Spherical Polyelectrolyte Brushes as Flocculants and Retention Aids in Wet-End Papermaking
Abstract
1. Introduction
2. Retention Systems
2.1. Single-Component Systems
2.2. Dual-Component Systems
3. Evaluation Methods of Retention Aids
4. Basic Theory in Wet-End Papermaking
4.1. Main Forces
4.2. Machinism of Interface Interaction
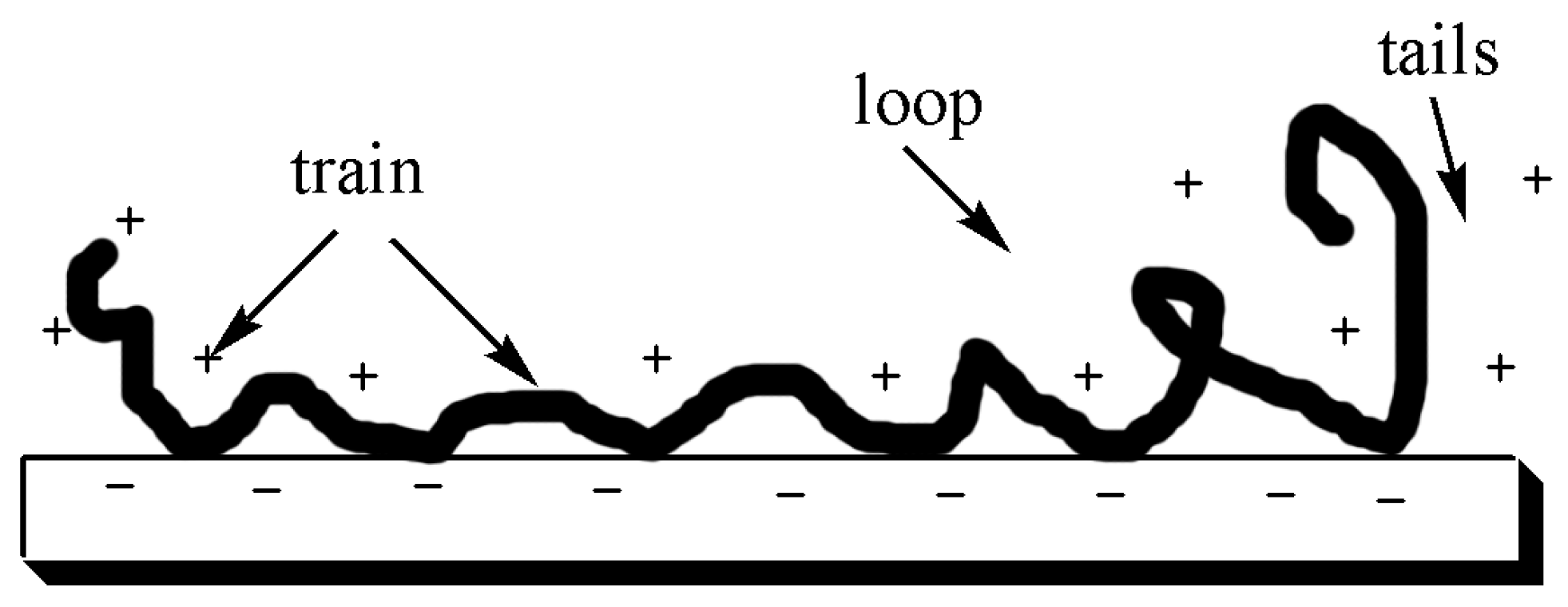
4.2.1. The Conformation of Polyelectrolytes
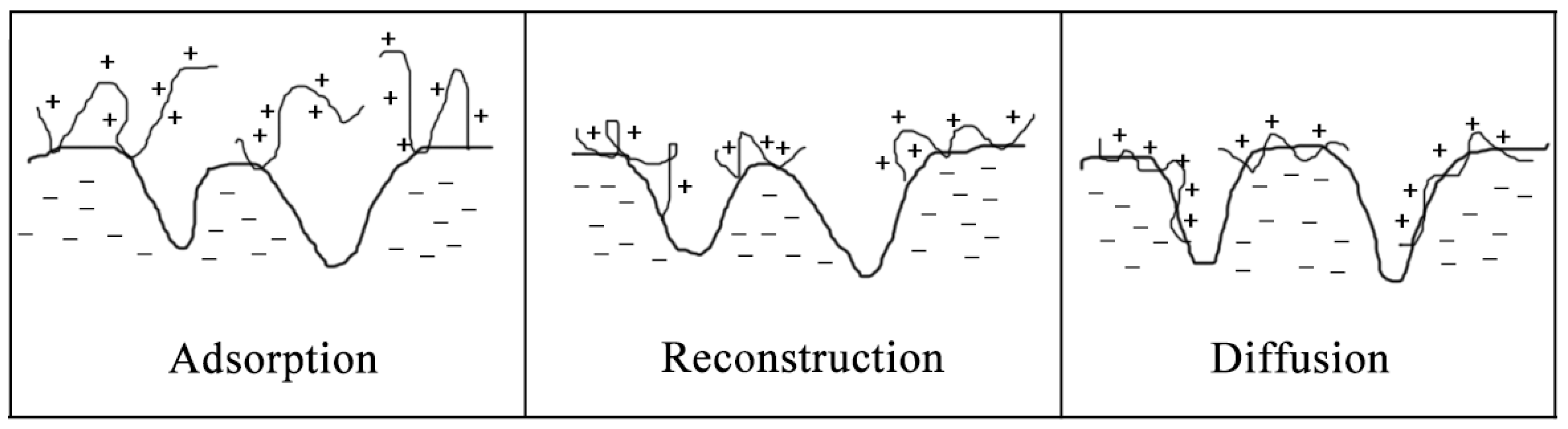
4.2.2. Adsorption between Polyelectrolytes and Fibers
4.2.3. Retention Mechanism
- (a)
- Single-component systems
- (b)
- Dual-polymeric retention system
- (c)
- Microparticle retention-aid system
5. Spherical Polyelectrolyte Brushes
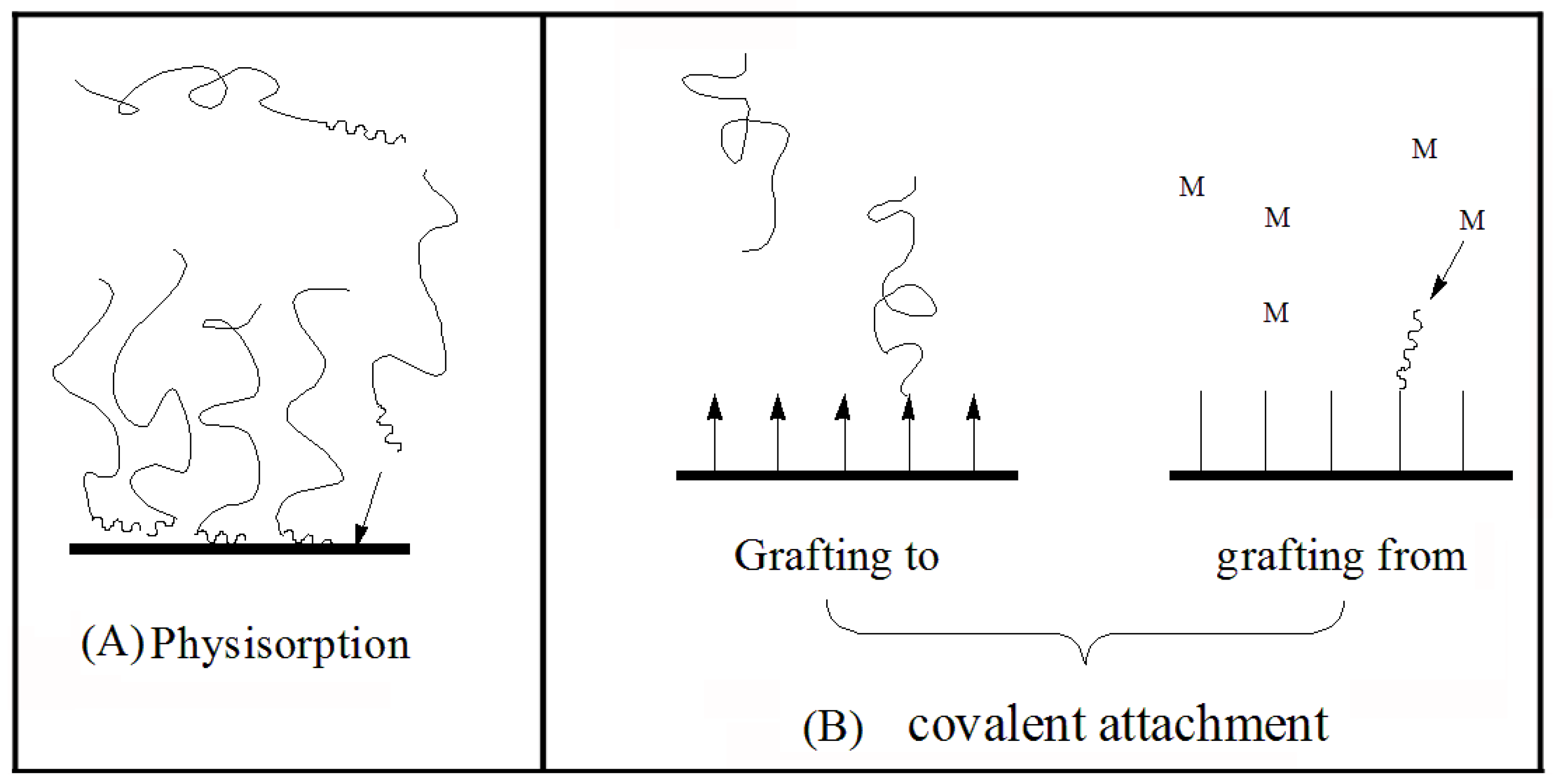
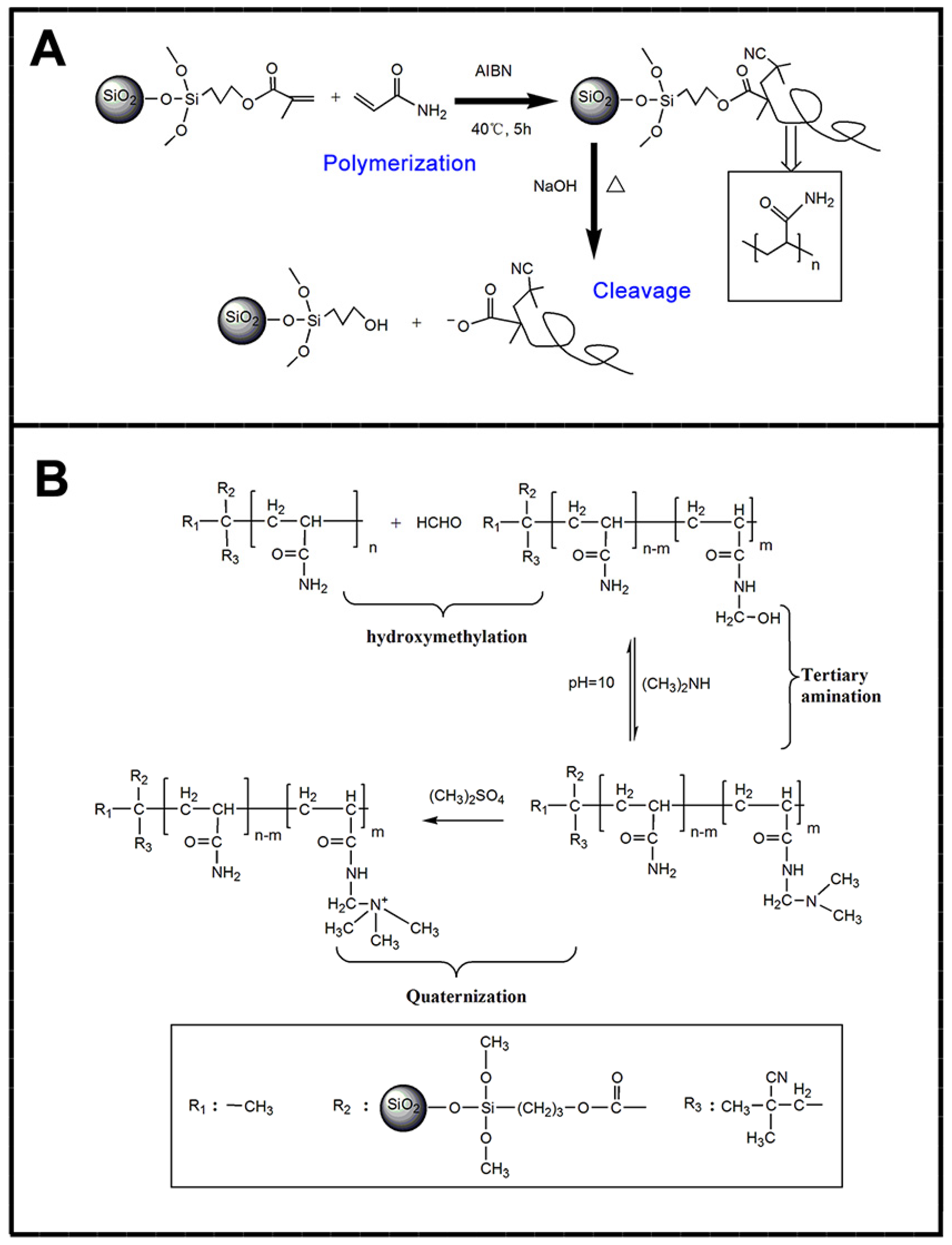
5.1. Synthesis Methods
5.1.1. Physisorption
5.1.2. Covalent Attachment
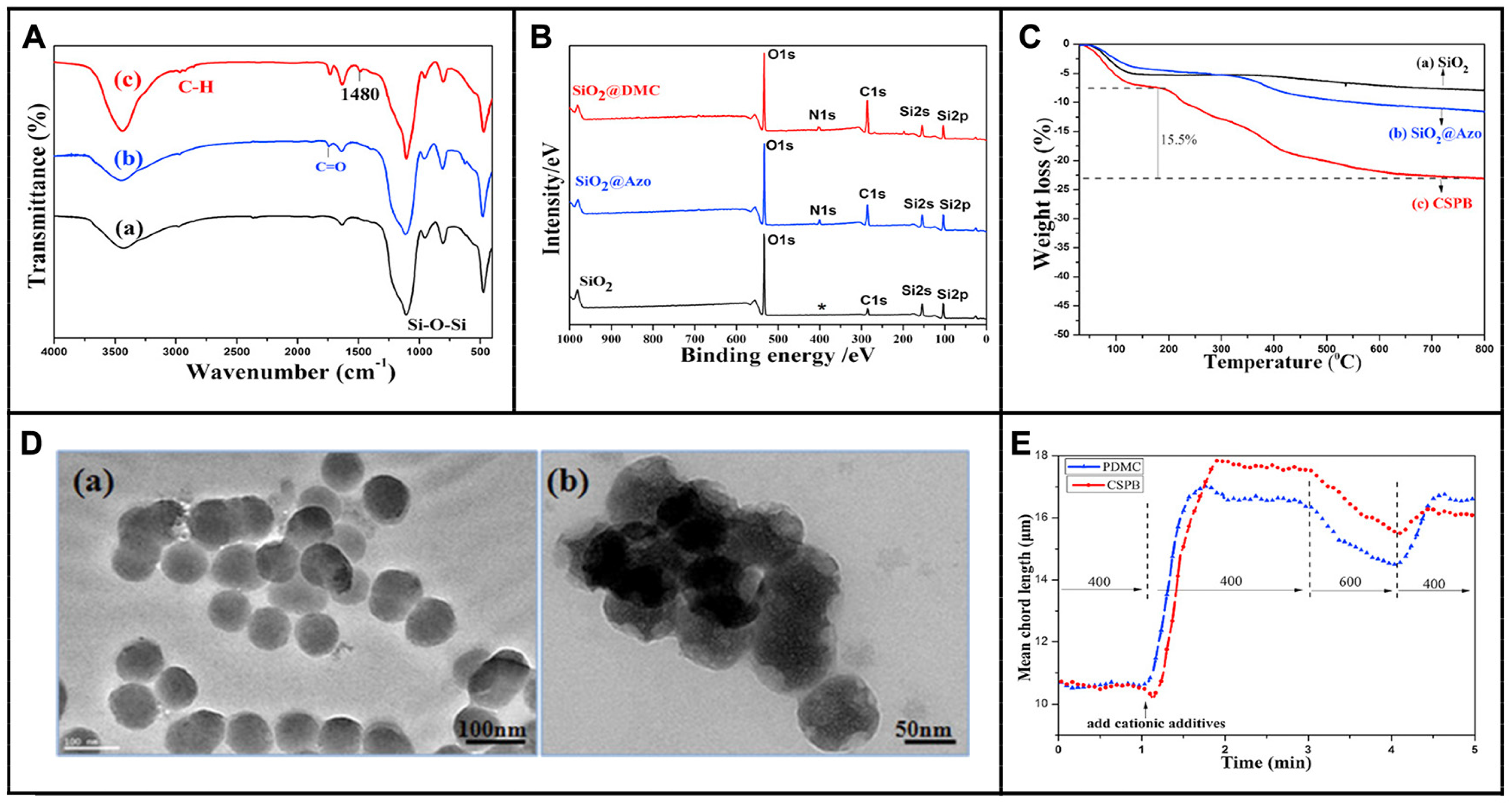
5.2. Characterization and Conformation
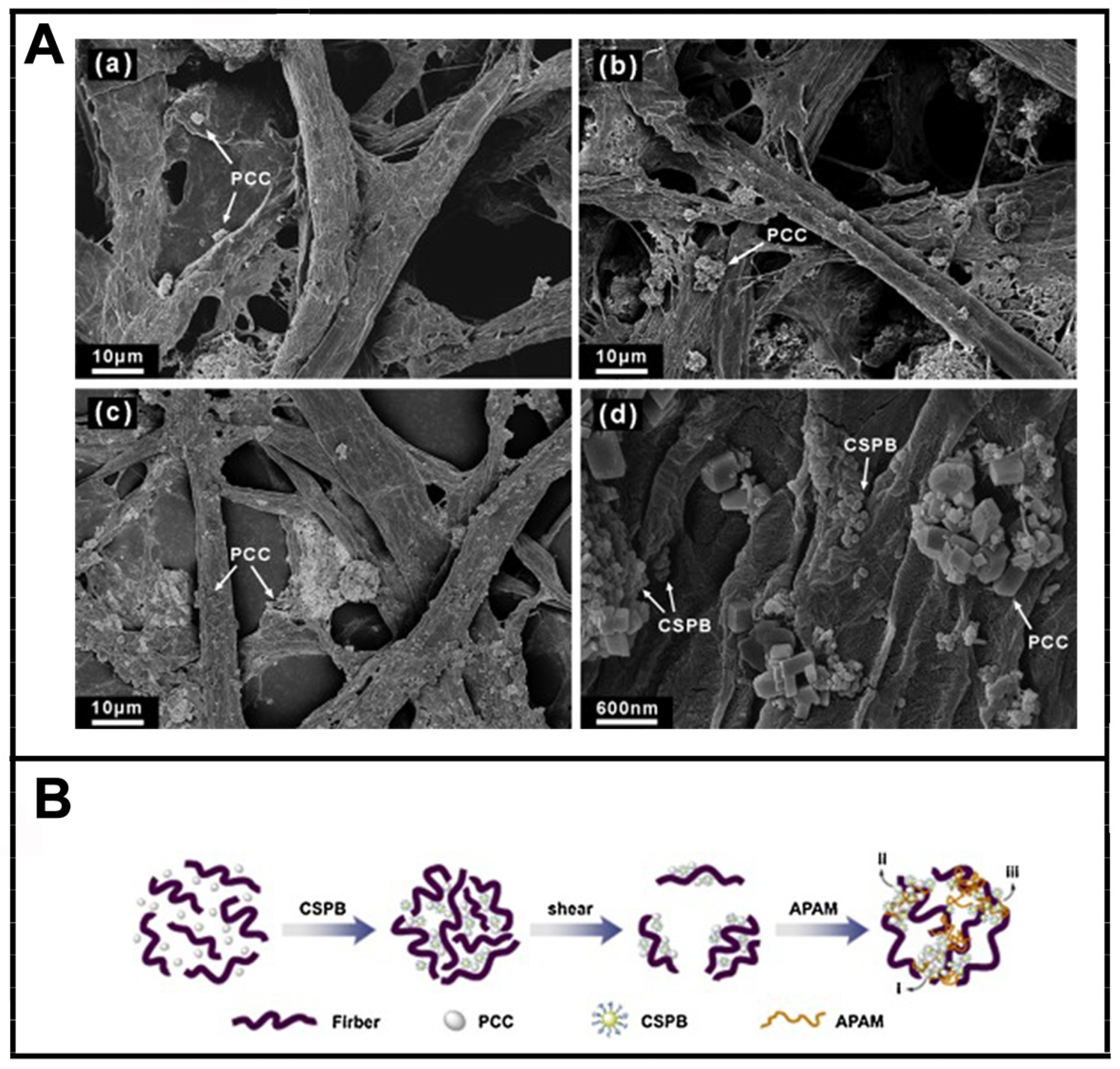
5.3. Application in Papermaking
6. Conclusions and Outlook
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Abbreviations | Name |
| GCC | Ground calcium carbonate |
| PCC | Precipitated calcium carbonate |
| SPBs | Spherical polyelectrolyte brushes |
| PAC | Polyaluminium chloride |
| PAM | Polyacrylamide |
| PEI | Polyethylene |
| CPAM | Cationic polyacrylamide |
| APAM | Anionic polyacrylamide |
| ACPAM | Amphoteric polyacrylamide |
| NPAM | Nonionic polyacrylamide |
| FBRM | Focused beam reflectance measurement |
| DAC | Acyloyloxyethyltrimethyl ammonium chloride |
| PEO | Polyethylene oxide |
| DDJ | Dynamic Drainage Jar |
| CPMP | Cationic Polymer Microparticle |
| ATRP | Atom transfer radical polymerization |
| RAFT | Reversible addition–fragmentation chain transfer |
| PS | Polystyrene |
| PMMA | Polymethyl methacrylate |
| PAA | Polyacrylic acid |
| Cryo-TEM | Cryogenic transmission electron microscopy |
| DLS | Dynamic light scattering |
| AFM | Atomic force microscopy |
| GPC | Gel permeation chromatography |
| NSCFT | Numerical self-consistent field theory |
| ASCFT | Analytical self-consistent field theory |
| MD | Molecular dynamics |
| BD | Brownian dynamics |
| EV | Extended volume |
| CSPAM | Cationic spherical polyacrylamide |
| CSPBs | Cationic spherical polyelectrolyte brushes |
| AM | Acrylamide |
| METAC | 2-(methacryloyloxy) ethyl trimethylammonium chloride |
| CEC | Cationic exchange capacity |
References
- Ma, H. A review of ways to adjust papermaking wet-end chemistry: Manipulation of cellulosic colloidal behavior. Lignocellulose 2014, 3, 69–107. [Google Scholar]
- Gibbs, A.; Pelton, R.; Cong, R. The influence of dextran derivatives on polyethylene oxide and polyacrylamide-induced calcium carbonate flocculation and floc strength. Colloids Surf. A 1999, 159, 31–35. [Google Scholar] [CrossRef]
- Lima, D.U.; Oliveira, R.C.; Buckeridge, M.C. Seed storage hemicelluloses as wet-end additives in papermaking. Carbohydr. Polym. 2003, 52, 367–373. [Google Scholar] [CrossRef]
- Fatemeh, N.; Hamidreza, R.; Hossein, R.; Hossein, J.T. Application of bio-based modified kaolin clay engineered as papermaking additive for improving the properties of filled recycled papers. Appl. Clay Sci. 2019, 182, 105258. [Google Scholar]
- Boufi, S.; González, I.; Delgado-Aguilar, M.; Tarrès, Q.; Pèlach, M.À.; Mutjé, P. Nanofibrillated cellulose as an additive in papermaking process: A review. Carbohydr. Polym. 2016, 154, 151–166. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.M.; Mobarak, F.; El-Din, E.I.; Ebaid, A.E.; Youssef, M.A. Modified Egyptian talc as internal sizing agent for papermaking. Carbohydr. Polym. 2009, 75, 130–134. [Google Scholar] [CrossRef]
- Lourenco, A.F.; Gamelas, J.A.F.; Sarmento, P.; Ferreira, P.J.T. Enzymatic nanocellulose in papermaking—The key role as filler flocculant and strengthening agent. Carbohydr. Polym. 2019, 224, 115200. [Google Scholar] [CrossRef] [PubMed]
- Koshani, R.; Tavakolian, M.; van de Ven, T.G. Cellulose-based dispersants and flocculants. J. Mater. Chem. B 2020, 8, 10502–10526. [Google Scholar] [CrossRef]
- Huang, C.; Li, H.; Liu, W.; Zhan, H. The retention- and drainage-aid behavior of quaternary chitosan in papermaking system. Colloids Surf. A 2007, 297, 147–153. [Google Scholar]
- Malik, S.; Rana, V.; Joshi, G.; Gupta, P.K.; Sharma, A. Valorization of Wheat Straw for the Paper Industry: Pre-extraction of Reducing Sugars and Its Effect on Pulping and Papermaking Properties. ACS Omega 2020, 5, 30704–30715. [Google Scholar] [CrossRef]
- Lossmann, K.; Hecht, R.; Saame, J.; Heering, A.; Leito, I.; Kipper, K. Retention mechanisms of acidic and basic analytes on the Pentafluorophenyl stationary phase using fluorinated eluent additives. J. Chromatogr. A 2022, 1666, 462850. [Google Scholar] [CrossRef] [PubMed]
- You, Y.; Zhang, J.; Sun, X. Fabrication of a novel high-performance leather waste-based composite retention aid. RSC Adv. 2019, 9, 16271–16277. [Google Scholar] [CrossRef] [PubMed]
- Karim, Z.; Svedberg, A. Controlled retention and drainage of microfibrillated cellulose in continuous paper production. New J. Chem. 2020, 44, 13796–13806. [Google Scholar] [CrossRef]
- Dichiara, A.B.; Song, A.; Goodman, S.M.; He, D.; Bai, J. Smart papers comprising carbon nanotubes and cellulose microfibers for multifunctional sensing applications. J. Mater. Chem. A 2017, 5, 20161–20169. [Google Scholar] [CrossRef]
- Xu, Q.; Kong, Q.; Liu, Z.; Zhang, J.; Wang, X.; Liu, R.; Yue, L.; Cui, G. Polydopamine-coated cellulose microfibrillated membrane as high performance lithium-ion battery separator. RSC Adv. 2014, 4, 7845–7850. [Google Scholar] [CrossRef]
- Baharvand, H.; Rabiee, A. Synthesis of polyelectrolyte brushes on spherical magnetic polymer particles. J. Polym. Res. 2014, 21, 596. [Google Scholar] [CrossRef]
