Synthesis, Structure, and Characterizations of a Volatile/Soluble Heterometallic Hexanuclear Precursor [NaMn2(thd)4(OAc)]2
Abstract
:1. Introduction
2. Results and Discussion
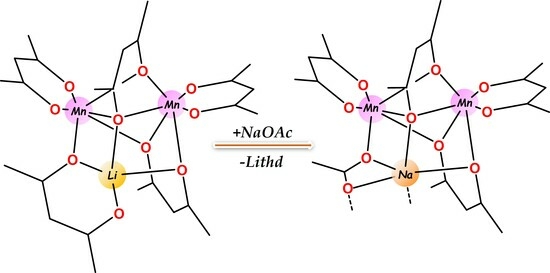
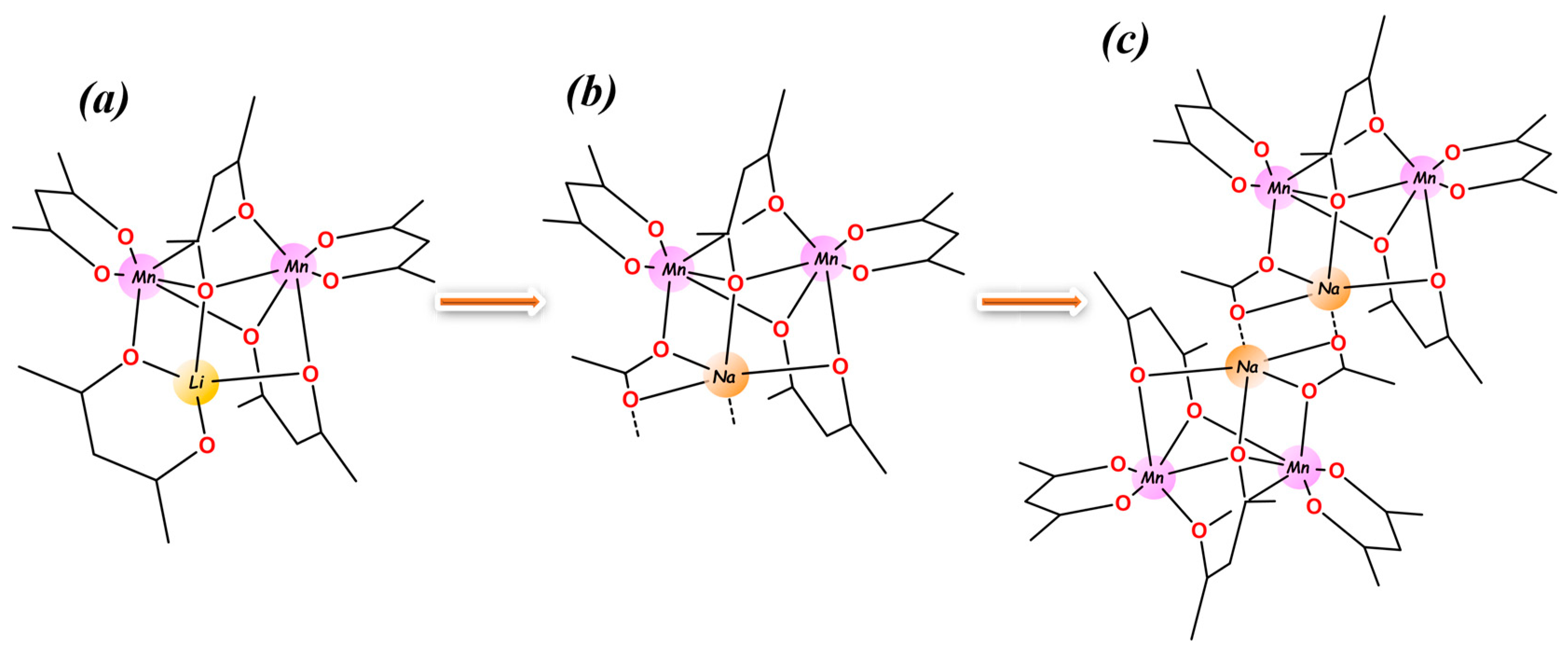
2.1. Design of the Heterometallic Precursor
2.2. Synthesis and Properties of Heterometallic Precursor
2.3. Molecular Structure of Hexanuclear Precursor 1
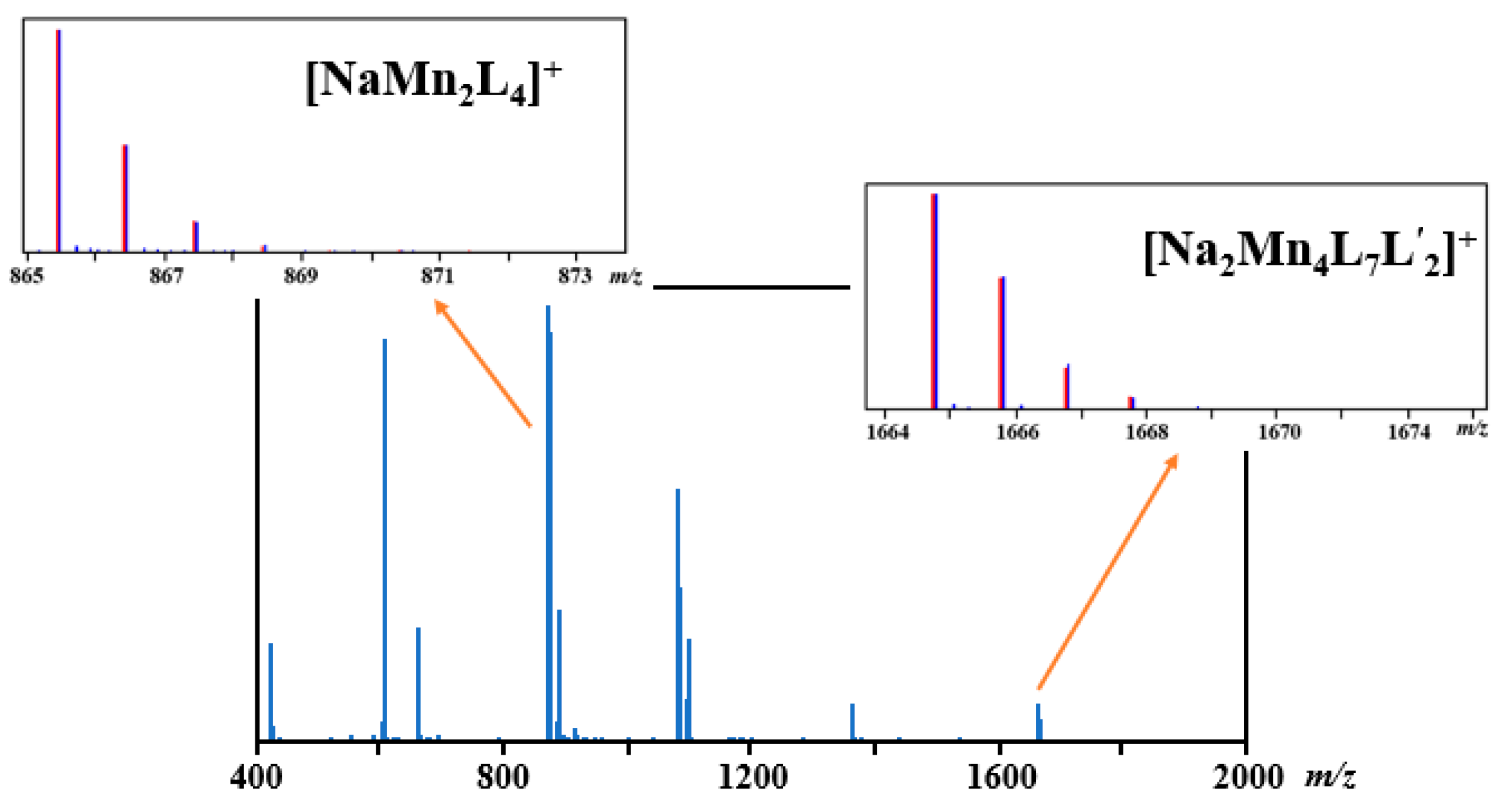
2.4. Direct Analysis of Real Time (DART) Mass Spectrometry of Complex 1
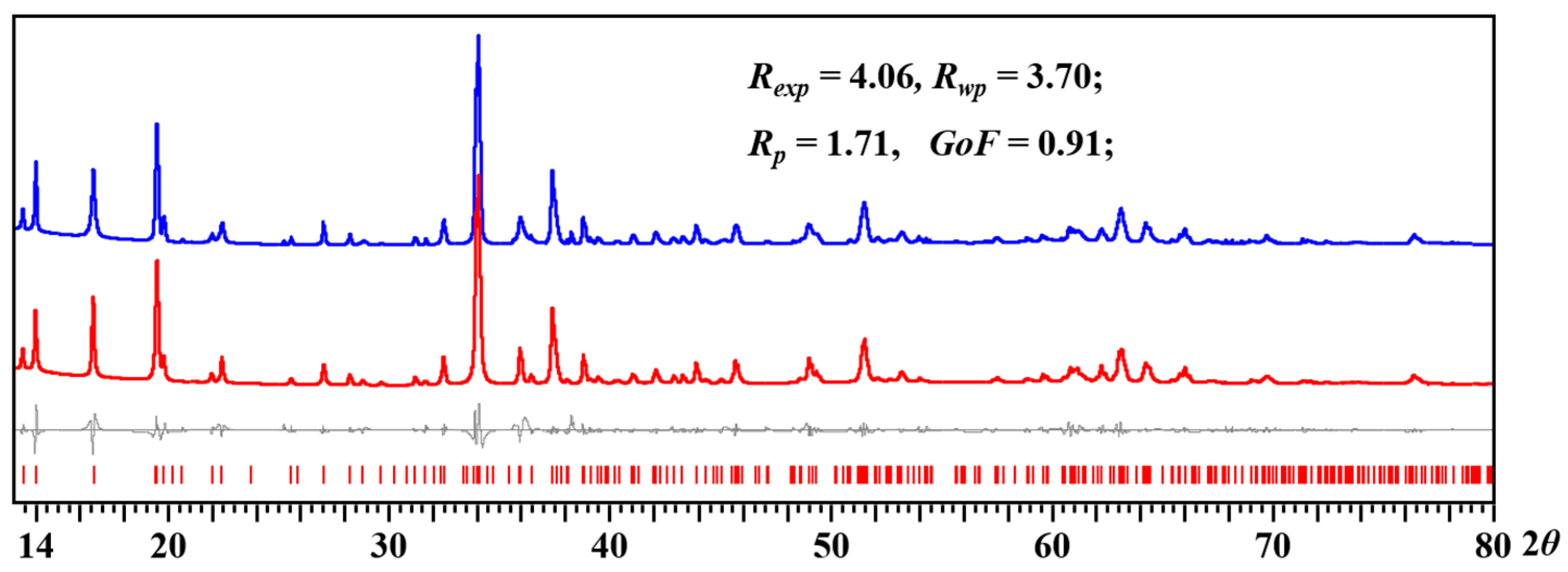
2.5. Thermal Decomposition of Heterometallic Precursor
3. Materials and Methods
3.1. Materials and Measurements
3.2. Synthesis of Hexanuclear Precursor
3.3. Single-Crystal X-ray Investigation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Navulla, A.; Huynh, L.; Wei, Z.; Filatov, A.S.; Dikarev, E.V. Volatile Single-Source Molecular Precursor for the Lithium-Ion Battery Cathode. J. Am. Chem. Soc. 2012, 134, 5762–5765. [Google Scholar] [CrossRef]
- Han, H.; Wei, Z.; Filatov, A.S.; Carozza, J.C.; Alkan, M.; Rogachev, A.Y.; Shevtsov, A.; Abakumov, A.M.; Pak, C.; Shatruk, M.; et al. Three to Tango Requires a Site-Specific Substitution: Hetero Tri Metallic Molecular Precursors for High-Voltage Rechargeable Batteries. Chem. Sci. 2019, 10, 524–534. [Google Scholar] [CrossRef] [PubMed]
- Shevtsov, A.; Han, H.; Morozov, A.; Carozza, J.C.; Savina, A.A.; Shakhova, I.; Khasanova, N.R.; Antipov, E.V.; Dikarev, E.V.; Abakumov, A.M. Protective Spinel Coating for Li1.17Ni0.17Mn0.50Co0.17O2 Cathode for Li-Ion Batteries through Single-Source Precursor Approach. Nanomaterials 2020, 10, 1870. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.L.; Chen, H.Y.; Wen, J.B.; Li, D.X. Electrochemical Properties of Spinel LiCoxMn2-xO4 Prepared by Sol–Gel Process. J. Alloys Compd. 2009, 474, 473–476. [Google Scholar] [CrossRef]
