Shaking Things from the Ground-Up: A Systematic Overview of the Mechanochemistry of Hard and High-Melting Inorganic Materials
Abstract
1. Introduction
- (1)
- adduct formation: particularly reactions in which there is no formal change in the oxidation number of the reactants.
- (2)
- dehydration reactions: mostly transformations of hydrated metal oxides and hydroxides into metal oxides, taking place under mechanical treatment at nominal room temperature.
- (3)
- reduction-oxidation (redox) reactions: such as the oxidation of copper metal into copper(II) sulphide by milling with elemental sulphur.
- (4)
- exchange (metathesis) reactions: for example, certain reactions observed upon pressing of KBr tablets for infrared spectroscopy analysis.
- (5)
- doping and structural rearrangements: including reactions with the mechanochemical reaction assembly (milling jar and/or balls).
- (6)
- acid–base reactions: the formation of mixed substances (e.g., phosphates, silicates, mixed metal oxides, inorganic frameworks), typically by reactions of acid and basic oxides
- (7)
- mixed reactions: for example, the synthesis of open zeolite frameworks through a combination of acid–base and dehydration processes; the formation of complex oxides (including battery materials, such as LiMn2O4) through combining different types of reactions; or the transformations of inorganic hydrides via the complex combinations of acid–base, metathesis, and redox chemistry, or even polymorphic transformations
2. Reactions of Adduct Formation
3. Dehydration Reactions
4. Redox Reactions
4.1. Reactions of Elements and Compounds with Oxygen and Other Chalcogens
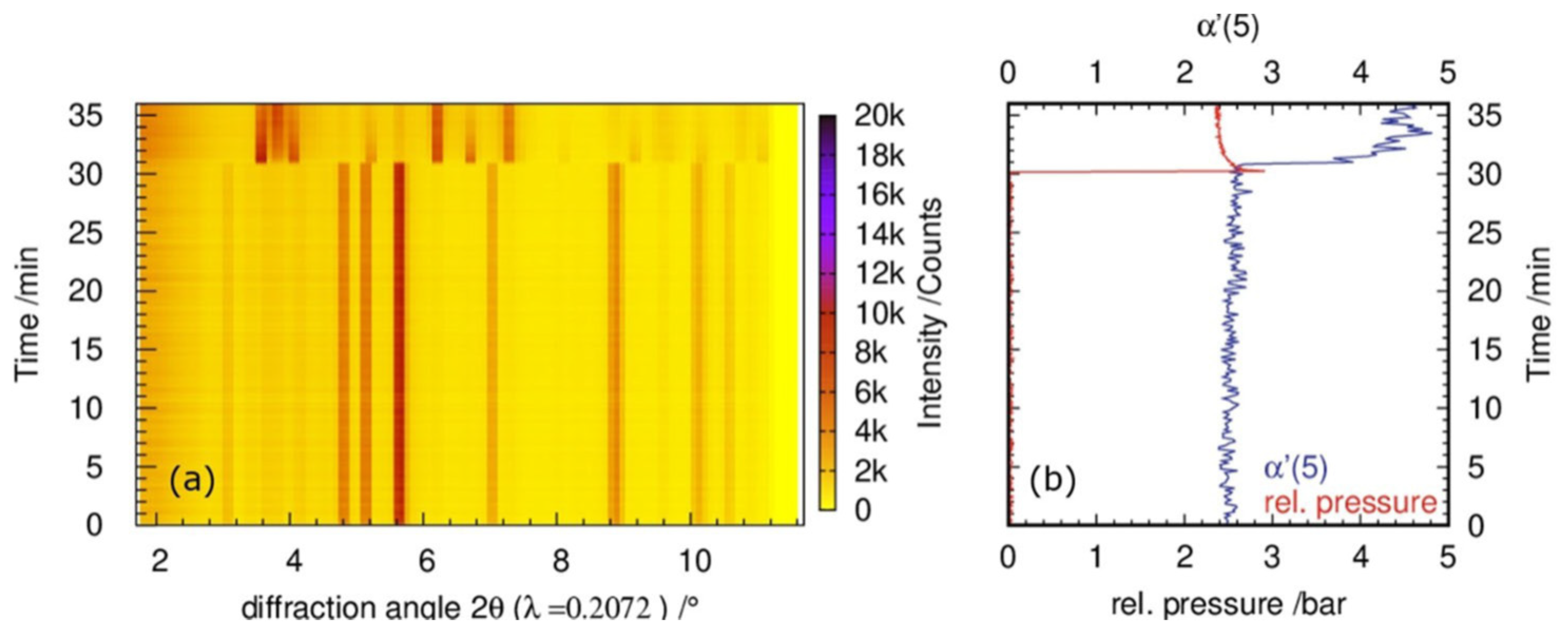
4.1.1. Mechanically Induced Self-Propagating Reactions (MSRs)
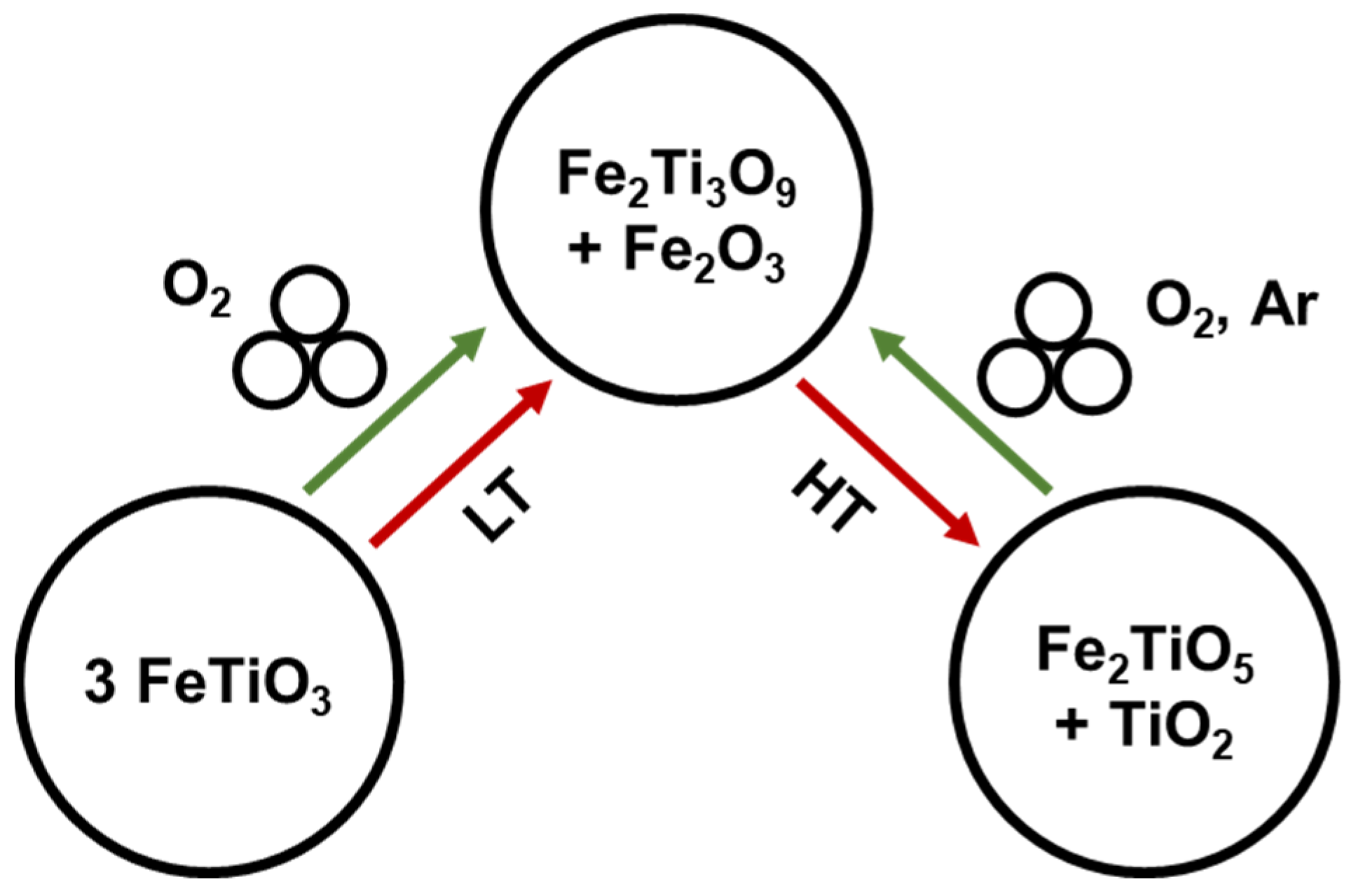
4.1.2. Reactivity of Metal Oxides with Molecular Oxygen
4.1.3. Reactivity with Peroxides, Peroxyacids, and Their Salts
4.2. Synthesis of Nitrides: Reactions with Elementary Nitrogen vs. Reactions with Ammonia
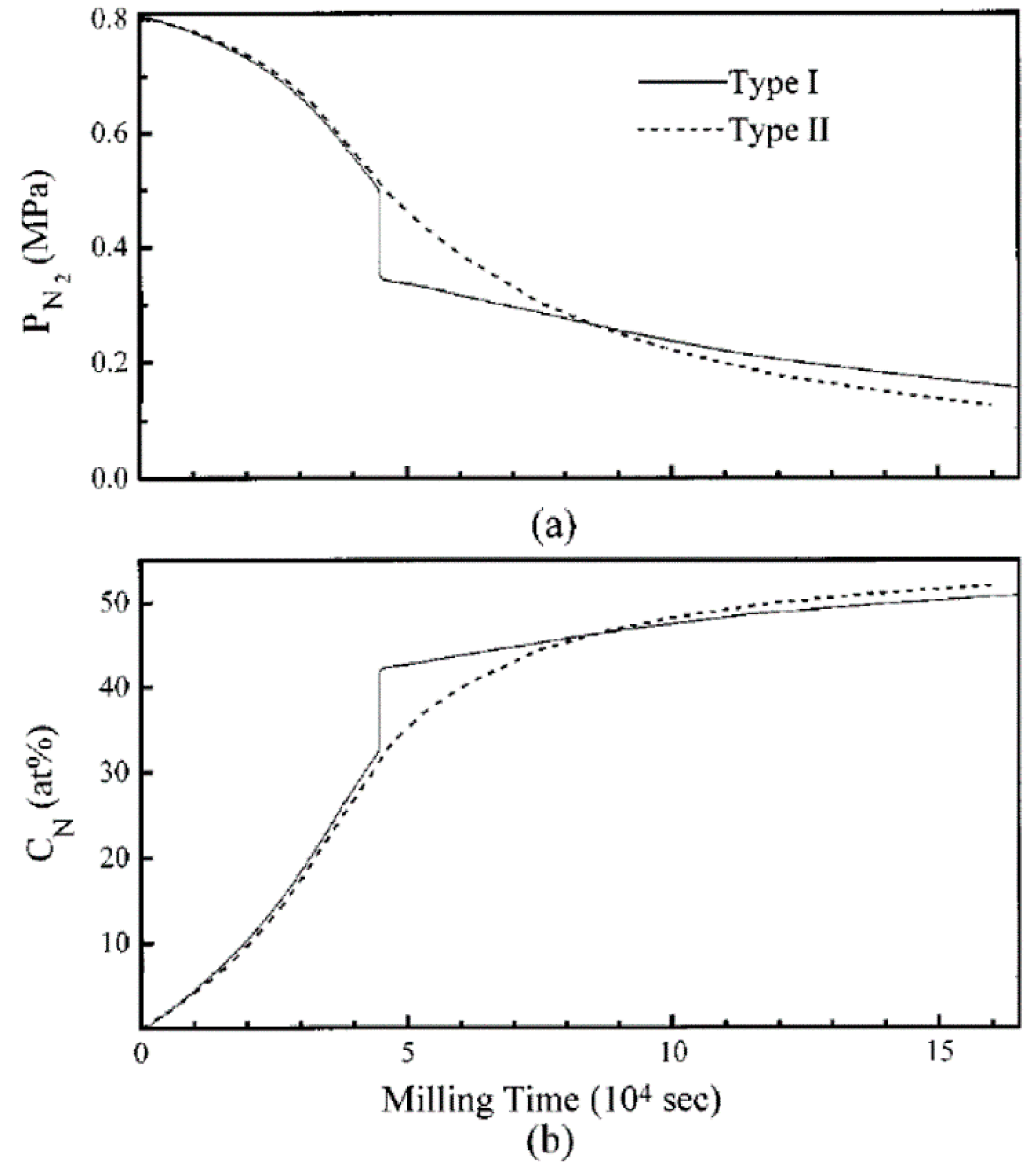
4.2.1. Mechanisms of Nitride Formation with Nitrogen Gas
4.2.2. Mechanisms of Nitride Formation with Ammonia Gas
4.3. The Synthesis of Phosphides from the Elements
4.4. The Synthesis of Borides and Carbides
4.5. The Synthesis of Hydrides from Hydrogen Gas
4.6. Synthesis of Ternary Compounds and Stoichiometric Control
4.7. Unexplained and “Stochastic” Reactions
4.8. Reduction of Materials by the Metallic Milling Vessel
5. Metathesis (Exchange) Reactions
