Lewis Acid-Base Adducts of α-Amino Acid-Derived Silaheterocycles and N-Methylimidazole
Abstract
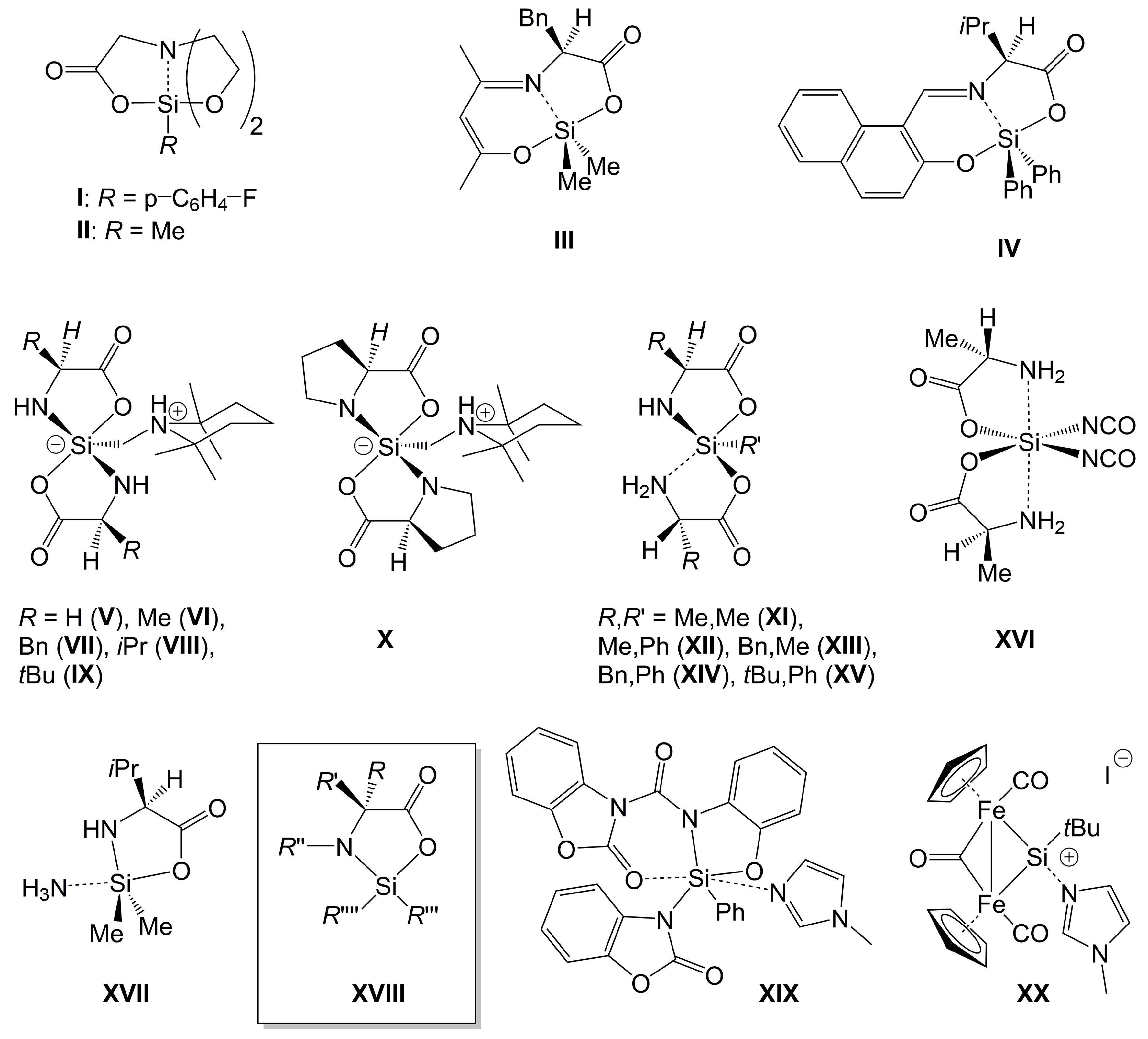
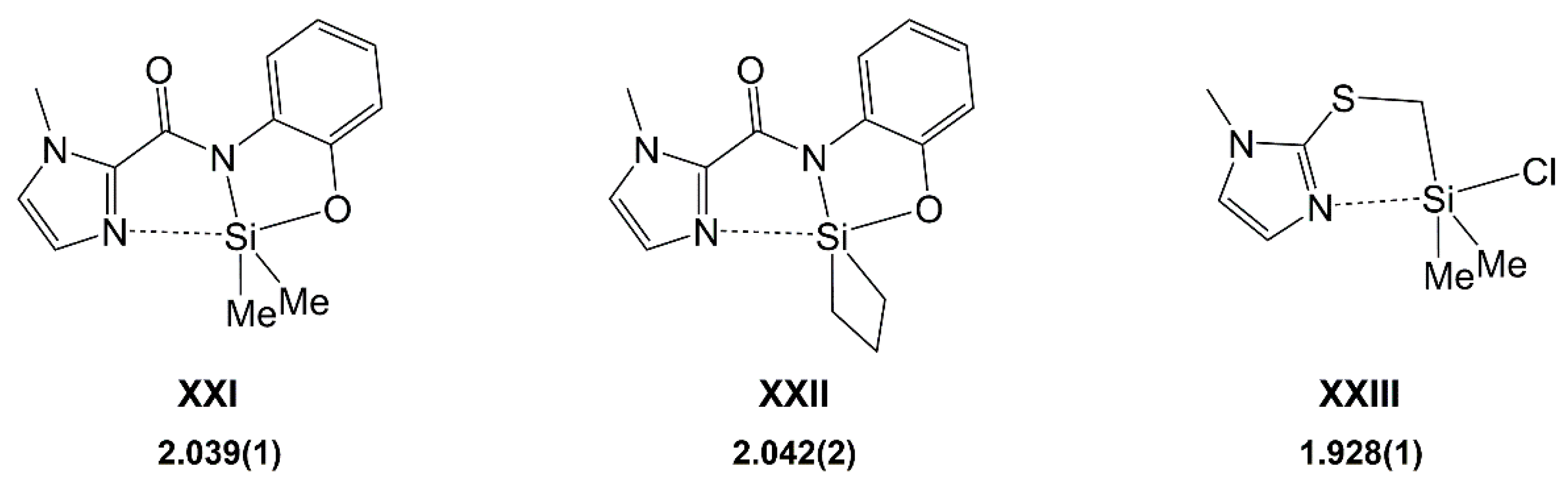
1. Introduction
2. Results and Discussion
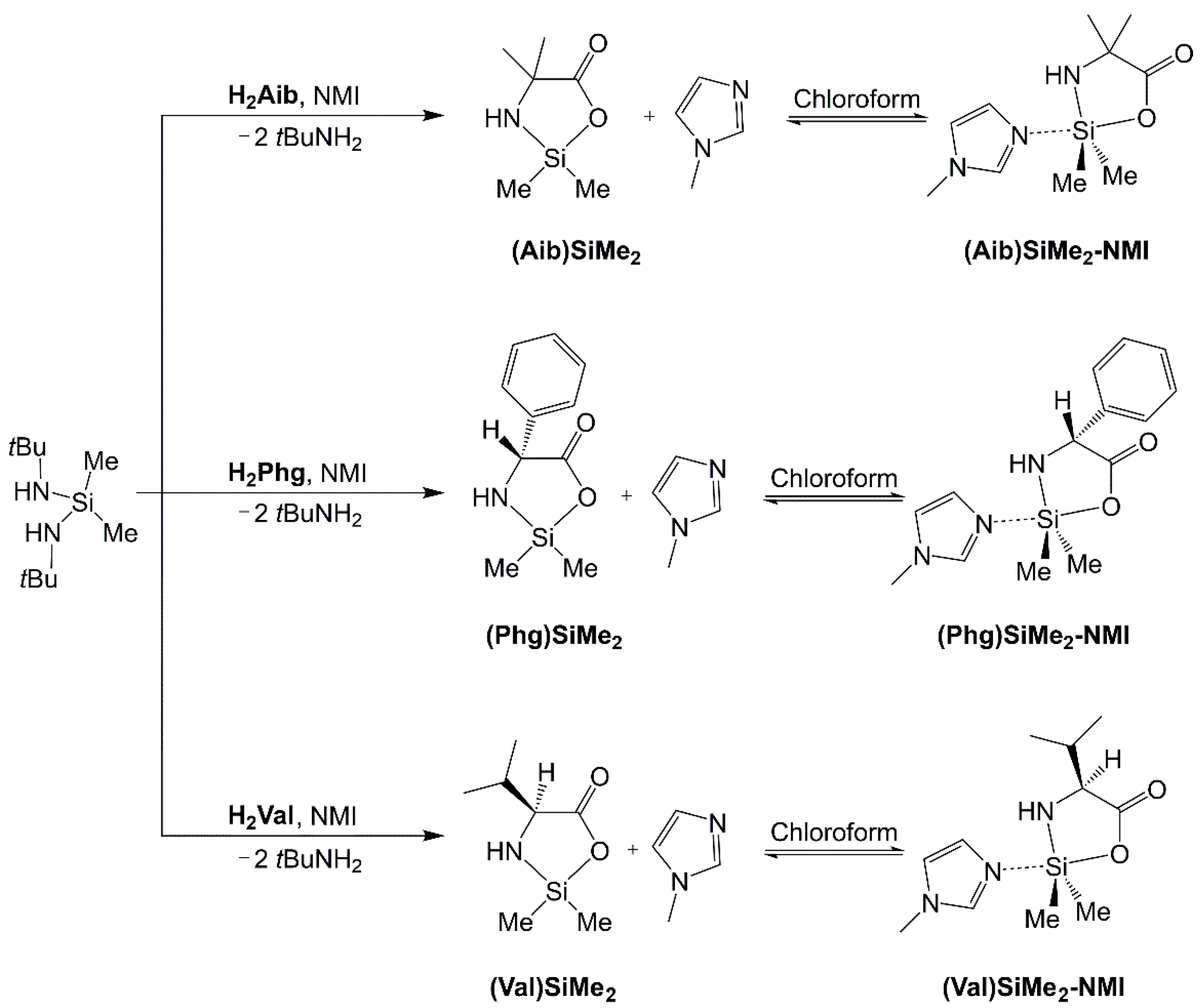
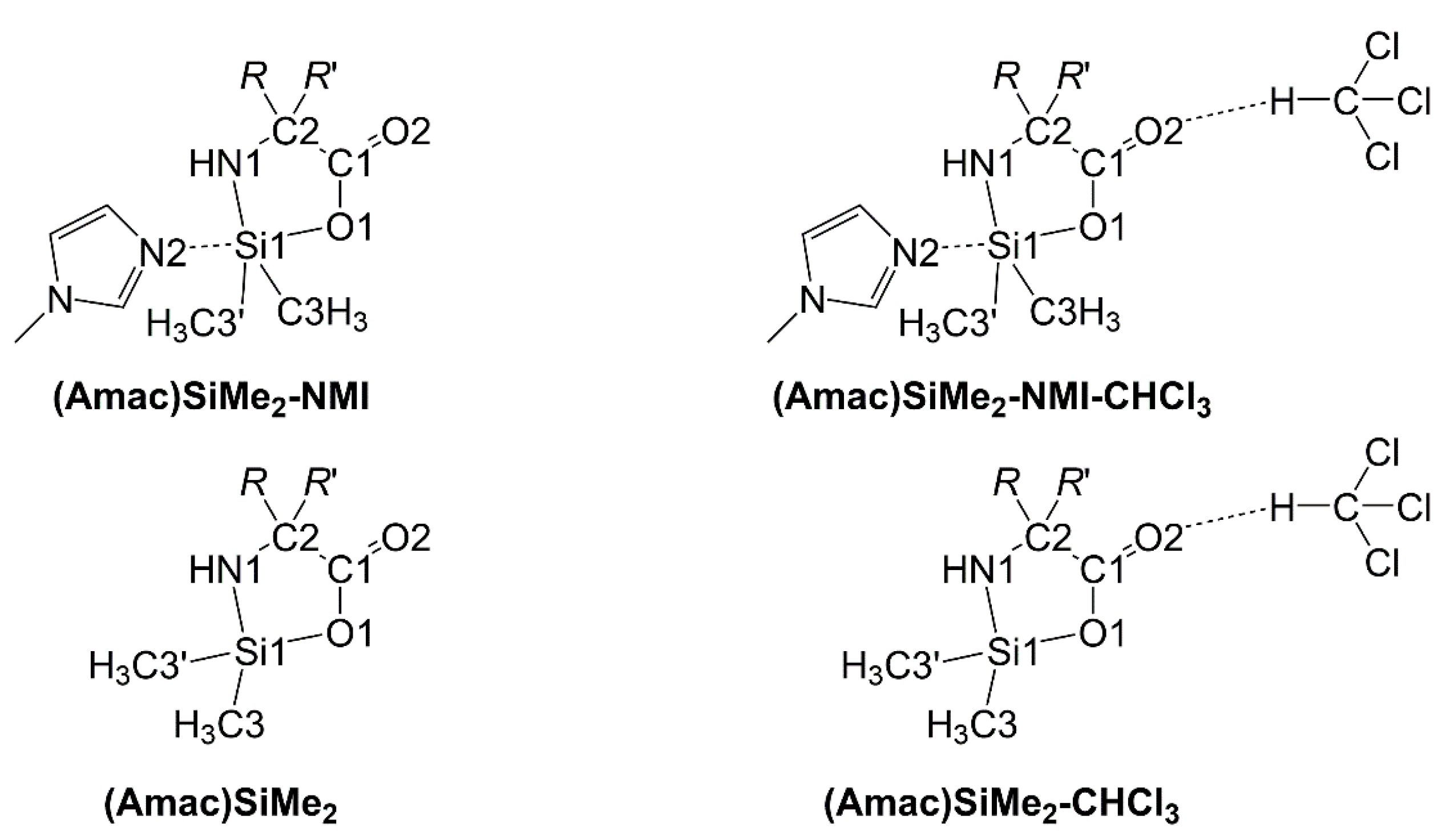
2.1. Compounds Overview
2.2. Single-Crystal X-ray Diffraction
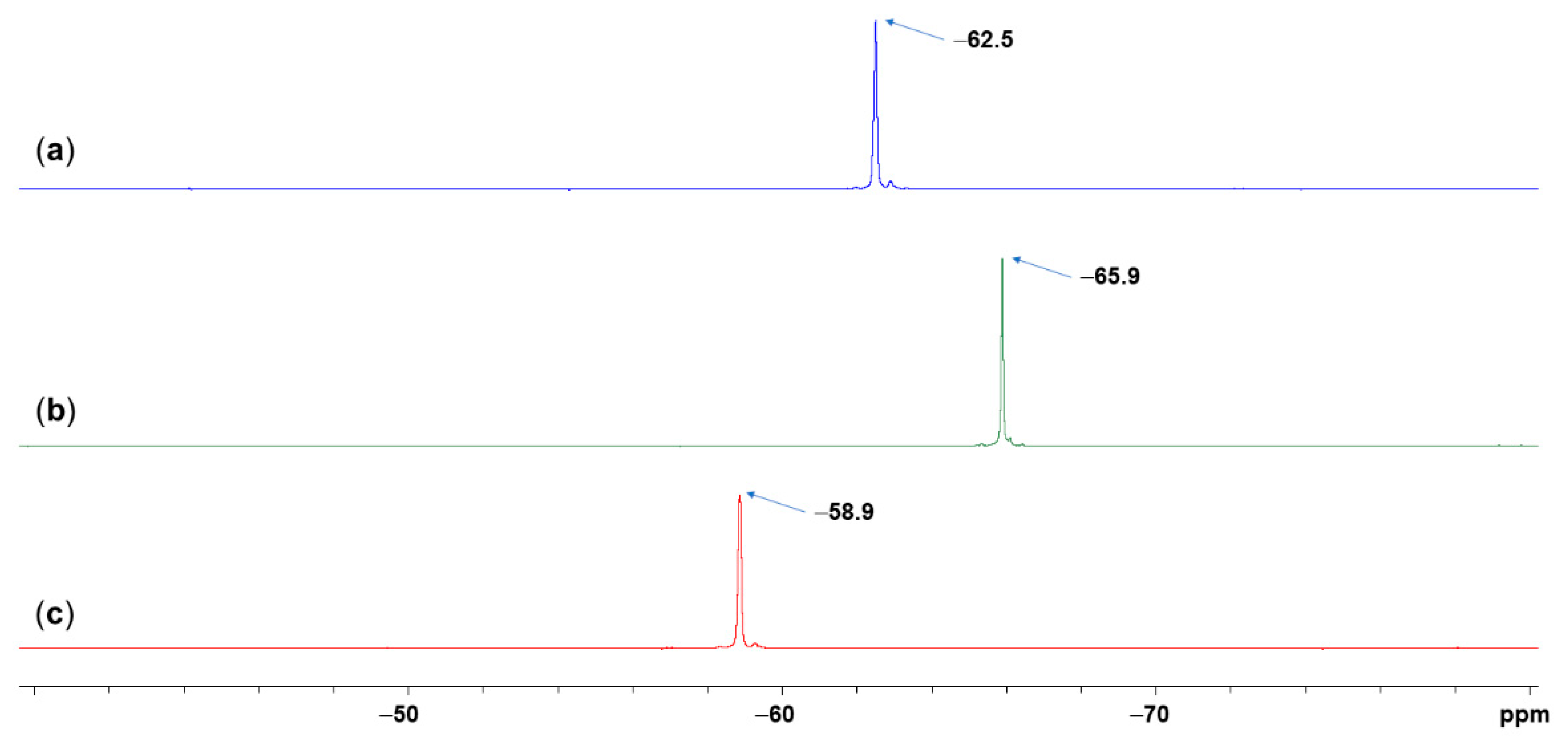
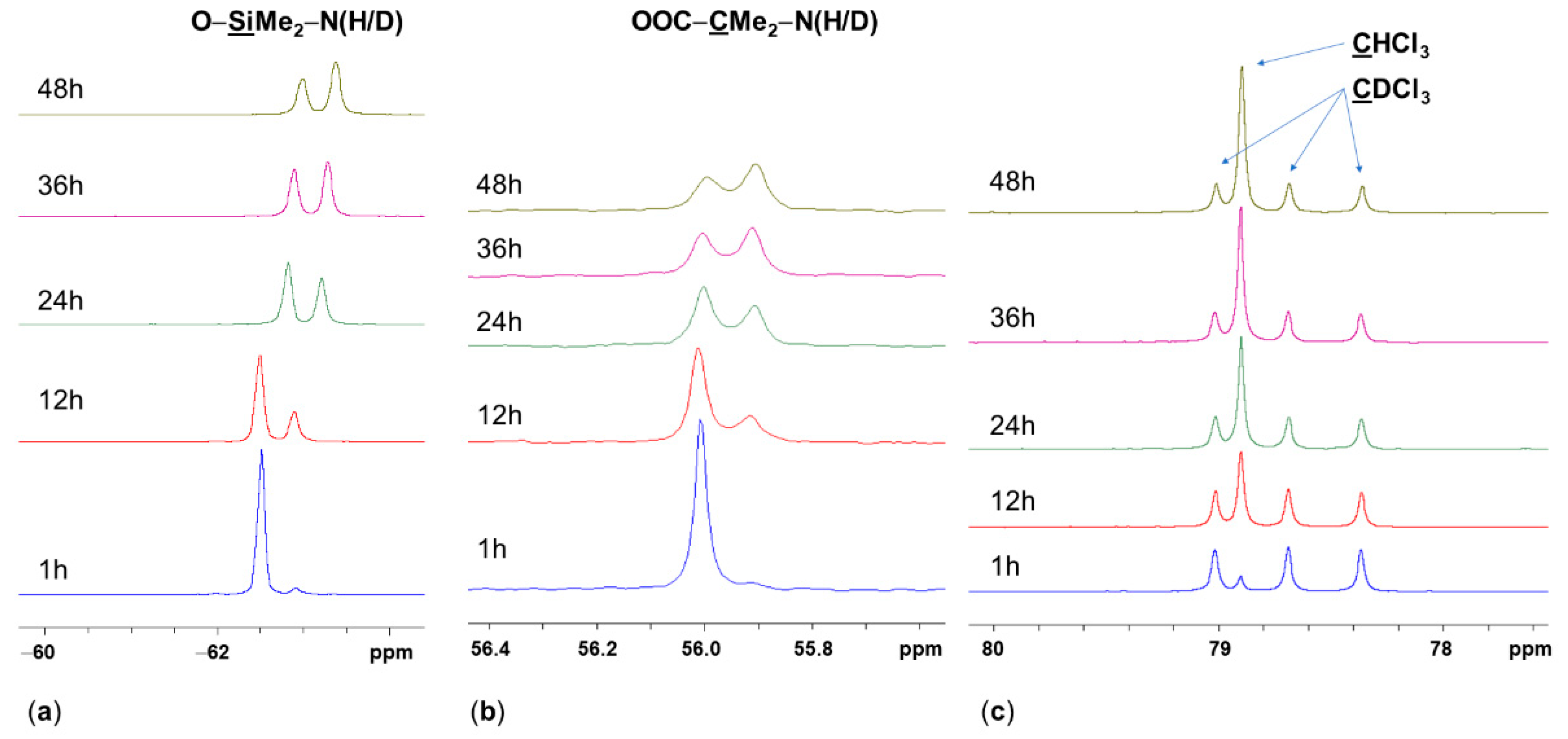
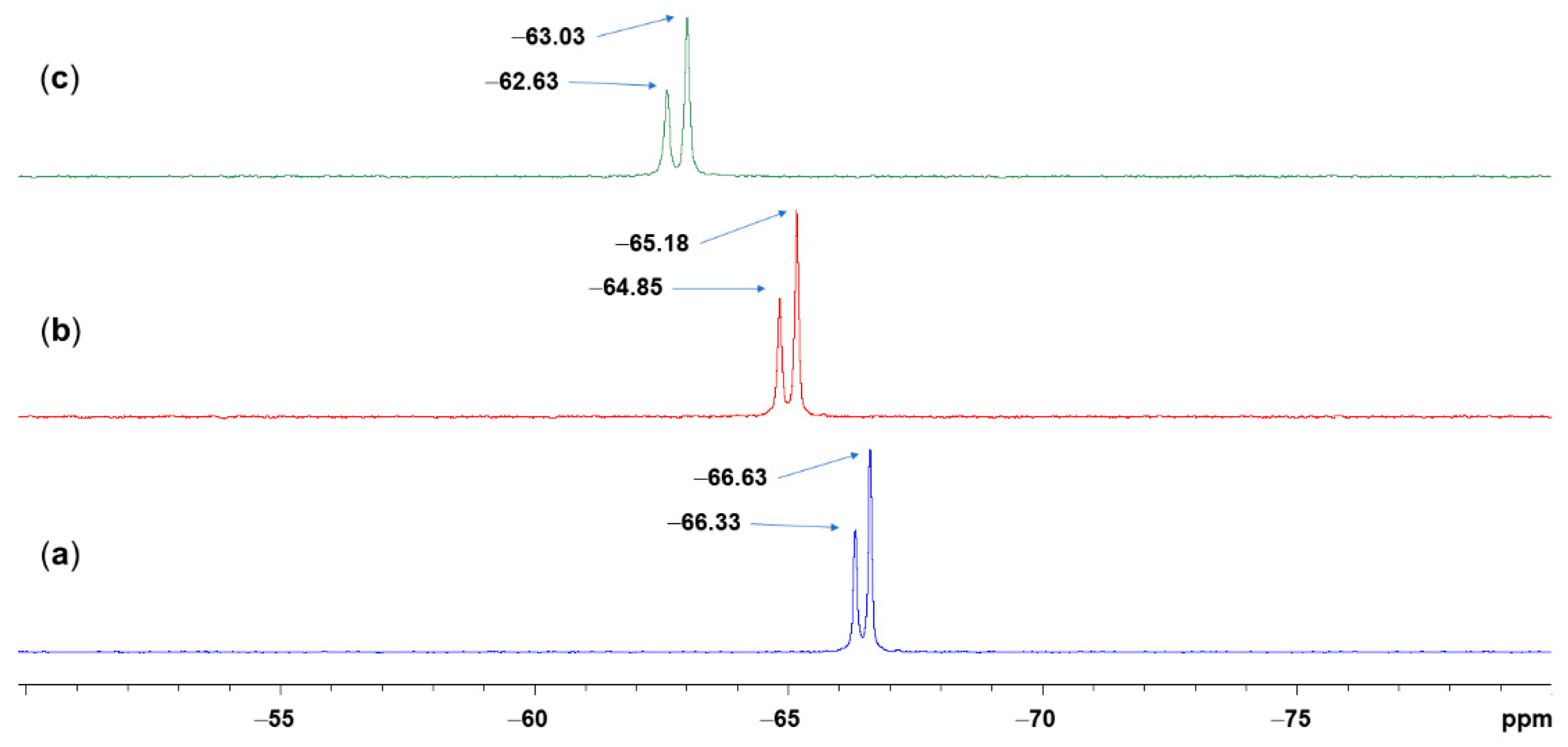
2.3. NMR Spectroscopic Analyses
2.4. Computational Analyses
2.4.1. Calculation of 29Si NMR Shifts
2.4.2. Evaluation of Structural Effects of NMI Coordination and CHCl3 Solvation
2.4.3. Evaluation of Energetic Effects of NMI Coordination and CHCl3 Solvation
3. Materials and Methods
3.1. General Considerations
3.2. Synthesis and Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
| Parameter | (Aib)SiMe2-NMI · CHCl3 1 | (Phg)SiMe2-NMI · 2CHCl3 2 |
|---|---|---|
| Formula | C11H20Cl3N3O2Si | C16H21Cl6N3O2Si |
| Mr | 360.74 | 528.15 |
| T (K) | 220(2) | 180(2) |
| λ (Å) | 0.71073 | 0.71073 |
| Crystal system | monoclinic | orthorhombic |
| Space group | P21/n | P212121 3 |
| a (Å) | 8.2985(3) | 10.1442(2) |
| b (Å) | 11.9564(2) | 15.5169(2) |
| c (Å) | 18.3551(6) | 15.9743(2) |
| β (°) | 96.346(3) | 90 |
| V (Å3) | 1810.04(9) | 2514.46(7) |
| Z | 4 | 4 |
| ρcalc (g·cm−1) | 1.32 | 1.40 |
| μMoKα (mm−1) | 0.6 | 0.7 |
| F(000) | 752 | 1080 |
| θmax(°), Rint | 26.0, 0.0507 | 28.0, 0.0446 |
| Completeness | 99.8% | 99.9% |
| Reflns collected | 26,805 | 65,042 |
| Reflns unique | 3543 | 6075 |
| Restraints | 165 | 206 |
| Parameters | 280 | 385 |
| GoF | 1.160 | 1.097 |
| R1, wR2 [I>2σ(I)] | 0.0472, 0.1185 | 0.0387, 0.0919 |
| R1, wR2 (all data) | 0.0562, 0.1224 | 0.0458, 0.0963 |
| Largest peak/hole (e·Å−3) | 0.19, −0.19 | 0.29, −0.18 |
References
- Davy, J. XVIII. An Account of some Experiments on different Combinations of Fluoric Acid. Philos. Trans. R. Soc. Lond. 1812, 102, 352–369. [Google Scholar] [CrossRef][Green Version]
- Bechstein, O.; Ziemer, B.; Hass, D.; Trojanov, S.I.; Rybakov, V.B.; Maso, G.N. Halogen Exchange on Silicon Halides. XIII. Structure and Reactivity of Silicon Halide-Pyridine Compounds. Z. Anorg. Allg. Chemie 1990, 582, 211–216. [Google Scholar] [CrossRef]
