Synthesis, Characterization, and Reactivity Studies of New Cyclam-Based Y(III) Complexes
Abstract
1. Introduction
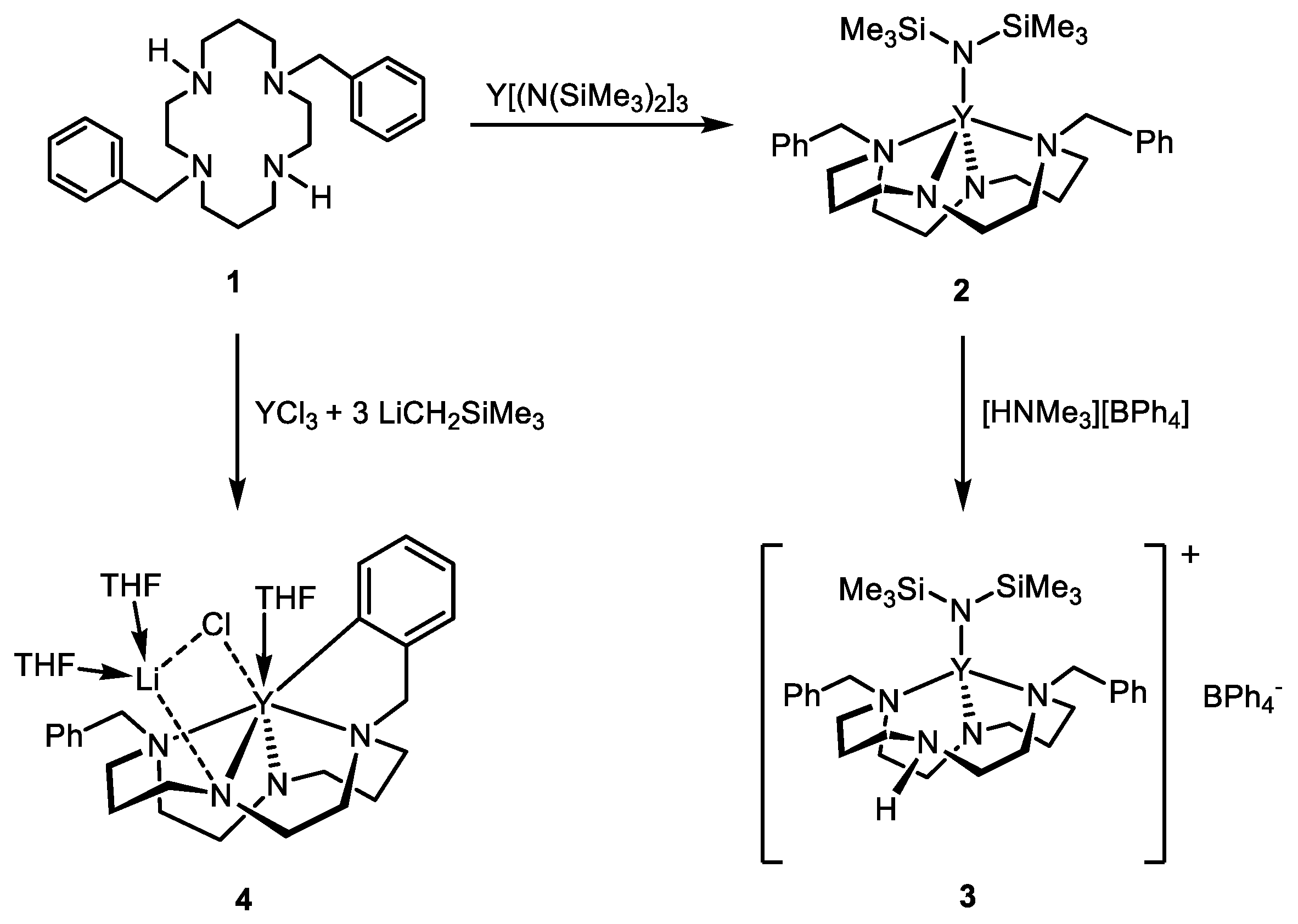
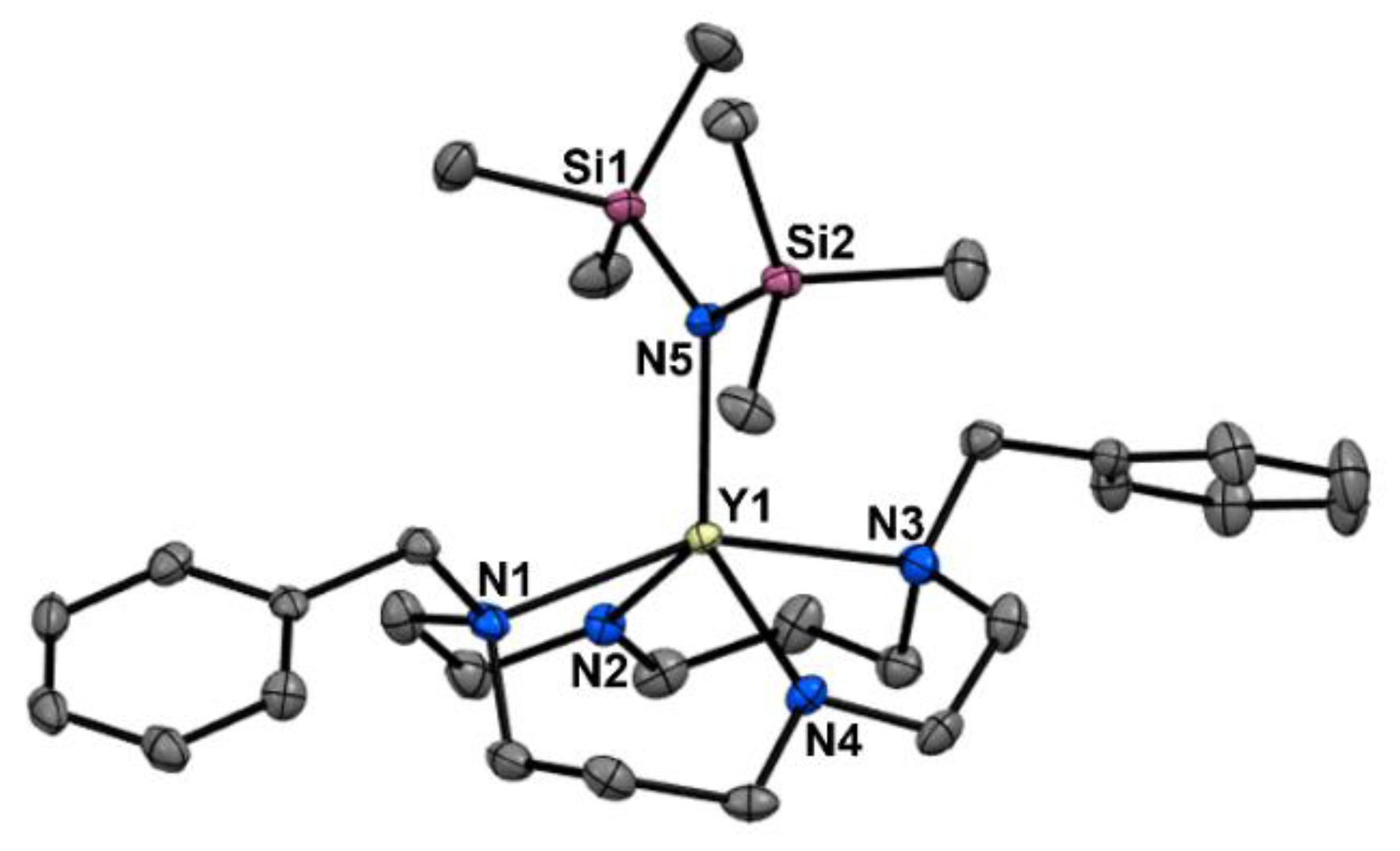
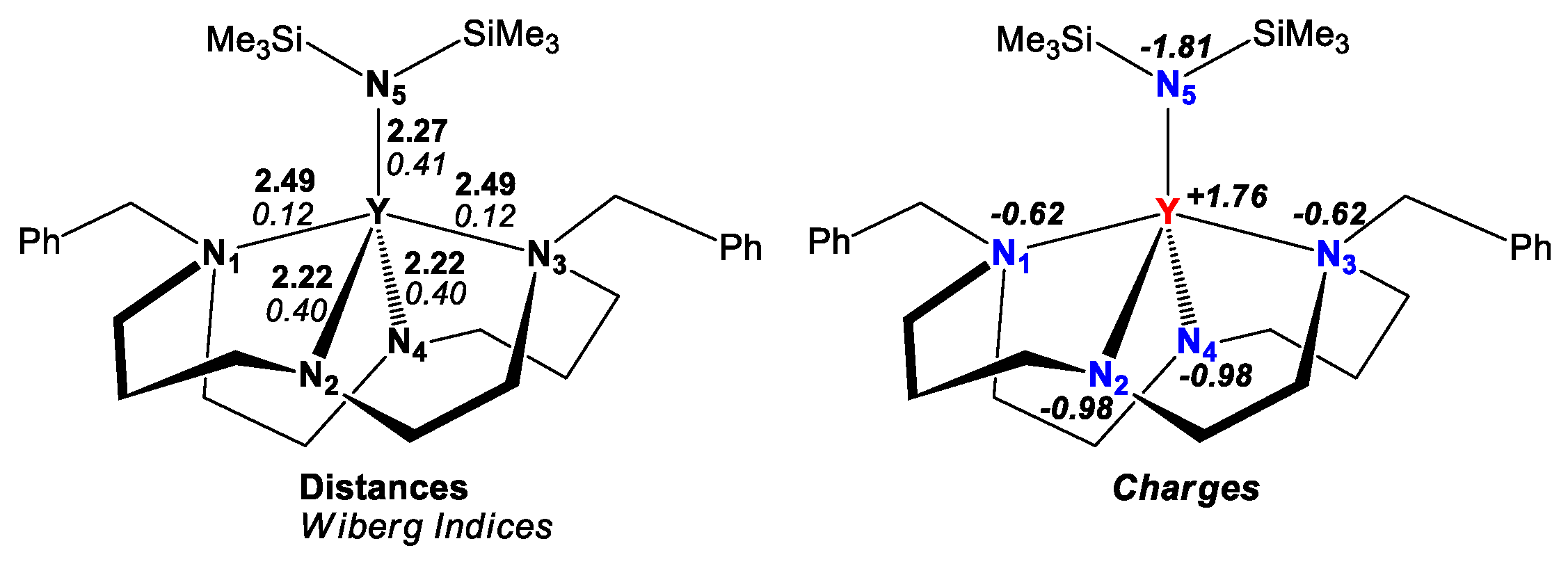
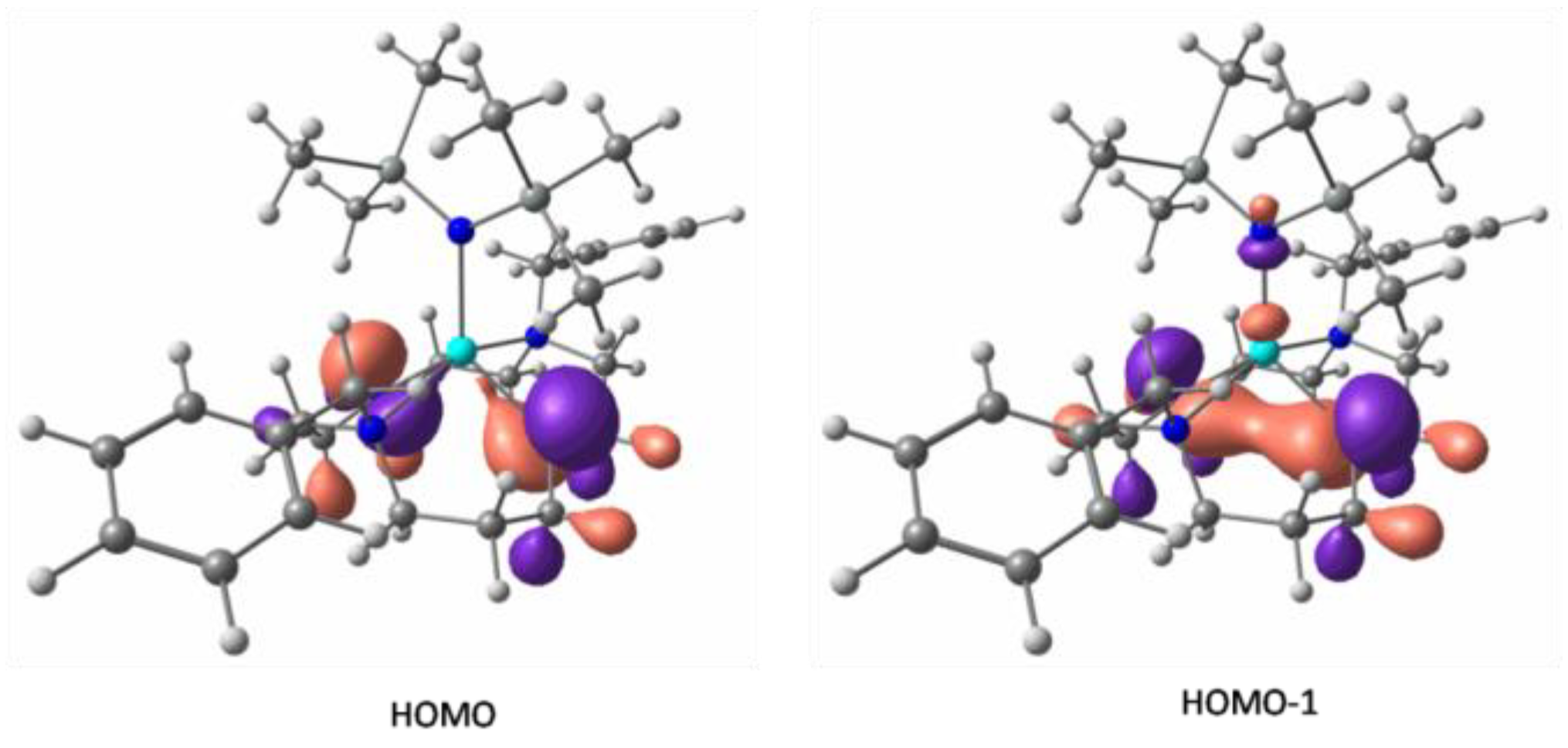
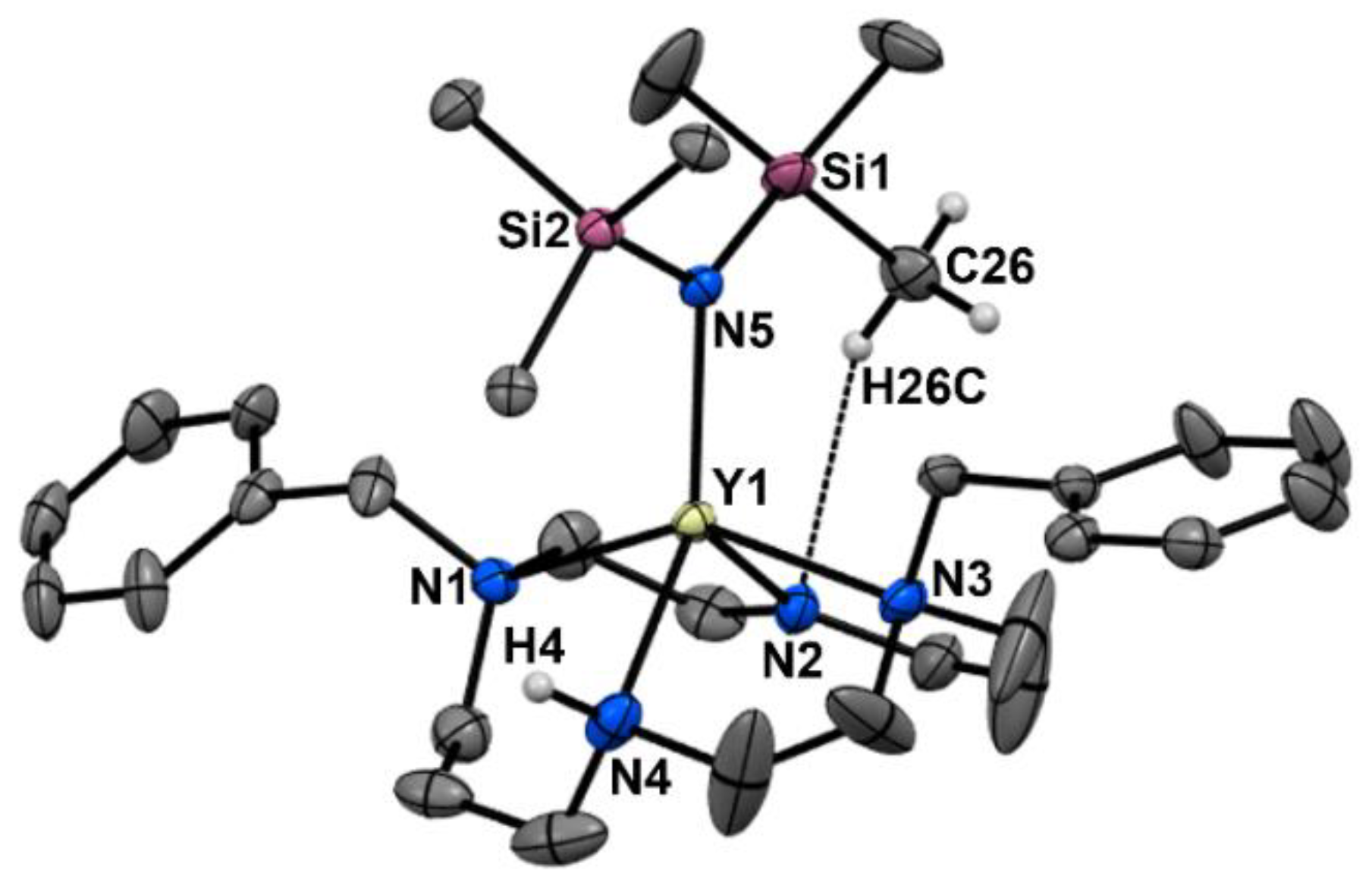
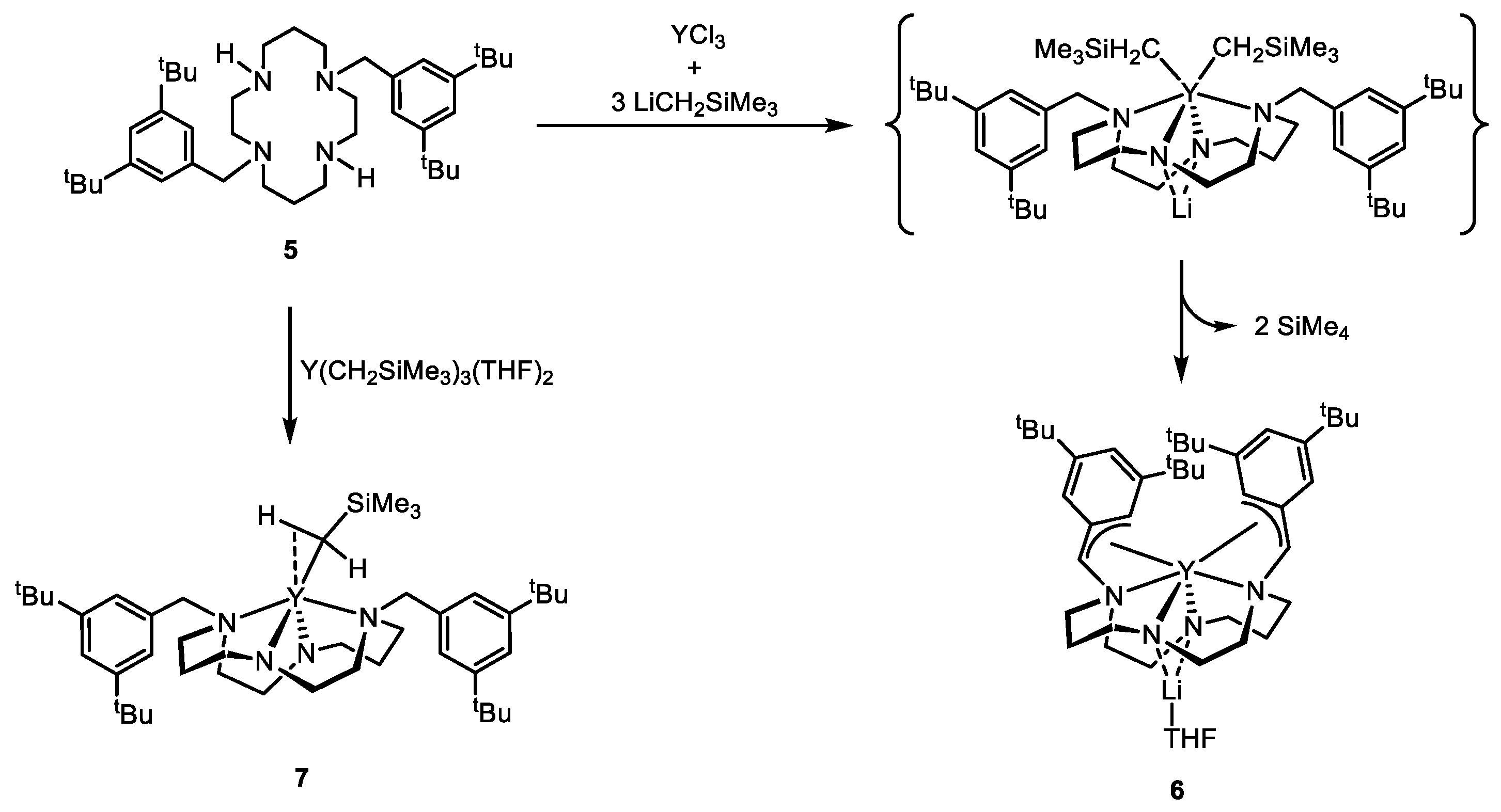
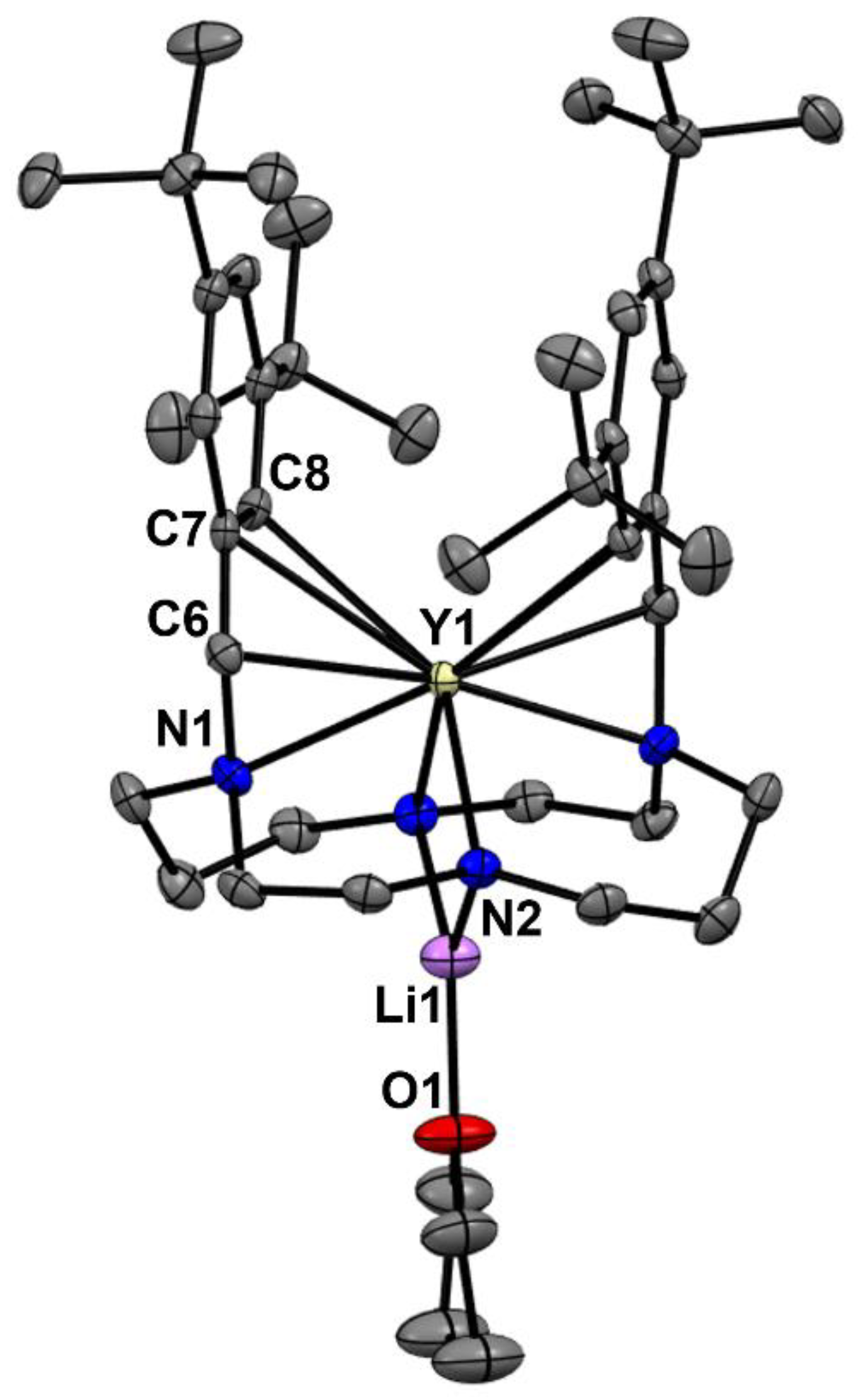
2. Results and Discussion
3. Materials and Methods
3.1. General Considerations
3.2. Synthetic Procedures
3.3. Catalytic Assays
3.4. Single-Crystal X-ray Diffraction Studies
3.5. Computational Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Trifonov, A.A.; Lyubov, D.M.A. A quarter-century long story of bis(alkyl) rare-earth (III) complexes. Coord. Chem. Rev. 2017, 340, 10–61. [Google Scholar] [CrossRef]
- Manβen, M.; Schafer, L.L. Early Transition Metal-Catalyzed Hydroamination. Trends Chem. 2021, 3, 428–429. [Google Scholar]
- Lovick, H.M.; An, D.K.; Livinghouse, T.S. Structure-activity relationships in group 3 metal catalysts for asymmetric intramolecular alkenehydroamination. An investigation of ligands based on the axially chiral 1,1′-binaphthyl-2,2′-diamine motif. Dalton Trans. 2011, 40, 7697–7700. [Google Scholar] [CrossRef] [PubMed]
- Chapurina, Y.; Guillot, R.; Lyubov, D.; Trifonov, A.; Hannedouche, J.; Schultz, E. LiCl-effect on asymmetric intramolecular hydroamination catalyzed by binaphthylamido yttrium complexes. Dalton Trans. 2013, 42, 507–520. [Google Scholar] [CrossRef] [PubMed]
- Lauterwasser, F.; Hayes, P.G.; Piers, W.E.; Schafer, L.L.; Bräse, S. Highly Active and Diastereoselective N,O- and N,N-Yttrium Complexes for Intramolecular Hydroamination. Adv. Synth. Catal. 2011, 353, 1384–1390. [Google Scholar] [CrossRef]
- Aillaud, I.; Collin, J.; Hannedouche, J.; Schulz, E.; Trifonov, A. Comparison of yttrium binaphthylamido alkyl and amide complexes for enantioselective intramolecular hydroamination. Tetrahedron Lett. 2010, 51, 4742–4745. [Google Scholar] [CrossRef]
- Reznichenko, A.L.; Hultzsch, K.C. C1-Symmetric Rare-Earth-Metal Aminodiolate Complexes for Intra- and Intermolecular Asymmetric Hydroamination of Alkenes. Organometallics 2013, 32, 1394–1408. [Google Scholar] [CrossRef]
- Nguyen, H.N.; Lee, H.; Audörsch, S.; Reznichenko, A.L.; Nawara-Hultzsch, A.J.; Schmidt, B.; Hultzsch, K.C. Asymmetric Intra- and Intermolecular Hydroamination Catalyzed by 3,3′-Bis(trisarylsilyl)- and 3,3′-Bis(arylalkylsilyl)-Substituted Binaphtholate Rare-Earth-Metal Complexes. Organometallics 2018, 37, 4358–4379. [Google Scholar] [CrossRef]
- Nguyen, H.N.; Hultzsch, K.C. Rare-Earth-Metal-Catalyzed Kinetic Resolution of Chiral Aminoalkenes via Hydroamination: The Effect of the Silyl Substituent of the Binaphtholate Ligand on Resolution Efficiency. Eur. J. Org. Chem. 2019, 2019, 2592–2601. [Google Scholar] [CrossRef] [PubMed]
- Nawara-Hultzsch, A.J.; Goswami, A.; Hultzsch, K.C. Effect of Additives in the Hydroamination/Cyclization of Aminoalkenes Catalyzed by a Binaphtholate Yttrium Catalyst. Adv. Syn. Cat. 2023, 365, 568–578. [Google Scholar] [CrossRef]