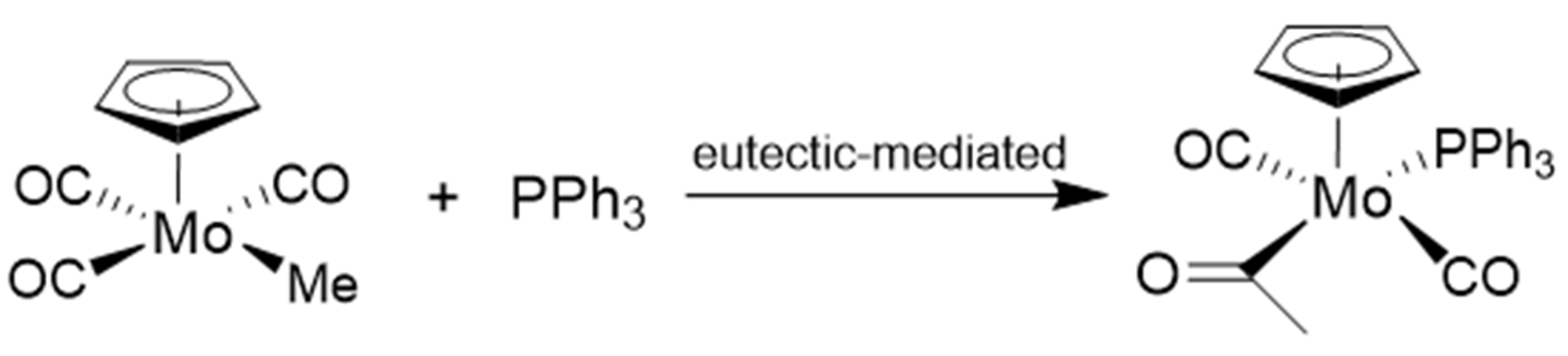
6. Doping and Structural Rearrangements, Including Reactions with the Mechanochemical Reaction Assembly (Milling Jar and Balls)
6.1. Doping with Inorganic Impurities from the Milling Vessel: Polymorphism
6.2. Doping with Molecular Precursors: Photoactive Materials
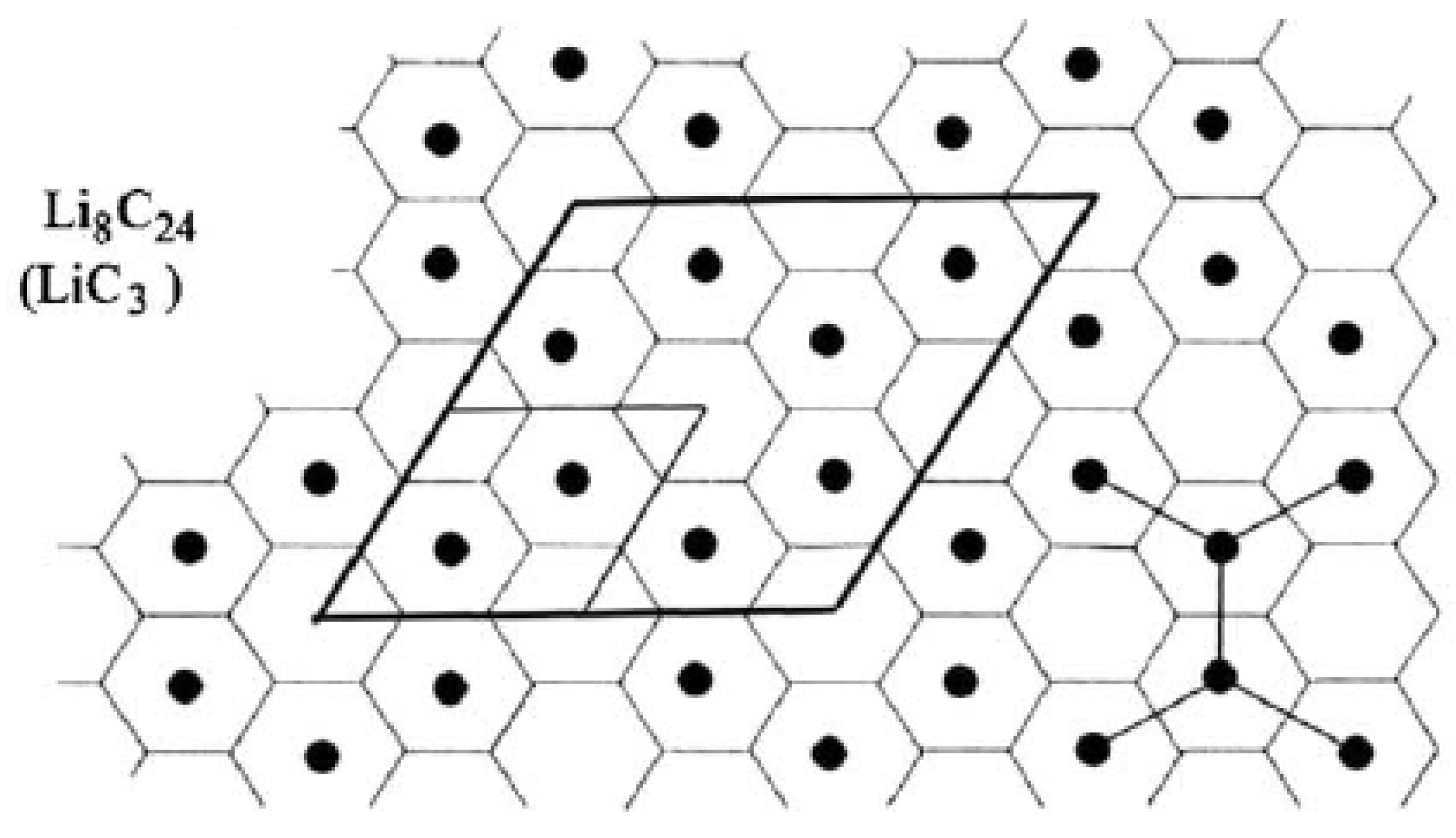
6.3. Energy Materials: Lithium-Graphite Intercalation Compounds
6.4. Structural Rearrangements and Mechanochemical Activation of Complex Oxides
7. Acid–Base Reactions: Synthesis of Mixed Metal Oxides
7.1. Synthesis of Normal Spinel Ferrites
7.2. Synthesis of Inverse Spinel Ferrites
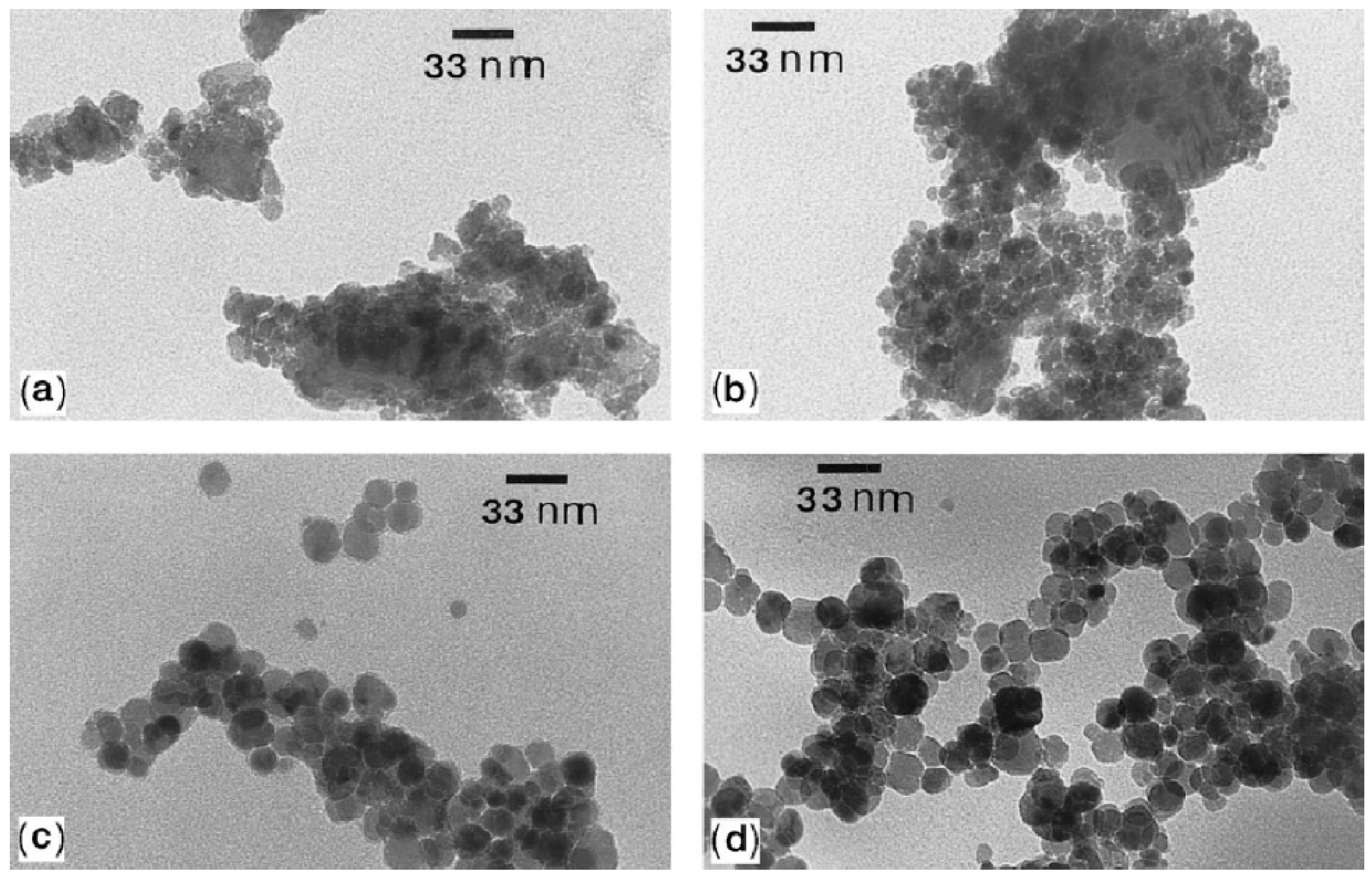
7.3. Particle Structure of Mechanochemically Synthesized Mixed Metal Oxides
7.4. Alternative Mechanochemical Procedures for the Synthesis of Mixed Metal Oxides
7.5. Synthesis of Oxides by Manual Grinding
8. Mixed Reactions
8.1. Solvent-Free Zeolite Synthesis and Structure Templating
8.2. Ferrite Synthesis through Iron Oxide Generated via Aerobic Oxidation of the Milling Assembly
8.3. Mechanochemical Reactions with Gaseous Reagents: Synthesis of the Battery Material LiMn2O4
8.4. Boranes and Borohydrides
8.5. Mixed Metal Hydrides and Borohydrides
8.6. Mechanochemical Activation and Transformation of LiAlH4
8.7. Catalytic Room-Temperature Dehydrogenation of LiAlH4
8.8. Mechanosynthesis of Complex Aluminium Hydrides
8.9. Complex Aluminium Hydrides with Octahedral Coordination
8.10. Mechanochemistry of Magnesium Borohydride
8.11. Screening for New Hydride and Hydrogen Release Materials by Mechanochemistry
9. Characterization Methods
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Takacs, L. Quicksilver from cinnabar: The first documented mechanochemical reaction? JOM 2000, 52, 12–13. [Google Scholar] [CrossRef]
- Marchini, M.; Gandolfi, M.; Maini, L.; Raggetti, L.; Martelli, M. Exploring the ancient chemistry of mercury. Proc. Natl. Acad. Sci. USA 2022, 119, e2123171119. [Google Scholar] [CrossRef]
- Takacs, L.M. Carey Lea, the first mechanochemist. J. Mater. Sci. 2004, 39, 4987–4993. [Google Scholar] [CrossRef]
- Friščić, T.; Mottillo, C.; Titi, H.M. Mechanochemistry for Synthesis. Angew. Chem. Int. Ed. 2020, 59, 1018–1029. [Google Scholar] [CrossRef] [PubMed]
- James, S.L.; Adams, C.J.; Bolm, C.; Braga, D.; Collier, P.; Friščić, T.; Grepioni, F.; Harris, K.D.; Hyett, G.; Jones, W.; et al. Mechanochemistry: Opportunities for new and cleaner synthesis. Chem. Soc. Rev. 2012, 41, 413–447. [Google Scholar] [CrossRef]