- Huang, Y.; Xue, X.; Fu, K. Application of spherical polyelectrolyte brushes microparticle system in flocculation and retention. Polymers 2020, 12, 746. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Hao, Q.H.; Xia, S.Y.; Yan, D.X.; Tan, H.G. Morphologies of spherical bidisperse polyelectrolyte brushes in the presence of trivalent counterions. Chem. Phys. 2020, 539, 110941. [Google Scholar] [CrossRef]
- Wang, W.; Li, L.; Henzler, K.; Lu, Y.; Wang, J.; Han, H.; Tian, Y.; Wang, Y.; Zhou, Z.; Lotze, G.; et al. Protein Immobilization onto Cationic Spherical Polyelectrolyte Brushes Studied by Small Angle X-ray Scattering. Biomacromolecules 2017, 18, 1574–1581. [Google Scholar] [CrossRef]
- Lqbal, D.; Yan, J.; Matyjaszewski, K.; Tilton, R.D. Swelling of muti-responsive spherical polyelectrolyte brush across a wide range of grafting densities. Colloid Polym. Sci. 2019, 298, 35–49. [Google Scholar]
- Azmana, M.; Mahmood, S.; Hilles, A.R.; Rahman, A.; Arifin, M.A.B.; Ahmed, S. A review on chitosan and chitosan-based bionanocomposites: Promising material for combatting global issues and its applications. Int. J. Biol. Macromol. 2021, 185, 832–848. [Google Scholar] [CrossRef]
- Lenze, C.J.; Peksa, C.A.; Sun, W.; Hoeger, I.C.; Salas, C.; Hubbe, M.A. Intact and broken cellulose nanocrystals as model nanoparticles to promote dewatering and fine-particle retention during papermaking. Cellulose 2016, 23, 3951–3962. [Google Scholar] [CrossRef]
- Moud, A.A. Polymer based flocculants: Review of water purification applications. J. Water Process Eng. 2022, 48, 102938. [Google Scholar] [CrossRef]
- Ballauff, M. Spherical polyelectrolyte brushes. Prog. Polym. Sci. 2007, 32, 1135–1151. [Google Scholar] [CrossRef]
- Nie, G.; Li, G.; Wang, L.; Zhang, X. Nanocomposites of polymer brush and inorganic nanoparticles: Preparation, characterization and application. Polym. Chem. 2016, 7, 753–769. [Google Scholar] [CrossRef]
- Yuan, H.; Liu, G. Ionic effects on synthetic polymers: From solutions to brushes and gels. Soft Matter 2020, 16, 4087–4104. [Google Scholar] [CrossRef] [PubMed]
- Giussi, J.M.; Cortez, L.; Marmisollé and Azzaroni, O. Practical use of polymer brushes in sustainable energy applications: Interfacial nanoarchitectonics for high-efficiency devices. Chem. Soc. Rev. 2019, 48, 814–849. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Ballauff, M. Spherical polyelectrolyte brushes as nanoreactors for the generation of metallic and oxidic nanoparticles: Synthesis and application in catalysis. Prog. Polym. Sci. 2016, 59, 86–90. [Google Scholar] [CrossRef]
- Bajza, E.; Hitrec, P.; Muic, M. Influence of different concentrations of Al2 (SO4)3 and anionic polyelectrolytes on tannery wastewater flocculation. Desalination 2005, 171, 13–20. [Google Scholar] [CrossRef]
- Dong, Y.; Shen, Y.; Ge, D.; Bian, C.; Yuan, H.; Zhu, N. A sodium dichloroisocyanurate-based conditioning process for the improvement of sludge dewaterability and mechanism studies. J. Environ. Manag. 2021, 284, 112020. [Google Scholar] [CrossRef]
- Zhao, C.; Shao, S.; Zhou, Y.; Yang, Y.; Shao, Y.; Zhang, L.; Zhou, Y.; Xie, L.; Luo, L. Optimization of flocculation conditions for soluble cadmium removal using the composite flocculant of green anion polyacrylamide and PAC by response surface methodology. Sci. Total Environ. 2018, 645, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Yang, Z.H.; Zeng, G.M.; Ruan, M.; Xu, H.Y.; Gao, W.C.; Luo, Y.L.; Xie, H.M. Influence of composite flocculant of PAC and MBFGA1 on residual aluminum species distribution. Chem. Eng. J. 2012, 191, 269–277. [Google Scholar] [CrossRef]
- Ren, J.; Wei, H.; Li, A.; Yang, H. Efficient removal of phosphorus from turbid water using chemical sedimentation by FeCl3 in conjunction with a starch-based flocculant. Water Res. 2020, 170, 115361. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yang, C.; Hu, J.; Huang, M.; Zhao, L.; He, J.; Zhang, S.; Shen, F.; Tian, D. Cascade utilization of crop straw through a FeCl3-mediated deep eutectic solvent biorefinery: Lignin-containing cellulose nanofibers flocculant fabrication followed by fertilizer production. Chem. Eng. J. 2023, 472, 144823. [Google Scholar] [CrossRef]
- Verma, A.K.; Dash, R.R.; Bhunia, P. A review on chemical coagulation/flocculation technologies for removal of color from textile wastewaters. J. Environ. Manag. 2012, 93, 154–168. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Y.; Wei, X.; Huang, L.; Zhang, J.; Wu, Y.; Zhang, Y.; Xiang, Y. A comprehensive study on the performance and mechanism of microplastics removal by enhanced coagulation methods. J. Water Process Eng. 2023, 56, 104238. [Google Scholar] [CrossRef]
- Rong, H.; Gao, B.; Dong, M.; Zhao, Y.; Sun, S.; Yue, Q.; Li, Q. Characterization of size, strength and structure of aluminum-polymer dual-coagulant flocs under different pH and hydraulic conditions. J. Hazard. Mater. 2013, 252–253, 330–337. [Google Scholar] [CrossRef]
- Kato, M.; Isogai, A.; Onabe, F. Adsorption behavior of aluminum compounds on pulp fibers at wet-end. J. Food Sci. 1998, 44, 361–368. [Google Scholar] [CrossRef]
- Dai, Q.; Ren, N.; Ning, P.; Ma, L.; Guo, Z.; Xie, L.; Yang, J.; Cai, Y. Inorganic flocculant for sludge treatment: Characterization, sludge properties, interaction mechanisms and heavy metals variations. J. Environ. Manag. 2020, 275, 111255. [Google Scholar] [CrossRef]
- Wei, X.; Tao, J.; Li, M.; Zhu, B.; Li, X.; Ma, Z.; Zhao, T.; Wang, B.; Suo, B.; Wang, H.; et al. Polyacrylamide-based inorganic hybrid flocculants with self-degradable property. Mater. Chem. Phys. 2017, 192, 72–77. [Google Scholar] [CrossRef]
- Huang, X.; Shen, J.; Qian, X. Filler modification for papermaking with starch/oleic acid complexes with the aid of calcium ions. Carbohydr. Polym. 2013, 98, 931–935. [Google Scholar] [CrossRef]
- Sharma, M.; Aguado, R.; Murtinho, D.; Valente, A.J.M.; Mendes, A.P.; Ferreira, P.J.T. A review on cationic starch and nanocellulose as paper coating components. Int. J. Biol. Macromol. 2020, 162, 578–598. [Google Scholar] [CrossRef]
- Kweon, M.; Sosulski, F.; Bhirud, P. Cationization of waxy and normal corn and barley starches by an aqueous alcohol process. Starch-Starke 1997, 49, 59–66. [Google Scholar] [CrossRef]