- Kissel, A.A.; Lyubov, D.M.; Mahrova, T.V.; Fukin, G.K.; Cherkasov, A.V.; Glukhova, T.A.; Cuib, D.; Trifonov, A.A. Rare-earth dichloro and bis(alkyl) complexes supported by bulky amido-iminoligand. Synthesis, structure, reactivity and catalytic activity in isoprene polymerization. Dalton Trans. 2013, 42, 9211–9225. [Google Scholar] [CrossRef] [PubMed]
- Rad’kova, N.Y.; Skvortsov, G.G.; Cherkasov, A.V.; Fukin, G.K.; Kovylina, T.A.; Ob’edkov, A.M.; Trifonov, A.A. Bis(alkyl) Sc and Y Complexes Supported by Tri- and Tetradentate Amidinate Ligands: Synthesis, Structure, and Catalytic Activity in α-Olefin and Isoprene Polymerization. Eur. J. Inorg. Chem. 2021, 2021, 2365–2373. [Google Scholar] [CrossRef]
- Tolpygin, A.O.; Sachkova, A.A.; Mikhailychev, A.D.; Ob’edkov, A.M.; Kovylina, T.A.; Cherkasov, A.V.; Fukin, G.K.; Trifonov, A.A. Sc and Y bis(alkyl) complexes supported by bidentate and tridentate amidinate ligands. Synthesis, structure and catalytic activity in polymerization of isoprene and 1-heptene. Dalton Trans. 2022, 51, 7723–7731. [Google Scholar] [CrossRef]
- Luconi, L.; Kissel, A.A.; Rossin, A.; Khamaletdinova, N.M.; Cherkasov, A.V.; Tuci, G.; Fukin, G.K.; Trifonov, A.A.; Giambastiani, G. C1 and CS 2-pyridylethylanilido zirconium(IV), yttrium(III) and lutetium(III) complexes: Synthesis, characterization and catalytic activity in the isoprene polymerization. New J. Chem. 2017, 41, 540–551. [Google Scholar] [CrossRef]
- Lyubov, D.M.; Tolpygin, A.O.; Trifonov, A.A. Rare-earth metal complexes as catalysts for ring-opening polymerization of cyclic esters. Coord. Chem. Rev. 2019, 392, 83–145. [Google Scholar] [CrossRef]
- Skvortsov, G.G.; Cherkasov, A.V.; Vorozhtsov, D.L.; Shchegravina, E.S.; Trifonov, A.A. Yttrium and Lithium Keto-β-Diketiminate Complexes [{2,6-Me2C6H3N=C(Me)}2CC(tert-Bu)=O]2Y(µ2-Cl)2Li(THF)2 and {[{2,6-Me2C6H3N=C(Me)}2CC(tert-Bu)=O]Li(THF)}n. Synthesis, and Catalytic Activity in ε-Caprolactone Polymerization. Russ. J. Coord. Chem. 2021, 47, 144–154. [Google Scholar] [CrossRef]
- Fanq, J.; Tschan, M.J.-L.; Roisnel, T.; Trivelli, X.; Gauvin, R.M.; Thomas, C.M.; Maron, L. Yttrium catalysts for syndioselective β-butyrolactone polymerization: On the origin of ligand-induced stereoselectivity. Polym. Chem. 2013, 4, 360–367. [Google Scholar] [CrossRef]
- Mazzeo, M.; Tramontano, R.; Lamberti, M.; Pilone, A.; Milione, S.; Pellecchia, C. Rare earth complexes of phenoxy-thioetherligands: Synthesis and reactivity in the ring opening polymerization of cyclic esters. Dalton Trans. 2013, 42, 9338–9351. [Google Scholar] [CrossRef]
- Li, G.; Lamberti, M.; Mazzeo, M.; Pappalardo, D.; Roviello, G.; Pellecchia, C. Anilidopyridyl-Pyrrolide and Anilidopyridyl-Indolide Group 3 Metal Complexes: Highly Active Initiators for the Ring-Opening Polymerization of rac-Lactide. Organometallics 2012, 31, 1180–1188. [Google Scholar] [CrossRef]
- Bakewell, C.; Cao, T.-P.-A.; Long, N.; Goff, X.F.L.; Auffrant, A.; Williams, C.K. Yttrium Phosphasalen Initiators for rac-Lactide Polymerization: Excellent Rates and High Iso-Selectives. J. Am. Chem. Soc. 2012, 134, 20577–20580. [Google Scholar] [CrossRef]
- Venugopal, A.; Fegler, W.; Spaniol, T.P.; Maron, L.; Okuda, J. Dihydrogen Addition in a Dinuclear Rare-Earth Metal Hydride Complex Supported by a Metalated TREN Ligand. J. Am. Chem. Soc. 2011, 133, 17574–17577. [Google Scholar] [CrossRef]
