Monometallic and Bimetallic Catalysts Supported on Praseodymium-Doped Ceria for the Water–Gas Shift Reaction
Abstract
1. Introduction
2. Results and Discussion
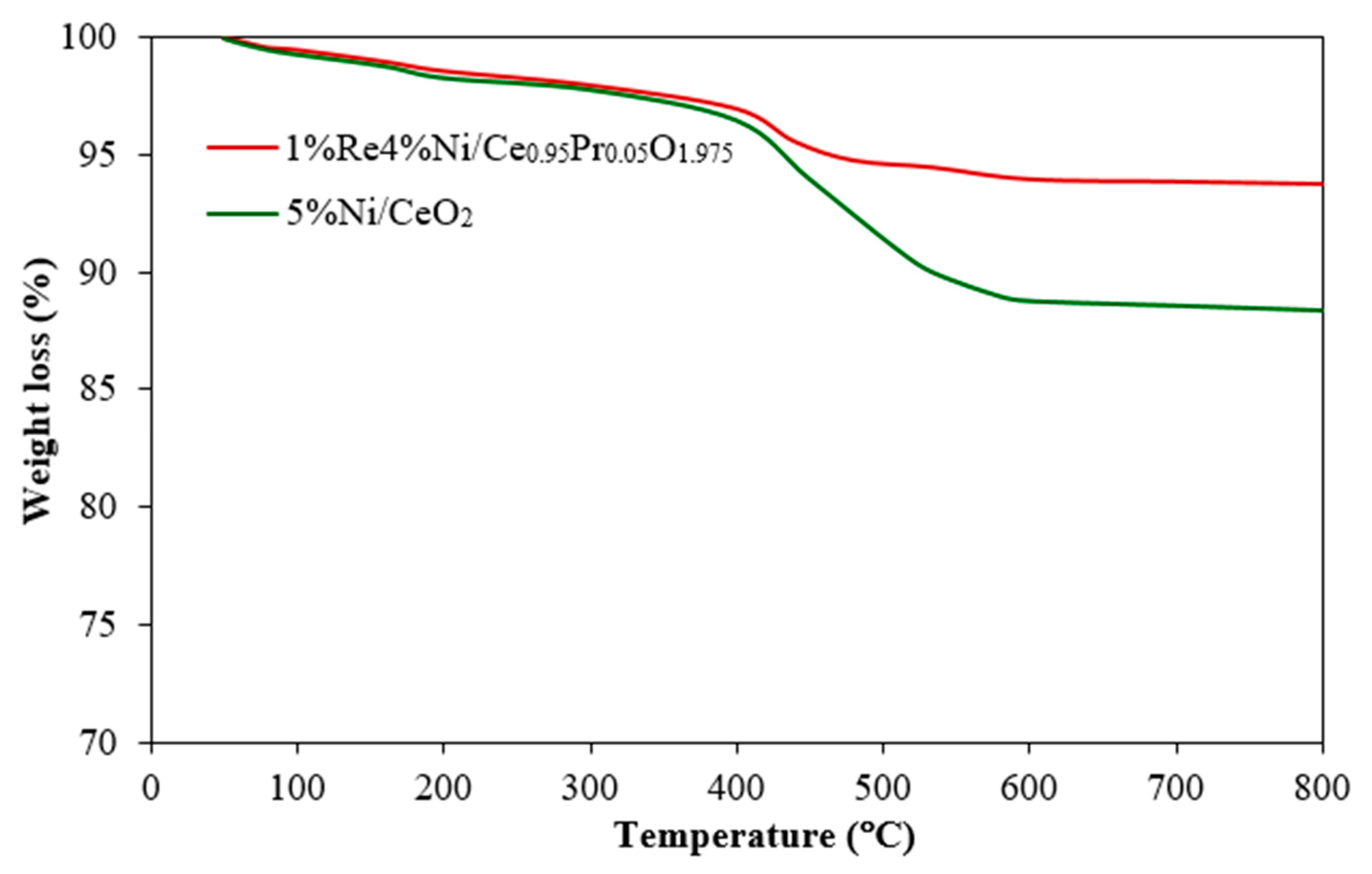
2.1. Catalysts Characterization
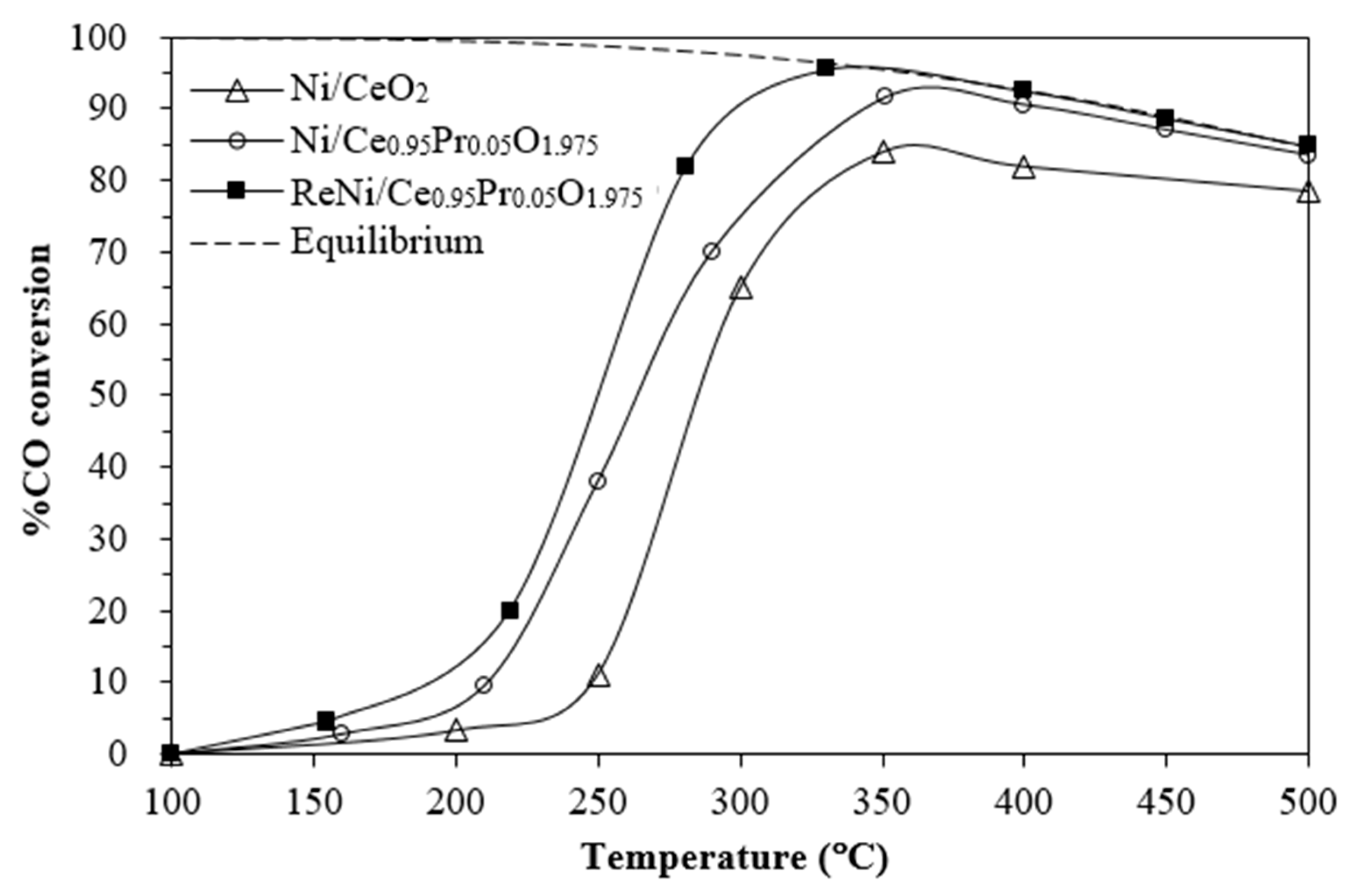
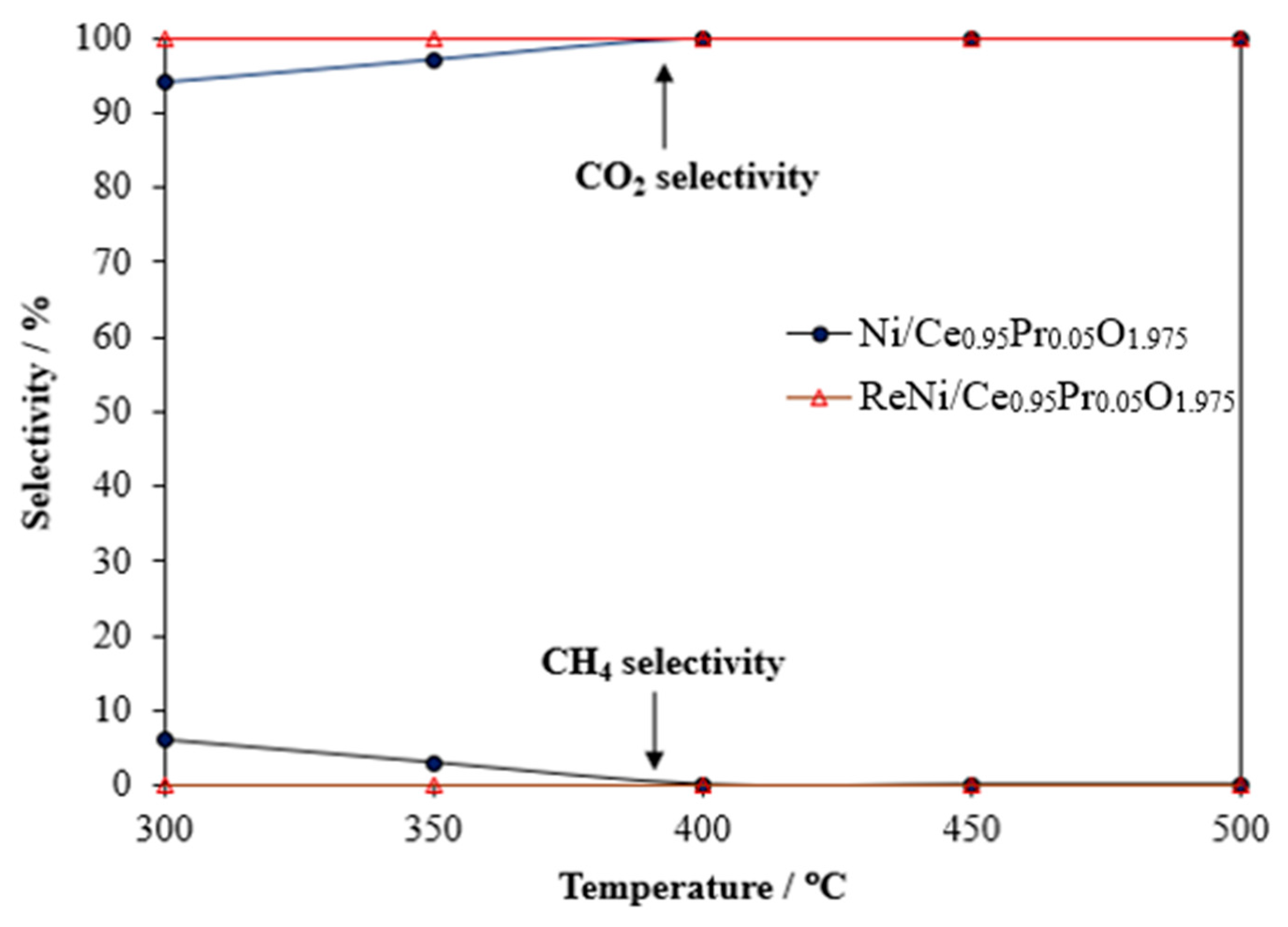
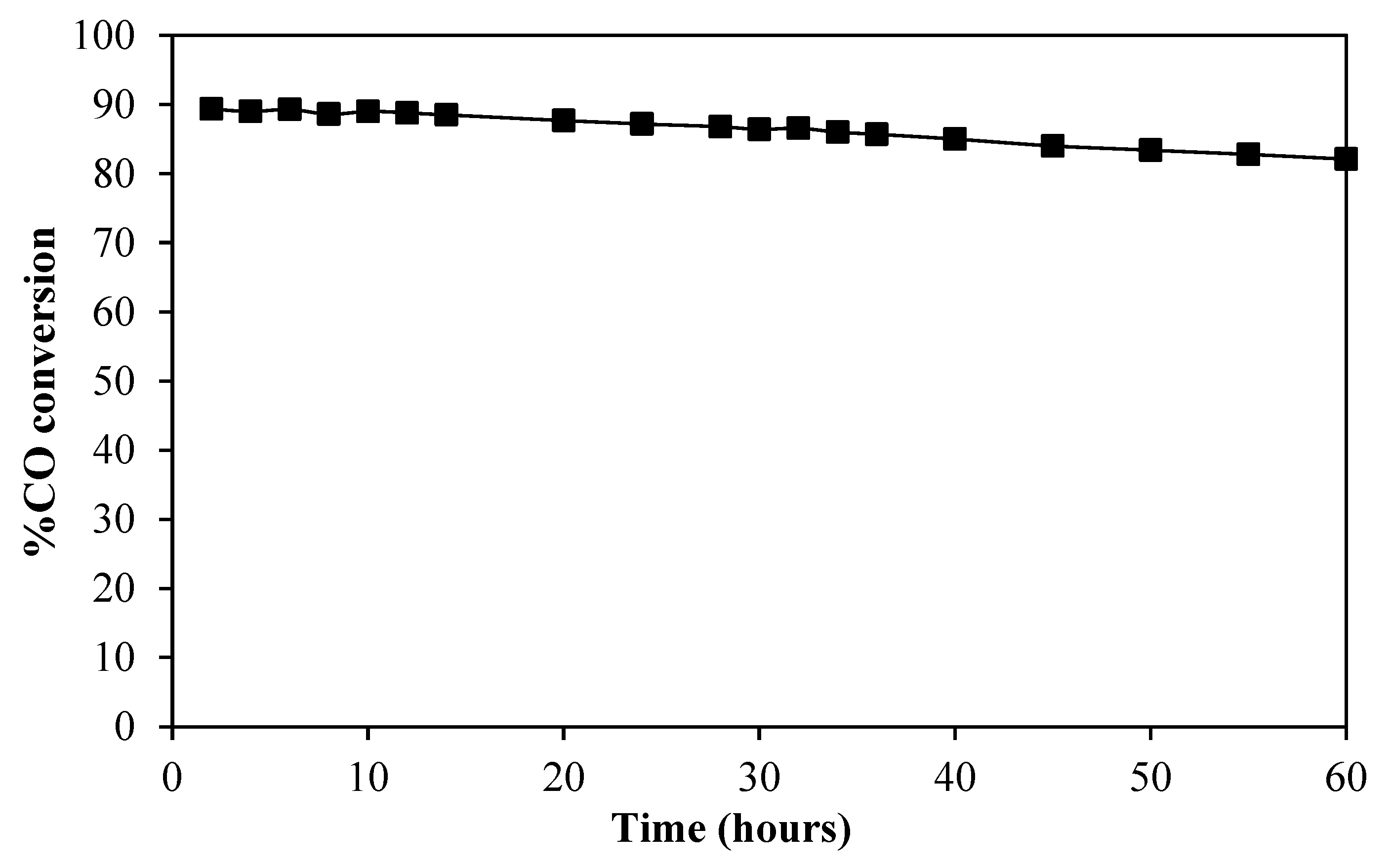
2.2. Water–Gas Shift Activity and Stability
3. Experimental Procedure
3.1. Catalysts Preparation
3.2. Catalyst Characterization
3.3. Water–Gas Shift Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, H.; Lei, Q.; Li, P.; Liu, C.; Xue, Y.; Zhang, X.; Li, C.; Yang, Z. Key CO2 capture technology of pure oxygen exhaust gas combustion for syngas-fueled high-temperature fuel cells. Int. J. Coal Sci. Technol. 2021, 8, 383–393. [Google Scholar] [CrossRef]
- Molochas, C.; Tsiakaras, P. Carbon Monoxide Tolerant Pt-Based Electrocatalysts for H2-PEMFC Applications: Current Progress and Challenges. Catalysts 2021, 11, 1127. [Google Scholar] [CrossRef]
- Kim, K.; Yoo, J.D.; Lee, S.; Bae, M.; Bae, J.; Jung, W.; Han, J.W. A Simple Descriptor to Rapidly Screen CO Oxidation Activity on Rare-Earth Metal-Doped CeO2: From Experiment to First-Principles. ACS Appl. Mater. Interfaces 2017, 9, 15449–15458. [Google Scholar] [CrossRef] [PubMed]
- Kayaalp, B.; Lee, S.; Klauke, K.; Seo, J.; Nodari, L.; Kornowski, A.; Jung, W.; Mascotto, S. Template-free mesoporous La0.3Sr0.7Ti1−xFexO3±δ for CH4 and CO oxidation catalysis. Appl. Catal. B Environ. 2019, 245, 536–545. [Google Scholar] [CrossRef]
- Montini, T.; Melchionna, M.; Monai, M.; Fornasiero, P. Fundamentals and Catalytic Applications of CeO2-Based Materials. Chem. Rev. 2016, 116, 5987–6041. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, J.A.; Grinter, D.C.; Liu, Z.; Palomino, R.M.; Senanayake, S.D. Ceria-based model catalysts: Fundamental studies on the importance of the metal–ceria interface in CO oxidation, the water–gas shift, CO2 hydrogenation, and methane and alcohol reforming. Chem. Soc. Rev. 2017, 46, 1824–1841. [Google Scholar] [CrossRef] [PubMed]