- Dokko, K.; Anzue, N.; Mohamedi, M.; Itoh, T.; Uchida, I. Raman Spectro-Electrochemistry of LiCoxMn2-xO4 Thin Film Electrodes for 5 V Lithium Batteries. Electrochem. Commun. 2004, 6, 384–388. [Google Scholar] [CrossRef]
- Mukai, K.; Uyama, T. Toward Positive Electrode Materials with High-Energy Density: Electrochemical and Structural Studies on LiCoxMn2–xO4 with 0 ≤ x ≤ 1. ACS Omega 2017, 2, 5142–5149. [Google Scholar] [CrossRef] [PubMed]
- Whitacre, J.F.; Tevar, A.; Sharma, S. Na4Mn9O18 as a Positive Electrode Material for an Aqueous Electrolyte Sodium-Ion Energy Storage Device. Electrochem. Commun. 2010, 12, 463–466. [Google Scholar] [CrossRef]
- Tevar, A.D.; Whitacre, J.F. Relating Synthesis Conditions and Electrochemical Performance for the Sodium Intercalation Compound Na4Mn9O18 in Aqueous Electrolyte. J. Electrochem. Soc. 2010, 157, A870–A875. [Google Scholar] [CrossRef]
- Sauvage, F.; Laffont, L.; Tarascon, J.-M.; Baudrin, E. Study of the Insertion/Deinsertion Mechanism of Sodium into Na0.44MnO2. Inorg. Chem. 2007, 46, 3289–3294. [Google Scholar] [CrossRef]
- Zhang, Y.; Ouyang, Y.; Liu, L.; Xia, J.; Nie, S.; Liu, W.; Wang, X.-Y. Synthesis and Characterization of Na0.44MnO2 Nanorods/Graphene Composite as Cathode Materials for Sodium-Ion Batteries. J. Cent. South Univ. 2019, 26, 1510–1520. [Google Scholar] [CrossRef]
- Hwang, J.-Y.; Myung, S.-T.; Sun, Y.-K. Sodium-Ion Batteries: Present and Future. Chem. Soc. Rev. 2017, 46, 3529–3614. [Google Scholar] [CrossRef] [PubMed]
