Chemical Patterning on Nanocarbons: Functionality Typewriting
Abstract
1. Introduction
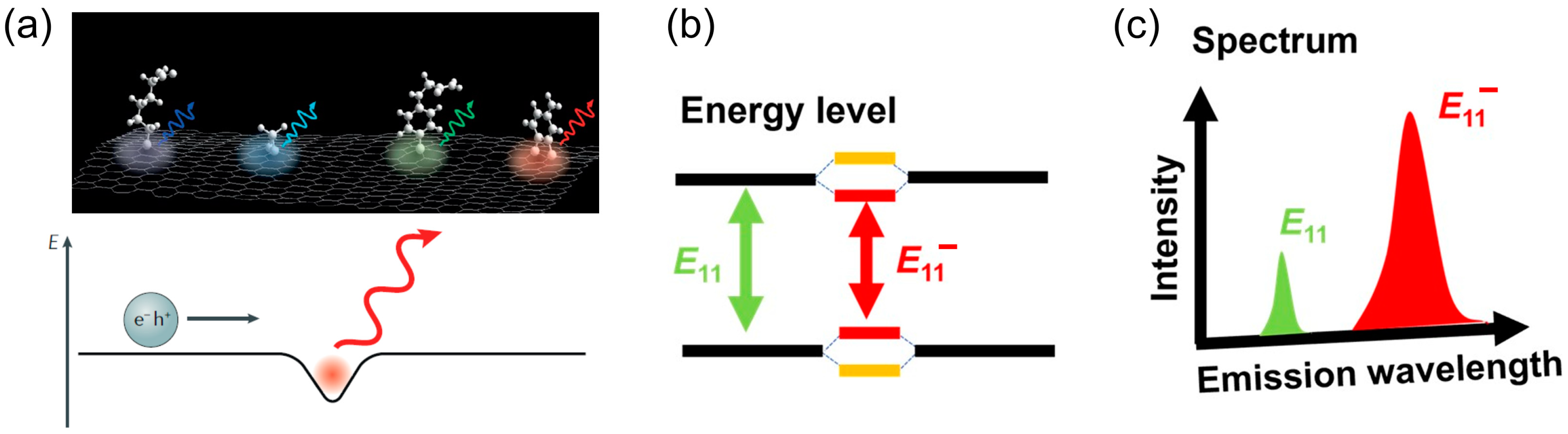
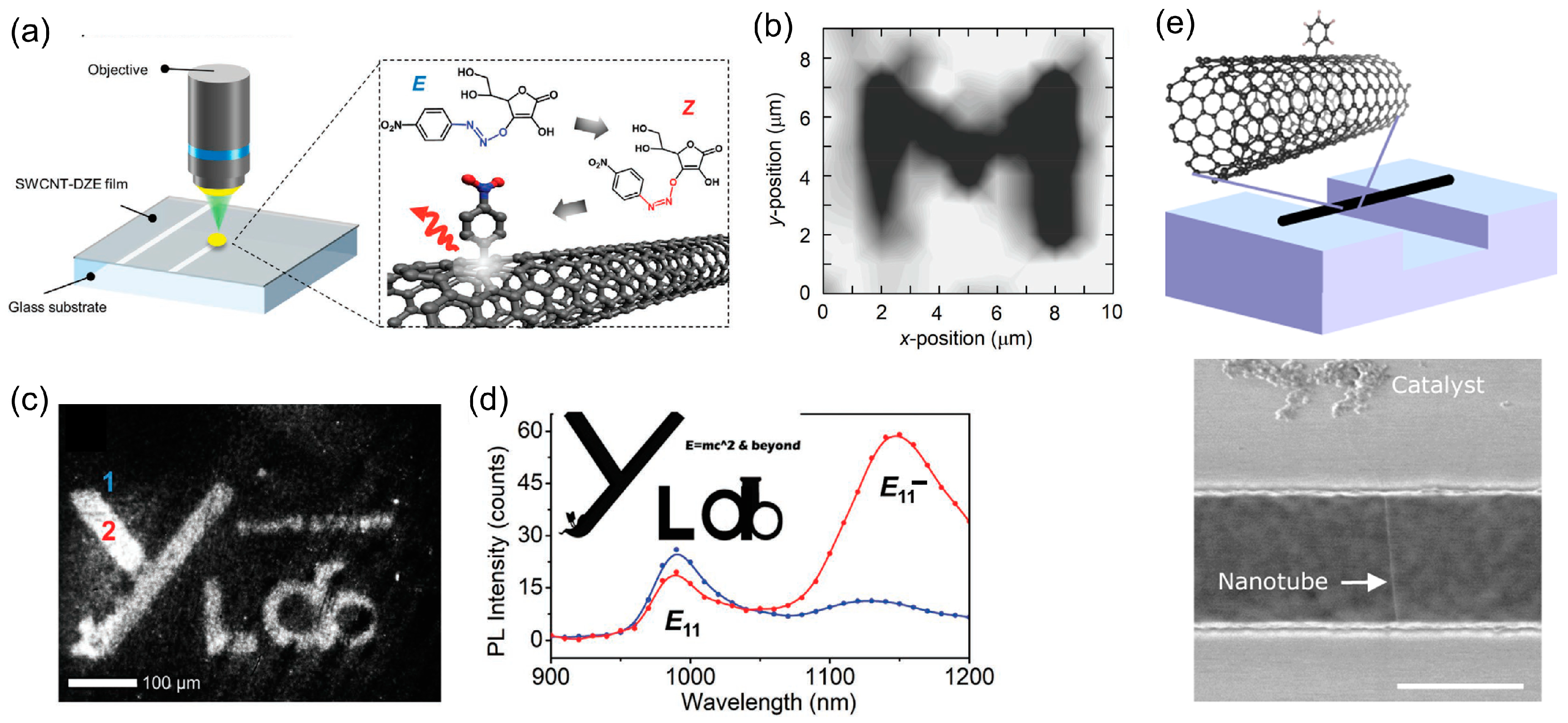
2. Chemical Patterning on CNTs
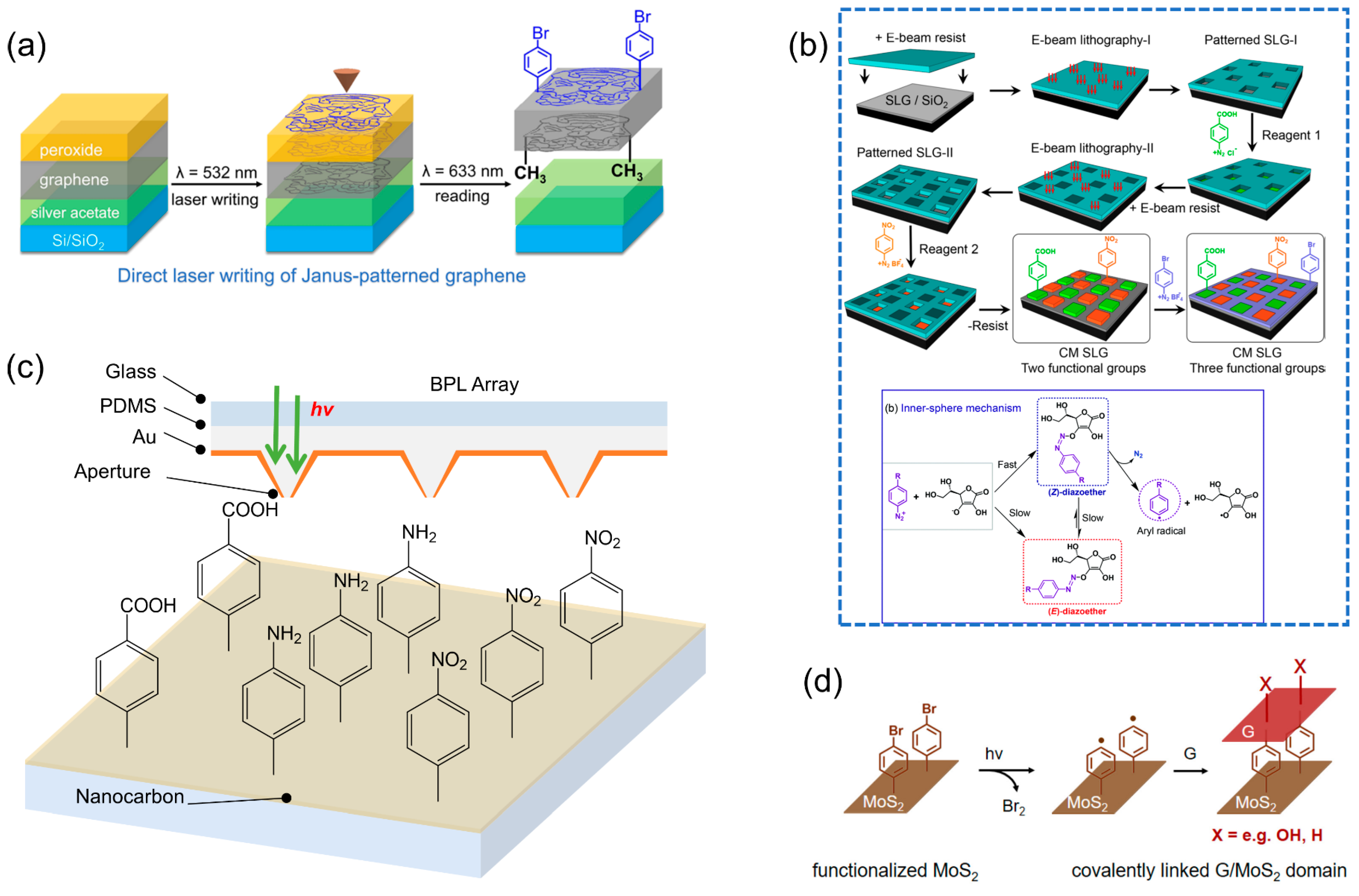
3. Chemical Patterning on Graphene
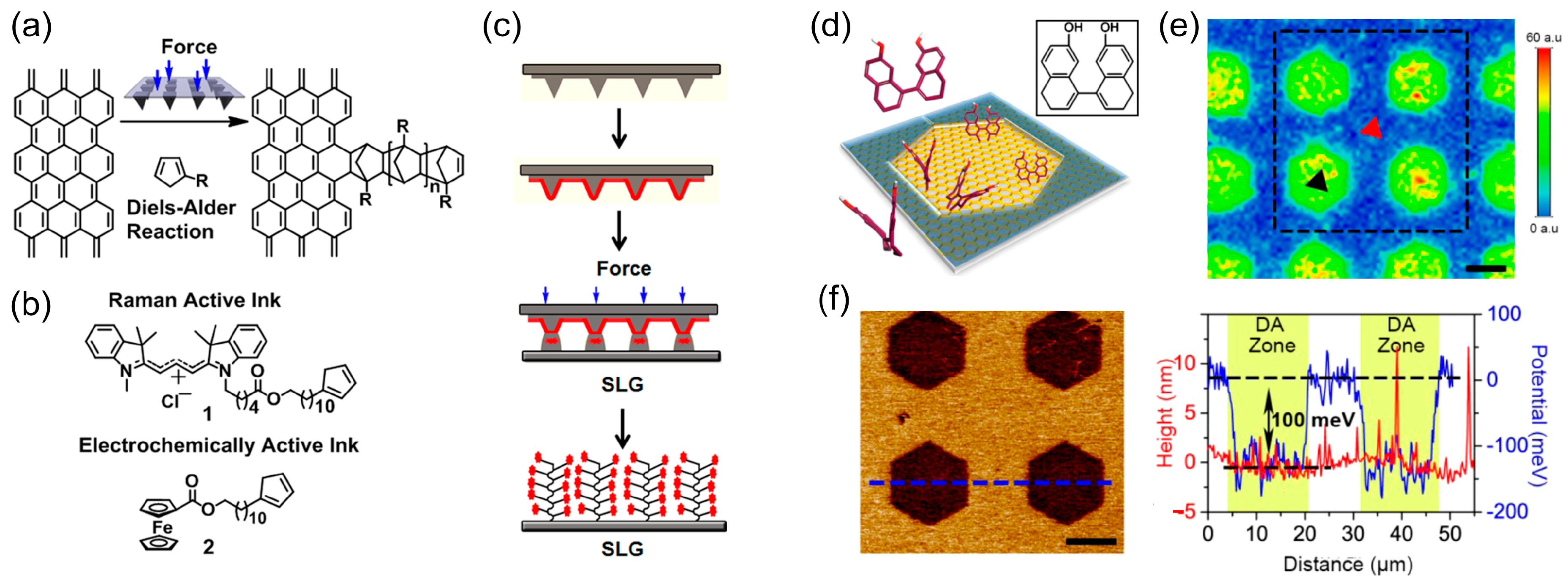
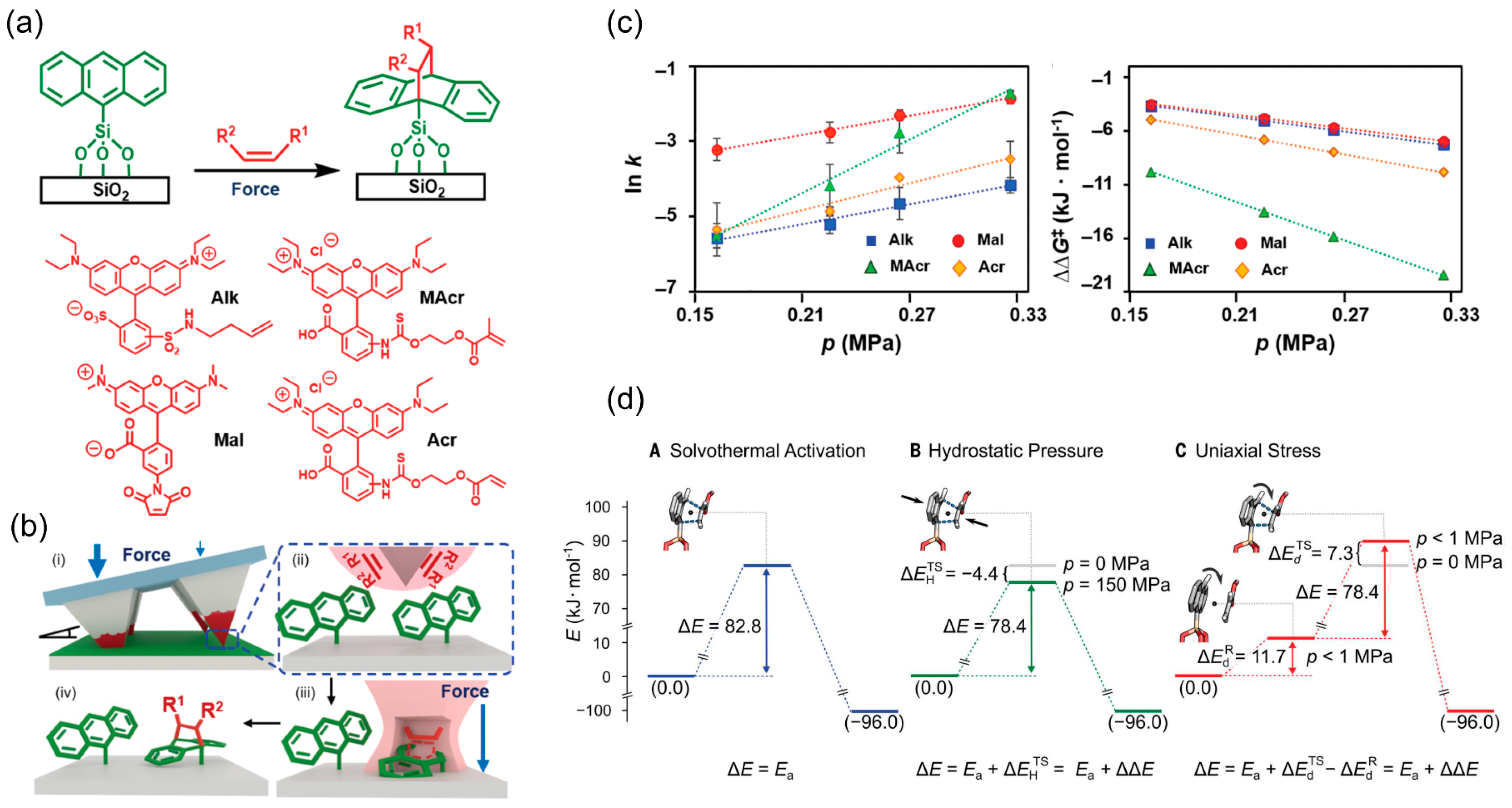
4. Chemical Patterning on Polycyclic Aromatic Rings
5. Outlook and Perspective
6. Summary
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rao, R.; Pint, C.L.; Islam, A.E.; Weatherup, R.S.; Hofmann, S.; Meshot, E.R.; Wu, F.Q.; Zhou, C.W.; Dee, N.; Amama, P.B.; et al. Carbon nanotubes and related nanomaterials: Critical advances and challenges for synthesis toward mainstream commercial applications. ACS Nano 2018, 12, 11756–11784. [Google Scholar] [CrossRef] [PubMed]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Geim, A.K. Graphene: Status and prospects. Science 2009, 324, 1530–1534. [Google Scholar] [CrossRef] [PubMed]
- Shulaker, M.M.; Hills, G.; Patil, N.; Wei, H.; Chen, H.-Y.; Wong, H.S.P.; Mitra, S. Carbon nanotube computer. Nature 2013, 501, 526–530. [Google Scholar] [CrossRef] [PubMed]
- Cao, Q.; Han, S.J. Single-walled carbon nanotubes for high-performance electronics. Nanoscale 2013, 5, 8852–8863. [Google Scholar] [CrossRef] [PubMed]
- Giacalone, F.; Martín, N. Fullerene polymers: Synthesis and properties. Chem. Rev. 2006, 106, 5136–5190. [Google Scholar] [CrossRef] [PubMed]
- Balog, R.; Jorgensen, B.; Nilsson, L.; Andersen, M.; Rienks, E.; Bianchi, M.; Fanetti, M.; Lægsgaard, E.; Baraldi, A.; Lizzit, S.; et al. Bandgap opening in graphene induced by patterned hydrogen adsorption. Nat. Mater. 2010, 9, 315–319. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, A.; Yuan, Z.; Benck, J.D.; Rajan, A.G.; Chu, X.S.; Wang, Q.H.; Strano, M.S. Current and future directions in electron transfer chemistry of graphene. Chem. Soc. Rev. 2017, 46, 4530–4571. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.J.; Wong, E.W.; Lieber, C.M. Probing electrical transport in nanomaterials: Conductivity of individual carbon nanotubes. Science 1996, 272, 523–526. [Google Scholar] [CrossRef]
- Ebbesen, T.W.; Lezec, H.J.; Hiura, H.; Bennett, J.W.; Ghaemi, H.F.; Thio, T. Electrical conductivity of individual carbon nanotubes. Nature 1996, 382, 54–56. [Google Scholar] [CrossRef]
- Odom, T.W.; Huang, J.L.; Kim, P.; Lieber, C.M. Atomic structure and electronic properties of single-walled carbon nanotubes. Nature 1998, 391, 62–64. [Google Scholar] [CrossRef]
