Recent Advances in Nanotechnology-Based Targeted Delivery Systems of Active Constituents in Natural Medicines for Cancer Treatment
Abstract
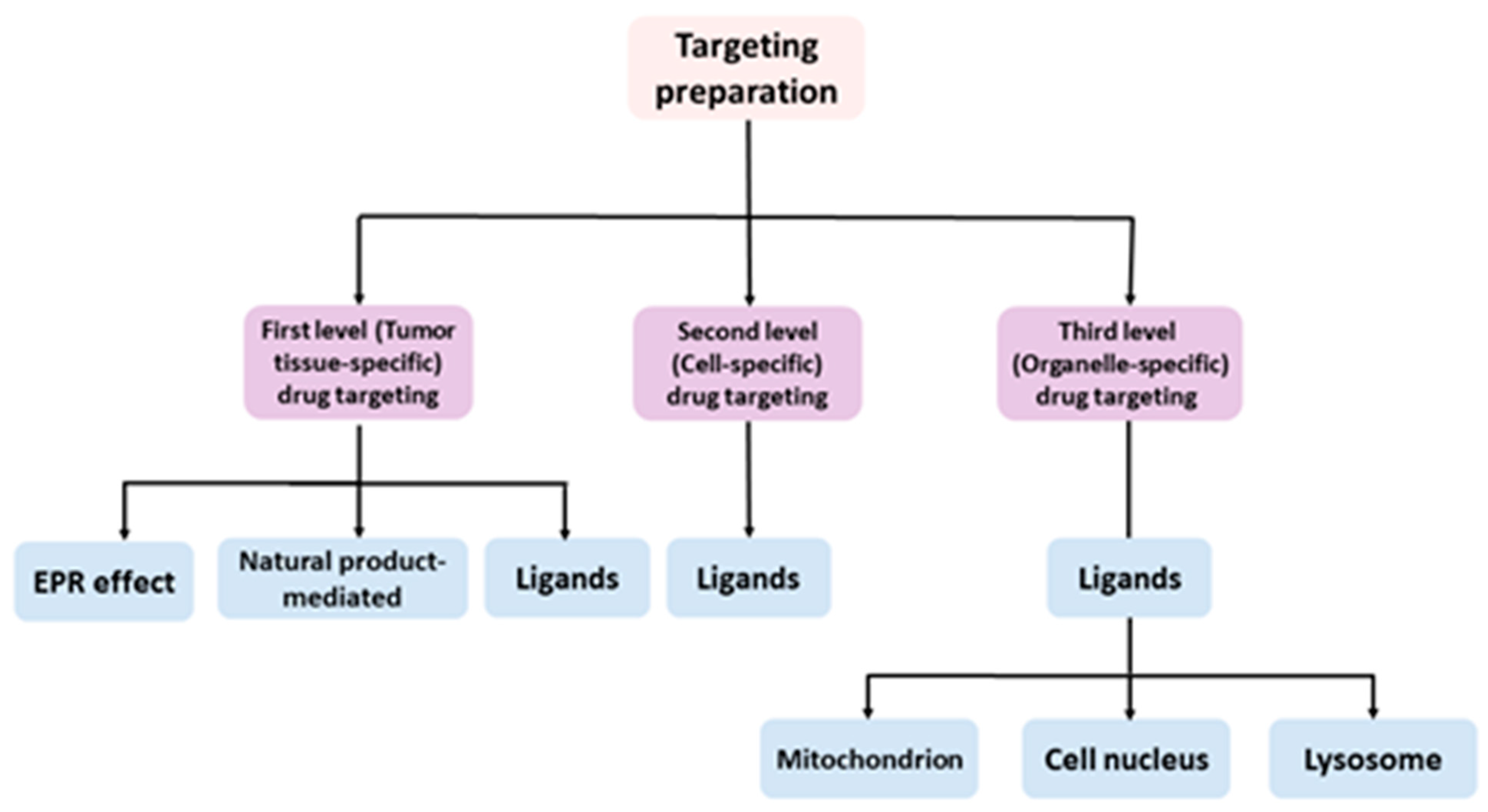
:1. Introduction
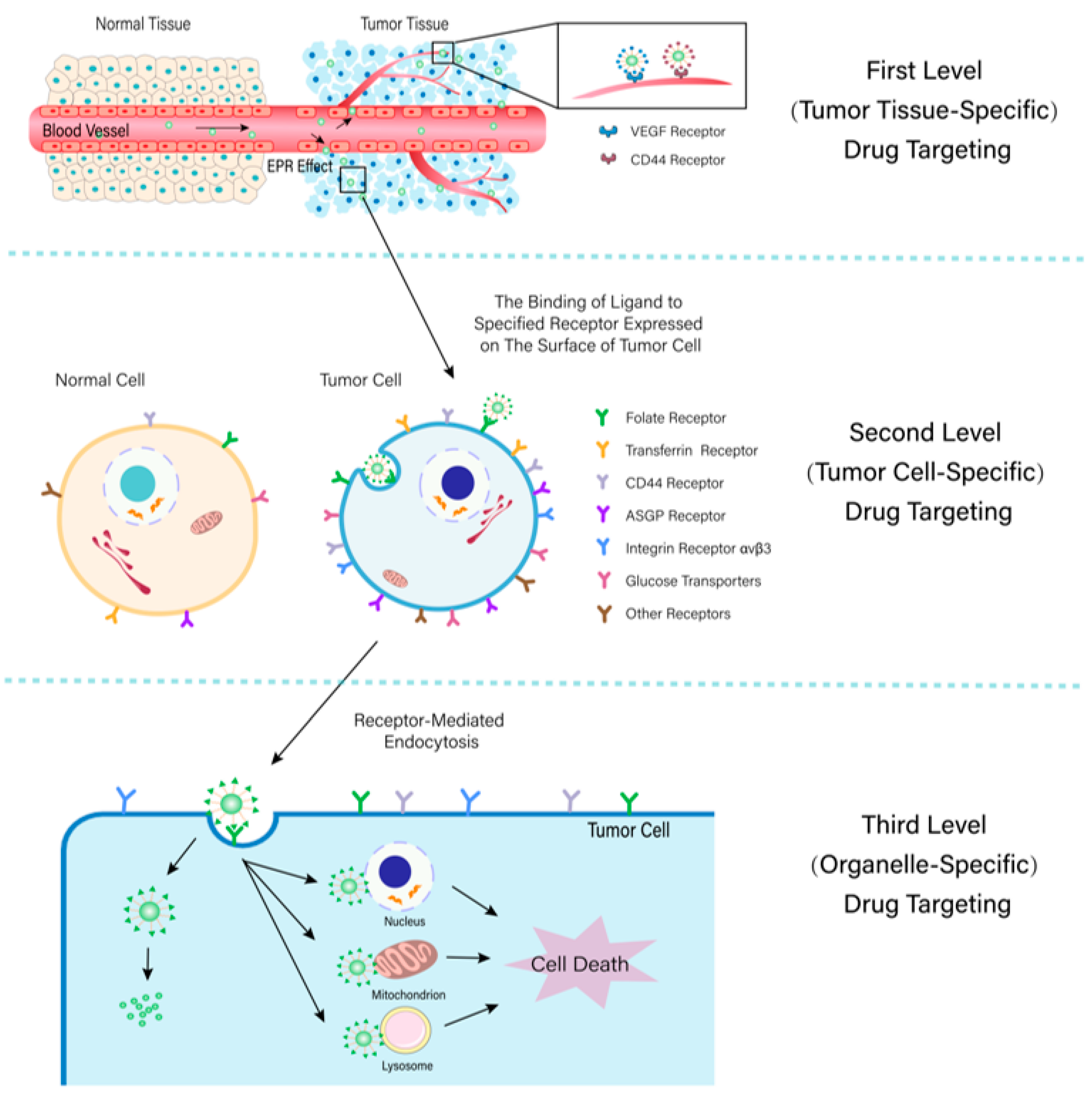
2. First-Level (Tumor Tissue-Specific) Drug Targeting
2.1. EPR Effect-Mediated Drug Targeting
2.1.1. Micelle
| Classifications | Polymers | Structures | Ref. |
|---|---|---|---|
| Hydrophilic polymers | Polyethylene glycol (PEG) | [34,36] | |
| Poly (vinyl pyrrolidone) | 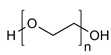 | [39] | |
| Poly(2-vinylpyridine) | 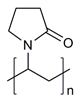 | [38] | |
| Hydrophobic polymers | Poly lactic-co-glycolic acid (PLGA) | 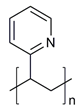 | [35,41] |
| Polycaprolactone (PCL) | 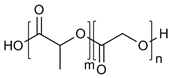 | [42,53] | |
| Polylactic acid (PLA) | 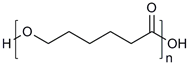 | [34,40] | |
| Amphiphilic block copolymers | D-alpha-tocopheryl polyethylene glycol succinate (TPGS) | 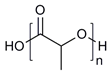 | [43,44] |
| Soluplus® |  | [47] | |
| Pluronic® (F127, F68, P123) | 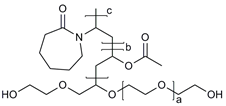 | [45,46,49,54] |
2.1.2. Liposome
2.1.3. Nanoemulsion
| Natural Products | Oily Phase | Surfactant | EE (%) | DL (%) | Zeta Potential (mV) | Size (nm) | Stability | Tumor Cell | IC50 | Ref. | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Free Drug | Nanoemulsion | ||||||||||
| Paclitaxel | Coix seed oil | Kolliphor® HS 15 | 98.8 | 0.978 | −4.40 ± 1.13 | 30.28 ± 0.36 | 30 days | HeLa | 3.101 ± 0.0375 µg/mL | 1.378 ± 0.0230 µg/mL | [90] |
| Quercetin (Q) and curcumin (C) | Soy lecithin | Polysorbate 80 | Q: 88.83 C: 85.37 | Q: 0.71 C: 0.83 | +26 | 25.9 ± 1.59 | 6 months | MCF-7 | - | 21.23 ± 2.16 µM | [95] |
| Mushroom polysaccharide | Isopropyl tetradecate | Tween 80 and Span 85 | 97.81 | - | - | 144.5 | 3 months | K562 | 4.22 mg/mL | 0.235 mg/mL | [85] |
| Pomegranate polysaccharides | Glycerylmonooleate | Cremophor RH 40 | 92.82 | - | −30.6 | 9.5 | - | HCT-116 | 287.5 µg/ml | 125.75 µg/mL | [96] |
| Quercetin | Olive oil | Span 60 and Tween 80 | - | - | −53.7 ± 0.52 | 21.7 ± 1.6 | - | HepG2 | 33.8 mM | 23.4 mM | [88] |
| Costunolide | Pumpkin oil | Replace with α-CD | - | - | - | 199.56 | - | A549 | 13.4 ± 1.5 µM | 6.1 ± 0.8 µM | [97] |
| Zingiber ottensii essential oil | Tween 80 | - | - | −4.44 ± 0.92 | 13.8 ± 0.2 | - | A549 | 43.37 ± 6.69 ng/mL | 18.45 ± 3.33 ng/mL | [89] | |
| MCF-7 | 9.77 ± 1.61 ng/mL | 1.08 ± 2.58 ng/mL | |||||||||
| HeLa | 23.25 ± 7.73 ng/mL | 5.81 ± 2.38 ng/mL | |||||||||
| K562 | 60.49 ± 9.41 ng/mL | 32.48 ± 1.21 ng/mL | |||||||||
| Heracleum persicum essential oil | Polysorbate 20 and 80, Tween 80 | - | - | −47.9 | 153 | - | MDA-MB-231 | - | 2.32 µg/mL | [98] | |
| Citronella essential oil | Tween 20 | 95.5 ± 4.775 | - | −12.6 | 130 ± 5 | 30 days | A549 | 41.20 µg/mL | 37.71 µg/mL | [99] | |
| Cinnamomum cassia essential oil | Polysorbate 80 | 63.65 ± 3.182 | - | −5.6 | 221.8 | 30 days | A549 | 50.21 µg/mL | 18.05 µg/mL | [91] | |
2.2. Active Ingredients in Natural Product-Mediated Drug Targeting
2.3. Ligand-Mediated Drug Targeting
2.3.1. APRPG Peptide Modified Nanocarrier
2.3.2. NGR Peptide-Modified Nanocarrier
3. Second-Level (Cell-Specific) Drug Targeting
3.1. Folate Modified Nanocarrier
3.2. Transferrin Modified Nanocarrier
3.3. Hyaluronic Acid Modified Nanocarrier
3.4. Galactose Modified Nanocarrier
3.5. Glycyrrhetinic Acid Modified Nanocarrier
3.6. RGD Peptide Modified Nanocarrier
3.7. Glucosyl Group/Glucose Derivative Modified Nanocarrier
3.8. Other Targeted Ligands Modified Nanocarriers
4. Third-Level (Organelle-Specific) Drug Targeting
4.1. Targeting to Mitochondria
4.1.1. Triphenylphosphine Modified Nanocarrier
4.1.2. Berberine Modified Nanocarrier
4.1.3. OPDMA Modified Nanocarrier
4.2. Targeting to Nucleus
4.2.1. Nuclear Localization Signal Peptide Modified Nanocarrier
4.2.2. Macrocyclic Polyamine Modified Nanocarrier
4.3. Targeting to Lysosome
5. Conclusions
- 1.