- Yabuuchi, N.; Kubota, K.; Dahbi, M.; Komaba, S. Research Development on Sodium-Ion Batteries. Chem. Rev. 2014, 114, 11636–11682. [Google Scholar] [CrossRef] [PubMed]
- Datta, M.K.; Kuruba, R.; Jampani, P.H.; Chung, S.J.; Saha, P.; Epur, R.; Kadakia, K.; Patel, P.; Gattu, B.; Manivannan, A.; et al. Electrochemical Properties of a New Nanocrystalline NaMn2O4 Cathode for Rechargeable Sodium Ion Batteries. Mater. Sci. Eng. B Solid State Mater. Adv. Technol. 2014, 188, 1–7. [Google Scholar] [CrossRef]
- Akimoto, J.; Awaka, J.; Kijima, N.; Takahashi, Y.; Maruta, Y.; Tokiwa, K.; Watanabe, T. High-Pressure Synthesis and Crystal Structure Analysis of NaMn2O4 with the Calcium Ferrite-Type Structure. J. Solid State Chem. 2006, 179, 169–174. [Google Scholar] [CrossRef]
- Han, H.; Carozza, J.C.; Colliton, A.P.; Zhang, Y.; Wei, Z.; Filatov, A.S.; Chen, Y.-S.; Alkan, M.; Rogachev, A.Y.; Dikarev, E.V. Heterotrimetallic Mixed-Valent Molecular Precursors Containing Periodic Table Neighbors: Assignment of Metal Positions and Oxidation States. Angew. Chem. Int. Ed. Engl. 2020, 59, 9624–9630. [Google Scholar] [CrossRef]
- Ta, A.T.; Nguyen, V.N.; Nguyen, T.T.O.; Le, H.C.; Le, D.T.; Dang, T.C.; Man, M.T.; Nguyen, S.H.; Pham, D.L. Hydrothermal Synthesis of Na4Mn9O18 Nanowires for Sodium Ion Batteries. Ceram. Int. 2019, 45, 17023–17028. [Google Scholar] [CrossRef]
- Yin, F.; Liu, Z.; Zhao, Y.; Feng, Y.; Zhang, Y. Electrochemical Properties of an Na4Mn9O18-Reduced Graphene Oxide Composite Synthesized via Spray Drying for an Aqueous Sodium-Ion Battery. Nanomaterials 2017, 7, 253. [Google Scholar] [CrossRef]
- Yuan, G.; Xiang, J.; Jin, H.; Jin, Y.; Wu, L.; Zhang, Y.; Mentbayeva, A.; Bakenov, Z. Flexible Free-Standing Na4Mn9O18/Reduced Graphene Oxide Composite Film as a Cathode for Sodium Rechargeable Hybrid Aqueous Battery. Electrochim. Acta 2018, 259, 647–654. [Google Scholar] [CrossRef]
