In Vitro and In Vivo Biological Evaluation of Indole-thiazolidine-2,4-dione Derivatives as Tyrosinase Inhibitors
Abstract
:1. Introduction
2. Results and Discussion
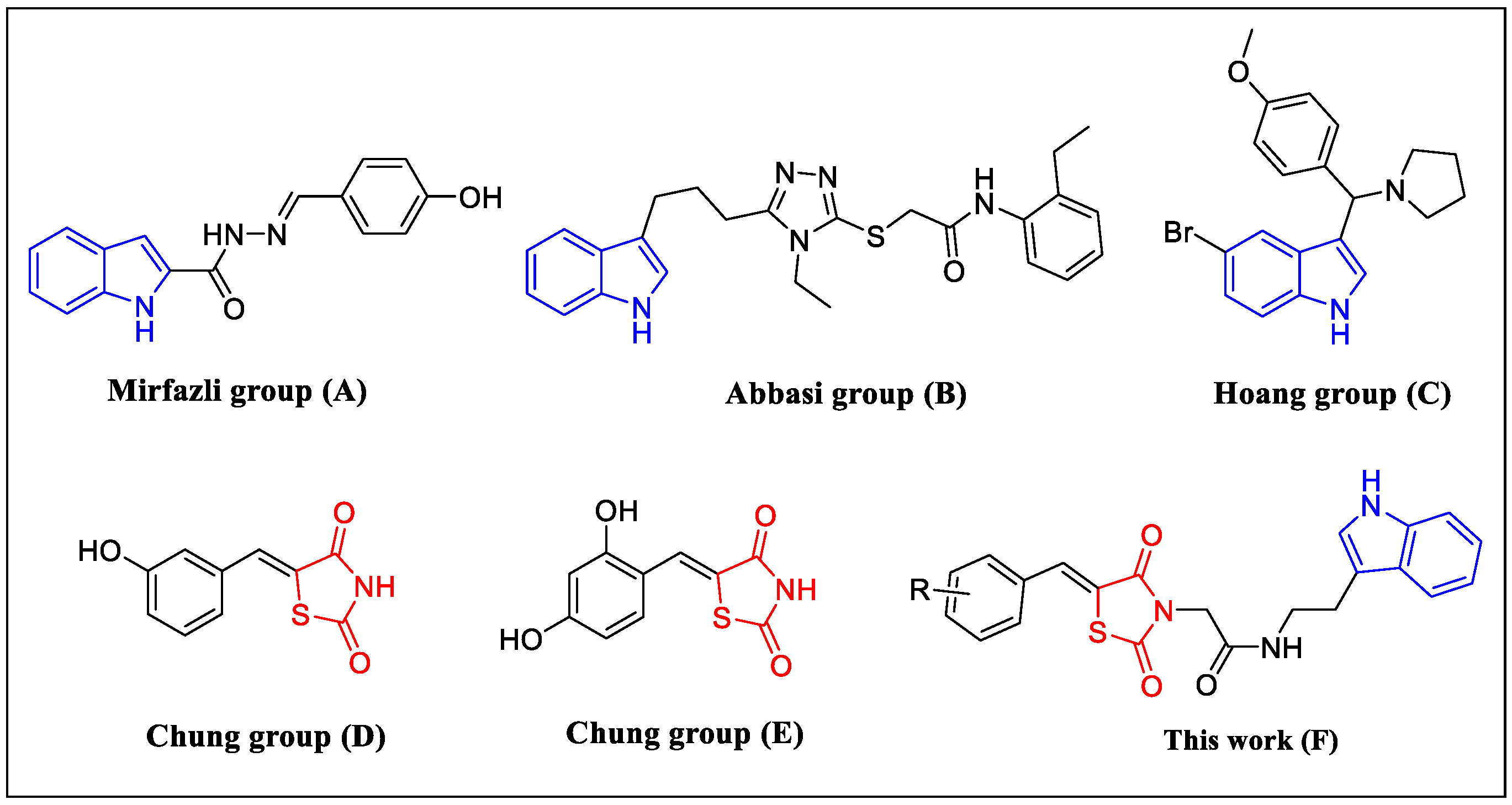
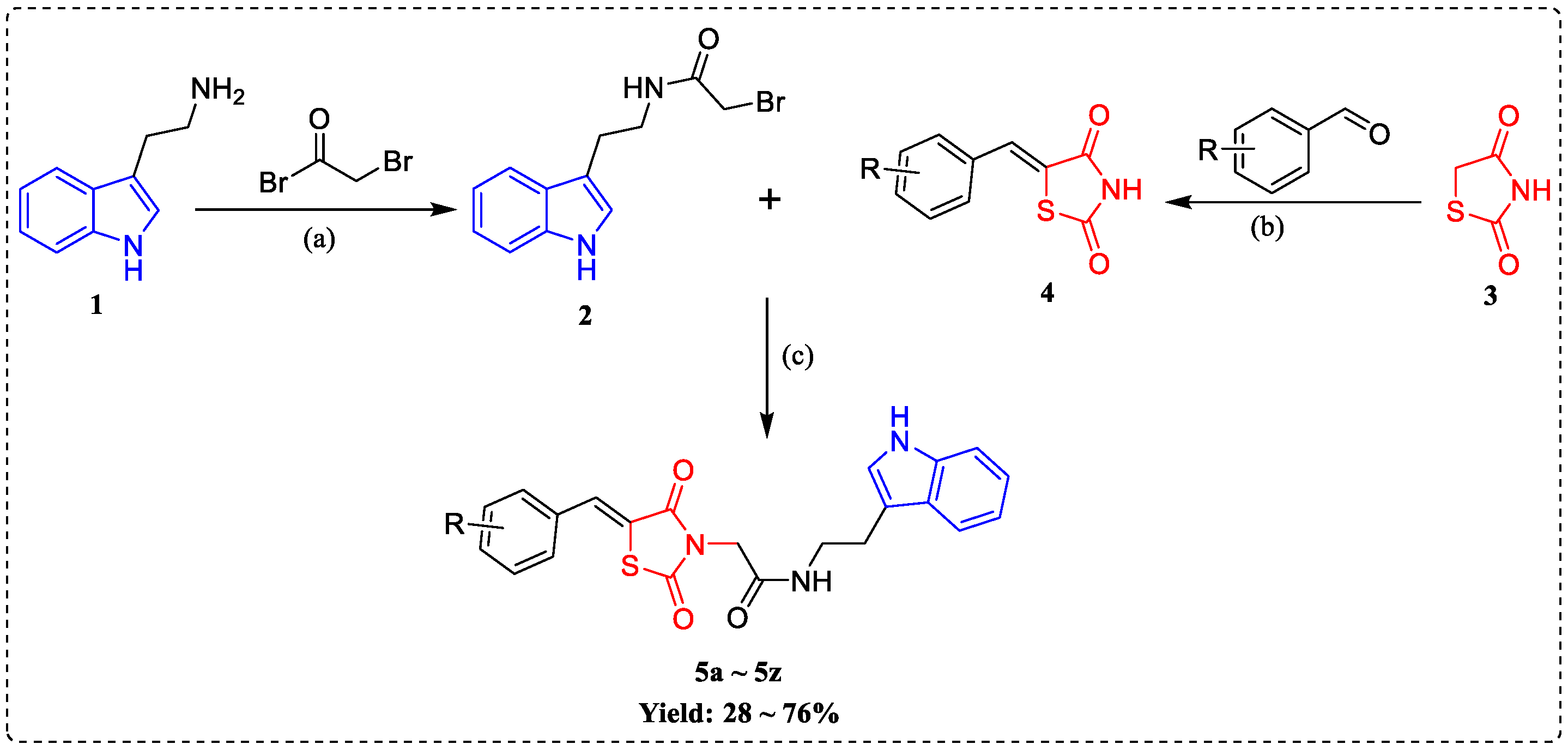
2.1. Chemistry
2.2. Tyrosinase Inhibitory Activity Assay
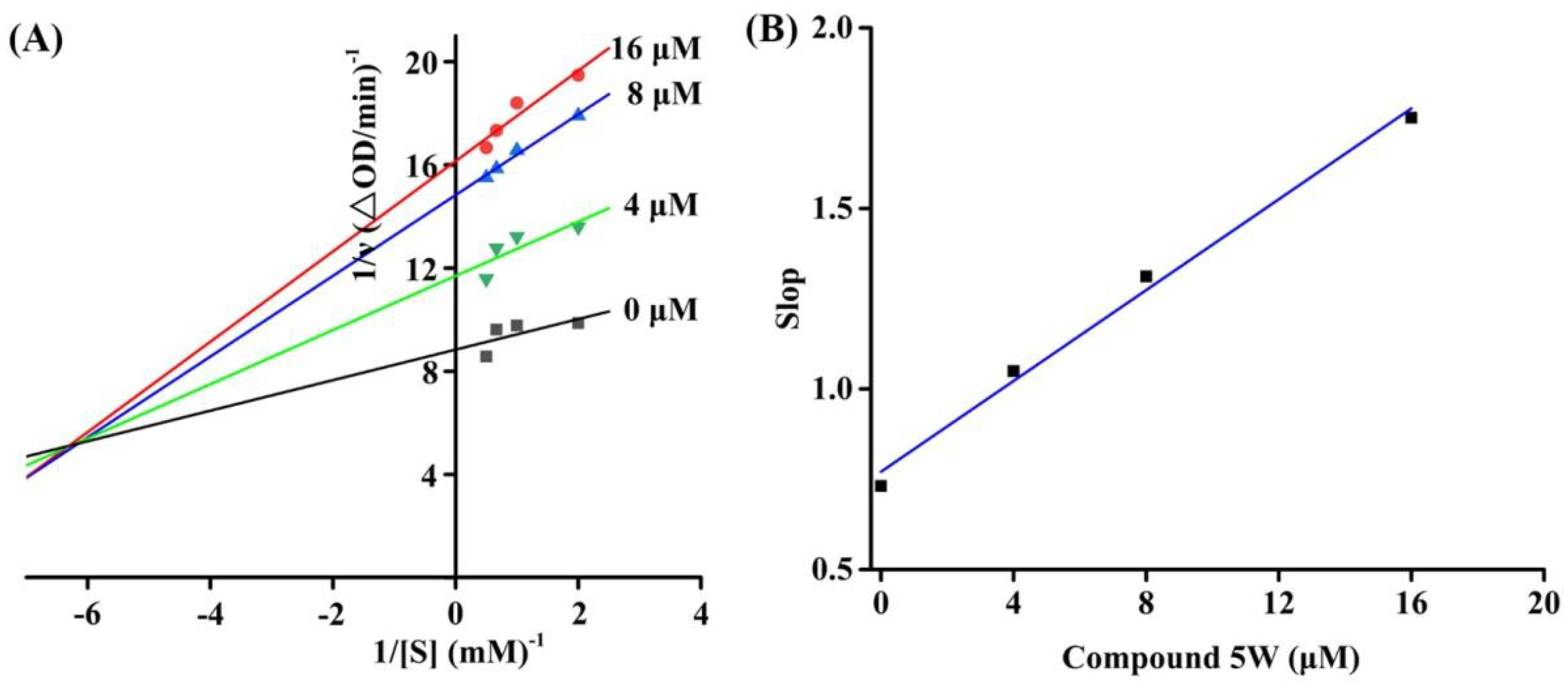
2.3. Inhibition Kinetics
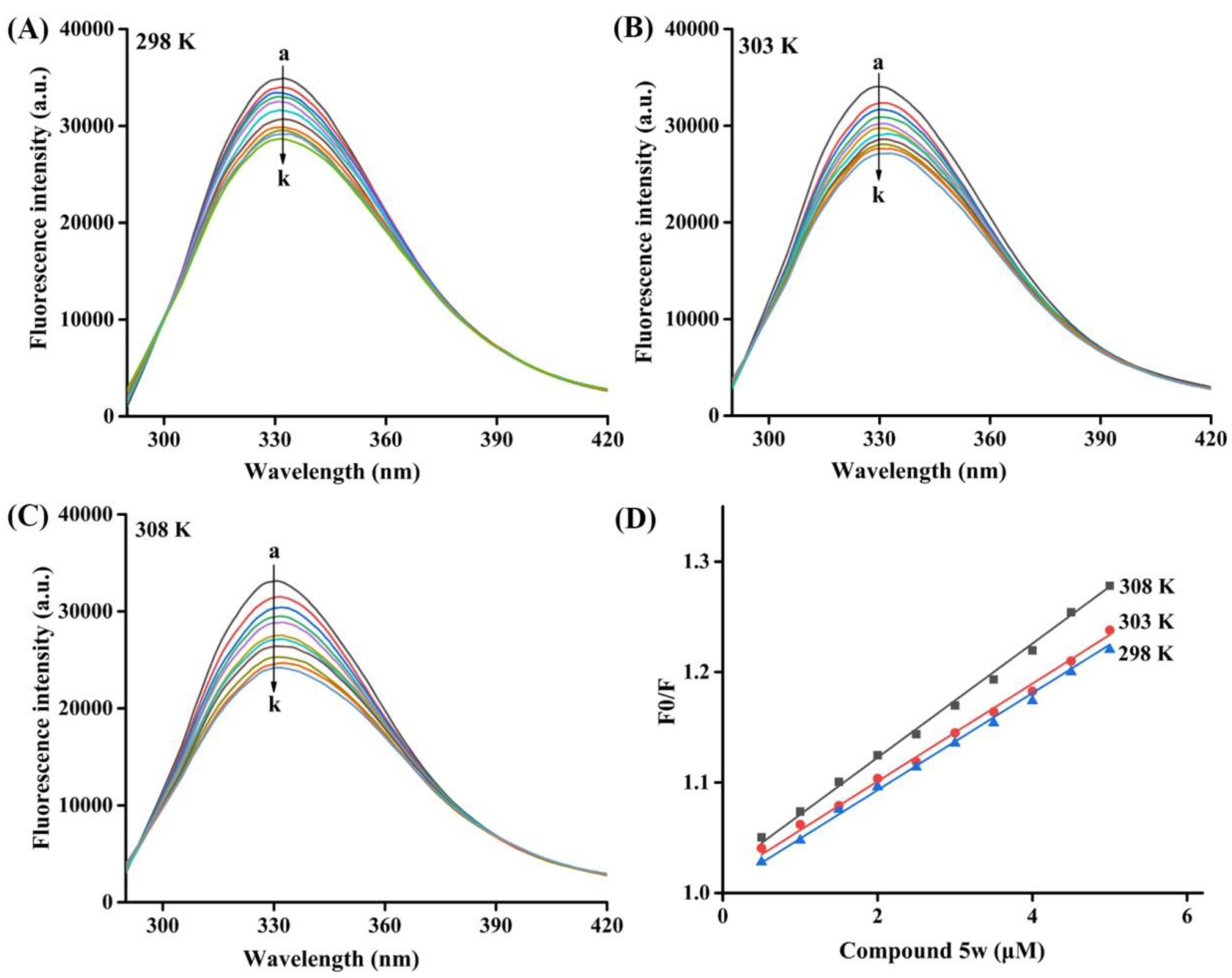
2.4. Fluorescence Quenching
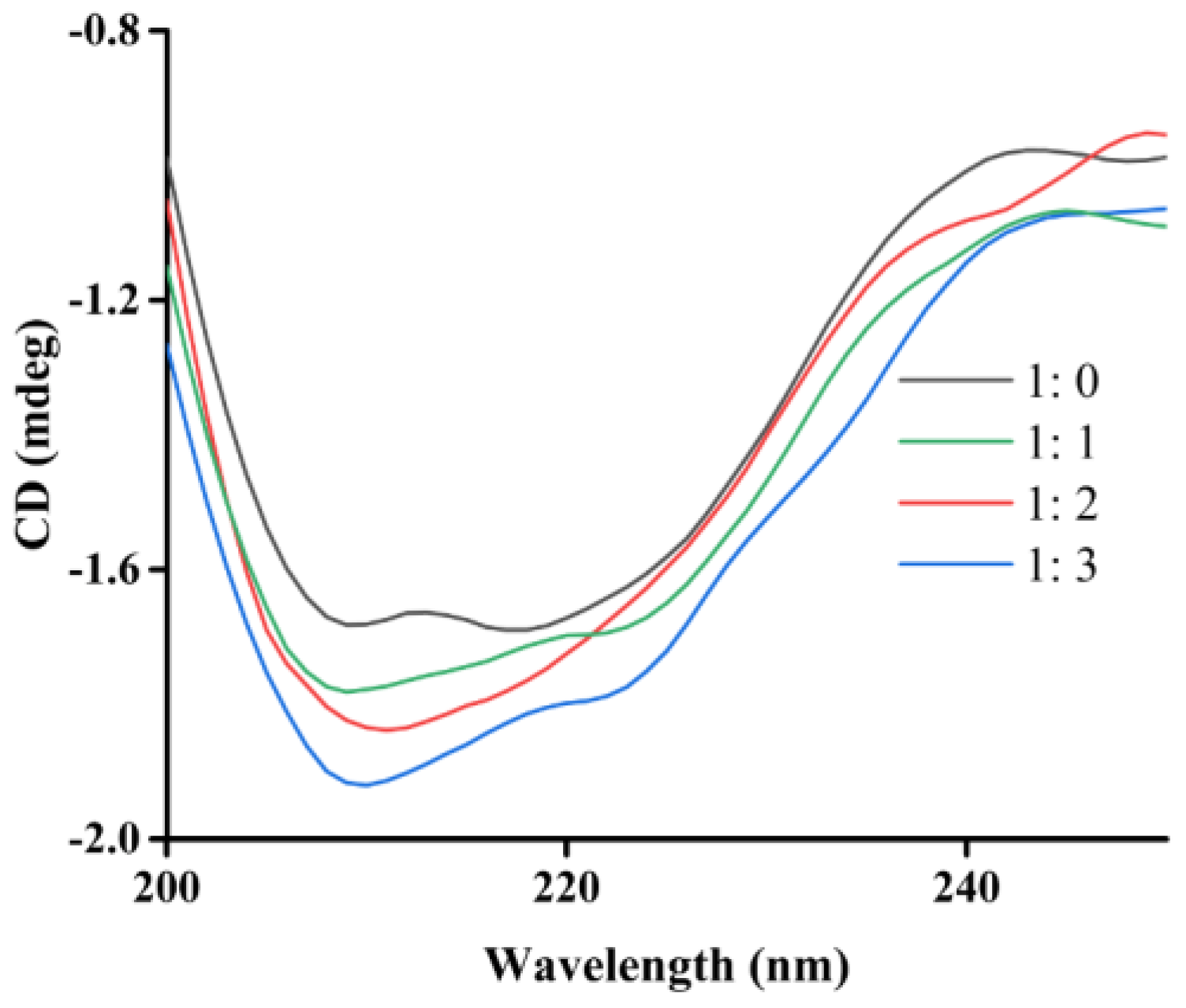
2.5. CD Spectra
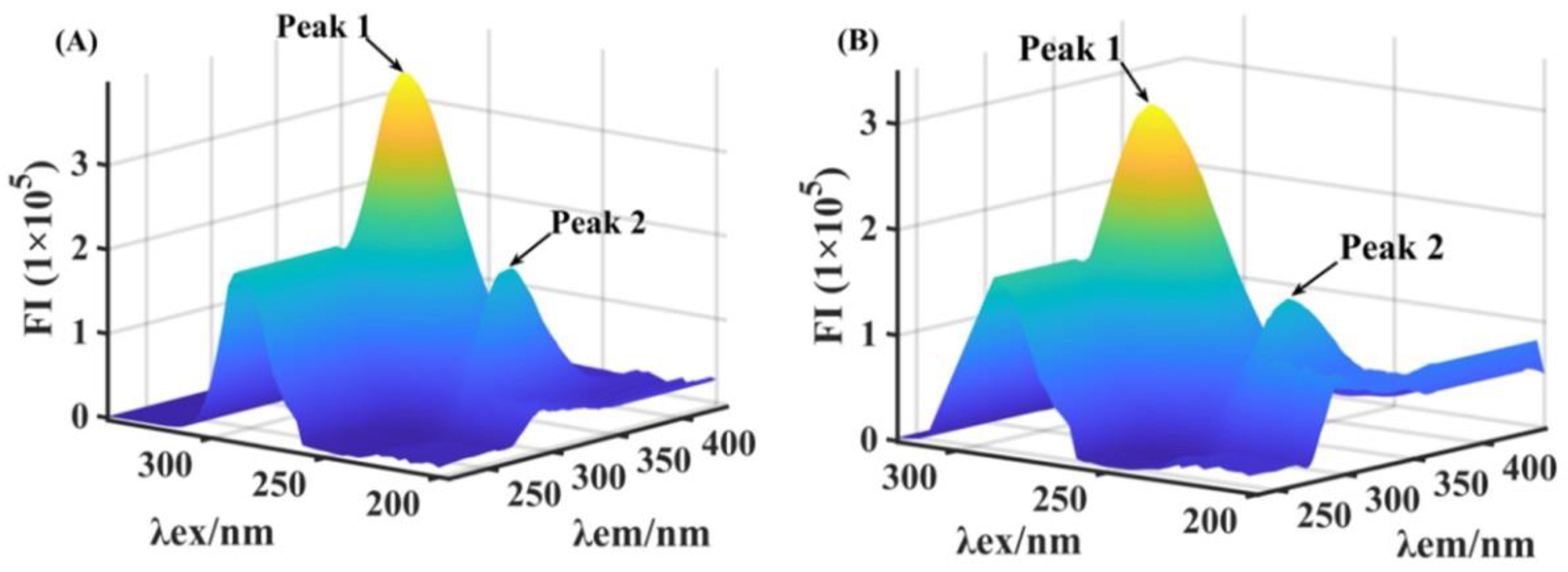
2.6. Three-Dimensional Fluorescence Spectra
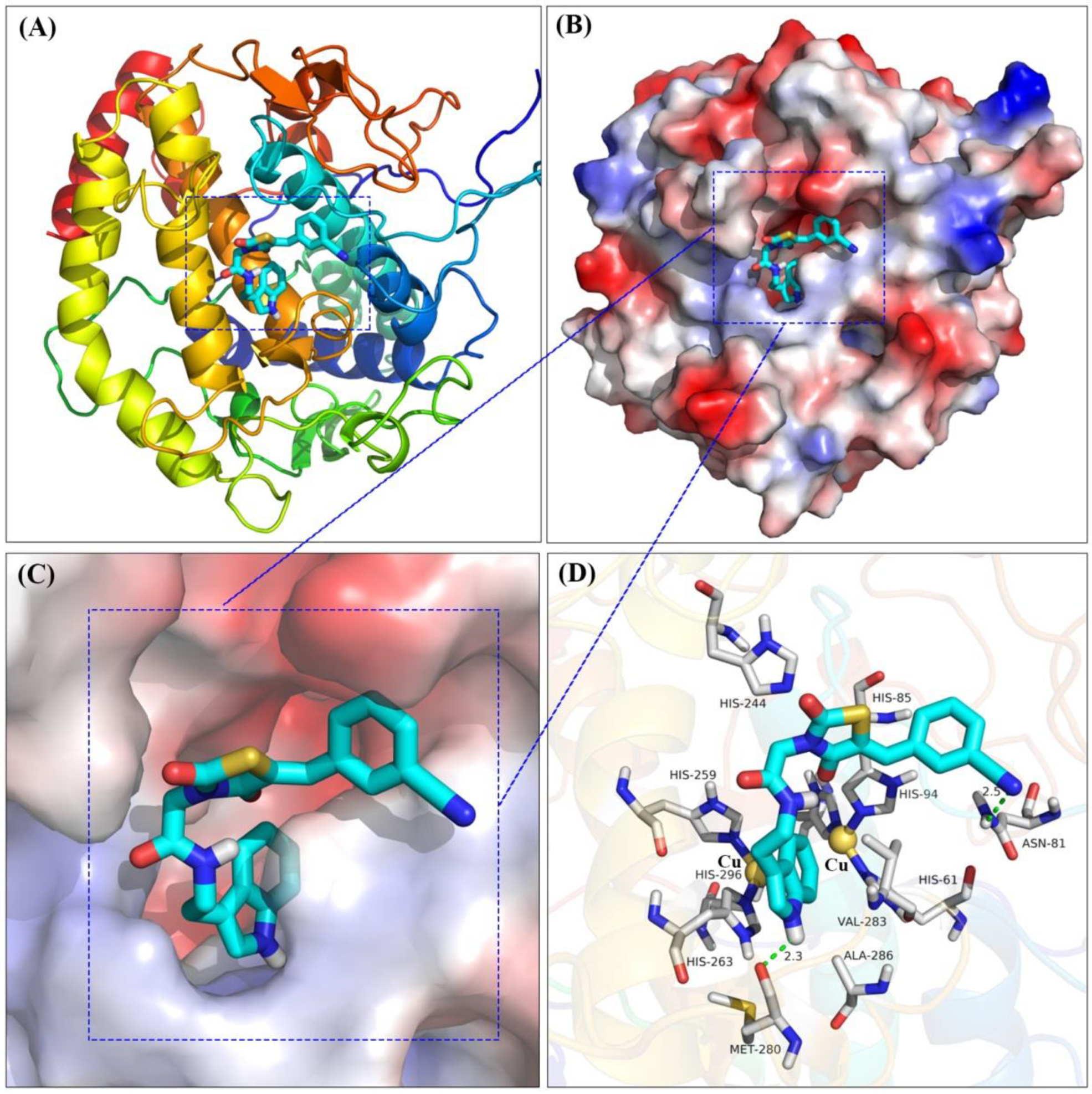
2.7. Molecular Docking
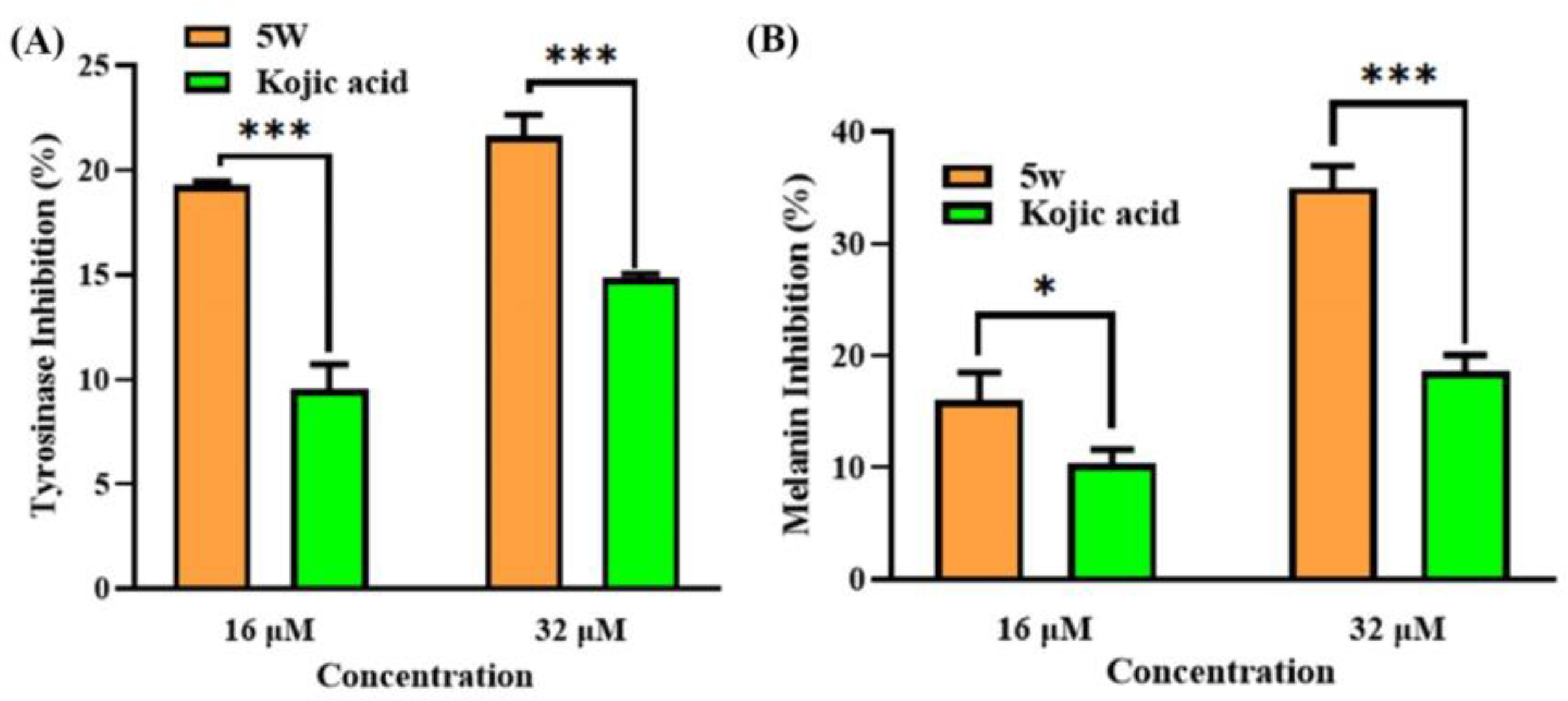
2.8. Tyrosinase Activity and Melanogenesis Assay in B16F10 Cells
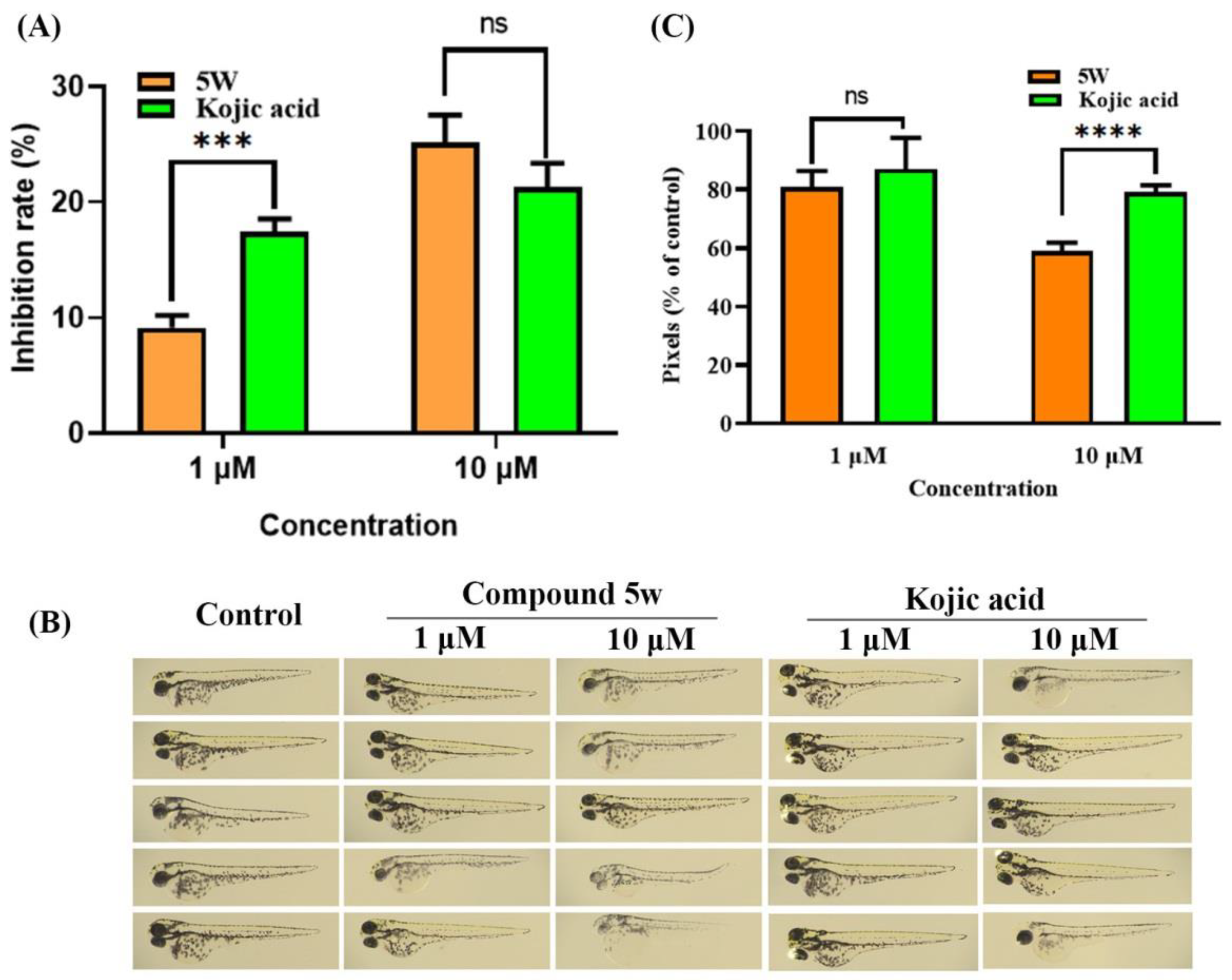
2.9. Tyrosinase Activity and Melanogenesis Assay in Zebrafish
3. Materials and Methods
3.1. Chemistry
3.2. Tyrosinase Inhibition and Kinetics Study
3.3. Fluorescence Quenching
3.4. CD Spectra
3.5. Three-Dimensional Fluorescence Spectra
3.6. Molecular Docking
3.7. Cell Assay
3.7.1. Cell Cytotoxicity
3.7.2. Tyrosinase Activity in B16F10 Cells
3.7.3. Melanin Content in B16F10 Cells
3.8. Zebrafish Assay
3.8.1. Acute Toxicity
3.8.2. Tyrosinase Activity in Zebrafish
3.8.3. Melanin Content in Zebrafish
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Romagnoli, R.; Oliva, P.; Prencipe, F.; Manfredini, S.; Germanò, M.P.; Luca, L.D.; Ricci, F.; Corallo, D.; Aveic, S.; Mariotto, E. Cinnamic acid derivatives linked to arylpiperazines as novel potent inhibitors of tyrosinase activity and melanin synthesis. Eur. J. Med. Chem. 2022, 231, 114147. [Google Scholar] [CrossRef] [PubMed]
- Moreiras, H.; Pereira, F.J.C.; Neto, M.V.; Seabra, M.C.; Barral, D.C. The exocyst is required for melanin exocytosis from melanocytes and transfer to keratinocytes. Pigm. Cell. Melanoma R. 2020, 33, 366–371. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Li, D.; Hu, Y.; You, X.; Guo, X.; Li, X.; Chen, B. A novel C6-sulfonated celastrol analog as a tyrosinase and melanin inhibitor: Design, synthesis, biological evaluation and molecular simulation. J. Mol. Struc. 2023, 1283, 135288. [Google Scholar] [CrossRef]
