Specifics of Pharmacokinetics and Biodistribution of 5-Fluorouracil Polymeric Complex
Abstract
1. Introduction
2. Results
2.1. Characterization of 5-FU Polymeric Complex
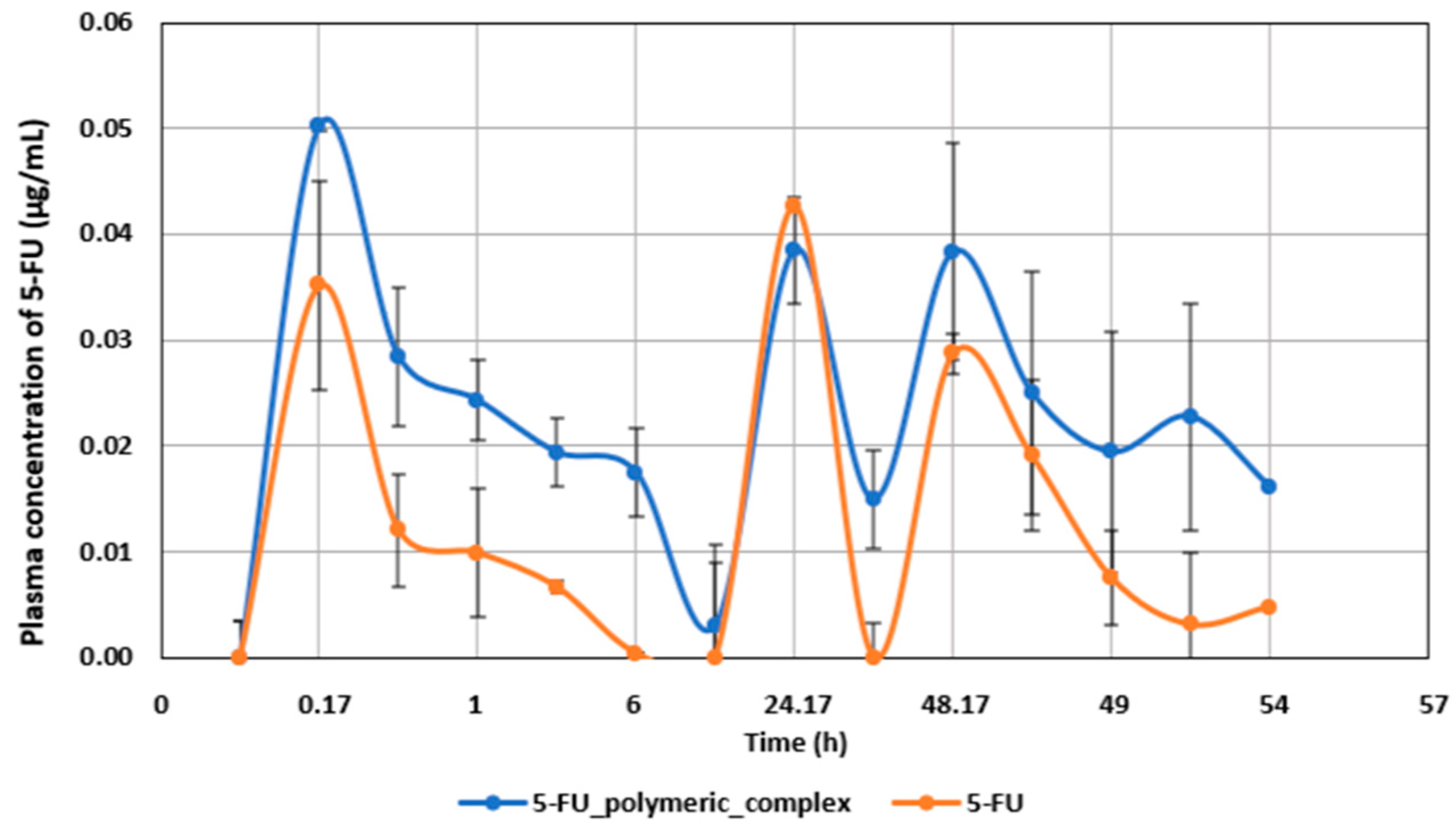
2.2. Pharmacokinetic Studies
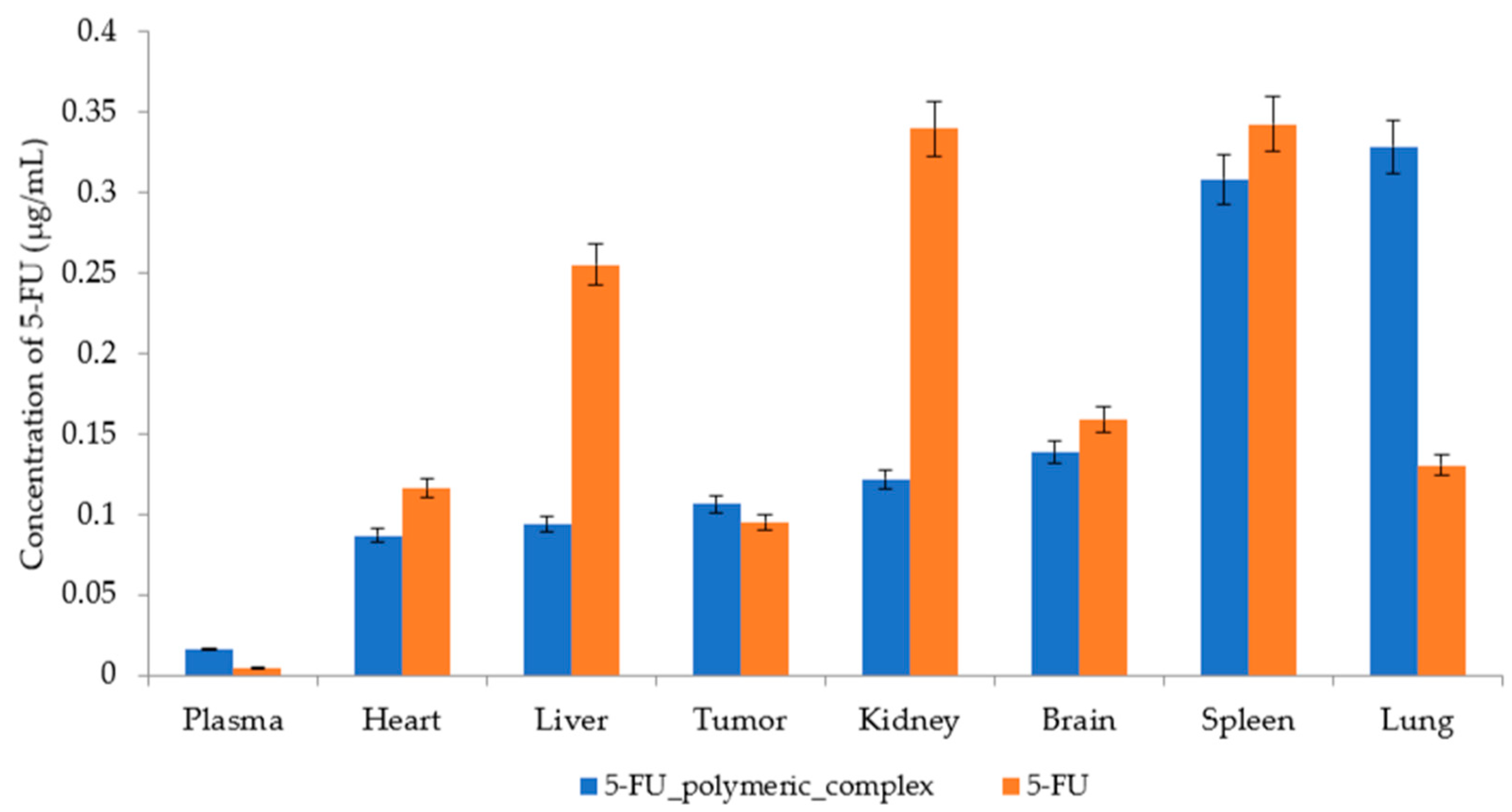
2.3. Biodistribution
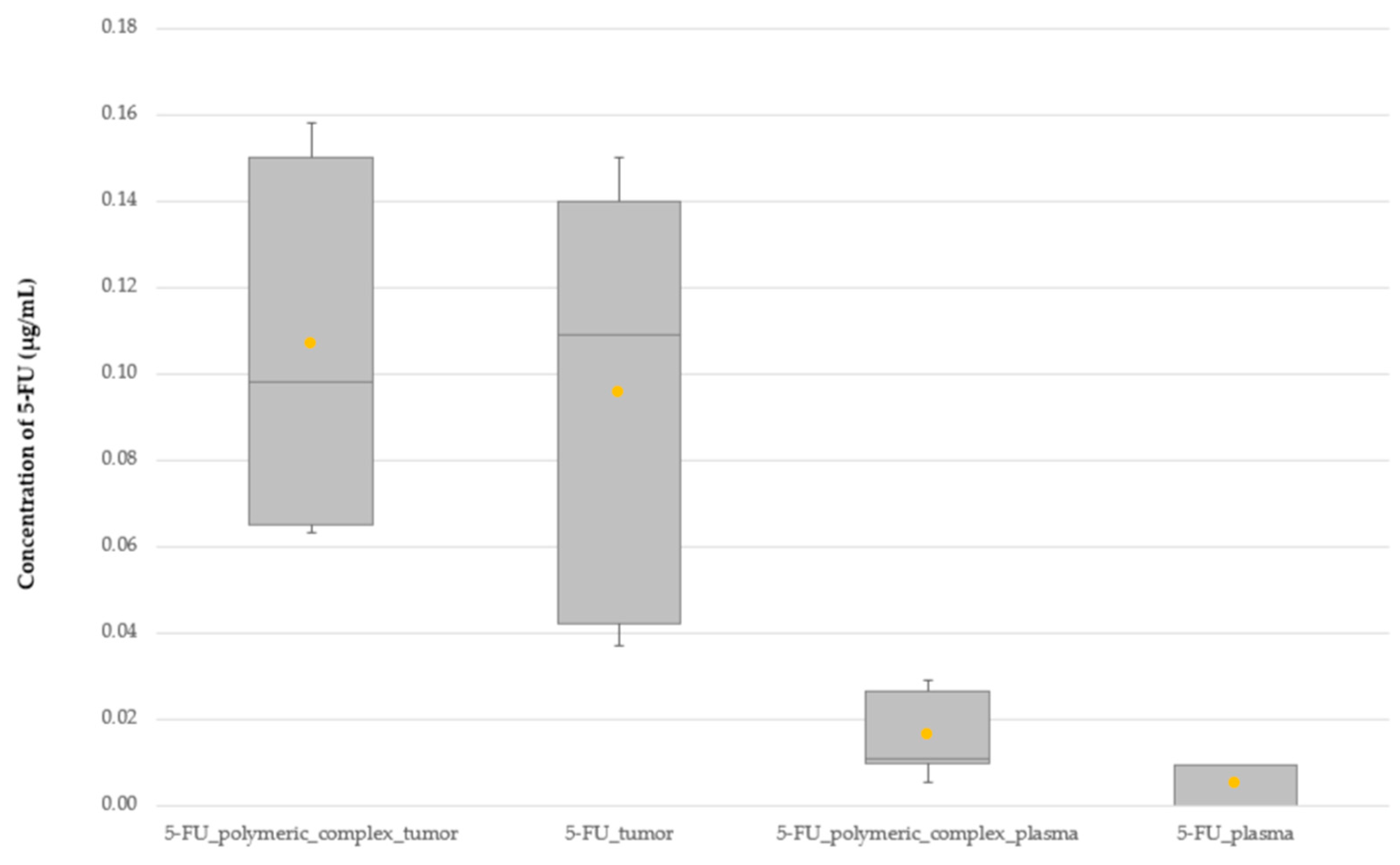
2.4. Comparison of the 5-FU Distribution Profile between Free 5-FU and the 5-FU Polymeric Complex
3. Discussion
- For what reason is there accumulation of the delivery system in the lungs, and is it possible to use this accumulation for the treatment of lung cancer;
- It is necessary to evaluate the component of immunotherapy due to the accumulation of a polymeric complex in the spleen and determine whether this is a side effect;
- To evaluate the possibility of reducing the course dose of 5-FU in the polymeric complex compared with an independent preparation by maintaining the concentration on the plateau.