- Jie, S.; Diaconescu, P.L. Reactions of Aromatic N-Heterocycles with Yttrium and Lutetium Benzyl Complexes Supported by a Pyridine-Diamine Ligand. Organometallics 2010, 29, 1222–1230. [Google Scholar] [CrossRef]
- Lu, E.; Gan, W.; Chen, Y. Monoalkyl and monoanilide yttrium complexes containing tridentate pyridyl-1-azaallyl dianionic ligands. Dalton Trans. 2011, 40, 2366–2374. [Google Scholar] [CrossRef] [PubMed]
- Basalov, I.V.; Kissel, A.A.; Nikolaevskaya, E.N.; Druzhkov, N.O.; Cherkasov, A.V.; Long, J.; Larionova, J.; Fukina, G.K.; Trifonov, A.A. 2-Imino-2,3-dihydrobenzoxazole-a useful platform for designing rare- and alkaline earth complexes with variable di- and trianionic O,N,N, ligands. Dalton Trans. 2022, 51, 1995–2004. [Google Scholar] [CrossRef] [PubMed]
- Fayoumi, A.; Lyubov, D.M.; Tolpygin, A.O.; Shavyrin, A.S.; Cherkasov, A.V.; Ob’edkov, A.M.; Trifonov, A.A. Sc and Y Heteroalkyl Complexes with a NCsp3N Pincer-Type Diphenylmethanido Ligand: Synthesis, Structure, and Reactivity. Eur. J. Inorg. Chem. 2020, 2020, 3259–3267. [Google Scholar] [CrossRef]
- Skvortsov, G.G.; Cherkasov, A.V.; Fukin, G.K.; Trifonov, A.A. Yttrium complexes containing heteroscorpionate ligands [(3,5-But2C3HN2)2CHC(Ph)2O]− and [o-Me2NC6H4CH2C(NCy)2]−. Russ. Chem. Bull. 2016, 65, 1189–1197. [Google Scholar] [CrossRef]
- Luconi, L.; Lyubov, D.M.; Bianchini, C.; Rossin, A.; Faggi, C.; Fukin, G.K.; Cherkasov, A.V.; Shavyrin, A.S.; Trifonov, A.A.; Giambastiani, G. Yttrium-Amidopyridinate Complexes: Synthesis and Characterization of Yttrium-Alkyl and Yttrium-Hydrido Derivatives. Eur. J. Inorg. Chem. 2010, 2010, 608–620. [Google Scholar] [CrossRef]
- Karpov, A.A.; Cherkasov, A.V.; Fukin, G.K.; Shavyrin, A.S.; Luconi, L.; Giambastini, G.; Trifonov, A.A. Yttrium Complexes Featuring Different Y-C Bonds. Comparative Reactivity Studies: Toward Terminal Imido Complexes. Organometallics 2013, 32, 2379–2388. [Google Scholar] [CrossRef]
- Cui, P.; Spaniol, T.P.; Okuda, J. Heterometallic Potassium Rare-Earth-Metal Allyl and Hydrido Complexes Stabilized by a Dianionic (NNNN)-Type Macrocyclic Ancillary Ligand. Organometallics 2013, 32, 1176–1182. [Google Scholar] [CrossRef]
- Cui, P.; Spaniol, T.P.; Maron, L.; Okuda, J. An ion pair scandium hydride supported by a dianionic (NNNN)-type macrocycle ligand. Chem. Commun. 2014, 50, 424–426. [Google Scholar] [CrossRef] [PubMed]
- Kulina, H.; Spaniol, T.P.; Okuda, J. Neutral and Cationic Zirconium Hydrides Supported by a Dianionic (NNNN)-Type Macrocycle Ligand. Organometallics 2015, 34, 2160–2164. [Google Scholar] [CrossRef]
- Munhá, R.F.; Ballmann, J.; Veiros, L.F.; Patrick, B.O.; Fryzuk, M.D.; Martins, A.M. Dinuclear Cationic Zirconium Hydrides Stabilized by the N,N-Dibenzylcyclam Ancillary Ligand. Organometallics 2012, 31, 4937–4940. [Google Scholar] [CrossRef]
- Munhá, R.F.; Veiros, L.F.; Duarte, M.T.; Fryzuk, M.D.; Martins, A.M. Synthesis and structural studies of amido, hydrazido and imido zirconium(IV) complexes incorporating a diamido/diamine cyclam-based ligand. Dalton Trans. 2009, 36, 7494–7508. [Google Scholar] [CrossRef]
