostimulatory Effects of Collagen from Jellyfish in Vivo. Cytotechnology 2011, 63, 481–492. [Google Scholar] [CrossRef]
- Sivaraman, K.; Shanthi, C. Role of Fish Collagen Hydrolysate in Attenuating Inflammation—An in Vitro Study. J. Food Biochem. 2021, 45, e13876. [Google Scholar] [CrossRef]
- Gupta, C.; Prakash, D. Nutraceuticals from Microbes of Marine Sources. In Nutraceuticals-Past, Present and Future; Intechopen: London, UK, 2019. [Google Scholar]
- Tomosugi, N.; Yamamoto, S.; Takeuchi, M.; Yonekura, H.; Ishigaki, Y.; Numata, N.; Katsuda, S.; Sakai, Y. Effect of Collagen Tripeptide on Atherosclerosis in Healthy Humans. J. Atheroscler. Thromb. 2017, 24, 530–538. [Google Scholar] [CrossRef]
- Tang, L.; Sakai, Y.; Ueda, Y.; Katsuda, S. Effects of Oral Administration of Tripeptides Derived from Type I Collagen (Collagen Tripeptide) on Atherosclerosis Development in Hypercholesterolemic Rabbits. J. Biosci. Bioeng. 2015, 119, 558–563. [Google Scholar] [CrossRef] [PubMed]
- Hatanaka, T.; Kawakami, K.; Uraji, M. Inhibitory Effect of Collagen-Derived Tripeptides on Dipeptidylpeptidase-IV Activity. J. Enzym. Inhib. Med. Chem. 2014, 29, 823–828. [Google Scholar] [CrossRef]
- Hsu, K.-C.; Tung, Y.-S.; Huang, S.-L.; Jao, C.-L.; Hsu, K.-C.; Tung, Y.-S.; Huang, S.-L.; Jao, C.-L. Dipeptidyl Peptidase-IV Inhibitory Activity of Peptides in Porcine Skin Gelatin Hydrolysates. In Bioactive Food Peptides in Health and Disease; IntechOpen: London, UK, 2013. [Google Scholar]
- Song, H.; Zhang, L.; Luo, Y.; Zhang, S.; Li, B. Effects of Collagen Peptides Intake on Skin Ageing and Platelet Release in Chronologically Aged Mice Revealed by Cytokine Array Analysis. J. Cell. Mol. Med. 2018, 22, 277–288. [Google Scholar] [CrossRef]
- Felim, J.; Chen, C.K.; Tsou, D.; Kuo, H.P.; Kong, Z.L. Effect of Different Collagen on Anterior Cruciate Ligament Transection and Medial Meniscectomy-Induced Osteoarthritis Male Rats. Front. Bioeng. Biotechnol. 2022, 10, 1034. [Google Scholar] [CrossRef]
- Song, W.H.; Kim, H.Y.; Lim, Y.S.; Hwang, S.Y.; Lee, C.; Lee, D.Y.; Moon, Y.; Song, Y.J.; Yoon, S. Fish collagen peptides protect against cisplatin-induced cytotoxicity and oxidative injury by inhibiting MAPK signaling pathways in mouse thymic epithelial cells. Mar. Drugs 2022, 20, 232. [Google Scholar] [CrossRef] [PubMed]
- Bălașa, A.F.; Chircov, C.; Grumezescu, A.M. Marine Biocompounds for Neuroprotection—A Review. Mar. Drugs 2020, 18, 290. [Google Scholar] [CrossRef] [PubMed]
- Bivona, G.; Iemmolo, M.; Piccoli, T.; Agnello, L.; lo Sasso, B.; Ciaccio, M.; Ghersi, G. High Cerebrospinal Fluid CX3CL1 Levels in Alzheimer’s Disease Patients but Not in Non-Alzheimer’s Disease Dementia. J. Clin. Med. 2022, 11, 5498. [Google Scholar] [CrossRef] [PubMed]
