Joining Forces: The Combined Application of Therapeutic Viruses and Nanomaterials in Cancer Therapy
Abstract
1. Introduction
2. The Formation of Tumors and Novel Treatment Strategies
2.1. The Occurrence of Tumors
2.2. Novel Therapies for Tumors
2.2.1. Gene Therapy
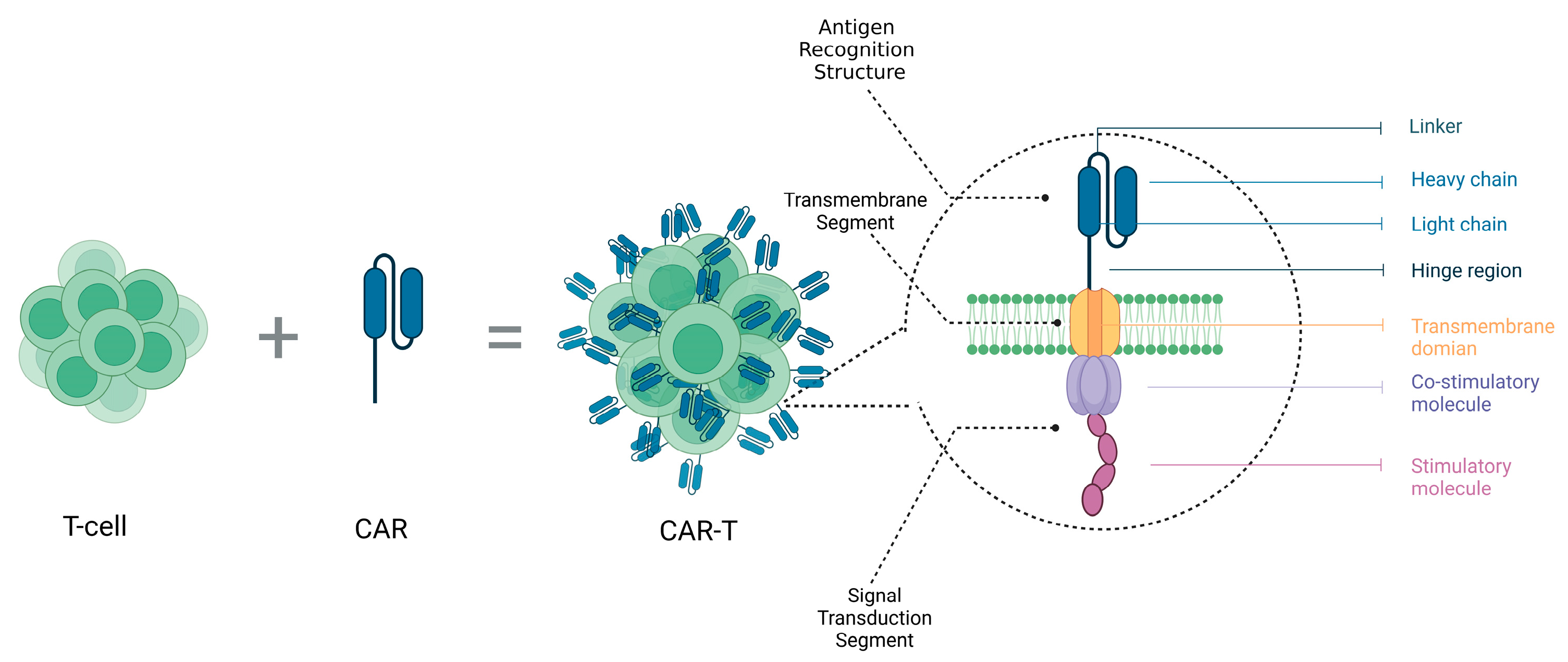
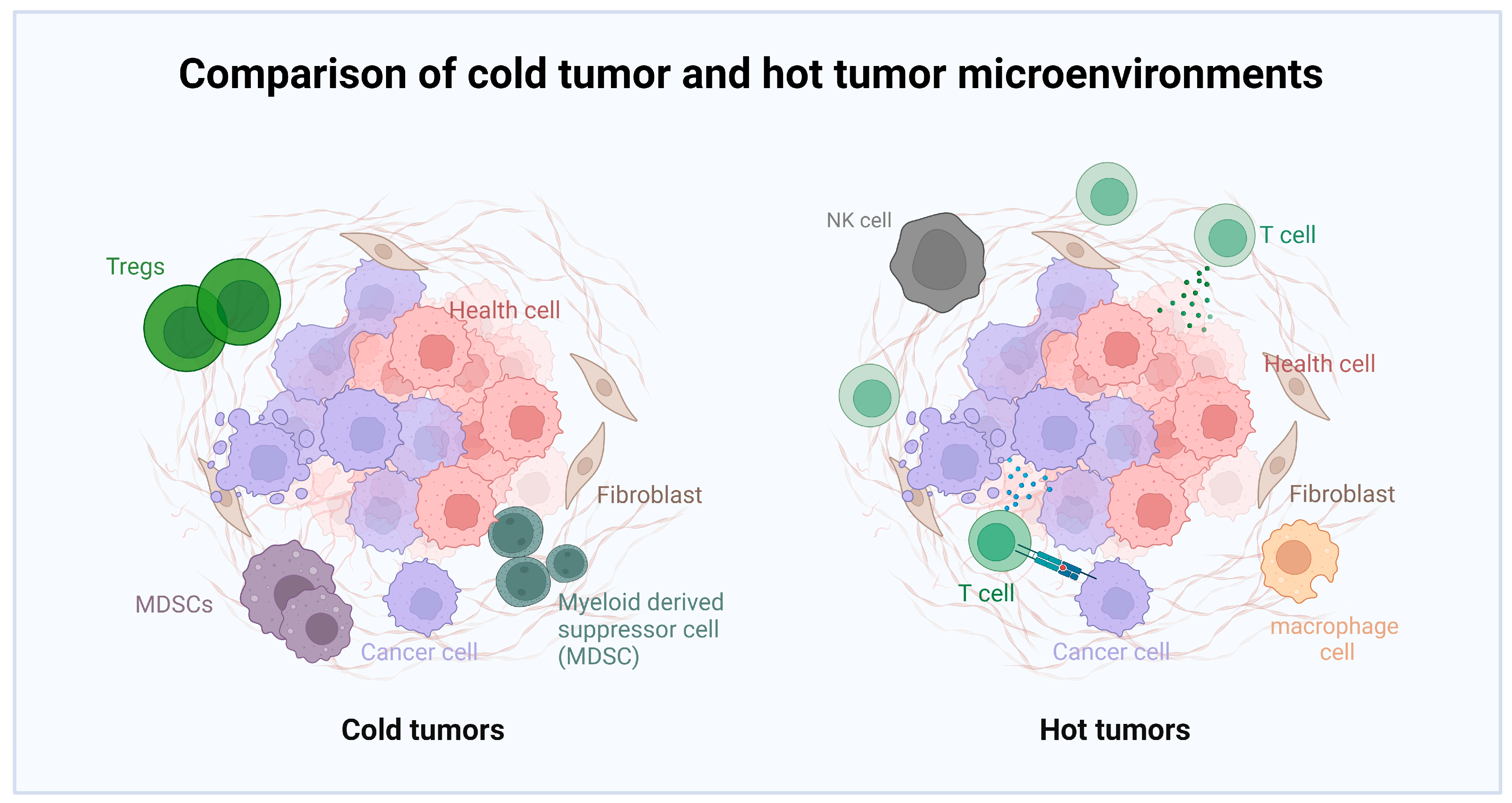
2.2.2. Immunotherapy
3. Therapeutic Viruses
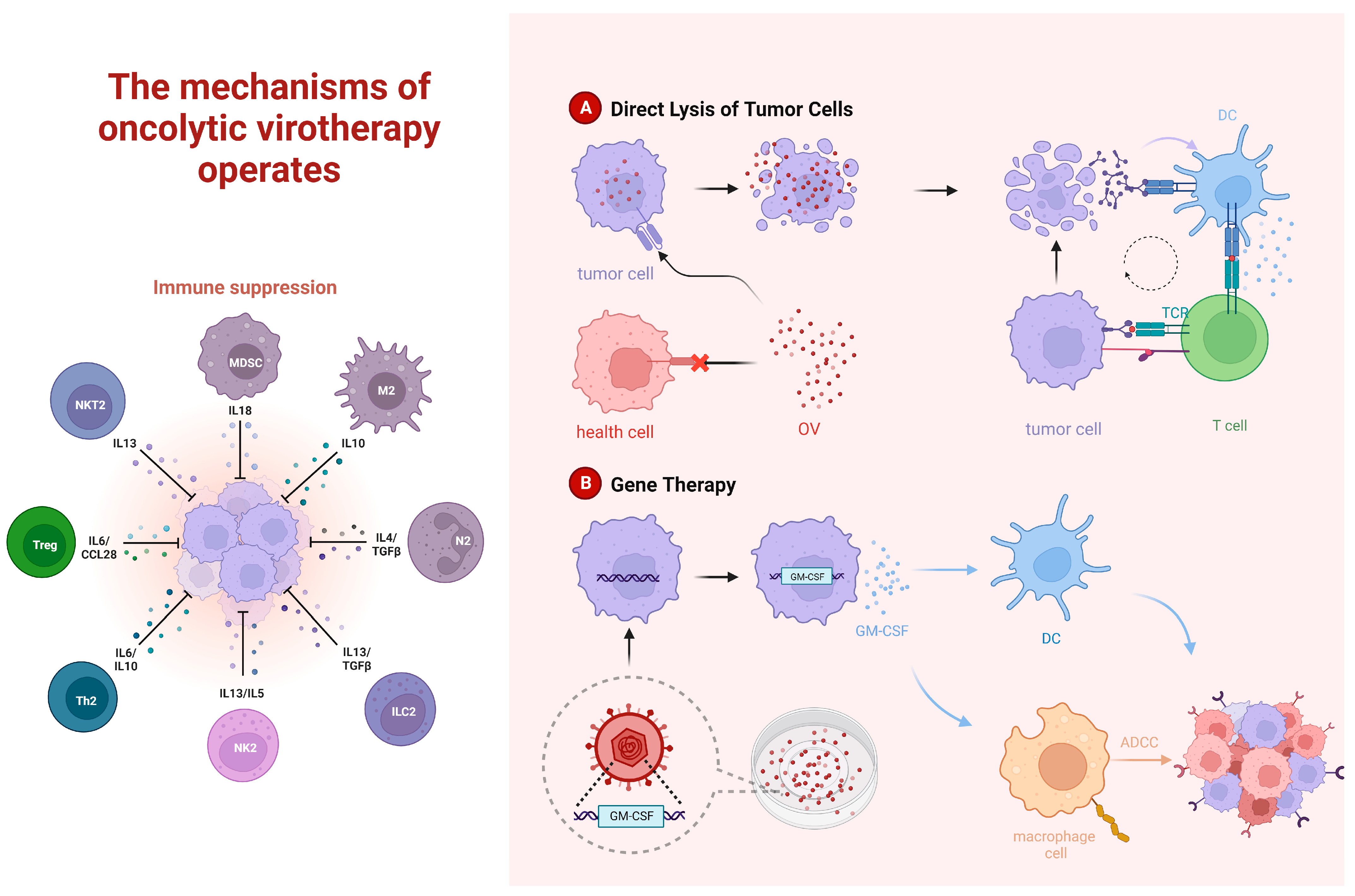
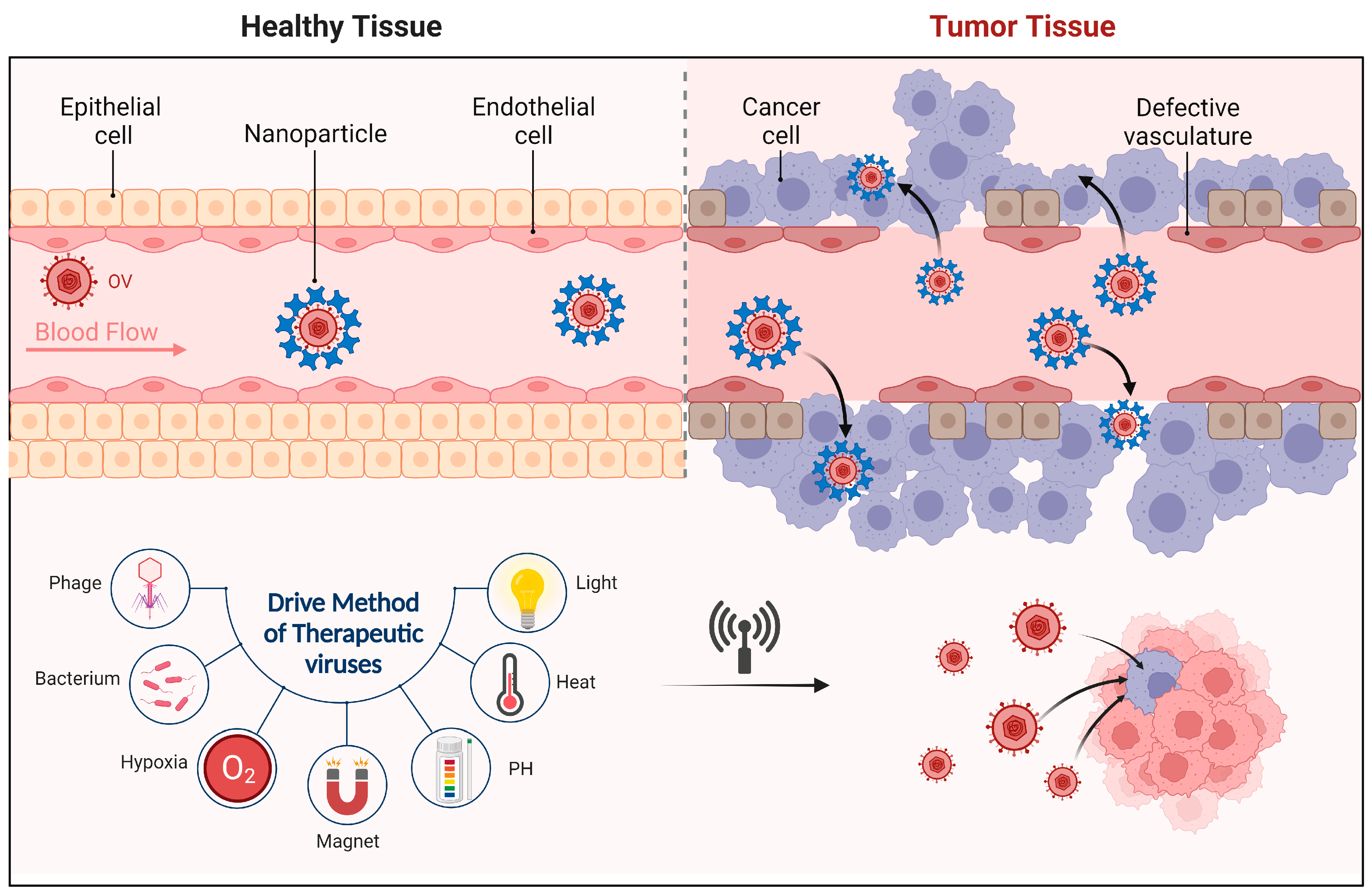
3.1. Oncolytic Viruses
3.1.1. Oncolytic Viruses Combined Radiochemotherapy
3.1.2. Oncolytic Viruses Combined with Immune Checkpoint Therapy
3.1.3. Oncolytic Viruses Combined with Adoptive Cell Therapy
3.1.4. Oncolytic Viruses Combined with Pretreatment of Nanomaterials
3.2. Viral Vectors
3.3. Immune Viruses and Virus-like Particles
4. Nanobiomaterials Delivery Systems
4.1. Definition and Advantages of Nanobiomaterials
4.2. Nanobiomaterials
4.3. Drawbacks and Limitations
5. Combination of Therapeutic Viruses and Nanobiomaterial Delivery Systems
5.1. Combination of OVs and Nanobiomaterials
5.1.1. Mechanism Principles and Advantages
5.1.2. Research Progress
5.2. Combination of Viral Vectors and Nanobiomaterial Delivery Systems
5.2.1. Advantages of Combination
5.2.2. Current Research on Combined Applications
5.3. Combination of Vaccine Viruses and Nanobiomaterial Delivery Systems
5.4. Challenges and Limitations of Combination Therapy
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2016. CA Cancer J. Clin. 2016, 66, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.X.; Massagué, J. Genetic determinants of cancer metastasis. Nat. Rev. Genet. 2007, 8, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Michels, B.E.; Mosa, M.H.; Streibl, B.I.; Zhan, T.; Menche, C.; Abou-El-Ardat, K.; Darvishi, T.; Członka, E.; Wagner, S.; Winter, J.; et al. Pooled In Vitro and In Vivo CRISPR-Cas9 Screening Identifies Tumor Suppressors in Human Colon Organoids. Cell Stem Cell 2020, 26, 782–792.e7. [Google Scholar] [CrossRef]
- Zhang, J.; Hu, Y.; Yang, J.; Li, W.; Zhang, M.; Wang, Q.; Zhang, L.; Wei, G.; Tian, Y.; Zhao, K.; et al. Non-viral, specifically targeted CAR-T cells achieve high safety and efficacy in B-NHL. Nature 2022, 609, 369–374. [Google Scholar] [CrossRef]
- Rosenblum, D.; Gutkin, A.; Kedmi, R.; Ramishetti, S.; Veiga, N.; Jacobi, A.M.; Schubert, M.S.; Friedmann-Morvinski, D.; Cohen, Z.R.; Behlke, M.A.; et al. CRISPR-Cas9 genome editing using targeted lipid nanoparticles for cancer therapy. Sci. Adv. 2020, 6, eabc9450. [Google Scholar] [CrossRef] [PubMed]
- Jo, S.; Das, S.; Williams, A.; Chretien, A.S.; Pagliardini, T.; Le Roy, A.; Fernandez, J.P.; Le Clerre, D.; Jahangiri, B.; Chion-Sotinel, I.; et al. Endowing universal CAR T-cell with immune-evasive properties using TALEN-gene editing. Nat. Commun. 2022, 13, 3453. [Google Scholar] [CrossRef]
- Das, S.; Valton, J.; Duchateau, P.; Poirot, L. Stromal depletion by TALEN-edited universal hypoimmunogenic FAP-CAR T cells enables infiltration and anti-tumor cytotoxicity of tumor antigen-targeted CAR-T immunotherapy. Front. Immunol. 2023, 14, 1172681. [Google Scholar] [CrossRef]
- Yun, W.; Kim, J.E.; Jin, Y.J.; Roh, Y.J.; Song, H.J.; Seol, A.; Kim, T.R.; Min, K.S.; Park, E.S.; Park, G.H.; et al. Chemosensitivity to doxorubicin in primary cells derived from tumor of FVB/N-Trp53(tm1Hw1) with TALEN-mediated Trp53 mutant gene. Lab. Anim. Res. 2023, 39, 23. [Google Scholar] [CrossRef]
- Herrmann, F.; Garriga-Canut, M.; Baumstark, R.; Fajardo-Sanchez, E.; Cotterell, J.; Minoche, A.; Himmelbauer, H.; Isalan, M. p53 Gene repair with zinc finger nucleases optimised by yeast 1-hybrid and validated by Solexa sequencing. PLoS ONE 2011, 6, e20913. [Google Scholar] [CrossRef]
- Ding, W.; Hu, Z.; Zhu, D.; Jiang, X.; Yu, L.; Wang, X.; Zhang, C.; Wang, L.; Ji, T.; Li, K.; et al. Zinc finger nucleases targeting the human papillomavirus E7 oncogene induce E7 disruption and a transformed phenotype in HPV16/18-positive cervical cancer cells. Clin. Cancer Res. 2014, 20, 6495–6503. [Google Scholar] [CrossRef]
- Vannocci, T.; Kurata, H.; de la Fuente, J.; Roberts, I.A.; Porter, A.C. Nuclease-stimulated homologous recombination at the human β-globin gene. J. Gene Med. 2014, 16, 1–10. [Google Scholar] [CrossRef]
- Tang, S.Y.; Zha, S.; Du, Z.; Zeng, J.; Zhu, D.; Luo, Y.; Wang, S. Targeted integration of EpCAM-specific CAR in human induced pluripotent stem cells and their differentiation into NK cells. Stem Cell Res. Ther. 2021, 12, 580. [Google Scholar] [CrossRef]
- Pham, T.; Carpinteri, S.; Sampurno, S.; Pereira, L.; Roth, S.; Narasimhan, V.; Darcy, P.; Desai, J.; Heriot, A.G.; Ramsay, R.G. Novel Vaccine Targeting Colonic Adenoma: A Pre-clinical Model. J. Gastrointest. Surg. 2019, 23, 626–633. [Google Scholar] [CrossRef] [PubMed]
- Yassin, M.; Sadowska, Z.; Tritsaris, K.; Kissow, H.; Hansen, C.H.F.; Forman, J.L.; Rogler, G.; Troelsen, J.T.; Pedersen, A.E.; Olsen, J. Rectal Insulin Instillation Inhibits Inflammation and Tumor Development in Chemically Induced Colitis. J. Crohns Colitis 2018, 12, 1459–1474. [Google Scholar] [CrossRef]
- Chen, S.Y.; Chen, K.L.; Ding, L.Y.; Yu, C.H.; Wu, H.Y.; Chou, Y.Y.; Chang, C.J.; Chang, C.H.; Wu, Y.N.; Wu, S.R.; et al. RNA bisulfite sequencing reveals NSUN2-mediated suppression of epithelial differentiation in pancreatic cancer. Oncogene 2022, 41, 3162–3176. [Google Scholar] [CrossRef] [PubMed]
- Loesch, R.; Caruso, S.; Paradis, V.; Godard, C.; Gougelet, A.; Renault, G.; Picard, S.; Tanaka, I.; Renoux-Martin, Y.; Perret, C.; et al. Deleting the β-catenin degradation domain in mouse hepatocytes drives hepatocellular carcinoma or hepatoblastoma-like tumor growth. J. Hepatol. 2022, 77, 424–435. [Google Scholar] [CrossRef]
- Kara, G.; Calin, G.A.; Ozpolat, B. RNAi-based therapeutics and tumor targeted delivery in cancer. Adv. Drug Deliv. Rev. 2022, 182, 114113. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.; Shin, M.; Lee, J.Y.; Lee, S.N.; Choi, J.H.; Choi, J.W. RNA interference (RNAi)-based plasmonic nanomaterials for cancer diagnosis and therapy. J. Control. Release 2022, 342, 228–240. [Google Scholar] [CrossRef]
- Cao, S.; Saw, P.E.; Shen, Q.; Li, R.; Liu, Y.; Xu, X. Reduction-responsive RNAi nanoplatform to reprogram tumor lipid metabolism and repolarize macrophage for combination pancreatic cancer therapy. Biomaterials 2022, 280, 121264. [Google Scholar] [CrossRef]
- Hastie, E.; Samulski, R.J. Adeno-associated virus at 50: A golden anniversary of discovery, research, and gene therapy success—A personal perspective. Hum. Gene Ther. 2015, 26, 257–265. [Google Scholar] [CrossRef]
- Fesnak, A.D.; June, C.H.; Levine, B.L. Engineered T cells: The promise and challenges of cancer immunotherapy. Nat. Rev. Cancer 2016, 16, 566–581. [Google Scholar] [CrossRef]
- Cyranoski, D. CRISPR gene-editing tested in a person for the first time. Nature 2016, 539, 479. [Google Scholar] [CrossRef] [PubMed]
- Hodi, F.S.; O’Day, S.J.; McDermott, D.F.; Weber, R.W.; Sosman, J.A.; Haanen, J.B.; Gonzalez, R.; Robert, C.; Schadendorf, D.; Hassel, J.C.; et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 2010, 363, 711–723. [Google Scholar] [CrossRef] [PubMed]