- Alves, L.G.; Martins, A.M. Synthesis and Characterization of New Cyclam-Based Zr(IV) Alkoxido Derivatives. Reactions 2021, 2, 323–332. [Google Scholar] [CrossRef]
- Alves, L.G.; Munhá, R.F.; Martins, A.M. Synthesis and reactivity of cyclam-based Zr(IV) complexes. Inorg. Chim. Acta 2019, 490, 204–214. [Google Scholar] [CrossRef]
- Munhá, R.F.; Antunes, M.A.; Alves, L.G.; Veiros, L.F.; Fryzuk, M.D.; Martins, A.M. Structure and Reactivity of Neutral and Cationic trans-N,N’-Dibenzylcyclam Zirconium Alkyl Complexes. Organometallics 2010, 29, 3753–3764. [Google Scholar] [CrossRef]
- Alves, L.G.; Antunes, M.A.; Matos, I.; Munhá, R.F.; Duarte, M.T.; Fernandes, A.C.; Marques, M.M.; Martins, A.M. Reactivity of a new family of diamido-diamine cyclam-based zirconium complexes in ethylene polymerization. Inorg. Chim. Acta 2010, 363, 1823–1830. [Google Scholar] [CrossRef]
- Alves, L.G.; Hild, F.; Munhá, R.F.; Veiros, L.F.; Dagorne, S.; Martins, A.M. Synthesis and structural characterization of novel cyclam-based zirconium complexes and their use in the controlled ROP of rac-lactide: Access to cyclam-functionalized polylactide materials. Dalton Trans. 2012, 41, 14288–14298. [Google Scholar] [CrossRef]
- Alves, L.G.; Madeira, F.; Munhá, R.F.; Barroso, S.; Veiros, L.F.; Martins, A.M. Reactions of heteroallenes with cyclam-based Zr(IV) complexes. Dalton Trans. 2015, 44, 1441–1455. [Google Scholar] [CrossRef]
- Alves, L.G.; Madeira, F.; Munhá, R.F.; Maulide, N.; Veiros, L.F.; Martins, A.M. Cooperative Metal-Ligand Hydroamination Catalysis Supported by C-H Activation in Cyclam Zr(IV) Complexes. Inorg. Chem. 2018, 57, 13034–13045. [Google Scholar] [CrossRef]
- Addison, A.W.; Rao, T.N.; Reedijk, J.; van Rijn, J.; Verschoor, G.C. Synthesis, structure, and spectroscopic properties of copper(II) compounds containing nitrogen-sulphur donor ligands; the crystal and molecular structure of aqua[1,7-bis(N-methylbenzimidazol-2′-yl)-2,6-dithiaheptane]copper(II) perchlorate. J. Chem. Soc. Dalton Trans. 1984, 1349–1356. [Google Scholar] [CrossRef]
- Lu, E.; Chen, Y.; Leng, X. Yttrium Anilido Hydride: Synthesis, Structure, and Reactivity. Organometallics 2011, 30, 5433–5441. [Google Scholar] [CrossRef]
- Skinner, M.E.G.; Mountford, P. Scandium and yttrium complexes of the diamide-diamine donor ligand (2-C5H4N)CH2N(CH2CH2NSiMe3)2: Chloride, primary and secondary amide, benzamidinate and alkyl functionalized derivatives. J. Chem. Soc. Dalton Trans. 2002, 1694–1703. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, S.; Fang, X.; Zhang, L.; Tao, G.; Wei, Y.; Zhu, X.; Cui, P.; Wang, S. Syntheses of Dianionic α-Iminopyridine Rare-Earth Metal Complexes and Their Catalytic Activities toward Dehydrogenative Coupling of Amines with Hydrosilanes. Inorg. Chem. 2020, 59, 9683–9692. [Google Scholar] [CrossRef] [PubMed]
- Vitanova, D.V.; Hampel, F.; Hultzsch, K.C. Rare earth metal complexes based on β-diketiminato and novel linked bis(β-diketiminato) ligands: Synthesis, structural characterization and catalytic application in epoxide/CO2-copolymerization. J. Organomet. Chem. 2005, 690, 5182–5197. [Google Scholar] [CrossRef]