4. Materials and Methods
4.1. Method of Obtaining Poly(methacrylic acid) (PMAA)
4.2. Molecular-Mass Characteristics of the Polymer
4.3. Laboratory Animals
4.4. In Vivo Tumor Graft Model
4.5. Production of a 5-FU Polymeric Complex
4.6. Pharmacokinetic Studies
4.7. Biodistribution
4.8. 5-FU Measurements in the Rat Plasma and Organs
4.9. Serum Sample Preparation
4.10. Tissue Sample Preparation for 5-FU Concentration Measurement by HPLC
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gan, B.K.; Rullah, K.; Yong, C.Y.; Ho, K.L.; Omar, A.R.; Alitheen, N.B.; Tan, W.S. Targeted delivery of 5-fluorouracil-1-acetic acid (5-FA) to cancer cells overexpressing epithelial growth factor receptor (EGFR) using virus-like nanoparticles. Sci. Rep. 2020, 10, 16867. [Google Scholar] [CrossRef] [PubMed]
- Bodoki, A.E.; Iacob, B.-C.; Bodoki, E. Perspectives of Molecularly Imprinted Polymer-Based Drug Delivery Systems in Cancer Therapy. Polymers 2019, 11, 2085. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Shan, C.; Liang, C.; Han, J.; He, B.; Sun, Y.; Luo, K.; Chang, J.; Wang, X.; Liang, Y. Smart Multistage “Trojan Horse”-Inspired Bovine Serum Albumin-Coated Liposomes for Enhancing Tumor Penetration and Antitumor Efficacy. Biomacromolecules 2022, 23, 5202–5212. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ma, Y.; Chen, H.; Wu, X.; Qian, H.; Yang, X.; Zha, Z. Novel doxorubicin load-ed PEGylated cuprous telluride nanocrystals for combined photothermal-chemo cancer treatment. Colloids Surf. B Biointerfaces 2017, 152, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Crintea, A.; Dutu, A.G.; Samasca, G.; Florian, I.A.; Lupan, I.; Craciun, A.M. The Nanosystems Involved in Treating Lung Cancer. Life 2021, 11, 682. [Google Scholar] [CrossRef] [PubMed]
- Bognanni, N.; Viale, M.; Distefano, A.; Tosto, R.; Bertola, N.; Loiacono, F.; Ponassi, M.; Spinelli, D.; Pappalardo, G.; Vecchio, G. Cyclodextrin Polymers as Delivery Systems for Targeted Anti-Cancer Chemotherapy. Molecules 2021, 26, 6046. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.-L.; Fan, B.-Y.; Li, Q.; Di, H.-X.; Meng, X.-Y.; Ling, N. Preparation of 5-fluorouracil-loaded Nanoparticles and Study of Interaction with Gastric Cancer Cells. Asian Pac. J. Cancer Prev. 2014, 15, 7611–7615. [Google Scholar] [CrossRef]
- Jubeen, F.; Jabeen, I.; Aftab, U.; Noor, S.; Hareem, M.e.; Sultan, M.; Kazi, M. Synthesis, Characterization, Theoretical and Experimental Anticancer Evaluation of Novel Co-crystals of 5-Fluorouracil and Schiff Bases against SW480 Colorectal Carcinoma. Pharmaceutics 2023, 15, 1929. [Google Scholar] [CrossRef]
- Dobryakova, N.V.; Zhdanov, D.D.; Sokolov, N.N.; Aleksandrova, S.S.; Pokrovskaya, M.V.; Kudryashova, E.V. Improvement of Biocatalytic Properties and Cytotoxic Activity of L-Asparaginase from Rhodospirillum rubrum by Conjugation with Chitosan-Based Cationic Polyelectrolytes. Pharmaceuticals 2022, 15, 406. [Google Scholar] [CrossRef]