- Daassi, D.; Mahoney, K.M.; Freeman, G.J. The importance of exosomal PDL1 in tumour immune evasion. Nat. Rev. Immunol. 2020, 20, 209–215. [Google Scholar] [CrossRef]
- Kornepati, A.V.R.; Vadlamudi, R.K.; Curiel, T.J. Programmed death ligand 1 signals in cancer cells. Nat. Rev. Cancer 2022, 22, 174–189. [Google Scholar] [CrossRef] [PubMed]
- Binnewies, M.; Roberts, E.W.; Kersten, K.; Chan, V.; Fearon, D.F.; Merad, M.; Coussens, L.M.; Gabrilovich, D.I.; Ostrand-Rosenberg, S.; Hedrick, C.C.; et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat. Med. 2018, 24, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Hajitou, A.; Trepel, M.; Lilley, C.E.; Soghomonyan, S.; Alauddin, M.M.; Marini, F.C., 3rd; Restel, B.H.; Ozawa, M.G.; Moya, C.A.; Rangel, R.; et al. A hybrid vector for ligand-directed tumor targeting and molecular imaging. Cell 2006, 125, 385–398. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Samulski, R.J. Engineering adeno-associated virus vectors for gene therapy. Nat. Rev. Genet. 2020, 21, 255–272. [Google Scholar] [CrossRef] [PubMed]
- Kallert, S.M.; Darbre, S.; Bonilla, W.V.; Kreutzfeldt, M.; Page, N.; Müller, P.; Kreuzaler, M.; Lu, M.; Favre, S.; Kreppel, F.; et al. Replicating viral vector platform exploits alarmin signals for potent CD8(+) T cell-mediated tumour immunotherapy. Nat. Commun. 2017, 8, 15327. [Google Scholar] [CrossRef] [PubMed]
- Kioussis, D.; Wilson, F.; Daniels, C.; Leveton, C.; Taverne, J.; Playfair, J.H. Expression and rescuing of a cloned human tumour necrosis factor gene using an EBV-based shuttle cosmid vector. EMBO J. 1987, 6, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.Y.; Gu, J.F. Targeting gene-virotherapy of cancer. Cell Res. 2006, 16, 25–30. [Google Scholar] [CrossRef][Green Version]
- Przystal, J.M.; Waramit, S.; Pranjol, M.Z.I.; Yan, W.; Chu, G.; Chongchai, A.; Samarth, G.; Olaciregui, N.G.; Tabatabai, G.; Carcaboso, A.M.; et al. Efficacy of systemic temozolomide-activated phage-targeted gene therapy in human glioblastoma. EMBO Mol. Med. 2019, 11, e8492. [Google Scholar] [CrossRef] [PubMed]
- Veeranarayanan, S.; Azam, A.H.; Kiga, K.; Watanabe, S.; Cui, L. Bacteriophages as Solid Tumor Theragnostic Agents. Int. J. Mol. Sci. 2021, 23, 402. [Google Scholar] [CrossRef]
- Russell, S.J.; Peng, K.W.; Bell, J.C. Oncolytic virotherapy. Nat. Biotechnol. 2012, 30, 658–670. [Google Scholar] [CrossRef]
- Thirukkumaran, C.M.; Nodwell, M.J.; Hirasawa, K.; Shi, Z.Q.; Diaz, R.; Luider, J.; Johnston, R.N.; Forsyth, P.A.; Magliocco, A.M.; Lee, P.; et al. Oncolytic viral therapy for prostate cancer: Efficacy of reovirus as a biological therapeutic. Cancer Res. 2010, 70, 2435–2444. [Google Scholar] [CrossRef]
- Lichty, B.D.; Breitbach, C.J.; Stojdl, D.F.; Bell, J.C. Going viral with cancer immunotherapy. Nat. Rev. Cancer 2014, 14, 559–567. [Google Scholar] [CrossRef]
- Kaufman, H.L.; Kohlhapp, F.J.; Zloza, A. Oncolytic viruses: A new class of immunotherapy drugs. Nat. Rev. Drug Discov. 2015, 14, 642–662. [Google Scholar] [CrossRef] [PubMed]
- Gujar, S.; Pol, J.G.; Kroemer, G. Heating it up: Oncolytic viruses make tumors ‘hot’ and suitable for checkpoint blockade immunotherapies. Oncoimmunology 2018, 7, e1442169. [Google Scholar] [CrossRef]
- Martin, N.T.; Bell, J.C. Oncolytic Virus Combination Therapy: Killing One Bird with Two Stones. Mol. Ther. 2018, 26, 1414–1422. [Google Scholar] [CrossRef] [PubMed]
- Ghonime, M.G.; Jackson, J.; Shah, A.; Roth, J.; Li, M.; Saunders, U.; Coleman, J.; Gillespie, G.Y.; Markert, J.M.; Cassady, K.A. Chimeric HCMV/HSV-1 and Δγ(1)34.5 oncolytic herpes simplex virus elicit immune mediated antigliomal effect and antitumor memory. Transl. Oncol. 2018, 11, 86–93. [Google Scholar] [CrossRef]
- Du, Y.N.; Wei, Q.; Zhao, L.J.; Fan, C.Q.; Guo, L.R.; Ye, J.F.; Li, Y. Hydrogel-based co-delivery of CIK cells and oncolytic adenovirus armed with IL12 and IL15 for cancer immunotherapy. Biomed. Pharmacother. 2022, 151, 113110. [Google Scholar] [CrossRef]
- Ribas, A.; Dummer, R.; Puzanov, I.; VanderWalde, A.; Andtbacka, R.H.I.; Michielin, O.; Olszanski, A.J.; Malvehy, J.; Cebon, J.; Fernandez, E.; et al. Oncolytic Virotherapy Promotes Intratumoral T Cell Infiltration and Improves Anti-PD-1 Immunotherapy. Cell 2017, 170, 1109–1119.e10. [Google Scholar] [CrossRef] [PubMed]
- Harrington, K.; Freeman, D.J.; Kelly, B.; Harper, J.; Soria, J.C. Optimizing oncolytic virotherapy in cancer treatment. Nat. Rev. Drug Discov. 2019, 18, 689–706. [Google Scholar] [CrossRef] [PubMed]
- Vile, R.; Ando, D.; Kirn, D. The oncolytic virotherapy treatment platform for cancer: Unique biological and biosafety points to consider. Cancer Gene Ther. 2002, 9, 1062–1067. [Google Scholar] [CrossRef]
- Alvarez-Breckenridge, C.A.; Yu, J.; Price, R.; Wojton, J.; Pradarelli, J.; Mao, H.; Wei, M.; Wang, Y.; He, S.; Hardcastle, J.; et al. NK cells impede glioblastoma virotherapy through NKp30 and NKp46 natural cytotoxicity receptors. Nat. Med. 2012, 18, 1827–1834. [Google Scholar] [CrossRef] [PubMed]
- Gholami, S.; Chen, C.H.; Lou, E.; De Brot, M.; Fujisawa, S.; Chen, N.G.; Szalay, A.A.; Fong, Y. Vaccinia virus GLV-1h153 is effective in treating and preventing metastatic triple-negative breast cancer. Ann. Surg. 2012, 256, 437–445. [Google Scholar] [CrossRef]
- Fan, J.; Jiang, H.; Cheng, L.; Ma, B.; Liu, R. Oncolytic herpes simplex virus and temozolomide synergistically inhibit breast cancer cell tumorigenesis in vitro and in vivo. Oncol. Lett. 2021, 21, 99. [Google Scholar] [CrossRef]
- Fares, J.; Ahmed, A.U.; Ulasov, I.V.; Sonabend, A.M.; Miska, J.; Lee-Chang, C.; Balyasnikova, I.V.; Chandler, J.P.; Portnow, J.; Tate, M.C.; et al. Neural stem cell delivery of an oncolytic adenovirus in newly diagnosed malignant glioma: A first-in-human, phase 1, dose-escalation trial. Lancet Oncol. 2021, 22, 1103–1114. [Google Scholar] [CrossRef]
- Wei, S.C.; Duffy, C.R.; Allison, J.P. Fundamental Mechanisms of Immune Checkpoint Blockade Therapy. Cancer Discov. 2018, 8, 1069–1086. [Google Scholar] [CrossRef] [PubMed]
- Granier, C.; De Guillebon, E.; Blanc, C.; Roussel, H.; Badoual, C.; Colin, E.; Saldmann, A.; Gey, A.; Oudard, S.; Tartour, E. Mechanisms of action and rationale for the use of checkpoint inhibitors in cancer. ESMO Open 2017, 2, e000213. [Google Scholar] [CrossRef]
- Robert, C.; Long, G.V.; Brady, B.; Dutriaux, C.; Maio, M.; Mortier, L.; Hassel, J.C.; Rutkowski, P.; McNeil, C.; Kalinka-Warzocha, E.; et al. Nivolumab in previously untreated melanoma without BRAF mutation. N. Engl. J. Med. 2015, 372, 320–330. [Google Scholar] [CrossRef]
- Ribas, A.; Puzanov, I.; Dummer, R.; Schadendorf, D.; Hamid, O.; Robert, C.; Hodi, F.S.; Schachter, J.; Pavlick, A.C.; Lewis, K.D.; et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): A randomised, controlled, phase 2 trial. Lancet Oncol. 2015, 16, 908–918. [Google Scholar] [CrossRef]