- Kang, S.J.; Kocabas, C.; Ozel, T.; Shim, M.; Pimparkar, N.; Alam, M.A.; Rotkin, S.V.; Rogers, J.A. High-performance electronics using dense, perfectly aligned arrays of single-walled carbon nanotubes. Nat. Nanotechnol. 2007, 2, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Dukovic, G.; Brus, L.E.; Heinz, T.F. The optical resonances in carbon nanotubes arise from excitons. Science 2005, 308, 838–841. [Google Scholar] [CrossRef]
- Noe, J.C.; Nutz, M.; Reschauer, J.; Morell, N.; Tsioutsios, I.; Reserbat-Plantey, A.; Watanabe, K.; Taniguch, T.; Bachtold, A.; Hogele, A. Environmental electrometry with luminescent carbon nanotubes. Nano Lett. 2018, 18, 4136–4140. [Google Scholar] [CrossRef] [PubMed]
- Nutz, M.; Zhang, J.; Kim, M.; Kwon, H.; Wu, X.; Wang, Y.; Högele, A. Photon correlation spectroscopy of luminescent quantum defects in carbon nanotubes. Nano Lett. 2019, 19, 7078–7084. [Google Scholar] [CrossRef]
- Coleman, J.N.; Khan, U.; Blau, W.J.; Gun’ko, Y.K. Small but strong: A review of the mechanical properties of carbon nanotube-polymer composites. Carbon 2006, 44, 1624–1652. [Google Scholar] [CrossRef]
- Song, S.Q.; Zhang, Y. Carbon nanotube/reduced graphene oxide hybrid for simultaneously enhancing the thermal conductivity and mechanical properties of styrene -butadiene rubber. Carbon 2017, 123, 158–167. [Google Scholar] [CrossRef]
- Ruoff, R.S.; Lorents, D.C. Mechanical and thermal-properties of carbon nanotubes. Carbon 1995, 33, 925–930. [Google Scholar] [CrossRef]
- Wang, P.; Barnes, B.; Huang, Z.J.; Wang, Z.Y.; Zheng, M.; Wang, Y.H. Beyond color: The new carbon ink. Adv. Mater. 2021, 33, 2005890. [Google Scholar] [CrossRef]
- Mochalin, V.N.; Shenderova, O.; Ho, D.; Gogotsi, Y. The properties and applications of nanodiamonds. Nat. Nanotechnol. 2012, 7, 11–23. [Google Scholar] [CrossRef]
- Sun, Y.P.; Zhou, B.; Lin, Y.; Wang, W.; Fernando, K.A.S.; Pathak, P.; Meziani, M.J.; Harruff, B.A.; Wang, X.; Wang, H.F.; et al. Quantum-sized carbon dots for bright and colorful photoluminescence. J. Am. Chem. Soc. 2006, 128, 7756–7757. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Wang, X.; Meziani, M.J.; Lu, F.S.; Wang, H.F.; Luo, P.J.G.; Lin, Y.; Harruff, B.A.; Veca, L.M.; Murray, D.; et al. Carbon dots for multiphoton bioimaging. J. Am. Chem. Soc. 2007, 129, 11318–11319. [Google Scholar] [CrossRef]