- Huang, X.; Qian, X.; Li, J.; Lou, S.; Shen, J. Starch/rosin complexes for improving the interaction of mineral filler particles with cellulosic fibers. Carbohydr. Polym. 2015, 117, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Bratskaya, S.; Schwarz, S.; Petzold, G.; Liebert, T.; Heinze, T. Cationic starches of high degree of functionalization: 12. Modification of cellulose fibers toward high filler technology in papermaking. Ind. Eng. Chem. Res. 2006, 45, 7374–7379. [Google Scholar] [CrossRef]
- Abdallah, A.F.; Jawaid, M.; Mohamed, A.Z.; Paridah, M.T.; Abdullah, U.H. Performance of nanofibrillated cellulose with chitosan as a wet-end additive for paper applications. Ind. Crop. Prod. 2023, 203, 117219. [Google Scholar] [CrossRef]
- Gal, M.R.; Rahmaninia, M.; Hubbe, M.A. A comprehensive review of chitosan applications in paper science and technologies. Carbohydr. Polym. 2023, 309, 120665. [Google Scholar] [CrossRef]
- Harikrishnan, M.P.; Thampi, A.; Nandhu Lal, A.M.; Warrier, A.S.; Basil, M.; Kothakota, A. Effect of chitosan-based bio coating on mechanical, structural and physical characteristics of microfiber based paper packaging: An alternative to wood pulp/plastic packaging. Int. J. Biol. Macromol. 2023, 253, 126888. [Google Scholar] [CrossRef] [PubMed]
- Miranda, R.; Nicu, R.; Latour, I.; Lupei, M.; Bobu, E.; Blanco, A. Efficiency of chitosans for the treatment of papermaking process water by dissolved air flotation. Chem. Eng. J. 2013, 231, 304–313. [Google Scholar] [CrossRef]
- Li, H.; Du, Y.; Wu, X.; Zhan, H. Effect of molecular weight and degree of substitution of quaternary chitosan on its adsorption and flocculation properties for potential retention-aids in alkaline papermaking. Colloids Surf. A 2004, 242, 1–8. [Google Scholar] [CrossRef]
- Musa, M.; Wolf, J.; Stephens, E.; Hankamer, B.; Brown, R.; Rainey, T.J. Cationic polyacrylamide induced flocculation and turbulent dewatering of microalgae on a Britt Dynamic Drainage Jar. Sep. Purif. Technol. 2020, 233, 116004. [Google Scholar] [CrossRef]
- Chakraborty, S.; Dutta, S.; Chatterjee, R.; Chanda, J.; Pal, S.; Bandyopadhyay, A. Flocculation of low concentration kaolin suspension using architecturally modified Xanthan gum: Effect of grafting to hyperbranching. J. Taiwan Inst. Chem. E 2023, 150, 105066. [Google Scholar] [CrossRef]
- Kutsevol, N.; Kuziv, Y.; Cabrera, T.; Husson, S.M.; DeVol, T.A.; Bliznyuk, V. Biodegradable star-like polymer flocculants for rapid, efficient purification of water contaminated with industrial radionuclides. Sep. Purif. Technol. 2021, 273, 118630. [Google Scholar] [CrossRef]
- Pöhler, T.; Mautner, A.; Aguilar-Sanchez, A.; Hansmann, B.; Kunnari, V.; Grönroos, A.; Rissanen, V.; Siqueira, G.; Mathew, A.P.; Tammelin, T. Pilot-scale modification of polyethersulfone membrane with a size and charge selective nanocellulose laye. Sep. Purif. Technol. 2022, 285, 120341. [Google Scholar] [CrossRef]
- Wu, S.; Li, G.; Liu, W.; Yu, D.; Li, G.; Liu, X.; Song, Z.; Wang, H.; Liu, H. Immobilization of pectinase on polyethyleneimine-coated pulp fiber for treatment of whitewater from papermaking. Fabrication of polyethyleneimine-paper composites with improved tribopositivity for triboelectric nanogenerators. Nano Energy 2022, 93, 106859. [Google Scholar] [CrossRef]
- Jiang, S.; Xi, J.; Dai, H.; Wu, W.; Xiao, H. Multifunctional cellulose paper-based materials and their application in complex wastewater treatment. Int. J. Biol. Macromol. 2022, 207, 414–423. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Zhao, Y.; Yin, C.; Tan, S.; Wang, X.; Yang, C.; Zhang, T.; Zhang, X.; Ye, F.; Xu, J.; et al. Polyvinylamine with moderate binding affinity as a highly effective vehicle for RNA delivery. J. Control. Release 2022, 345, 20–37. [Google Scholar] [CrossRef]
- Carrasco, F.; Mutjé, P.; Pelach, M. Control of retention in paper-making by colloid titration and zeta potential techniques. Wood Sci. Technol. 1998, 32, 145–155. [Google Scholar] [CrossRef]
- Kumar, A.; Bhardwaj, N.K.; Singh, S.P. Polyacrylamide stabilized alkenyl succinic anhydride emulsion as sizing agent for various cellulosic pulps and fillers. Carbohydr. Polym. 2020, 236, 116069. [Google Scholar] [CrossRef]
- Mcliesh, H.; Sharman, S.; Garnier, G. Effect of cationic polyelectrolytes on the performance of paper diagnostics for blood typing. Colloids Surf. B 2015, 133, 189–197. [Google Scholar] [CrossRef]
- Zhang, B.; Yang, X.; Fan, C.; Hao, W.; Zhao, Y.; Zeng, Y. Cationic polyacrylamide alleviated the inhibitory impact of ZnO nanoparticles on anaerobic digestion of waste activated sludge through reducing reactive oxygen species induced. Water Res. 2021, 205, 117651. [Google Scholar] [CrossRef]
- Chen, B.; Zheng, C.; Bi, J.; Qu, X.; Zhang, R.; Liu, X.; Yang, Y. Improved emulsified oil removal approach for industrial coal pyrolysis wastewater by flocculation under pressurized CO2 atmosphere. J. Clean. Prod. 2023, 418, 138213. [Google Scholar] [CrossRef]
- Raghuwanshi, V.S.; Garusinghe, U.M.; Raj, P.; Kirby, N.; Hoell, A.; Batchelor, W.; Garnier, G. Cationic polyacrylamide induced nanoparticles assembly in a cellulose nanofiber network. J. Colloid Interf. Sci. 2018, 529, 180–186. [Google Scholar] [CrossRef]
- Heo, J.W.; An, L.; Kim, M.S.; Youn, D.H. Preparation and characterization of zwitterion-substituted lignin/Nafion composite membranes. Int. J. Biol. Macromol. 2023, 253, 127421. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, S.M.R.; Nasser, M.S.; Hussein, I.A.; Benamor, A. Investigation of the effect of polyelectrolyte structure and type on the electrokinetics and flocculation behavior of bentonite dispersions. Chem. Eng. J. 2017, 311, 265–276. [Google Scholar] [CrossRef]
- Wang, Q.; Oshita, K.; Takaoka, M. Flocculation properties of eight microalgae induced by aluminum chloride, chitosan, amphoteric polyacrylamide, and alkaline: Life-cycle assessment for screening species and harvesting methods. Algal Res. 2021, 54, 102226. [Google Scholar] [CrossRef]