- Taube, J.M.; Klein, A.; Brahmer, J.R.; Xu, H.; Pan, X.; Kim, J.H.; Chen, L.; Pardoll, D.M.; Topalian, S.L.; Anders, R.A. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin. Cancer Res. 2014, 20, 5064–5074. [Google Scholar] [CrossRef] [PubMed]
- Chesney, J.; Puzanov, I.; Collichio, F.; Singh, P.; Milhem, M.M.; Glaspy, J.; Hamid, O.; Ross, M.; Friedlander, P.; Garbe, C.; et al. Randomized, Open-Label Phase II Study Evaluating the Efficacy and Safety of Talimogene Laherparepvec in Combination With Ipilimumab Versus Ipilimumab Alone in Patients With Advanced, Unresectable Melanoma. J. Clin. Oncol. 2018, 36, 1658–1667. [Google Scholar] [CrossRef]
- Joyce, J.A.; Fearon, D.T. T cell exclusion, immune privilege, and the tumor microenvironment. Science 2015, 348, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Postow, M.A.; Sidlow, R.; Hellmann, M.D. Immune-Related Adverse Events Associated with Immune Checkpoint Blockade. N. Engl. J. Med. 2018, 378, 158–168. [Google Scholar] [CrossRef]
- Chen, L.; Zhou, C.; Chen, Q.; Shang, J.; Liu, Z.; Guo, Y.; Li, C.; Wang, H.; Ye, Q.; Li, X.; et al. Oncolytic Zika virus promotes intratumoral T cell infiltration and improves immunotherapy efficacy in glioblastoma. Mol. Ther. Oncolytics 2022, 24, 522–534. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, A.; Chaurasiya, S.; Park, A.K.; Lu, J.; Kim, S.I.; Warner, S.G.; Yuan, Y.C.; Liu, Z.; Han, H.; et al. CF33-hNIS-antiPDL1 virus primes pancreatic ductal adenocarcinoma for enhanced anti-PD-L1 therapy. Cancer Gene Ther. 2022, 29, 722–733. [Google Scholar] [CrossRef] [PubMed]
- Zuo, S.; Wei, M.; Xu, T.; Kong, L.; He, B.; Wang, S.; Wang, S.; Wu, J.; Dong, J.; Wei, J. An engineered oncolytic vaccinia virus encoding a single-chain variable fragment against TIGIT induces effective antitumor immunity and synergizes with PD-1 or LAG-3 blockade. J. Immunother. Cancer 2021, 9, e002843. [Google Scholar] [CrossRef]
- Bommareddy, P.K.; Shettigar, M.; Kaufman, H.L. Integrating oncolytic viruses in combination cancer immunotherapy. Nat. Rev. Immunol. 2018, 18, 498–513. [Google Scholar] [CrossRef]
- Evgin, L.; Kottke, T.; Tonne, J.; Thompson, J.; Huff, A.L.; van Vloten, J.; Moore, M.; Michael, J.; Driscoll, C.; Pulido, J.; et al. Oncolytic virus-mediated expansion of dual-specific CAR T cells improves efficacy against solid tumors in mice. Sci. Transl. Med. 2022, 14, eabn2231. [Google Scholar] [CrossRef]
- Huang, J.; Zheng, M.; Zhang, Z.; Tang, X.; Chen, Y.; Peng, A.; Peng, X.; Tong, A.; Zhou, L. Interleukin-7-loaded oncolytic adenovirus improves CAR-T cell therapy for glioblastoma. Cancer Immunol. Immunother. 2021, 70, 2453–2465. [Google Scholar] [CrossRef] [PubMed]
- Park, A.K.; Fong, Y.; Kim, S.I.; Yang, J.; Murad, J.P.; Lu, J.; Jeang, B.; Chang, W.C.; Chen, N.G.; Thomas, S.H.; et al. Effective combination immunotherapy using oncolytic viruses to deliver CAR targets to solid tumors. Sci. Transl. Med. 2020, 12, eaaz1863. [Google Scholar] [CrossRef] [PubMed]
- Wenthe, J.; Naseri, S.; Labani-Motlagh, A.; Enblad, G.; Wikström, K.I.; Eriksson, E.; Loskog, A.; Lövgren, T. Boosting CAR T-cell responses in lymphoma by simultaneous targeting of CD40/4-1BB using oncolytic viral gene therapy. Cancer Immunol. Immunother. 2021, 70, 2851–2865. [Google Scholar] [CrossRef]
- Jackman, J.A.; Lee, J.; Cho, N.J. Nanomedicine for Infectious Disease Applications: Innovation towards Broad-Spectrum Treatment of Viral Infections. Small 2016, 12, 1133–1139. [Google Scholar] [CrossRef] [PubMed]
- Iscaro, A.; Jones, C.; Forbes, N.; Mughal, A.; Howard, F.N.; Janabi, H.A.; Demiral, S.; Perrie, Y.; Essand, M.; Weglarz, A.; et al. Targeting circulating monocytes with CCL2-loaded liposomes armed with an oncolytic adenovirus. Nanomedicine 2022, 40, 102506. [Google Scholar] [CrossRef]
- Mendez, N.; Herrera, V.; Zhang, L.; Hedjran, F.; Feuer, R.; Blair, S.L.; Trogler, W.C.; Reid, T.R.; Kummel, A.C. Encapsulation of adenovirus serotype 5 in anionic lecithin liposomes using a bead-based immunoprecipitation technique enhances transfection efficiency. Biomaterials 2014, 35, 9554–9561. [Google Scholar] [CrossRef]
- Ito, K.; Hamamichi, S.; Asano, M.; Hori, Y.; Matsui, J.; Iwata, M.; Funahashi, Y.; Umeda, I.O.; Fujii, H. Radiolabeled liposome imaging determines an indication for liposomal anticancer agent in ovarian cancer mouse xenograft models. Cancer Sci. 2016, 107, 60–67. [Google Scholar] [CrossRef]
- Aoyama, K.; Kuroda, S.; Morihiro, T.; Kanaya, N.; Kubota, T.; Kakiuchi, Y.; Kikuchi, S.; Nishizaki, M.; Kagawa, S.; Tazawa, H.; et al. Liposome-encapsulated plasmid DNA of telomerase-specific oncolytic adenovirus with stealth effect on the immune system. Sci. Rep. 2017, 7, 14177. [Google Scholar] [CrossRef]
- Ran, H.; Quan, G.; Huang, Y.; Zhu, C.; Lu, C.; Liu, W.; Pan, X.; Wu, C. The practical self-targeted oncolytic adenoviral nanosphere based on immuno-obstruction method via polyprotein surface precipitation technique enhances transfection efficiency for virotherapy. Biochem. Biophys. Res. Commun. 2019, 508, 791–796. [Google Scholar] [CrossRef]
- Choi, J.H.; Jonsson-Schmunk, K.; Qiu, X.; Shedlock, D.J.; Strong, J.; Xu, J.X.; Michie, K.L.; Audet, J.; Fernando, L.; Myers, M.J.; et al. A Single Dose Respiratory Recombinant Adenovirus-Based Vaccine Provides Long-Term Protection for Non-Human Primates from Lethal Ebola Infection. Mol. Pharm. 2015, 12, 2712–2731. [Google Scholar] [CrossRef]
- Levine, A.G.; Mendoza, A.; Hemmers, S.; Moltedo, B.; Niec, R.E.; Schizas, M.; Hoyos, B.E.; Putintseva, E.V.; Chaudhry, A.; Dikiy, S.; et al. Stability and function of regulatory T cells expressing the transcription factor T-bet. Nature 2017, 546, 421–425. [Google Scholar] [CrossRef] [PubMed]
- Naseer, F.; Ahmad, T.; Kousar, K.; Kakar, S.; Gul, R.; Anjum, S.; Shareef, U. Formulation for the Targeted Delivery of a Vaccine Strain of Oncolytic Measles Virus (OMV) in Hyaluronic Acid Coated Thiolated Chitosan as a Green Nanoformulation for the Treatment of Prostate Cancer: A Viro-Immunotherapeutic Approach. Int. J. Nanomed. 2023, 18, 185–205. [Google Scholar] [CrossRef] [PubMed]
- Kakiuchi, Y.; Kuroda, S.; Kanaya, N.; Kagawa, S.; Tazawa, H.; Fujiwara, T. Exosomes as a drug delivery tool for cancer therapy: A new era for existing drugs and oncolytic viruses. Expert Opin. Ther. Targets 2023, 27, 807–816. [Google Scholar] [CrossRef] [PubMed]
- Lucas, T.; Benihoud, K.; Vigant, F.; Schmidt, C.Q.; Wortmann, A.; Bachem, M.G.; Simmet, T.; Kochanek, S. Hexon modification to improve the activity of oncolytic adenovirus vectors against neoplastic and stromal cells in pancreatic cancer. PLoS ONE 2015, 10, e0117254. [Google Scholar] [CrossRef] [PubMed]
- Jung, B.K.; Oh, E.; Hong, J.; Lee, Y.; Park, K.D.; Yun, C.O. A hydrogel matrix prolongs persistence and promotes specific localization of an oncolytic adenovirus in a tumor by restricting nonspecific shedding and an antiviral immune response. Biomaterials 2017, 147, 26–38. [Google Scholar] [CrossRef] [PubMed]
- Kwon, O.J.; Kang, E.; Choi, J.W.; Kim, S.W.; Yun, C.O. Therapeutic targeting of chitosan-PEG-folate-complexed oncolytic adenovirus for active and systemic cancer gene therapy. J. Control. Release 2013, 169, 257–265. [Google Scholar] [CrossRef]