- The optimal usage contents of different targeted ligands still need further research;
- 2.
- In a further study of a nano-targeted drug delivery system, more attention should be paid to the combination of drugs acting on different targets. Ligands targeting different sites could be combined to design double-layer or multifunctional formulations, achieving programmed drug release and accurate drug delivery. There are relatively few studies on this aspect. Some multiple levels targeting of nano-preparations of natural medicines are shown in Table 5;
- 3.
- At present, the research on targeted formulations of natural medicines is mostly in the laboratory research stage, with few clinical applications. Therefore, when designing formulations, actual production requirements and clinical needs should also be taken into account to promote the industrial and clinical transformation of research results and better serve clinical needs;
- 4.
- Some targeted formulations have relatively low drug-loading capacities. Increasing the drug-loading capacity and reducing the number of doses is of great significance in reducing a drug’s toxicity and side effects.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Baskar, R.; Lee, K.A.; Yeo, R.; Yeoh, K.W. Cancer and radiation therapy: Current advances and future directions. Int. J. Med. Sci. 2012, 9, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Kong, F.; Cai, Y. Study Insights into Gastrointestinal Cancer through the Gut Microbiota. Biomed. Res. Int. 2019, 2019, 8721503. [Google Scholar] [CrossRef] [PubMed]
- Brodowicz, T.; O’Byrne, K.; Manegold, C. Bone matters in lung cancer. Ann. Oncol. 2012, 23, 2215–2222. [Google Scholar] [CrossRef]
- Li, F.; Liang, Y.; Wang, M.; Xu, X.; Zhao, F.; Wang, X.; Sun, Y.; Chen, W. Multifunctional nanoplatforms as cascade-responsive drug-delivery carriers for effective synergistic chemo-photodynamic cancer treatment. J. Nanobiotechnol. 2021, 19, 140. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, E.; Sarkar, D. Emerging Therapies for Hepatocellular Carcinoma (HCC). Cancers 2022, 14, 2798. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.; Abuwatfa, W.H.; Awad, N.S.; Sabouni, R.; Husseini, G.A. Encapsulation, Release, and Cytotoxicity of Doxorubicin Loaded in Liposomes, Micelles, and Metal-Organic Frameworks: A Review. Pharmaceutics 2022, 14, 254. [Google Scholar] [CrossRef]
- Pfister, C.; Gravis, G.; Fléchon, A.; Chevreau, C.; Mahammedi, H.; Laguerre, B.; Guillot, A.; Joly, F.; Soulié, M.; Allory, Y.; et al. Dose-Dense Methotrexate, Vinblastine, Doxorubicin, and Cisplatin or Gemcitabine and Cisplatin as Perioperative Chemotherapy for Patients With Nonmetastatic Muscle-Invasive Bladder Cancer: Results of the GETUG-AFU V05 VESPER Trial. J. Clin. Oncol. 2022, 40, 2013–2022. [Google Scholar]
- Dash, A.; Pettus, J.A.; Herr, H.W.; Bochner, B.H.; Dalbagni, G.; Donat, S.M.; Russo, P.; Boyle, M.G.; Milowsky, M.I.; Bajorin, D.F. A role for neoadjuvant gemcitabine plus cisplatin in muscle-invasive urothelial carcinoma of the bladder: A retrospective experience. Cancer 2008, 113, 2471–2477. [Google Scholar] [CrossRef]
- Knezevic, C.E.; Clarke, W. Cancer Chemotherapy: The Case for Therapeutic Drug Monitoring. Ther. Drug Monit. 2020, 42, 6–19. [Google Scholar] [CrossRef]
- Wei, G.; Wang, Y.; Yang, G.; Wang, Y.; Ju, R. Recent progress in nanomedicine for enhanced cancer chemotherapy. Theranostics 2021, 11, 6370–6392. [Google Scholar] [CrossRef]
- Casey, S.C.; Amedei, A.; Aquilano, K.; Azmi, A.S.; Benencia, F.; Bhakta, D.; Bilsland, A.E.; Boosani, C.S.; Chen, S.; Ciriolo, M.R.; et al. Cancer prevention and therapy through the modulation of the tumor microenvironment. Semin. Cancer Biol. 2015, 35, S199–S223. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Liu, Y.; Lin, L.; Zhao, Y.; Wang, X.; Zhong, M.; Xie, T.; Luo, Y.; Li, S.; Yang, R.; et al. Glycyrrhetinic acid modified and pH-sensitive mixed micelles improve the anticancer effect of curcumin in hepatoma carcinoma cells. RSC Adv. 2019, 9, 40131–40145. [Google Scholar] [CrossRef] [PubMed]
- Poonia, N.; Kaur Narang, J.; Lather, V.; Beg, S.; Sharma, T.; Singh, B.; Pandita, D. Resveratrol loaded functionalized nanostructured lipid carriers for breast cancer targeting: Systematic development, characterization and pharmacokinetic evaluation. Colloids Surf. B Biointerfaces 2019, 181, 756–766. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Feng, Q.; Zhang, H.; Liu, Q.; Gong, J. Curcumin enhances drug sensitivity of gemcitabine-resistant lung cancer cells and inhibits metastasis. Pharmazie 2021, 76, 538–543. [Google Scholar] [PubMed]
- Xu, T.; Guo, P.; He, Y.; Pi, C.; Wang, Y.; Feng, X.; Hou, Y.; Jiang, Q.; Zhao, L.; Wei, Y. Application of curcumin and its derivatives in tumor multidrug resistance. Phytother. Res. 2020, 34, 2438–2458. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wang, M.; Ning, S.; Yang, Z.; Zhou, L.; Xia, X. Development of Glycyrrhetinic Acid and Folate Modified Cantharidin Loaded Solid Lipid Nanoparticles for Targeting Hepatocellular Carcinoma. Molecules 2022, 27, 6786. [Google Scholar] [CrossRef]
- Lei, M.; Sha, S.; Wang, X.; Wang, J.; Du, X.; Miao, H.; Zhou, H.; Bai, E.; Shi, J.; Zhu, Y. Co-delivery of paclitaxel and gemcitabine via a self-assembling nanoparticle for targeted treatment of breast cancer. RSC Adv. 2019, 9, 5512–5520. [Google Scholar] [CrossRef]
- Wu, H.; Yu, T.; Tian, Y.; Wang, Y.; Zhao, R.; Mao, S. Enhanced liver-targeting via coadministration of 10-Hydroxycamptothecin polymeric micelles with vinegar baked Radix Bupleuri. Phytomedicine 2018, 44, 1–8. [Google Scholar] [CrossRef]
- Song, H.; Xing, W.; Shi, X.; Zhang, T.; Lou, H.; Fan, P. Antitumor and toxicity study of mitochondria-targeted triptolide derivatives using triphenylphosphine (TPP(+)) as a carrier. Bioorg. Med. Chem. 2021, 50, 116466. [Google Scholar] [CrossRef]
- Wang, W.Y.; Cao, Y.X.; Zhou, X.; Wei, B. Delivery of folic acid-modified liposomal curcumin for targeted cervical carcinoma therapy. Drug Des. Devel. Ther. 2019, 13, 2205–2213. [Google Scholar] [CrossRef] [PubMed]
- Wu, J. The Enhanced Permeability and Retention (EPR) Effect: The Significance of the Concept and Methods to Enhance Its Application. J. Pers. Med. 2021, 11, 771. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Choi, Y.; Chang, H.; Um, W.; Ryu, J.H.; Kwon, I.C. Alliance with EPR Effect: Combined Strategies to Improve the EPR Effect in the Tumor Microenvironment. Theranostics 2019, 9, 8073–8090. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; van der Meel, R.; Chen, X.; Lammers, T. The EPR effect and beyond: Strategies to improve tumor targeting and cancer nanomedicine treatment efficacy. Theranostics 2020, 10, 7921–7924. [Google Scholar] [CrossRef]
- Vaidya, F.U.; Sharma, R.; Shaikh, S.; Ray, D.; Aswal, V.K.; Pathak, C. Pluronic micelles encapsulated curcumin manifests apoptotic cell death and inhibits pro-inflammatory cytokines in human breast adenocarcinoma cells. Cancer Rep. 2019, 2, e1133. [Google Scholar] [CrossRef]
- Jiao, W.W.; Zhang, S.J.; Zhang, Y.; Gao, X.R.; Han, G. Preparation of Andrographolide Derivative ISA-Loaded Bile Salt-Phosphatidy-I Choline-Mixed Micelles and Pharmacokinetics Evaluation in Rats. Chin. Pharm. J. 2012, 47, 1643–1648. [Google Scholar]
- Zhang, Z.; Huang, Y.; Gao, F.; Gao, Z.; Bu, H.; Gu, W.; Li, Y. A self-assembled nanodelivery system enhances the oral bioavailability of daidzein: In vitro characteristics and in vivo performance. Nanomedicine 2011, 6, 1365–1379. [Google Scholar] [CrossRef]
- Duan, Y.; Wang, J.; Yang, X.; Du, H.; Xi, Y.; Zhai, G. Curcumin-loaded mixed micelles: Preparation, optimization, physicochemical properties and cytotoxicity in vitro. Drug Deliv. 2015, 22, 50–57. [Google Scholar] [CrossRef]
- Liang, H.; Yang, Q.; Deng, L.; Lu, J.; Chen, J. Phospholipid-Tween 80 mixed micelles as an intravenous delivery carrier for paclitaxel. Drug Dev. Ind. Pharm. 2011, 37, 597–605. [Google Scholar] [CrossRef]
- Yang, R.; Lao, Q.C.; Yu, H.P.; Zhang, Y.; Liu, H.C.; Luan, L.; Sun, H.M.; Li, C.Q. Tween-80 and impurity induce anaphylactoid reaction in zebrafish. J. Appl. Toxicol. 2015, 35, 295–301. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, C.; Dou, D.; Wang, Q. Evaluation of anaphylactoid constituents in vitro and in vivo. Int. Immunopharmacol. 2017, 43, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Gao, T.; Fang, F.; Sun, S.; Yang, D.; Li, Y.; Lv, S. A novel polymer micelle as a targeted drug delivery system for 10-hydroxycamptothecin with high drug-loading properties and anti-tumor efficacy. Biophys. Chem. 2021, 279, 106679. [Google Scholar] [CrossRef]
- Ren, Q.; Li, M.; Deng, Y.; Lu, A.; Lu, J. Triptolide delivery: Nanotechnology-based carrier systems to enhance efficacy and limit toxicity. Pharmacol. Res. 2021, 165, 105377. [Google Scholar] [CrossRef] [PubMed]
- Alami-Milani, M.; Zakeri-Milani, P.; Valizadeh, H.; Fathi, M.; Salatin, S.; Salehi, R.; Jelvehgari, M. PLA-PCL-PEG-PCL-PLA based micelles for improving the ocular permeability of dexamethasone: Development, characterization, and in vitro evaluation. Pharm. Dev. Technol. 2020, 25, 704–719. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.; Gao, J.; Kwon, G.S. PEG-b-PLA micelles and PLGA-b-PEG-b-PLGA sol-gels for drug delivery. J. Control Release 2016, 240, 191–201. [Google Scholar] [CrossRef]
- Ferrentino, N.; Romano, M.P.; Zappavigna, S.; Abate, M.; Del Vecchio, V.; Romano, D.; Germinario, C.; Grifa, C.; Filosa, R.; Pappalardo, D. Poly(ε-caprolactone)-poly(ethylene glycol) Tri-Block Copolymer as Quercetin Delivery System for Human Colorectal Carcinoma Cells: Synthesis, Characterization and In Vitro Study. Polymers 2023, 15, 1179. [Google Scholar] [CrossRef]