- Atribak, I.; Such-Basáñez, I.; Bueno-López, A.; García, A. Comparison of the catalytic activity of MO2 (M = Ti, Zr, Ce) for soot oxidation under NOx/O2. J. Catal. 2007, 250, 75–84. [Google Scholar] [CrossRef]
- Aneggi, E.; de Leitenburg, C.; Trovarelli, A. On the role of lattice/surface oxygen in ceria–zirconia catalysts for diesel soot combustion. Catal. Today 2012, 181, 108–115. [Google Scholar] [CrossRef]
- Damyanova, S.; Pawelec, B.; Arishtirova, K.; Huerta, M.M.; Fierro, J. Study of the surface and redox properties of ceria–zirconia oxides. Appl. Catal. A Gen. 2008, 337, 86–96. [Google Scholar] [CrossRef]
- Hernández, W.Y.; Centeno, M.A.; Romero-Sarria, F.; Odriozola, J.A. Synthesis and Characterization of Ce1−xEuxO2−x/2 Mixed Oxides and Their Catalytic Activities for CO Oxidation. J. Phys. Chem. C 2009, 113, 5629–5635. [Google Scholar] [CrossRef]
- Lomonaco, J.G.; Sesuk, T.; Charojrochkul, S.; Tepamatr, P. Effect of Re Addition on the Water–Gas Shift Activity of Ni Catalyst Supported by Mixed Oxide Materials for H2 Production. Catalysts 2023, 13, 959. [Google Scholar] [CrossRef]
- Tojira, O.; Tepamatr, P. Catalytic Activity of Ni Based Materials Prepared by Different Methods for Hydrogen Production via the Water Gas Shift Reaction. Catalysts 2023, 13, 176. [Google Scholar] [CrossRef]
- Atribak, I.; Bueno-López, A.; García-García, A. Thermally stable ceria–zirconia catalysts for soot oxidation by O2. Catal. Commun. 2008, 9, 250–255. [Google Scholar] [CrossRef]
- Atribak, I.; Bueno-López, A.; García-García, A. Role of yttrium loading in the physico-chemical properties and soot combustion activity of ceria and ceria–zirconia catalysts. J. Mol. Catal. A Chem. 2009, 300, 103–110. [Google Scholar] [CrossRef]
- Kırkgeçit, R.; Torun, H.; Dokan, F.K.; Öztürk, E. Optical and electrical conductivity properties of rare earth elements (Sm, Y, La, Er) co-doped CeO2. J. Rare Earths 2021, 40, 1619–1627. [Google Scholar] [CrossRef]