- Nasab, N.H.; Raza, H.; Eom, Y.S.; Hassan, M.; Kloczkowski, A.; Kim, S.J. Synthesis and discovery of potential tyrosinase inhibitor of new coumarin-based thiophenyl-pyrazolylthiazole nuclei: In-vitro evaluation, cytotoxicity, kinetic and computational studies. Chem. Biol. Drug. Des. 2023, 101, 13–88. [Google Scholar]
- Pillaiyar, T.; Namasivayam, V.; Manickam, M.; Jung, S.H. Inhibitors of melanogenesis: An updated review. J. Med. Chem. 2018, 61, 7395–7418. [Google Scholar] [CrossRef] [PubMed]
- Najafi, Z.; Rafiei, F.; Ghafouri-Khosrowshahi, A.; Mahdavi, M.; Dianatpour, M.; Iraji, A. Design, synthesis, and molecular dynamics simulation studies of new chalcone-based 2-arylidene-1,3-indandiones as tyrosinase inhibitors. Chemistryselect 2023, 33, e202302192. [Google Scholar] [CrossRef]
- Ielo, L.; Deri, B.; Germano, M.P.; Vittorio, S.; Mirabile, S.; Gitto, R.; Rapisarda, A.; Ronsisvalle, S.; Floris, S.; Pazy, Y. Exploiting the 1-(4-fluorobenzyl) piperazine fragment for the development of novel tyrosinase inhibitors as anti-melanogenic agents: Design, synthesis, structural insights and biological profile. Eur. J. Med. Chem. 2019, 178, 380–389. [Google Scholar] [CrossRef]
- Roberts, N.B.; Curtis, S.A.; Milan, A.M.; Ranganath, L.R. The pigment in alkaptonuria relationship to melanin and other coloured substances: A review of metabolism, composition and chemical analysis. JIMD Rep. 2015, 24, 51–66. [Google Scholar]
- Chortani, S.; Hajlaoui, A.; Jlizi, S.; Harrath, A.H.; Jannet, H.B.; Romdhane, A. Access to new phosphonate-and imidazolidine-benzopyrimidinone derivatives as antityrosinase and anti-acetylcholinesterase agents: Design, synthesis and molecular docking. J. Mol. Struct. 2022, 1268, 133693. [Google Scholar] [CrossRef]