- Cui, Y.; Jin, R.; Zhou, Y.; Yu, M.; Ling, Y.; Wang, L.Q. Crystallization enhanced thermal-sensitive hydrogels of PCL-PEG-PCL triblock copolymer for 3D printing. Biomed. Mater. 2021, 16, 035006. [Google Scholar] [CrossRef]
- Brewer, K.; Bai, F.; Blencowe, A. pH-Responsive Poly(ethylene glycol)-b-poly(2-vinylpyridine) Micelles for the Triggered Release of Therapeutics. Pharmaceutics 2023, 15, 977. [Google Scholar] [CrossRef]
- Liang, R.; Ma, L.; Zhang, L.; Li, C.; Liu, W.; Wei, M.; Yan, D.; Evans, D.G.; Duan, X. A monomeric photosensitizer for targeted cancer therapy. Chem. Commun. 2014, 50, 14983–14986. [Google Scholar] [CrossRef]
- Repp, L.; Skoczen, S.L.; Rasoulianboroujeni, M.; Stern, S.T.; Kwon, G.S. Plasma Stability and Plasma Metabolite Concentration-Time Profiles of Oligo(Lactic Acid)(8)-Paclitaxel Prodrug Loaded Polymeric Micelles. AAPS J. 2023, 25, 39. [Google Scholar] [CrossRef]
- Wang, S.; Xie, L.; Liu, Y.; Yang, Q.; Jia, W.; Zhao, D.; Zhao, X. Study on the preparation and activity of intelligent response poly(lactic-co-glycolic acid)-ss-polyethylene glycol copolymer micelles. J. Biomater. Appl. 2022, 37, 259–274. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Bao, J.; Duan, T.; Hu, M.; He, Y.; Wang, J.; Hu, R.; Tang, J. Nanomicelle-Microsphere Composite as a Drug Carrier to Improve Lung-Targeting Specificity for Lung Cancer. Pharmaceutics 2022, 14, 510. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Du, X.; Fu, M.; Khan, A.R.; Ji, J.; Liu, W.; Zhai, G. Galactosamine-modified PEG-PLA/TPGS micelles for the oral delivery of curcumin. Int. J. Pharm. 2021, 595, 120227. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhou, Q.; Jia, S.; Lin, K.; Fan, G.; Yuan, J.; Yu, S.; Shi, J. Specific Modification with TPGS and Drug Loading of Cyclodextrin Polyrotaxanes and the Enhanced Antitumor Activity Study in Vitro and in Vivo. ACS Appl. Mater. Interfaces 2019, 11, 46427–46436. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Fu, S.; Lin, L.; Cao, Y.; Xie, X.; Yu, H.; Chen, M.; Li, H. Redox-sensitive Pluronic F127-tocopherol micelles: Synthesis, characterization, and cytotoxicity evaluation. Int. J. Nanomed. 2017, 12, 2635–2644. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.; Ujalambkar, V.; Rathore, A.; Rojatkar, S.; Pokharkar, V. Galangin loaded galactosylated pluronic F68 polymeric micelles for liver targeting. Biomed. Pharmacother. 2019, 112, 108691. [Google Scholar] [CrossRef]
- Zhao, J.; Xu, Y.; Wang, C.; Ding, Y.; Chen, M.; Wang, Y.; Peng, J.; Li, L.; Lv, L. Soluplus/TPGS mixed micelles for dioscin delivery in cancer therapy. Drug Dev. Ind. Pharm. 2017, 43, 1197–1204. [Google Scholar] [CrossRef]
- Nicoud, M.B.; Ospital, I.A.; Taquez Delgado, M.A.; Riedel, J.; Fuentes, P.; Bernabeu, E.; Rubinstein, M.R.; Lauretta, P.; Martinez Vivot, R.; Aguilar, M.L.A.; et al. Nanomicellar Formulations Loaded with Histamine and Paclitaxel as a New Strategy to Improve Chemotherapy for Breast Cancer. Int. J. Mol. Sci. 2023, 24, 3546. [Google Scholar] [CrossRef]
- de Oliveira, A.C.V.; de Morais, F.A.P.; Campanholi, K.; Bidoia, D.L.; Balbinot, R.B.; Nakamura, C.V.; Caetano, W.; Hioka, N.; Monteiro, O.D.S.; da Rocha, C.Q.; et al. Melanoma-targeted photodynamic therapy based on hypericin-loaded multifunctional P123-spermine/folate micelles. Photodiagnosis. Photodyn. Ther. 2022, 40, 103103. [Google Scholar] [CrossRef]
- Li, B.; Shao, H.; Gao, L.; Li, H.; Sheng, H.; Zhu, L. Nano-drug co-delivery system of natural active ingredients and chemotherapy drugs for cancer treatment: A review. Drug Deliv. 2022, 29, 2130–2161. [Google Scholar] [CrossRef]
- Ren, X.; Xu, W.; Sun, J.; Dong, B.; Awala, H.; Wang, L. Current Trends on Repurposing and Pharmacological Enhancement of Andrographolide. Curr. Med. Chem. 2021, 28, 2346–2368. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Y.; Gao, W.; Repka, M.A.; Wang, Y.; Chen, M. Andrographolide-loaded PLGA-PEG-PLGA micelles to improve its bioavailability and anticancer efficacy. Expert Opin. Drug Deliv. 2014, 11, 1367–1380. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Guo, D.; Zhang, W.; Yan, Q.; Yang, Y.; Hong, W.; Yang, G. Preparation of curcumin-loaded PCL-PEG-PCL triblock copolymeric nanoparticles by a microchannel technology. Eur. J. Pharm. Sci. 2017, 99, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, Q.; Yang, L.; Xia, X.; Zhu, R.; Chen, S.; Wang, M.; Cheng, L.; Wu, X.; Wang, S. Curcumin-Loaded TPGS/F127/P123 Mixed Polymeric Micelles for Cervical Cancer Therapy: Formulation, Characterization, and InVitro and InVivo Evaluation. J. Biomed. Nanotechnol. 2017, 13, 1631–1646. [Google Scholar] [CrossRef] [PubMed]
- Manjappa, A.S.; Kumbhar, P.S.; Patil, A.B.; Disouza, J.I.; Patravale, V.B. Polymeric Mixed Micelles: Improving the Anticancer Efficacy of Single-Copolymer Micelles. Crit. Rev. Ther. Drug Carrier Syst. 2019, 36, 1–58. [Google Scholar] [CrossRef] [PubMed]
- Giuliani, C. The Flavonoid Quercetin Induces AP-1 Activation in FRTL-5 Thyroid Cells. Antioxidants 2019, 8, 112. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yan, L.; Tang, E.K.Y.; Zhang, Z.; Chen, W.; Liu, G.; Mo, J. Synthesis of TPGS/Curcumin Nanoparticles by Thin-Film Hydration and Evaluation of Their Anti-Colon Cancer Efficacy In Vitro and In Vivo. Front. Pharmacol. 2019, 10, 769. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.P.; Li, Y.; Zhang, Y.; Li, G.W. Formulation, Characterization And Evaluation Of Curcumin- Loaded PLGA- TPGS Nanoparticles For Liver Cancer Treatment. Drug Des. Devel. Ther. 2019, 13, 3569–3578. [Google Scholar] [CrossRef]
- Du, X.; Yin, S.; Zhou, F.; Du, X.; Xu, J.; Gu, X.; Wang, G.; Li, J. Reduction-sensitive mixed micelles for selective intracellular drug delivery to tumor cells and reversal of multidrug resistance. Int. J. Pharm. 2018, 550, 1–13. [Google Scholar] [CrossRef]
- Mozafari, M.R. Nanoliposomes: Preparation and analysis. Methods Mol. Biol. 2010, 605, 29–50. [Google Scholar]
- Talens-Visconti, R.; Diez-Sales, O.; de Julian-Ortiz, J.V.; Nacher, A. Nanoliposomes in Cancer Therapy: Marketed Products and Current Clinical Trials. Int. J. Mol. Sci. 2022, 23, 4249. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Pi, C.; Fu, S.; Yang, H.; Zheng, X.; Hou, Y.; Wang, Y.; Zhang, X.; Zhao, L.; Wei, Y. Combination of Curcumin and Paclitaxel Liposomes Exhibits Enhanced Cytotoxicity Towards A549/A549-T Cells and Unaltered Pharmacokinetics. J. Biomed. Nanotechnol. 2020, 16, 1304–1313. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Tang, X. Novel Long-Circulating Liposomes Consisting of PEG Modified beta-Sitosterol for Gambogic Acid Delivery. J. Nanosci. Nanotechnol. 2016, 16, 3115–3121. [Google Scholar] [CrossRef] [PubMed]
- Tahara, Y.; Yoshikawa, T.; Sato, H.; Mori, Y.; Zahangir, M.H.; Kishimura, A.; Mori, T.; Katayama, Y. Encapsulation of a nitric oxide donor into a liposome to boost the enhanced permeation and retention (EPR) effect. Medchemcomm 2017, 8, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.; Zhang, J.; Pan, H.; Shi, J.; Wang, J.; Chen, L. Preparation and evaluation of long circulating erythrocyte membrane-cloaked anti-cancer drug delivery system. Drug Deliv. Transl. Res. 2020, 10, 1278–1287. [Google Scholar] [CrossRef]
- Marshall, S.K.; Angsantikul, P.; Pang, Z.; Nasongkla, N.; Hussen, R.S.D.; Thamphiwatana, S.D. Biomimetic Targeted Theranostic Nanoparticles for Breast Cancer Treatment. Molecules 2022, 27, 6473. [Google Scholar] [CrossRef]
- Ferrel, C.; Rayamajhi, S.; Nguyen, T.; Marasini, R.; Saravanan, T.; Deba, F.; Aryal, S. Re-engineering a Liposome with Membranes of Red Blood Cells for Drug Delivery and Diagnostic Applications. ACS Appl. Bio Mater. 2021, 4, 6974–6981. [Google Scholar] [CrossRef]
- Zhong, X.; Xian, J.; Shi, J.; Wu, Y.; Chen, J.; Lin, J. Preparation and Characterization of Biomimetic Liposomes Coated with Erythrocyte Membrane Co-Loading Triptolide and Celastrol. Acta Pharm. Sin. 2021, 56, 3252–3260. [Google Scholar]
- Wei, X.Q.; Ba, K. Construction a Long-Circulating Delivery System of Liposomal Curcumin by Coating Albumin. ACS Omega 2020, 5, 16502–16509. [Google Scholar] [CrossRef]
- Wang, R.; Zou, L.; Yi, Z.; Zhang, Z.; Zhao, M.; Shi, S. PLGA nanoparticles loaded with curcumin produced luminescence for cell bioimaging. Int. J. Pharm. 2023, 639, 122944. [Google Scholar] [CrossRef]
- Song, J.W.; Liu, Y.S.; Guo, Y.R.; Zhong, W.X.; Guo, Y.P.; Guo, L. Nano-Liposomes Double Loaded with Curcumin and Tetrandrine: Preparation, Characterization, Hepatotoxicity and Anti-Tumor Effects. Int. J. Mol. Sci. 2022, 23, 6858. [Google Scholar] [CrossRef] [PubMed]
- Alemi, A.; Zavar Reza, J.; Haghiralsadat, F.; Zarei Jaliani, H.; Haghi Karamallah, M.; Hosseini, S.A.; Haghi Karamallah, S. Paclitaxel and curcumin coadministration in novel cationic PEGylated niosomal formulations exhibit enhanced synergistic antitumor efficacy. J. Nanobiotechnol. 2018, 16, 28. [Google Scholar] [CrossRef] [PubMed]
- Carson, J.A.S.; Lichtenstein, A.H.; Anderson, C.A.M.; Appel, L.J.; Kris-Etherton, P.M.; Meyer, K.A.; Petersen, K.; Polonsky, T.; Van Horn, L.; American Heart Association Nutrition Committee of the Council on, L; et al. Dietary Cholesterol and Cardiovascular Risk: A Science Advisory From the American Heart Association. Circulation 2020, 141, e39–e53. [Google Scholar] [CrossRef] [PubMed]
- Bae, Y.; Heo, J.; Chung, Y.; Shin, S.Y.; Lee, S.W. Effect of Total Cholesterol Level Variabilities on Cerebrovascular Disease. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 544–557. [Google Scholar] [PubMed]
- Wu, Q.; Wang, Q.; Fu, J.; Ren, R. Polysaccharides derived from natural sources regulate triglyceride and cholesterol metabolism: A review of the mechanisms. Food Funct. 2019, 10, 2330–2339. [Google Scholar] [CrossRef] [PubMed]