- Sivaramakrishna, A.; Pete, S.; Mhaskar, C.M.; Ramann, H.; Ramanaiah, D.V.; Arbaaz, M.; Niyaz, M.; Janardan, S.; Suman, P. Role of hypercoordinated silicon(IV) complexes in activation of carbon–silicon bonds: An overview on utility in synthetic chemistry. Coord. Chem. Rev. 2023, 485, 215140. [Google Scholar] [CrossRef]
- Singh, G.; Kaur, G.; Singh, J. Progressions in hyper–coordinate silicon complexes. Inorg. Chem. Commun. 2018, 88, 11–20. [Google Scholar] [CrossRef]
- Lemière, G.; Millanvois, A.; Ollivier, C.; Fensterbank, L. A Parisian Vision of the Chemistry of Hypercoordinated Silicon Derivatives. Chem. Rec. 2021, 21, 1119–1129. [Google Scholar] [CrossRef] [PubMed]
- Wagler, J.; Böhme, U.; Kroke, E. Higher-Coordinated Molecular Silicon Compounds. In Functional Molecular Silicon Compounds I—Regular Oxidation States; Scheschkewitz, D., Ed.; Springer: Berlin/Heidelberg, Germany, 2013; Volume 115, pp. 29–105. [Google Scholar] [CrossRef]
- Chuit, C.; Corriu, R.J.P.; Reye, C.; Young, J.C. Reactivity of Penta- and Hexacoordinate Silicon Compounds and Their Role as Reaction Intermediates. Chem. Rev. 1993, 93, 1371–1448. [Google Scholar] [CrossRef]
- Peloquin, D.M.; Schmedake, T.A. Recent advances in hexacoordinate silicon with pyridine-containing ligands: Chemistry and emerging applications. Coord. Chem. Rev. 2016, 323, 107–119. [Google Scholar] [CrossRef]
- Párkányi, L.; Hencsei, P.; Popowski, E. The crystal structures of m-trifluoromethylphenylsilatranone and p-fluorophenylsilatranone. J. Organomet. Chem. 1980, 197, 275–283. [Google Scholar] [CrossRef]
- Fülöp, V.; Kálmán, A.; Hencsei, P.; Csonka, G.; Kovács, I. Structure of l-Methylsilatranone, CH3Si(OCOCH2)(OCH2CH2)2N. Acta Crystallogr. C 1988, 44, 720–723. [Google Scholar] [CrossRef]
- Böhme, U.; Fels, F. A new type of chiral pentacoordinated silicon compounds with azomethine ligands made from acetylacetone and amino acids. Inorg. Chim. Acta 2013, 406, 251–255. [Google Scholar] [CrossRef]
- Schwarzer, S.; Böhme, U.; Fels, S.; Günther, B.; Brendler, E. (S)-N-[(2-hydroxynaphthalen-1-yl)methylidene]valine—A valuable ligand for the preparation of chiral complexes. Inorg. Chim. Acta 2018, 483, 136–147. [Google Scholar] [CrossRef]