- Teleanu, R.I.; Chircov, C.; Grumezescu, A.M.; Volceanov, A.; Teleanu, D.M. Antioxidant therapies for neuroprotection—A review. J. Clin. Med. 2019, 8, 1659. [Google Scholar] [CrossRef]
- Zargarzadeh, M.; Amaral, A.J.R.; Custódio, C.A.; Mano, J.F. Biomedical Applications of Laminarin. Carbohydr. Polym. 2020, 232, 115774. [Google Scholar] [CrossRef]
- Anyanwu, R.C.; Rodriguez, C.; Durrant, A.; Ramadan, M.; Olabi, A.G. Micro-Macroalgae Properties and Applications. Encycl. Smart Mater. 2022, 2, 732–758. [Google Scholar]
- Abdallah, M. Extraction of Hyaluronic Acid and Chondroitin Sulfate from Marine Biomass for Their Application in the Treatment of the Dry Eye Disease. Acta Ophthalmol. 2019, 97, S263. [Google Scholar] [CrossRef]
- Vázquez, J.A.; Fraguas, J.; Novoa-Carvallal, R.; Reis, R.L.; Antelo, L.T.; Pérez-Martín, R.I.; Valcarcel, J. Isolation and Chemical Characterization of Chondroitin Sulfate from Cartilage By-Products of Blackmouth Cat-shark ( Galeus melastomus). Mar. Drugs 2018, 16, 344. [Google Scholar] [CrossRef]
- Araújo, J.R.C.; Coelho, C.B.; Campos, A.R.; de Azevedo Moreira, R.; de Oliveira Monteiro-Moreira, A.C. Ani-mal Galectins and Plant Lectins as Tools for Studies in Neurosciences. Curr. Neuropharmacol. 2020, 18, 202–215. [Google Scholar] [CrossRef]
- Spector, A.A.; Kim, H.Y. Emergence of Omega-3 Fatty Acids in Biomedical Research. Prostaglandins Leukot. Essent. Fat. Acids 2019, 140, 47. [Google Scholar] [CrossRef]
- Ouyang, W.C.; Sun, G.C.; Hsu, M.C. Omega-3 Fatty Acids in Cause, Prevention and Management of Violence in Schizophrenia: Conceptualization and Application. Aggress. Violent Behav. 2020, 50, 101347. [Google Scholar] [CrossRef]
- Wang, H. A Review of the Effects of Collagen Treatment in Clinical Studies. Polymer 2021, 13, 3868. [Google Scholar] [CrossRef]
- Armiento, A.R.; Stoddart, M.J.; Alini, M.; Eglin, D. Biomaterials for Articular Cartilage Tissue Engineering: Learning from Biology. Acta Biomater. 2018, 65, 1–20. [Google Scholar] [CrossRef]
- Lopresti, F.; Campora, S.; Tirri, G.; Capuana, E.; Carfì Pavia, F.; Brucato, V.; Ghersi, G.; la Carrubba, V. Core-Shell PLA/Kef Hybrid Scaffolds for Skin Tissue Engineering Applications Prepared by Direct Kefiran Coating on PLA Electrospun Fibers Optimized via Air-Plasma Treatment. Mater. Sci. Eng. C. 2021, 127, 112248. [Google Scholar] [CrossRef] [PubMed]
