Thermal Treatment to Obtain 5-Hydroxymethyl Furfural (5-HMF), Furfural and Phenolic Compounds from Vinasse Waste from Agave
Abstract
1. Introduction
2. Results and Discussion
2.1. Vinasses Characterization
2.2. COD Removal
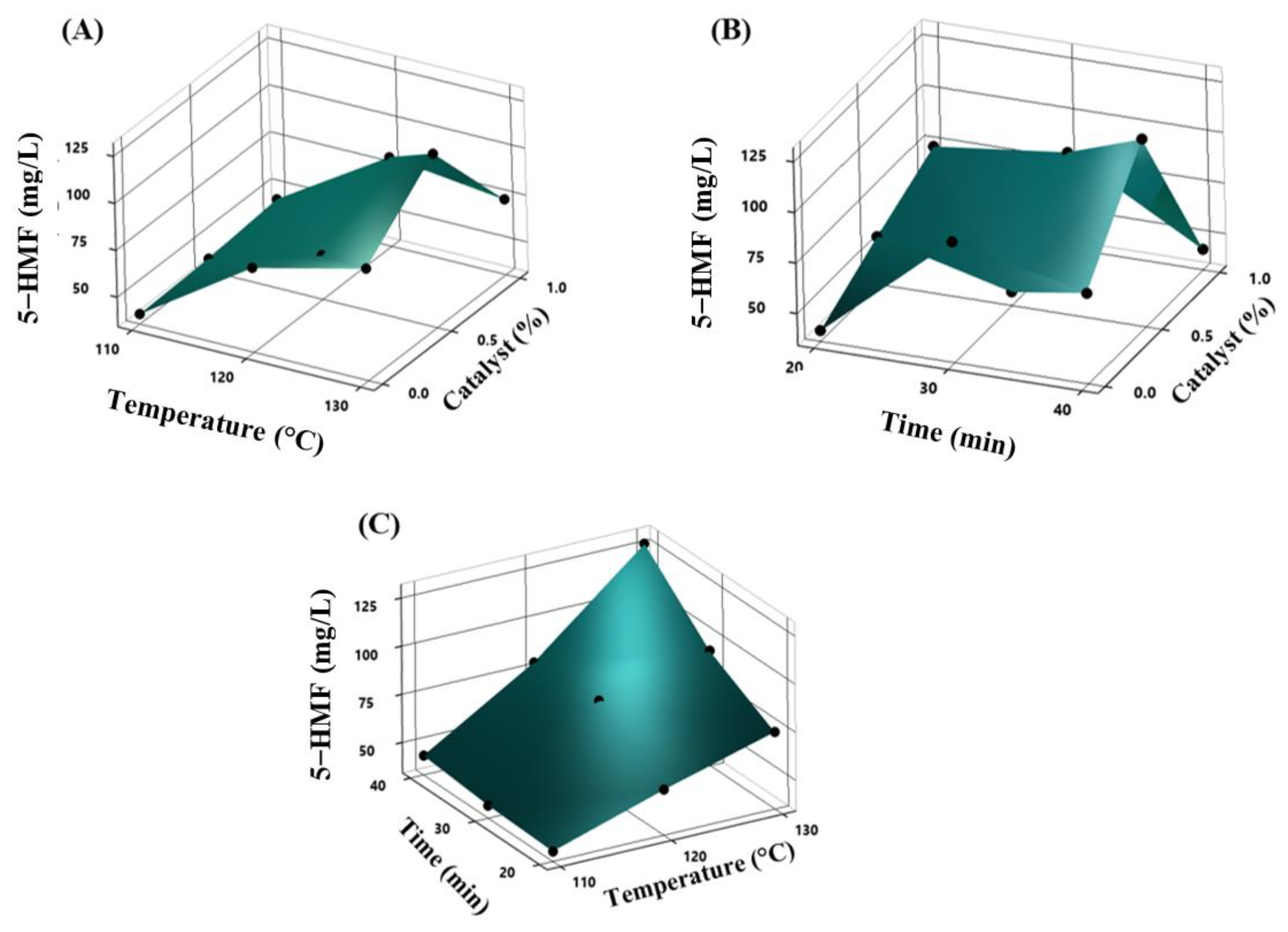
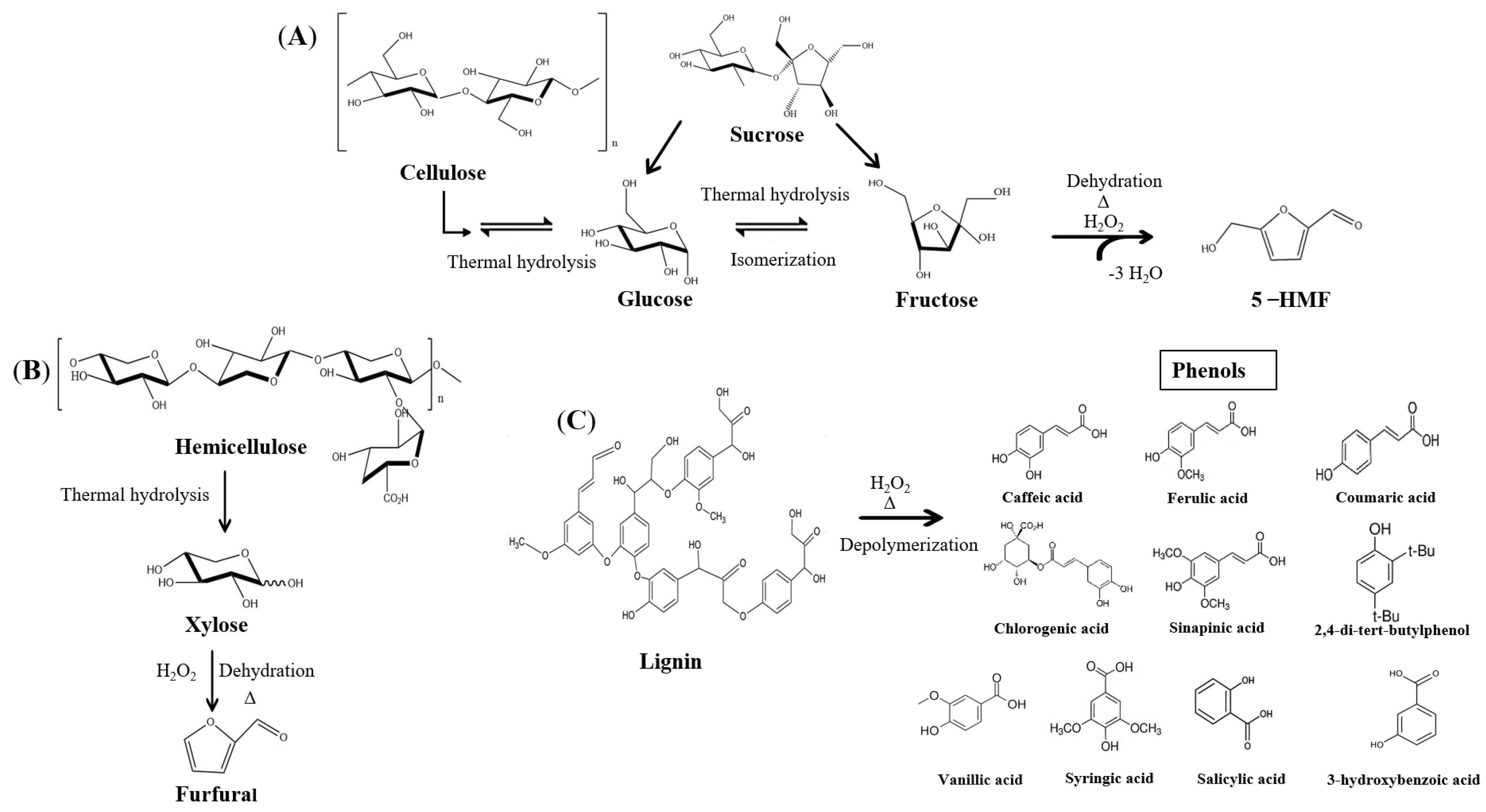
2.3. Furans Increase
2.4. Sugar Removal
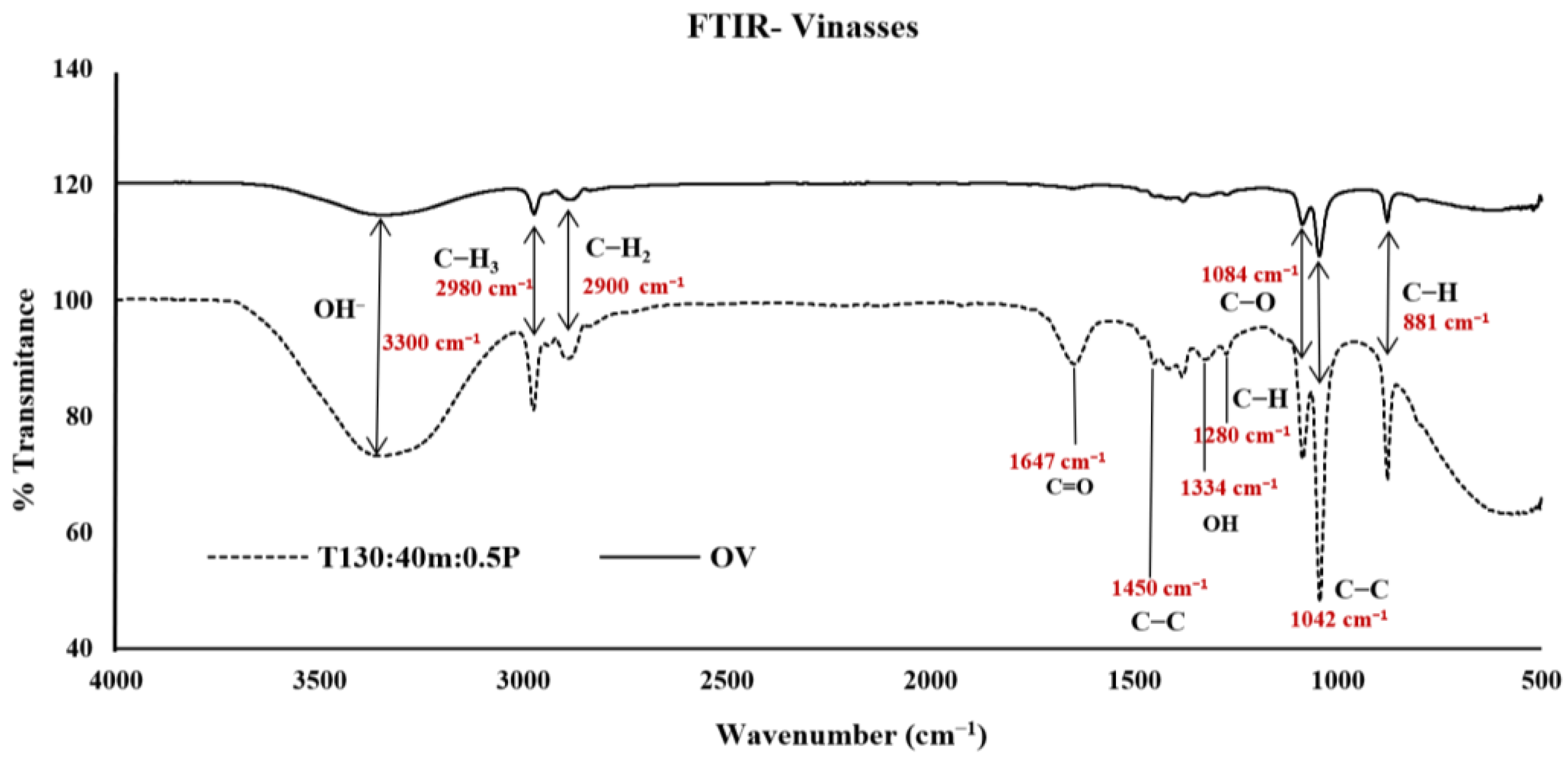
2.5. FTIR from Tequila Vinasses
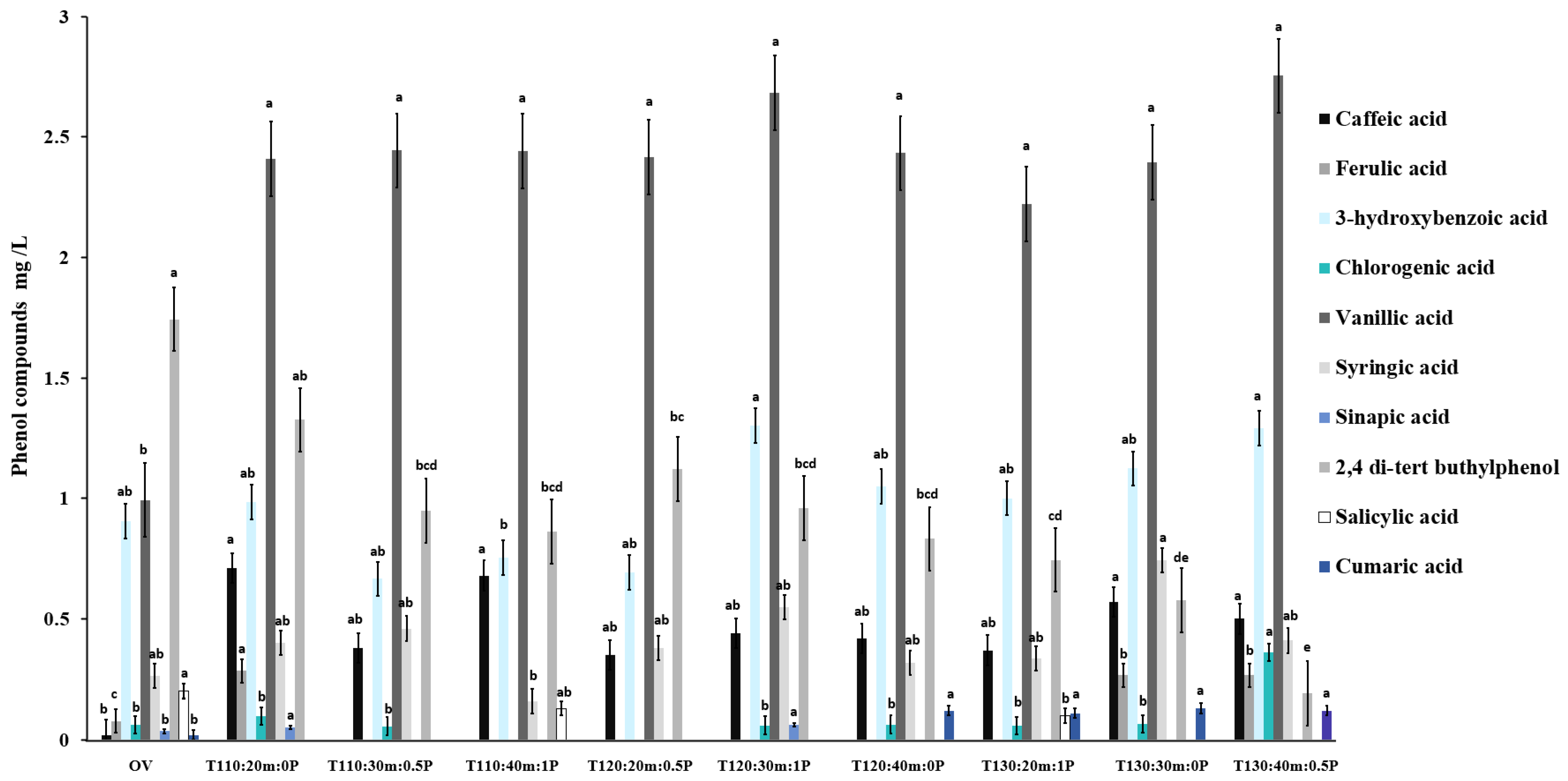
2.6. Phenol Compounds Identification
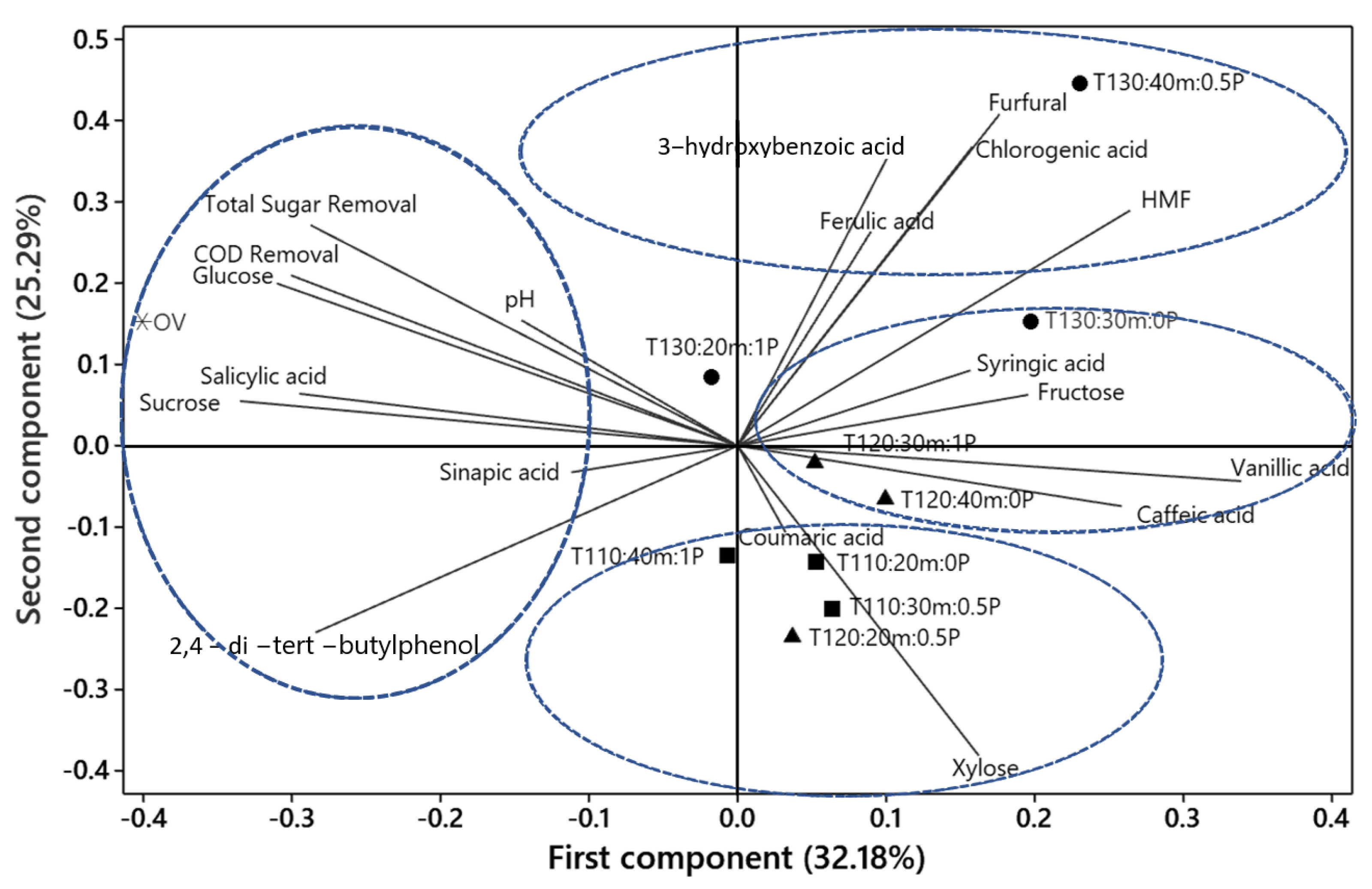
2.7. Analysis of Principal Components
3. Materials and Methods
3.1. Original Vinasse (OV)
3.2. Experimental Design
3.3. COD and Total Sugars
3.4. Furans Determination
3.5. Phenolic Compounds Determination
3.6. Sugar (Glucose, Fructose, Sucrose and Xylose) Determination
3.7. FTIR of Tequila Vinasses