- Tacke, R.; Bertermann, R.; Burschka, C.; Dragota, S.; Penka, M.; Richter, I. Spirocyclic Zwitterionic λ5Si-Silicates with Two Bidentate Ligands Derived from α-Amino Acids or α-Hydroxycarboxylic Acids: Synthesis, Structure, and Stereodynamics. J. Am. Chem. Soc. 2004, 126, 14493–14505. [Google Scholar] [CrossRef] [PubMed]
- Dragota, S.; Bertermann, R.; Burschka, C.; Penka, M.; Tacke, R. Diastereo- and Enantiomerically Pure Zwitterionic Spirocyclic λ5Si-[(Ammonio)methyl]silicates with an SiO2N2C Skeleton Containing Two Bidentate Chelate Ligands Derived from α-Amino Acids. Organometallics 2005, 24, 5560–5568. [Google Scholar] [CrossRef]
- Cota, S.; Beyer, M.; Bertermann, R.; Burschka, C.; Götz, K.; Kaupp, M.; Tacke, R. Neutral Penta- and Hexacoordinate Silicon(IV) Complexes Containing Two Bidentate Ligands Derived from the α-Amino Acids (S)-Alanine, (S)-Phenylalanine, and (S)-tert-Leucine. Chem. Eur. J. 2010, 16, 6582–6589. [Google Scholar] [CrossRef]
- Kowalke, J.; Brendler, E.; Wagler, J. Valinate and SiMe2—An interesting couple in pentacoordinate Si-complexes: Templated generation of the dipeptide val-val and formation of an organosilicon-ammonia-adduct. J. Organomet. Chem. 2021, 956, 122126. [Google Scholar] [CrossRef]
- van Leeuwen, S.H.; Quaedflieg, P.J.L.M.; Broxterman, Q.B.; Liskamp, R.M.J. Synthesis of amides from unprotected amino acids by a simultaneous protection–activation strategy using dichlorodialkyl silanes. Tetrahedron Lett. 2002, 43, 9203–9207. [Google Scholar] [CrossRef]
- Eleftheriou, S.; Gatos, D.; Panagopoulos, A.; Stathopoulos, S.; Barlos, K. Attachment of Histidine, Histamine and Urocanic acid to Resins of the Trityl-Type. Tetrahedron Lett. 1999, 40, 2825–2828. [Google Scholar] [CrossRef]
- Hensen, K.; Zengerly, T.; Müller, T.; Pickel, P. Ionic Structures of 4- and 5-coordinated Silicon. Novel Ionic Crystal Structures of 4- and 5-coordinated Silicon: [Me3Si(NMI)]+ Cl–, [Me2HSi(NMI)2]+ Cl–, [Me2Si(NMI)3]2+ 2 Cl–·NMI. Z. Anorg. Allg. Chem. 1988, 558, 21–27. [Google Scholar] [CrossRef]
- Burger, H.; Hensen, K.; Pickel, P. Crystal Structure Determination of N-Trimethylsilyl-N’-Methylimidazolium Bromide. Z. Anorg. Allg. Chem. 1992, 617, 93–95. [Google Scholar] [CrossRef]