- Vaithilingam, J.; Sanjuan-Alberte, P.; Campora, S.; Rance, G.A.; Jiang, L.; Thorpe, J.; Burroughs, L.; Tuck, C.J.; Denning, C.; Wildman, R.D.; et al. Multifunctional Bioinstructive 3D Architectures to Modulate Cellular Behavior. Adv. Funct. Mater. 2019, 29, 38–1902016. [Google Scholar] [CrossRef]
- Lanzalaco, S.; Campora, S.; Brucato, V.; Carfì Pavia, F.; di Leonardo, E.R.; Ghersi, G.; Scialdone, O.; Galia, A. Sterilization of Macroscopic Poly(l-Lactic Acid) Porous Scaffolds with Dense Carbon Dioxide: Investigation of the Spatial Penetration of the Treatment and of Its Effect on the Properties of the Matrix. J. Supercrit. Fluids 2016, 111, 83–90. [Google Scholar] [CrossRef]
- Zhang, E.; Zhu, C.; Yang, J.; Sun, H.; Zhang, X.; Li, S.; Wang, Y.; Sun, L.; Yao, F. Electrospun PDLLA/PLGA Composite Membranes for Potential Application in Guided Tissue Regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 58, 278–285. [Google Scholar] [CrossRef]
- Scaffaro, R.; Lopresti, F.; Botta, L.; Rigogliuso, S.; Ghersi, G. Preparation of Three-Layered Porous PLA/PEG Scaffold: Relationship between Morphology, Mechanical Behavior and Cell Permeability. J. Mech. Behav. Biomed. Mater. 2016, 54, 8–20. [Google Scholar] [CrossRef]
- Ghersi, G.; Carfì Pavia, F.; Conoscenti, G.; Mannella, G.A.; Greco, S.; Rigogliuso, S.; La Carrubba, V.; Brucato, V. PLLA Scaffold via TIPS for Bone Tissue Engineering. Chem. Eng. Trans. 2016, 49, 301–306. [Google Scholar]
- Scaffaro, R.; Lopresti, F.; Maio, A.; Botta, L.; Rigogliuso, S.; Ghersi, G. Electrospun PCL/GO-g-PEG Structures: Processing-Morphology-Properties Relationships. Compos. Part A Appl. Sci. Manuf. 2017, 92, 97–107. [Google Scholar] [CrossRef]
- Scaffaro, R.; Lopresti, F.; Botta, L.; Rigogliuso, S.; Ghersi, G. Melt Processed PCL/PEG Scaffold With Discrete Pore Size Gradient for Selective Cellular Infiltration. Macromol. Mater. Eng. 2016, 301, 182–190. [Google Scholar] [CrossRef]
- Rigogliuso, S.; Carfi Pavia, F.; Brucato, V.; la Carrubba, V.; Favia, P.; Intranuovo, F.; Gristina, R.; Ghersi, G. Use of Modified 3D Scaffolds to Improve Cell Adhesion and Drive Desired Cell Responses. Chem. Eng. Trans. 2012, 27, 415–420. [Google Scholar]
- Ghersi, G. Roles of Molecules Involved in Epithelial/Mesenchymal Transition during Angiogenesis. Front. Biosci. 2008, 13, 2335–2355. [Google Scholar] [CrossRef]
- Wang, J.K.; Yeo, K.P.; Chun, Y.Y.; Tan, T.T.Y.; Tan, N.S.; Angeli, V.; Choong, C. Fish Scale-Derived Collagen Patch Promotes Growth of Blood and Lymphatic Vessels in Vivo. Acta Biomater. 2017, 63, 246–260. [Google Scholar] [CrossRef]
- Scaffaro, R.; Re, G.L.; Rigogliuso, S.; Ghersi, G. 3D Polylactide-Based Scaffolds for Studying Human Hepato-carcinoma Processes in Vitro. Sci. Technol. Adv. Mater. 2012, 13, 045003. [Google Scholar] [CrossRef] [PubMed]