- Liu, M.; Qin, M.; Fang, G.; Liang, S.; Wang, X.; Luo, Z. Realization restrain vanadium dissolution in aqueous zinc-ion batteries with amphoteric ionic polyacrylamide gel electrolyte. J. Alloys Compd. 2023, 959, 170455. [Google Scholar] [CrossRef]
- Lu, S.; Liu, R.; Sun, X. A study on the synthesis and application of an inverse emulsion of amphoteric polyacrylamide as a retention aid in papermaking. J. Appl. Polym. Sci. 2002, 84, 343–350. [Google Scholar] [CrossRef]
- Lai, N.; Li, S.; Liu, L.; Li, Y.; Li, J.; Zhao, M. Synthesis and rheological property of various modified nano-SiO2/AM/AA hyperbranched polymers for oil displacement. Russ. J. Appl. Chem. 2017, 90, 480–491. [Google Scholar] [CrossRef]
- Pu, W.; Du, R.; Liu, R.; Gu, J.; Li, K.; Zhang, Y.; Liu, P. Synthesis and characterization of hyperbranched associative polyacrylamide. RSC Adv. 2016, 6, 39522–39529. [Google Scholar] [CrossRef]
- Li, S.N.; Li, B.; Gong, L.X.; Yu, Z.R.; Feng, Y.; Jia, D.; Zhou, Y.; Tang, L.C. Enhanced mechanical properties of polyacrylamide/chitosan hydrogels by tuning the molecular structure of hyperbranched polysiloxane. Mater. Design 2019, 162, 162–170. [Google Scholar] [CrossRef]
- Zhao, T.; Zhang, Y.; Peng, G.; Chen, Y. A branched hydrophobicity associated with polyacrylamide based on silica: Synthesis and solution properties. J. Polym. Res. 2019, 26, 250. [Google Scholar] [CrossRef]
- Mahdavi, H.; Amirsadeghi, M. Synthesis and applications of quaternized highly branched polyacrylamide as a novel multi-site polymeric phase transfer catalyst. J. Iran. Chem. Soc. 2013, 10, 791–797. [Google Scholar] [CrossRef]
- Shin, J.H.; Sin, H.N.; Sohn, C.; Ow, S.K.; Mah, S. Highly branched cationic polyelectrolytes: Fines retention. Tappi J. 1997, 80, 185–189. [Google Scholar]
- Liu, Z.; Liu, T.; Zhang, J.; Li, Y.; Luo, J.; Li, J.; Shi, S.Q.; Gao, Q.; Mao, A. Preparation of a strong, high prepressing intensity, and multifunction soybean protein adhesive by using hyperbranched functional polymer. Polym. Test. 2023, 119, 107931. [Google Scholar] [CrossRef]
- Lai, N.; Wu, T.; Ye, Z.; Zhou, N.; Xu, Q.; Zeng, F. Preparation and properties of hyperbranched polymer containing functionalized Nano-SiO2 for low-moderate permeability reservoirs. Russ. J. Appl. Chem. 2016, 89, 1681–1693. [Google Scholar] [CrossRef]
- Blanco, Á.; Fuente, E.; Monte, M.C.; Cortés, N.; Negro, C. Polymeric branched flocculant effect on the flocculation process of pulp suspensions in the papermaking industry. Ind. Eng. Chem. Res. 2009, 48, 4826–4836. [Google Scholar] [CrossRef]
- England, R.M.; Moss, J.I.; Gunnarsson, A.; Parker, J.S.; Ashford, M.B. Synthesis and characterization of dendrimer-based polysarcosine star polymers: Well-defined, versatile platforms designed for drug-delivery applications. Biomacromolecules 2020, 21, 3332–3341. [Google Scholar] [CrossRef]
- Fuller, K.M.; Clay, D.; Almahdali, S.R.; Paterson, A.; Barratt, C.M.; Desyatkin, V.D.; Rodionov, V.O. Arm-first synthesis of hyperbranched-core star polymers via copper-catalyzed azide-alkyne cycloaddition. Eur. Polym. J. 2023, 190, 111987. [Google Scholar] [CrossRef]
- Yao, X.; Zhang, S.; Qian, L.; Du, M. Dendrimer-assisted boronate affinity cellulose foams for the efficient and selective separation of glycoproteins. Carbohydr. Polym. 2021, 265, 118082. [Google Scholar] [CrossRef]
- Yan, L.; Dang, X.; Yang, M.; Zhang, M.; Rui, L.; Han, W.; Li, Y. One-pot synthesis of PAMAM-grafted hyperbranched cellulose towards enhanced thermal stability and antibacterial activity. Prog. Biophys. 2022, 121, 78–86. [Google Scholar] [CrossRef]
- Wever, D.A.Z.; Picchioni, F.; Broekhuis, A.A. Branched polyacrylamides: Synthesis and effect of molecular architecture on solution rheology. Eur. Polym. J. 2013, 49, 3289–3301. [Google Scholar] [CrossRef]
- Shan, Y.Y.; Fu, Y.J.; Qin, M.H. Synthesis of star cationic polyacrylamide and its application in the retention and drainage system of papermaking. Adv. Mater. Res. Trans. Tech. Publ. 2012, 476, 2256–2259. [Google Scholar] [CrossRef]
- Ovenden, C.; Xiao, H. Flocculation behavior and mechanisms of cationic inorganic microparticle/polymer systems. Colloids Surf. A 2002, 197, 225–234. [Google Scholar] [CrossRef]
- Yoon, D.H.; Jang, J.W.; Cheong, I.W. Synthesis of cationic polyacrylamide/silica nanocomposites from inverse emulsion polymerization and their flocculation property for papermaking. Colloids Surf. A. 2012, 411, 18–23. [Google Scholar] [CrossRef]
- Dai, K.; Chen, P.; Wang, Z.; Yang, P.; Li, M.; Tang, C.; Zhang, W.; Zhu, C.; Ying, H.; Wu, J. Magnetic composite Ca(OH)2/Fe3O4 for highly efficient flocculation in papermaking black liquor without pH neutralization. Adv. Powder Technol. 2021, 32, 2457–2468. [Google Scholar] [CrossRef]
- Yan, Z.; Deng, Y. Cationic microparticle based flocculation and retention system. Chem. Eng. J. 2000, 80, 31–36. [Google Scholar] [CrossRef]
- Peng, X.; Shen, J.; Xiao, H. Preparation and retention of poly (ethylene oxide)-grafted cationic polyacrylamide microparticles. J. Appl. Polym. Sci. 2006, 101, 359–363. [Google Scholar] [CrossRef]
- Feng, X.; Zhang, Y.; Wang, G.; Miao, M.; Shi, L. Dual-surface modification of calcium sulfate whisker with sodium hexametaphosphate/silica and use as new water-resistant reinforcing fillers in papermaking. Powder Technol. 2015, 271, 1–6. [Google Scholar] [CrossRef]
- Mohamed, D.; Denis, C.; Nabila, E.; Mohammed, L.H.; Ibrahim, F.Z.; Evelyne, M. Biobased polymers and cationic microfibrillated cellulose as retention and drainage aids in papermaking: Comparison between softwood and bagasse pulps. Ind. Crop. Prod. 2015, 72, 34–45. [Google Scholar]
- Ornanong, S.K.; Nisit, K. Preparation and physicomechanical properties of co-precipitated rice starch-colloidal silicon dioxide. Powder Technol. 2012, 271, 377–382. [Google Scholar]