- Köckerling, M.; Peppel, T.; Thiele, P.; Verevkin, S.P.; Emel’yanenko, V.N.; Samarov, A.A.; Ruth, W. Easily Vaporizable Ionic Liquids—No Contradiction! Eur. J. Inorg. Chem. 2015, 4032–4037. [Google Scholar] [CrossRef]
- Burger, H.; Hensen, K.; Pickel, P. Synthesis and Crystal Structure Determination of Dimethyldi-(N-Methylimidazo1ium)silicon Bromide. Z. Anorg. Allg. Chem. 1995, 621, 101–104. [Google Scholar] [CrossRef]
- Hensen, K.; Kettner, M.; Pickel, P.; Bolte, M. New Dicationic Silicon Complexes with N-Methylimidazole. Z. Naturforschung B 1999, 54, 200–208. [Google Scholar] [CrossRef]
- Hensen, K.; Gebhardt, F.; Bolte, M. 3,3′-(1-Silacyclohexane-l,l-diyl)bis(1-methylimidazolium) Dibromide Acetonitrile Solvate at 173 K. Acta Crystallogr. C 1998, 54, 1462–1464. [Google Scholar] [CrossRef]
- Hensen, K.; Spangenberg, B.; Bolte, M. The addition-reaction product of 1,1,1,4,4,4-hexachloro-1,4-disilabutane with N-methylimidazole. Acta Crystallogr. C 2000, 56, 1245–1246. [Google Scholar] [CrossRef] [PubMed]
- Hensen, K.; Mayr-Stein, R.; Stumpf, T.; Pickel, P.; Bolte, M.; Fleischer, H. Halogen exchange and expulsion: Ligand stabilized dihalogen silicon dications. J. Chem. Soc., Dalton Trans. 2000, 29, 473–477. [Google Scholar] [CrossRef]
- Hensen, K.; Gebhardt, F.; Bolte, M. Synthesis and Crystal Structure Determination of Tetrakis-(N-Methylimidazolium)-silacyclopentanedichloride·2 NMI. Z. Anorg. Allg. Chem. 1997, 623, 633–636. [Google Scholar] [CrossRef]
- Wagler, J.; Kaempfe, A. (TU Bergakademie Freiberg, Germany). Private Communication to the Cambridge Structure Database. Private Communication, 2014. [Google Scholar] [CrossRef]
- Wagler, J.; Hill, A.F. Templated Rearrangement of Silylated Benzoxazolin-2-ones: A Novel Tridentate (ONO)2– Chelating Ligand System. Organometallics 2007, 26, 3630–3632. [Google Scholar] [CrossRef]
- Herzog, U.; Richter, R.; Brendler, E.; Roewer, G. Methylchlorooligosilanes as products of the basecatalysed disproportionation of various methylchlorodisilanes. J. Organomet. Chem. 1996, 507, 221–228. [Google Scholar] [CrossRef]
- Trommer, K.; Herzog, U.; Schulze, N.; Roewer, G. Disproportionation of chlorodisilanes containing vinyl, diethylamino or phenyl substituents. Main Group Met. Chem. 2001, 24, 425–433. [Google Scholar] [CrossRef]