- Hong, C.; Liang, J.; Xia, J.; Zhu, Y.; Guo, Y.; Wang, A.; Lu, C.; Ren, H.; Chen, C.; Li, S.; et al. One Stone Four Birds: A Novel Liposomal Delivery System Multi-functionalized with Ginsenoside Rh2 for Tumor Targeting Therapy. Nanomicro Lett. 2020, 12, 129. [Google Scholar] [CrossRef]
- Hong, C.; Wang, D.; Liang, J.; Guo, Y.; Zhu, Y.; Xia, J.; Qin, J.; Zhan, H.; Wang, J. Novel ginsenoside-based multifunctional liposomal delivery system for combination therapy of gastric cancer. Theranostics 2019, 9, 4437–4449. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, A.; Zhang, S.; Kim, J.; Xia, J.; Zhang, F.; Wang, D.; Wang, Q.; Wang, J. Paclitaxel-loaded ginsenoside Rg3 liposomes for drug-resistant cancer therapy by dual targeting of the tumor microenvironment and cancer cells. J. Adv. Res. 2022, 49, 159–173. [Google Scholar] [CrossRef]
- Chen, C.; Xia, J.; Ren, H.; Wang, A.; Zhu, Y.; Zhang, R.; Gan, Z.; Wang, J. Effect of the structure of ginsenosides on the in vivo fate of their liposomes. Asian J. Pharm. Sci. 2022, 17, 219–229. [Google Scholar] [CrossRef]
- Ma, J.; Zhao, D.; Yu, D.; Song, W.; Yang, X.; Yin, H. Ginsenoside Rh2 attenuates the progression of non-small cell lung cancer by sponging miR-28-5p/STK4 axis and inactivating Wnt/beta-catenin signaling. Cancer Med. 2023, 12, 12653–12667. [Google Scholar] [CrossRef]
- Tang, M.; Deng, H.; Zheng, K.; He, J.; Yang, J.; Li, Y. Ginsenoside 3beta-O-Glc-DM (C3DM) suppressed glioma tumor growth by downregulating the EGFR/PI3K/AKT/mTOR signaling pathway and modulating the tumor microenvironment. Toxicol. Appl. Pharmacol. 2023, 460, 116378. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, F.; Jin, D. Ginsenosides are Promising Medicine for Tumor and Inflammation: A Review. Am. J. Chin. Med. 2023, 51, 883–908. [Google Scholar] [CrossRef]
- Asasutjarit, R.; Sooksai, N.; Fristiohady, A.; Lairungruang, K.; Ng, S.F.; Fuongfuchat, A. Optimization of Production Parameters for Andrographolide-Loaded Nanoemulsion Preparation by Microfluidization and Evaluations of Its Bioactivities in Skin Cancer Cells and UVB Radiation-Exposed Skin. Pharmaceutics 2021, 13, 1290. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.Y.; Chen, B.H. A Comparative Study on Inhibition of Breast Cancer Cells and Tumors in Mice by Carotenoid Extract and Nanoemulsion Prepared from Sweet Potato (Ipomoea batatas L.) Peel. Pharmaceutics 2022, 14, 980. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Han, X.; Bao, R.; Hao, Y.; Li, S. Preparation and properties of water-in-oil shiitake mushroom polysaccharide nanoemulsion. Int. J. Biol. Macromol. 2019, 140, 343–349. [Google Scholar] [CrossRef]
- Gupta, A.; Eral, H.B.; Hatton, T.A.; Doyle, P.S. Nanoemulsions: Formation, properties and applications. Soft Matter 2016, 12, 2826–2841. [Google Scholar] [CrossRef]
- Altamimi, M.A.; Hussain, A.; Alshehri, S.; Imam, S.S.; Alnemer, U.A. Development and Evaluations of Transdermally Delivered Luteolin Loaded Cationic Nanoemulsion: In Vitro and Ex Vivo Evaluations. Pharmaceutics 2021, 13, 1218. [Google Scholar] [CrossRef]
- Ahmadi Oskooei, F.; Mehrzad, J.; Asoodeh, A.; Motavalizadehkakhky, A. Olive oil-based quercetin nanoemulsion (QuNE)’s interactions with human serum proteins (HSA and HTF) and its anticancer activity. J. Biomol. Struct. Dyn. 2023, 41, 778–791. [Google Scholar] [CrossRef]
- Panyajai, P.; Chueahongthong, F.; Viriyaadhammaa, N.; Nirachonkul, W.; Tima, S.; Chiampanichayakul, S.; Anuchapreeda, S.; Okonogi, S. Anticancer activity of Zingiber ottensii essential oil and its nanoformulations. PLoS ONE 2022, 17, e0262335. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, S.; Hu, Q.; Zhou, L. Self-emulsifying System Co-loaded with Paclitaxel and Coix Seed Oil Deeply Penetrated to Enhance Efficacy in Cervical Cancer. Curr. Drug Deliv. 2023, 20, 919–926. [Google Scholar] [CrossRef]
- Alam, A.; Ansari, M.J.; Alqarni, M.H.; Salkini, M.A.; Raish, M. Antioxidant, Antibacterial, and Anticancer Activity of Ultrasonic Nanoemulsion of Cinnamomum Cassia L. Essential Oil. Plants 2023, 12, 834. [Google Scholar] [CrossRef]
- Zhou, W.-J.; Wang, S.; Hu, Z.; Zhou, Z.-Y.; Song, C.-J. Angelica sinensis polysaccharides promotes apoptosis in human breast cancer cells via CREB-regulated caspase-3 activation. Biochem. Biophys. Res. Commun. 2015, 467, 562–569. [Google Scholar] [CrossRef]
- Ye, G.; Li, J.; Zhang, J.; Liu, H.; Ye, Q.; Wang, Z. Structural characterization and antitumor activity of a polysaccharide from Dendrobium wardianum. Carbohydr. Polym. 2021, 269, 118253. [Google Scholar] [CrossRef]
- Li, X.; Ma, L.; Zhang, L. Molecular basis for Poria cocos mushroom polysaccharide used as an antitumor drug in China. Prog. Mol. Biol. Transl Sci. 2019, 163, 263–296. [Google Scholar]
- Rahman, M.A.; Mittal, V.; Wahab, S.; Alsayari, A.; Bin Muhsinah, A.; Almaghaslah, D. Intravenous Nanocarrier for Improved Efficacy of Quercetin and Curcumin against Breast Cancer Cells: Development and Comparison of Single and Dual Drug-Loaded Formulations Using Hemolysis, Cytotoxicity and Cellular Uptake Studies. Membranes 2022, 12, 713. [Google Scholar] [CrossRef] [PubMed]
- Hoseny, S.S.; Soliman, A.M.; Fahmy, S.R.; Sadek, S.A. Development of a Novel Pomegranate Polysaccharide Nanoemulsion Formulation with Anti-Inflammatory, Antioxidant, and Antitumor Properties. Curr. Drug Deliv. 2023, 20, 575–586. [Google Scholar] [PubMed]
- Alhakamy, N.A.; Badr-Eldin, S.M.; Ahmed, O.A.A.; Aldawsari, H.M.; Okbazghi, S.Z.; Alfaleh, M.A.; Abdulaal, W.H.; Neamatallah, T.; Al-Hejaili, O.D.; Fahmy, U.A. Green Nanoemulsion Stabilized by In Situ Self-Assembled Natural Oil/Native Cyclodextrin Complexes: An Eco-Friendly Approach for Enhancing Anticancer Activity of Costunolide against Lung Cancer Cells. Pharmaceutics 2022, 14, 227. [Google Scholar] [CrossRef] [PubMed]
- Bashlouei, S.G.; Karimi, E.; Zareian, M.; Oskoueian, E.; Shakeri, M. Heracleum persicum Essential Oil Nanoemulsion: A Nanocarrier System for the Delivery of Promising Anticancer and Antioxidant Bioactive Agents. Antioxidants 2022, 11, 831. [Google Scholar] [CrossRef] [PubMed]
- Jawaid, T.; Alaseem, A.M.; Khan, M.M.; Mukhtar, B.; Kamal, M.; Anwer, R.; Ahmed, S.; Alam, A. Preparation and Evaluation of Nanoemulsion of Citronella Essential Oil with Improved Antimicrobial and Anti-Cancer Properties. Antibiotics 2023, 12, 478. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Liu, L.; Zhao, Y.; Zhao, R. Saikosaponins A, C and D enhance liver-targeting effects of anticancer drugs by modulating drug transporters. Oncotarget 2017, 8, 110092–110102. [Google Scholar] [CrossRef] [PubMed]
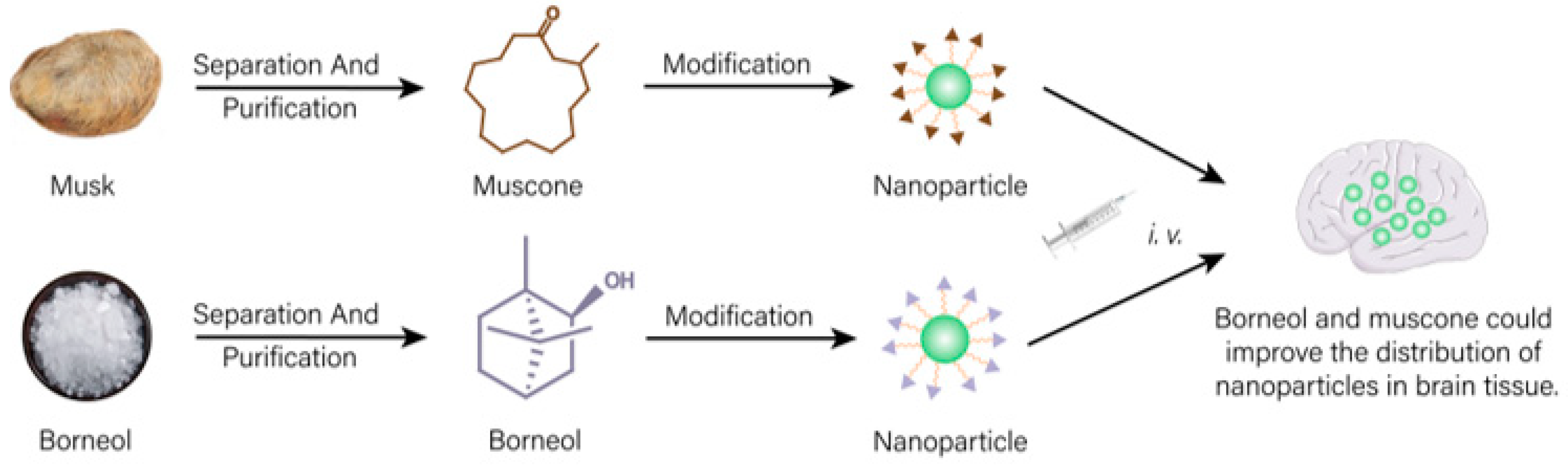
- Kang, S.; Duan, W.; Zhang, S.; Chen, D.; Feng, J.; Qi, N. Muscone/RI7217 co-modified upward messenger DTX liposomes enhanced permeability of blood-brain barrier and targeting glioma. Theranostics 2020, 10, 4308–4322. [Google Scholar] [CrossRef]
- Wang, L.; Xu, L.; Du, J.; Zhao, X.; Liu, M.; Feng, J.; Hu, K. Nose-to-brain delivery of borneol modified tanshinone IIA nanoparticles in prevention of cerebral ischemia/reperfusion injury. Drug Deliv. 2021, 28, 1363–1375. [Google Scholar] [CrossRef]
- Guo, X.; Wu, G.; Wang, H.; Chen, L. Pep-1&borneol-Bifunctionalized Carmustine-Loaded Micelles Enhance Anti-Glioma Efficacy Through Tumor-Targeting and BBB-Penetrating. J. Pharm. Sci. 2019, 108, 1726–1735. [Google Scholar] [PubMed]
- Frezzetti, D.; Gallo, M.; Maiello, M.R.; D’Alessio, A.; Esposito, C.; Chicchinelli, N.; Normanno, N.; De Luca, A. VEGF as a potential target in lung cancer. Expert. Opin. Ther. Targets 2017, 21, 959–966. [Google Scholar] [CrossRef]
- Zhao, Y.; Adjei, A.A. Targeting Angiogenesis in Cancer Therapy: Moving Beyond Vascular Endothelial Growth Factor. Oncologist 2015, 20, 660–673. [Google Scholar] [CrossRef] [PubMed]
- Lai, K.; Li, Y.; Gong, Y.; Li, L.; Huang, C.; Xu, F.; Zhong, X.; Jin, C. Triptolide-nanoliposome-APRPG, a novel sustained-release drug delivery system targeting vascular endothelial cells, enhances the inhibitory effects of triptolide on laser-induced choroidal neovascularization. Biomed. Pharmacother. 2020, 131, 110737. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, P.; Duan, Y.; Yin, X.; Wang, Q.; Liu, X.; Wang, X.; Zhou, J.; Wang, W.; Qiu, L.; et al. Specific cell targeting with APRPG conjugated PEG-PLGA nanoparticles for treating ovarian cancer. Biomaterials 2014, 35, 983–992. [Google Scholar] [CrossRef]
- Xie, M.H.; Fu, Z.L.; Hua, A.L.; Zhou, J.F.; Chen, Q.; Li, J.B.; Yao, S.; Cai, X.J.; Ge, M.; Zhou, L.; et al. A new core-shell-type nanoparticle loaded with paclitaxel/norcantharidin and modified with APRPG enhances anti-tumor effects in hepatocellular carcinoma. Front. Oncol. 2022, 12, 932156. [Google Scholar] [CrossRef]
- Liu, H.; Tang, L.; Li, X.; Li, H. Triptolide inhibits vascular endothelial growth factor-mediated angiogenesis in human breast cancer cells. Exp. Ther. Med. 2018, 16, 830–836. [Google Scholar] [CrossRef]
- Cheng, X.; Wang, F.; Qiao, Y.; Chen, T.; Fan, L.; Shen, X.; Yu, D.; Huang, Y.; Wei, M. Honokiol inhibits interleukin-induced angiogenesis in the NSCLC microenvironment through the NF-κB signaling pathway. Chem.-Biol. Interact. 2023, 370, 110295. [Google Scholar] [CrossRef]
- Wang, T.; Liu, J.; Xiao, X.-Q. Cantharidin inhibits angiogenesis by suppressing VEGF-induced JAK1/STAT3, ERK and AKT signaling pathways. Arch. Pharmacal Res. 2014, 38, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.J.; Fei, W.D.; Xu, Y.Y.; Xu, H.; Yang, G.Y.; Cao, J.W.; Ni, J.J.; Wang, Z. Combination of metronomic administration and target delivery strategies to improve the anti-angiogenic and anti-tumor effects of triptolide. Drug Deliv. Transl. Res. 2020, 10, 93–107. [Google Scholar] [CrossRef] [PubMed]