- You, Y.; Sun, X.; Yang, W.; Dai, L.; He, L.; Wang, H.; Zhang, J.; Xiang, W. A high-performance and low-cost strategy to harvest saltwater Chlorella vulgaris using cationic polyacrylamide coupled with bentonite. Algal Res. 2019, 41, 101579. [Google Scholar] [CrossRef]
- Alince, B.; Bednar, F.; van de Ven, T.G.M. Deposition of calcium carbomate particles on fiber surfaces induced by cationic polyelectrolyte and bentonite. Colloids Surf. A 2001, 190, 71–80. [Google Scholar] [CrossRef]
- Cezar, N.; Xiao, H. Novel retention system based on (2, 3-epoxypropyl) trimethylammonium chloride modified silica nanoparticles and anionic polymer. Ind. Eng. Chem. Res. 2005, 44, 539–545. [Google Scholar] [CrossRef]
- Guo, K.; Gao, B.; Tian, X.; Yue, Q.; Zhang, P.; Shen, X.; Xu, X. Synthesis of polyaluminium chloride/papermaking sludge-based organic polymer composites for removal of disperse yellow and reactive blue by flocculation. Chemosphere 2019, 231, 337–348. [Google Scholar] [CrossRef]
- Escamilla-Lara, K.A.; Lopez-Tellez, J.; Rodriguez, J.A. Adsorbents obtained from recycled polymeric materials for retention of different pollutants: A review. Chemosphere 2023, 335, 139159. [Google Scholar] [CrossRef]
- Gibson, T.F.; Watanabe, W.O.; Losordo, T.M.; Whitehead, R.F.; Carroll, P.M. Evaluation of chemical polymers as coagulation aids to remove suspended solids from marine finfish recirculating aquaculture system discharge using a geotextile bag. Aquacult. Eng. 2020, 90, 102065. [Google Scholar] [CrossRef]
- Gardi, I.; Mishael, Y.G.; Lindahl, M.; Muro-Pastor, A.M.; Undabeytia, T. Coagulation-flocculation of Microcystis aeruginosa by polymer-clay based composites. J. Clean. Prod. 2023, 394, 136356. [Google Scholar] [CrossRef]
- Xiao, H.; Liu, Z.; Wiseman, N. Synergetic effect of cationic polymer microparticles and anionic polymer on fine clay flocculation. J. Colloid Inter. Sci. 1999, 216, 409–417. [Google Scholar] [CrossRef]
- Ono, H.; Deng, Y. Flocculation and retention of precipitated calcium carbonate by cationic polymeric microparticle flocculants. J. Colloid Inter. Sci. 1997, 188, 183–192. [Google Scholar] [CrossRef][Green Version]
- Hämäläinen, J.; Lindström, S.B.; Hämäläinen, T.; Niskanen, H. Papermaking fibre-suspension flow simulations at multiple scales. J. Eng. Math. 2011, 71, 55–79. [Google Scholar] [CrossRef]
- Nissan, A.H.; Sternstein, S.S. Cellulose-fiber bonding. Tappi J. 1964, 47, 1–5. [Google Scholar]
- Samanta, M.; Chaudhury, S. Coarse-grained molecular dynamics simulations study of the conformational properties of single polyelectrolyte diblock copolymer. Biphys. Chem. 2020, 266, 106437. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Wu, M.; Guo, Y.; Zhang, H. Comprehensive physical visualisation of the chain conformation and solution property of carboxymethylated konjac glucomannan: Comparison of charged and uncharged polyelectrolytes. Food Hydrocoll. 2021, 118, 106725. [Google Scholar] [CrossRef]
- Quezada, G.R.; Jeldres, R.I.; Fawell, P.D.; Toledo, P.G. Use of molecular dynamics to study the conformation of an anionic polyelectrolyte in saline medium and its adsorption on a quartz surface. Miner. Eng. 2018, 129, 102–105. [Google Scholar] [CrossRef]
- Lee, H. Effect of polyelectrolyte size on multilayer conformation and dynamics at different temperatures and salt concentrations. J. Mol. Graph. Model. 2016, 70, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Böhmer, M.R.; Evers, O.A.; Scheutjens, J.M.H.M. Weak Polyelectrolytes two surfaces: Adsorption and stabilization. Macromolecules 1990, 23, 2288–2301. [Google Scholar] [CrossRef]
- Falk, M.; Ödberg, L.; Wågberg, L.; Risinger, G. Adsorption kinetics for cationic polyelectrolytes onto pulp fibers in turbulent flow. Colloids Surf. 1989, 40, 115–124. [Google Scholar] [CrossRef]
- Nasser, M.S.; Twaiq, F.A.; Onaizi, S.A. Effect of polyelectrolytes on the degree of flocculation of papermaking suspensions. Sep. Purif. Technol. 2013, 103, 43–52. [Google Scholar] [CrossRef]
- Lai, M.; Lin, H.; Luo, Y.; Li, H.; Wang, X.; Sun, R. Interaction between chitosan-based clay nanocomposites and cellulose in a chemical pulp suspension. Carbohydr. Polym. 2016, 137, 375–381. [Google Scholar] [CrossRef]
- Cui, J.; Niu, X.; Zhang, D.; Ma, J.; Zhu, X.; Zheng, X.; Lin, Z.; Fu, M. The novel chitosan-amphoteric starch dual flocculants for enhanced removal of Microcystis aeruginosa and algal organic matter. Carbohydr. Polym. 2023, 304, 120474. [Google Scholar] [CrossRef]
- Wang, D.; Wang, D.; Deng, C.; Wang, K.; Tan, X. Flocculation of quartz by a dual polymer system containing tannic acid and poly (ethylene oxide): Effect of polymer chemistry and hydrodynamic conditions. Chem. Eng. J. 2022, 446, 137403. [Google Scholar] [CrossRef]
- An, Z.; Hou, X.; Zhou, P.; Zhang, R.; Fang, D. A novel flexible, layered, recoverable SiO2 fiber skeleton and aerogel composites material prepared by papermaking process. Ceram. Int. 2021, 47, 12963–12969. [Google Scholar] [CrossRef]
- Bhayo, A.M.; Yang, Y.; He, X. Polymer brushes: Synthesis, characterization, properties and applications. Prog. Mater. Sci. 2022, 130, 101000. [Google Scholar] [CrossRef]
- Yang, R.; Wang, X.; Yan, S.; Dong, A.; Luan, S.; Yin, J. Advances in design and biomedical application of hierarchical polymer. Prog. Polym. Sci. 2021, 118, 101409. [Google Scholar] [CrossRef]
- Zhao, K.; Gao, Z.; Song, D.; Zhang, P.; Cui, J. Assembly of catechol-modified polymer brushes for drug delivery. Polym. Chem. 2022, 13, 373–378. [Google Scholar] [CrossRef]
- Chang, L.; Yan, H.; Chang, J.; Gautrot, J.E. Cationic polymer brush-coated bioglass nanoparticles for the design of bioresorbable RNA delivery vectors. Euro. Polym. J. 2021, 156, 110593. [Google Scholar] [CrossRef]
- Cheng, Y.; Xia, Q.; Liu, H.; Solomon, M.B.; Ling, C.D.; Müllner, M. Polymer brush-grafted cellulose nanocrystals for the synthesis of porous carbon-coated titania nanocomposites. Polym. Chem. 2023, 14, 2181–2189. [Google Scholar] [CrossRef]
- Jia, H.; Cao, J.; Lu, Y. Design and fabrication of functional hybrid materials for catalytic applications. Curr. Opin. Green Sust. 2017, 4, 16–22. [Google Scholar] [CrossRef]