- Reddy, B.M.; Saikia, P.; Bharali, P.; Yamada, Y.; Kobayashi, T.; Muhler, M.; Grünert, W. Structural Characterization and Catalytic Activity of Nanosized Ceria-Terbia Solid Solutions. J. Phys. Chem. C 2008, 112, 16393–16399. [Google Scholar] [CrossRef]
- Somacescu, S.; Parvulescu, V.; Calderon-Moreno, J.M.; Suh, S.-H.; Osiceanu, P.; Su, B.-L. Uniform nanoparticles building Ce1−xPrxO2−δ mesoarchitectures: Structure, morphology, surface chemistry, and catalytic performance. J. Nanoparticle Res. 2012, 14, 885. [Google Scholar] [CrossRef]
- Zhang, G.; Zhao, Z.; Xu, J.; Zheng, J.; Liu, J.; Jiang, G.; Duan, A.; He, H. Comparative study on the preparation, characterization and catalytic performances of 3DOM Ce-based materials for the combustion of diesel soot. Appl. Catal. B Environ. 2011, 107, 302–315. [Google Scholar] [CrossRef]
- Rodriguez, J.A.; Hanson, J.C.; Kim, J.-Y.; Liu, G.; Iglesias-Juez, A.; Fernández-García, M. Properties of CeO2 and Ce1−xZrxO2 Nanoparticles: X-ray Absorption Near-Edge Spectroscopy, Density Functional, and Time-Resolved X-ray Diffraction Studies. J. Phys. Chem. B 2003, 107, 3535–3543. [Google Scholar] [CrossRef]
- Wang, X.; Hanson, J.C.; Liu, G.; Rodriguez, J.A.; Iglesias-Juez, A.; Fernández-García, M. The behavior of mixed-metal oxides: Physical and chemical properties of bulk Ce1−xTbxO2 and nanoparticles of Ce1−xTbxOy. J. Chem. Phys. 2004, 121, 5434–5444. [Google Scholar] [CrossRef]
- Ryan, K.M.; McGrath, J.P.; Farrell, R.A.; O’Neill, W.M.; Barnes, C.J.; Morris, M.A. Morris, Measurements of the lattice constant of ceria when doped with lanthana and praseodymia-the possibility of local defect ordering and the observation of extensive phase separation. J. Phys. Condens. Matter 2003, 15, L49. [Google Scholar] [CrossRef]
- Poggio-Fraccari, E.; Irigoyen, B.; Baronetti, G.; Mariño, F. Ce-Pr mixed oxides as active supports for Water-gas Shift reaction: Experimental and density functional theory characterization. Appl. Catal. A Gen. 2014, 485, 123–132. [Google Scholar] [CrossRef]