- Mateti, S.; Mathesh, M.; Liu, Z.; Tao, T.; Ramireddy, T.; Glushenkov, A.M.; Yang, W.; Chen, Y.I. Mechanochemistry: A force in disguise and conditional effects towards chemical reactions. Chem. Commun. 2021, 57, 1080–1092. [Google Scholar] [CrossRef]
- O’Neill, R.T.; Boulatov, R. The many flavours of mechanochemistry and its plausible conceptual underpinnings. Nat. Rev. Chem. 2021, 5, 148–167. [Google Scholar] [CrossRef]
- Michalchuk, A.A.L.; Boldyreva, E.V.; Belenguer, A.M.; Emmerling, F.; Boldyrev, V.V. Tribochemistry, Mechanical Alloying, Mechanochemistry: What is in a Name? Front. Chem. 2021, 9, 685789. [Google Scholar] [CrossRef]
- Braga, D.; Grepioni, F. Reactions between or within molecular crystals. Angew. Chem. Int. Ed. 2004, 43, 4002–4011. [Google Scholar] [CrossRef] [PubMed]
- Braga, D.; Maini, L.; Grepioni, F. Mechanochemical preparation of co-crystals. Chem. Soc. Rev. 2013, 42, 7638–7648. [Google Scholar] [CrossRef]
- Friščić, T. Supramolecular concepts and new techniques in mechanochemistry: Cocrystals, cages, rotaxanes, open metal-organic frameworks. Chem. Soc. Rev. 2012, 41, 3493–3510. [Google Scholar] [CrossRef]
- Andersen, J.; Mack, J. Mechanochemistry and organic synthesis: From mystical to practical. Green Chem. 2018, 20, 1435–1443. [Google Scholar] [CrossRef]
- Crawford, D.E.; Miskimmin, C.K.; Cahir, J.; James, S.L. Continuous multi-step synthesis by extrusion—Telescoping solvent-free reactions for greater efficiency. Chem. Commun. 2017, 53, 13067–13070. [Google Scholar] [CrossRef] [PubMed]
- Howard, J.L.; Cao, Q.; Browne, D.L. Mechanochemistry as an emerging tool for molecular synthesis: What can it offer? Chem. Sci. 2018, 9, 3080–3094. [Google Scholar] [CrossRef]
- Rodríguez, B.; Bruckmann, A.; Rantanen, T.; Bolm, C. Solvent-Free Carbon-Carbon Bond Formations in Ball Mills. Adv. Synth. Catal. 2007, 349, 2213–2233. [Google Scholar] [CrossRef]
- Wang, G.W. Mechanochemical organic synthesis. Chem. Soc. Rev. 2013, 42, 7668–7700. [Google Scholar] [CrossRef] [PubMed]
- Declerck, V.; Nun, P.; Martinez, J.; Lamaty, F. Solvent-free synthesis of peptides. Angew. Chem. Int. Ed. 2009, 48, 9318–9321. [Google Scholar] [CrossRef] [PubMed]
- Maurin, O.; Verdie, P.; Subra, G.; Lamaty, F.; Martinez, J.; Metro, T.X. Peptide synthesis: Ball-milling, in solution, or on solid support, what is the best strategy? Beilstein J. Org. Chem. 2017, 13, 2087–2093. [Google Scholar] [CrossRef] [PubMed]
