Selective Adsorption of Gadolinium by Nitrogen-Doped Carboxymethylated Cellulose Nanocrystalline Carbon Aerogels Functionalized in the Ammonia–Urea System
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization Results
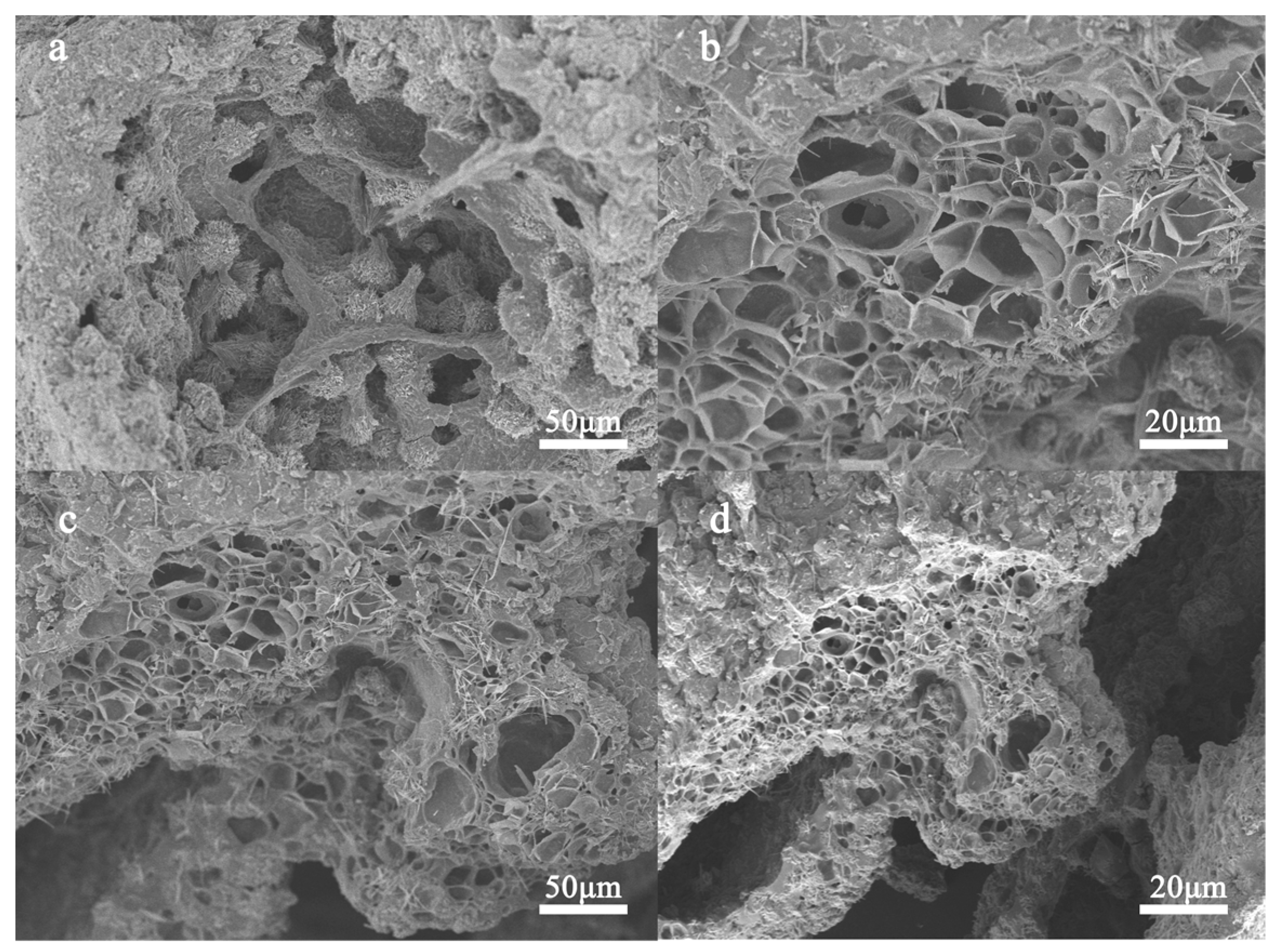
2.1.1. SEM Analysis of Aerogel Materials
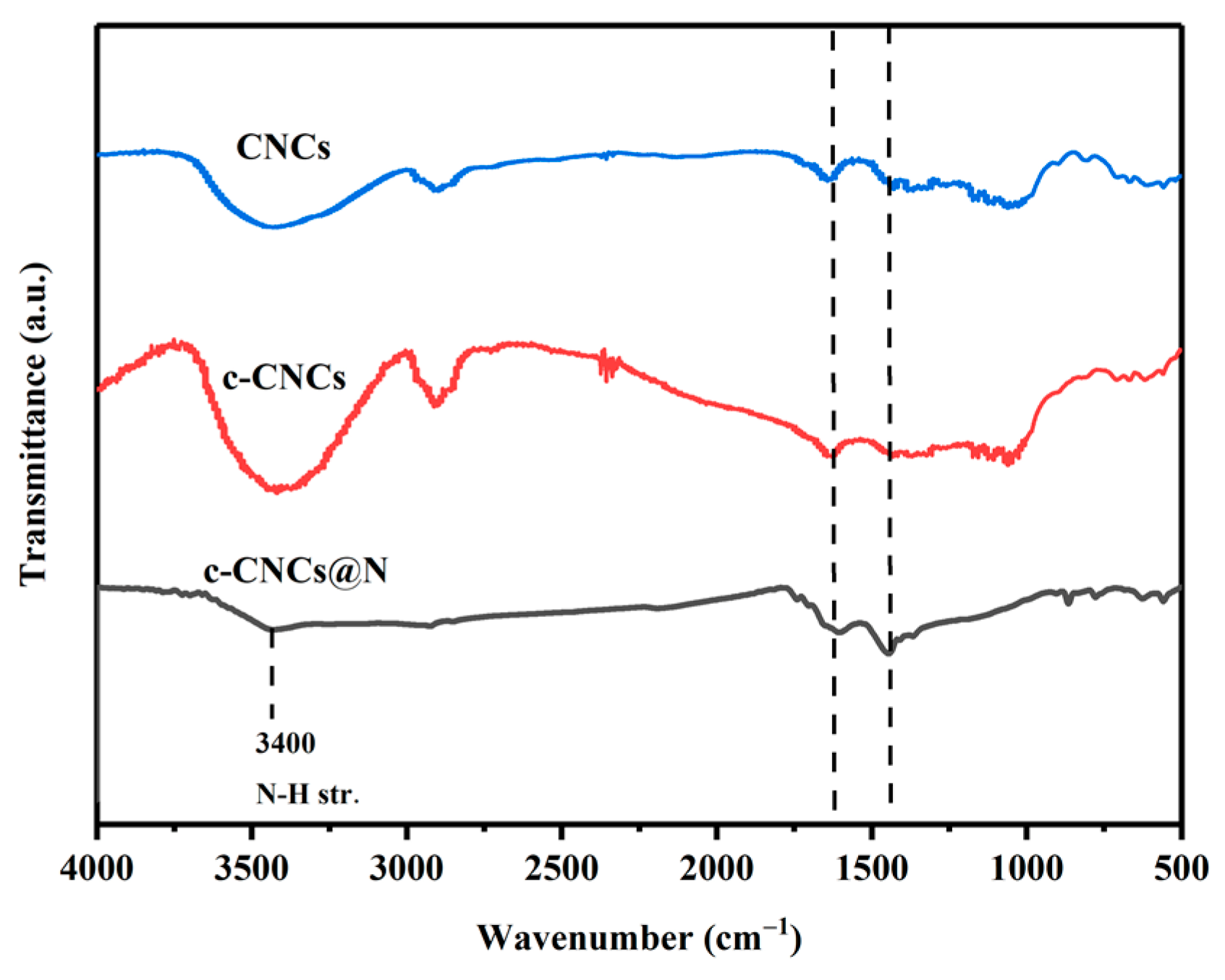
2.1.2. FTIR Analysis of Aerogel Materials
2.1.3. N2 Adsorption–Desorption
2.1.4. Zeta Potential Analysis of Aerogel Materials
2.2. Adsorption Experimental Analysis
2.2.1. pH Experiments
2.2.2. Adsorption Kinetics Experiment
2.2.3. Adsorption Isotherm Experiment
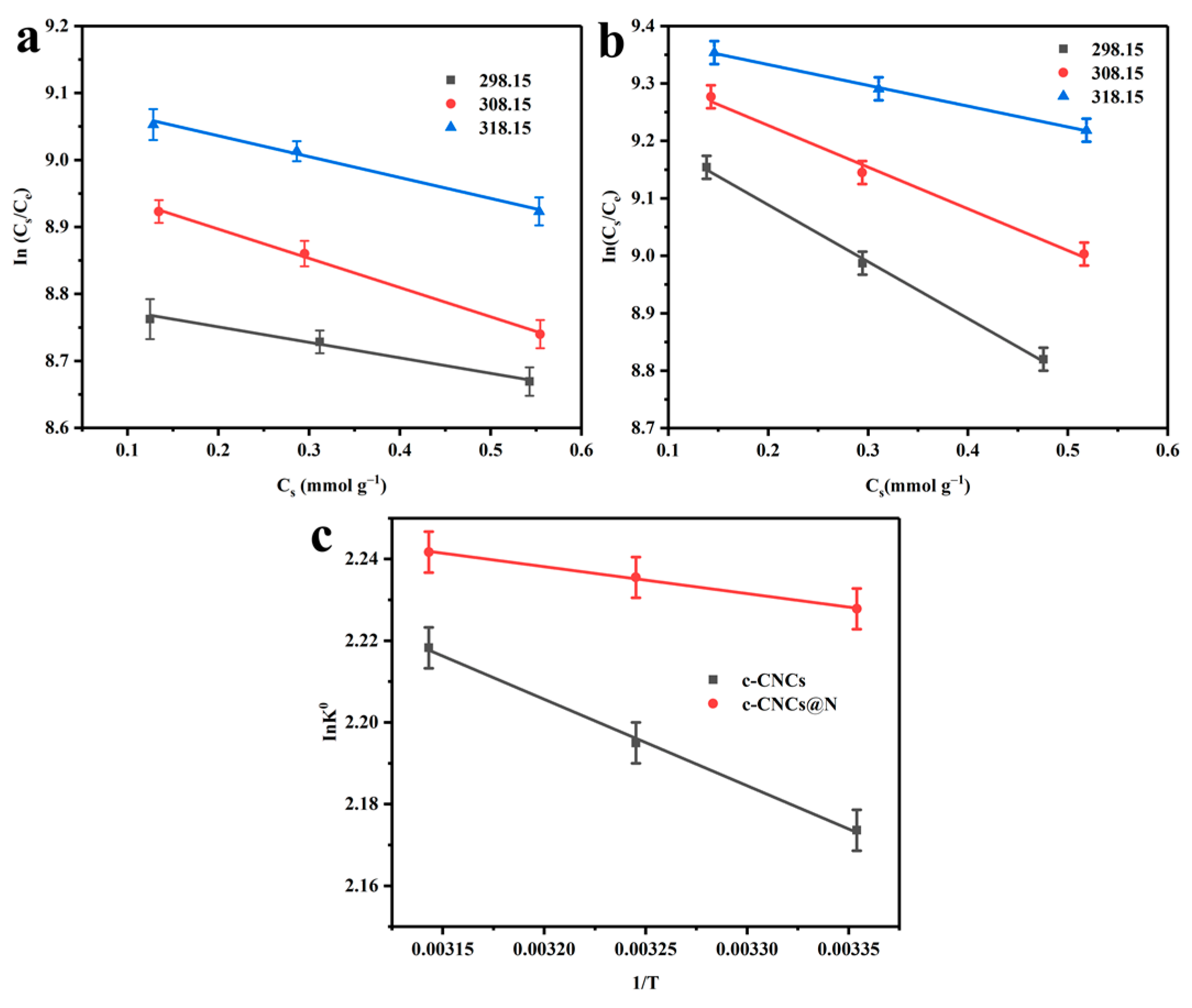
2.2.4. Adsorption Thermodynamics Experiment
2.2.5. Repeatable Adsorption Experiment
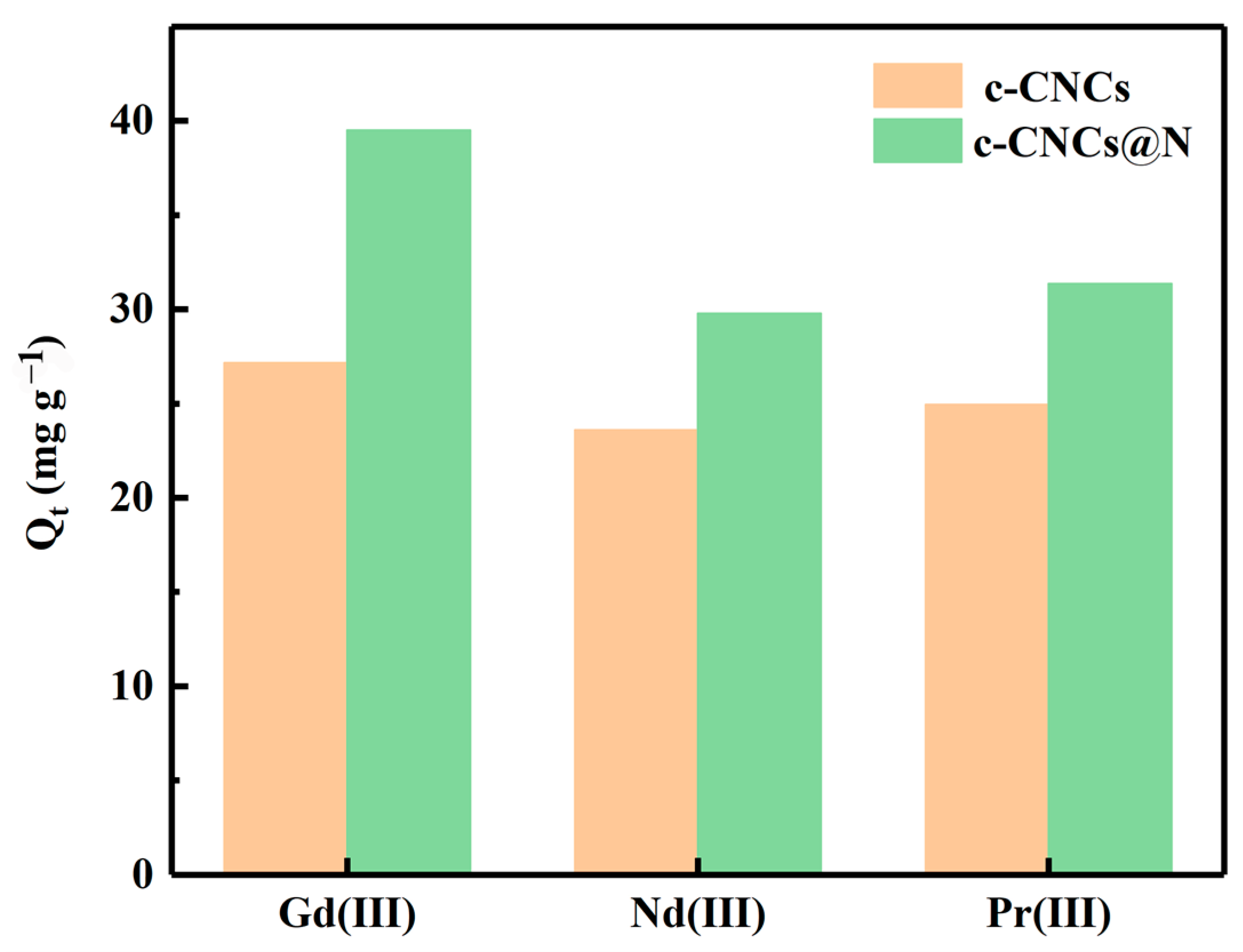
2.2.6. Selective Adsorption Experiment
3. Experimental and Methods
3.1. Materials
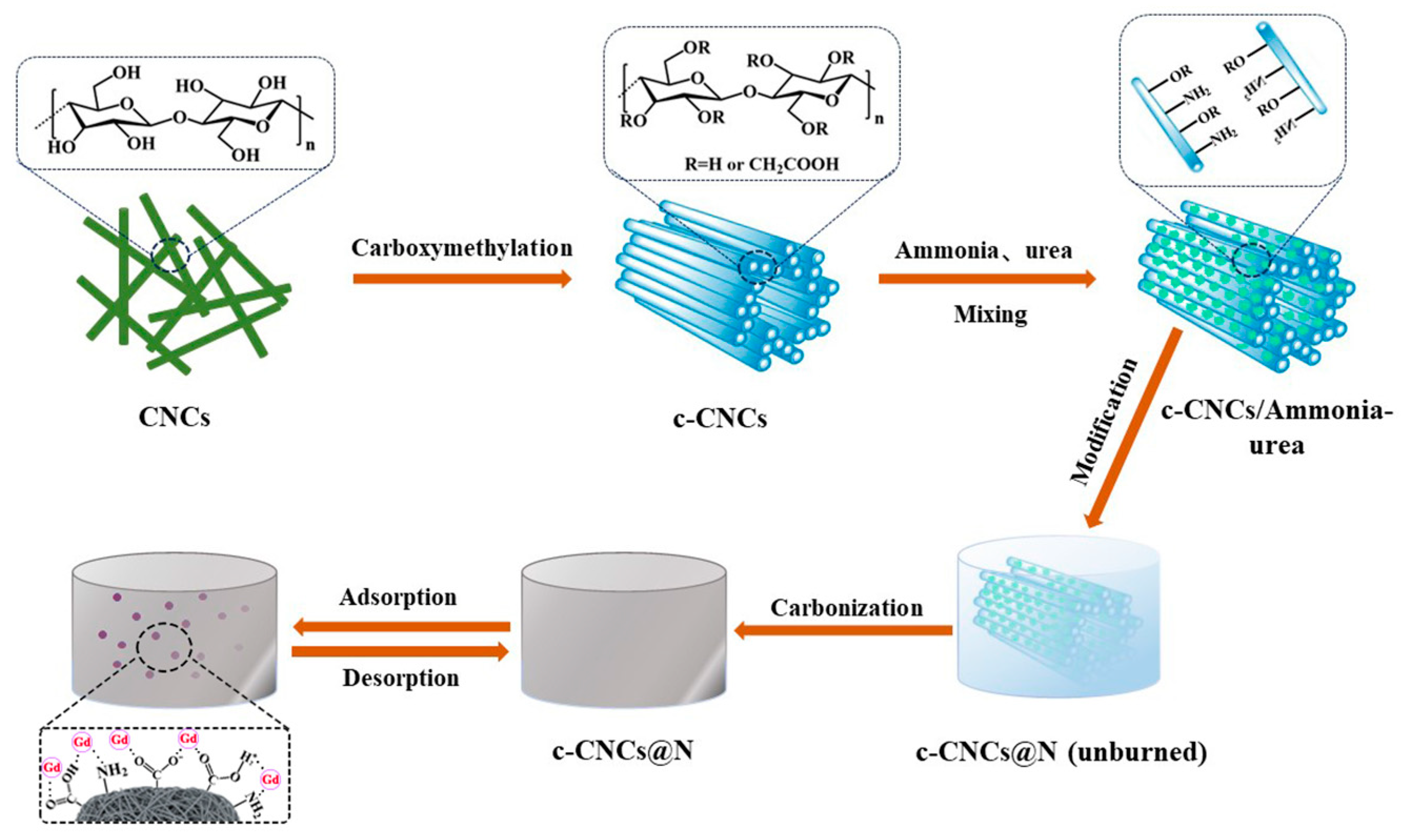
3.2. Preparation of Cellulose Nanocrystal (CNCs)
3.3. Preparation of Carboxymethylated Cellulose Nanocrystals (c-CNCs)
3.4. Preparation of Nitrogen-Doped Carboxymethyl Cellulose Nanocrystals (c-CNCs@N)
3.5. Adsorption Experiment
3.6. Characterization Instruments