- Yang, Q.; Li, L.; Zhao, F.; Wang, Y.; Ye, Z.; Guo, X. Generation of MnO2 nanozyme in spherical polyelectrolyte brush for colorimetric detection of glutathione. Mater. Lett. 2019, 248, 89–92. [Google Scholar] [CrossRef]
- Wang, X.; Chen, X.; Cowling, S.; Wang, L.; Liu, X. Polymer brushes tethered ZnO crystal on cotton fiber and the application on durable and washable UV protective clothing. Adv. Mater. Interfaces 2019, 6, 1900564. [Google Scholar] [CrossRef]
- Hu, Q.; Wang, W.; Ma, T.; Zhang, C.; Kuang, J.; Wang, R. Anti-UV and hydrophobic dual-functional coating fabrication for flame retardant polyester fabrics by surface-initiated PET RAFT technique. Eur. Polym. J. 2022, 173, 111275. [Google Scholar] [CrossRef]
- Cozens, E.J.; Kong, D.; Roohpour, N.; Gautrot, J.E. The physic-chemistry of adhesions of protein resistant and weak polyelectrolyte brushes to cells and tissues. Soft Matter 2020, 16, 505–522. [Google Scholar] [CrossRef] [PubMed]
- Su, N.; Li, H.; Zheng, H.; Yi, S.; Liu, X. Synthesis and characterization of poly(sodium-p-styrenesulfonate)/modified SiO2 spherical brushes. Express. Polym. Lett. 2012, 8, 680–686. [Google Scholar] [CrossRef]
- Hirai, T.; Kobayashi, M.; Takahara, A. Control of the primary and secondary structure of polymer brushes by surface-initiated living/controlled polymerization. Polym. Chem. 2017, 8, 5456–5468. [Google Scholar] [CrossRef]
- Khan, M.; Wu, Z.; Mao, S.; Shah, S.N.; Lin, J.M. Controlled grafted poly (quaternized-4-vinylpyridine-co-acrylic acid) brushes attract bacteria for effective antimicrobial surfaces. J. Mater. Chem. B 2018, 6, 3782–3791. [Google Scholar] [CrossRef] [PubMed]
- Su, N. Polyaniline-doped spherical polyelectrolyte brush nanocomposites with enhanced electrical conductivity, thermal stability, and solubility property. Polymers 2015, 7, 1599–1616. [Google Scholar] [CrossRef]
- Irigoyen, J.; Arekalyan, V.B.; Navoyan, Z.; Iturri, J.; Moya, S.E.; Donath, E. Spherical polyelectrolyte brushes’ constant zeta potential with varying ionic strength: An electrophoretic study using a hairy layer approach. Soft Matter 2013, 9, 11609–11617. [Google Scholar] [CrossRef]
- Cao, Q.; Bachmann, M. Polyelectrolyte adsorption on an oppositely charged spherical polyelectrolyte brush. Soft Matter 2013, 9, 5087–5098. [Google Scholar] [CrossRef]
- Hao, Q.; Xia, G.; Tan, H.; Chen, E.; Yang, S. Surface morphologies of spherical polyelectrolyte brushes induced by trivalent salt ions. Phys. Chem. Chem. Phys. 2018, 20, 26542–26551. [Google Scholar] [CrossRef]
- Zhang, R.; Yu, Z.; Hou, X.; Shen, Z.; Deng, J.; Zhou, Z.; Guo, X.; Wang, J.; Zhu, X. Highly selective separation of dyes using compressed CO2 and spherical polyelectrolyte brushes. RSC Adv. 2016, 6, 42693–42700. [Google Scholar] [CrossRef]
- Masoomi, H.; Wang, Y.; Fang, X.; Wang, P.; Chen, C.; Liu, K.; Gu, H.; Xu, H. Ultrabright dye-loaded spherical polyelectrolyte brushes and their fundamental structure-fluorescence tuning principles. Nanoscale 2019, 11, 14050–14059. [Google Scholar] [CrossRef] [PubMed]
- Bakhshandeh, A.; Segala, M.; Colla, T.E. Equilibrium conformations and surface charge regulation of spherical polymer brushes in stretched regimes. Macromolecules 2022, 55, 35–48. [Google Scholar] [CrossRef]
- Parsonage, E.; Tirrell, M.; Watanabe, H. Adsorption of poly (2-vinylpyridine)-poly (styrene) block copolymers from toluene solutions. Macromolecules 1991, 24, 1987–1995. [Google Scholar] [CrossRef]
- Motschmann, H.; Stamm, M.; Toprakcioglu, C. Adsorption kinetics of block copolymers from a good solvent: A two-stage process. Macromolecules 1991, 24, 3681–3688. [Google Scholar] [CrossRef]
- Li, C.; Mao, J.; Li, S.; Wang, Y.; Liu, H. A long chain-induced depletion effect for abnormal grafting in the preparation of bimodal bidisperse polymer-grafted nanoparticles. Phys. Chem. Chem. Phys. 2023, 25, 5627–5637. [Google Scholar] [CrossRef]
- Tan, H.; Xia, G.; Liu, L.; Miao, B. Morphologies of a polyelectrolyte brush grafted onto a cubic colloid in the presence of trivalent ions. Phys. Chem. Chem. Phys. 2019, 21, 20031–20044. [Google Scholar] [CrossRef]
- Tran, Y.; Auory, P. Synthesis of poly (styrene sulfonate) brushes. J. Am. Chem. Soc. 2001, 123, 3644–3654. [Google Scholar] [CrossRef]
- Su, N. Synthesis of poly (2-Acrylamido-2-methylpropanesulfnoinc Salt) modified carbon spheres. Polymers 2023, 15, 3510. [Google Scholar] [CrossRef]
- Wolski, K.; Szuwarzyński, M.; Zapotoczny, S. A facile route electronically conductive polyelectrolyte brushes as platforms of molecular wires. Chem. Sci. 2015, 6, 1754–1760. [Google Scholar] [CrossRef]
- Neri-Cruz, C.E.; Teixeira, F.M.E.; Gautrot, J.E. A guide to functionalisation and bioconjugation strategies to surface-initiated polymer brushes. Chem. Commun. 2023, 59, 7534–7558. [Google Scholar] [CrossRef] [PubMed]
- Banerijee, S.; Paira, T.K.; Mandal, T.K. Surface confined atom transfer radical polymerization: Access to custom library of polymer-based hybrid materials for speciality applications. Polym. Chem. 2014, 5, 4153–4167. [Google Scholar] [CrossRef]
- Zhang, H.; Ruhe, J. Weak Polyelectrolyte brushes as substrates for the formation of surface-attached polyelectrolyte-polyelectrolyte complexes and polyelectrolyte multilayers. Macromolecules 2005, 38, 10743–10749. [Google Scholar] [CrossRef]
- Wang, S.; Song, J.; Li, Y.; Zhao, X.; Chen, L.; Li, G.; Wang, L.; Jia, Z.; Ge, X. Grafting antibacterial polymer brushes from titanium surface via polydopamine chemistry and activators regenerated by electron transfer ATRP. React. Funct. Polym. 2019, 140, 48–55. [Google Scholar] [CrossRef]
- Kalelkar, P.P.; Geng, Z.; Cox, B.; Finn, M.G.; Collard, D.M. Surface-initiated atom-transfer radical polymerization (SI-ATRP) of bactericidal polymer brushes on poly (lactic acid) surfaces. Colloids Surf. B 2022, 211, 112242. [Google Scholar] [CrossRef] [PubMed]