- Peng, T.; Liu, K.; Gao, L.; Gao, L.; Chen, J.; Wang, J.; Liu, Y.; Wang, Y.; Yan, Z.; Yu, L. Poly (l-γ-glutamylglutamine) Polymer Enhances Doxorubicin Accumulation in Multidrug Resistant Breast Cancer Cells. Molecules 2016, 21, 720. [Google Scholar] [CrossRef]
- Ogiso, T.; Noda, N.; Asai, N.; Kato, Y. Antitumor agents. I. Effect of 5-fluorouracil and cyclophosphamide on liver microsomes and thymus of rat. Jpn. J. Pharmacol. 1976, 26, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Ogiso, T.; Noda, N.; Masuda, H.; Kato, Y. Antitumor agents. II. Effect of 5-fluorouracil and cyclophosphamide on immunological parameters and liver microsomes of tumor-bearing rats. Jpn. J. Pharmacol. 1978, 28, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Yassin, A.E.; Anwer, M.K.; Mowafy, H.A.; El-Bagory, I.M.; Bayomi, M.A.; Alsarra, I.A. Optimization of 5-flurouracil solid-lipid nanoparticles: A preliminary study to treat colon cancer. Int. J. Med. Sci. 2010, 7, 398–408. [Google Scholar] [CrossRef] [PubMed]
- Akinyelu, J.; Singh, M. Folate-tagged chitosan-functionalized gold nanoparticles for enhanced delivery of 5-fluorouracil to cancer cells. Appl. Nanosci. 2019, 9, 7–17. [Google Scholar] [CrossRef]
- Sun, L.; Chen, Y.; Zhou, Y.; Guo, D.; Fan, Y.; Guo, F.; Zheng, Y.; Chen, W. Preparation of 5-fluorouracil-loaded chitosan nanoparticles and study of the sustained release in vitro and in vivo. Asian J. Pharm. Sci. 2017, 12, 418–423. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Yin, Y.; Xu, S.-J.; Chen, W.-S. 5-Fluorouracil: Mechanisms of Resistance and Reversal Strategies. Molecules 2008, 13, 1551–1569. [Google Scholar] [CrossRef] [PubMed]
- Sommer, J.; Mahli, A.; Freese, K.; Schiergens, T.S.; Kuecuekoktay, F.S.; Teufel, A.; Thasler, W.E.; Müller, M.; Bosserhoff, A.K.; Hellerbrand, C. Analysis of molecular mechanisms of 5-fluorouracil-induced steatosis and inflammation in vitro and in mice. Oncotarget 2017, 8, 13059–13072. [Google Scholar] [CrossRef] [PubMed]
- Papanastasopoulos, P.; Stebbing, J. Molecular basis of 5-fluorouracil-related toxicity: Lessons from clinical practice. Anticancer Res. 2014, 34, 1531–1535. [Google Scholar]
- Entezar-Almahdi, E.; Mohammadi-Samani, S.; Tayebi, L.; Farjadian, F. Recent Advances in Designing 5-Fluorouracil Delivery Systems: A Stepping Stone in the Safe Treatment of Colorectal Cancer. Int. J. Nanomed. 2020, 15, 5445–5458. [Google Scholar] [CrossRef]
- Ghafouri-Fard, S.; Abak, A.; Tondro Anamag, F.; Shoorei, H.; Fattahi, F.; Javadinia, S.A.; Basiri, A.; Taheri, M. 5-Fluorouracil: A Narrative Review on the Role of Regulatory Mechanisms in Driving Resistance to This Chemotherapeutic Agent. Front. Oncol. 2021, 11, 1–21. [Google Scholar] [CrossRef]
- Longley, D.B.; Harkin, D.P.; Johnston, P.G. 5-Fluorouracil: Mechanisms of action and clinical strategies. Nat. Rev. Cancer 2003, 3, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, J.S. Toxicity of 5-fluorouracil. Oncology 1999, 13, 33–34. [Google Scholar] [PubMed]