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, G.; Long, W.; Luo, W.; Yang, Y.; Liu, X.; Wu, B.; Wei, X.; Li, C. A detailed research on allanite in weathered sphere from the Xiajialing REE deposit in Xiangshan, Jiangxi, and its metallogenic significance. Acta Petrol. Sin. 2022, 38, 2067–2079. [Google Scholar]
- Zhao, Z.; Wang, D.; Zou, X. The genesis and diversity of ion adsorption REE mineralization in the Zhaibei deposit, Jiangxi Province, South China. Acta Petrol. Sin. 2022, 38, 356–370. [Google Scholar] [CrossRef]
- Zeng, C.-X.; Guan, Q.-J.; Sui, Y.; Yu, W.-J.; Bu, Y.-J.; Liu, C.-F.; Zhang, Z.-Y. Kinetics of nitric acid leaching of low-grade rare earth elements from phosphogypsum. J. Cent. South Univ. 2022, 29, 1869–1880. [Google Scholar] [CrossRef]
- Zhong, S.-L.; Qiu, J.-H.; Luo, W.-W.; Wu, M.-S. First-principles study of properties of rare-earth-doped LiFePO4. Acta Phys. Sin. 2021, 70, 158203. [Google Scholar] [CrossRef]
- Xue, J.-Q.; Liu, J.-X.; Huang, D.-Z.; Zhou, W.-J.; Liu, C.-M.; Cao, Y.-S.; Cao, C.-H. Sources of rare earth elements REE plus Y (REY) in Bayili Coal Mine from Wensu County of Xinjiang, China. Trans. Nonferrous Met. Soc. China 2021, 31, 3105–3115. [Google Scholar] [CrossRef]
- Cai, W.; Shen, Z.; Zhan, Z. Effect of Rare Earth Y on High Temperature Oxidation Resistance of AISI430 Stainless Steel. Rare Met. Mater. Eng. 2021, 50, 2495–2501. [Google Scholar]