- Kwamen, A.C.N.; Jenniches, J.; Oppel, I.M.; Albrecht, M. Solvent Dependence of the Monomer–Dimer Equilibrium of Ketone-substituted Triscatecholate Titanium(IV) Complexes. Chemistry 2020, 26, 10550–10554. [Google Scholar] [CrossRef]
- Piarulli, U.; Rogers, A.J.; Floriani, C.; Gervasio, G.; Viterbo, D. Metallohosts Derived from the Assembly of Sugars around Transition Metals: The Complexation of Alkali Metal Cations. Inorg. Chem. 1997, 36, 6127–6133. [Google Scholar] [CrossRef]
- Marsh, R.E.; Clemente, D.A. A Survey of Crystal Structures Published in the Journal of the American Chemical Society. Inorg. Chim. Acta 2007, 360, 4017–4024. [Google Scholar] [CrossRef]
- Kochelakov, D.V.; Vikulova, E.S.; Kuratieva, N.V.; Sukhikh, A.S.; Gromilov, S.A. Study of Potassium, Rubidium Hexafluoroacetylacetonates and by-Products of Their Synthesis and Crystallization. J. Struct. Chem. 2023, 64, 82–96. [Google Scholar] [CrossRef]
- Roitershtein, D.M.; Puntus, L.N.; Lyssenko, K.A.; Taidakov, I.V.; Varaksina, E.A.; Minyaev, M.E.; Gerasin, V.A.; Guseva, M.A.; Vinogradov, A.A.; Savchenko, M.S.; et al. An Efficient Route for Design of Luminescent Composite Materials Based on Polyethylene Containing Europium Dibenzoylmethanate. New J. Chem. 2017, 41, 13663–13672. [Google Scholar] [CrossRef]
- Bilyachenko, A.N.; Gutsul, E.I.; Khrustalev, V.N.; Astakhov, G.S.; Zueva, A.Y.; Zubavichus, Y.V.; Kirillova, M.V.; Shul’pina, L.S.; Ikonnikov, N.S.; Dorovatovskii, P.V.; et al. Acetone Factor in the Design of Cu4-, Cu6-, and Cu9-Based Cage Coppersilsesquioxanes: Synthesis, Structural Features, and Catalytic Functionalization of Alkanes. Inorg. Chem. 2022, 61, 14800–14814. [Google Scholar] [CrossRef] [PubMed]