- Fang, M.; Yuan, J.; Peng, C.; Li, Y. Collagen as a Double-Edged Sword in Tumor Progression. Tumour Biol. 2014, 35, 2871–2882. [Google Scholar] [CrossRef]
- Qi, Y.; Xu, R. Roles of PLODs in Collagen Synthesis and Cancer Progression. Front. Cell Dev. Biol. 2018, 6, 66. [Google Scholar] [CrossRef]
- Paradiso, F.; Fitzgerald, J.; Yao, S.; Barry, F.; Taraballi, F.; Gonzalez, D.; Conlan, R.S.; Francis, L. Marine Collagen Substrates for 2D and 3D Ovarian Cancer Cell Systems. Front. Bioeng. Biotechnol. 2019, 7, 343. [Google Scholar] [CrossRef]
- Raabe, O.; Reich, C.; Wenisch, S.; Hild, A.; Burg-Roderfeld, M.; Siebert, H.C.; Arnhold, S. Hydrolyzed Fish Collagen Induced Chondrogenic Differentiation of Equine Adipose Tissue-Derived Stromal Cells. Histochem. Cell Biol. 2010, 134, 545–554. [Google Scholar] [CrossRef]
- Hoyer, B.; Bernhardt, A.; Lode, A.; Heinemann, S.; Sewing, J.; Klinger, M.; Notbohm, H.; Gelinsky, M. Jellyfish Collagen Scaffolds for Cartilage Tissue Engineering. Acta Biomater. 2014, 10, 883–892. [Google Scholar] [CrossRef]
- Pustlauk, W.; Paul, B.; Gelinsky, M.; Bernhardt, A. Jellyfish Collagen and Alginate: Combined Marine Materials for Superior Chondrogenesis of HMSC. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 64, 190–198. [Google Scholar] [CrossRef]
- Pugliano, M.; Vanbellinghen, X.; Schwinté, P.; Benkirane-Jessel, N.; Keller, L. Combined Jellyfish Collagen Type II, Human Stem Cells and Tgf-ÃŽÂ23 as a Therapeutic Implant for Cartilage Repair. J. Stem. Cell. Res. Ther. 2017, 7, 1–9. [Google Scholar]
- Sewing, J.; Klinger, M.; Notbohm, H. Jellyfish Collagen Matrices Conserve the Chondrogenic Phenotype in Two- and Three-Dimensional Collagen Matrices. J. Tissue Eng. Regen. Med. 2017, 11, 916–925. [Google Scholar] [CrossRef]
- Bornes, T.D.; Adesida, A.B.; Jomha, N.M. Mesenchymal Stem Cells in the Treatment of Traumatic Articular Cartilage Defects: A Comprehensive Review. Arthritis Res. Ther. 2014, 16, 432. [Google Scholar] [CrossRef] [PubMed]
- Costa, E.; González-García, C.; Gómez Ribelles, J.L.; Salmerón-Sánchez, M. Maintenance of Chondrocyte Phenotype during Expansion on PLLA. J. Tissue Eng. 2018, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Miao, Z.; Lu, Z.; Wu, H.; Liu, H.; Li, M.; Lei, D.; Zheng, L.; Zhao, J. Collagen, Agarose, Alginate, and Matrigel Hydrogels as Cell Substrates for Culture of Chondrocytes in Vitro: A Comparative Study. J. Cell Biochem. 2018, 119, 7924–7933. [Google Scholar] [CrossRef]
- Liu, C.; Sun, J. Hydrolyzed Tilapia Fish Collagen Induces Osteogenic Differentiation of Human Periodontal Ligament Cells. Biomed. Mater. 2015, 10, 065020. [Google Scholar] [CrossRef]