- Yeboue, Y.; Gallard, B.; Le Moigne, N.; Jean, M.; Lamaty, F.; Martinez, J.; Métro, T.-X. Peptide Couplings by Reactive Extrusion: Solid-Tolerant and Free from Carcinogenic, Mutagenic and Reprotoxic Chemicals. ACS Sustain. Chem. Eng. 2018, 6, 16001–16004. [Google Scholar] [CrossRef]
- Krusenbaum, A.; Gratz, S.; Tigineh, G.T.; Borchardt, L.; Kim, J.G. The mechanochemical synthesis of polymers. Chem. Soc. Rev. 2022, 51, 2873–2905. [Google Scholar] [CrossRef]
- Garay, A.L.; Pichon, A.; James, S.L. Solvent-free synthesis of metal complexes. Chem. Soc. Rev. 2007, 36, 846–855. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Lim, G.K.; Harris, K.D.M.; Apperley, D.C.; Horton, P.N.; Hursthouse, M.B.; James, S.L. Efficient, Scalable, and Solvent-free Mechanochemical Synthesis of the OLED Material Alq3 (q = 8-Hydroxyquinolinate). Cryst. Growth Des. 2012, 12, 5869–5872. [Google Scholar] [CrossRef]
- Pichon, A.; Lazuen-Garay, A.; James, S.L. Solvent-free synthesis of a microporous metal–organic framework. CrystEngComm 2006, 8, 211–214. [Google Scholar] [CrossRef]
- Prochowicz, D.; Saski, M.; Yadav, P.; Gratzel, M.; Lewinski, J. Mechanoperovskites for Photovoltaic Applications: Preparation, Characterization, and Device Fabrication. Acc. Chem. Res. 2019, 52, 3233–3243. [Google Scholar] [CrossRef]
- Tan, D.; García, F. Main group mechanochemistry: From curiosity to established protocols. Chem. Soc. Rev. 2019, 48, 2274–2292. [Google Scholar] [CrossRef]
- Andre, V.; Quaresma, S.; da Silva, J.L.F.; Duarte, M.T. Exploring mechanochemistry to turn organic bio-relevant molecules into metal-organic frameworks: A short review. Beilstein J. Org. Chem. 2017, 13, 2416–2427. [Google Scholar] [CrossRef]
- Sepelak, V.; Duvel, A.; Wilkening, M.; Becker, K.D.; Heitjans, P. Mechanochemical reactions and syntheses of oxides. Chem. Soc. Rev. 2013, 42, 7507–7520. [Google Scholar] [CrossRef] [PubMed]
- Rightmire, N.R.; Hanusa, T.P. Advances in organometallic synthesis with mechanochemical methods. Dalton Trans. 2016, 45, 2352–2362. [Google Scholar] [CrossRef]
- Cook, T.L.; Walker, J.A.; Mack, J. Scratching the catalytic surface of mechanochemistry: A multi-component CuAAC reaction using a copper reaction vial. Green Chem. 2013, 15, 617–619. [Google Scholar] [CrossRef]
- Haley, R.A.; Zellner, A.R.; Krause, J.A.; Guan, H.; Mack, J. Nickel Catalysis in a High Speed Ball Mill: A Recyclable Mechanochemical Method for Producing Substituted Cyclooctatetraene Compounds. ACS Sustain. Chem. Eng. 2016, 4, 2464–2469. [Google Scholar] [CrossRef]