- Liu, C.H.; Wong, S.H.; Tai, C.J.; Tai, C.J.; Pan, Y.C.; Hsu, H.Y.; Richardson, C.D.; Lin, L.T. Ursolic Acid and Its Nanoparticles Are Potentiators of Oncolytic Measles Virotherapy against Breast Cancer Cells. Cancers 2021, 13, 136. [Google Scholar] [CrossRef]
- Xu, H.N.; Huang, W.D.; Cai, Y.; Ding, M.; Gu, J.F.; Wei, N.; Sun, L.Y.; Cao, X.; Li, H.G.; Zhang, K.J.; et al. HCCS1-armed, quadruple-regulated oncolytic adenovirus specific for liver cancer as a cancer targeting gene-viro-therapy strategy. Mol. Cancer 2011, 10, 133. [Google Scholar] [CrossRef]
- Katsura, T.; Iwai, S.; Ota, Y.; Shimizu, H.; Ikuta, K.; Yura, Y. The effects of trichostatin A on the oncolytic ability of herpes simplex virus for oral squamous cell carcinoma cells. Cancer Gene Ther. 2009, 16, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Puig-Saus, C.; Rojas, L.A.; Laborda, E.; Figueras, A.; Alba, R.; Fillat, C.; Alemany, R. iRGD tumor-penetrating peptide-modified oncolytic adenovirus shows enhanced tumor transduction, intratumoral dissemination and antitumor efficacy. Gene Ther. 2014, 21, 767–774. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Samoranos, K.T.; Krisiewicz, A.L.; Karpinecz, B.C.; Glover, P.A.; Gale, T.V.; Chehadeh, C.; Ashshan, S.; Koya, R.; Chung, E.Y.; Lim, H.L. pH Sensitive Erythrocyte-Derived Membrane for Acute Systemic Retention and Increased Infectivity of Coated Oncolytic Vaccinia Virus. Pharmaceutics 2022, 14, 1810. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.W.; Park, J.W.; Na, Y.; Jung, S.J.; Hwang, J.K.; Choi, D.; Lee, K.G.; Yun, C.O. Using a magnetic field to redirect an oncolytic adenovirus complexed with iron oxide augments gene therapy efficacy. Biomaterials 2015, 65, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Douglas, J.T.; Kim, M.; Sumerel, L.A.; Carey, D.E.; Curiel, D.T. Efficient oncolysis by a replicating adenovirus (ad) in vivo is critically dependent on tumor expression of primary ad receptors. Cancer Res. 2001, 61, 813–817. [Google Scholar]
- Rein, D.T.; Breidenbach, M.; Curiel, D.T. Current developments in adenovirus-based cancer gene therapy. Future Oncol. 2006, 2, 137–143. [Google Scholar] [CrossRef]
- Cong, Z.; Tang, S.; Xie, L.; Yang, M.; Li, Y.; Lu, D.; Li, J.; Yang, Q.; Chen, Q.; Zhang, Z.; et al. Magnetic-Powered Janus Cell Robots Loaded with Oncolytic Adenovirus for Active and Targeted Virotherapy of Bladder Cancer. Adv. Mater. 2022, 34, e2201042. [Google Scholar] [CrossRef]
- Ring, S.S.; Cupovic, J.; Onder, L.; Lütge, M.; Perez-Shibayama, C.; Gil-Cruz, C.; Scandella, E.; De Martin, A.; Mörbe, U.; Hartmann, F.; et al. Viral vector-mediated reprogramming of the fibroblastic tumor stroma sustains curative melanoma treatment. Nat. Commun. 2021, 12, 4734. [Google Scholar] [CrossRef]
- Ghosh, S.; Banerjee, M. A smart viral vector for targeted delivery of hydrophobic drugs. Sci. Rep. 2021, 11, 7030. [Google Scholar] [CrossRef]
- Wang, T.; Narayanaswamy, R.; Ren, H.; Gillespie, J.W.; Petrenko, V.A.; Torchilin, V.P. Phage-derived protein-mediated targeted chemotherapy of pancreatic cancer. J. Drug Target. 2018, 26, 505–515. [Google Scholar] [CrossRef]
- Manivannan, A.C.; Dhandapani, R.; Velmurugan, P.; Thangavelu, S.; Paramasivam, R.; Ragunathan, L.; Saravanan, M. Phage in cancer treatment-Biology of therapeutic phage and screening of tumor targeting peptide. Expert Opin. Drug Deliv. 2022, 19, 873–882. [Google Scholar] [CrossRef] [PubMed]
- Petrenko, V.A.; Jayanna, P.K. Phage protein-targeted cancer nanomedicines. FEBS Lett. 2014, 588, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Mohsen, M.O.; Bachmann, M.F. Virus-like particle vaccinology, from bench to bedside. Cell. Mol. Immunol. 2022, 19, 993–1011. [Google Scholar] [CrossRef]
- Nooraei, S.; Bahrulolum, H.; Hoseini, Z.S.; Katalani, C.; Hajizade, A.; Easton, A.J.; Ahmadian, G. Virus-like particles: Preparation, immunogenicity and their roles as nanovaccines and drug nanocarriers. J. Nanobiotechnology 2021, 19, 59. [Google Scholar] [CrossRef]
- Alexis, F.; Pridgen, E.; Molnar, L.K.; Farokhzad, O.C. Factors affecting the clearance and biodistribution of polymeric nanoparticles. Mol. Pharm. 2008, 5, 505–515. [Google Scholar] [CrossRef]
- Kamaly, N.; Yameen, B.; Wu, J.; Farokhzad, O.C. Degradable Controlled-Release Polymers and Polymeric Nanoparticles: Mechanisms of Controlling Drug Release. Chem. Rev. 2016, 116, 2602–2663. [Google Scholar] [CrossRef] [PubMed]
- Murphy, E.A.; Majeti, B.K.; Barnes, L.A.; Makale, M.; Weis, S.M.; Lutu-Fuga, K.; Wrasidlo, W.; Cheresh, D.A. Nanoparticle-mediated drug delivery to tumor vasculature suppresses metastasis. Proc. Natl. Acad. Sci. USA 2008, 105, 9343–9348. [Google Scholar] [CrossRef]
- Chen, Q.; Nan, Y.; Yang, Y.; Xiao, Z.; Liu, M.; Huang, J.; Xiang, Y.; Long, X.; Zhao, T.; Wang, X.; et al. Nanodrugs alleviate acute kidney injury: Manipulate RONS at kidney. Bioact. Mater. 2023, 22, 141–167. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, B.; Tang, L.; Romero, G. Nanoparticles-Mediated Combination Therapies for Cancer Treatment. Adv. Ther. 2019, 2, 1900076. [Google Scholar] [CrossRef]
- Li, Y.; Humphries, B.; Yang, C.; Wang, Z. Nanoparticle-Mediated Therapeutic Agent Delivery for Treating Metastatic Breast Cancer-Challenges and Opportunities. Nanomaterials 2018, 8, 361. [Google Scholar] [CrossRef] [PubMed]
- Candela, F.; Quarta, E.; Buttini, F.; Ancona, A.; Bettini, R.; Sonvico, F. Recent Patents on Nasal Vaccines Containing Nanoadjuvants. Recent Adv. Drug Deliv. Formul. 2022, 16, 103–121. [Google Scholar] [CrossRef] [PubMed]
- De Serrano, L.O.; Burkhart, D.J. Liposomal vaccine formulations as prophylactic agents: Design considerations for modern vaccines. J. Nanobiotechnol. 2017, 15, 83. [Google Scholar] [CrossRef] [PubMed]
- Cho, C.S.; Hwang, S.K.; Gu, M.J.; Kim, C.G.; Kim, S.K.; Ju, D.B.; Yun, C.H.; Kim, H.J. Mucosal Vaccine Delivery Using Mucoadhesive Polymer Particulate Systems. Tissue Eng. Regen. Med. 2021, 18, 693–712. [Google Scholar] [CrossRef] [PubMed]
- Martín-Illana, A.; Cazorla-Luna, R.; Notario-Pérez, F.; Ruiz-Caro, R.; Rubio, J.; Tamayo, A.; Veiga, M.D. Silicon Oxycarbide Porous Particles and Film Coating as Strategies for Tenofovir Controlled Release in Vaginal Tablets for HIV Prevention. Pharmaceutics 2022, 14, 1567. [Google Scholar] [CrossRef]
- Khurana, A.; Tekula, S.; Saifi, M.A.; Venkatesh, P.; Godugu, C. Therapeutic applications of selenium nanoparticles. Biomed. Pharmacother. 2019, 111, 802–812. [Google Scholar] [CrossRef]
- Bozzuto, G.; Molinari, A. Liposomes as nanomedical devices. Int. J. Nanomed. 2015, 10, 975–999. [Google Scholar] [CrossRef] [PubMed]
- Torchilin, V.P. Recent advances with liposomes as pharmaceutical carriers. Nat. Rev. Drug Discov. 2005, 4, 145–160. [Google Scholar] [CrossRef] [PubMed]
- Sercombe, L.; Veerati, T.; Moheimani, F.; Wu, S.Y.; Sood, A.K.; Hua, S. Advances and Challenges of Liposome Assisted Drug Delivery. Front. Pharmacol. 2015, 6, 286. [Google Scholar] [CrossRef]