- Cui, H.; Zhang, M.; Wang, Y.; Wang, X.; Xin, B. Preparation of Rare-earth Ytterbium-doped Zr-based Amorphous Alloy Thin Film by Co-sputtering. Rare Met. Mater. Eng. 2021, 50, 3295–3303. [Google Scholar]
- Guo, N.; Liu, S.; Chen, Z.; Jiang, S.; Li, H. Mechanism of Nb and REE enrichment in the Tiemuli alkali-feldspar granite, Chongyi County, Jiangxi Province. Acta Petrol. Sin. 2022, 38, 371–392. [Google Scholar] [CrossRef]
- Shen, T.; Ni, X.-F.; Ling, J. Recent Progress in Ring-opening Polymerizations Catalyzed by Rare Earth Catalysts. Acta Polym. Sin. 2021, 52, 445–455. [Google Scholar] [CrossRef]
- Lu, F.; Zhao, T.; Sun, X.; Fan, Q.; Huang, W. Design of NIR-II Emissive Rare-earth Nanoparticles and Their Applications for Bio-imaging. Prog. Chem. 2022, 34, 1348–1358. [Google Scholar] [CrossRef]
- Li, H.-D.; Zhai, L.-J.; Song, Y.-B.; Nil, Y.-L. Two Nitronyl Nitroxide Biradical. Bridged Lanthanide One-Dimensional Chains: Crystal Structure, Magnetic Properties and Luminescent Behavior. Chin. J. Inorg. Chem. 2021, 37, 914–920. [Google Scholar] [CrossRef]
- Chen, C.; Xi, Y.; Weng, Y. Recent Advances in Cellulose-Based Hydrogels for Tissue Engineering Applications. Polymers 2022, 14, 3335. [Google Scholar] [CrossRef] [PubMed]