- Pickhardt, W.; Gratz, S.; Borchardt, L. Direct Mechanocatalysis: Using Milling Balls as Catalysts. Chem. Eur. J. 2020, 26, 12903–12911. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Venegas, M.; Juaristi, E. Mechanochemical and Mechanoenzymatic Synthesis of Pharmacologically Active Compounds: A Green Perspective. ACS Sustain. Chem. Eng. 2020, 8, 8881–8893. [Google Scholar] [CrossRef]
- Bilke, M.; Losch, P.; Vozniuk, O.; Bodach, A.; Schuth, F. Methane to Chloromethane by Mechanochemical Activation: A Selective Radical Pathway. J. Am. Chem. Soc. 2019, 141, 11212–11218. [Google Scholar] [CrossRef]
- Bolm, C.; Hernandez, J.G. Mechanochemistry of Gaseous Reactants. Angew. Chem. Int. Ed. 2019, 58, 3285–3299. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, J.G.; Bolm, C. Altering Product Selectivity by Mechanochemistry. J. Org. Chem. 2017, 82, 4007–4019. [Google Scholar] [CrossRef]
- Kubota, K.; Pang, Y.; Miura, A.; Ito, H. Redox reactions of small organic molecules using ball milling and piezoelectric materials. Science 2019, 366, 1500–1504. [Google Scholar] [CrossRef]
- Strukil, V. Mechanochemical synthesis of thioureas, ureas and guanidines. Beilstein J. Org. Chem. 2017, 13, 1828–1849. [Google Scholar] [CrossRef]
- Toda, F. Solid State Organic Chemistry: Efficient Reactions, Remarkable Yields, and Stereoselectivity. Acc. Chem. Res. 1995, 28, 480–486. [Google Scholar] [CrossRef]
- Zhu, S.E.; Li, F.; Wang, G.W. Mechanochemistry of fullerenes and related materials. Chem. Soc. Rev. 2013, 42, 7535–7570. [Google Scholar] [CrossRef]
- Andersen, J.; Mack, J. Insights into Mechanochemical Reactions at Targetable and Stable, Sub-ambient Temperatures. Angew. Chem. Int. Ed. 2018, 57, 13062–13065. [Google Scholar] [CrossRef]
- Carta, M.; Colacino, E.; Delogu, F.; Porcheddu, A. Kinetics of mechanochemical transformations. Phys. Chem. Chem. Phys. 2020, 22, 14489–14502. [Google Scholar] [CrossRef]
- Colacino, E.; Carta, M.; Pia, G.; Porcheddu, A.; Ricci, P.C.; Delogu, F. Processing and Investigation Methods in Mechanochemical Kinetics. ACS Omega 2018, 3, 9196–9209. [Google Scholar] [CrossRef]
- Boldyreva, E. Mechanochemistry of inorganic and organic systems: What is similar, what is different? Chem. Soc. Rev. 2013, 42, 7719–7738. [Google Scholar] [CrossRef]
- Yang, L.; Moores, A.; Friščić, T.; Provatas, N. Thermodynamics Model for Mechanochemical Synthesis of Gold Nanoparticles: Implications for Solvent-Free Nanoparticle Production. ACS Appl. Nano Mater. 2021, 4, 1886–1897. [Google Scholar] [CrossRef]