- Yin, R.; Wang, Z.; Bockstaller, M.R.; Matyjaszewski, K. Tuning dispersity of linear polymers and polymeric brushes grown from nanoparticles by atom transfer radical polymerization. Polym. Chem. 2021, 12, 6071–6082. [Google Scholar] [CrossRef]
- Zhou, J.; Sun, Y.; Huang, Z.; Luo, Z.; Yu, B.; Zou, X.; Hu, H. Growing antifouling fluorinated polymer brushes on polyvinyl alcohol hydrogel surface via g-C3N4@InVO4 catalyzed surface-initiated photo atom transfer radical polymerization. Colloids Surf. A 2021, 622, 126598. [Google Scholar] [CrossRef]
- Lee, Y.; Boyer, C.; Kwon, M.S. Photocontrolled RAFT polymerization: Past, present, and future. Chem. Soc. Rev. 2023, 52, 3035–3097. [Google Scholar] [CrossRef]
- Hu, L.; Hao, Q.; Wang, L.; Cui, Z.; Fu, P.; Liu, M.; Qiao, X.; Pang, X. The in situ “grafting from” approach for the synthesis of polymer brushes on upconversion nanoparticles via NIR-mediated RAFT polymerization. Polym. Sci. 2021, 12, 545–553. [Google Scholar] [CrossRef]
- Cho, M.K.; Seo, H.; Lee, J.; Cho, W.K.; Son, K. Polymer brush growth by oxygen-initiated RAFT polymerization on various substrates. Polym. Chem. 2021, 12, 7023–7030. [Google Scholar] [CrossRef]
- Xing, Y.; Li, Q.; Chen, X.; Li, M.; Wang, S.; Li, Y.; Wang, T.; Sun, X.; Li, X. Preparation of isoelectric point-switchable polymer brush-grafted mesoporous silica using RAFT polymerization with high performance for Ni(II) adsorption. Powder Technol. 2022, 412, 117980. [Google Scholar] [CrossRef]
- Zhao, B.; Brittain, W.J. Polymer brushes: Surface-immobilized macromolecules. Prog. Polym. Sci. 2000, 25, 677–710. [Google Scholar] [CrossRef]
- Baum, M.; Brittain, W.J. Synthesis of polymer brushes on silicate substrates via reversible addition fragmentation chain transfer technique. Macromolecules 2002, 35, 610. [Google Scholar] [CrossRef]
- Wittemann, A.; Drechsler, M.; Talmon, Y.; Ballauff, M. High elongation of polyelectrolyte chains in the osmotic limit of spherical polyelectrolyte brushes: A study by cryogenic transmition electron microscopy. J. Am. Chem. Soc. 2005, 127, 9688–9689. [Google Scholar] [CrossRef]
- Lu, Y.; Proch, S.; Scrinner, M.; Drechsler, M.; Kempe, R.; Ballauff, M. Thermosensitive core-shell microgel as a “nanoreactor” for catalytic active metal nanoparticles. J. Mater. Chem. 2009, 19, 3955–3961. [Google Scholar] [CrossRef]
- Sharma, G.; Mei, Y.; Ballauff, M.; Irrgang, T.; Proch, S.; Kempe, R. Spherical polyelectrolyte brushes as carriers for platinum nanoparticles in heterogeneous hydrogenation reactions. J. Catal. 2007, 246, 10–14. [Google Scholar] [CrossRef]
- Newcomb, C.J.; Moyer, T.J.; Lee, S.S.; Stupp, S.I. Advances in cryogenic transmission electron microscopy for the characterization of dynamic self-assembling nanostructures. Curr. Opin. Colloid Interface Sci. 2012, 17, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Henzler, K.; Haupt, B.; Rosenfeldt, S.; Harnau, L.; Narayanan, T.; Ballauff, M. Interaction strength between proteins and polyelectrolyte brushes: A small angle X-ray scattering study. Phys. Chem. Chem. Phys. 2011, 13, 17599. [Google Scholar] [CrossRef] [PubMed]
- Penfold, J.; Thomas, R.K. Neutron reflectivity and small angle neutron scattering: An introduction and perspective on recent progress. Curr. Opin. Colloid Interface Sci. 2014, 19, 198–206. [Google Scholar] [CrossRef]
- Reese, C.J.; Boyes, S.G. New methods in polymer brush synthesis: Non-vinyl-based semiflexible and rigid-rod polymer brushes. Prog. Polym. Sci. 2021, 114, 101361. [Google Scholar] [CrossRef]
- Kilbey, S.M.; Ankner, J.F. Neutron reflectivity as a tool to understand polyelectrolyte brushes. Curr. Opin. Colloid Interface Sci. 2012, 17, 83–89. [Google Scholar] [CrossRef]
- Yoshioka, H.; Aoki, Y.; Nonaka, K.; Yamada, N.L.; Kobayashi, M. Effect of molecular weight distribution on the thermal adhesion of polystyrene and PMMA brushes. Polymer 2023, 264, 125561. [Google Scholar] [CrossRef]
- Jalili, K.; Abbasi, F.; Behboodpour, L. In situ probing of switchable nanomechanical properties of responsive high-density polymer brushes on poly (dimethylsiloxane): An AFM nanoindentation approach. J. Mech. Behav. Biomed. 2019, 93, 118–129. [Google Scholar] [CrossRef] [PubMed]
- Eskhan, A.; Johnson, D. Microscale characterization of abiotic surfaces and prediction of their biofouling/anti-biofouling potential using the AFM colloidal probe technique. Adv. Colloid Interface Sci. 2022, 310, 102796. [Google Scholar] [CrossRef] [PubMed]
- Riley, J.K.; Matyjaszewski, K.; Tilton, R.D. Friction and adhesion control between adsorbed layers of polyelectrolyte brush-grafted nanoparticles via pH-triggered bridging interactions. J. Colloid Interface Sci. 2018, 526, 114–1123. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Wunder, S.; Warmuth, F. Spherical polymer brushes with vinylimidazolium-type poly (ionic liquid) chains as support for metallic nanoparticles. Polymer 2012, 53, 43–49. [Google Scholar] [CrossRef]
- Qin, X.; Chen, K.; Cao, L.; Zhang, Y.; Li, L.; Guo, X. Antifouling performance of nano-sized spherical poly (N-hydroxyethyl acrylamide) brush. Colloids Surf. B 2017, 155, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Joafshan, M.; Shakeri, A.; Razavi, S.R.; Salehi, H. Gas responsive magnetic nanoparticle as novel draw agent for removal of Rhodamine B via forward osmosis: High water flux and easy regeneration. Sep. Purif. Technol. 2022, 282, 119998. [Google Scholar] [CrossRef]
- Li, T.H.; Robertson, M.L.; Conrad, J.C. Molecular weight and dispersity affect chain conformation and pH-response in weak polyelectrolyte brushes. Polym. Chem. 2021, 12, 6737–6744. [Google Scholar] [CrossRef]
- Conrad, J.C.; Robertson, M.L. Shaping the structure and response of surface-grafted polymer brushes via the molecular weight distribution. JACS Au 2023, 3, 333–343. [Google Scholar] [CrossRef]
- Ramesh, A.; Neelaveni, M.; Tamizhdurai, P.; Ramya, R.; Sasirekha, N.; Shanthi, K. Facile synthesis of poly (benzylamine) brushes stabilized silver nanoparticle catalyst for the abatement of environmental pollutant methylene blue. Mater. Chem. Phys. 2019, 229, 42–430. [Google Scholar] [CrossRef]