- Hassan, M.; Shahzadi, S.; Kloczkowski, A. Tyrosinase inhibitors naturally present in plants and synthetic modifications of these natural products as anti-agents: A review. Molecules 2023, 28, 378. [Google Scholar] [CrossRef]
- Li, J.; Feng, L.; Liu, L.; Wang, F.; Ouyang, L.; Zhang, L.; Hu, X.; Wang, G. Recent advances in the design and discovery of synthetic tyrosinase inhibitors. Eur. J. Med. Chem. 2021, 224, 113744. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; He, M.; Huang, Y.; Peng, Z. Synthesis and biological evaluation of new kojic acid-1, 3, 4-oxadiazole hybrids as tyrosinase inhibitors and their application in the anti-browning of fresh-cut mushrooms. Food Chem. 2023, 409, 135275. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Fan, M.; Yang, W.; Peng, Z.; Wang, G. Novel kojic acid-1, 2, 4-triazine hybrids as anti-tyrosinase agents: Synthesis, biological evaluation, mode of action, and anti-browning studies. Food Chem. 2023, 419, 136047. [Google Scholar] [CrossRef] [PubMed]
- Xue, S.; Li, Z.; Ze, X.; Wu, X.; He, C.; Shuai, W.; Marlow, M.; Chen, J.; Scurr, D.; Zhu, Z. Design, synthesis, and biological evaluation of novel hybrids containing dihydrochalcone as tyrosinase inhibitors to treat skin hyperpigmentation. J. Med. Chem. 2023, 66, 5099–5117. [Google Scholar] [CrossRef]
- Barros, M.R.; Menezes, T.M.; Garcia, Y.S.; Neves, J.L. Inhibitory effects of iron-based carbonaceous nanocomposites on mushroom tyrosinase activity: Molecular aspects and mechanistic insights. New J. Chem. 2023, 47, 9134–9142. [Google Scholar] [CrossRef]
- Najafi, Z.; Ebadi, A.; Chehardoli, G.; Ziaei, M.; Akbarzadeh, T.; Saeedi, M.; Gholamhoseini, P.; Mahdavi, M. Design, synthesis, in vitro, and in silico studies of novel benzylidene 6-methoxy-1-tetralone linked to benzyloxy and benzyl-1, 2, 3-triazole rings as potential tyrosinase inhibitors. J. Mol. Struct. 2023, 1271, 134018. [Google Scholar] [CrossRef]