- Conte, C.; Longobardi, G.; Barbieri, A.; Palma, G.; Luciano, A.; Dal Poggetto, G.; Avitabile, C.; Pecoraro, A.; Russo, A.; Russo, G.; et al. Non-covalent strategies to functionalize polymeric nanoparticles with NGR peptides for targeting breast cancer. Int. J. Pharm. 2023, 633, 122618. [Google Scholar] [CrossRef] [PubMed]
- Tripodi, A.A.P.; Randelovic, I.; Biri-Kovacs, B.; Szeder, B.; Mezo, G.; Tovari, J. In Vivo Tumor Growth Inhibition and Antiangiogenic Effect of Cyclic NGR Peptide-Daunorubicin Conjugates Developed for Targeted Drug Delivery. Pathol. Oncol. Res. 2020, 26, 1879–1892. [Google Scholar] [CrossRef]
- Duan, H.; Liu, C.; Hou, Y.; Liu, Y.; Zhang, Z.; Zhao, H.; Xin, X.; Liu, W.; Zhang, X.; Chen, L.; et al. Sequential Delivery of Quercetin and Paclitaxel for the Fibrotic Tumor Microenvironment Remodeling and Chemotherapy Potentiation via a Dual-Targeting Hybrid Micelle-in-Liposome System. ACS Appl. Mater. Interfaces 2022, 14, 10102–10116. [Google Scholar] [CrossRef] [PubMed]
- Kunjachan, S.; Pola, R.; Gremse, F.; Theek, B.; Ehling, J.; Moeckel, D.; Hermanns-Sachweh, B.; Pechar, M.; Ulbrich, K.; Hennink, W.E.; et al. Passive versus active tumor targeting using RGD- and NGR-modified polymeric nanomedicines. Nano Lett. 2014, 14, 972–981. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.M.; Huang, Y.; Zhao, B.X.; Zhao, X.; Duan, Y.; Du, R.; Yu, K.F.; Song, P.; Zhao, Y.; Zhang, X.; et al. Anti-tumor and anti-angiogenic effect of metronomic cyclic NGR-modified liposomes containing paclitaxel. Biomaterials 2013, 34, 1102–1114. [Google Scholar] [CrossRef]
- Alavi, M.; Hamidi, M. Passive and active targeting in cancer therapy by liposomes and lipid nanoparticles. Drug Metab. Pers. Ther. 2019, 34, 20180032. [Google Scholar] [CrossRef]
- Muhamad, N.; Plengsuriyakarn, T.; Na-Bangchang, K. Application of active targeting nanoparticle delivery system for chemotherapeutic drugs and traditional/herbal medicines in cancer therapy: A systematic review. Int. J. Nanomed. 2018, 13, 3921–3935. [Google Scholar] [CrossRef]
- Leamon, C.P.; Reddy, J.A. Folate-targeted chemotherapy. Adv. Drug Deliv. Rev. 2004, 56, 1127–1141. [Google Scholar] [CrossRef]
- Sudimack, J.; Lee, R.J. Targeted Drug Delivery via The Folate Receptor. Adv. Drug Deliv. Rev. 2000, 41, 147–162. [Google Scholar] [CrossRef]
- Grigoletto, A.; Martinez, G.; Gabbia, D.; Tedeschini, T.; Scaffidi, M.; Martin, S.; Pasut, G. Folic Acid-Targeted Paclitaxel-Polymer Conjugates Exert Selective Cytotoxicity and Modulate Invasiveness of Colon Cancer Cells. Pharmaceutics 2021, 13, 929. [Google Scholar] [CrossRef]
- Song, B.; Wu, S.; Li, W.; Chen, D.; Hu, H. Folate Modified Long Circulating Nano-Emulsion as a Promising Approach for Improving the Efficiency of Chemotherapy Drugs in Cancer Treatment. Pharm. Res. 2020, 37, 242. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.C.; Xiong, X.Y.; Ge, X.J.; Li, Z.L.; Li, Y.P. Effect of the Folate Ligand Density on the Targeting Property of Folated-Conjugated Polymeric Nanoparticles. Macromol. Biosci. 2019, 19, e1800348. [Google Scholar] [CrossRef]
- Yu, L.; Xu, M.; Xu, W.; Xiao, W.; Jiang, X.H.; Wang, L.; Gao, H. Enhanced Cancer-targeted Drug Delivery Using Precoated Nanoparticles. Nano Lett. 2020, 20, 8903–8911. [Google Scholar] [CrossRef] [PubMed]
- Rao, L.; Meng, Q.F.; Bu, L.L.; Cai, B.; Huang, Q.; Sun, Z.J.; Zhang, W.F.; Li, A.; Guo, S.S.; Liu, W.; et al. Erythrocyte Membrane-Coated Upconversion Nanoparticles with Minimal Protein Adsorption for Enhanced Tumor Imaging. ACS Appl. Mater. Interfaces 2017, 9, 2159–2168. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, Y.; Yu, Y.; Li, J.; Pan, W.; Yang, X.; Zhang, Z.; Jiang, S.; Yang, X.; Wang, X. Transferrin Modified Dioscin Loaded PEGylated Liposomes: Characterization and In Vitro Antitumor Effect. J. Nanosci. Nanotechnol. 2020, 20, 1321–1331. [Google Scholar] [CrossRef]
- Liu, X.; Dong, S.; Dong, M.; Li, Y.; Sun, Z.; Zhang, X.; Wang, Y.; Teng, L.; Wang, D. Transferrin-conjugated liposomes loaded with carnosic acid inhibit liver cancer growth by inducing mitochondria-mediated apoptosis. Int. J. Pharm. 2021, 607, 121034. [Google Scholar] [CrossRef]
- Li, S.; Guo, J.; Tian, Z.; Chen, J.; Gou, G.; Niu, Y.; Li, L.; Yang, J. Piperine-Loaded Glycyrrhizic Acid- and PLGA-Based Nanoparticles Modified with Transferrin for Antitumor: Piperine-Loaded Glycyrrhizic Acid- and PLGA-Based Nanoparticles. AAPS PharmSciTech 2021, 22, 239. [Google Scholar] [CrossRef]
- Kesharwani, P.; Chadar, R.; Sheikh, A.; Rizg, W.Y.; Safhi, A.Y. CD44-Targeted Nanocarrier for Cancer Therapy. Front. Pharmacol. 2021, 12, 800481. [Google Scholar] [CrossRef]
- Mattheolabakis, G.; Milane, L.; Singh, A.; Amiji, M.M. Hyaluronic acid targeting of CD44 for cancer therapy: From receptor biology to nanomedicine. J. Drug Target 2015, 23, 605–618. [Google Scholar] [CrossRef]
- Sun, L.; Fang, Y.; Wang, X.; Han, Y.; Du, F.; Li, C.; Hu, H.; Liu, H.; Liu, Q.; Wang, J.; et al. miR-302a Inhibits Metastasis and Cetuximab Resistance in Colorectal Cancer by Targeting NFIB and CD44. Theranostics 2019, 9, 8409–8425. [Google Scholar] [CrossRef]
- Teng, C.; Chai, Z.; Yuan, Z.; Ren, L.; Lin, C.; Yan, Z.; He, W.; Qin, C.; Yang, L.; Han, X.; et al. Desirable PEGylation for improving tumor selectivity of hyaluronic acid-based nanoparticles via low hepatic captured, long circulation times and CD44 receptor-mediated tumor targeting. Nanomedicine 2020, 24, 102105. [Google Scholar] [CrossRef]
- Sun, J.; Li, M.; Lin, K.; Liu, Z.; Wang, Z.; Wang, W.; Zhao, Y.; Zhen, Y.; Zhang, S. Delivery of quercetin for breast cancer and targeting potentiation via hyaluronic nano-micelles. Int. J. Biol. Macromol. 2023, 242 Pt 2, 124736. [Google Scholar] [CrossRef]
- Li, Y.; Wu, J.; Lu, Q.; Liu, X.; Wen, J.; Qi, X.; Liu, J.; Lian, B.; Zhang, B.; Sun, H.; et al. GA&HA-Modified Liposomes for Co-Delivery of Aprepitant and Curcumin to Inhibit Drug-Resistance and Metastasis of Hepatocellular Carcinoma. Int. J. Nanomed. 2022, 17, 2559–2575. [Google Scholar]
- Ma, Y.; Liu, J.; Cui, X.; Hou, J.; Yu, F.; Wang, J.; Wang, X.; Chen, C.; Tong, L. Hyaluronic Acid Modified Nanostructured Lipid Carrier for Targeting Delivery of Kaempferol to NSCLC: Preparation, Optimization, Characterization, and Performance Evaluation In Vitro. Molecules 2022, 27, 4553. [Google Scholar] [CrossRef] [PubMed]
- Kotla, N.G.; Burke, O.; Pandit, A.; Rochev, Y. An Orally Administrated Hyaluronan Functionalized Polymeric Hybrid Nanoparticle System for Colon-Specific Drug Delivery. Nanomaterials 2019, 9, 1246. [Google Scholar] [CrossRef] [PubMed]
- Ji, P.; Wang, L.; Chen, Y.; Wang, S.; Wu, Z.; Qi, X. Hyaluronic acid hydrophilic surface rehabilitating curcumin nanocrystals for targeted breast cancer treatment with prolonged biodistribution. Biomater. Sci. 2020, 8, 462–472. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Tian, L.; Gao, M.; Xu, H.; Zhang, C.; Lv, L.; Zhang, J.; Wang, C.; Tian, Y.; Ma, X. Promising galactose-decorated biodegradable poloxamer 188-PLGA diblock copolymer nanoparticles of resibufogenin for enhancing liver cancer therapy. Drug Deliv. 2017, 24, 1302–1316. [Google Scholar] [CrossRef]
- Wu, F.; Li, X.; Jiang, B.; Yan, J.; Zhang, Z.; Qin, J.; Yu, W.; Gao, Z. Glycyrrhetinic Acid Functionalized Nanoparticles for Drug Delivery to Liver Cancer. J. Biomed. Nanotechnol. 2018, 14, 1837–1852. [Google Scholar] [CrossRef]
- Speciale, A.; Muscara, C.; Molonia, M.S.; Cristani, M.; Cimino, F.; Saija, A. Recent Advances in Glycyrrhetinic Acid-Functionalized Biomaterials for Liver Cancer-Targeting Therapy. Molecules 2022, 27, 1775. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.; Zhou, L.; Zou, M.; Ning, S.; Liu, S.; Zhou, Y.; Du, K.; Zhang, X.; Xia, X. 18-GA-Suc Modified Liposome Loading Cantharidin for Augmenting Hepatic Specificity: Preparation, Characterization, Antitumor Effects, and Liver-Targeting Efficiency. J. Pharm. Sci. 2020, 109, 2038–2047. [Google Scholar] [CrossRef] [PubMed]
- Li, X.T.; Tang, W.; Xie, H.J.; Liu, S.; Song, X.L.; Xiao, Y.; Wang, X.; Cheng, L.; Chen, G.R. The efficacy of RGD modified liposomes loaded with vinorelbine plus tetrandrine in treating resistant brain glioma. J. Liposome Res. 2019, 29, 21–34. [Google Scholar] [CrossRef]
- Shi, J.; Liu, S.; Yu, Y.; He, C.; Tan, L.; Shen, Y.M. RGD peptide-decorated micelles assembled from polymer-paclitaxel conjugates towards gastric cancer therapy. Colloids Surf. B Biointerfaces 2019, 180, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, R.; Ashraf Mirahmadi-Babaheidri, S.; Delaviz, H.; Fouani, M.H.; Alipour, M.; Jafari Barmak, M.; Christiansen, G.; Bardania, H. RGD peptide-mediated liposomal curcumin targeted delivery to breast cancer cells. J. Biomater. Appl. 2021, 35, 743–753. [Google Scholar] [CrossRef]
- Long, Q.; Zhu, W.; Guo, L.; Pu, L. RGD-Conjugated Resveratrol HSA Nanoparticles as a Novel Delivery System in Ovarian Cancer Therapy. Drug Des. Devel. Ther. 2020, 14, 5747–5756. [Google Scholar] [CrossRef]
- Liu, M.; Jiang, X.; Fu, C.; Zhao, R.; Jin, T.; Ma, J.; Qin, S.; Li, L.A.; Hu, Y.; Zhang, X. Molecular design and anti-melanoma activity of a novel bullfrog antibacterial peptide RGD-chimera. Oncol. Lett. 2021, 21, 115. [Google Scholar] [CrossRef]
- Zambrano, A.; Molt, M.; Uribe, E.; Salas, M. Glut 1 in Cancer Cells and the Inhibitory Action of Resveratrol as A Potential Therapeutic Strategy. Int. J. Mol. Sci. 2019, 20, 3374. [Google Scholar] [CrossRef]