- Zhou, T.; Liu, X.; Sui, B.; Liu, C.; Mo, X.; Sun, J. Development of Fish Collagen/Bioactive Glass/Chitosan Composite Nanofibers as a GTR/GBR Membrane for Inducing Periodontal Tissue Regeneration. Biomed. Mater. 2017, 12, 055004. [Google Scholar] [CrossRef]
- Terada, M.; Izumi, K.; Ohnuki, H.; Saito, T.; Kato, H.; Yamamoto, M.; Kawano, Y.; Nozawa-Inoue, K.; Kashiwazaki, H.; Ikoma, T.; et al. Construction and Characterization of a Tissue-Engineered Oral Mucosa Equivalent Based on a Chitosan-Fish Scale Collagen Composite. J. Biomed. Mater. Res. B Appl. Biomater. 2012, 100B, 1792–1802. [Google Scholar] [CrossRef]
- Yuan, N.; Tian, W.; Sun, L.; Yuan, R.; Tao, J.; Chen, D. Neural Stem Cell Transplantation in a Double-Layer Collagen Membrane with Unequal Pore Sizes for Spinal Cord Injury Repair. Neural Regen. Res. 2014, 9, 1014–1019. [Google Scholar] [PubMed]
- Xu, L.; Dong, W.; Zhao, J.; Xu, Y. Effect of Marine Collagen Peptides on Physiological and Neurobehavioral Development of Male Rats with Perinatal Asphyxia. Mar. Drugs 2015, 13, 3653–3671. [Google Scholar] [CrossRef]
- Hu, Z.; Yang, P.; Zhou, C.; Li, S.; Hong, P. Marine Collagen Peptides from the Skin of Nile Tilapia (Oreo-chromis Niloticus): Characterization and Wound Healing Evaluation. Mar. Drugs 2017, 15, 4. [Google Scholar] [CrossRef] [PubMed]
- Muthukumar, T.; Prabu, P.; Ghosh, K.; Sastry, T.P. Fish Scale Collagen Sponge Incorporated with Macrotyloma Uniflorum Plant Extract as a Possible Wound/Burn Dressing Material. Colloids Surf. B Biointerfaces 2014, 113, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Oslan, S.N.H.; Li, C.X.; Shapawi, R.; Mokhtar, R.A.M.; Noordin, W.N.M.; Huda, N. Extraction and Characterization of Bioactive Fish By-Product Collagen as Promising for Potential Wound Healing Agent in Pharmaceutical Applications: Current Trend and Future Perspective. Int. J. Food Sci. 2022, 2022, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Huibertus van Essen, T.; Lin, C.C.; Hussain, A.K.; Maas, S.; Lai, H.J.; Linnartz, H.; van den Berg, T.J.T.P.; Salvatori, D.C.F.; Luyten, G.P.M.; Jager, M.J. A Fish Scale–Derived Collagen Matrix as Artificial Cornea in Rats: Properties and Potential. Investig. Ophthalmol. Vis. Sci. 2013, 54, 3224–3233. [Google Scholar] [CrossRef] [PubMed]
- Campora, S.; Ghersi, G. Recent Developments and Applications of Smart Nanoparticles in Biomedicine. Nanotechnol. Rev. 2022, 11, 2595–2631. [Google Scholar] [CrossRef]
- Campora, S.; Mauro, N.; Griffiths, P.; Giammona, G.; Ghersi, G. Graphene Nanosystems as Supports in SiRNA Delivery. Chem. Eng. Trans. 2018, 64, 415–420. [Google Scholar]