- Chen, D.; Feng, H.B.; Li, J.H. Graphene oxide: Preparation, functionalization, and electrochemical applications. Chem. Rev. 2012, 112, 6027–6053. [Google Scholar] [CrossRef] [PubMed]
- Itami, K.; Maekawa, T. Molecular nanocarbon science: Present and future. Nano Lett. 2020, 20, 4718–4720. [Google Scholar] [CrossRef] [PubMed]
- Bahr, J.L.; Yang, J.P.; Kosynkin, D.V.; Bronikowski, M.J.; Smalley, R.E.; Tour, J.M. Functionalization of carbon nanotubes by electrochemical reduction of aryl diazonium salts: A bucky paper electrode. J. Am. Chem. Soc. 2001, 123, 6536–6542. [Google Scholar] [CrossRef]
- Singh, K.V.; Pandey, R.R.; Wang, X.; Lake, R.; Ozkan, C.S.; Wang, K.; Ozkan, M. Covalent functionalization of single walled carbon nanotubes with peptide nucleic acid: Nanocomponents for molecular level electronics. Carbon 2006, 44, 1730–1739. [Google Scholar] [CrossRef]
- Mansor, M.R.; Fadzullah, S.H.S.M.; Masripan, N.A.B.; Omar, G.; Akop, M.Z. Comparison between functionalized graphene and carbon nanotubes: Effect of morphology and surface group on mechanical, electrical, and thermal properties of nanocomposites. In Functionalized Graphene Nanocomposites and Their Derivatives; Elsevier: Amsterdam, The Netherlands, 2019; pp. 177–204. [Google Scholar]
- Xing, W.Y.; Yang, W.; Yang, W.J.; Hu, Q.H.; Si, J.Y.; Lu, H.D.; Yang, B.H.; Song, L.; Hu, Y.; Yuen, R.K.K. Functionalized carbon nanotubes with phosphorus- and nitrogen-containing agents: Effective reinforcer for thermal, mechanical, and flame-retardant properties of polystyrene nanocomposites. ACS Appl. Mater. Inter. 2016, 8, 26266–26274. [Google Scholar] [CrossRef]
- Qiu, S.; Wu, K.J.; Gao, B.; Li, L.Q.; Jin, H.H.; Li, Q.W. Solution-processing of high-purity semiconducting single-walled carbon nanotubes for electronics devices. Adv. Mater. 2019, 31, 1800750. [Google Scholar] [CrossRef]
- Sarukhanyan, E.; Milano, G.; Roccatano, D. Coating mechanisms of single-walled carbon nanotube by linear polyether surfactants: Insights from computer simulations. J. Phys. Chem. C 2014, 118, 18069–18078. [Google Scholar] [CrossRef][Green Version]
- Ajayan, P.M.; Tour, J.M. Nanotube composites. Nature 2007, 447, 1066–1068. [Google Scholar] [CrossRef]
- Ahir, S.V.; Terentjev, E.M. Photomechanical actuation in polymer-nanotube composites. Nat. Mater. 2005, 4, 491–495. [Google Scholar] [CrossRef]
- Yang, Y.K.; Zhan, W.J.; Peng, R.G.; He, C.G.; Pang, X.C.; Shi, D.; Jiang, T.; Lin, Z.Q. Graphene-enabled superior and tunable photomechanical actuation in liquid crystalline elastomer nanocomposites. Adv. Mater. 2015, 27, 6376–6381. [Google Scholar] [CrossRef] [PubMed]