- Poggio-Fraccari, E.; Baronetti, G.; Mariño, F. Pr3+ surface fraction in CePr mixed oxides determined by XPS analysis. J. Electron Spectrosc. Relat. Phenom. 2018, 222, 1–4. [Google Scholar] [CrossRef]
- Mariño, F.; Descorme, C.; Duprez, D. Supported base metal catalysts for the preferential oxidation of carbon monoxide in the presence of excess hydrogen (PROX). Appl. Catal. B Environ. 2005, 58, 175–183. [Google Scholar] [CrossRef]
- Hung, C.-M. The effect of the calcination temperature on the activity of Cu–La–Ce composite metal catalysts for the catalytic wet oxidation of ammonia solution. Powder Technol. 2009, 191, 21–26. [Google Scholar] [CrossRef]
- Chen, Y.-Z.; Liaw, B.-J.; Chen, H.-C. Selective oxidation of CO in excess hydrogen over CuO/CexZr1−xO2 catalysts. Int. J. Hydrogen Energy 2006, 31, 427–435. [Google Scholar] [CrossRef]
- Ratnasamy, C.; Wagner, J.P. Water Gas Shift Catalysis. Catal. Rev. 2009, 51, 325–440. [Google Scholar] [CrossRef]
- Kim, S.H.; Chung, J.H.; Kim, Y.T.; Han, J.; Yoon, S.P.; Nam, S.W.; Lim, T.-H.; Lee, H.-I. SiO2/Ni and CeO2/Ni catalysts for single-stage water gas shift reaction. Int. J. Hydrogen Energy 2010, 35, 3136–3140. [Google Scholar] [CrossRef]
- Hilaire, S.; Wang, X.; Luo, T.; Gorte, R.; Wagner, J. A comparative study of water-gas-shift reaction over ceria supported metallic catalysts. Appl. Catal. A Gen. 2001, 215, 271–278. [Google Scholar] [CrossRef]
- Poggio-Fraccari, E.; Mariño, F.; Laborde, M.; Baronetti, G. Copper and nickel catalysts supported on praseodymium-doped ceria (PDC) for the water-gas shift reaction. Appl. Catal. A Gen. 2013, 460–461, 15–20. [Google Scholar] [CrossRef]
- Wang, T.; Porosoff, M.D.; Chen, J.G. Effects of oxide supports on the water-gas shift reaction over PtNi bimetallic catalysts: Activity and methanation inhibition. Catal. Today 2014, 233, 61–69. [Google Scholar] [CrossRef]