- Coseri, S. Insights on Cellulose Research in the Last Two Decades in Romania. Polymers 2021, 13, 689. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Liu, K.; Du, H.; Zheng, T.; Zhang, N.; Xu, T.; Pang, B.; Zhang, X.; Si, C.; Zhang, K. Cellulose Nanopaper: Fabrication, Functionalization, and Applications. Nano-Micro Lett. 2022, 14, 104. [Google Scholar] [CrossRef]
- Yan, Z.; Meng, L.; Huang, X.; Wei, Q.; Liu, J.; Sun, Z.; Ding, S. Preparation and properties of silanation-modified nanocellulose-reinforced polyvinyl alcohol nanocomposites. J. Text. Institute 2023, 114, 1881–1886. [Google Scholar] [CrossRef]
- Zhang, K.; Zhou, M.; Cheng, F.; Lin, Y.; Zhu, P.; Li, J.; Tang, K. Preparation and characterization of starch-based nanocomposites reinforced by graphene oxide self-assembled on the surface of silane coupling agent modified cellulose nanocrystals. Int. J. Biol. Macromol. 2022, 198, 187–193. [Google Scholar] [CrossRef]
- Jia, P.; Xu, J.; Wang, X.; Chen, Z.; Xie, Z.; Jiang, H. Comparison of characteristics of the cellulose nanocrystal aerogels aminosilane-functionalized through gas-phase reaction. J. Porous Mater. 2022, 29, 745–758. [Google Scholar] [CrossRef]
- Sun, L.; Ren, X.; Du, T.; Luo, Y.; Zhang, J.; Wang, J. High Entropy Engineering: New Strategy for the Critical Property Optimizations of Rare Earth Silicates. J. Inorg. Mater. 2021, 36, 339–346. [Google Scholar] [CrossRef]