- Amrute, A.P.; De Bellis, J.; Felderhoff, M.; Schuth, F. Mechanochemical Synthesis of Catalytic Materials. Chem. Eur. J. 2021, 27, 6819–6847. [Google Scholar] [CrossRef]
- Balaz, P.; Achimovicova, M.; Balaz, M.; Billik, P.; Cherkezova-Zheleva, Z.; Criado, J.M.; Delogu, F.; Dutkova, E.; Gaffet, E.; Gotor, F.J.; et al. Hallmarks of mechanochemistry: From nanoparticles to technology. Chem. Soc. Rev. 2013, 42, 7571–7637. [Google Scholar] [CrossRef] [PubMed]
- Schreyer, H.; Eckert, R.; Immohr, S.; de Bellis, J.; Felderhoff, M.; Schuth, F. Milling Down to Nanometers: A General Process for the Direct Dry Synthesis of Supported Metal Catalysts. Angew. Chem. Int. Ed. 2019, 58, 11262–11265. [Google Scholar] [CrossRef]
- Xu, C.; De, S.; Balu, A.M.; Ojeda, M.; Luque, R. Mechanochemical synthesis of advanced nanomaterials for catalytic applications. Chem. Commun. 2015, 51, 6698–6713. [Google Scholar] [CrossRef]
- Mucsi, G. A review on mechanical activation and mechanical alloying in stirred media mill. Chem. Eng. Res. Des. 2019, 148, 460–474. [Google Scholar] [CrossRef]
- Baláž, P. Mechanochemistry in Nanoscience and Minerals Engineering; Springer: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- Bellosta von Colbe, J.M.; Felderhoff, M.; Bogdanovic, B.; Schuth, F.; Weidenthaler, C. One-step direct synthesis of a Ti-doped sodium alanate hydrogen storage material. Chem. Commun. 2005, 4732–4734. [Google Scholar] [CrossRef] [PubMed]
- Gratz, S.; Beyer, D.; Tkachova, V.; Hellmann, S.; Berger, R.; Feng, X.; Borchardt, L. The mechanochemical Scholl reaction—A solvent-free and versatile graphitization tool. Chem. Commun. 2018, 54, 5307–5310. [Google Scholar] [CrossRef] [PubMed]
- Brekalo, I.; Yuan, W.; Mottillo, C.; Lu, Y.; Zhang, Y.; Casaban, J.; Holman, K.T.; James, S.L.; Duarte, F.; Williams, P.A.; et al. Manometric real-time studies of the mechanochemical synthesis of zeolitic imidazolate frameworks. Chem. Sci. 2020, 11, 2141–2147. [Google Scholar] [CrossRef] [PubMed]
- Trost, B.M. On inventing reactions for atom economy. Acc. Chem. Res. 2002, 35, 695–705. [Google Scholar] [CrossRef]
- Adeyemi, O.G.; Coville, N.J. Solvent-Free Organometallic Migratory Insertion Reactions. Organometallics 2003, 22, 2284–2290. [Google Scholar] [CrossRef]
- Klissurski, D.; Blaskov, V. Phase transition during mechanical dehydration of γ-FeOOH. J. Chem. Soc. Chem. Commun. 1983, 863–864. [Google Scholar] [CrossRef]