- Jagdale, S.; Narwade, M.; Sheikh, A.; Md, S.; Salve, R.; Gajbhiye, V.; Kesharwani, P.; Gajbhiye, K.R. GLUT1 transporter-facilitated solid lipid nanoparticles loaded with anti-cancer therapeutics for ovarian cancer targeting. Int. J. Pharm. 2023, 637, 122894. [Google Scholar] [CrossRef]
- Meng, L.; Liu, F.; Du, C.; Zhu, J.; Xiong, Q.; Li, J.; Sun, W. Glucosamine-Modified Reduction-Responsive Polymeric Micelles for Liver Cancer Therapy. Molecules 2023, 28, 3824. [Google Scholar] [CrossRef]
- Shen, S.; Du, M.; Liu, Q.; Gao, P.; Wang, J.; Liu, S.; Gu, L. Development of GLUT1-targeting alkyl glucoside-modified dihydroartemisinin liposomes for cancer therapy. Nanoscale 2020, 12, 21901–21912. [Google Scholar] [CrossRef] [PubMed]
- Hong, W.; Guo, F.; Yu, N.; Ying, S.; Lou, B.; Wu, J.; Gao, Y.; Ji, X.; Wang, H.; Li, A.; et al. A Novel Folic Acid Receptor-Targeted Drug Delivery System Based on Curcumin-Loaded beta-Cyclodextrin Nanoparticles for Cancer Treatment. Drug Des. Devel. Ther. 2021, 15, 2843–2855. [Google Scholar] [CrossRef]
- Riaz, M.K.; Zhang, X.; Wong, K.H.; Chen, H.; Liu, Q.; Chen, X.; Zhang, G.; Lu, A.; Yang, Z. Pulmonary delivery of transferrin receptors targeting peptide surface-functionalized liposomes augments the chemotherapeutic effect of quercetin in lung cancer therapy. Int. J. Nanomed. 2019, 14, 2879–2902. [Google Scholar] [CrossRef]
- Setayesh, A.; Bagheri, F.; Boddohi, S. Self-assembled formation of chondroitin sulfate-based micellar nanogel for curcumin delivery to breast cancer cells. Int. J. Biol. Macromol. 2020, 161, 771–778. [Google Scholar] [CrossRef]
- Qu, D.; Liu, M.; Huang, M.; Wang, L.; Chen, Y.; Liu, C.; Liu, Y. Octanoyl galactose ester-modified microemulsion system self-assembled by coix seed components to enhance tumor targeting and hepatoma therapy. Int. J. Nanomed. 2017, 12, 2045–2059. [Google Scholar] [CrossRef]
- Rofeal, M.G.; Elzoghby, A.O.; Helmy, M.W.; Khalil, R.; Khairy, H.; Omar, S. Dual Therapeutic Targeting of Lung Infection and Carcinoma Using Lactoferrin-Based Green Nanomedicine. ACS Biomater. Sci. Eng. 2020, 6, 5685–5699. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zhao, M.; Zhao, L. Lysine-mediated hydroxyethyl starch-10-hydroxy camptothecin micelles for the treatment of liver cancer. Drug Deliv. 2020, 27, 519–529. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Wong, B.C.K.; Chen, H.; Bian, Z.; Zhang, G.; Zhang, X.; Kashif Riaz, M.; Tyagi, D.; Lin, G.; Zhang, Y.; et al. Pulmonary delivery of triptolide-loaded liposomes decorated with anti-carbonic anhydrase IX antibody for lung cancer therapy. Sci. Rep. 2017, 7, 1097. [Google Scholar] [CrossRef]
- Wang, C.; Liu, B.; Xu, X.; Zhuang, B.; Li, H.; Yin, J.; Cong, M.; Xu, W.; Lu, A. Toward Targeted Therapy in Chemotherapy-resistant Pancreatic Cancer with a Smart Triptolide Nanomedicine. Oncotarget 2016, 7, 8360–8372. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Wang, X.; Teng, B.; Wang, Z.; Li, F.; Zhao, Y.; Guo, Y.; Zeng, Q. Peptide-Targeted High-Density Lipoprotein Nanoparticles for Combinatorial Treatment against Metastatic Breast Cancer. ACS Appl. Mater. Interfaces 2021, 13, 35248–35265. [Google Scholar] [CrossRef] [PubMed]
- Lan, M.; Kong, Z.; Liu, F.; Zou, T.; Li, L.; Cai, T.; Tian, H.; Cai, Y. Activating caspase-8/Bid/ROS signaling to promote apoptosis of breast cancer cells by folate-modified albumin baicalin-loaded nanoparticles. Nanotechnology 2022, 33, 435101. [Google Scholar] [CrossRef] [PubMed]
- Patra, A.; Satpathy, S.; Naik, P.K.; Kazi, M.; Hussain, M.D. Folate receptor-targeted PLGA-PEG nanoparticles for enhancing the activity of genistein in ovarian cancer. Artif. Cells Nanomed. Biotechnol. 2022, 50, 228–239. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Wu, C.; Duan, J.; Miao, J.; Ren, H.; Liu, J. Anti-Glioma Effect with Targeting Therapy Using Folate Modified Nano-Micelles Delivery Curcumin. J. Biomed. Nanotechnol. 2020, 16, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; He, Y.; Ji, J.; Zheng, S.; Cheng, Y. Tumor Targeted Curcumin Delivery by Folate-Modified MPEG-PCL Self-Assembly Micelles for Colorectal Cancer Therapy. Int. J. Nanomed. 2020, 15, 1239–1252. [Google Scholar] [CrossRef] [PubMed]
- Solanki, R.; Jangid, A.K.; Jadav, M.; Kulhari, H.; Patel, S. Folate Functionalized and Evodiamine-Loaded Pluronic Nanomicelles for Augmented Cervical Cancer Cell Killing. Macromol. Biosci. 2023, 23, e2300077. [Google Scholar] [CrossRef] [PubMed]
- Cai, M.; Zeng, Y.; Liu, M.; You, L.; Huang, H.; Hao, Y.; Yin, X.; Qu, C.; Ni, J.; Dong, X. Construction of a Multifunctional Nano-Scale Metal-Organic Framework-Based Drug Delivery System for Targeted Cancer Therapy. Pharmaceutics 2021, 13, 1945. [Google Scholar] [CrossRef]
- Kumari, P.; Swami, M.O.; Nadipalli, S.K.; Myneni, S.; Ghosh, B.; Biswas, S. Curcumin Delivery by Poly(Lactide)-Based Co-Polymeric Micelles: An In Vitro Anticancer Study. Pharm. Res. 2016, 33, 826–841. [Google Scholar] [CrossRef]
- Xu, R.; Yang, J.; Qian, Y.; Deng, H.; Wang, Z.; Ma, S.; Wei, Y.; Yang, N.; Shen, Q. Ferroptosis/pyroptosis dual-inductive combinational anti-cancer therapy achieved by transferrin decorated nanoMOF. Nanoscale Horiz. 2021, 6, 348–356. [Google Scholar] [CrossRef]
- RS, P.; Mal, A.; Valvi, S.K.; Srivastava, R.; De, A.; Bandyopadhyaya, R. Noninvasive Preclinical Evaluation of Targeted Nanoparticles for the Delivery of Curcumin in Treating Pancreatic Cancer. ACS Appl. Bio Mater. 2020, 3, 4643–4654. [Google Scholar]
- Zhang, Q.; Tian, X.; Cao, X. Transferrin-functionalised microemulsion co-delivery of beta-elemene and celastrol for enhanced anti-lung cancer treatment and reduced systemic toxicity. Drug Deliv. Transl. Res. 2019, 9, 667–678. [Google Scholar] [CrossRef]
- Sun, S.; Shang, E.; Ju, A.; Li, Y.; Wu, Q.; Li, Q.; Yang, Y.; Guo, Y.; Yang, D.; Lv, S. Tumor-targeted hyaluronic acid-mPEG modified nanostructured lipid carriers for cantharidin delivery: An in vivo and in vitro study. Fitoterapia 2021, 155, 105033. [Google Scholar] [CrossRef]
- Xu, F.; Li, M.; Que, Z.; Su, M.; Yao, W.; Zhang, Y.; Luo, B.; Li, Y.; Zhang, Z.; Tian, J. Combined chemo-immuno-photothermal therapy based on ursolic acid/astragaloside IV-loaded hyaluronic acid-modified polydopamine nanomedicine inhibiting the growth and metastasis of non-small cell lung cancer. J. Mater. Chem. B 2023, 11, 3453–3472. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, H.; Zhang, Y.; Huang, Q.; Feng, J.; Xing, H.; Fu, X.; Yan, X.; Zhang, Y.; Xu, Q.; et al. HA-DOPE-Modified Honokiol-Loaded Liposomes Targeted Therapy for Osteosarcoma. Int. J. Nanomed. 2022, 17, 5137–5151. [Google Scholar] [CrossRef]
- Ganguly, S.; Dewanjee, S.; Sen, R.; Chattopadhyay, D.; Ganguly, S.; Gaonkar, R.; Debnath, M.C. Apigenin-loaded galactose tailored PLGA nanoparticles: A possible strategy for liver targeting to treat hepatocellular carcinoma. Colloids Surf. B Biointerfaces 2021, 204, 111778. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.; Zhang, H.; Cheng, Y.; Cao, P.; Cui, J.; Yin, X.; Fan, S.; Li, Y. Fluorescent Imaging-Guided Chemo- and Photodynamic Therapy of Hepatocellular Carcinoma with HCPT@NMOFs-RGD Nanocomposites. Int. J. Nanomed. 2022, 17, 1381–1395. [Google Scholar] [CrossRef]
- Ma, T.; Jiang, J.L.; Qi, W.X.; Chen, J.Y.; Xu, H.P. A Novel Delivery System of RGD-HSA Loaded GEM/CUR Nanoparticles for the Treatment of Pancreatic Cancer Therapy. Drug Des. Devel. Ther. 2022, 16, 2395–2406. [Google Scholar] [CrossRef]
- Cheng, X.; Feng, D.; Lv, J.; Cui, X.; Wang, Y.; Wang, Q.; Zhang, L. Application Prospects of Triphenylphosphine-Based Mitochondria-Targeted Cancer Therapy. Cancers 2023, 15, 666. [Google Scholar] [CrossRef]
- Zielonka, J.; Joseph, J.; Sikora, A.; Hardy, M.; Ouari, O.; Vasquez-Vivar, J.; Cheng, G.; Lopez, M.; Kalyanaraman, B. Mitochondria-Targeted Triphenylphosphonium-Based Compounds: Syntheses, Mechanisms of Action, and Therapeutic and Diagnostic Applications. Chem. Rev. 2017, 117, 10043–10120. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Lin, C.; Yang, X.; Xie, Y.; Hu, P.; Li, H.; Zhu, W.; Hu, H. Mitochondrial targeting nanodrugs self-assembled from 9-O-octadecyl substituted berberine derivative for cancer treatment by inducing mitochondrial apoptosis pathways. J. Control Release 2019, 294, 27–42. [Google Scholar] [CrossRef]
- Kolb, D.; Kolishetti, N.; Surnar, B.; Sarkar, S.; Guin, S.; Shah, A.S.; Dhar, S. Metabolic Modulation of the Tumor Microenvironment Leads to Multiple Checkpoint Inhibition and Immune Cell Infiltration. ACS Nano 2020, 14, 11055–11066. [Google Scholar] [CrossRef] [PubMed]
- Vasan, K.; Werner, M.; Chandel, N.S. Mitochondrial Metabolism as a Target for Cancer Therapy. Cell Metab. 2020, 32, 341–352. [Google Scholar] [CrossRef]
- Kong, F.; Xie, C.; Zhao, X.; Zong, X.; Bu, L.; Zhang, B.; Tian, H.; Ma, S. Resveratrol regulates PINK1/Parkin-mediated mitophagy via the lncRNA ZFAS1-miR-150-5p-PINK1 axis, and enhances the antitumor activity of paclitaxel against non-small cell lung cancer. Toxicol. Res. 2022, 11, 962–974. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Zhang, Y.; Dong, L.; Qu, X.Y.; Tao, L.N.; Zhang, Y.M.; Zhai, J.H.; Song, Y.Q. Triptolide induces autophagy and apoptosis through ERK activation in human breast cancer MCF-7 cells. Exp. Ther. Med. 2018, 15, 3413–3419. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Yu, H.; Wang, C.; He, F.; Shi, Z.; Tu, H.; Ning, N.; Duan, S.; Zhao, Y. The Anti-Cancer Effects of Mitochondrial-Targeted Triphenylphosphonium-Resveratrol Conjugate on Breast Cancer Cells. Pharmaceuticals 2022, 15, 1271. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Chen, Y.; He, K.; Cao, T.; Song, D.; Yang, H.; Li, L.; Lin, J. The Apoptosis of Liver Cancer Cells Promoted by Curcumin/TPP-CZL Nanomicelles With Mitochondrial Targeting Function. Front. Bioeng. Biotechnol. 2022, 10, 804513. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, F.; Wen, H.; Shi, W.; Huang, Q.; Huang, Y.; Xie, J.; Li, P.; Chen, J.; Qin, L.; et al. Tumor- and mitochondria-targeted nanoparticles eradicate drug resistant lung cancer through mitochondrial pathway of apoptosis. J. Nanobiotechnol. 2020, 18, 8. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; In, I.; Park, S.Y. pH-Responsive NIR-Absorbing Fluorescent Polydopamine with Hyaluronic Acid for Dual Targeting and Synergistic Effects of Photothermal and Chemotherapy. Biomacromolecules 2017, 18, 1825–1835. [Google Scholar] [CrossRef] [PubMed]