- Zanjanijam, A.R.; Hajian, M.; Koohmareh, G.A. Fabrication of single wall carbon nanotubes-based poly(vinyl butyral) nanocomposites with enhanced mechanical and thermal properties. J. Macromol. Sci. A 2014, 51, 369–377. [Google Scholar] [CrossRef]
- Feng, J.; Li, W.B.; Qian, X.F.; Qi, J.S.; Qi, L.; Li, J. Patterning of graphene. Nanoscale 2012, 4, 4883–4899. [Google Scholar] [CrossRef]
- Shukla, S.; Kang, S.Y.; Saxena, S. Synthesis and patterning of graphene: Strategies and prospects. Appl. Phys. Rev. 2019, 6, 021311. [Google Scholar] [CrossRef]
- Khare, K.S.; Khabaz, F.; Khare, R. Effect of carbon nanotube functionalization on mechanical and thermal properties of cross-linked epoxy-carbon nanotube nanocomposites: Role of strengthening the interfacial interactions. ACS Appl. Mater. Inter. 2014, 6, 6098–6110. [Google Scholar] [CrossRef]
- Huang, Z.; Li, L.; Zhang, X.A.; Alsharif, N.; Wu, X.; Peng, Z.; Cheng, X.; Wang, P.; Brown, K.A.; Wang, Y. Photoactuated pens for molecular printing. Adv. Mater. 2018, 30, 1705303. [Google Scholar] [CrossRef]
- Ham, E.K.; Choi, W.K.; Kim, Y.K.; Seo, M.K. Influence of functional groups on the surface of carbon nanotube on mechanical and thermal properties of carbon nanotube/polymer composites. Polym. Korea 2015, 39, 909–916. [Google Scholar] [CrossRef]
- Sa, K.; Mahakul, P.C.; Nanda, K.K.; Mahanandia, P. Effect of ionic liquid functionalized carbon nanotubes on mechanical, thermal and electrical properties of carbon nanotubes-reduced graphene oxide/PMMA nanocomposites. Chem. Phys. Lett. 2018, 706, 76–81. [Google Scholar] [CrossRef]
- Huang, Z.J.; Li, L.; Yin, T.S.; Brown, K.A.; Wang, Y.H. Rational structural design of polymer pens for energy-efficient photoactuation. Polymers 2023, 15, 3595. [Google Scholar] [CrossRef]
- Spearman, S.S.; Irin, F.; Rivero, I.V.; Green, M.J.; Abidi, N. Effect of dsDNA wrapped single-walled carbon nanotubes on the thermal and mechanical properties of polycaprolactone and polyglycolide fiber blend composites. Polymer 2015, 56, 476–481. [Google Scholar] [CrossRef]
- Li, S.P.; Zhang, J.Q.; He, J.; Liu, W.P.; Wang, Y.H.; Huang, Z.J.; Pang, H.; Chen, Y.W. Functional PDMS elastomers: Bulk composites, surface engineering, and precision fabrication. Adv. Sci. 2023, 10, 20230456. [Google Scholar] [CrossRef]
- Piao, Y.M.; Meany, B.; Powell, L.R.; Valley, N.; Kwon, H.; Schatz, G.C.; Wang, Y.H. Brightening of carbon nanotube photoluminescence through the incorporation of sp3 defects. Nat. Chem. 2013, 5, 840–845. [Google Scholar] [CrossRef]