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- RFA—Renewable Fuels Association, Market & Statistics: World Fuel Ethanol Production. Available online: http://ethanolrfa.org/resources/industry/statistics/ (accessed on 30 November 2022).
- Sica, P.; Carvalho, R.; Das, K.C.; Baptista, A.S. Biogas and Biofertilizer from Vinasse: Making Sugarcane Ethanol Even More Sustainable. J. Mater. Cycles Waste Manag. 2020, 22, 1427–1433. [Google Scholar] [CrossRef]
- Rojas Álvarez, O.E.; Nicolás Vázquez, M.I.; Oñate-Garzón, J.; Arango, C.A. Validation by Molecular Dynamics of the Major Components of Sugarcane Vinasse, On a Surface of Calcium Carbonate (Calcite). Molecules 2021, 26, 2353. [Google Scholar] [CrossRef] [PubMed]
- Barcelos, S.T.V.; Ferreira, I.F.L.; Costa, R.B.; Magalhães Filho, F.J.C.; Ribeiro, A.A.; Cereda, M.P. Startup of UASB Reactor with Limestone Fixed Bed Operating in the Thermophilic Range Using Vinasse as Substrate. Renew Energy 2022, 196, 610–616. [Google Scholar] [CrossRef]
- Rodrigues Reis, C.E.; Hu, B. Vinasse from Sugarcane Ethanol Production: Better Treatment or Better Utilization? Front Energy Res. 2017, 5, 7. [Google Scholar] [CrossRef]
- Garcia, C.F.H.; Souza, R.B.d.; de Souza, C.P.; Christofoletti, C.A.; Fontanetti, C.S. Toxicity of Two Effluents from Agricultural Activity: Comparing the Genotoxicity of Sugar Cane and Orange Vinasse. Ecotoxicol. Environ. Saf. 2017, 142, 216–221. [Google Scholar] [CrossRef]
- Parsaee, M.; Kiani Deh Kiani, M.; Karimi, K. A Review of Biogas Production from Sugarcane Vinasse. Biomass Bioenergy 2019, 122, 117–125. [Google Scholar] [CrossRef]
- Rastogi, M.; Sharma, S.; Kumar, S. Sugarcane Vinasse Biogas Generation: An Evaluation. Asian J. Multidimens. Res. 2021, 10, 125–131. [Google Scholar] [CrossRef]
- Sousa, R.M.O.F.; Amaral, C.; Fernandes, J.M.C.; Fraga, I.; Semitela, S.; Braga, F.; Coimbra, A.M.; Dias, A.A.; Bezerra, R.M.; Sampaio, A. Hazardous Impact of Vinasse from Distilled Winemaking By-Products in Terrestrial Plants and Aquatic Organisms. Ecotoxicol. Environ. Saf. 2019, 183, 109493. [Google Scholar] [CrossRef]
- Díaz-Vázquez, D.; Carrillo-Nieves, D.; Orozco-Nunnelly, D.A.; Senés-Guerrero, C.; Gradilla-Hernández, M.S. An Integrated Approach for the Assessment of Environmental Sustainability in Agro-Industrial Waste Management Practices: The Case of the Tequila Industry. Front Environ. Sci 2021, 9, 682093. [Google Scholar] [CrossRef]
- Singh, A.; Rodríguez-Jasso, R.M.; Saxena, R.; Cerda, R.B.; Singhania, R.R.; Ruiz, H.A. Subcritical Water Pretreatment for Agave Bagasse Fractionation from Tequila Production and Enzymatic Susceptibility. Bioresour. Technol. 2021, 338, 125536. [Google Scholar] [CrossRef]
- CRT. Producción Total de Tequila y Tequila 100%. Available online: https://www.crt.org.mx/EstadisticasCRTweb/ (accessed on 25 November 2022).