- Dong, H.H.; Wang, X.Y.; Li, G.; Zhao, L.X.; Zhao, C.H. Synthesis, characterization, antioxidant and tyrosinase inhibitory activities of hesperetin derivatives. Synth. Commun. 2023, 53, 1579–1587. [Google Scholar] [CrossRef]
- Roulier, B.; Rush, I.; Lazinski, L.M.; Pérès, B.; Olleik, H.; Royal, G.; Fishman, A.; Maresca, M.; Haudecoeur, R. Resorcinol-based hemiindigoid derivatives as human tyrosinase inhibitors and melanogenesis suppressors in human melanoma cells. Eur. J. Med. Chem. 2023, 246, 114972. [Google Scholar] [CrossRef]
- Mermer, A.; Demirci, S. Recent advances in triazoles as tyrosinase inhibitors. Eur. J. Med. Chem. 2023, 259, 115655. [Google Scholar] [CrossRef]
- Vittorio, S.; Dank, C.; Ielo, L. Heterocyclic compounds as synthetic tyrosinase inhibitors: Recent advances. Int. J. Mol. Sci. 2023, 24, 9097. [Google Scholar] [CrossRef]
- Obaid, R.J.; Mughal, E.U.; Naeem, N.; Sadiq, A.; Alsantali, R.I.; Jassas, R.S.; Moussa, Z.; Ahmed, S.A. Natural and synthetic flavonoid derivatives as new potential tyrosinase inhibitors: A systematic review. RSC Adv. 2021, 11, 22159–22198. [Google Scholar] [CrossRef] [PubMed]
- Ubeid, A.A.; Do, S.; Nye, C.; Hantash, B.M. Potent low toxicity inhibition of human melanogenesis by novel indole-containing octapeptides. BBA-Gen. Subj. 2012, 1820, 1481–1489. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, N.K.; Kaushik, N.; Attri, P.; Kumar, N.; Kim, C.H.; Verma, A.K.; Choi, E.H. Biomedical importance of indoles. Molecules 2013, 18, 6620–6662. [Google Scholar] [CrossRef] [PubMed]
- Kudličková, Z.; Michalková, R.; Salayová, A.; Ksiažek, M.; Vilková, M.; Bekešová, S.; Mojžiš, J. Design, synthesis, and evaluation of novel indole hybrid chalcones and their antiproliferative and antioxidant activity. Molecules 2023, 28, 6583. [Google Scholar] [CrossRef]
- Citarella, A.; Moi, D.; Pedrini, M.; Pérez-Peña, H.; Pieraccini, S.; Dimasi, A.; Stagno, C.; Micale, N.; Schirmeister, T.; Sibille, G.; et al. Synthesis of SARS-CoV-2 Mpro inhibitors bearing a cinnamic ester warhead with in vitro activity against human coronaviruses. Org. Biomol. Chem. 2023, 21, 3811. [Google Scholar] [CrossRef]