- Brozena, A.H.; Kim, M.; Powell, L.R.; Wang, Y. Controlling the optical properties of carbon nanotubes with organic colour-centre quantum defects. Nat. Rev. Chem. 2019, 3, 375–392. [Google Scholar] [CrossRef]
- Kwon, H.; Furmanchuk, M.; Kim, M.; Meany, B.; Guo, Y.; Schatz, G.C.; Wang, Y.H. Molecularly tunable fluorescent quantum defects. J. Am. Chem. Soc. 2016, 138, 6878–6885. [Google Scholar] [CrossRef]
- Powell, L.R.; Kim, M.; Wang, Y.H. Chirality-selective functionalization of semiconducting carbon nanotubes with a reactivity-switchable molecule. J. Am. Chem. Soc. 2017, 139, 12533–12540. [Google Scholar] [CrossRef]
- Huang, Z.; Powell, L.R.; Wu, X.; Kim, M.; Qu, H.; Wang, P.; Fortner, J.L.; Xu, B.; Ng, A.L.; Wang, Y. Photolithographic patterning of organic color-centers. Adv. Mater. 2020, 32, 1906517. [Google Scholar] [CrossRef]
- Dresselhaus, M.S.; Dresselhaus, G.; Saito, R.; Jorio, A. Raman spectroscopy of carbon nanotubes. Phys. Rep. 2005, 409, 47–99. [Google Scholar] [CrossRef]
- Costa, S.; Borowiak-Palen, E.; Kruszynska, M.; Bachmatiuk, A.; Kalenczuk, R.J. Characterization of carbon nanotubes by Raman spectroscopy. Mater. Sci. 2008, 26, 433–441. [Google Scholar]
- Rebelo, S.L.H.; Guedes, A.; Szefczyk, M.E.; Pereira, A.M.; Araujo, J.P.; Freire, C. Progress in the Raman spectra analysis of covalently functionalized multiwalled carbon nanotubes: Unraveling disorder in graphitic materials. Phys. Chem. Chem. Phys. 2016, 18, 12784–12796. [Google Scholar] [CrossRef] [PubMed]
- Kozawa, D.; Wu, X.J.; Ishii, A.; Fortner, J.; Otsuka, K.; Xiang, R.; Inoue, T.; Maruyama, S.; Wang, Y.H.; Kato, Y.K. Formation of organic color centers in air-suspended carbon nanotubes using vapor-phase reaction. Nat. Commun. 2022, 13, 2814. [Google Scholar] [CrossRef] [PubMed]
- Ng, A.L.; Piao, Y.M.; Wang, Y.H. Laser lithography of a tube-in-a-tube nanostructure. ACS Nano 2017, 11, 3320–3327. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, N.F.; Yalcin, S.E.; Adamska, L.; Hároz, E.H.; Ma, X.D.; Tretiak, S.; Htoon, H.; Doorn, S.K. Photoluminescence imaging of solitary dopant sites in covalently doped single-wall carbon nanotubes. Nanoscale 2015, 7, 20521–20530. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Katsnelson, M.I.; Grigorieva, I.V.; Dubonos, S.V.; Firsov, A.A. Two-dimensional gas of massless Dirac fermions in graphene. Nature 2005, 438, 197–200. [Google Scholar] [CrossRef]