- Campora, S.; Mohsen, R.; Passaro, D.; Samir, H.; Ashraf, H.; Al-Mofty, S.E.D.; Diab, A.A.; El-Sherbiny, I.M.; Snowden, M.J.; Ghersi, G. Functionalized Poly(N-Isopropylacrylamide)-Based Microgels in Tumor Targeting and Drug Delivery. Gels 2021, 7, 203. [Google Scholar] [CrossRef]
- Mauro, N.; Campora, S.; Adamo, G.; Scialabba, C.; Ghersi, G.; Giammona, G. Polyaminoacid-Doxorubicin Prodrug Micelles as Highly Selective Therapeutics for Targeted Cancer Therapy. RSC Adv. 2016, 6, 77256–77266. [Google Scholar] [CrossRef]
- Adamo, G.; Grimaldi, N.; Campora, S.; Bulone, D.; Bondì, M.L.; Al-Sheikhly, M.; Sabatino, M.A.; Dispenza, C.; Ghersi, G. Multi-Functional Nanogels for Tumor Targeting and Redox-Sensitive Drug and SiRNA Delivery. Molecules 2016, 21, 1594. [Google Scholar] [CrossRef]
- Adamo, G.; Grimaldi, N.; Campora, S.; Sabatino, M.A.; Dispenza, C.; Ghersi, G. Glutathione-Sensitive Nanogels for Drug Release. Chem. Eng. Trans. 2014, 38, 457–462. [Google Scholar]
- Mauro, N.; Campora, S.; Scialabba, C.; Adamo, G.; Licciardi, M.; Ghersi, G.; Giammona, G. Self-Organized Environment-Sensitive Inulin-Doxorubicin Conjugate with a Selective Cytotoxic Effect towards Cancer Cells. RSC Adv. 2015, 5, 32421–32430. [Google Scholar] [CrossRef]
- Di Prima, G.; Saladino, S.; Bongiovì, F.; Adamo, G.; Ghersi, G.; Pitarresi, G.; Giammona, G. Novel Inulin-Based Mucoadhesive Micelles Loaded with Corticosteroids as Potential Transcorneal Permeation Enhancers. Eur. J. Pharm. Biopharm. 2017, 117, 385–399. [Google Scholar] [CrossRef]
- Dispenza, C.; Rigogliuso, S.; Grimaldi, N.; Sabatino, M.A.; Bulone, D.; Bondì, M.L.; Ghersi, G. Structure and Biological Evaluation of Amino-Functionalized PVP Nanogels for Fast Cellular Internalization. React. Funct. Polym. 2013, 73, 1103–1113. [Google Scholar] [CrossRef]
- Dispenza, C.; Sabatino, M.A.; Grimaldi, N.; Spadaro, G.; Bulone, D.; Bondi, M.L.; Adamo, G.; Rigogliuso, S. Large-Scale Radiation Manufacturing of Hierarchically Assembled Nanogels. Chem. Eng. Trans. 2012, 27, 229–234. [Google Scholar]
- Verma, A.K. Collagen-Based Biomaterial as Drug Delivery Module. In Collagen Biomaterials; IntechOpen: London, UK, 2022. [Google Scholar]
- Calejo, M.T.; Almeida, A.J.; Fernandes, A.I. Exploring a New Jellyfish Collagen in the Production of Micro-particles for Protein Delivery. J. Microencapsul. 2012, 29, 520–531. [Google Scholar] [CrossRef]
- Kozlowska, J.; Stachowiak, N.; Sionkowska, A. Collagen/Gelatin/Hydroxyethyl Cellulose Composites Containing Microspheres Based on Collagen and Gelatin: Design and Evaluation. Polymers 2018, 10, 456. [Google Scholar] [CrossRef]