- Garrepalli, S.; Gudipati, R.; Kapavarapu, R.; Ravindhranath, K.; Pal, M. Synthesis and characterization of two known and one new impurities of dolutegravir: In silico evaluation of certain intermediates against SARS-CoV-2 O-ribose methyltransferase (OMTase). J. Mol. Struct. 2023, 1271, 133992. [Google Scholar] [CrossRef]
- Iraji, A.; Sheikhi, N.; Attarroshan, M.; Ardani, G.R.S.; Kabiri, M.; Bafghi, A.N.; Kobarfard, F.; Rezaei, Z.; Khoshneviszadeh, M.; Foroumadi, A.; et al. Design, synthesis, spectroscopic characterization, in vitro tyrosinase inhibition, antioxidant evaluation, in silico and kinetic studies of substituted indole-carbohydrazides. Bioorg. Chem. 2022, 129, 106140. [Google Scholar] [CrossRef]
- Shakila; Abbasi, M.A.; Aziz-ur-Rehman; Siddiqui, S.Z.; Nazir, M.; Raza, H.; Zafar, A.; Shah, S.A.A.; Shahid, M.; Seo, S. Multi-step synthesis of indole-N -ethyltriazole hybrids amalgamated with N-arylated ethanamides: Structure-activity relationship and mechanistic explorations through tyrosinase inhibition, kinetics and computational ascriptions. J. Mol. Struct. 2022, 126, 1132953. [Google Scholar] [CrossRef]
- Huynh, T.; Ngo, K.; Nguyen, H.; Dang, H.; Phung, V.; Thaie, K.; Hoang, T. Catalyst-free and multicomponent synthesis of 3-aminoalkylated indoles via a Mannich-type reaction: Multitargeted anticancer, tyrosinase and a-glucosidase inhibitory activities. New J. Chem. 2021, 45, 18183. [Google Scholar] [CrossRef]
- Asati, V.; Mahapatra, D.K.; Bharti, S.K. Thiazolidine-2,4-diones as multi-targeted scaffold in medicinal chemistry: Potential anticancer agents. Eur. J. Med. Chem. 2014, 87, 814–833. [Google Scholar] [CrossRef]