- Allen, T.M.; Cullis, P.R. Liposomal drug delivery systems: From concept to clinical applications. Adv. Drug Deliv. Rev. 2013, 65, 36–48. [Google Scholar] [CrossRef]
- Saraiva, C.; Praça, C.; Ferreira, R.; Santos, T.; Ferreira, L.; Bernardino, L. Nanoparticle-mediated brain drug delivery: Overcoming blood-brain barrier to treat neurodegenerative diseases. J. Control. Release 2016, 235, 34–47. [Google Scholar] [CrossRef]
- Mura, S.; Nicolas, J.; Couvreur, P. Stimuli-responsive nanocarriers for drug delivery. Nat. Mater. 2013, 12, 991–1003. [Google Scholar] [CrossRef] [PubMed]
- Kiaie, S.H.; Majidi Zolbanin, N.; Ahmadi, A.; Bagherifar, R.; Valizadeh, H.; Kashanchi, F.; Jafari, R. Recent advances in mRNA-LNP therapeutics: Immunological and pharmacological aspects. J. Nanobiotechnol. 2022, 20, 276. [Google Scholar] [CrossRef] [PubMed]
- Ullah, I.; Subbarao, R.B.; Rho, G.J. Human mesenchymal stem cells-current trends and future prospective. Biosci. Rep. 2015, 35, e00191. [Google Scholar] [CrossRef]
- Burrello, J.; Monticone, S.; Gai, C.; Gomez, Y.; Kholia, S.; Camussi, G. Stem Cell-Derived Extracellular Vesicles and Immune-Modulation. Front. Cell Dev. Biol. 2016, 4, 83. [Google Scholar] [CrossRef] [PubMed]
- Tkach, M.; Théry, C. Communication by Extracellular Vesicles: Where We Are and Where We Need to Go. Cell 2016, 164, 1226–1232. [Google Scholar] [CrossRef]
- Mulcahy, L.A.; Pink, R.C.; Carter, D.R. Routes and mechanisms of extracellular vesicle uptake. J. Extracell. Vesicles 2014, 3, 24641. [Google Scholar] [CrossRef]
- van Niel, G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef]
- Tian, T.; Zhu, Y.L.; Zhou, Y.Y.; Liang, G.F.; Wang, Y.Y.; Hu, F.H.; Xiao, Z.D. Exosome uptake through clathrin-mediated endocytosis and macropinocytosis and mediating miR-21 delivery. J. Biol. Chem. 2014, 289, 22258–22267. [Google Scholar] [CrossRef]
- Lener, T.; Gimona, M.; Aigner, L.; Börger, V.; Buzas, E.; Camussi, G.; Chaput, N.; Chatterjee, D.; Court, F.A.; Del Portillo, H.A.; et al. Applying extracellular vesicles based therapeutics in clinical trials-an ISEV position paper. J. Extracell. Vesicles 2015, 4, 30087. [Google Scholar] [CrossRef] [PubMed]
- Kamerkar, S.; LeBleu, V.S.; Sugimoto, H.; Yang, S.; Ruivo, C.F.; Melo, S.A.; Lee, J.J.; Kalluri, R. Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature 2017, 546, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Nehra, M.; Uthappa, U.T.; Kumar, V.; Kumar, R.; Dixit, C.; Dilbaghi, N.; Mishra, Y.K.; Kumar, S.; Kaushik, A. Nanobiotechnology-assisted therapies to manage brain cancer in personalized manner. J. Control. Release 2021, 338, 224–243. [Google Scholar] [CrossRef]
- Panda, S.; Hajra, S.; Kaushik, A.; Rubahn, H.G.; Mishra, Y.K.; Kim, H.J. Smart nanomaterials as the foundation of a combination approach for efficient cancer theranostics. Mater. Today Chem. 2022, 26, 101182. [Google Scholar] [CrossRef]
- Lee, O.; Jeong, S.H.; Shin, W.U.; Lee, G.; Oh, C.; Son, S.W. Influence of surface charge of gold nanorods on skin penetration. Skin Res. Technol. 2013, 19, e390–e396. [Google Scholar] [CrossRef]
- Zeng, X.; Wang, Y.; Huang, Y.S.; Han, J.; Sun, W.; Butt, H.J.; Liang, X.J.; Wu, S. Amphiphilic Metallodrug Assemblies with Red-Light-Enhanced Cellular Internalization and Tumor Penetration for Anticancer Phototherapy. Small 2022, 18, e2205461. [Google Scholar] [CrossRef]
- Parayath, N.N.; Gandham, S.K.; Amiji, M.M. Tumor-targeted miRNA nanomedicine for overcoming challenges in immunity and therapeutic resistance. Nanomedicine 2022, 17, 1355–1373. [Google Scholar] [CrossRef] [PubMed]
- Lv, C.; Ao, J.; Wang, J.; Tang, M.; Liu, A.A.; Pang, D.W. Host-cell-assisted construction of a folate-engineered nanocarrier based on viral light particles for targeted cancer therapy. Nanoscale 2021, 13, 17881–17889. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, T.; Ujiie, H.; Chen, J.; Ding, L.; Chan, H.; Gregor, A.; Bernards, N.; McVeigh, P.Z.; Fujino, K.; Lee, C.Y.; et al. Evaluation of Novel Imaging Devices for Nanoparticle-Mediated Fluorescence-Guided Lung Tumor Therapy. Ann. Thorac. Surg. 2019, 107, 1613–1620. [Google Scholar] [CrossRef]
- Simón, M.; Jørgensen, J.T.; Norregaard, K.; Kjaer, A. (18)F-FDG positron emission tomography and diffusion-weighted magnetic resonance imaging for response evaluation of nanoparticle-mediated photothermal therapy. Sci. Rep. 2020, 10, 7595. [Google Scholar] [CrossRef]
- Guglielmelli, A.; Rosa, P.; Contardi, M.; Prato, M.; Mangino, G.; Miglietta, S.; Petrozza, V.; Pani, R.; Calogero, A.; Athanassiou, A.; et al. Biomimetic keratin gold nanoparticle-mediated in vitro photothermal therapy on glioblastoma multiforme. Nanomedicine 2021, 16, 121–138. [Google Scholar] [CrossRef]
- Zhu, X.; Chen, S.; Hu, X.; Zhao, L.; Wang, Y.; Huang, J.; Chen, J.; Qiu, Y.; Zhang, X.; Wang, M.; et al. Near-Infrared Nano-Optogenetic Activation of Cancer Immunotherapy via Engineered Bacteria. Adv. Mater. 2023, 35, e2207198. [Google Scholar] [CrossRef]
- Gao, M.; Yang, T.; Qin, W.; Wang, Q.; Huang, M.; Peng, H.; Shao, M.; Yao, W.; Yi, X.; Sun, G.; et al. Cell Membrane-Anchoring Nano-Photosensitizer for Light-Controlled Calcium-Overload and Tumor-Specific Synergistic Therapy. Small 2022, 18, e2204689. [Google Scholar] [CrossRef]
- Chirivì, M.; Bearzi, C.; Rosa, P.; Miglietta, S.; Petronella, F.; De Falco, E.; Calogero, A.; Pani, R.; Petrozza, V.; Perotto, G.; et al. Biomimetic Keratin-Coated Gold Nanoparticles for Photo-Thermal Therapy in a 3D Bioprinted Glioblastoma Tumor Model. Int. J. Mol. Sci. 2022, 23, 9528. [Google Scholar] [CrossRef]
- Tu, S.; Ren, W.; Han, J.; Cui, H.; Dai, T.; Lu, H.; Xie, Y.; He, W.; Wu, A. Polydopamine nanoparticle-mediated mild photothermal therapy for inhibiting atherosclerotic plaque progression by regulating lipid metabolism of foam cells. Regen. Biomater. 2023, 10, rbad031. [Google Scholar] [CrossRef]
- Ingle, J.; Uttam, B.; Panigrahi, R.; Khatua, S.; Basu, S. Dog-bone shaped gold nanoparticle-mediated chemo-photothermal therapy impairs the powerhouse to trigger apoptosis in cancer cells. J. Mater. Chem. B 2023, 11, 9732–9741. [Google Scholar] [CrossRef]
- Yu, Z.; Gong, H.; Xu, J.; Li, Y.; Xue, F.; Zeng, Y.; Liu, X.; Tang, D. Liposome-Embedded Cu(2−x)Ag(x)S Nanoparticle-Mediated Photothermal Immunoassay for Daily Monitoring of cTnI Protein Using a Portable Thermal Imager. Anal. Chem. 2022, 94, 7408–7416. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Cao, J.; Chen, X.; Yang, K.; Zhu, L.; Fu, G.; Huang, X.; Chen, X. Noninvasive Dynamic Imaging of Tumor Early Response to Nanoparticle-mediated Photothermal Therapy. Theranostics 2015, 5, 1444–1455. [Google Scholar] [CrossRef]
- Li, S.; Jiang, Q.; Liu, S.; Zhang, Y.; Tian, Y.; Song, C.; Wang, J.; Zou, Y.; Anderson, G.J.; Han, J.Y.; et al. A DNA nanorobot functions as a cancer therapeutic in response to a molecular trigger in vivo. Nat. Biotechnol. 2018, 36, 258–264. [Google Scholar] [CrossRef]
- Ventola, C.L. Progress in Nanomedicine: Approved and Investigational Nanodrugs. Pharm. Ther. 2017, 42, 742–755. [Google Scholar]