- Wei, T.; Hauke, F.; Hirsch, A. Evolution of graphene patterning: From dimension regulation to molecular engineering. Adv. Mater. 2021, 33, 2104060. [Google Scholar] [CrossRef]
- Zheng, Y.Q.; Wang, H.; Hou, S.F.; Xia, D.Y. Lithographically defined graphene patterns. Adv. Mater. Technol. 2017, 2, 201600237. [Google Scholar] [CrossRef]
- Goronzy, D.P.; Ebrahim, M.; Rosei, F.; Arramel; Fang, Y.; De Feyter, S.; Tait, S.L.; Wang, C.; Beton, P.H.; Wee, A.T.S.; et al. Supramolecular assemblies on surfaces: Nanopatterning, functionality, and reactivity. ACS Nano 2018, 12, 7445–7481. [Google Scholar] [CrossRef]
- Greenwood, J.; Phan, T.H.; Fujita, Y.; Li, Z.; Lvasenko, O.; Vanderlinden, W.; Van Gorp, H.; Frederickx, W.; Lu, G.; Tahara, K.; et al. Covalent modification of graphene and graphite using diazonium chemistry: Tunable grafting and nanomanipulation. ACS Nano 2015, 9, 5520–5535. [Google Scholar] [CrossRef]
- Wei, T.; Bao, L.P.; Hauke, F.; Hirsch, A. Recent advances in graphene patterning. Chempluschem 2020, 85, 1655–1668. [Google Scholar] [CrossRef]
- Wei, T.; Kohring, M.; Chen, M.Q.; Yang, S.F.; Weber, H.B.; Hauke, F.; Hirsch, A. Highly efficient and reversible covalent patterning of graphene: 2D-management of chemical information. Angew. Chem. Int. Ed. 2020, 59, 5602–5606. [Google Scholar] [CrossRef]
- Bao, L.P.; Zhao, B.L.; Lloret, V.; Halik, M.; Hauke, F.; Hirsch, A. Spatially sesolved bottom-side fluorination of graphene by two-dimensional substrate patterning. Angew. Chem. Int. Ed. 2020, 59, 6700–6705. [Google Scholar] [CrossRef] [PubMed]
- Wei, T.; Al-Fogra, S.; Hauke, F.; Hirsch, A. Direct laser writing on graphene with unprecedented efficiency of covalent two-dimensional functionalization. J. Am. Chem. Soc. 2020, 142, 21926–21931. [Google Scholar] [CrossRef] [PubMed]
- Bao, L.P.; Kohring, M.; Weber, H.B.; Hauke, F.; Hirsch, A. Covalently doped graphene superlattices: Spatially resolved supratopic- and Janus-binding. J. Am. Chem. Soc. 2020, 142, 16016–16022. [Google Scholar] [CrossRef] [PubMed]
- Wei, T.; Liu, X.; Al-Fogra, S.; Bachmann, J.; Hauke, F.; Hirsch, A. A general concept for highly efficient covalent laser patterning of graphene based on silver carboxylates. Chem. Commun. 2021, 57, 4654–4657. [Google Scholar] [CrossRef]
- Dierke, T.; Dasler, D.; Nagel, T.; Hauke, F.; Hirsch, A.; Maultzsch, J. Spatial control of graphene functionalization by patterning a 2D substrate: Implications for graphene based Van-der-Waals heterostructures. ACS Appl. Nano Mater. 2022, 5, 4966–4971. [Google Scholar] [CrossRef]