- Zhang, W.; Zhu, H.; Liu, S.-F.; Ning, S.-Y.; Wei, Y.-Z. Scandium recovery from ion-adsorption rare earth concentrate with HEHEHP as extractant. J. Cent. South Univ. 2021, 28, 679–689. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, S.; Li, C.; Li, W.; Kou, S.; Mao, X.; Yan, F. Effect of Rare Earth Elements Addition on Corrosion Behavior of Fe-based Amorphous Composites. Rare Met. Mater. Eng. 2021, 50, 2592–2598. [Google Scholar]
- Dortez, S.; Sierra, T.; Alvarez-Sanchez, M.A.; Gonzalez-Dominguez, J.M.; Benito, A.M.; Maser, W.K.; Crevillen, A.G.; Escarpa, A. Effect of nanocellulose polymorphism on electrochemical analytical performance in hybrid nanocomposites with non-oxidized single-walled carbon nanotubes. Microchim. Acta 2022, 189, 62. [Google Scholar] [CrossRef] [PubMed]
- Munier, P.; Di, A.; Hadi, S.E.; Kapuscinski, M.; Segad, M.; Bergstrom, L. Assembly of cellulose nanocrystals and clay nanoplatelets studied by time-resolved X-ray scattering. Soft Matter 2021, 17, 5747–5755. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Wang, W.; Wang, X.; Zhao, X.; Xia, N.; Kong, F.; Wang, S. Easy way to prepare dispersible CNC dry powder by precipitation and conventional evaporation. Cellulose 2021, 28, 9661–9676. [Google Scholar] [CrossRef]