- Vitanova, D.V.; Hampel, F.; Hultzsch, K.C. Linked bis(β-diketiminato) yttrium and lanthanum complexes as catalysts in asymmetric hydroamination/cyclization of aminoalkenes (AHA). J. Organomet. Chem. 2011, 696, 321–330. [Google Scholar] [CrossRef]
- Matsuo, Y.; Mashima, K.; Tani, K. Selective Formation of Homoleptic and Heteroleptic 2,5-Bis(N-aryliminomathyl)pyrrolyl Yttrium Complexes and Their Performance as Initiators of ε-Caprolactone Polymerization. Organometallics 2001, 20, 3510–3518. [Google Scholar] [CrossRef]
- Benndorf, P.; Kratsh, J.; Hartenstein, L.; Preuss, C.M.; Roesky, P.W. Chiral Benzamidinate Ligands in Rare-Earth-Metal Coordination Chemistry. Chem. Eur. J. 2012, 18, 14454–14463. [Google Scholar] [CrossRef]
- Arnold, P.L.; Cadenbach, T.; Marr, I.H.; Fyfe, A.A.; Bell, N.L.; Bellabarba, R.; Tooze, R.P.; Love, J.B. Homo- and heteroleptic alkoxycarbene f-element complexes and their reactivity towards acidic N-H and C-H bonds. Dalton Trans. 2014, 43, 14346–14358. [Google Scholar] [CrossRef]
- Deelman, B.-J.; Booij, M.; Meetsma, A.; Teuben, J.H.; Kooijman, H.; Spek, A.L. Activation of Ethers and Sulfides by Organolanthanide Hydrides. Molecular Structures of (Cp*2Y)2(.mu.-OCH2CH2O)(THF)2 and (Cp*2Ce)2(.mu.-O)(THF)2. Organometallics 1995, 14, 2306–2317. [Google Scholar] [CrossRef][Green Version]
- Carpenter, J.E.; Weinhold, F. Analysis of the geometry of the hydroxymethyl radical by the “different hybrids for different spins” natural bond orbital procedure. J. Mol. Struct. (Theochem) 1988, 169, 41–62. [Google Scholar] [CrossRef]
- Foster, J.P.; Weinhold, F. Natural hybrid orbitals. J. Am. Chem. Soc. 1980, 102, 7211–7218. [Google Scholar] [CrossRef]
- Reed, A.E.; Weinhold, F. Natural bond orbital analysis of near-Hartree-Fock water dimer. J. Chem. Phys. 1983, 78, 4066–4073. [Google Scholar] [CrossRef]
- Reed, A.E.; Weinstock, R.B.; Weinhold, F. Natural population analysis. J. Chem. Phys. 1985, 83, 735–746. [Google Scholar] [CrossRef]
- Reed, A.E.; Curtis, L.A.; Weinhold, F. Intermolecular interactions from a natural bond orbital, donor-acceptor viewpoint. Chem. Rev. 1988, 88, 899–926. [Google Scholar] [CrossRef]
- Weinhold, F.; Carpenter, J.E. The Natural Bond Orbital Lewis Structure Concept for Molecules, Radicals, and Radical Ions. In The Structure of Small Molecules and Ions; Naaman, R., Vager, Z., Eds.; Springer: Boston, MA, USA, 1988; pp. 227–236. [Google Scholar]
- Lin, M.-H.; RajanBabu, T.V. Ligand-assisted rate acceleration in transacylation by a yttrium-salen complex. Demonstration of a conceptually new strategy for metal-catalyzed kinetic resolution of alcohols. Org. Lett. 2002, 4, 1607–1610. [Google Scholar] [CrossRef] [PubMed]
- Martins, A.M.; Ascenso, J.R.; Costa, S.M.B.; Dias, A.R.; Ferreira, H.; Ferreira, J.A. Ion Pairing in Ti(IV) Trisamidotriazacyclononane Compounds. Inorg. Chem. 2005, 44, 9017–9022. [Google Scholar] [CrossRef]
- Meyer, E.A.; Castellano, R.K.; Diederich, F. Interactions with Aromatic Rings in Chemical and Biological Recognition. Angew. Chemie Int. Ed. 2003, 42, 1210–1250. [Google Scholar] [CrossRef]