- Rodríguez-Félix, E.; Contreras-Ramos, S.; Davila-Vazquez, G.; Rodríguez-Campos, J.; Marino-Marmolejo, E. Identification and Quantification of Volatile Compounds Found in Vinasses from Two Different Processes of Tequila Production. Energies 2018, 11, 490. [Google Scholar] [CrossRef]
- Carpanez, T.G.; Moreira, V.R.; Assis, I.R.; Amaral, M.C.S. Sugarcane Vinasse as Organo-Mineral Fertilizers Feedstock: Opportunities and Environmental Risks. Sci. Total Environ. 2022, 832, 154998. [Google Scholar] [CrossRef] [PubMed]
- Muñiz-Valencia, R.; Portillo-Pérez, G.; Ceballos-Magaña, S.G.; Cortés-Quintero, G.C.; Nava-García, A.Y.; Dumont, M.-J.; Pineda-Urbina, K. Utilization of Mango Wastes as a Potential Feedstock for the Production of HMF. Biomass Convers. Biorefin. 2020, 12, 5145–5152. [Google Scholar] [CrossRef]
- Yu, I.K.M.; Tsang, D.C.W. Conversion of Biomass to Hydroxymethylfurfural: A Review of Catalytic Systems and Underlying Mechanisms. Bioresour. Technol. 2017, 238, 716–732. [Google Scholar] [CrossRef]
- Hakeem, I.G.; Halder, P.; Marzbali, M.H.; Patel, S.; Kundu, S.; Paz-Ferreiro, J.; Surapaneni, A.; Shah, K. Research Progress on Levoglucosan Production via Pyrolysis of Lignocellulosic Biomass and Its Effective Recovery from Bio-Oil. J. Environ. Chem. Eng. 2021, 9, 105614. [Google Scholar] [CrossRef]
- Moses, V.; Narula, A.; Chetan, N.; Kumar Mishra, R. Hydroxymethyl Furfural (HMF) a High Strength Cellulose Resin for Wood Composite Laminates. Heliyon 2022, 8, e12081. [Google Scholar] [CrossRef]
- Lee, K.W.; Cho, J.K.; Park, C.; Kim, B.-J. Step-by-Step Hybrid Conversion of Glucose to 5-Acetoxymethyl-2-Furfural Using Immobilized Enzymes and Cation Exchange Resin. Processes 2022, 10, 2086. [Google Scholar] [CrossRef]
- Arellano-García, L.; Velázquez-Fernández, J.B.; Macías-Muro, M.; Marino-Marmolejo, E.N. Continuous Hydrogen Production and Microbial Community Profile in the Dark Fermentation of Tequila Vinasse: Response to Increasing Loading Rates and Immobilization of Biomass. Biochem. Eng. J. 2021, 172, 108049. [Google Scholar] [CrossRef]
- García-Becerra, M.; Macías-Muro, M.; Arellano-García, L.; Aguilar-Juárez, O. Bio-Hydrogen Production from Tequila Vinasses: Effect of Detoxification with Activated Charcoal on Dark Fermentation Performance. Int. J. Hydrog. Energy 2019, 44, 31860–31872. [Google Scholar] [CrossRef]
- Hernández, D.; Rebolledo-Leiva, R.; Fernández-Puratich, H.; Quinteros-Lama, H.; Cataldo, F.; Muñoz, E.; Tenreiro, C. Recovering Apple Agro-Industrial Waste for Bioethanol and Vinasse Joint Production: Screening the Potential of Chile. Fermentation 2021, 7, 203. [Google Scholar] [CrossRef]
- Primasari, B.; Tamin, M.Z.A.; Mustafa, M.A.H. Effects of Different Pre-Treatment Methods on Anaerobic Mixed Microflora for Hydrogen Production and COD Reduction from Domestic Effluent. IOP Conf. Ser. Mater. Sci. Eng. 2019, 602, 012061. [Google Scholar] [CrossRef]
- Ghosh Ray, S.; Ghangrekar, M.M. Comprehensive Review on Treatment of High-Strength Distillery Wastewater in Advanced Physico-Chemical and Biological Degradation Pathways. IJEST 2019, 16, 527–546. [Google Scholar] [CrossRef]
- Bundhoo, Z.M.A. Effects of Microwave and Ultrasound Irradiations on Dark Fermentative Bio-Hydrogen Production from Food and Yard Wastes. Int. J. Hydrog. Energy 2017, 42, 4040–4050. [Google Scholar] [CrossRef]
- Rodríguez-Romero, J.d.J.; Aceves-Lara, C.A.; Silva, C.F.; Gschaedler, A.; Amaya-Delgado, L.; Arrizon, J. 2-Phenylethanol and 2-Phenylethylacetate Production by Nonconventional Yeasts Using Tequila Vinasses as a Substrate. Biotechnol. Rep. 2020, 25, e00420. [Google Scholar] [CrossRef] [PubMed]
- Shen, F.; Sun, S.; Zhang, X.; Yang, J.; Qiu, M.; Qi, X. Mechanochemical-Assisted Production of 5-Hydroxymethylfurfural from High Concentration of Cellulose. Cellulose 2020, 27, 3013–3023. [Google Scholar] [CrossRef]
- Jeong, G.-T. Valorization of Microalgae into 5-Hydroxymethylfurfural by Two-Step Conversion with Ferric Sulfate. J. Environ. Manag. 2021, 293, 112919. [Google Scholar] [CrossRef]
- Wang, X.; Qiu, M.; Tang, Y.; Yang, J.; Shen, F.; Qi, X.; Yu, Y. Synthesis of Sulfonated Lignin-Derived Ordered Mesoporous Carbon for Catalytic Production of Furfural from Xylose. Int. J. Biol. Macromol. 2021, 187, 232–239. [Google Scholar] [CrossRef]