- Ha, Y.M.; Park, Y.J.; Kim, J.A.; Park, D.; Park, J.Y.; Lee, H.J.; Lee, J.Y.; Moon, H.R.; Chung, H.Y. Design and synthesis of 5-(substituted benzylidene) thiazolidine-2,4-dione derivatives as novel tyrosinase inhibitors. Eur. J. Med. Chem. 2012, 49, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Ha, Y.M.; Moon, K.M.; Choi, Y.J.; Park, Y.J.; Jeong, H.O.; Chung, K.W.; Lee, H.J.; Chun, P.; Moon, H.R.; et al. Anti-melanogenic effect of (Z)-5-(2,4-dihydroxybenzylidene) thiazolidine-2,4-dione, a novel tyrosinase inhibitor. Arch. Pharm. Res. 2013, 36, 1189–1197. [Google Scholar] [CrossRef] [PubMed]
- Roulier, B.; Pérès, B.; Haudecoeur, R. Advances in the Design of Genuine Human Tyrosinase Inhibitors for Targeting Melanogenesis and Related Pigmentations. J. Med. Chem. 2020, 63, 13428–13443. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Zhang, X.; Kang, Y.; Xiong, Z.; Zhang, K.; Xu, X.T.; Bai, L.P.; Li, H.G. Novel coumarin derivatives as potential tyrosinase inhibitors: Synthesis, binding analysis and biological evaluation. Arab. J. Chem. 2023, 16, 104724. [Google Scholar] [CrossRef]
- Li, J.; Min, X.; Zheng, X.; Wang, S.; Xu, X.; Peng, J. Synthesis, anti-tyrosinase activity, and spectroscopic inhibition mechanism of cinnamic acid-eugenol esters. Molecules 2023, 28, 5969. [Google Scholar] [CrossRef]
- Zargaham, M.K.; Ahmed, M.; Akhtar, N.; Ashraf, Z.; Abdel-Maksoud, M.A.; Aufy, M.; Nadeem, H. Synthesis, in silico studies, and antioxidant and tyrosinase inhibitory potential of 2-(substituted phenyl) thiazolidine-4-carboxamide derivatives. Pharmaceuticals 2023, 16, 835. [Google Scholar] [CrossRef]
- Nunes, J.A.; de Araújo, R.S.A.; da Silva, F.N.; Cytarska, J.; Łączkowski, K.Z.; Cardoso, S.H.; Mendonça-Júnior, F.J.B.; da Silva-Júnior, E.F. Coumarin-based compounds as inhibitors of tyrosinase/tyrosine hydroxylase: Synthesis, kinetic studies, and in silico approaches. Int. J. Mol. Sci. 2023, 24, 5216. [Google Scholar] [CrossRef]
- Peng, Z.; Wang, G.; Zeng, Q.; Li, Y.; Wu, Y.; Liu, H.; Wang, J.; Zhao, Y. Synthesis, antioxidant and anti-tyrosinase activity of 1,2,4-triazole hydrazones as antibrowning agents. Food Chem. 2021, 34, 1128265. [Google Scholar] [CrossRef]