- Xia, Y.Z.; Sun, L.; Eyley, S.; Daelemans, B.; Thielemans, W.; Seibel, J.; De Feyter, S. Grafting ink for direct writing: Solvation activated covalent functionalization of graphene. Adv. Sci. 2022, 9, 2105017. [Google Scholar] [CrossRef]
- Bian, S.D.; Scott, A.M.; Cao, Y.; Liang, Y.; Osuna, S.; Houk, K.N.; Braunschweig, A.B. Covalently patterned graphene surfaces by a force-accelerated Diels-Alder reaction. J. Am. Chem. Soc. 2013, 135, 9240–9243. [Google Scholar] [CrossRef]
- Huo, F.; Zheng, Z.; Zheng, G.; Giam, L.R.; Zhang, H.; Mirkin, C.A. Polymer pen lithography. Science 2008, 321, 1658–1660. [Google Scholar] [CrossRef]
- Braunschweig, A.B.; Huo, F.; Mirkin, C.A. Molecular printing. Nat. Chem. 2009, 1, 353–358. [Google Scholar] [CrossRef]
- Li, J.; Li, M.; Zhou, L.L.; Lang, S.Y.; Lu, H.Y.; Wang, D.; Chen, C.F.; Wan, L.J. Click and patterned functionalization of graphene by Diels-Alder reaction. J. Am. Chem. Soc. 2016, 138, 7448–7451. [Google Scholar] [CrossRef]
- Lian, J.X.; Lherbier, A.; Wang, L.J.; Charlier, J.C.; Beljonne, D.; Olivier, Y. Electronic structure and charge transport in nanostripped graphene. J. Phys. Chem. C 2016, 120, 20024–20032. [Google Scholar] [CrossRef]
- Dyck, O.; Yeom, S.; Lupini, A.R.; Swett, J.L.; Hensley, D.; Yoon, M.; Jesse, S. Top-down fabrication of atomic patterns in twisted bilayer graphene. Adv. Mater. 2023, 35, 2302906. [Google Scholar] [CrossRef] [PubMed]
- Zholdassov, Y.S.; Yuan, L.; Garcia, S.R.; Kwok, R.W.; Boscoboinik, A.; Valles, D.J.; Marianski, M.; Martini, A.; Carpick, R.W.; Braunschweig, A.B. Acceleration of Diels-Alder reactions by mechanical distortion. Science 2023, 380, 1053–1058. [Google Scholar] [CrossRef]
- Weiss, P.S. A push for mechanochemistry. Science 2023, 380, 1013. [Google Scholar] [CrossRef]
- Al-Fogra, S.; Yang, B.W.; Jurkiewicz, L.; Hauke, F.; Hirsch, A.; Wei, T. Spatially resolved Janus patterning of graphene by direct laser writing. J. Am. Chem. Soc. 2022, 144, 19825–19831. [Google Scholar] [CrossRef]
- González, M.C.R.; Leonhardt, A.; Stadler, H.; Eyley, S.; Thielemans, W.; De Gendt, S.; Mali, K.S.; De Feyter, S. Multicomponent covalent chemical patterning of graphene. ACS Nano 2021, 15, 10618–10627. [Google Scholar] [CrossRef]
- González, M.C.R.; Brown, A.; Eyley, S.; Thielemans, W.; Mali, K.S.; De Feyter, S. Self-limiting covalent modification of carbon surfaces: Diazonium chemistry with a twist. Nanoscale 2020, 12, 18782–18789. [Google Scholar] [CrossRef]