- Bao, J.; Zhao, Y.; Xu, J.; Guo, Y. Design and construction of IR780- and EGCG-based and mitochondrial targeting nanoparticles and their application in tumor chemo-phototherapy. J. Mater. Chem. B 2021, 9, 9932–9945. [Google Scholar] [CrossRef]
- Fu, S.; Xie, Y.; Tuo, J.; Wang, Y.; Zhu, W.; Wu, S.; Yan, G.; Hu, H. Discovery of mitochondria-targeting berberine derivatives as the inhibitors of proliferation, invasion and migration against rat C6 and human U87 glioma cells. MedChemComm 2015, 6, 164–173. [Google Scholar] [CrossRef]
- Cheng, Y.; Ji, Y. Mitochondria-targeting nanomedicine self-assembled from GSH-responsive paclitaxel-ss-berberine conjugate for synergetic cancer treatment with enhanced cytotoxicity. J. Control Release 2020, 318, 38–49. [Google Scholar] [CrossRef]
- Geng, Y.; Zhong, Y.; Zhou, Q.; Chen, S.; Piao, Y.; Yin, W.; Lu, H.; Shen, Y. A neutral water-soluble mitochondria-targeting polymer. Chem. Commun. 2019, 55, 10015–10018. [Google Scholar] [CrossRef] [PubMed]
- Geng, Y.; Xiang, J.; Shao, S.; Tang, J.; Shen, Y. Mitochondria-targeted polymer-celastrol conjugate with enhanced anticancer efficacy. J. Control Release 2022, 342, 122–133. [Google Scholar] [CrossRef] [PubMed]
- Pommier, Y.; Leo, E.; Zhang, H.; Marchand, C. DNA Topoisomerases and Their Poisoning by Anticancer and Antibacterial Drugs. Chem. Biol. 2010, 17, 421–433. [Google Scholar] [CrossRef]
- D’Yakonov, V.A.; Dzhemileva, L.U.; Dzhemilev, U.M. Advances in the Chemistry of Natural and Semisynthetic Topoisomerase I/II Inhibitors. Stud. Nat. Prod. Chem. 2017, 54, 21–86. [Google Scholar]
- Chen, Y.; Wang, Z.; Wang, X.; Su, M.; Xu, F.; Yang, L.; Jia, L.; Zhang, Z. Advances in Antitumor Nano-Drug Delivery Systems of 10-Hydroxycamptothecin. Int. J. Nanomed. 2022, 17, 4227–4259. [Google Scholar] [CrossRef]
- Pommier, Y. Topoisomerase I inhibitors: Camptothecins and beyond. Nat. Rev. Cancer 2006, 6, 789–802. [Google Scholar] [CrossRef]
- López-Lázaro, M.; Willmore, E.; Jobson, A.; Gilroy, K.L.; Curtis, H.; Padget, K.; Austin, C.A. Curcumin induces high levels of topoisomerase I- and II-DNA complexes in K562 leukemia cells. J. Nat. Prod. 2007, 70, 1884–1888. [Google Scholar] [CrossRef]
- Bensasson, R.V.; Zoete, V.; Jossang, A.; Bodo, B.; Arimondo, P.B.; Land, E.J. Potency of inhibition of human DNA topoisomerase I by flavones assessed through physicochemical parameters. Free Radic. Biol. Med. 2011, 51, 1406–1410. [Google Scholar] [CrossRef]
- Yoneda, Y.; Hieda, M.; Nagoshi, E.; Miyamoto, Y. Nucleocytoplasmic Protein Transport and Recycling of Ran. Cell Struct. Funct. 1999, 24, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Schlenstedt, G. Protein Import into the Nucleus. FEBS Lett. 1996, 389, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Fang, Z.; Zhang, M.; Yang, D.; Wang, S.; Liu, K. A Co-Delivery System of Curcumin and p53 for Enhancing the Sensitivity of Drug-Resistant Ovarian Cancer Cells to Cisplatin. Molecules 2020, 25, 2621. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Shi, W.; Gu, J.; Lee, R.J.; Zhang, Y. Design of a Novel Nucleus-Targeted NLS-KALA-SA Nanocarrier to Delivery Poorly Water-Soluble Anti-Tumor Drug for Lung Cancer Treatment. J. Pharm. Sci. 2021, 110, 2432–2441. [Google Scholar] [CrossRef]
- Huang, Z.; Liu, Y.H.; Zhang, Y.M.; Zhang, J.; Liu, Q.; Yu, X.Q. Cyclen-based cationic lipids containing a pH-sensitive moiety as gene delivery vectors. Org. Biomol. Chem. 2015, 13, 620–630. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Liu, Y.; Wang, Z.; Zhang, J.; Mu, G.; Wang, W.; Liu, J. Supramolecular nanofibers increase the efficacy of 10-hydroxycamptothecin by enhancing nuclear accumulation and depleting cellular ATP. Acta Biomater. 2021, 122, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Zhang, J.; Guo, L.; Du, C.; Song, P.; Zhao, B.; Li, L.; Li, C.; Qiao, R. Cyclen Grafted with poly[(Aspartic acid)-co-Lysine]: Preparation, Assembly with Plasmid DNA, and in Vitro Transfection Studies. Mol. Pharm. 2016, 13, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Piao, S.; Amaravadi, R.K. Targeting the lysosome in cancer. Ann. N. Y. Acad. Sci. 2016, 1371, 45–54. [Google Scholar] [CrossRef]
- Lin, J.; Yang, K.; New, E.J. Strategies for organelle targeting of fluorescent probes. Org. Biomol. Chem. 2021, 19, 9339–9357. [Google Scholar] [CrossRef]
- Khaket, T.P.; Singh, M.P.; Khan, I.; Kang, S.C. In vitro and in vivo studies on potentiation of curcumin-induced lysosomal-dependent apoptosis upon silencing of cathepsin C in colorectal cancer cells. Pharmacol. Res. 2020, 161, 105156. [Google Scholar] [CrossRef]
- Owa, C.; Messina, M.E., Jr.; Halaby, R. Triptolide induces lysosomal-mediated programmed cell death in MCF-7 breast cancer cells. Int. J. Womens Health 2013, 5, 557–569. [Google Scholar]
- Abbasi, S.; Yousefi, G.; Tamaddon, A.M.; Firuzi, O. Paclitaxel-loaded polypeptide-polyacrylamide nanomicelles overcome drug-resistance by enhancing lysosomal membrane permeability and inducing apoptosis. J. Biomed. Mater. Res. A 2021, 109, 18–30. [Google Scholar] [CrossRef]
- Wang, Y.; Ding, R.; Zhang, Z.; Zhong, C.; Wang, J.; Wang, M. Curcumin-loaded liposomes with the hepatic and lysosomal dual-targeted effects for therapy of hepatocellular carcinoma. Int. J. Pharm. 2021, 602, 120628. [Google Scholar] [CrossRef]
- Honndorf, V.S.; WSRolle, A.M.; Schmitt, J.; Kreft, L.; Quintanilla-Martinez, L.; Kohlhofer, U.; Reischl, G.; Maurer, A.; Boldt, K.; Schwarz, M.; et al. Preclinical evaluation of the anti-tumor effects of the natural isoflavone genistein in two xenograft mouse models monitored by [18F]FDG, [18F]FLT, and [64Cu]NODAGA-cetuximab small animal PET. Oncotarget 2016, 7, 28247–28261. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, T.V.; Stein, R.; de Andrade, D.F.; Beck, R.C.R. Preclinical studies of the antitumor effect of curcumin-loaded polymeric nanocapsules: A systematic review and meta-analysis. Phytother. Res. 2022, 36, 3202–3214. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.; Lin, Y.; Ni, Z.; Chen, T.; Wang, X. Therapeutic effects of natural polyphenols on colorectal adenomas: Focus on preclinical studies (Review). Oncol. Rep. 2023, 49, 112. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Yang, Z.; Wang, S.; Chen, J.; Liu, Q.; Tianle, H.; Hai, L.; Lu, R.; Wu, Y. Berberine and folic acid co-modified pH-sensitive cascade-targeted PTX-liposomes coated with Tween 80 for treating glioma. Bioorg. Med. Chem. 2022, 69, 116893. [Google Scholar] [CrossRef]
| Receptors | Targeting Ligands | Ref. |
|---|---|---|
| Folate (FA) receptor | FA | [152] |
| Transferrin (Tf) receptor | Tf | [129] |
| T7 peptide | [153] | |
| Cluster of differentiation-44 (CD44) receptor | Hyaluronic acid (HA) | [134] |
| Chondroitin sulfate (CS) | [154] | |
| Asialoglycoprotein (ASGP) receptor | Galactose (Gal) | [155] |
| Glycyrrhetinic acid (GA) receptor | GA | [13] |
| Integrin receptor αvβ3 | RGD peptide | [143] |
| Glucose transporters | Glucose dervative | [76] |
| Membrane internalization receptors (LRP1 and LRP2) and Tf receptor | Lactoferrin (Lf) | [156] |
| Amino acids transporters | Lysine | [157] |
| Carbonic anhydrase IX (CA IX) | Anti-carbonic anhydrase IX (CA IX) antibody | [158] |
| Nucleolin | Nucleolin-specific aptamer (AS1411) | [159] |
| Scavenger receptor B | High-density lipoprotein (HDL) | [160] |
| Ligand | Drug Delivery System | Natural Products | Cancer Type | In Vitro and In Vivo Evaluations | Ref. |
|---|---|---|---|---|---|
| FA | FA-BAS NPS/BA | Baicalin | Breast cancer | (1) Increasing targeted uptake efficiency and cytotoxicity; (2) Promoting apoptosis by increasing the expression of caspase-8 and reactive oxygen species (ROS) and decreasing the level of Bid. | [161] |
| PLGA-PEG-FA NPs | Genistein | Ovarian cancer | (1) Exhibiting sustained release of drug for around six days; (2) Increasing cellular uptake. | [162] | |
| FA-Cur-NPs | Curcumin | Cervical cancer | (1) Showing superior cytotoxic activity and drug uptake through FR-mediated endocytosis pathways; (2) Distributing mainly in in tumor sites of Hela xenograft mouse model and significantly inhibiting tumor growth; (3) Possessing a high safety that there was no significant change in body weight of mouse after 30 days of treatment. | [152] | |
| Cur/FA-PEG-PLA | Curcumin | Glioblastoma multiforme | (1) Suppressing the growth of GL261 cells and promoting apoptotic rate; (2) The tumor growth of tumor-bearing mice processed with Cur/FA-PEG-PLA were repressed via suppressing angiogenesis and facilitating apoptosis. | [163] | |
| FA-MPEG-PCL/CUR | Curcumin | Colorectal cancer | (1) Increasing the t1/2 and AUC; (2) Showing the most significant inhibitory and apoptotic effects on cell growth; (3) Having a stronger effect on inhibiting tumor growth, promoting tumor apoptosis, and weakening tumor angiogenesis than free Cur and unmodified micelles. | [164] | |
| FA-F108 | Evodiamine | Cervical cancer | (1) Showing dose-dependent and time-dependent cytotoxicity against Hela cells; (2) Greatly inducing apoptosis as compared to pure drug; (3) Improving intracellular delivery of evodiamine through overexpressed folate receptors. | [165] | |