- Hu, L.; Wu, Z.; Jiang, Y.; Wang, X.; He, A.; Song, J.; Xu, J.; Zhou, S.; Zhao, Y.; Xu, J. Recent Advances in Catalytic and Autocatalytic Production of Biomass-Derived 5-Hydroxymethylfurfural. Renew. Sust. Energ. Rev. 2020, 134, 110317. [Google Scholar] [CrossRef]
- Sweygers, N.; Kamali, M.; Aminabhavi, T.M.; Dewil, R.; Appels, L. Efficient Microwave-Assisted Production of Furanics and Hydrochar from Bamboo (Phyllostachys Nigra “Boryana”) in a Biphasic Reaction System: Effect of Inorganic Salts. Biomass Convers Biorefin. 2022, 12, 173–181. [Google Scholar] [CrossRef]
- Wang, J.; Wang, J.; Yu, Y. Effective and Safer Catalyst KHSO4 for Producing Furfural: A Platform Compound. Biomass Convers Biorefin. 2021, 11, 1293–1300. [Google Scholar] [CrossRef]
- Iryani, D.A.; Kumagai, S.; Nonaka, M.; Sasaki, K.; Hirajima, T. Production of 5-Hydroxymethyl Furfural from Sugarcane Bagasse under Hot Compressed Water. Procedia Earth Planet. Sci. 2013, 6, 441–447. [Google Scholar] [CrossRef]
- Santos, J.F.d.; Canettieri, E.V.; Souza, S.M.A.; Rodrigues, R.C.L.B.; Martínez, E.A. Treatment of Sugarcane Vinasse from Cachaça Production for the Obtainment of Candida Utilis CCT 3469 Biomass. Biochem Eng. J. 2019, 148, 131–137. [Google Scholar] [CrossRef]
- Candido, J.P.; Almeida, É.C.; de Oliveira Leite, D.N.; Brienzo, M.; de Franceschi de Angelis, D. Vinasse from Sugarcane Bagasse (Hemicellulose) Acid Hydrolysate and Molasses Supplemented: Biodegradability and Toxicity. Ecotoxicology 2021, 30, 818–827. [Google Scholar] [CrossRef] [PubMed]
- Komolwanich, T.; Tatijarern, P.; Prasertwasu, S.; Khumsupan, D.; Chaisuwan, T.; Luengnaruemitchai, A.; Wongkasemjit, S. Comparative Potentiality of Kans Grass (Saccharum Spontaneum) and Giant Reed (Arundo Donax) as Lignocellulosic Feedstocks for the Release of Monomeric Sugars by Microwave/Chemical Pretreatment. Cellulose 2014, 21, 1327–1340. [Google Scholar] [CrossRef]
- Kreissl, H.T.; Nakagawa, K.; Peng, Y.-K.; Koito, Y.; Zheng, J.; Tsang, S.C.E. Niobium Oxides: Correlation of Acidity with Structure and Catalytic Performance in Sucrose Conversion to 5-Hydroxymethylfurfural. J. Catal. 2016, 338, 329–339. [Google Scholar] [CrossRef]
- Li, D.; Sun, Y.; Li, R.; Ao, T.; Liu, X.; Luo, Y. Selective Conversion of Corncob Hemicellulose to Xylose via Hydrothermal Treatment with Fe2(SO4)3 and NaCl. Biomass Convers Biorefin. 2021, 12, 1231–1240. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, Y.-J.; Hao, X.; Peng, P.; Shi, J.-Y.; Peng, F.; Sun, R.-C. Hydrothermal Synthesis and Applications of Advanced Carbonaceous Materials from Biomass: A Review. Adv. Compos. Hybrid Mater. 2020, 3, 267–284. [Google Scholar] [CrossRef]
- Morales-Leal, F.J.; Rivera de la Rosa, J.; Lucio-Ortiz, C.J.; de Haro-Del Rio, D.A.; Solis Maldonado, C.; Wi, S.; Casabianca, L.B.; Garcia, C.D. Dehydration of Fructose over Thiol– and Sulfonic– Modified Alumina in a Continuous Reactor for 5–HMF Production: Study of Catalyst Stability by NMR. Appl. Catal. B 2019, 244, 250–261. [Google Scholar] [CrossRef]
- Paksung, N.; Matsumura, Y. Decomposition of Xylose in Sub- and Supercritical Water. Ind. Eng. Chem. Res. 2015, 54, 7604–7613. [Google Scholar] [CrossRef]
- Tang, Z.; Su, J. Direct Conversion of Cellulose to 5-Hydroxymethylfurfural (HMF) Using an Efficient and Inexpensive Boehmite Catalyst. Carbohydr. Res. 2019, 481, 52–59. [Google Scholar] [CrossRef]
- Wei, S.; Li, Z.; Sun, Y.; Zhang, J.; Ge, Y.; Li, Z. A Comprehensive Review on Biomass Humification: Recent Advances in Pathways, Challenges, New Applications, and Perspectives. Renew. Sustain. Energy Rev. 2022, 170, 112984. [Google Scholar] [CrossRef]
- Yoshikawa, T.; Yagi, T.; Shinohara, S.; Fukunaga, T.; Nakasaka, Y.; Tago, T.; Masuda, T. Production of Phenols from Lignin via Depolymerization and Catalytic Cracking. Fuel Process. Technol. 2013, 108, 69–75. [Google Scholar] [CrossRef]
- Catauro, M.; Barrino, F.; Dal Poggetto, G.; Crescente, G.; Piccolella, S.; Pacifico, S. New SiO2/Caffeic Acid Hybrid Materials: Synthesis, Spectroscopic Characterization, and Bioactivity. Materials 2020, 13, 394. [Google Scholar] [CrossRef] [PubMed]
- Kun, D.; Pukánszky, B. Polymer/Lignin Blends: Interactions, Properties, Applications. Eur. Polym. J. 2017, 93, 618–641. [Google Scholar] [CrossRef]