- Huo, F.; Zheng, G.; Liao, X.; Giam, L.R.; Chai, J.; Chen, X.; Shim, W.; Mirkin, C.A. Beam pen lithography. Nat. Nanotechnol. 2010, 5, 637–640. [Google Scholar] [CrossRef]
- Liao, X.; Brown, K.A.; Schmucker, A.L.; Liu, G.L.; He, S.; Shim, W.; Mirkin, C.A. Desktop nanofabrication with massively multiplexed beam pen lithography. Nat. Commun. 2013, 4, 2103. [Google Scholar] [CrossRef]
- Carbonell, C.; Valles, D.J.; Wong, A.M.; Tsui, M.W.; Niang, M.; Braunschweig, A.B. Massively multiplexed tip-based photochemical lithography under continuous capillary flow. Chem 2018, 4, 857–867. [Google Scholar] [CrossRef]
- Chen, X.; Assebban, M.; Kohring, M.; Bao, L.P.; Weber, H.B.; Knirsch, K.C.; Hirsch, A. Laser-triggered bottom-up transcription of chemical information: Toward patterned Graphene/MoS2 heterostructures. J. Am. Chem. Soc. 2022, 144, 9645–9650. [Google Scholar] [CrossRef] [PubMed]
- Payne, N.A.; Mauzeroll, J. Identifying nanoscale pinhole defects in nitroaryl Layers with scanning electrochemical cell microscopy. Chemelectrochem 2019, 6, 5439–5445. [Google Scholar] [CrossRef]
- Zhou, M.; Yu, Y.; Blanchard, P.Y.; Mirkin, M.V. Surface patterning using diazonium ink filled nanopipette. Anal. Chem. 2015, 87, 10956–10962. [Google Scholar] [CrossRef]
- Zhang, G.H.; Kirkman, P.M.; Patel, A.N.; Cuharuc, A.S.; McKelvey, K.; Unwin, P.R. Molecular functionalization of graphite surfaces: Basal plane versus step edge electrochemical activity. J. Am. Chem. Soc. 2014, 136, 11444–11451. [Google Scholar] [CrossRef]
- Zhang, H.; Bekyarova, E.; Huang, J.-W.; Zhao, Z.; Bao, W.; Wang, F.; Haddon, R.C.; Lau, C.N. Aryl functionalization as a route to band gap engineering in single layer graphene devices. Nano Lett. 2011, 11, 4047–4051. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Z. Chemical Patterning on Nanocarbons: Functionality Typewriting. Molecules 2023, 28, 8104. https://doi.org/10.3390/molecules28248104
Huang Z. Chemical Patterning on Nanocarbons: Functionality Typewriting. Molecules. 2023; 28(24):8104. https://doi.org/10.3390/molecules28248104
Chicago/Turabian StyleHuang, Zhongjie. 2023. "Chemical Patterning on Nanocarbons: Functionality Typewriting" Molecules 28, no. 24: 8104. https://doi.org/10.3390/molecules28248104
APA StyleHuang, Z. (2023). Chemical Patterning on Nanocarbons: Functionality Typewriting. Molecules, 28(24), 8104. https://doi.org/10.3390/molecules28248104