- van Kuilenburg, A.B.; Meinsma, R.; van Gennip, A.H. Pyrimidine degradation defects and severe 5-fluorouracil toxicity. Nucleosides Nucleotides Nucleic Acids 2004, 23, 1371–1375. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, A.; Jiang, W.; Guan, Z. Pharmacokinetic characteristics and anticancer effects of 5-Fluorouracil loaded nanoparticles. BMC Cancer 2008, 8, 103. [Google Scholar] [CrossRef]
- Ning, S.; Dai, X.; Tang, W.; Guo, Q.; Lyu, M.; Zhu, D.; Zhang, W.; Qian, H.; Yao, X.; Wang, X. Cancer cell membrane-coated C-TiO2 hollow nanoshells for combined sonodynamic and hypoxia-activated chemotherapy. Acta Biomater. 2022, 152, 562–574. [Google Scholar] [CrossRef]
- Guo, W.; Wang, T.; Huang, C.; Ning, S.; Guo, Q.; Zhang, W.; Yang, H.; Zhu, D.; Huang, Q.; Qian, H.; et al. Platelet membrane-coated C-TiO2 hollow nano-spheres for combined sonodynamic and alkyl-radical cancer therapy. Nano Res. 2023, 16, 782–791. [Google Scholar] [CrossRef]
- He, G.; Ma, Y.; Zhou, H.; Sun, S.; Wang, X.; Qian, H.; Xu, Y.; Miao, Z.; Zha, Z. Mesoporous NiS2 nanospheres as a hydrophobic anticancer drug delivery vehicle for synergis-tic photothermal-chemotherapy. J. Mater. Chem. B 2019, 7, 143–149. [Google Scholar] [CrossRef]
- Zhukova, O.V.; Ryabov, S.A.; Zaitsev, S.D.; Kuznetsova, O.V.; Gavrilova, D.M.; Archipova, E.V.; Golovacheva, A.A.; Volkova, Y.S. Water-soluble polymeric ionic 5-fluorouracil complex based on methacrylic acid copolymers. Int. J. Appl. Pharm. 2019, 11, 214–219. [Google Scholar] [CrossRef]
- Shen, J.; Yan, B.; Li, T.; Long, Y.; Li, N.; Ye, M. Mechanical, thermal and swelling properties of poly(acrylic acid)–graphene oxide composite hydrogels. Soft Matter 2012, 8, 1831–1836. [Google Scholar] [CrossRef]
- Zhukova, O.V.; Arkhipova, E.V.; Kovaleva, T.F.; Ryabov, S.A.; Ivanova, I.P.; Golovacheva, A.A.; Zykova, D.A.; Zaitsev, S.D. Immunopharmacological Properties of Methacrylic Acid Polymers as Potential Polymeric Carrier Constituents of Anticancer Drugs. Molecules 2021, 26, 4855. [Google Scholar] [CrossRef]
- Yalabik-Kas, H.S.; Kreuter, J.; Hincal, A.A.; Speiser, P.P. Sorption of 5-fluorouracil from aqueous solutions onto methyl methacrylate nanoparticles. J. Microencapsul. 1986, 3, 71–75. [Google Scholar] [CrossRef]
- Hariharan, M.S.; Sivaraj, R.; Ponsubha, S.; Jagadeesh, R.; Enoch, I.V.M.V. 5-Fluorouracil-loaded β-cyclodextrin-carrying polymeric poly(methylmethacrylate)-coated samarium ferrite nanoparticles and their anticancer activity. J. Mater. Sci. 2018, 54, 4942–4951. [Google Scholar] [CrossRef]
- Zheng, X.F.; Lian, Q.; Yang, H.; Wang, X. Surface Molecularly Imprinted Polymer of Chitosan Grafted Poly(methyl methacrylate) for 5-Fluorouracil and Controlled Release. Sci. Rep. 2016, 6, 21409. [Google Scholar] [CrossRef] [PubMed]
- Rao, K.K.; Chung, I.; Reddy, K.M.; Ha, C.S. PMMA-Based Microgels for Controlled Release of an Anticancer Drug. J. Appl. Polym. Sci. 2008, 111, 845–853. [Google Scholar] [CrossRef]
- Pandey, S.P.; Shukla, T.; Dhote, V.K.; Mishra, D.; Maheshwari, R.; Tekade, R.K. Use of Polymers in Controlled Release of Active Agents. In Basic Fundamentals of Drug Delivery; Academic Press: Cambridge, MA, USA, 2019; pp. 113–172. [Google Scholar] [CrossRef]