- Kawano, Y.; Tobita, H.; Ogino, H. [Cp2Fe2(CO)3(μ-SitBu·NMI)]I: The First Silanetriyldiiron Complex. Angew. Chem. Int. Ed. 1991, 30, 843–844. [Google Scholar] [CrossRef]
- Addison, A.W.; Rao, T.N.; Reedijk, J.; van Rijn, J.; Verschoor, G.C. Synthesis, Structure, and Spectroscopic Properties of Copper(II) Compounds containing Nitrogen-Sulphur Donor Ligands; the Crystal and Molecular Structure of Aqua [1,7-bis(N-methylbenzimidazol-2′-yl)-2,6-dithiaheptane]copper(II) Perchlorate. J. Chem. Soc. Dalton. Trans. 1984, 13, 1349–1356. [Google Scholar] [CrossRef]
- Bent, H.A. An Appraisal of Valence-bond Structures and Hybridization in Compounds of the First-row elements. Chem. Rev. 1961, 61, 275–311. [Google Scholar] [CrossRef]
- Ackermann, H.; Leo, R.; Massa, W.; Dehnicke, K. Octaethyl-triphosphadiazenium dimethyltrifluorosilicate, [Et3PNPEt2NPEt3]+[SiF3Me2]−. Z. Anorg. Allg. Chem. 2004, 630, 1205–1209. [Google Scholar] [CrossRef]
- Robertson, A.P.M.; Friedmann, J.N.; Jenkins, H.A.; Burford, N. Exploring structural trends for complexes of Me2E(OSO2CF3)2 (E = Si, Ge, Sn) with pyridine derivatives. Chem. Commun. 2014, 50, 7979–7981. [Google Scholar] [CrossRef]
- Wagler, J.; Hill, A.F. Ring Opening of Organosilicon-Substituted Benzoxazolinone: A Convenient Route to Chelating Ureato and Carbamido Ligands. Organometallics 2008, 27, 6579–6586. [Google Scholar] [CrossRef]
- Gericke, R.; Gerlach, D.; Wagler, J. Ring-Strain-Formation Lewis Acidity? A Pentacoordinate Silacyclobutane Comprising Exclusively Equatorial Si-C Bonds. Organometallics 2009, 28, 6831–6834. [Google Scholar] [CrossRef]
- Brendler, E.; Heine, T.; Hill, A.F.; Wagler, J. A Pentacoordinate Chlorotrimethylsilane Derivative: A very Polar Snapshot of a Nucleophilic Substitution and its Influence on 29Si Solid State NMR Properties. Z. Anorg. Allg. Chem. 2009, 635, 1300–1305. [Google Scholar] [CrossRef]
- Trouton, F. On molecular latent heat. Philos. Mag. 1884, 18, 54–57. [Google Scholar] [CrossRef]
- Rakipov, I.T.; Varfolomeev, M.A.; Kirgizov, A.Y.; Solomonov, B.N. Thermodynamics of the Hydrogen Bonding of Nitrogen-Containing Cyclic and Aromatic Compounds with Proton Donors: The Structure–Property Relationship. Russ. J. Phys. Chem. A 2014, 88, 2023–2028. [Google Scholar] [CrossRef]