- Gromov, N.V.; Medvedeva, T.B.; Taran, O.P.; Bukhtiyarov, A.V.; Aymonier, C.; Prosvirin, I.P.; Parmon, V.N. Hydrothermal Solubilization–Hydrolysis–Dehydration of Cellulose to Glucose and 5-Hydroxymethylfurfural Over Solid Acid Carbon Catalysts. Top Catal. 2018, 61, 1912–1927. [Google Scholar] [CrossRef]
- Freitas, P.V.; da Silva, D.R.; Beluomini, M.A.; da Silva, J.L.; Stradiotto, N.R. Determination of Phenolic Acids in Sugarcane Vinasse by HPLC with Pulse Amperometry. J. Anal. Methods Chem. 2018, 2018, 4869487. [Google Scholar] [CrossRef] [PubMed]
- Molina-Cortés, A.; Sánchez-Motta, T.; Tobar-Tosse, F.; Quimbaya, M. Spectrophotometric Estimation of Total Phenolic Content and Antioxidant Capacity of Molasses and Vinasses Generated from the Sugarcane Industry. Waste Biomass Valorization 2020, 11, 3453–3463. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, H.; Zhang, D.; Liu, J.; Wang, J.; Wang, S.; Sun, B. Baijiu Vinasse Extract Scavenges Glyoxal and Inhibits the Formation of Nε-Carboxymethyllysine in Dairy Food. Molecules 2019, 24, 1526. [Google Scholar] [CrossRef]
- Chatterjee, N.S.; Panda, S.K.; Navitha, M.; Asha, K.K.; Anandan, R.; Mathew, S. Vanillic Acid and Coumaric Acid Grafted Chitosan Derivatives: Improved Grafting Ratio and Potential Application in Functional Food. J. Food Sci. Technol. 2015, 52, 7153–7162. [Google Scholar] [CrossRef]
- Silva, T.; Oliveira, C.; Borges, F. Caffeic Acid Derivatives, Analogs and Applications: A Patent Review (2009–2013). Expert Opin. Pat 2014, 24, 1257–1270. [Google Scholar] [CrossRef]
- Stanely Mainzen Prince, P.; Rajakumar, S.; Dhanasekar, K. Protective Effects of Vanillic Acid on Electrocardiogram, Lipid Peroxidation, Antioxidants, Proinflammatory Markers and Histopathology in Isoproterenol Induced Cardiotoxic Rats. Eur. J. Pharm. 2011, 668, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Zhou, X.; Guo, K.; Zhou, F.; Yang, H. Use of Chlorogenic Acid against Diabetes Mellitus and Its Complications. J. Immunol. Res. 2020, 2020, 9680508. [Google Scholar] [CrossRef] [PubMed]
- Cea Barcia, G.E.; Imperial Cervantes, R.A.; Torres Zuniga, I.; van den Hende, S. Converting Tequila Vinasse Diluted with Tequila Process Water into Microalgae-Yeast Flocs and Dischargeable Effluent. Bioresour. Technol. 2020, 300, 122644. [Google Scholar] [CrossRef] [PubMed]
- Dubois, M.; Gilles, K.; Hamilton, J.K.; Rebers, P.A.; Smith, F. A Colorimetric Method for the Determination of Sugars. Nature 1951, 168, 167. [Google Scholar] [CrossRef] [PubMed]
| Treatment | COD Removal (%) | Total Sugars Removal (%) | HMF mg/L | Furfural mg/L | Sucrose mg/L | Fructose mg/L | Xylose mg/L | Glucose mg/L |
|---|---|---|---|---|---|---|---|---|
| OV | - | - | 10.45 ± 0.01 g | 0.08 ± 0.01 d | 30.95 ± 0.02 a | 26.80 ± 0.04 cd | 18.49 ± 0.04 c | 100.1 ± 0.10 a |
| T110:20m:0P | 14.1 ± 2.0 c | 31.1 ± 3.7 c | 39.41 ± 1.2 f | n.d. | 5.1 ± 0.2 b | 62.8 ± 0.2 abc | 161.4 ± 0.2 ab | 23.9 ±0.107 b |
| T110:30m:0.5P | 16.2 ± 4.4 c | 34.2 ± 3.4 bc | 40.84 ± 0.7 f | n.d. | 4.4 ± 0.3 bc | 86.4 ±0.07 ab | 177.8 ± 0.04 a | 15.9 ± 0.019 b |
| T110:40m:1P | 14.1 ± 0.9 c | 36.5 ± 4.3 bc | 44.23 ± 1.3 e | 0.17 ± 10.1 c | 2.8 ± 0.1 cd | 77.9 ± 0.1 ab | 95.0 ± 0.7 abc | 16.21 ± 0.059 b |
| T120:20m:0.5P | 24.9 ± 4.5 b | 31.1 ± 4.4 c | 57.66 ± 1.35 d | n.d. | 2.0 ± 0.5 de | 67.3 ± 0.05 ab | 184.5 ±0.246 a | 15.2 ± 0.053 b |
| T120:30m:1P | 28.1 ±0.7 ab | 42.6 ± 0.4 b | 81.73 ± 1.45 c | 0.88 ± 1.3 b | 0.4 ± 0.1 ef | 22.8 ± 0.03 d | 159.5 ± 0.430 ab | 21.7 ±0.008 b |
| T120:40m:0P | 29.3 ± 0.5 ab | 34.9 ± 4.0 bc | 79.11 ± 1.60 c | 1.33 ± 0.5 ab | 0.1 ± 0.1 f | 48.6 ± 0.2 bcd | 177.0 ± 0.357 a | 17.7 ± 0.023 b |
| T130:20m:1P | 28.6 ±1.1 ab | 55.1 ± 0.9 a | 74.16 ± 2.96 c | 0.90 ± 2.6 b | 0.2 ± 0.2 f | 75.3 ± 0.1 ab | 71.5 ± 0.445 bc | 21.1 ± 0.062 b |
| T130:30m:0P | 31.1 ± 4.5 ab | 34.6 ± 1.6 bc | 93.80 ± 2.49 b | 1.68 ± 0.4 ab | 0.2± 0.04 f | 64.4 ± 0.2 ab | 125.5 ± 0.197 ab | 24.5 ± 0.032 b |
| T130:40m:0.5P | 32.9 ± 2.4 a | 57.3 ± 2.7 a | 127.0 ± 9.71 a | 3.07 ± 0.3 a | 0.02 ± 0.01 f | 99.7 ± 0.03 a | 29.47 ± 0.117 c | 31.7 ± 0.018 b |
| Compounds | Temperature | Time | Catalyst | Temperature × Time | Catalyst × Temperature | Catalyst × Time | Temperature × Time × Catalyst |