- Liu, X.; Shang, X.; Tang, T.; Hu, N.; Pei, F.; Cui, D.; Chen, X.; Jing, X. Achiral Lanthanide Alkyl Complexes Bearing N,O Multidentate Ligands. Synthesis and Catalysis of Highly Heteroselective Ring-Opening Polymerization of rac-Lactide. Organometallics 2007, 26, 2747–2757. [Google Scholar] [CrossRef]
- Maria, L.; Santos, I.C.; Alves, L.G.; Marçalo, J.; Martins, A.M. Rare earth metal complexes anchored on a new dianionic bis(phenolate)dimethylamine Cyclam ligand. J. Organomet. Chem. 2013, 728, 57–67. [Google Scholar] [CrossRef]
- Song, G.; Luo, G.; Oyamada, J.; Luo, Y.; Hou, Z. ortho-Selective C-H addition of N,N-dimethyl anilides to alkenes by a yttrium catalyst. Chem. Sci. 2016, 7, 5265–5270. [Google Scholar] [CrossRef] [PubMed]
- Wong, E.W.Y.; Das, A.K.; Katz, M.J.; Nishimura, Y.; Batchelor, R.J.; Onishi, M.; Leznoff, D.B. Diamidosilylether complexes of yttrium(III) and chromium(III): Synthetic challenges and surprises. Inorg. Chim. Acta 2006, 359, 2826–2834. [Google Scholar] [CrossRef]
- Rastätter, M.; Muterle, R.B.; Roesky, P.W.; Thiele, S.K.-H. Bis(amido)cyclodiphosph(III)azane Complexes of Yttrium and the Lanthanides. Chem. Eur. J. 2009, 15, 474–481. [Google Scholar] [CrossRef]
- Hultzsch, K.C.; Hampel, F.; Wagner, T. New yttrium complexes bearing diamidoamine ligands as efficient and diastereoselective catalysts for the intramolecular hydroamination of alkenes and alkynes. Organometallics 2004, 23, 2601–2612. [Google Scholar] [CrossRef]
- Barroso, S.; Cui, J.; Carretas, J.M.; Cruz, A.; Santos, I.C.; Duarte, M.T.; Telo, J.P.; Marques, N.; Martins, A.M. Diamine Bis(phenolate) M(III) (Y, Ti) Complexes: Synthesis, Structures, and Reactivity. Organometallics 2009, 28, 3449–3458. [Google Scholar] [CrossRef]
- Zhu, L.; Dong, Y.; Yin, B.; Ma, P.; Li, D. Improving the single-molecule magnet properties of two pentagonal bipyramidal Dy3+ compounds by the introduction of both electron-withdrawing and -donating groups. Dalton Trans. 2021, 50, 12607–12618. [Google Scholar] [CrossRef] [PubMed]
- Wells, A.F. Structural Inorganic Chemistry, 5th ed.; Oxford University Press: Oxford, UK, 1984. [Google Scholar]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst. 1976, A32, 751–767. [Google Scholar] [CrossRef]
- Mahrova, T.V.; Fukin, G.K.; Cherkasov, A.V.; Trifonov, A.A.; Ajellal, N.; Carpentier, J.-F. Yttrium Complexes Supported by Linked Bis(amide) Ligand: Synthesis, Structure, and Catalytic Activity in the Ring-Opening Polymerization of Cyclic Esters. Inorg. Chem. 2009, 48, 4258–4266. [Google Scholar] [CrossRef]
- Ge, S.; Bambirra, S.; Meetsma, A.; Hessen, B. The 6-amino-6-methyl-1,4-diazepine group as na ancillary ligand framework forneutral and cationic scandium and yttrium alkyls. Chem. Commun. 2006, 3320–3322. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liu, D.; Cui, D. NNN-Tridentate Pyrrolyl Rare-Earth Metal Complexes: Structure and Catalysis on Specific Selective Living Polymerization of Isoprene. Organometallics 2012, 31, 6014–6021. [Google Scholar] [CrossRef]
- Hillesheim, N.S.; Elfferding, M.; Linder, T.; Sundermeyer, J. New Cyclopentadienyl-N-Silylphosphazene Complexes of Rare-Earth Metals Yttrium and Lutetium. Z. Anorg. Allg. Chem. 2010, 636, 1776–1782. [Google Scholar] [CrossRef]