- Bondancia, T.J.; Batista, G.; de Aguiar, J.; Lorevice, M.V.; Cruz, A.J.G.; Marconcini, J.M.; Mattoso, L.H.C.; Farinas, C.S. Cellulose Nanocrystals from Sugar Cane Bagasse Using Organic and/or Inorganic Acids: Techno-Economic Analysis and Life Cycle Assessment. ACS Sustain. Chem. Eng. 2022, 10, 4660–4676. [Google Scholar] [CrossRef]
- Zheng, X.; Sun, W.; Li, A.; Zhang, Y.; Li, Z. Bacterial cellulose nanofibrous ion imprinted aerogel for highly efficient recognition and adsorption of Dy(III). Process Saf. Environ. Prot. 2022, 160, 70–79. [Google Scholar] [CrossRef]
- Zheng, X.; Zhang, Y.; Bian, T.; Zhang, Y.; Li, Z.; Pan, J. Oxidized carbon materials cooperative construct ionic imprinted cellulose nanocrystals films for efficient adsorption of Dy(III). Chem. Eng. J. 2020, 381, 122669. [Google Scholar] [CrossRef]
- Zheng, X.; Bian, T.; Zhang, Y.; Zhang, Y.; Li, Z. Construction of ion-imprinted nanofiber chitosan films using low-temperature thermal phase separation for selective and efficiency adsorption of Gd (III). Cellulose 2020, 27, 455–467. [Google Scholar] [CrossRef]
- Zheng, X.; Sun, W.; Li, A.; Wang, B.; Jiang, R.; Song, Z.; Zhang, Y.; Li, Z. Graphene oxide and polyethyleneimine cooperative construct ionic imprinted cellulose nanocrystal aerogel for selective adsorption of Dy (III). Cellulose 2021, 29, 469–481. [Google Scholar] [CrossRef]