- Passarelli, V.; Benetollo, F.; Zanella, P.; Carta, G.; Rossetto, G. Synthesis and characterisation of novel zirconium(IV) derivatives containing the bis-amido ligand SiMe2(NRR′)2. Dalton Trans. 2003, 32, 1411–1418. [Google Scholar] [CrossRef]
- Wagler, J.; Gericke, R. Molecular structures of various alkyldichlorosilanes in the solid state. Dalton Trans. 2017, 46, 8875–8882. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. A 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Program for the Refinement of Crystal Structures; SHELXL-2018/3; University of Göttingen: Göttingen, Germany, 2018. [Google Scholar]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. C 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Farrugia, L.J. ORTEP-3 for windows—A version of ORTEP-III with a graphical user interface (GUI). J. Appl. Crystallogr. 1997, 30, 565. [Google Scholar] [CrossRef]
- Farrugia, L.J. WinGX and ORTEP for Windows: An update. J. Appl. Crystallogr. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- POV-RAY. Version 3.7. Trademark of Persistence of Vision Raytracer Pty. Ltd.: Williamstown, Australia; Copyright Hallam Oaks Pty. Ltd.: Melbourne, Australia, 1994–2004. Available online: http://www.povray.org/download/ (accessed on 28 June 2021).
- Neese, F. Software update: The ORCA program system—Version 5.0. WIREs Comput. Mol. Sci. 2022, 8, e1606. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef] [PubMed]
- Pantazis, D.A.; Neese, F. All-electron basis sets for heavy elements. WIREs Comput. Mol. Sci. 2014, 4, 363–374. [Google Scholar] [CrossRef]
- van Lenthe, E.; Baerends, E.J.; Snijders, J.G. Relativistic regular two-component Hamiltonians. J. Chem. Phys. 1993, 99, 4597. [Google Scholar] [CrossRef]
- van Wüllen, C. Molecular density functional calculations in the regular relativistic approximation: Method, application to coinage metal diatomics, hydrides, fluorides and chlorides, and comparison with first-order relativistic calculations. J. Chem. Phys. 1998, 109, 392. [Google Scholar] [CrossRef]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [PubMed]
- Chemcraft, Version 1.8 (Build 164). 2016. Available online: http://www.chemcraftprog.com/ (accessed on 19 September 2015).