- Barrans, Y.; Alléaume, M.; David, L. Complexe de Sodium de l’ionophore Nigéricine. Acta Crystallogr. B 1980, 36, 936–938. [Google Scholar] [CrossRef]
- Han, H.; Zhou, Z.; Carozza, J.C.; Lengyel, J.; Yao, Y.; Wei, Z.; Dikarev, E.V. From Lithium to Sodium: Design of Heterometallic Molecular Precursors for the NaMO2 Cathode Materials. Chem. Commun. 2019, 55, 7243–7246. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Carozza, J.C.; Zhou, Z.; Zhang, Y.; Wei, Z.; Abakumov, A.M.; Filatov, A.S.; Chen, Y.-S.; SantaLucia, D.J.; Berry, J.F.; et al. Heterotrimetallic Precursor with 2:2:1 Metal Ratio Requiring at Least a Pentanuclear Molecular Assembly. J. Am. Chem. Soc. 2020, 142, 12767–12776. [Google Scholar] [CrossRef]
- Lam, T.C.H.; Mak, W.-L.; Wong, W.-L.; Kwong, H.-L.; Sung, H.H.Y.; Lo, S.M.F.; Williams, I.D.; Leung, W.-H. Synthesis and Crystal Structure of a Chiral C3-Symmetric Oxygen Tripodal Ligand and Its Applications to Asymmetric Catalysis. Organometallics 2004, 23, 1247–1252. [Google Scholar] [CrossRef]
- Barrios, L.A.; Borilovic, I.; Salinas Uber, J.; Aguilà, D.; Roubeau, O.; Aromí, G. A New Type of Paddle-Wheel Coordination Complex. Dalton Trans. 2013, 42, 12185. [Google Scholar] [CrossRef]
- Dorkov, P.; Pantcheva, I.N.; Sheldrick, W.S.; Mayer-Figge, H.; Petrova, R.; Mitewa, M. Synthesis, Structure and Antimicrobial Activity of Manganese(II) and Cobalt(II) Complexes of the Polyether Ionophore Antibiotic Sodium Monensin A. J. Inorg. Biochem. 2008, 102, 26–32. [Google Scholar] [CrossRef]