|---|---|---|---|---|---|---|---|
| COD | <0.0001 | 0.156 | 0.759 | <0.0001 | 0.002 | 0.508 | 0.017 |
| Total sugars | 0.012 | 0.071 | 0.224 | 0.090 | 0.033 | 0.204 | 0.096 |
| 5-HMF | <0.0001 | <0.0001 | 0.556 | <0.0001 | <0.0001 | 0.010 | 0.002 |
| Furfural | 0.349 | 0.475 | 0.309 | 0.263 | 0.431 | 0.305 | 0.005 |
| Fructose | 0.086 | 0.415 | 0.189 | 0.240 | 0.048 | 0.435 | 0.085 |
| Glucose | <0.0001 | <0.0001 | 0.490 | 0.002 | 0.002 | 0.006 | 0.063 |
| Sucrose | <0.0001 | <0.0001 | 0.525 | <0.0001 | <0.0001 | 0.005 | 0.001 |
| Xylose | 0.037 | 0.297 | 0.956 | 0.058 | 0.119 | 0.570 | 0.127 |
| Caffeic acid | 0.605 | 0.550 | 0.311 | 0.639 | 0.608 | 0.562 | 0.679 |
| ferulic acid | 0.477 | 0.999 | 0.312 | 0.843 | 0.347 | 0.821 | 0.767 |
| 3-hidroxybenzoic acid | 0.325 | 0.863 | 0.760 | 0.581 | 0.586 | 0.945 | 0.825 |
| Chlorogenic acid | 0.482 | 0.706 | 0.514 | 0.653 | 0.642 | 0.809 | 0.809 |
| Vanillic acid | 0.002 | 0.001 | 0.426 | 0.015 | 0.025 | 0.012 | 0.161 |
| Syringic acid | 0.606 | 0.068 | 0.817 | 0.108 | 0.780 | 0.147 | 0.153 |
| Sinapic acid | 0.663 | 0.663 | 0.516 | 0.810 | 0.810 | 0.810 | 0.922 |
| 2,4-di-tert-buthylphenol acid | 0.018 | 0.082 | 0.542 | 0.003 | 0.120 | 0.327 | 0.003 |
| Salicylic acid | 0.094 | 0.094 | 0.481 | 0.293 | 0.062 | 0.062 | 0.206 |
| Cumaric acid | 0.050 | 0.828 | 0.799 | 0.137 | 0.109 | 0.909 | 0.237 |
| Treatment Code | Temperature (°C) | Time (min) | Catalyst H2O2 (%) |
|---|---|---|---|
| T110:20m:0P | 110 | 20 | 0 |
| T110:30m:0.5P | 110 | 30 | 0.5 |
| T110:40m:1P | 110 | 40 | 1.0 |
| T120:20m:0.5P | 120 | 20 | 0.5 |
| T120:30m:1P | 120 | 30 | 1.0 |
| T120:40m:0P | 120 | 40 | 0 |
| T130:20m:1P | 130 | 20 | 1.0 |
| T130:30m:0P | 130 | 30 | 0 |
| T130:40m:0.5P | 130 | 40 | 0.5 |
| Phenolic Compounds | tR (min) | Ion Mode | MRM (m/z) | Cone (V) | Collision (ev) |
|---|---|---|---|---|---|
| Caffeic acid | 8 | - | 179 > 135 | 20 | 10 |
| ferulic acid | 13.9 | - | 193 > 134 | 30 | 15 |
| 3-hydroxybenzoic acid | 8 | - | 137 > 93 | 10 | 10 |
| Chlorogenic acid | 6.5 | - | 353 > 191 | 20 | 15 |
| Vanillic acid | 13 | - | 167 > 108 | 15 | 15 |
| Syringic acid | 13.9 | - | 197 > 123 | 15 | 22 |
| Sinapic acid | 13.9 | - | 223 > 164 | 15 | 15 |
| 2,4-di-tert-buthylphenol acid | 14 | + | 207 > 207 | 10 | 0 |
| Salicylic acid | 14 | - | 137 > 65 | 25 | 18 |
| Cumaric acid | 13.9 | - | 163 > 119 | 20 | 10 |
| Furans | tR (min) | Ion Mode | MRM (m/z) | Cone (V) | Collision (ev) |
| Furfural | 1 | + | 97 > 41 | 25 | 15 |
| 5-HMF | 4.4 | + | 127 > 81 | 20 | 20 |
| Reducing Sugars | tR (min) | Ion Mode | SIR (m/z) | Cone (V) | Collision (ev) |
|---|---|---|---|---|---|
| Xylose | 8 | - | 149 > 59 | 20 | 15 |
| Glucose | 7 | - | 179 > 89 | 10 | 12 |
| Fructose | 7 | - | 341 > 179 | 22 | 5 |
| Sucrose | 7 | - | 341 > 179 | 22 | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lorenzo-Santiago, M.A.; Rodríguez-Campos, J.; Rendón-Villalobos, R.; García-Hernández, E.; Vallejo-Cardona, A.A.; Contreras-Ramos, S.M. Thermal Treatment to Obtain 5-Hydroxymethyl Furfural (5-HMF), Furfural and Phenolic Compounds from Vinasse Waste from Agave. Molecules 2023, 28, 1063. https://doi.org/10.3390/molecules28031063
Lorenzo-Santiago MA, Rodríguez-Campos J, Rendón-Villalobos R, García-Hernández E, Vallejo-Cardona AA, Contreras-Ramos SM. Thermal Treatment to Obtain 5-Hydroxymethyl Furfural (5-HMF), Furfural and Phenolic Compounds from Vinasse Waste from Agave. Molecules. 2023; 28(3):1063. https://doi.org/10.3390/molecules28031063
Chicago/Turabian StyleLorenzo-Santiago, Miguel Angel, Jacobo Rodríguez-Campos, Rodolfo Rendón-Villalobos, Edgar García-Hernández, Alba Adriana Vallejo-Cardona, and Silvia Maribel Contreras-Ramos. 2023. "Thermal Treatment to Obtain 5-Hydroxymethyl Furfural (5-HMF), Furfural and Phenolic Compounds from Vinasse Waste from Agave" Molecules 28, no. 3: 1063. https://doi.org/10.3390/molecules28031063
APA StyleLorenzo-Santiago, M. A., Rodríguez-Campos, J., Rendón-Villalobos, R., García-Hernández, E., Vallejo-Cardona, A. A., & Contreras-Ramos, S. M. (2023). Thermal Treatment to Obtain 5-Hydroxymethyl Furfural (5-HMF), Furfural and Phenolic Compounds from Vinasse Waste from Agave. Molecules, 28(3), 1063. https://doi.org/10.3390/molecules28031063