- Wang, D.; Li, Y.; Wen, L.; Xi, J.; Liu, P.; Hansen, T.W.; Li, P. Ni-Pd-incorporated Fe3O4 yolk-shelled nanospheres as efficient magnetically recyclable catalysts for reduction of n-containing unsaturated compounds. Catalysts 2023, 13, 190. [Google Scholar] [CrossRef]
- Wen, L.; Wang, D.; Xi, J.; Tian, F.; Liu, P.; Bai, Z.-W. Heterometal modified Fe3O4 hollow nanospheres as efficient catalysts for organic transformations. J. Catal. 2022, 413, 779–785. [Google Scholar] [CrossRef]
- Guo, X.; Li, M.; Qiu, L.; Tian, F.; He, L.; Geng, S.; Liu, Y.; Song, Y.; Yang, W.; Yu, Y. Engineering electron redistribution of bimetallic phosphates with CeO2 enables high-performance overall water splitting. Chem. Eng. J. 2023, 453, 139796. [Google Scholar] [CrossRef]
- Iida, H.; Igarashi, A. Difference in the reaction behavior between Pt–Re/TiO2 (Rutile) and Pt–Re/ZrO2 catalysts for low-temperature water gas shift reactions. Appl. Catal. A Gen. 2006, 303, 48–55. [Google Scholar] [CrossRef]
- Lomonaco, J.G.; Tojira, O.; Charojrochkul, S.; Tepamatr, P. Structure-Activity Relationship of Ceria Based Catalyst for Hydrogen Production. Chiang Mai J. Sci. 2022, 49, 1129–1134. [Google Scholar] [CrossRef]
- Tepamatr, P.; Laosiripojana, N.; Charojrochkul, S. Water gas shift reaction over monometallic and bimetallic catalysts supported by mixed oxide materials. Appl. Catal. A Gen. 2016, 523, 255–262. [Google Scholar] [CrossRef]
- Tojira, O.; Lomonaco, J.G.; Sesuk, T.; Charojrochkul, S.; Tepamatr, P. Enhancement of hydrogen production using Ni catalysts supported by Gd-doped ceria. Heliyon 2021, 7, e08202. [Google Scholar] [CrossRef]
- Saw, E.T.; Oemar, U.; Ang, M.L.; Kus, H.; Kawi, S. High-temperature water gas shift reaction on Ni–Cu/CeO2 catalysts: Effect of ceria nanocrystal size on carboxylate formation. Catal. Sci. Technol. 2016, 6, 5336–5349. [Google Scholar] [CrossRef]