- Wei, Z.; Han, H.; Filatov, A.S.; Dikarev, E.V. Changing the Bridging Connectivity Pattern within a Heterometallic Assembly: Design of Single-Source Precursors with Discrete Molecular Structures. Chem. Sci. 2014, 5, 813–818. [Google Scholar] [CrossRef]
- Han, H.; Zhang, Y.; Zhou, Z.; Carozza, J.C.; Wei, Z.; Filatov, A.S.; Shevtsov, A.; Abakumov, A.M.; Dikarev, E.V. Multi-Functional Single-Source Molecular Precursors for Carbon-Coated Mixed-Metal Phosphates. Inorg. Chem. 2023, 62, 12931–12939. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Yang, K.; Liu, J.; Hu, X.; Kong, D.; Liu, T.; Zhang, M.; Pan, F. A Heterobimetallic Single-Source Precursor Enabled Layered Oxide Cathode for Sodium-Ion Batteries. Chem. Commun. 2018, 54, 10714–10717. [Google Scholar] [CrossRef] [PubMed]
- Troyanov, S.I.; Gorbenko, O.Y.; Bosak, A.A. Synthesis, Crystal Structure and Properties of Manganese(II) Hexafluoroacetylacetonates Mn(Hfa)2(H2O)2 and KMn(Hfa)3. Polyhedron 1999, 18, 3505–3509. [Google Scholar] [CrossRef]
- Alvarez, S.; Avnir, D.; Llunell, M.; Pinsky, M. Continuous Symmetry Maps and Shape Classification. The Case of Six-Coordinated Metal Compounds. New J. Chem. 2002, 26, 996–1009. [Google Scholar] [CrossRef]
- Llunell, M. SHAPE, Version 2.1; Universitat de Barcelona: Barcelona, Spain, 2013.
- Alvarez, S.; Llunell, M. Continuous Symmetry Measures of Penta-Coordinate Molecules: Berry and Non-Berry Distortions of the Trigonal Bipyramid. J. Chem. Soc. Dalton Trans. 2000, 19, 3288–3303. [Google Scholar] [CrossRef]
- Chu, Q.; Wang, X.; Li, Q.; Liu, X. The Tunnel Manganese Oxide Na4.32Mn9O18: A New Na+Site Discovered by Single-Crystal X-Ray Diffraction. Acta Crystallogr. C Struct. Chem. 2011, 67, i10–i12. [Google Scholar] [CrossRef]
- SAINT, version 2017.3-0. Part of Bruker APEX3 Software Package. Bruker AXS: Billerica, MA, USA, 2017.