- Obeidat, W.M.; Qasim, D.; Nokhodchi, A.; Al-Jabery, A.; Sallam, A.S. Novel Salted An-ionic-Cationic Polymethacrylate Polymer Blends for Sustained Release of Acidic and Basic Drugs. Curr. Drug Deliv. 2017, 14, 109–122. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Victor, S.P.; Sharma, C.P. Stimuli Sensitive Polymethacrylic Acid Microparticles (PMAA)—Oral Insulin Delivery. J. Biomater. Appl. 2002, 17, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, G.; Demir, B.; Timur, S.; Becer, C.R. Poly(methacrylic acid)-Coated Gold Nanoparticles: Functional Platforms for Theranostic Applications. Biomacromolecules 2016, 17, 2901–2911. [Google Scholar] [CrossRef]
- Lin, Y.; Xu, J.; Lan, H. Tumor-associated macrophages in tumor metastasis: Biological roles and clinical therapeutic applications. J. Hematol. Oncol. 2019, 12, 76. [Google Scholar] [CrossRef]
- Zhukova, O.V.; Arkhipova, E.V.; Kovaleva, T.F.; Zykova, D.A.; Dubovskaya, N.A. Effect of Poly(methacrylic acid) on the Cytokine Level in an In Vivo Tumor Model. Molecules 2022, 27, 4572. [Google Scholar] [CrossRef]
- Diasio, R.B.; Harris, B.E. Clinical Pharmacology of 5-Fluorouracil. Clin. Pharmacokinet. 1989, 16, 215–237. [Google Scholar] [CrossRef]
- Fraile, R.J.; Baker, L.H.; Buroker, T.R.; Horwitz, J.; Vaitkevicius, V.K. Pharmacokinetics of 5-fluorouracil administered orally, by rapid intravenous and by slow infusion. Cancer Res. 1980, 40, 2223–2228. [Google Scholar] [PubMed]
- Li, M.; Liang, Z.; Sun, X.; Gong, T.; Zhang, Z. A polymeric prodrug of 5-fluorouracil-1-acetic acid using a multi-hydroxyl polyethylene glycol derivative as the drug carrier. PLoS ONE 2014, 9, e112888. [Google Scholar] [CrossRef] [PubMed]
- Kuznecova, I.G.; Dubovik, E.G.; Dubovik, N.S.; Komarov, T.N.; Medvedev, Y.V.; Men’shikova, L.A.; Severin, S.E.; Shohin, I.E.; Yarushok, T.A. Bioraspredelenie polimernoj transportnoj formy rifabutina. Vestn. RAMN 2015, 70, 366–371. [Google Scholar] [CrossRef]
- Xu, R.; Shi, S.J.; Zhou, S.C.; Zheng, J.W.; Chen, H.; Zou, S.Q.; Zeng, F.D. Pharmacokinetics and distribution of 5-Fu magnetic albumin deutomicrosphere in normal and tumor-bearing mice. Acta Pharm. Sin. 2007, 42, 66–70. [Google Scholar]
- Jin, Y.; Li, J.; Rong, L.F.; Lü, X.W.; Huang, Y.; Xu, S.Y. Pharmacokinetics and tissue dis-tribution of 5-fluorouracil encapsulated by galactosylceramide liposomes in mice. Acta Pharmacol. Sin. 2005, 26, 250–256. [Google Scholar] [CrossRef] [PubMed][Green Version]
- De Clercq, E.; De Somer, P. Effect of interferon, polyacrylin acid, and polymethacrylic acid on tail lesions on mice infected with vaccinia virus. Appl. Microbiol. 1968, 16, 1314–1319. [Google Scholar] [CrossRef] [PubMed]
- De Somer, P.; De Clercq, E.; Billiau, A.; Schonne, E.; Claesen, M. Antiviral activity of polyacrylic and polymethacrylic acids. I. Mode of action in vitro. J. Virol. 1968, 2, 878–885. [Google Scholar] [CrossRef] [PubMed]
- De Somer, P.; De Clercq, E.; Billiau, A.; Schonne, E.; Claesen, M. Antiviral activity of polyacrylic and polymethacrylic acids. II. Mode of action in vivo. J. Virol. 1968, 2, 886–893. [Google Scholar] [CrossRef]