- Xiao, D.; Lu, L.; Liang, B.; Xiong, Z.; Xu, X.T.; Chen, W.H. Identification of 1,3,4-oxadiazolyl-containing β-carboline derivatives as novel α-glucosidase inhibitors with antidiabetic activity. Eur. J. Med. Chem. 2023, 261, 115795. [Google Scholar] [CrossRef]
- Lin, J.; Xiao, D.; Lu, L.; Liang, B.W.; Xiong, Z.; Xu, X.T. New β-carboline derivatives as potential α-glucosidase inhibitor: Synthesis and biological activity evaluation. J. Mol. Struct. 2023, 1283, 135279. [Google Scholar] [CrossRef]
- Wu, X.Z.; Zhu, W.J.; Lu, L.; Hu, C.M.; Zheng, Y.Y.; Zhang, X.; Lin, J.; Wu, J.Y.; Xiong, Z.; Zhang, K.; et al. Synthesis and anti- α-glucosidase activity evaluation of be-tulinic acid derivatives. Arab. J. Chem. 2023, 16, 104659. [Google Scholar] [CrossRef]
- Pakhare, D.; Radhika, K. Application of Horner-Wadsworth-Emmons olefination for the synthesis of granulatamide A, its E isomer and other amides of tryptamine. New J. Chem. 2016, 40, 5428–5431. [Google Scholar] [CrossRef]
- Touaibia, M.; St-Coeur, P.; Duff, P.; Faye, D.C.; Pichaud, N. 5-Benzylidene, 5-benzyl, and 3-benzylthiazolidine-2,4-diones as potential inhibitors of the mitochondrial pyruvate carrier: Effects on mitochondrial functions and survival in Drosophila melanogaster. Eur. J. Pharmacol. 2021, 913, 174627. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, F.M.; Patel, N.B.; Sanna, G.; Busonera, B.; Colla, P.L.; Rajani, D.P. Synthesis of some new 2-amino-6-thiocyanato benzothiazole derivatives bearing 2,4-thiazolidinediones and screening of their in vitro antimicrobial, antitubercular and antiviral activities. Med. Chem. Res. 2015, 24, 3129–3142. [Google Scholar] [CrossRef]
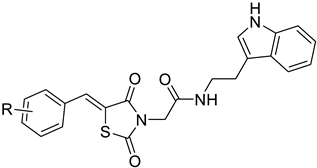 | |||||||
|---|---|---|---|---|---|---|---|
| No. | R | Inhibition (%) | IC50 (μM) | No. | R | Inhibition (%) | IC50 (μM) |
| 5a | H | 14.2 | - | 5n | 2-CF3 | 3.8 | - |
| 5b | 2-Me | 19.3 | - | 5o | 3-CF3 | 4.5 | - |