- SADABS, version 2017.3-0. Part of Bruker APEX3 Software Package. Bruker AXS: Billerica, MA, USA, 2017.
- Sheldrick, G.M. SHELXT—Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
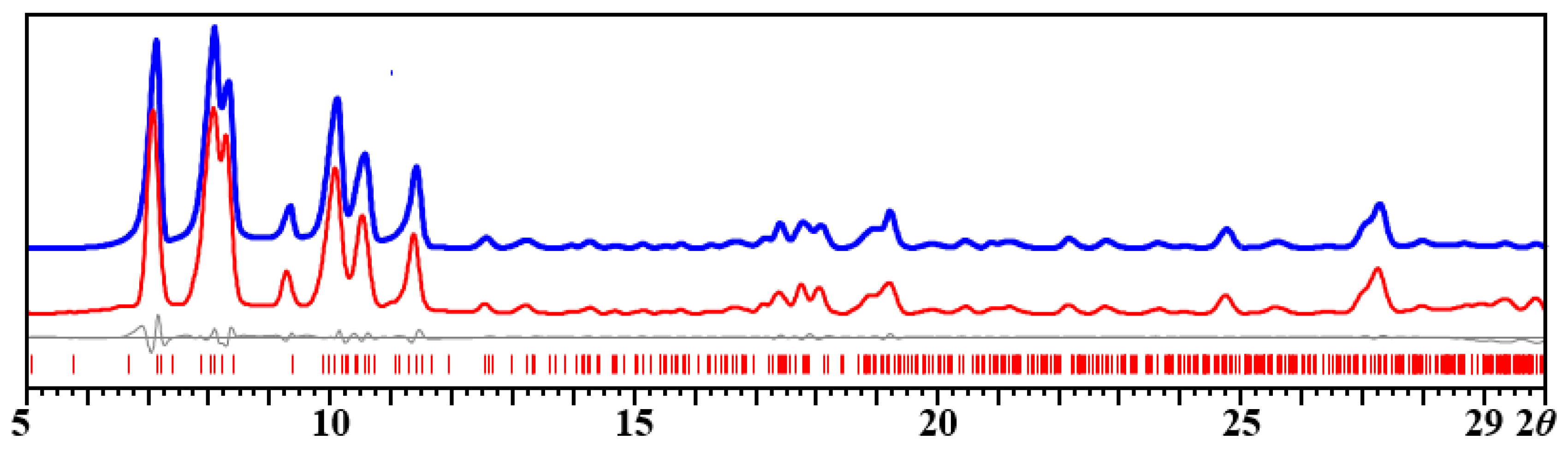


| Single Crystal Data (−173 °C) | Le Bail Fit (20 °C) | |
|---|---|---|
| Space group | P-1 | P-1 |
| a (Å) | 12.466 (2) | 12.9279 (14) |
| b (Å) | 18.285 (4) | 18.531 (2) |
| c (Å) | 24.847 (5) | 25.105 (3) |
| α (°) | 81.650 (2) | 81.533 (10) |
| β (°) | 89.291 (2) | 90.033 (12) |
| γ (°) | 71.240 (2) | 71.758 (10) |
| V (Å3) | 5302.6 (18) | 5642.7 (11) |
| Average Distances (Å) | [NaMn2(thd)4(OAc)]2 | [LiMn2(thd)5] |
|---|---|---|
| Mn1–O * | 2.069 (3) | 2.069 (3) |
| Mn1–O ** | 2.290 (3) | 2.253 (3) |
| Mn2–O * | 2.061 (3) | 2.060 (3) |
| Mn2–O ** | 2.195 (3) | 2.209 (3) |
| A–O | 2.367 (3) | 1.966 (7) |
| Mn1···Mn2 | 3.105 (3) | 3.161 (3) |
| Mn1···A | 3.424 (3) | 2.884 (3) |
| Mn2···A | 3.405 (2) | 2.939 (3) |
| Angles (°) | ||
| Mn1–A–Mn2 | 54.08 (3) | 65.76 (2) |
| A–Mn1–Mn2 | 62.56 (3) | 57.95 (2) |
| A–Mn2–Mn1 | 63.27 (3) | 56.29 (2) |
| Fragments | Calc. m/z | Exp. m/z | Δ | Relative Int. (%) |
|---|---|---|---|---|
| [Na2Mn4L7L’2]+ | 1665.728 | 1665.738 | 0.010 | 9.0 |
| [Na2Mn3L6L’]+ | 1368.638 | 1368.647 | 0.009 | 8.9 |
| [Mn3L5(H2O)]+ | 1098.517 | 1098.550 | 0.033 | 24.1 |
| [Mn3L5]+ | 1080.507 | 1080.521 | 0.014 | 58.4 |
| [NaMn2L4(H2O)]+ | 883.430 | 883.850 | 0.010 | 30.4 |
| [NaMn2L4]+ | 865.420 | 865.424 | 0.004 | 100 |
| [Mn2L3]+ | 659.292 | 659.304 | 0.012 | 26.6 |
| [H2MnL3]+ | 606.369 | 606.377 | 0.012 | 92.5 |
| [HMnL2]+ | 422.223 | 422.239 | 0.016 | 22.5 |
| Le Bail Fit (20 °C) | PDF-2 Database [38] | |
|---|---|---|
| Space group | Pbam | Pbam |
| a (Å) | 9.096 (2) | 9.084 (4) |
| b (Å) | 26.371 (10) | 26.311 (10) |
| c (Å) | 2.826 (3) | 2.8223 (11) |
| V (Å3) | 677.9 (7) | 674.6 (8) |
| Compound | [NaMn2(thd)4(OAc)]2 |