- He, B.; Sui, X.; Yu, B.; Wang, S.; Shen, Y.; Cong, H. Recent advances in drug delivery systems for enhancing drug penetration into tumors. Drug Deliv. 2020, 27, 1474–1490. [Google Scholar] [CrossRef] [PubMed]
- Kozielski, K.L.; Tzeng, S.Y.; Green, J.J. Bioengineered nanoparticles for siRNA delivery. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2013, 5, 449–468. [Google Scholar] [CrossRef] [PubMed]
- Hajeri, P.B.; Sharma, N.S.; Yamamoto, M. Oncolytic Adenoviruses: Strategies for Improved Targeting and Specificity. Cancers 2020, 12, 1504. [Google Scholar] [CrossRef] [PubMed]
- Baker, A.T.; Aguirre-Hernández, C.; Halldén, G.; Parker, A.L. Designer Oncolytic Adenovirus: Coming of Age. Cancers 2018, 10, 201. [Google Scholar] [CrossRef]
- Barenholz, Y. Doxil®--the first FDA-approved nano-drug: Lessons learned. J. Control. Release 2012, 160, 117–134. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Wu, J.; Sawa, T.; Matsumura, Y.; Hori, K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: A review. J. Control. Release 2000, 65, 271–284. [Google Scholar] [CrossRef]
- Maeda, H.; Nakamura, H.; Fang, J. The EPR effect for macromolecular drug delivery to solid tumors: Improvement of tumor uptake, lowering of systemic toxicity, and distinct tumor imaging in vivo. Adv. Drug Deliv. Rev. 2013, 65, 71–79. [Google Scholar] [CrossRef]
- Eguchi, M.; Hirata, S.; Ishigami, I.; Shuwari, N.; Ono, R.; Tachibana, M.; Tanuma, M.; Kasai, A.; Hashimoto, H.; Ogawara, K.I.; et al. Pre-treatment of oncolytic reovirus improves tumor accumulation and intratumoral distribution of PEG-liposomes. J. Control. Release 2023, 354, 35–44. [Google Scholar] [CrossRef]
- Wang, J.Y.; Chen, H.; Dai, S.Z.; Huang, F.Y.; Lin, Y.Y.; Wang, C.C.; Li, L.; Zheng, W.P.; Tan, G.H. Immunotherapy combining tumor and endothelium cell lysis with immune enforcement by recombinant MIP-3α Newcastle disease virus in a vessel-targeting liposome enhances antitumor immunity. J. Immunother. Cancer 2022, 10, e003950. [Google Scholar] [CrossRef] [PubMed]
- Yoon, A.R.; Rivera-Cruz, C.; Gimble, J.M.; Yun, C.O.; Figueiredo, M.L. Immunotherapy by mesenchymal stromal cell delivery of oncolytic viruses for treating metastatic tumors. Mol. Ther. Oncolytics 2022, 25, 78–97. [Google Scholar] [CrossRef]
- Tahir, M.; Ahmad, N.; Lei, D.; Ali, S. Emerging role of oncolytic viruses and stem cells in gene therapy: Should they be integrated? Drug Discov. Today 2022, 27, 2244–2251. [Google Scholar] [CrossRef]
- Shimizu, Y.; Gumin, J.; Gao, F.; Hossain, A.; Shpall, E.J.; Kondo, A.; Parker Kerrigan, B.C.; Yang, J.; Ledbetter, D.; Fueyo, J.; et al. Characterization of patient-derived bone marrow human mesenchymal stem cells as oncolytic virus carriers for the treatment of glioblastoma. J. Neurosurg. 2022, 136, 757–767. [Google Scholar] [CrossRef]
- Lu, Y.; Aimetti, A.A.; Langer, R.; Gu, Z. Bioresponsive materials. Nat. Rev. Mater. 2016, 2, 16075. [Google Scholar] [CrossRef]
- Rani, S.; Ryan, A.E.; Griffin, M.D.; Ritter, T. Mesenchymal Stem Cell-derived Extracellular Vesicles: Toward Cell-free Therapeutic Applications. Mol. Ther. 2015, 23, 812–823. [Google Scholar] [CrossRef] [PubMed]
- Garofalo, M.; Villa, A.; Brunialti, E.; Crescenti, D.; Dell’Omo, G.; Kuryk, L.; Vingiani, A.; Mazzaferro, V.; Ciana, P. Cancer-derived EVs show tropism for tissues at early stage of neoplastic transformation. Nanotheranostics 2021, 5, 1–7. [Google Scholar] [CrossRef]
- Wu, A.T.H.; Srivastava, P.; Yadav, V.K.; Tzeng, D.T.W.; Iamsaard, S.; Su, E.C.; Hsiao, M.; Liu, M.C. Ovatodiolide, isolated from Anisomeles indica, suppresses bladder carcinogenesis through suppression of mTOR/β-catenin/CDK6 and exosomal miR-21 derived from M2 tumor-associated macrophages. Toxicol. Appl. Pharmacol. 2020, 401, 115109. [Google Scholar] [CrossRef] [PubMed]
- Ilett, E.; Kottke, T.; Donnelly, O.; Thompson, J.; Willmon, C.; Diaz, R.; Zaidi, S.; Coffey, M.; Selby, P.; Harrington, K.; et al. Cytokine conditioning enhances systemic delivery and therapy of an oncolytic virus. Mol. Ther. 2014, 22, 1851–1863. [Google Scholar] [CrossRef]
- Kakiuchi, Y.; Kuroda, S.; Kanaya, N.; Kumon, K.; Tsumura, T.; Hashimoto, M.; Yagi, C.; Sugimoto, R.; Hamada, Y.; Kikuchi, S.; et al. Local oncolytic adenovirotherapy produces an abscopal effect via tumor-derived extracellular vesicles. Mol. Ther. 2021, 29, 2920–2930. [Google Scholar] [CrossRef] [PubMed]
- Saari, H.; Turunen, T.; Lõhmus, A.; Turunen, M.; Jalasvuori, M.; Butcher, S.J.; Ylä-Herttuala, S.; Viitala, T.; Cerullo, V.; Siljander, P.R.M.; et al. Extracellular vesicles provide a capsid-free vector for oncolytic adenoviral DNA delivery. J. Extracell. Vesicles 2020, 9, 1747206. [Google Scholar] [CrossRef]
- Wedge, M.E.; Jennings, V.A.; Crupi, M.J.F.; Poutou, J.; Jamieson, T.; Pelin, A.; Pugliese, G.; de Souza, C.T.; Petryk, J.; Laight, B.J.; et al. Virally programmed extracellular vesicles sensitize cancer cells to oncolytic virus and small molecule therapy. Nat. Commun. 2022, 13, 1898. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhang, T.; Ma, X.; Yang, Q.C.; Yang, L.L.; Yang, S.C.; Liang, M.; Xu, Z.; Sun, Z.J. Microenvironment-Responsive Prodrug-Induced Pyroptosis Boosts Cancer Immunotherapy. Adv. Sci. 2021, 8, e2101840. [Google Scholar] [CrossRef]
- Tang, D.; Kang, R.; Berghe, T.V.; Vandenabeele, P.; Kroemer, G. The molecular machinery of regulated cell death. Cell Res. 2019, 29, 347–364. [Google Scholar] [CrossRef]
- Kovacs, S.B.; Miao, E.A. Gasdermins: Effectors of Pyroptosis. Trends Cell Biol. 2017, 27, 673–684. [Google Scholar] [CrossRef] [PubMed]
- Su, W.; Qiu, W.; Li, S.J.; Wang, S.; Xie, J.; Yang, Q.C.; Xu, J.; Zhang, J.; Xu, Z.; Sun, Z.J. Dual-Responsive STAT3 Inhibitor Nanoprodrug Combined with Oncolytic Virus Elicits Synergistic Antitumor Immune Responses by Igniting Pyroptosis. Adv. Mater. 2022, 35, e2209379. [Google Scholar] [CrossRef] [PubMed]
- Kasala, D.; Lee, S.H.; Hong, J.; Oh, E.; Yoon, A.R.; Yun, C.O. Bioreducible polymer-mediated delivery of oncolytic adenovirus can attenuate antiviral immune response and concurrently enhance the induction of antitumor immune response to effectively prevent metastasis. Biomater. Sci. 2022, 10, 4293–4308. [Google Scholar] [CrossRef] [PubMed]
- Lv, P.; Liu, X.; Chen, X.; Liu, C.; Zhang, Y.; Chu, C.; Wang, J.; Wang, X.; Chen, X.; Liu, G. Genetically Engineered Cell Membrane Nanovesicles for Oncolytic Adenovirus Delivery: A Versatile Platform for Cancer Virotherapy. Nano Lett. 2019, 19, 2993–3001. [Google Scholar] [CrossRef]
- Huang, L.L.; Li, X.; Zhang, J.; Zhao, Q.R.; Zhang, M.J.; Liu, A.A.; Pang, D.W.; Xie, H.Y. MnCaCs-Biomineralized Oncolytic Virus for Bimodal Imaging-Guided and Synergistically Enhanced Anticancer Therapy. Nano Lett. 2019, 19, 8002–8009. [Google Scholar] [CrossRef]
- Howard, F.H.N.; Al-Janabi, H.; Patel, P.; Cox, K.; Smith, E.; Vadakekolathu, J.; Pockley, A.G.; Conner, J.; Nohl, J.F.; Allwood, D.A.; et al. Nanobugs as Drugs: Bacterial Derived Nanomagnets Enhance Tumor Targeting and Oncolytic Activity of HSV-1 Virus. Small 2022, 18, e2104763. [Google Scholar] [CrossRef]