- Tsakoumis, N.E.; Walmsley, J.C.; Rønning, M.; van Beek, W.; Rytter, E.; Holmen, A. Evaluation of Reoxidation Thresholds for γ-Al2O3-Supported Cobalt Catalysts under Fischer–Tropsch Synthesis Conditions. J. Am. Chem. Soc. 2017, 139, 3706–3715. [Google Scholar] [CrossRef]
- D’angelo, A.M.; Chaffee, A.L. Correlations between Oxygen Uptake and Vacancy Concentration in Pr-Doped CeO2. ACS Omega 2017, 2, 2544–2551. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, T.; Watanabe, T.; Sugiyama, N.; Subramani, A.K.; Wagata, H.; Matsushita, N.; Yoshimura, M. Identifying Defects in Ceria-Based Nanocrystals by UV Resonance Raman Spectroscopy. J. Phys. Chem. C 2009, 113, 19789–19793. [Google Scholar] [CrossRef]
- Lee, Y.; He, G.; Akey, A.J.; Si, R.; Flytzani-Stephanopoulos, M.; Herman, I.P. Raman Analysis of Mode Softening in Nanoparticle CeO2−δ and Au-CeO2−δ during CO Oxidation. J. Am. Chem. Soc. 2011, 133, 12952–12955. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Lu, J.; Wu, Y.; Wang, Y.; Luo, M. UV and Visible Raman Studies of Oxygen Vacancies in Rare-Earth-Doped Ceria. Langmuir 2011, 27, 3872–3877. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.J.; Virkar, A.V. Lattice Parameters and Densities of Rare-Earth Oxide Doped Ceria Electrolytes. J. Am. Ceram. Soc. 1995, 78, 433–439. [Google Scholar] [CrossRef]
- Guo, J.; Lou, H.; Zhao, H.; Chai, D.; Zheng, X. Dry reforming of methane over nickel catalysts supported on magnesium aluminate spinels. Appl. Catal. A Gen. 2004, 273, 75–82. [Google Scholar] [CrossRef]
- Akiki, E.; Akiki, D.; Italiano, C.; Vita, A.; Abbas-Ghaleb, R.; Chlala, D.; Ferrante, G.D.; Laganà, M.; Pino, L.; Specchia, S. Production of hydrogen by methane dry reforming: A study on the effect of cerium and lanthanum on Ni/MgAl2O4 catalyst performance. Int. J. Hydrogen Energy 2020, 45, 21392–21408. [Google Scholar] [CrossRef]
- Parastaev, A.; Muravev, V.; Osta, E.H.; van Hoof, A.J.F.; Kimpel, T.F.; Kosinov, N.; Hensen, E.J.M. Boosting CO2 hydrogenation via size-dependent metal–support interactions in cobalt/ceria-based catalysts. Nat. Catal. 2020, 3, 526–533. [Google Scholar] [CrossRef]