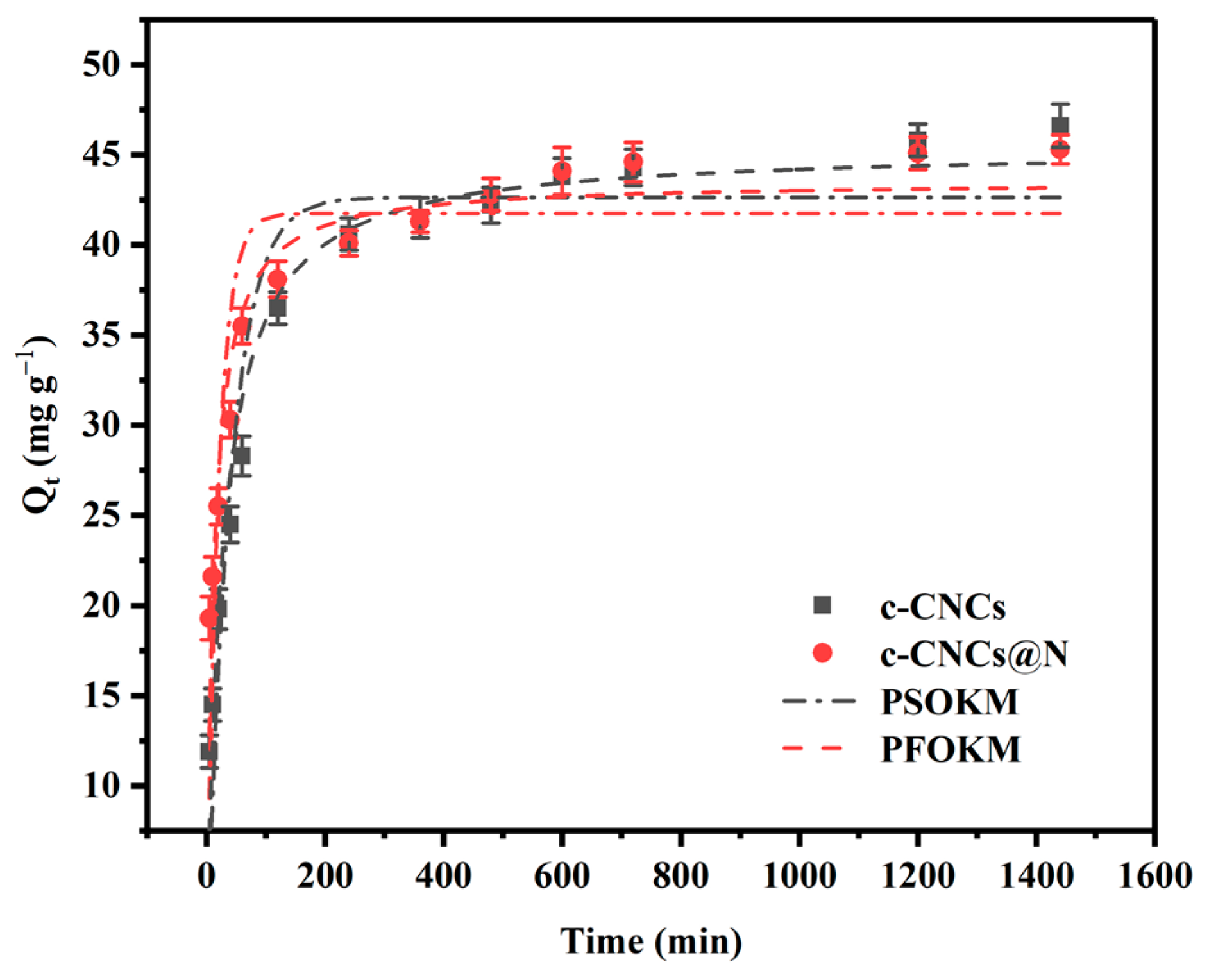


| Absorbents | Qe,exp (mg g−1) | Quasi-First-Order Kinetic Model | Quasi-Second-Order Kinetic Model | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Qe,c (mg g−1) | K1 (min−1) | R2 | Qe,c (mg g−1) | K2 × 10−3(g mg−1 min−1) | h (mg g−1 min−1) | t1/2 (min) | R2 | ||
| c-CNCs | 46.6 | 42.64 | 0.0248 | 0.904 | 45.36 | 0.083 | 1.709 | 26.53 | 0.991 |
| c-CNCs@N | 45.3 | 41.75 | 0.05047 | 0.763 | 43.53 | 0.192 | 3.636 | 11.97 | 0.992 |
| Absorbents | Langmuir Isotherm Equation | Freundlich Isotherm Equation | ||||
|---|---|---|---|---|---|---|
| Qm (mg g−1) | KL (L mg−1) | R2 | KF (mg g−1) | 1/n | R2 | |
| c-CNCs | 95.63 | 0.0373 | 0.996 | 21.455 | 0.334 | 0.848 |
| c-CNCs@N | 99.65 | 0.12967 | 0.998 | 32.134 | 0.259 | 0.798 |
| Absorbents | ΔH° (kJ mol−1) | ΔS° (J mol−1) | T (K) | K° | ΔG° (kJ mol−1) | R2 |
|---|---|---|---|---|---|---|
| c-CNCs | 211.69 | 2.88 | 298.15 | 8.79 | −5.39 | 0.998 |
| 308.15 | 8.98 | −5.63 | ||||
| 318.15 | 9.10 | −5.84 | ||||
| c-CNCs@N | 65.98 | 2.45 | 298.15 | 9.28 | −5.52 | 0.998 |
| 308.15 | 9.37 | −5.73 | ||||
| 318.15 | 9.41 | −5.93 |
| Cation | c-CNCs | c-CNCs@N | ||
|---|---|---|---|---|
| Cf (mg L−1) | Kd (mL g−1) | Cf (mg L−1) | Kd (mL g−1) | |