- Tseng, S.J.; Huang, K.Y.; Kempson, I.M.; Kao, S.H.; Liu, M.C.; Yang, S.C.; Liao, Z.X.; Yang, P.C. Remote Control of Light-Triggered Virotherapy. ACS Nano 2016, 10, 10339–10346. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, S.; Wang, J.; Li, N.; Zhou, J.; Chen, H. Application of Nano Drug Delivery System (NDDS) in Cancer Therapy: A Perspective. Recent Pat. Anticancer Drug Discov. 2022, 18, 125–132. [Google Scholar] [CrossRef]
- Sasso, E.; D’Alise, A.M.; Zambrano, N.; Scarselli, E.; Folgori, A.; Nicosia, A. New viral vectors for infectious diseases and cancer. Semin. Immunol. 2020, 50, 101430. [Google Scholar] [CrossRef]
- Kurup, D.; Wirblich, C.; Feldmann, H.; Marzi, A.; Schnell, M.J. Rhabdovirus-based vaccine platforms against henipaviruses. J. Virol. 2015, 89, 144–154. [Google Scholar] [CrossRef]
- Vyas, K.; Rathod, M.; Patel, M.M. Insight on nano drug delivery systems with targeted therapy in treatment of oral cancer. Nanomedicine 2023, 49, 102662. [Google Scholar] [CrossRef]
- Lin, G.; Zhang, H.; Huang, L. Smart polymeric nanoparticles for cancer gene delivery. Mol. Pharm. 2015, 12, 314–321. [Google Scholar] [CrossRef]
- Albuquerque, T.; Neves, A.R.; Quintela, T.; Costa, D. Exploring the link between chronobiology and drug delivery: Effects on cancer therapy. J. Mol. Med. 2021, 99, 1349–1371. [Google Scholar] [CrossRef]
- Ganeson, K.; Alias, A.H.; Murugaiyah, V.; Amirul, A.A.; Ramakrishna, S.; Vigneswari, S. Microneedles for Efficient and Precise Drug Delivery in Cancer Therapy. Pharmaceutics 2023, 15, 744. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.C. Baculoviral vectors for gene delivery: A review. Curr. Gene Ther. 2008, 8, 54–65. [Google Scholar] [CrossRef]
- Bhattacharjee, B.; Hallan, V. Geminivirus-Derived Vectors as Tools for Functional Genomics. Front. Microbiol. 2022, 13, 799345. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Yang, Z.; Zheng, M.; Shi, L.; Gu, M.; Liu, G.; Miao, F.; Chang, Y.; Huang, F.; Tang, N. Recombinant adeno-associated virus 8 vector in gene therapy: Opportunities and challenges. Genes Dis. 2023, 11, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Malogolovkin, A.; Egorov, A.D.; Karabelsky, A.; Ivanov, R.A.; Verkhusha, V.V. Optogenetic technologies in translational cancer research. Biotechnol. Adv. 2022, 60, 108005. [Google Scholar] [CrossRef]
- Roesch, A.; Zölls, S.; Stadler, D.; Helbig, C.; Wuchner, K.; Kersten, G.; Hawe, A.; Jiskoot, W.; Menzen, T. Particles in Biopharmaceutical Formulations, Part 2: An Update on Analytical Techniques and Applications for Therapeutic Proteins, Viruses, Vaccines and Cells. J. Pharm. Sci. 2022, 111, 933–950. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; La Salvia, S.; Liang, Y.; Adamiak, M.; Kohlbrenner, E.; Jeong, D.; Chepurko, E.; Ceholski, D.; Lopez-Gordo, E.; Yoon, S.; et al. Extracellular Vesicle-Encapsulated Adeno-Associated Viruses for Therapeutic Gene Delivery to the Heart. Circulation 2023, 148, 405–425. [Google Scholar] [CrossRef]
- Singh, R.; Al-Jamal, K.T.; Lacerda, L.; Kostarelos, K. Nanoengineering artificial lipid envelopes around adenovirus by self-assembly. ACS Nano 2008, 2, 1040–1050. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Balasundaram, G.; Lo, S.L.; Guang, E.C.; Xue, J.M.; Song, J.; Wan, A.C.; Ying, J.Y.; Wang, S. Microfibers fabricated by non-covalent assembly of peptide and DNA for viral vector encapsulation and cancer therapy. Adv. Mater. 2012, 24, 3280–3284. [Google Scholar] [CrossRef]
- Marín-Caba, L.; Chariou, P.L.; Pesquera, C.; Correa-Duarte, M.A.; Steinmetz, N.F. Tobacco Mosaic Virus-Functionalized Mesoporous Silica Nanoparticles, a Wool-Ball-like Nanostructure for Drug Delivery. Langmuir 2019, 35, 203–211. [Google Scholar] [CrossRef]
- Barkovich, K.J.; Wu, Z.; Zhao, Z.; Simms, A.; Chang, E.Y.; Steinmetz, N.F. Physalis Mottle Virus-Like Nanocarriers with Expanded Internal Loading Capacity. Bioconjug. Chem. 2023, 34, 1585–1595. [Google Scholar] [CrossRef]
- Kim, S.Y.; Lee, S.J.; Kim, J.K.; Choi, H.G.; Lim, S.J. Optimization and physicochemical characterization of a cationic lipid-phosphatidylcholine mixed emulsion formulated as a highly efficient vehicle that facilitates adenoviral gene transfer. Int. J. Nanomed. 2017, 12, 7323–7335. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kernan, D.L.; Wen, A.M.; Pitek, A.S.; Steinmetz, N.F. Featured Article: Delivery of chemotherapeutic vcMMAE using tobacco mosaic virus nanoparticles. Exp. Biol. Med. 2017, 242, 1405–1411. [Google Scholar] [CrossRef] [PubMed]
- Tockary, T.A.; Foo, W.; Dirisala, A.; Chen, Q.; Uchida, S.; Osawa, S.; Mochida, Y.; Liu, X.; Kinoh, H.; Cabral, H.; et al. Single-Stranded DNA-Packaged Polyplex Micelle as Adeno-Associated-Virus-Inspired Compact Vector to Systemically Target Stroma-Rich Pancreatic Cancer. ACS Nano 2019, 13, 12732–12742. [Google Scholar] [CrossRef]
- Gonzalez-Pastor, R.; Hernandez, Y.; Gimeno, M.; de Martino, A.; Man, Y.K.S.; Hallden, G.; Quintanilla, M.; de la Fuente, J.M.; Martin-Duque, P. Coating an adenovirus with functionalized gold nanoparticles favors uptake, intracellular trafficking and anti-cancer therapeutic efficacy. Acta Biomater. 2021, 134, 593–604. [Google Scholar] [CrossRef]
- Unchwaniwala, N.; Zhan, H.; den Boon, J.A.; Ahlquist, P. Cryo-electron microscopy of nodavirus RNA replication organelles illuminates positive-strand RNA virus genome replication. Curr. Opin. Virol. 2021, 51, 74–79. [Google Scholar] [CrossRef]
- Tornesello, A.L.; Tagliamonte, M.; Buonaguro, F.M.; Tornesello, M.L.; Buonaguro, L. Virus-like Particles as Preventive and Therapeutic Cancer Vaccines. Vaccines 2022, 10, 227. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.N.; Kouzi, S.A.; Hussain, M.D. Strategies for Developing Oral Vaccines for Human Papillomavirus (HPV) Induced Cancer using Nanoparticle mediated Delivery System. J. Pharm. Pharm. Sci. 2015, 18, 220–234. [Google Scholar] [CrossRef] [PubMed]
- Davodabadi, F.; Sarhadi, M.; Arabpour, J.; Sargazi, S.; Rahdar, A.; Díez-Pascual, A.M. Breast cancer vaccines: New insights into immunomodulatory and nano-therapeutic approaches. J. Control. Release 2022, 349, 844–875. [Google Scholar] [CrossRef]
- Hills, R.A.; Howarth, M. Virus-like particles against infectious disease and cancer: Guidance for the nano-architect. Curr. Opin. Biotechnol. 2022, 73, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Heinimäki, S.; Lampinen, V.; Tamminen, K.; Hankaniemi, M.M.; Malm, M.; Hytönen, V.P.; Blazevic, V. Antigenicity and immunogenicity of HA2 and M2e influenza virus antigens conjugated to norovirus-like, VP1 capsid-based particles by the SpyTag/SpyCatcher technology. Virology 2022, 566, 89–97. [Google Scholar] [CrossRef]
- Rana, I.; Oh, J.; Baig, J.; Moon, J.H.; Son, S.; Nam, J. Nanocarriers for cancer nano-immunotherapy. Drug Deliv. Transl. Res. 2022, 13, 1936–1954. [Google Scholar] [CrossRef]
- Lundstrom, K. Alphaviruses in cancer immunotherapy. Int. Rev. Cell Mol. Biol. 2023, 379, 143–168. [Google Scholar] [CrossRef] [PubMed]