- Cao, H.; Chen, M.M.; Liu, Y.; Liu, Y.Y.; Huang, Y.Q.; Wang, J.H.; Chen, J.D.; Zhang, Q.Q. Fish Collagen-Based Scaffold Containing PLGA Microspheres for Controlled Growth Factor Delivery in Skin Tissue Engineering. Colloids Surf. B Biointerfaces 2015, 136, 1098–1106. [Google Scholar] [CrossRef] [PubMed]
- Adamo, G.; Campora, S.; Ghersi, G. Functionalization of Nanoparticles in Specific Targeting and Mechanism Release. In Micro and Nano Technologies, Nanostructures for Novel Therapy; Ficai, D., Mihai Grumezescu, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 57–80. [Google Scholar]
- Hermida-Merino, C.; Cabaleiro, D.; Lugo, L.; Valcarcel, J.; Vázquez, J.A.; Bravo, I.; Longo, A.; Salloum-Aboujaoude, G.; Solano, E.; Gracia-Fernández, C.; et al. Characterization of Tuna Gelatin-Based Hy-drogels as a Matrix for Drug Delivery. Gels 2022, 8, 237. [Google Scholar] [CrossRef]
- Lynn, A.K.; Yannas, I.V.; Bonfield, W. Antigenicity and Immunogenicity of Collagen. J. Biomed. Mater. Res. B Appl. Biomater. 2004, 71B, 343–354. [Google Scholar] [CrossRef]
- Collinge, J. Prion Diseases of Humans and Animals: Their Causes and Molecular Basis. Annu. Rev. Neurosci. 2001, 24, 519–550. [Google Scholar] [CrossRef]
- Sugiura, H.; Yunoki, S.; Kondo, E.; Ikoma, T.; Tanaka, J.; Yasuda, K. In Vivo Biological Responses and Bioresorption of Tilapia Scale Collagen as a Potential Biomaterial. J. Biomater. Sci. Polym. Ed. 2012, 20, 1353–1368. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Elango, J.; Wang, S.; Hou, C.; Miao, M.; Li, J.; Na, L.; Wu, W. Characterization of Immunogenicity Associated with the Biocompatibility of Type I Collagen from Tilapia Fish Skin. Polymers 2022, 14, 2300. [Google Scholar] [CrossRef] [PubMed]
- Lukáč, P.; Hartinger, J.M.; Mlček, M.; Popková, M.; Suchý, T.; Šupová, M.; Závora, J.; Adámková, V.; Benáko-vá, H.; Slanař, O.; et al. A Novel Gentamicin-Releasing Wound Dressing Prepared from Freshwater Fish Cyprinus Carpio Collagen Cross-Linked with Carbodiimide. J. Bioact. Compat. Polym. 2019, 34, 246–262. [Google Scholar] [CrossRef]
- Maczynska, B.; Secewicz, A.; Smutnicka, D.; Szymczyk, P.; Dudek-Wicher, R.; Junka, A.; Bartoszewicz, M. In Vitro Efficacy of Gentamicin Released from Collagen Sponge in Eradication of Bacterial Biofilm Preformed on Hydroxyapatite Surface. PLoS ONE 2019, 14, e0217769. [Google Scholar] [CrossRef] [PubMed]
- Hartinger, J.M.; Lukáč, P.; Mitáš, P.; Mlček, M.; Popková, M.; Suchý, T.; Šupová, M.; Závora, J.; Adámková, V.; Benáková, H.; et al. Vancomycin-Releasing Cross-Linked Collagen Sponges as Wound Dressings. Bosn. J. Basic Med. Sci. 2021, 21, 61–70. [Google Scholar] [CrossRef]