| 5-FAM/FA/TP@Fe-MIL-101 | Triptolide | Liver cancer | (1) Showing better targeted therapy efficiency and reducing the systemic toxicity of triptolide; (2) The modification of 5-FAM facilitated fluorescence imaging of the tumor site and realized the construction of an integrated nano-platform for fluorescence imaging and treatment. | [166] | |
| C-RSV-FER-FA-SLNs | Resveratrol and ferulic acid | Colon cancer | Compared with free drugs, FA-modified formulations increased the cytotoxicity of cancer cells, thereby inducing cell apoptosis. | [167] | |
| RSV-FA-NLCs | Resveratrol | Breast cancer | (1) Compared with unmodified NLCs, folate-modified NLCs exhibited higher cytotoxicity on MCF-7 cells overexpressing folate receptors at high levels; (2) The AUC value of RSV-FA-NLCs increased by 9 times compared to free drug (57.92 ± 4.15, 6.37 ± 1.16 (μg/mL)·h, respectively). | [14] | |
| Tf | Tf-PIP-NPs | Piperine | Liver cancer and breast cancer | (1) Compared with unmodified preparations, Tf-PIP-NPs had strong cytotoxicity; (2) Tf-PIP-NPs could reduce mitochondrial membrane potential and induce apoptosis through mitochondrial pathway. | [129] |
| Tf-LipoMof@PL | Piperlongumine | Breast cancer | The modification of Tf enhanced the endocytosis of cells towards the formulation, thereby strengthening the ferroptotic cell death. | [168] | |
| MSN-NH2-Cur-PEG-Tf | Curcumin | Pancreatic cancer | (1) The uptake of the formulation by tumor cells was 7 times higher than that of free drugs; (2) The cytotoxicity of the preparation was 3 times higher than that of free curcumin; (3) MSN-NH2-Cur-PEG-Tf could inhibit tumor growth and reduce tumor metastasis to non-tumor sites. | [169] | |
| Tf-functionalised microemulsion (Tf-EC-MEs) | ꞵ-Elemene and celastrol | Lung cancer | (1) Improving cell uptake of A549, exhibiting stronger anti-proliferative effects and higher cell apoptosis rates; (2) In the xenograft mouse tumor model carrying A549, Tf-EC-MEs showed enhanced anti-tumor activity compared to all other treatments and did not cause significant systemic toxicity. | [170] | |
| Tf-LP-CA | Carnosic acid | Liver cancer | (1) Inducing higher levels of apoptosis and reducing the mitochondrial membrane potential more effectively in HepG2- and SMMC-7721 cells; (2) Regulating the expressions of cleaved poly(ADP-ribose) polymerase, caspase-3 and -9, and B-cell lymphoma 2 (Bcl2) family members. | [128] | |
| HA | dHAD-QT | Quercetin | Breast cancer | (1) Compared with unmodified formulations, the CD44 targeting ability of dHAD-QT micelles was significantly improved; (2) Exhibiting high cytotoxicity and the ability to induce cell apoptosis; (3) Effectively inhibiting tumor growth in tumor-bearing mice, with a tumor inhibition rate of 91.8%. | [134] |
| HA-KA-NLCs | Kaempferol | Non-small-cell lung cancer | (1) Inhibiting the proliferation, migration and invasion of A549 cells, promoting cell apoptosis and increasing cell uptake; (2) Activating the epithelial–mesenchymal transition (EMT)-related signaling pathway and regulating the expression of E-cadherin, N-cadherin, and Vimentin in A549 cells. | [136] | |
| HA-mPEG-CTD-NLC | Cantharidin | Liver cancer | (1) The t1/2, AUC, and plasma clearance rates of the formulation were higher than those of the cantharidin solution; (2) Having superior cytotoxicity and targeting effects on SMMC-7721 cells; (3) Significantly inhibiting tumor growth and prolonged survival in tumor-bearing mice, with a tumor inhibition rate of 65.96%. | [171] | |
| Cur-HA NPs | Curcumin | Colon cancer | (1) Nanoparticles can effectively orally deliver drugs to the lower gastrointestinal tract to treat local colon diseases; (2) The modification of HA can effectively increase the uptake of the formulation by HT-29 cells. | [137] | |
| UA/(AS-IV)@PDA-HA | Ursolic acid and astragaloside IV | Non-small-cell lung cancer | UA/(AS-IV)@PDA-HA could be used for chemotherapy, photothermal therapy, and immunotherapy simultaneously. In this nanosystem, HA could improve drug targeting, ursolic acid exerted cytotoxic effects, astragaloside IV mediated autoimmune response, and polydopamine-mediated photothermal therapy inhibits tumor growth. | [172] | |
| HA-DOPE@Lips/HNK | Honokiol | Osteosarcoma | (1) HA-DOPE@Lips/HNK could inhibit cell proliferation, cause apoptosis, arrest the cell cycle in the G1 phase, and disrupt mitochondrial activity. (2) In vivo experiments indicated that HA-DOPE@Lips/HNK specially delivered the drug into the tumor and inhibited tumor growth and showed no evident toxicity to normal tissues. | [173] | |
| Gal | Gal-SP188–PLGA | Resibufogenin | Liver cancer | Both cellular and animal experiments had shown that the preparation had strong liver targeting properties. | [139] |
| API-GAL-NPs | Apigenin | Liver cancer | (1) API-GAL-NPs could better improve the intracellular internalization of drugs, thereby significantly increasing the cytotoxicity and apoptotic potential of HepG2 cells; (2) By the significant reduction in nodule formation, downregulation of matrix metalloproteinases (MMP-2 and MMP-9), and induction of apoptosis in the liver, API-GAL-NPs had a better protective effect on DEN-induced liver cancer in rats. | [174] | |
| Microemulsion (Gal(oct)-C-ME) | Coix seed oil, coixan | Liver cancer | (1) The internalized Gal(oct)-C-ME was 2.28 times higher than that of formulations without Gal modification; (2) Tumor-bearing mice were gavaged with Gal(oct)-C-ME for 14 days, which had the strongest inhibitory effect on tumor growth and the lowest toxicity to the liver and kidneys. | [155] | |
| GF68-Gal | Galangin | Liver cancer | (1) Increasing the accumulation of the preparation in the liver; (2) Inhibiting P-pg and cytochrome enzyme, thus inhibiting drug effluence and metabolism | [46] | |
| RGD | HCPT@NMOFs-RGD | 10-Hydroxycamptothecin | Liver cancer | (1) HCPT@NMOFs-RGD were specifically enriched in the tumor by binding specifically to integrin αvβ3 and led to a reduction in tumor volume; (2) The xenografts in mice were eliminated remarkably following HCPT@NMOFs-RGD treatment with laser irradiation. | [175] |
| RGD-EG-SS-PTX | Paclitaxel | Gastric cancer | (1) This micelle had a controlled release function and could decompose and ultimately release PTX under the reduction in glutathione (GSH) in tumor cells; (2) It could target gastric cancer cells and inhibit cell proliferation by inducing apoptosis; (3) In vivo experiments had shown that PTX micelles could be effectively delivered to the tumor site and inhibit tumor growth. | [144] | |
| RGD modified liposomes (phosphatidylcholine/cholesterol/DSPE-PEG2000-RGD) | Vinorelbine and tetrandrine | Brain glioma | It could significantly enhance the transport between brain barriers, accumulate significantly in glioma cells, and have a significant inhibitory effect on mouse glioma. | [143] | |
| RGD-Lip-Cur | Curcumin | Breast cancer | Promoting apoptosis by activating caspase 3/7. | [145] | |
| RGD-HSA-GEM/CUR NPs | Gemcitabine and curcumin | Pancreatic cancer | In vivo research indicated that RGD-HSA-GEM/CUR NPs had significant targeting effects on subcutaneous tumors. | [176] |
| Drug | Targeting Ligands | Cancer Type | Study Interest | Ref. |
|---|---|---|---|---|
| Quercetin (Que) and paclitaxel (PTX) | NGR and RGD | Breast cancer | It was a matrix metalloproteinase-triggered dual-targeting hybrid micelle-in-liposome system. Que was delivered to tumor tissue under the guidance of NGR to exert anti-fibrotic effects, whereas PTX was delivered to tumor cells as a chemotherapy agent. | [115] |
| PTX | HA and TPP | Lung cancer | The modification of HA enabled micelles to target tumor cells through CD44 receptors. TPP promoted the accumulation of micelles in mitochondria, which was beneficial for enhancing the anti-tumor effect of PTX and reversing the multiple drug resistance. | [186] |
| PTX | HA and TPP | Breast cancer | HA and TPP targeted cell membranes and mitochondria, respectively, and boric acid had pH and photothermal responsiveness. This preparation could respond to infrared signals and regulate the release of PTX, which had great potential in tumor imaging and chemical photothermal therapy. | [187] |
| PTX | FA and BBR | Brain glioma | Liposomes modified by FA could be effectively targeted to glioma cells. BBR could be attracted by mitochondrial membrane potential and concentrate on mitochondria to achieve mitochondrial targeting and induce cell apoptosis. | [215] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Y.; Song, J.; Feng, A.; Li, J.; Li, M.; Shi, Y.; Sun, W.; Li, L. Recent Advances in Nanotechnology-Based Targeted Delivery Systems of Active Constituents in Natural Medicines for Cancer Treatment. Molecules 2023, 28, 7767. https://doi.org/10.3390/molecules28237767
Hu Y, Song J, Feng A, Li J, Li M, Shi Y, Sun W, Li L. Recent Advances in Nanotechnology-Based Targeted Delivery Systems of Active Constituents in Natural Medicines for Cancer Treatment. Molecules. 2023; 28(23):7767. https://doi.org/10.3390/molecules28237767
Chicago/Turabian StyleHu, Yu, Jizheng Song, Anjie Feng, Jieyu Li, Mengqi Li, Yu Shi, Wenxiu Sun, and Lingjun Li. 2023. "Recent Advances in Nanotechnology-Based Targeted Delivery Systems of Active Constituents in Natural Medicines for Cancer Treatment" Molecules 28, no. 23: 7767. https://doi.org/10.3390/molecules28237767
APA StyleHu, Y., Song, J., Feng, A., Li, J., Li, M., Shi, Y., Sun, W., & Li, L. (2023). Recent Advances in Nanotechnology-Based Targeted Delivery Systems of Active Constituents in Natural Medicines for Cancer Treatment. Molecules, 28(23), 7767. https://doi.org/10.3390/molecules28237767