- Venkataraman, S.; Hefferon, K. Application of Plant Viruses in Biotechnology, Medicine, and Human Health. Viruses 2021, 13, 1697. [Google Scholar] [CrossRef] [PubMed]
- Zheng, P.; Yang, Y.; Fu, Y.; He, J.; Hu, Y.; Zheng, X.; Duan, B.; Wang, M.; Liu, Q.; Li, W.; et al. Engineered Norovirus-Derived Nanoparticles as a Plug-and-Play Cancer Vaccine Platform. ACS Nano 2023, 17, 3412–3429. [Google Scholar] [CrossRef]
- Li, J.; Wang, Y.; Zhu, Y.; Oupický, D. Recent advances in delivery of drug-nucleic acid combinations for cancer treatment. J. Control. Release 2013, 172, 589–600. [Google Scholar] [CrossRef]
- Wang, Y.; Ye, M.; Xie, R.; Gong, S. Enhancing the In Vitro and In Vivo Stabilities of Polymeric Nucleic Acid Delivery Nanosystems. Bioconjug. Chem. 2019, 30, 325–337. [Google Scholar] [CrossRef]
- Chakraborty, D.; Ghosh, D.; Kumar, S.; Jenkins, D.; Chandrasekaran, N.; Mukherjee, A. Nano-diagnostics as an emerging platform for oral cancer detection: Current and emerging trends. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2023, 15, e1830. [Google Scholar] [CrossRef] [PubMed]
| Virus | Structure | Characteristic | Advantage | Related Treatments | Tumor Associations | Refs. |
|---|---|---|---|---|---|---|
| Adeno-Associated Virus |  | The helper virus replicates, infects, and lyses host cells only in the presence of the helper virus. | It has the advantages of strong safety, simple structure, and wide host, and as a prokaryotic virus, it is easier to eliminate the natural tropism of mammalian cells and bind to its receptors. | Luxturna, Zolgensma | Bladder cancer, prostate cancer, Kaposi’s sarcoma | [28,29] |
| Lymphocytic Choriomeningitis Virus |  | It replicates, and transcription and replication are mainly dependent on the polymerase synthesized by the virus itself. | The simplicity and rapid ability of vector generation to stabilize attenuation could elicit CTL responses of high intensity and cytolytic capacity. | Junin arenavirus live attenuated vaccine Candidate #1 | Lung cancer, Colorectal cancer, Breast cancer, Pancreatic cancer | [30] |
| Epstein-Barr Virus |  | The ability to cause sarcoma necrosis, regression of transplantable tumors, and death of several tumor cell lines. | Leaving most normal cells unaffected and with little species specificity. Efficient transfection of relevant clay particles. | Gene modification | Nasopharyngeal carcinoma, Lymphoma | [31] |
| Adenovirus Vector ZD55 |  | ZD55 has one cloning site to insert recombinant genes, ZD55 has elements of only Adv5. | The weak toxic effect of the virus was enhanced by the expression of the antitumor transgene. | ZD55-IL-24 | Hepatoma, Breast cancer | [32] |
| M13 Bacteriophage |  | It infects only bacterial cells and lacks native tropism to normal tissues in humans and eukaryotes in general. | It is safe and has good tumor selectivity. | Gene modification | Soft tissue sar coma, Melanoma, Breast cancer, prostate cancer, pancreatic cancer | [33] |
| Phage |  | Phages pervade the human body, transcytosing human tissues efficiently and crossing the blood-brain barrier. | Safe and non-pathogenic nature. Most phages range in the nanoscale diameter. Phages in general lack diversity in their surface architecture. Phages are known to modulate innate as well as humoral immunity. | SP94-targeted virus, T-VEC | Breast cancer, prostate cancer, Chondrosarcoma | [34] |
| Name | Diagram | Features | Advantage | Related Treatments | Tumor | Ref. |
|---|---|---|---|---|---|---|
| Immune stimulating Nano adjuvants |  | Influence immune response through the effect of antigen availability over time | Enhance antigenicity with weak antigenic substances, increase the level of specific circulating antibodies or produce effective protective immunity, change the produced, enhance cell-mediated hypersensitivity response, protect antigens from the decomposition of enzymes in the body | Nasal vaccines | Enhance the efficacy of cancer immunotherapy | [101] |
| Liposomal vaccine |  | Developed to target specific immune cell types to induce certain immune responses | Ability to encapsulate and deliver vaccines to specific locations in the body and release their contents at specific | For the treatment of important viral, bacterial, fungal, and parasitic infections (including tuberculosis, tuberculosis) | A promising mRNA vaccine delivery vector that can effectively elicit tumor-specific cytotoxic T lymphocyte responses | [102] |
| Polymer vaccine |  | Protect antigens from degradation and prolong the residence time of antigens at the target site | It can prove the safety of vaccines and can be used to deliver immune adjuvants and achieve sustained release of vaccine | As a therapeutic agent delivery system for oncology treatment | The most attractive candidate for polymer nanoparticles in tumor immunotherapy | [103] |
| Virus-like particles |  | A virus-derived structure consisting of one or more different molecules with self-assembly ability, with the ability to self-assemble, mimicking the form and size of viral particles, but lacking genetic material | Storing immunogenicity and biological activity | CRISPR/Cas9 mRNA can be delivered for safe and efficient in vivo gene editing | It plays an important role in the prevention and treatment of infectious diseases and cancer | [94] |
| Porous silicon particles |  | Sustained release of antiretroviral drugs | It can be used as a vehicle for the continuous release and processing of tumor antigens | Incorporated into tablets, thereby providing a sustained release of the drug | Prevention of HIV | [104] |
| Selenium nanoparticles |  | Has significantly reduced toxicity | Nanoselenium has better antioxidant capacity than other chemical forms of selenium and has important antibacterial activity against pathogenic bacteria, fungi, and parasites | It is used for various oxidative stress and inflammation-mediated diseases such as arthritis, cancer, diabetes, and kidney disease | Achieve precise anti-cancer treatment | [105] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Zhu, Y.; Wang, X.; Feng, Y.; Qian, Y.; Ma, Q.; Li, X.; Chen, Y.; Chen, K. Joining Forces: The Combined Application of Therapeutic Viruses and Nanomaterials in Cancer Therapy. Molecules 2023, 28, 7679. https://doi.org/10.3390/molecules28227679
Li H, Zhu Y, Wang X, Feng Y, Qian Y, Ma Q, Li X, Chen Y, Chen K. Joining Forces: The Combined Application of Therapeutic Viruses and Nanomaterials in Cancer Therapy. Molecules. 2023; 28(22):7679. https://doi.org/10.3390/molecules28227679
Chicago/Turabian StyleLi, Hongyu, Yunhuan Zhu, Xin Wang, Yilu Feng, Yuncheng Qian, Qiman Ma, Xinyuan Li, Yihan Chen, and Keda Chen. 2023. "Joining Forces: The Combined Application of Therapeutic Viruses and Nanomaterials in Cancer Therapy" Molecules 28, no. 22: 7679. https://doi.org/10.3390/molecules28227679
APA StyleLi, H., Zhu, Y., Wang, X., Feng, Y., Qian, Y., Ma, Q., Li, X., Chen, Y., & Chen, K. (2023). Joining Forces: The Combined Application of Therapeutic Viruses and Nanomaterials in Cancer Therapy. Molecules, 28(22), 7679. https://doi.org/10.3390/molecules28227679