- Zhu, X.; Li, Y.; Wei, Y.; Wang, S.; Zhou, S.; Zhang, L. Reactivity of 3-Imino-Functionalized Indoles with Rare-Earth-Metal Amides: Unexpected Substituent Effects on C-H Activation Pathways and Assembly of Rare-Earth-Metal Complexes. Organometallics 2016, 35, 1838–1846. [Google Scholar] [CrossRef]
- Hultzsch, K.C.; Spaniol, T.P.; Okuda, J. Synthesis and Characterization of Yttrium Complexes Containing a Tridentate Linked Amido-Cyclopentadienyl Ligand. Organometallics 1998, 17, 485–488. [Google Scholar] [CrossRef]
- Martins, A.M.; Munhá, R.F.; Alves, L.G.; Bharathi, S. A new family of zirconium complexes anchored by dianionic cyclam-based ligands: Syntheses, Structures, and Catalytic Applications. In Advances in Organometallic Chemistry: The Silver/Gold Jubilee International Conference on Organometallic Chemistry Celebratory Book; Pombeiro, J.L., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2004; pp. 315–323. [Google Scholar]
- Westerhausen, M.; Hartmann, M.; Pfitzner, A.; Schwarz, W. Bis(trimethylsilyl)amide und -methanide des Yttriums—Molekülstrukturen von Tris(diethylether-O)lithium-(µ-chloro)-tris[bis(trimethylsilyl)methyl]yttriat, solvensfreiem Yttrium-tris[bis(trimethylsilyl)amid] sowie dem Bis(benzonitrile)-Komplex. Z. Anorg. Allg. Chem. 1995, 621, 837–850. [Google Scholar] [CrossRef]
- Evans, W.J.; Shreeve, J.L.; Broomhall-Dillard, R.N.R.; Ziller, J.W. Isolation and structure of a homoleptic yttrium trimethylsilylmethyl complex. J. Organomet. Chem. 1995, 501, 7–11. [Google Scholar] [CrossRef]
- Wei, X.; Cheng, Y.; Hitchcock, P.B.; Lappert, M.F. Syntheses, structures and reactions of a series of β-diketiminatoyttrium compounds. Dalton Trans. 2008, 5235–5246. [Google Scholar] [CrossRef] [PubMed]
- Behrle, A.C.; Schmidt, J.A.R. Synthesis and Reactivity of Homoleptic α-Metalated N,N-Dimethylbenzylamine Rare-Earth-Metal Complexes. Organometallics 2011, 30, 3915–3918. [Google Scholar] [CrossRef]
- Bambirra, S.; van Leusen, D.; Meetsma, A.; Hessen, B.; Teuben, J.H. Neutral and cationic yttrium alkyl complexes with linked 1,4,7-triazacyclononane-amide monoanionic ancillary ligands: Synthesis and catalytic ethene polymerization. Chem. Commun. 2001, 637–638. [Google Scholar] [CrossRef]
- Bambirra, S.; Boot, S.J.; van Leusen, D.; Meetsma, A.; Hessen, B. Yttrium Alkyl Complexes with Triamido-Amide Ligands. Organometallics 2004, 23, 1891–1898. [Google Scholar] [CrossRef]
- Royal, G.; Dahaoui-Gindrey, V.; Dahaoui, S.; Tabard, A.; Guilard, R.; Pullumbi, P.; Lecomte, C. New Synthesis of trans-Disubstituted Cyclam Macrocycles—Elucidation of the Disubstitution Mechanism on the Basis of X-ray Data and Molecular Modeling. Eur. J. Org. Chem. 1998, 1998, 1971–1975. [Google Scholar] [CrossRef]
- Munhá, R.F.; Alves, L.G.; Maulide, N.; Duarte, M.T.; Markó, I.E.; Fryzuk, M.D.; Martins, A.M. trans-Disubstituted diamido/diamine cyclam zirconium complexes. Inorg. Chem. Commun. 2008, 11, 1174–1176. [Google Scholar] [CrossRef]
- Hong, S.; Tian, S.; Metz, M.V.; Marks, T.J. C2-Symmetric Bis(oxazolinato)lanthanide Catalysts for Enantioselective Intramolecular Hydroamination/Cyclization. J. Am. Chem. Soc. 2003, 125, 14768–14783. [Google Scholar] [CrossRef] [PubMed]
- SAINT, version 7.03A; Bruker AXS Inc.: Madison, WI, USA, 1997–2003.