- Braddock, D.C.; Davies, J.J.; Lickiss, P.D. Methyltrimethoxysilane (MTM) as a Reagent for Direct Amidation of Carboxylic Acids. Org. Lett. 2022, 24, 1175–1179. [Google Scholar] [CrossRef] [PubMed]
- Braddock, D.C.; Rowley, B.C.; Lickiss, P.D.; Fussell, S.J.; Qamar, R.; Pugh, D.; Rzepa, H.S.; White, A.J.P. On the Use of Triarylsilanols as Catalysts for Direct Amidation of Carboxylic Acids. J. Org. Chem. 2023, 88, 9853–9869. [Google Scholar] [CrossRef]

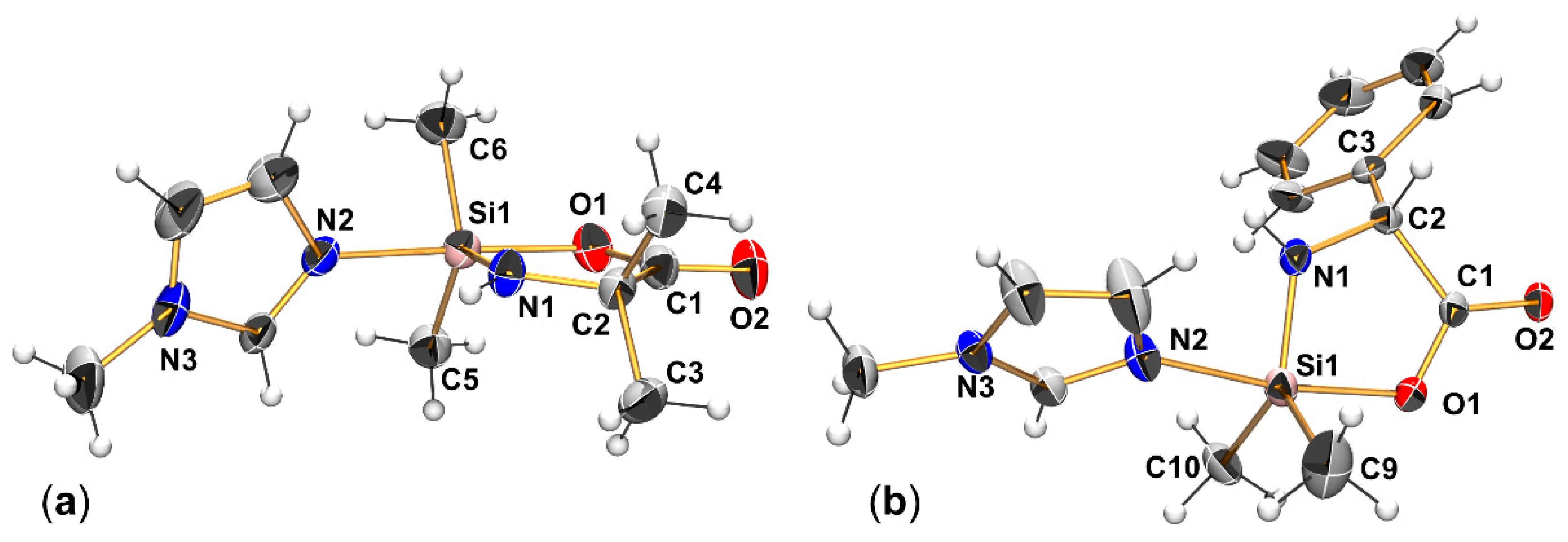
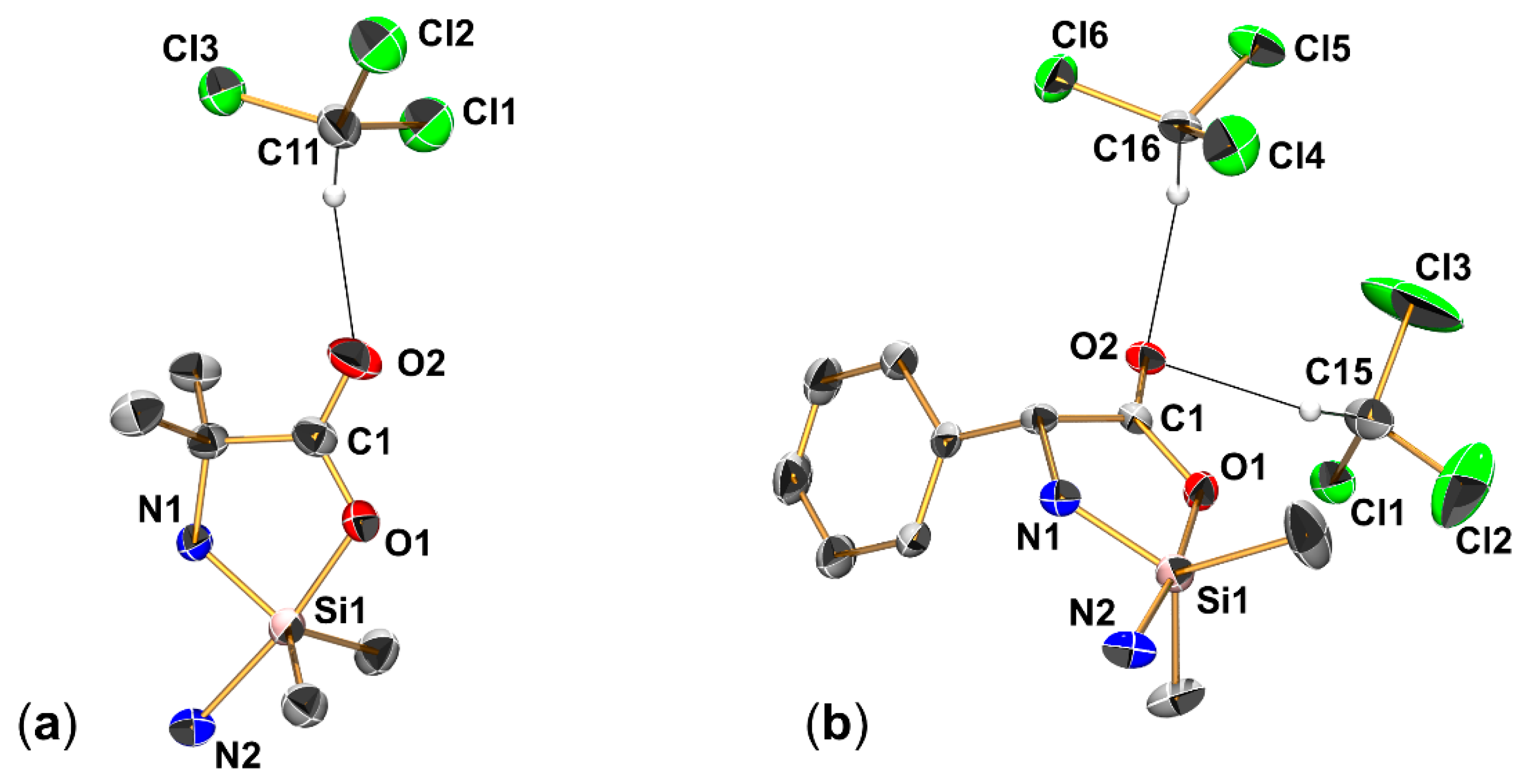

| (Aib)SiMe2-NMI | (Phg)SiMe2-NMI | XVII | |
|---|---|---|---|
| Si1–O1 | 1.852(2) | 1.873(2) | 1.877(1) |
| Si1–N1 | 1.713(2) | 1.716(3) | 1.717(2) |
| Si1–N2 | 2.036(2) | 2.011(2) | 2.014(2) |
| Si1–C 1 | 1.864(3) | 1.859(4) | 1.870(2) |
| 1.867(3) | 1.873(3) | 1.876(2) | |
| C1–O1 | 1.291(3) | 1.290(3) | 1.302(2) |
| C1–O2 | 1.227(3) | 1.223(3) | 1.226(2) |
| O1-Si1-N1 | 85.25(9) | 84.69(10) | 84.93(5) |
| O1-Si1-N2 | 172.33(16) | 171.27(10) | 171.08(6) |
| C-Si1-C 1 | 113.92(14) | 115.2(3) | 113.44(9) |
| N1-Si1-C 1 | 122.69(13) | 120.98(16) | 121.93(8) |
| 123.38(12) | 123.8(2) | 124.63(8) | |
| τ5 | 0.816 | 0.791 | 0.774 |
| Amino Acid | (tBuNH)2SiMe2 1 | NMI | |
|---|---|---|---|
| (Aib)SiMe2-NMI-2 | 300, 2.90 | 621, 3.07 | 504, 6.10 |
| (Aib)SiMe2-NMI-4 | 300, 2.90 | 625, 3.09 | 1002, 12.20 |
| (Aib)SiMe2-NMI-4-dil 2 | 300, 2.90 | 625, 3.09 | 1002, 12.20 |
| (Phg)SiMe2-NMI-4 | 438, 2.90 | 633, 3.13 | 1005, 12.24 |
| (Val)SiMe2-NMI-4 | 340, 2.90 | 629, 3.11 | 1012, 12.33 |
| (Amac) | δ29Sicalc((Amac)SiMe2-CHCl3) | δ29Sicalc((Amac)SiMe2-NMI-CHCl3) | δ29Siexp | X((Amac)SiMe2-NMI-CHCl3) |
|---|---|---|---|---|
| (Aib) | +25.0 | −77.6 | −62.5 | 0.853 |
| (Phg) | +29.8 | −77.1 | −65.9 | 0.895 |
| (Val) | +32.6 | −74.6 | −58.9 | 0.854 |
| (Aib)SiMe2 | (Aib)SiMe2-CHCl3 | (Phg)SiMe2 | (Phg)SiMe2-CHCl3 | (Val)SiMe2 | (Val)SiMe2-CHCl3 | ØΔ | |
|---|---|---|---|---|---|---|---|
| C1–O2 | 1.201 | 1.205 | 1.199 | 1.202 | 1.201 | 1.205 | +0.004 |
| C1–O1 | 1.337 | 1.330 | 1.335 | 1.330 | 1.340 | 1.333 | −0.006 |
| C1–C2 | 1.534 | 1.533 | 1.536 | 1.533 | 1.526 | 1.524 | −0.002 |
| Si1–O1 | 1.704 | 1.706 | 1.704 | 1.708 | 1.702 | 1.707 | +0.004 |
| Si1–N1 | 1.712 | 1.710 | 1.711 | 1.710 | 1.714 | 1.712 | −0.002 |