- Tudoroiu, E.E.; Dinu-Pîrvu, C.E.; Kaya, M.G.A.; Popa, L.; Anuța, V.; Prisada, R.M.; Ghica, M.V. An Overview of Cellulose Derivatives-Based Dressings for Wound-Healing Management. Pharmaceuticals 2021, 14, 1215. [Google Scholar] [CrossRef] [PubMed]
- Pathan, I.B.; Munde, S.J.; Shelke, S.; Ambekar, W.; Mallikarjuna Setty, C. Curcumin Loaded Fish Scale Collagen-HPMC Nanogel for Wound Healing Application: Ex-Vivo and In-Vivo Evaluation. Int. J. Polym. Mater. Polym. Biomater. 2018, 68, 165–174. [Google Scholar] [CrossRef]
- Nguyen, C.T.; Vu, M.Q.; Phan, T.T.; Vu, T.Q.; Vo, Q.A.; Bach, G.L.; Thai, H. Novel PH-Sensitive Hydrogel Beads Based on Carrageenan and Fish Scale Collagen for Allopurinol Drug Delivery. J. Polym. Environ. 2020, 28, 1795–1810. [Google Scholar] [CrossRef]
- Chen, M.M.; Huang, Y.Q.; Cao, H.; Liu, Y.; Guo, H.; Chen, L.S.; Wang, J.H.; Zhang, Q.Q. Collagen/Chitosan Film Containing Biotinylated Glycol Chitosan Nanoparticles for Localized Drug Delivery. Colloids Surf. B Biointerfaces 2015, 128, 339–346. [Google Scholar] [CrossRef]
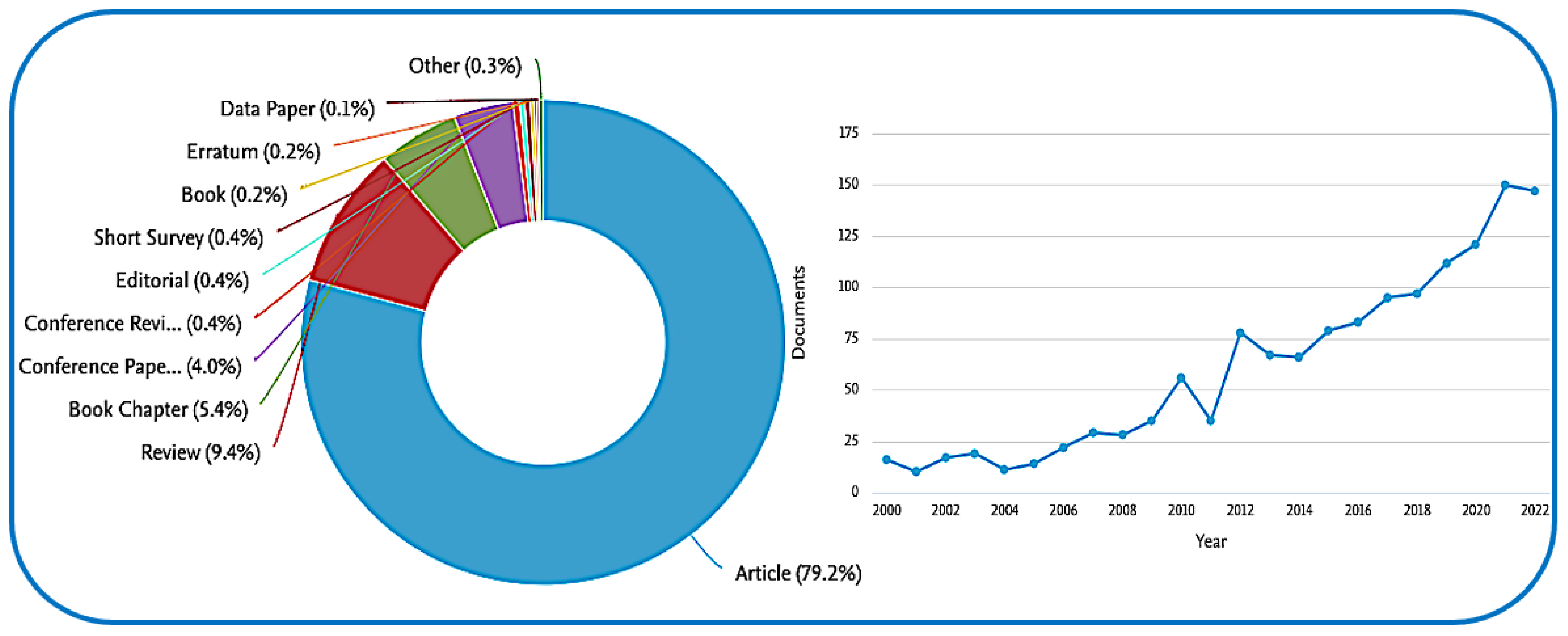


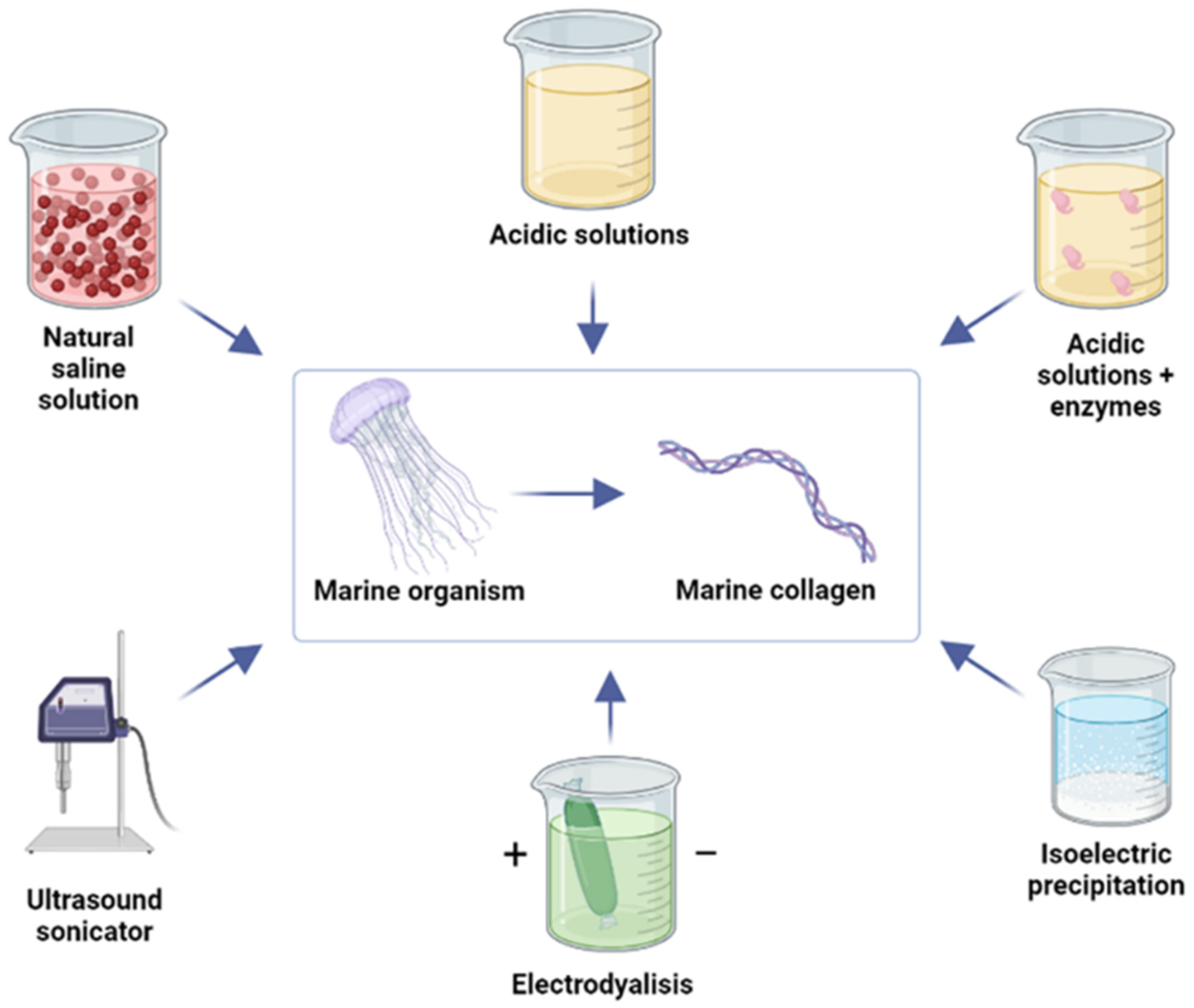
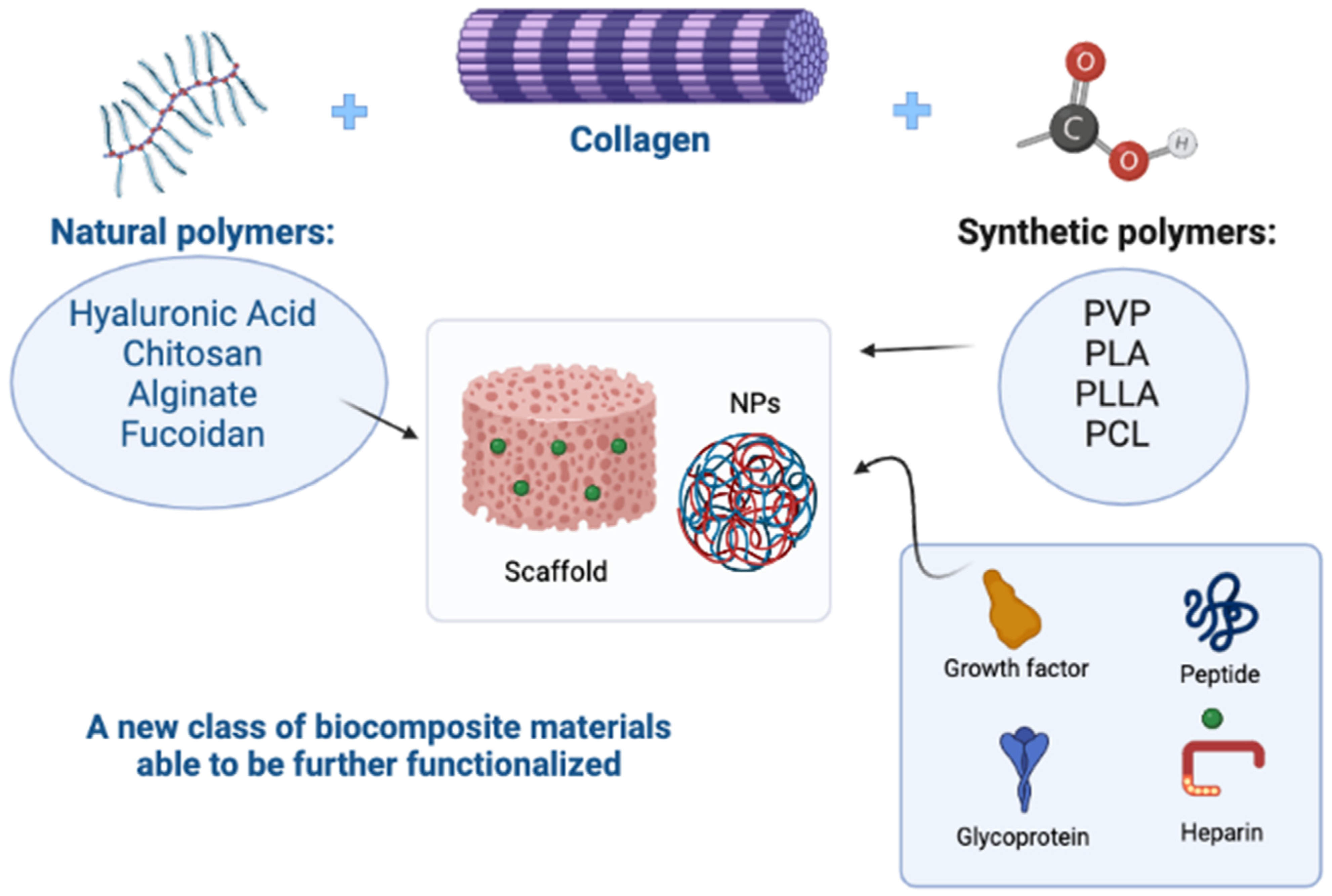

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rigogliuso, S.; Campora, S.; Notarbartolo, M.; Ghersi, G. Recovery of Bioactive Compounds from Marine Organisms: Focus on the Future Perspectives for Pharmacological, Biomedical and Regenerative Medicine Applications of Marine Collagen. Molecules 2023, 28, 1152. https://doi.org/10.3390/molecules28031152
Rigogliuso S, Campora S, Notarbartolo M, Ghersi G. Recovery of Bioactive Compounds from Marine Organisms: Focus on the Future Perspectives for Pharmacological, Biomedical and Regenerative Medicine Applications of Marine Collagen. Molecules. 2023; 28(3):1152. https://doi.org/10.3390/molecules28031152
Chicago/Turabian StyleRigogliuso, Salvatrice, Simona Campora, Monica Notarbartolo, and Giulio Ghersi. 2023. "Recovery of Bioactive Compounds from Marine Organisms: Focus on the Future Perspectives for Pharmacological, Biomedical and Regenerative Medicine Applications of Marine Collagen" Molecules 28, no. 3: 1152. https://doi.org/10.3390/molecules28031152
APA StyleRigogliuso, S., Campora, S., Notarbartolo, M., & Ghersi, G. (2023). Recovery of Bioactive Compounds from Marine Organisms: Focus on the Future Perspectives for Pharmacological, Biomedical and Regenerative Medicine Applications of Marine Collagen. Molecules, 28(3), 1152. https://doi.org/10.3390/molecules28031152