- Šubrt, J.; Pérez-Maqueda, L.A.; Criado, J.M.; Real, C.; Boháček, J.; Večerníková, E. Preparation of Nanosized Hematite Particles by Mechanical Activation of Goethite Samples. J. Am. Ceram. Soc. 2000, 83, 294–298. [Google Scholar] [CrossRef]
- Blaskov, V.; Radev, D.D.; Klissurski, D.; Yordanov, N.D. Behaviour of Cu(II) hydroxide during mechanical treatment. J. Alloys Compd. 1994, 206, 267–270. [Google Scholar] [CrossRef]
- Štefanić, G.; Popović, S.; Musić, S. The effect of mechanical treatment of zirconium(IV) hydroxide on its thermal behaviour. Thermochim. Acta 1995, 259, 225–234. [Google Scholar] [CrossRef]
- Liao, J.; Senna, M. Mechanochemical dehydration and amorphization of hydroxides of Ca, Mg and Al on grinding with and without SiO2. Solid State Ion. 1993, 66, 313–319. [Google Scholar] [CrossRef]
- Sanchez, C.; Rozes, L.; Ribot, F.; Laberty-Robert, C.; Grosso, D.; Sassoye, C.; Boissiere, C.; Nicole, L. “Chimie douce”: A land of opportunities for the designed construction of functional inorganic and hybrid organic-inorganic nanomaterials. C. R. Chim. 2010, 13, 3–39. [Google Scholar] [CrossRef]
- Senna, M. Grinding of mixture under mild condition for mechanochemical complexation. Int. J. Miner. Process. 1996, 44–45, 187–195. [Google Scholar] [CrossRef]
- Etter, M.C.; Reutzel, S.M.; Choo, C.G. Self-organization of adenine and thymine in the solid state. J. Am. Chem. Soc. 1993, 115, 4411–4412. [Google Scholar] [CrossRef]
- Chen, Y.; Halstead, T.; Williams, J.S. Influence of milling temperature and atmosphere on the synthesis of iron nitrides by ball milling. Mater. Sci. Eng. A 1996, 206, 24–29. [Google Scholar] [CrossRef]
- Baláž, M.; Zorkovská, A.; Urakaev, F.; Baláž, P.; Briančin, J.; Bujňáková, Z.; Achimovičová, M.; Gock, E. Ultrafast mechanochemical synthesis of copper sulfides. RSC Adv. 2016, 6, 87836–87842. [Google Scholar] [CrossRef]
- Baláž, P.; Baláž, M.; Achimovičová, M.; Bujňáková, Z.; Dutková, E. Chalcogenide mechanochemistry in materials science: Insight into synthesis and applications (a review). J. Mater. Sci. 2017, 52, 11851–11890. [Google Scholar] [CrossRef]
- Petersen, H.; Reichle, S.; Leiting, S.; Losch, P.; Kersten, W.; Rathmann, T.; Tseng, J.; Etter, M.; Schmidt, W.; Weidenthaler, C. In Situ Synchrotron X-ray Diffraction Studies of the Mechanochemical Synthesis of ZnS from its Elements. Chem. Eur. J. 2021, 27, 12558–12565. [Google Scholar] [CrossRef]