- Ataullakhanov, R.I.; Khaitov, R.M.; Petrov, R.V.; Abdullaev, D.M.; Ataullakhanov, F.I. Interaction of polyanion molecules with the plasma membrane of lymphocytes differing in density of charged groups on the cell surface. Biull. Eksp. Biol. Med. 1984, 97, 588–590. [Google Scholar] [CrossRef]
- Hao, X.; Cheng, G.; Zou, M.; Sun, J.; Cui, F. Organ distribution of 5-fluorouracil loaded gelatine microspheres in mice. Pharmazie 2004, 59, 709–712. [Google Scholar]
- Borchard, G.; Kreuter, J. The role of serum complement on the organ distribution of in-travenously administered poly (methyl methacrylate) nanoparticles: Effects of pre-coating with plasma and with serum complement. Pharm. Res. 1996, 13, 1055–1058. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Joyce, P.; Bremmell, K.; Milne, R.; Prestidge, C.A. Liposomal 5-Fluorouracil Polymer Complexes Facilitate Tumor-Specific Delivery: Pharmaco-Distribution Kinet-ics Using Microdialysis. Pharmaceutics 2022, 14, 221. [Google Scholar] [CrossRef]
- Yan, C.; Gu, J.; Guo, Y.; Chen, D. In vivo biodistribution for tumor targeting of 5-fluorouracil (5-FU) loaded N-succinyl-chitosan (Suc-Chi) nanoparticles. Yakugaku Zasshi 2010, 130, 801–804. [Google Scholar] [CrossRef] [PubMed]
- Rana, A.; Adhikary, M.; Singh, P.K.; Das, B.C.; Bhatnagar, S. “Smart” drug delivery: A window to future of translational medicine. Front. Chem. 2023, 10, 1095598. [Google Scholar] [CrossRef] [PubMed]
- Ulbrich, K.; Holá, K.; Šubr, V.; Bakandritsos, A.; Tuček, J.; Zbořil, R. Targeted Drug De-livery with Polymers and Magnetic Nanoparticles: Covalent and Noncovalent Approaches, Release Control, and Clinical Studies. Chem. Rev. 2016, 116, 5338–5431. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Wu, J.; Sawa, T.; Matsumura, Y.; Hori, K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: A review. J. Control. Release 2000, 65, 271–284. [Google Scholar] [CrossRef] [PubMed]
- Seymour, L.W.; Miyamoto, Y.; Maeda, H.; Brereton, M.; Strohalm, J.; Ulbrich, K.; Duncan, R. Influence of molecular weight on passive tumour accumulation of a soluble macromolecular drug carrier. Eur. J. Cancer 1995, 31A, 766–770. [Google Scholar] [CrossRef] [PubMed]
- Krasnopeeva, E.L.; Melenevskaya, E.Y.; Klapshina, L.G.; Shilyagina, N.Y.; Balalaeva, I.V.; Smirnov, N.N.; Smirnov, M.A.; Yakimansky, A.V. Poly(methacrylic Acid)-Cellulose Brushes as Anticancer Porphyrazine Carrier. Nanomaterials 2021, 11, 1997. [Google Scholar] [CrossRef]
- Alsehli, M. Polymeric nanocarriers as stimuli-responsive systems for targeted tumor (cancer) therapy: Recent advances in drug delivery. Saudi Pharm. J. 2020, 28, 255–265. [Google Scholar] [CrossRef]
- Shalviri, A.; Raval, G.; Prasad, P.; Chan, C.; Liu, Q.; Heerklotz, H.; Rauth, A.M.; Wu, X.Y. pH-Dependent doxorubicin release from terpolymer of starch, polymethacrylic acid and polysorbate 80 nanoparticles for overcoming multi-drug resistance in human breast cancer cells. Eur. J. Pharm. Biopharm. 2012, 82, 587–597. [Google Scholar] [CrossRef]