| Si1–C3/3′ | 1.850 | 1.849 | 1.850 | 1.849 | 1.849 | 1.848 | −0.001 |
| 1.853 | 1.852 | 1.851 | 1.849 | 1.854 | 1.852 | ||
| Cl3CH···O2 | - | 2.057 | - | 2.107 | - | 2.051 | - |
| (Aib)SiMe2-NMI | (Aib)SiMe2-NMI-CHCl3 | (Phg)SiMe2-NMI | (Phg)SiMe2-NMI-CHCl3 | (Val)SiMe2-NMI | (Val)SiMe2-NMI-CHCl3 | ØΔ | |
|---|---|---|---|---|---|---|---|
| C1–O2 | 1.215 | 1.220 | 1.213 | 1.218 | 1.215 | 1.220 | +0.005 |
| C1–O1 | 1.301 | 1.295 | 1.299 | 1.293 | 1.304 | 1.297 | −0.006 |
| C1–C2 | 1.529 | 1.528 | 1.533 | 1.529 | 1.523 | 1.521 | −0.002 |
| Si1–O1 | 1.820 | 1.828 | 1.825 | 1.835 | 1.815 | 1.830 | +0.011 |
| Si1–N1 | 1.728 | 1.728 | 1.732 | 1.731 | 1.731 | 1.734 | +0.001 |
| Si1–N2 | 2.079 | 2.061 | 2.067 | 2.055 | 2.077 | 2.063 | −0.015 |
| Si1–C3/3′ | 1.878 | 1.878 | 1.878 | 1.876 | 1.878 | 1.876 | −0.001 |
| 1.880 | 1.879 | 1.879 | 1.877 | 1.879 | 1.879 | ||
| Cl3CH···O2 1 | - | 1.966 (0.091) | - | 2.017 (0.090) | - | 1.984 (0.067) | - |
| (Amac) | (Amac)SiMe2-NMI | (Amac)SiMe2-CHCl3 | (Amac)SiMe2-NMI-CHCl3 | (Amac)SiMe2-NMI-CHCl3 Eff. 1 | (Amac)SiMe2-NMI-CHCl3 Coop. 2 |
|---|---|---|---|---|---|
| (Aib) | −10.9 (3.5) | −4.9 (7.7) | −16.8 (10.9) | −6.8 (−3.8) | −1.0 (−0.3) |
| (Phg) | −11.9 (3.2) | −5.6 (7.7) | −18.6 (10.2) | −7.9 (−4.4) | −1.1 (−0.7) |
| (Val) | −11.1 (4.0) | −5.3 (8.3) | −17.5 (10.2) | −7.0 (−5.1) | −1.1 (−2.1) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seidel, A.; Gericke, R.; Kutzner, B.; Wagler, J. Lewis Acid-Base Adducts of α-Amino Acid-Derived Silaheterocycles and N-Methylimidazole. Molecules 2023, 28, 7816. https://doi.org/10.3390/molecules28237816
Seidel A, Gericke R, Kutzner B, Wagler J. Lewis Acid-Base Adducts of α-Amino Acid-Derived Silaheterocycles and N-Methylimidazole. Molecules. 2023; 28(23):7816. https://doi.org/10.3390/molecules28237816
Chicago/Turabian StyleSeidel, Anne, Robert Gericke, Beate Kutzner, and Jörg Wagler. 2023. "Lewis Acid-Base Adducts of α-Amino Acid-Derived Silaheterocycles and N-Methylimidazole" Molecules 28, no. 23: 7816. https://doi.org/10.3390/molecules28237816
APA StyleSeidel, A., Gericke, R., Kutzner, B., & Wagler, J. (2023). Lewis Acid-Base Adducts of α-Amino Acid-Derived Silaheterocycles and N-Methylimidazole. Molecules, 28(23), 7816. https://doi.org/10.3390/molecules28237816