| 5c | 3-Me | 33.2 | - | 5p | 4-CF3 | 3.0 | - |
| 5d | 4-Me | 15.1 | - | 5q | 2-OMe | 1 | - |
| 5e | 2-F | 16.5 | - | 5r | 3-OMe | 2 | - |
| 5f | 3-F | 32.6 | - | 5s | 4-OMe | 1 | - |
| 5g | 4-F | 27.4 | - | 5t | 2-OH | 26.9 | - |
| 5h | 2-Cl | 16.8 | - | 5u | 3-OH | 36.2 | - |
| 5i | 3-Cl | 37.6 | - | 5v | 4-OH | 24.6 | - |
| 5j | 4-Cl | 36.6 | - | 5w | 3-CN | 71.5 | 11.2 |
| 5k | 2-Br | 31.7 | - | 5x | 4-CN | 24.6 | - |
| 5l | 3-Br | 70.3 | 13.3 | 5y | 3-NO2 | 19.8 | - |
| 5m | 4-Br | 19.5 | - | 5z | 4-NO2 | 19.1 | - |
| Kojic acid | 15.6 | ||||||
| T | KSV (×104 Lmol−1) | Kq (×1012 Lmol−1) | n |
|---|---|---|---|
| 298 | 3.77 | 3.77 | 0.72 |
| 303 | 3.84 | 3.84 | 0.74 |
| 308 | 4.47 | 4.47 | 0.82 |
| [Enzyme]: [5w] | α-Helix (%) | β-Sheet (%) | β-Turn (%) | Random Coil (%) |
|---|---|---|---|---|
| 1: 0 | 8.7 | 34.0 | 19.8 | 30.6 |
| 1: 1 | 8.8 | 33.6 | 19.9 | 32.5 |
| 1: 2 | 8.9 | 33.1 | 20.0 | 34.5 |
| 1: 3 | 9.0 | 32.7 | 20.1 | 35.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, L.; Hu, C.; Min, X.; Liu, Z.; Xu, X.; Gan, L. In Vitro and In Vivo Biological Evaluation of Indole-thiazolidine-2,4-dione Derivatives as Tyrosinase Inhibitors. Molecules 2023, 28, 7470. https://doi.org/10.3390/molecules28227470
Lu L, Hu C, Min X, Liu Z, Xu X, Gan L. In Vitro and In Vivo Biological Evaluation of Indole-thiazolidine-2,4-dione Derivatives as Tyrosinase Inhibitors. Molecules. 2023; 28(22):7470. https://doi.org/10.3390/molecules28227470
Chicago/Turabian StyleLu, Li, Chunmei Hu, Xiaofeng Min, Zhong Liu, Xuetao Xu, and Lishe Gan. 2023. "In Vitro and In Vivo Biological Evaluation of Indole-thiazolidine-2,4-dione Derivatives as Tyrosinase Inhibitors" Molecules 28, no. 22: 7470. https://doi.org/10.3390/molecules28227470
APA StyleLu, L., Hu, C., Min, X., Liu, Z., Xu, X., & Gan, L. (2023). In Vitro and In Vivo Biological Evaluation of Indole-thiazolidine-2,4-dione Derivatives as Tyrosinase Inhibitors. Molecules, 28(22), 7470. https://doi.org/10.3390/molecules28227470