- Mishra, R.K.; Ramasamy, K.; Ahmad, N.A.; Eshak, Z.; Majeed, A.B. pH dependent poly [2-(methacryloyloxyethyl)trimetylammonium chloride-co-methacrylic acid] hydrogels for enhanced targeted delivery of 5-fluorouracil in colon cancer cells. J. Mater. Sci. Mater. Med. 2014, 25, 999–1012. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Cao, D.; Liu, Y.; Wang, H.; Ke, X.; Ci, T. Low molecular weight heparin-based reduction sensitive nanoparticles for antitumor and antimetastasis of orthotopic breast cancer. Biomater. Sci. 2018, 6, 2172–2188. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Tang, Z.; Li, M.; Song, W.; Zhang, D.; Zhang, Y.; Yang, Y.; Sun, H.; Deng, M.; Chen, X. Cisplatin Loaded Poly(L-glutamic acid)-g-Methoxy Poly(ethylene glycol) Complex Nanoparticles for Potential Cancer Therapy: Preparation, In Vitro and In Vivo Evaluation. J. Biomed. Nanotechnol. 2016, 12, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Tang, Z.; Song, W.; Zhang, D.; Zhang, Y.; Chen, X. Co-administration of iRGD enhancing the anticancer efficacy of cisplatin-loaded polypeptide nanoparticles. J. Control. Release 2015, 205, e145–e146. [Google Scholar] [CrossRef]
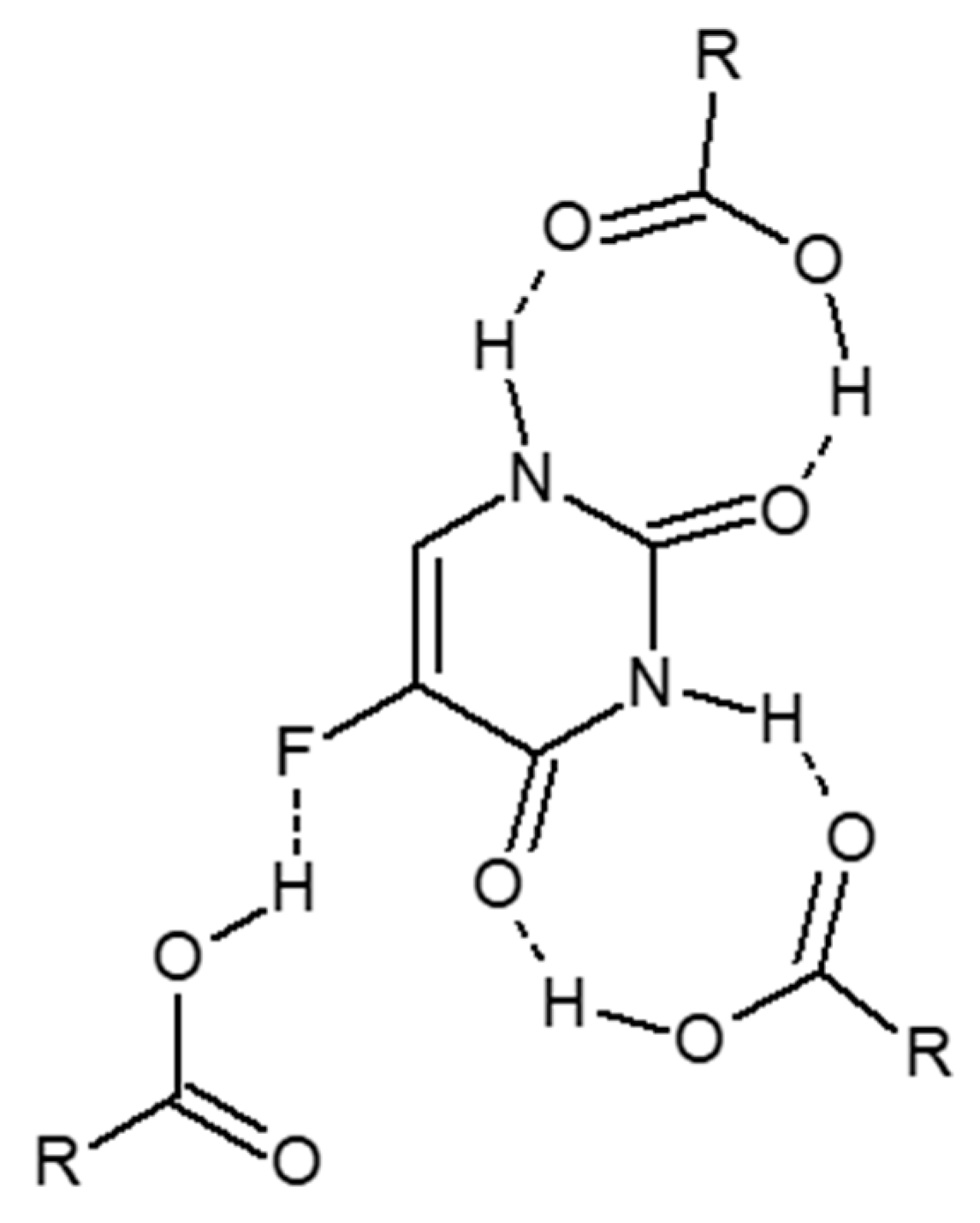
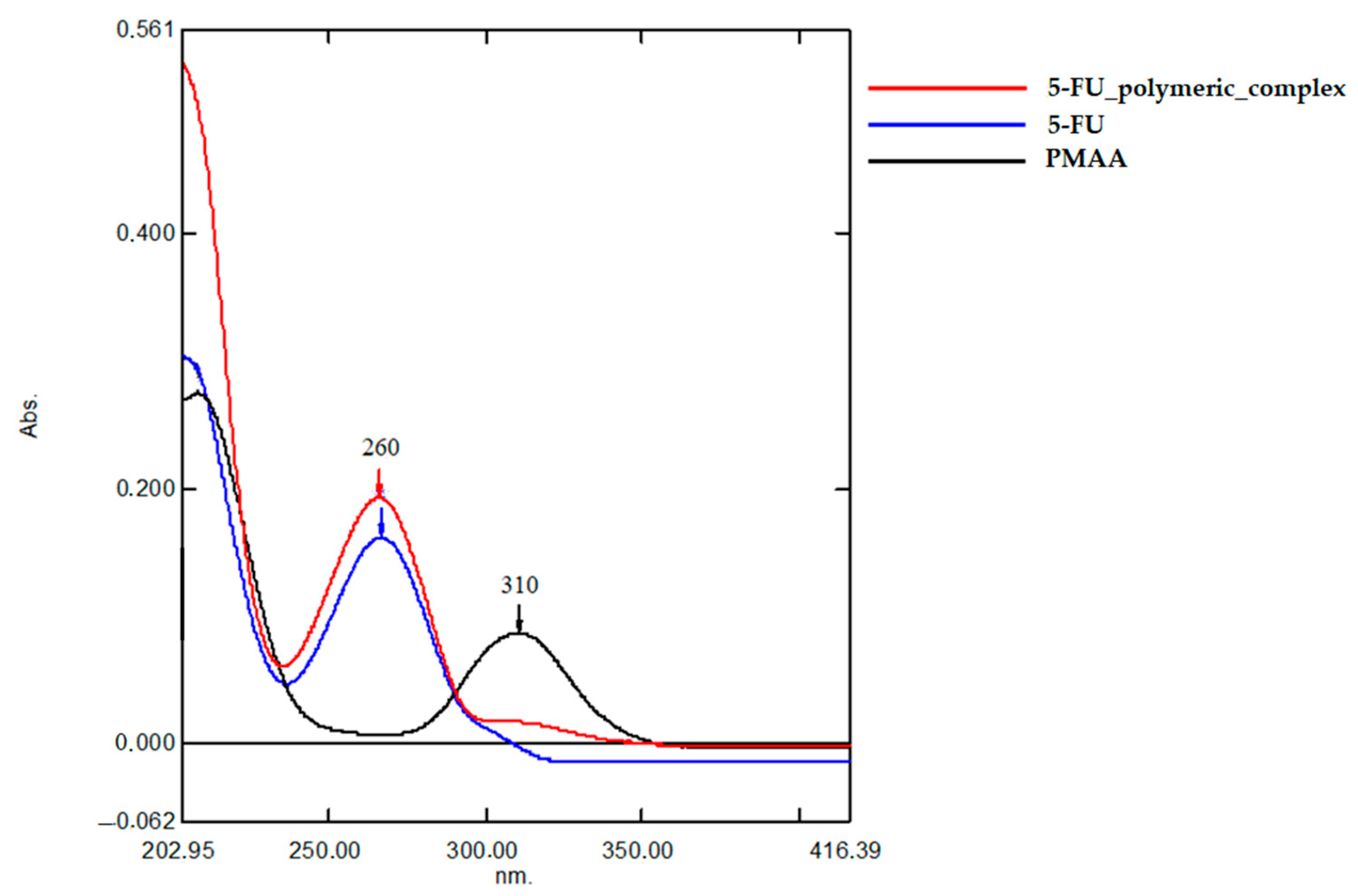
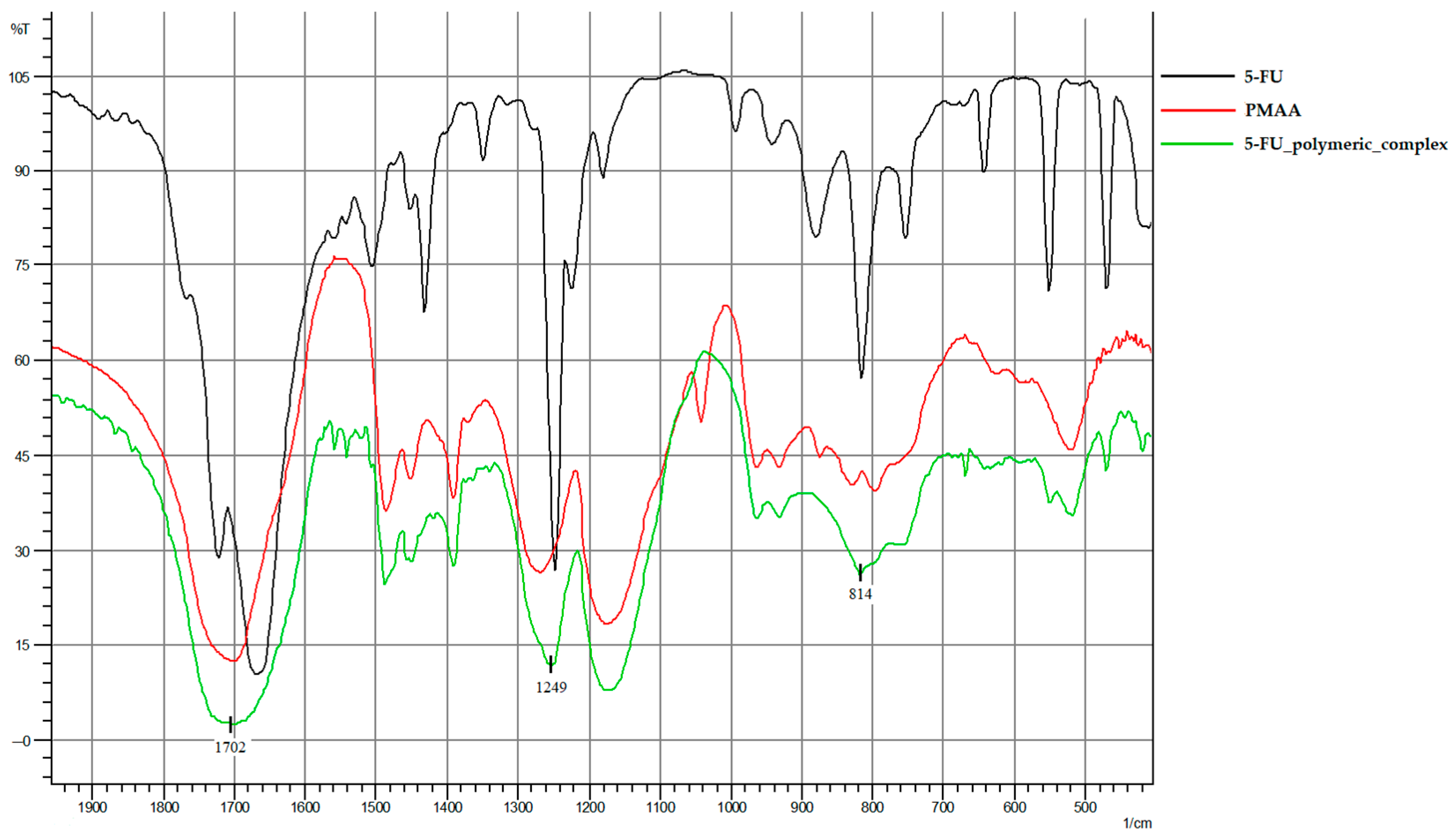

| IR Spectrum Peaks | PMAA | 5-FU | 5-FU Polymeric Complex |
|---|---|---|---|
| 1702 cm−1 | -C=O carbonyl group | - | -C=O carbonyl group |
| 1249 cm−1 | - | C-F | |
| 814 cm−1 | - | deformation vibrations of the C-H pyrimidine ring | |
| Intact Control | 5-FU Polymeric Complex | |
|---|---|---|
| Average tumor volume (mm3) | 3069 | 989.80 |
| Average tumor weight (g) | 4.87 | 2.67 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhukova, O.V.; Dubovskaya, N.A.; Zykova, D.A.; Arkhipova, E.V.; Vorobeva, O.A.; Zaborskaya, O.G.; Zaitsev, S.D.; Grigoreva, A.O.; Chicharov, A.A.; Ryabov, S.A. Specifics of Pharmacokinetics and Biodistribution of 5-Fluorouracil Polymeric Complex. Molecules 2023, 28, 8096. https://doi.org/10.3390/molecules28248096
Zhukova OV, Dubovskaya NA, Zykova DA, Arkhipova EV, Vorobeva OA, Zaborskaya OG, Zaitsev SD, Grigoreva AO, Chicharov AA, Ryabov SA. Specifics of Pharmacokinetics and Biodistribution of 5-Fluorouracil Polymeric Complex. Molecules. 2023; 28(24):8096. https://doi.org/10.3390/molecules28248096
Chicago/Turabian StyleZhukova, Olga V., Natalya A. Dubovskaya, Daria A. Zykova, Evgenia V. Arkhipova, Olga A. Vorobeva, Olga G. Zaborskaya, Sergey D. Zaitsev, Alexandra O. Grigoreva, Aleksandr A. Chicharov, and Sergey A. Ryabov. 2023. "Specifics of Pharmacokinetics and Biodistribution of 5-Fluorouracil Polymeric Complex" Molecules 28, no. 24: 8096. https://doi.org/10.3390/molecules28248096
APA StyleZhukova, O. V., Dubovskaya, N. A., Zykova, D. A., Arkhipova, E. V., Vorobeva, O. A., Zaborskaya, O. G., Zaitsev, S. D., Grigoreva, A. O., Chicharov, A. A., & Ryabov, S. A. (2023). Specifics of Pharmacokinetics and Biodistribution of 5-Fluorouracil Polymeric Complex. Molecules, 28(24), 8096. https://doi.org/10.3390/molecules28248096