| Gd3+ | 22.86 | 1187.23 | 10.51 | 3757.37 |
| Nd3+ | 26.41 | 893.22 | 20.23 | 1471.58 |
| Pr3+ | 25.07 | 994.32 | 19.64 | 1545.82 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, T.; Zheng, X.; Li, A.; Ji, B. Selective Adsorption of Gadolinium by Nitrogen-Doped Carboxymethylated Cellulose Nanocrystalline Carbon Aerogels Functionalized in the Ammonia–Urea System. Molecules 2023, 28, 7965. https://doi.org/10.3390/molecules28247965
Xu T, Zheng X, Li A, Ji B. Selective Adsorption of Gadolinium by Nitrogen-Doped Carboxymethylated Cellulose Nanocrystalline Carbon Aerogels Functionalized in the Ammonia–Urea System. Molecules. 2023; 28(24):7965. https://doi.org/10.3390/molecules28247965
Chicago/Turabian StyleXu, Tongtong, Xudong Zheng, Ang Li, and Biao Ji. 2023. "Selective Adsorption of Gadolinium by Nitrogen-Doped Carboxymethylated Cellulose Nanocrystalline Carbon Aerogels Functionalized in the Ammonia–Urea System" Molecules 28, no. 24: 7965. https://doi.org/10.3390/molecules28247965
APA StyleXu, T., Zheng, X., Li, A., & Ji, B. (2023). Selective Adsorption of Gadolinium by Nitrogen-Doped Carboxymethylated Cellulose Nanocrystalline Carbon Aerogels Functionalized in the Ammonia–Urea System. Molecules, 28(24), 7965. https://doi.org/10.3390/molecules28247965