|---|---|
| CCDC | 2,301,967 |
| Empirical formula | C92H158Mn4Na2O20 |
| Formula weight | 1849.91 |
| Temperature (K) | 100 (2) |
| Wavelength (Ǻ) | 0.71073 |
| Crystal system | Triclinic |
| Space group | P-1 |
| a (Å) | 12.466 (2) |
| b (Å) | 18.285 (3) |
| c (Å) | 24.847 (4) |
| α (°) | 81.650 (2) |
| β (°) | 89.291 (3) |
| γ (°) | 71.240 (2) |
| V (Å3) | 5302.6 (16) |
| Z | 2 |
| ρcalcd (g·cm−3) | 1.159 |
| μ (mm−1) | 0.533 |
| F (000) | 1984 |
| Crystal size (mm3) | 0.15 × 0.06 × 0.03 |
| θ range for data collection (°) | 1.548–28.069 |
| Reflections collected | 45,149 |
| Independent reflections | 23,476 |
| Transmission factors (min/max) | 0.6255/0.7457 |
| Completeness to full θ (%) | 98.4 |
| Data/restraints/params. | 23,476/729/1378 |
| R1, a wR2 b (I > 2σ(I)) | 0.0510/0.1240 |
| R1, a wR2 b (all data) | 0.0940/0.1432 |
| Quality-of-fit c | 0.975 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Wei, Z.; Dikarev, E.V. Synthesis, Structure, and Characterizations of a Volatile/Soluble Heterometallic Hexanuclear Precursor [NaMn2(thd)4(OAc)]2. Molecules 2023, 28, 7795. https://doi.org/10.3390/molecules28237795
Zhang Y, Wei Z, Dikarev EV. Synthesis, Structure, and Characterizations of a Volatile/Soluble Heterometallic Hexanuclear Precursor [NaMn2(thd)4(OAc)]2. Molecules. 2023; 28(23):7795. https://doi.org/10.3390/molecules28237795
Chicago/Turabian StyleZhang, Yuxuan, Zheng Wei, and Evgeny V. Dikarev. 2023. "Synthesis, Structure, and Characterizations of a Volatile/Soluble Heterometallic Hexanuclear Precursor [NaMn2(thd)4(OAc)]2" Molecules 28, no. 23: 7795. https://doi.org/10.3390/molecules28237795
APA StyleZhang, Y., Wei, Z., & Dikarev, E. V. (2023). Synthesis, Structure, and Characterizations of a Volatile/Soluble Heterometallic Hexanuclear Precursor [NaMn2(thd)4(OAc)]2. Molecules, 28(23), 7795. https://doi.org/10.3390/molecules28237795