- Li, H.; Chen, G.; Das, S. Electric double layer electrostatics of pH-responsive spherical polyelectrolyte brushes in the decoupled regime. Colloids Surf. B 2016, 147, 180–190. [Google Scholar] [CrossRef] [PubMed]
- Su, N.; Li, H.; Huang, Y.; Zhang, X. Synthesis of salt responsive spherical polymer brushes. J. Nanomater. 2015, 2015, 956819. [Google Scholar] [CrossRef]
- Currie, E.P.K.; Wagemaker, M.; Cohen Stuart, M.A.; Van Well, A.A. Structure of monodisperse and bimodal brushes. Macromolecules 1999, 32, 9041–9050. [Google Scholar] [CrossRef]
- Guo, X.; Ballauff, M. Spatial Dimensions of colloidal polyelectrolyte brushes as determined by dynamic light scattering. Langmuir 2000, 16, 8719–8726. [Google Scholar] [CrossRef]
- Prucker, O.; Ruhe, J. Synthesis of poly(styrene) monolayers attach to high surface area silica gels through self-assembled monolayers of azo initiators. Nacromolecules 1998, 31, 592–601. [Google Scholar] [CrossRef]
- Polanowski, P.; Jeszka, J.K.; Matyjaszewski, K. Polymer brushes in pores by ATRP: Monte Carlo simulations. Polymer 2020, 211, 123124. [Google Scholar] [CrossRef]
- Utz, M.; Begley, M.R. Scaling theory of adsorption-induced stresses in polymer brushes grafted onto compliant structures. J. Mech. Phys. Solids 2008, 56, 801–804. [Google Scholar] [CrossRef]
- Marsh, D. Scaling and mean-field theories applied to polymer brushes. Biophys. J. 2004, 86, 2630–2633. [Google Scholar] [CrossRef]
- Manav, M.; Anilkumar, P.; Phani, A.S. Mechanics of polymer brush based soft active materials–theory and experiments. J. Mech. Phys. Solids 2018, 121, 296–312. [Google Scholar] [CrossRef]
- Adeli, F.; Abbasi, F.; Ghandforoushan, P.; Külahli, H.E.; Meran, M.; Abedi, F.; Ghamkhari, A.; Afif, S. Recent advances in formulation and application of molecular polymer brushes in biomedicine: Therapeutic, diagnostic, and theranostics capabilities. Nanotoday 2023, 53, 102010. [Google Scholar] [CrossRef]
- Ackerman, D.M.; Delaney, K.; Fredrickson, G.H.; Ganapathysubramanian, B. A finite element approach to self-consistent field theory calculations of multiblock polymers. J. Comput. Phys. 2017, 331, 280–296. [Google Scholar] [CrossRef]
- Wiebe, M.; Leermakers, F.A.M. Modeling the structure of a polydisperse polymer brush. Polymer 2009, 50, 305–316. [Google Scholar]
- Zhulina, E.B.; Amoskov, V.M.; Polotsky, A.A.; Birshtein, T.M. Analytical self-consistent field model of arm-grafted starlike polymers in nonlinear elasticity regime. Polymer 2014, 55, 5160–5167. [Google Scholar] [CrossRef]
- Kritikos, G.; Terzis, A.F. Variable density self consistent field study on bounded polymer layer around spherical nanoparticles. Eur. Polym. J. 2013, 49, 613–629. [Google Scholar] [CrossRef]
- Daniel, J.S.; Jan-Michael, Y.C.; Andrey, V.D. Molecular dynamics simulations of polyelectrolyte brushes: From single chains to bundles of chains. Langmuir 2007, 23, 12716–12728. [Google Scholar]
- Zhang, Q.; Xiang, X. Adsorption of a spherical nanoparticle in polymer brushes: Brownian dynamics investigation. Physica A 2013, 392, 3857–3862. [Google Scholar] [CrossRef]
- Sugimura, N.; Ohno, K. A Monte Carlo simulation of water + oil + ABA triblock copolymer ternary system II. Rheology under shear flow field by Monte Carlo Brownian Dynamics method. Chem. Phys. Lett. 2021, 777, 138382. [Google Scholar] [CrossRef]
- Hariharan, R.; Biver, C.; Mays, J.; Russel, W.B. Ionic strength and curvature effects in flat and highly curved polyelectrolyte brushes. Macromolecules 1998, 31, 7506–7513. [Google Scholar] [CrossRef]
- Luo, M.B.; Huang, J.H. Influence of excluded volume on the conformational property of tail-like chain on the simple cubic lattice. Eur. Polym. J. 2003, 39, 135–141. [Google Scholar] [CrossRef]
- Guo, X.; Ballauff, M. Spherical polyelectrolyte brushes: Comparison between annealed and quenched brushes. Phys. Rev. E 2001, 64, 051406. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Karlheinz, L.; Martin, H.; Borisov, O.V.; Ballauff, M.; Jusufi, A. Collapse of spherical polyelectrolyte brushes in the presence of multivalent counterions. Phys. Rev. Lett. 2006, 97, 158301–158304. [Google Scholar]
- Su, N. Preparation and performance of retention and drainage aid made of cationic spherical polyelectrolyte brushes. E-Polymers 2022, 22, 676–685. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, Y.; Fu, K.; Yuan, S.; Huang, C.; Li, H. Preparation and performance of cationic flocculant for papermaking based on the graft polymerization of cationic chains from colloidal silica particles. Colloids Surf. A 2016, 491, 29–36. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, X.; Fu, K.; Li, H.; Huang, C.; Yuan, S. Synthesis and application of cationic spherical polyelectrolyte brushes as retention and drainage aid in bleached eucalyptus kraft pulp. J. Ind. Eng. Chem. 2015, 31, 309–316. [Google Scholar] [CrossRef]
- Mei, Y.; Abetz, C.; Birkert, O.; Schädler, V.; Leyrer, R.J.; Ballauff, M. Interaction of spherical polyelectrolyte brushes with calcium carbonate and cellulose fibers: Mechanistic studies and their application in papermaking. J. Appl. Polym. Sci. 2006, 102, 233–241. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, N. Spherical Polyelectrolyte Brushes as Flocculants and Retention Aids in Wet-End Papermaking. Molecules 2023, 28, 7984. https://doi.org/10.3390/molecules28247984
Su N. Spherical Polyelectrolyte Brushes as Flocculants and Retention Aids in Wet-End Papermaking. Molecules. 2023; 28(24):7984. https://doi.org/10.3390/molecules28247984
Chicago/Turabian StyleSu, Na. 2023. "Spherical Polyelectrolyte Brushes as Flocculants and Retention Aids in Wet-End Papermaking" Molecules 28, no. 24: 7984. https://doi.org/10.3390/molecules28247984
APA StyleSu, N. (2023). Spherical Polyelectrolyte Brushes as Flocculants and Retention Aids in Wet-End Papermaking. Molecules, 28(24), 7984. https://doi.org/10.3390/molecules28247984