- Sartoretti, E.; Novara, C.; Chiodoni, A.; Giorgis, F.; Piumetti, M.; Bensaid, S.; Russo, N.; Fino, D. Nanostructured ceria-based catalysts doped with La and Nd: How acid-base sites and redox properties determine the oxidation mechanisms. Catal. Today 2021, 390–391, 117–134. [Google Scholar] [CrossRef]
- Sultana, S.; Mansingh, S.; Scurrell, M.; Parida, K.M. Controlled Synthesis of CeO2NS-Au-CdSQDs Ternary Nanoheterostructure: A Promising Visible Light Responsive Photocatalyst for H2 Evolution. Inorg. Chem. 2017, 56, 12297–12307. [Google Scholar] [CrossRef]
- Mansingh, S.; Padhi, D.K.; Parida, K.M. Enhanced visible light harnessing and oxygen vacancy promoted N, S co-doped CeO2 nanoparticle: A challenging photocatalyst for Cr(vi) reduction. Catal. Sci. Technol. 2017, 7, 2772–2781. [Google Scholar] [CrossRef]
- Wang, L.; Li, D.; Koike, M.; Koso, S.; Nakagawa, Y.; Xu, Y.; Tomishige, K. Catalytic performance and characterization of Ni-Fe catalysts for the steam reforming of tar from biomass pyrolysis to synthesis gas. Appl. Catal. A Gen. 2011, 392, 248–255. [Google Scholar] [CrossRef]
- Sahoo, S.K.; Ray, S.S.; Singh, I. Structural characterization of coke on spent hydroprocessing catalysts used for processing of vacuum gas oils. Appl. Catal. A Gen. 2004, 278, 83–91. [Google Scholar] [CrossRef]
- Chayakul, K.; Srithanratana, T.; Hengrasmee, S. Effect of Re addition on the activities of Co/CeO2 catalysts for water gas shift reaction. J. Mol. Catal. A Chem. 2011, 340, 39–47. [Google Scholar] [CrossRef]
- Takeda, T.; Kato, K.; Kikkawa, S. Gel Combustion Synthesis of Rare Earth Aluminate Using Glycine or Urea. J. Ceram. Soc. Jpn. 2007, 115, 588–591. [Google Scholar] [CrossRef][Green Version]
- Tepamatr, P.; Laosiripojana, N.; Sesuk, T.; Charojrochkul, S. Effect of samarium and praseodymium addition on water gas shift performance of Co/CeO2 catalysts. J. Rare Earths 2020, 38, 1201–1206. [Google Scholar] [CrossRef]