- Sheldrick, G.M. SADABS, Software for Empirical Absorption Corrections; University of Göttingen: Göttingen, Germany, 1996. [Google Scholar]
- Altomare, A.; Cascarano, G.; Giacovazzo, C.; Guagliardi, A.; Burla, M.C.; Polidori, G.; Camalli, M. SIR92—A program for automic solution of crystal structures by direct methods. J. Appl. Cryst. 1994, 27, 435. [Google Scholar]
- Altomare, A.; Burla, M.C.; Camalli, M.; Cascarano, G.L.; Giacovazzo, C.; Guagliardi, A.; Moliterni, A.G.G.; Polidori, G.; Spagna, R. SIR97: A new tool for crystal structure determination and refinement. J. Appl. Cryst. 1999, 32, 115–119. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar]
- Farrugia, L.J. WinGX and ORTEP for Windows: An update. J. Appl. Cryst. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Spek, A.L. PLATON SQUEEZE: A tool for the calculation of the disordered solvent contribution to the calculated structure factors. Acta Cryst. 2015, C71, 9–18. [Google Scholar]
- Macrae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.A.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M.; et al. Mercury 4.0: From visualization to analysis, design and prediction. J. Appl. Cryst. 2020, 53, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Parr, R.G.; Young, W. Density Functional Theory of Atoms and Molecules; Oxford University Press: New York, NY, USA, 1989. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. GAUSSIAN 09 (Revision A.01); Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Zhao, Y.; Truhlar, D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar]
- Zhao, Y.; Truhlar, D.G. Density Functionals with Broad Applicability in Chemistry. Theor. Chem. Acc. 2008, 41, 157–167. [Google Scholar] [CrossRef]
- Zhao, Y.; Truhlar, D.G. Applications and validations of the Minnesota density functionals. Chem. Phys. Lett. 2011, 502, 1–13. [Google Scholar] [CrossRef]
- Dunning, T.H., Jr.; Hay, P.J. Modern Theoretical Chemistry; Plenum Press: New York, NY, USA, 1977; pp. 1–28. [Google Scholar]
- Hay, P.J.; Wadt, W.R. Ab initio effective core potentials for molecular calculations. Potentials for the transition metal atoms Sc to Hg. J. Chem. Phys. 1985, 82, 270–283. [Google Scholar] [CrossRef]
- Wadt, W.R.; Hay, P.J. Ab initio effective core potentials for molecular calculations. Potentials for the main group elements Na to Bi. J. Chem. Phys. 1985, 82, 284–298. [Google Scholar] [CrossRef]
- Hay, P.J.; Wadt, W.R. Ab initio effective core potentials for molecular calculations. Potentials for K to Au including the outermost core orbitals. J. Chem. Phys. 1985, 82, 299–310. [Google Scholar] [CrossRef]
- Ehlers, A.W.; Böhme, M.; Dapprich, S.; Gobbi, A.; Höllwarth, A.; Jonas, V.; Köhler, F.; Stegmann, R.; Veldkamp, A.; Frenking, G. A set of f-polarization functions for pseudo-potential basis sets of the transition metals Sc-Cu, Y-Ag and La-Au. Chem. Phys. Lett. 1993, 208, 111–114. [Google Scholar] [CrossRef]
- Ditchfield, R.; Hehre, W.J.; Pople, J.A. Self-Consistent Molecular-Orbital Methods. IX. An Extended Gaussian-Type Basis for Molecular-Orbital Studies of Organic Molecules. J. Chem. Phys. 1971, 54, 724–728. [Google Scholar] [CrossRef]
- Hehre, W.J.; Ditchfield, R.; Pople, J.A. Self-Consistent Molecular Orbital Methods. XII. Further Extensions of Gaussian-Type Basis Sets for Use in Molecular Orbital Studies of Organic Molecules. J. Chem. Phys. 1972, 56, 2257–2261. [Google Scholar] [CrossRef]
- Hariharan, P.C.; Pople, J.A. Accuracy of Ahn equilibrium geometries by single determinant molecular orbital theory. Mol. Phys. 1974, 27, 209–214. [Google Scholar] [CrossRef]
- Gordon, M.S. The isomers of silacyclopropane. Chem. Phys. Lett. 1980, 76, 163–168. [Google Scholar] [CrossRef]
- Hariharan, P.C.; Pople, J.A. The influence of polarization functions on molecular orbital hydrogenation energies. Theor. Chim. Acta 1973, 28, 213–222. [Google Scholar] [CrossRef]
- Wiberg, K.B. Application of the people-santry-segal CNDO method to the cyclopropylcarbinyl and cyclobutyl cation and to bicyclobutane. Tetrahedron 1968, 24, 1083–1096. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Madeira, F.; Veiros, L.F.; Alves, L.G.; Martins, A.M. Synthesis, Characterization, and Reactivity Studies of New Cyclam-Based Y(III) Complexes. Molecules 2023, 28, 7998. https://doi.org/10.3390/molecules28247998
Madeira F, Veiros LF, Alves LG, Martins AM. Synthesis, Characterization, and Reactivity Studies of New Cyclam-Based Y(III) Complexes. Molecules. 2023; 28(24):7998. https://doi.org/10.3390/molecules28247998
Chicago/Turabian StyleMadeira, Filipe, Luis F. Veiros, Luis G. Alves, and Ana M. Martins. 2023. "Synthesis, Characterization, and Reactivity Studies of New Cyclam-Based Y(III) Complexes" Molecules 28, no. 24: 7998. https://doi.org/10.3390/molecules28247998
APA StyleMadeira, F., Veiros, L. F., Alves, L. G., & Martins, A. M. (2023). Synthesis, Characterization, and Reactivity Studies of New Cyclam-Based Y(III) Complexes. Molecules, 28(24), 7998. https://doi.org/10.3390/molecules28247998