- Tuti, S.; Luisetto, I.; Laverdura, U.P.; Marconi, E. Dry Reforming of Methane on Ni/Nanorod-CeO2 Catalysts Prepared by One-Pot Hydrothermal Synthesis: The Effect of Ni Content on Structure, Activity, and Stability. Reactions 2022, 3, 333–351. [Google Scholar] [CrossRef]
- Di Michele, A.; Dell’Angelo, A.; Tripodi, A.; Bahadori, E.; Sànchez, F.; Motta, D.; Dimitratos, N.; Rossetti, I.; Ramis, G. Steam reforming of ethanol over Ni/MgAl2O4 catalysts. Int. J. Hydrogen Energy 2018, 44, 952–964. [Google Scholar] [CrossRef]

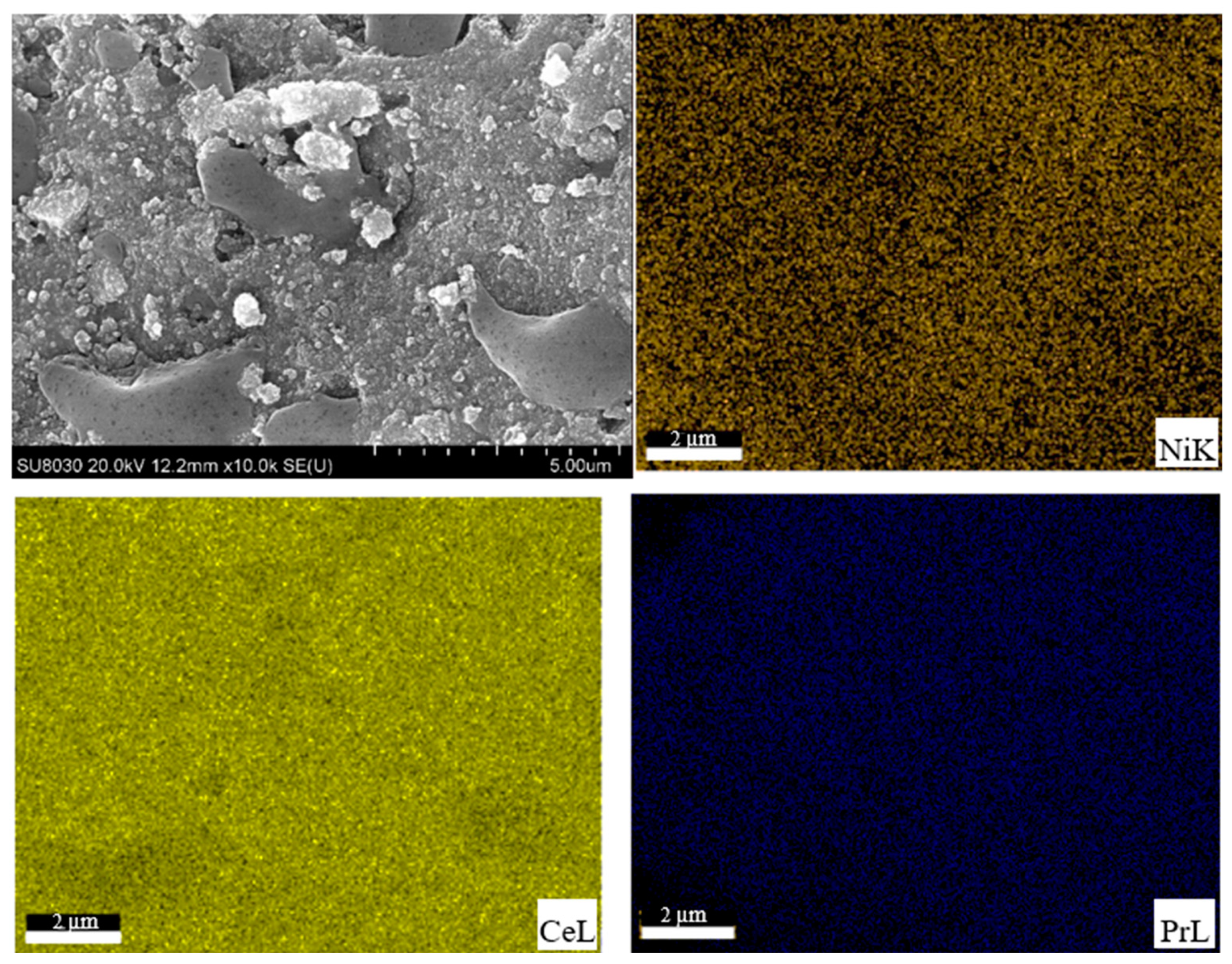
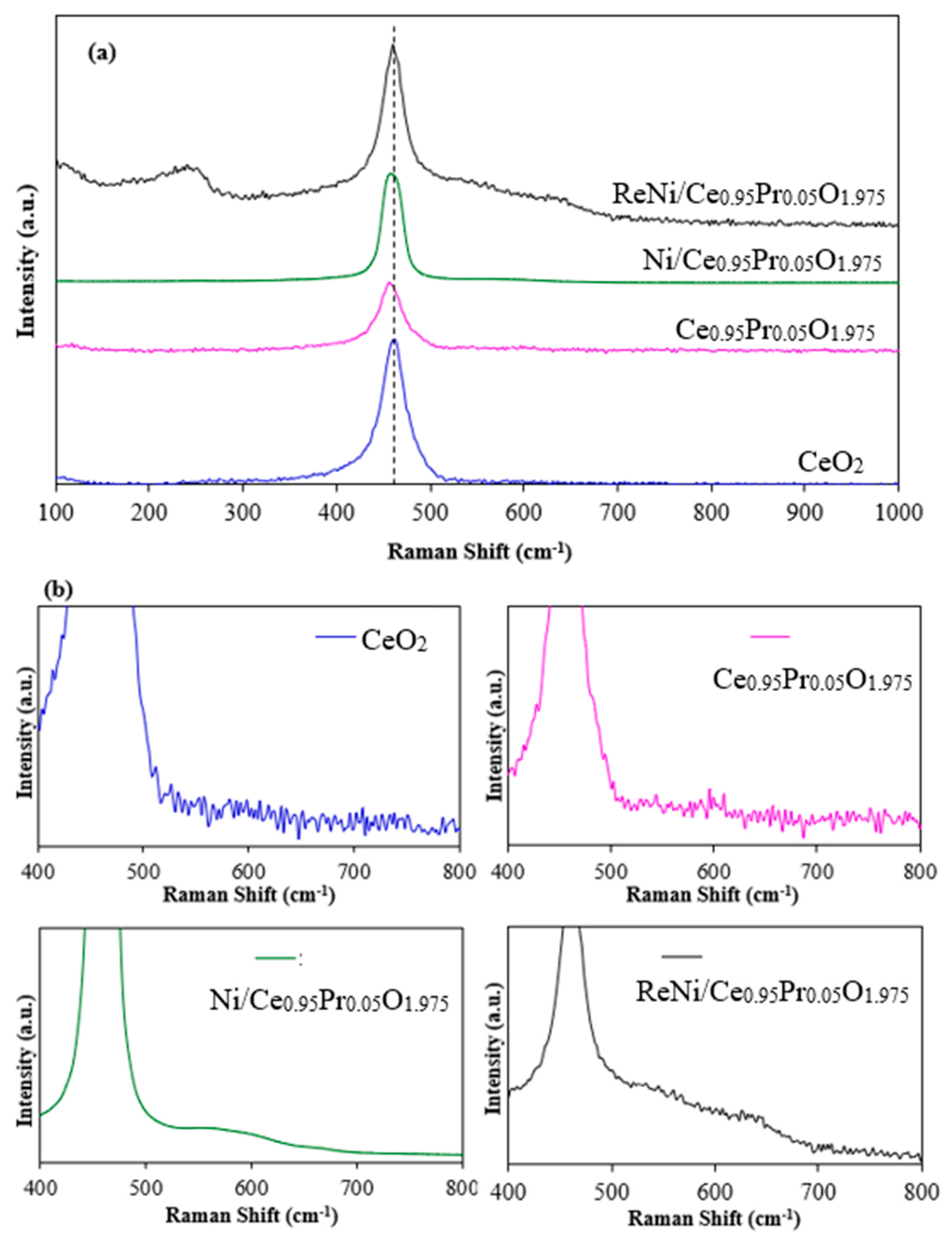
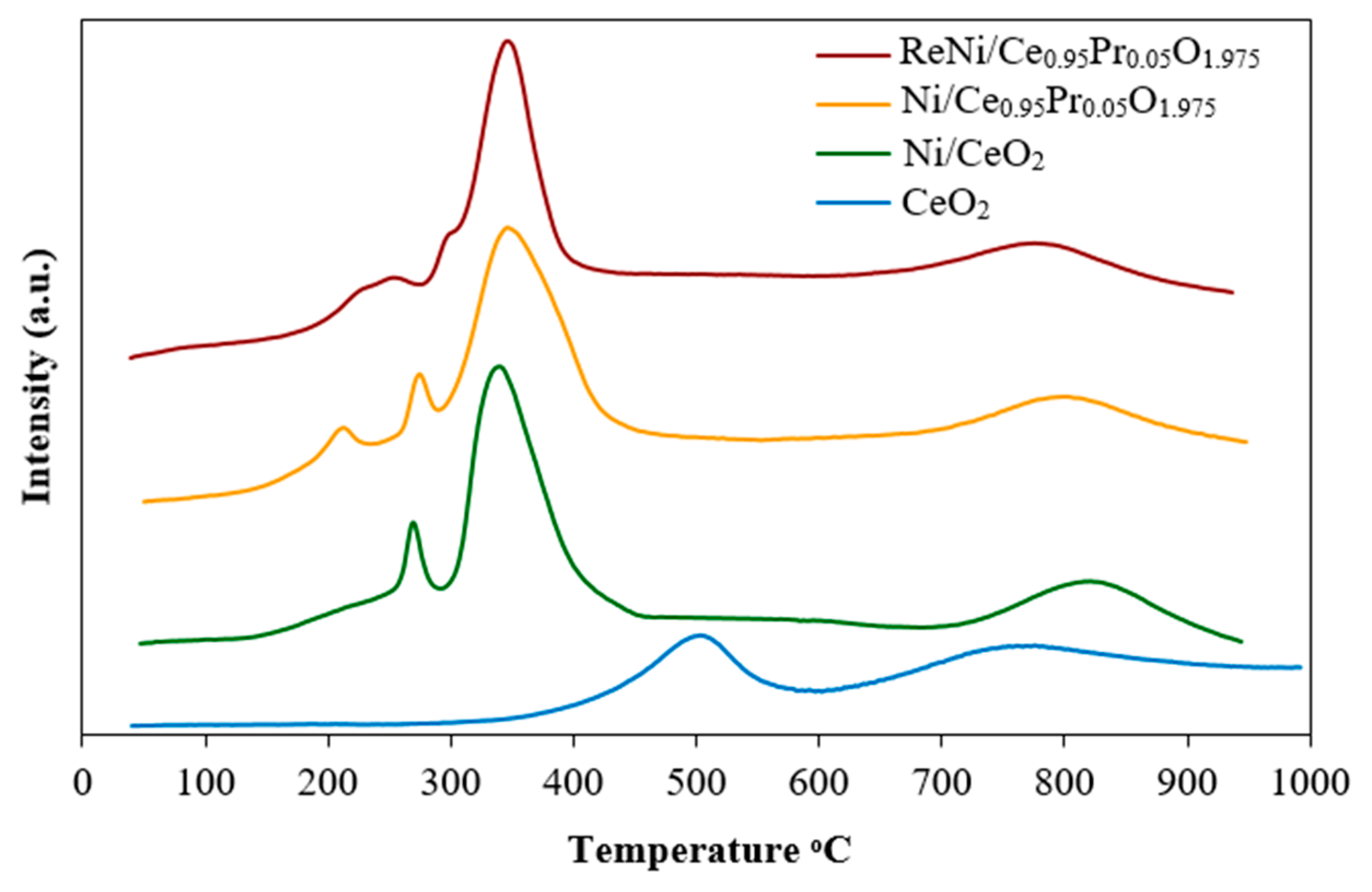
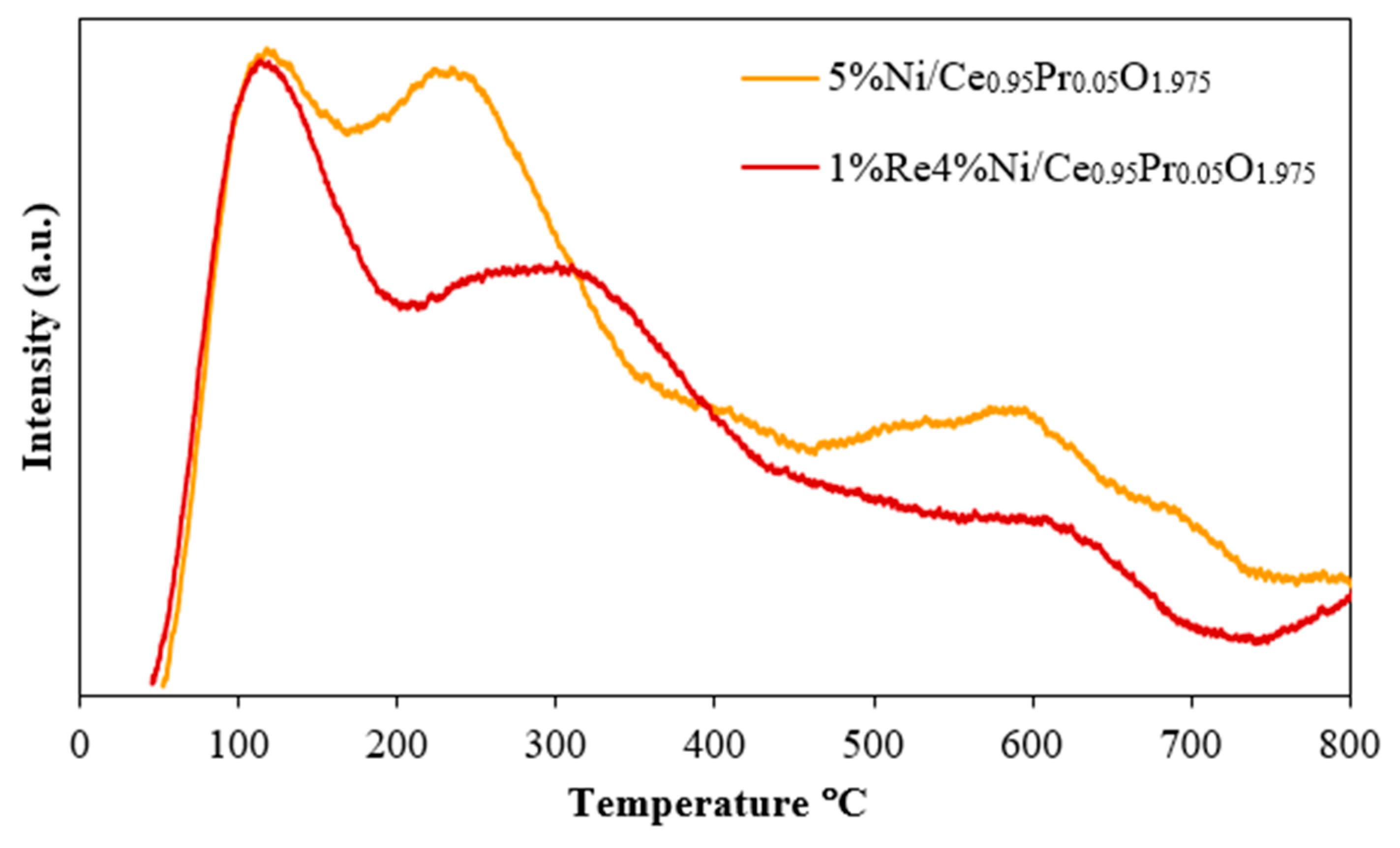


| Catalysts | Crystallite Size a (nm) | BET Surface Area b (m2/g) | Ni Dispersion c (%) | Ni Particle Size c (nm) | Ni Surface Area c (m2/g) |
|---|---|---|---|---|---|
| CeO2 | 9.8 | 68 | - | - | - |
| 5%Ni/CeO2 | 13.35 | 45 | 0.17 | 35.1 | 0.95 |
| 5%Ni/CePrO | 8.55 | 64 | 0.30 | 19.6 | 1.70 |
| 1%Re4%Ni/CePrO | 8.01 | 60 | 1.15 | 5.50 | 6.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Srichaisiriwech, W.; Tepamatr, P. Monometallic and Bimetallic Catalysts Supported on Praseodymium-Doped Ceria for the Water–Gas Shift Reaction. Molecules 2023, 28, 8146. https://doi.org/10.3390/molecules28248146
Srichaisiriwech W, Tepamatr P. Monometallic and Bimetallic Catalysts Supported on Praseodymium-Doped Ceria for the Water–Gas Shift Reaction. Molecules. 2023; 28(24):8146. https://doi.org/10.3390/molecules28248146
Chicago/Turabian StyleSrichaisiriwech, Weerayut, and Pannipa Tepamatr. 2023. "Monometallic and Bimetallic Catalysts Supported on Praseodymium-Doped Ceria for the Water–Gas Shift Reaction" Molecules 28, no. 24: 8146. https://doi.org/10.3390/molecules28248146
APA StyleSrichaisiriwech, W., & Tepamatr, P. (2023). Monometallic and Bimetallic Catalysts Supported on Praseodymium-Doped Ceria for the Water–Gas Shift Reaction. Molecules, 28(24), 8146. https://doi.org/10.3390/molecules28248146