- Takacs, L. Self-sustaining reactions induced by ball milling. Prog. Mater Sci. 2002, 47, 355–414. [Google Scholar] [CrossRef]
- Pei, Z.; Xu, H.; Zhang, Y. Preparation of CrO2 nanoparticles via oxidation method. Mater. Lett. 2012, 76, 205–207. [Google Scholar] [CrossRef]
- Li, Y.X.; Zhou, X.Z.; Wang, Y.; You, X.Z. Preparation of nano-sized CeO2 by mechanochemical reaction of cerium carbonate with sodium hydroxide. Mater. Lett. 2003, 58, 245–249. [Google Scholar] [CrossRef]
- Chen, Y. Low-temperature oxidation of ilmenite (FeTiO3) induced by high energy ball milling at room temperature. J. Alloys Compd. 1997, 257, 156–160. [Google Scholar] [CrossRef]
- Li, C.; Liang, B. Study on the mechanochemical oxidation of ilmenite. J. Alloys Compd. 2008, 459, 354–361. [Google Scholar] [CrossRef]
- Chen, Y. Different oxidation reactions of ilmenite induced by high energy ball milling. J. Alloys Compd. 1998, 266, 150–154. [Google Scholar] [CrossRef]
- Temuujin, J.; MacKenzie, K.J.D.; Burmaa, G.; Tsend-Ayush, D.; Jadambaa, T.; Riessen, A.v. Mechanical activation of MoS2+Na2O2 mixtures. Miner. Eng. 2009, 22, 415–418. [Google Scholar] [CrossRef]
- Kravchuk, D.V.; Forbes, T.Z. Mechanochemical activation and oxidation of U(IV)O(2). Chem. Commun. 2022, 58, 4528–4531. [Google Scholar] [CrossRef] [PubMed]
- Friščić, T.; Childs, S.L.; Rizvi, S.A.A.; Jones, W. The role of solvent in mechanochemical and sonochemical cocrystal formation: A solubility-based approach for predicting cocrystallisation outcome. CrystEngComm 2009, 11, 418–426. [Google Scholar] [CrossRef]
- Do, J.L.; Tan, D.; Friščić, T. Oxidative Mechanochemistry: Direct, Room-Temperature, Solvent-Free Conversion of Palladium and Gold Metals into Soluble Salts and Coordination Complexes. Angew. Chem. Int. Ed. 2018, 57, 2667–2671. [Google Scholar] [CrossRef]
- Roldan, M.A.; López-Flores, V.; Alcala, M.D.; Ortega, A.; Real, C. Mechanochemical synthesis of vanadium nitride. J. Eur. Ceram. Soc. 2010, 30, 2099–2107. [Google Scholar] [CrossRef]
- Ogino, Y.; Yamasaki, T.; Miki, M.; Atsumi, N.; Yoshioka, K. Synthesis of TiN and (Ti, Al)N powders by mechanical alloying in nitrogen gas. Scr. Metall. Mater. 1993, 28, 967–971. [Google Scholar] [CrossRef]
- Criado, J.; Alacala, M.D.; Real, C. Influence of the atmosphere control during the grinding of titanium powder on its reactivity towards the conversion into titanium nitride. Solid State Ion. 1997, 101–103, 1387–1391. [Google Scholar] [CrossRef]
- Chin, Z.H.; Perng, T.P. In situ observation of combustion to form TiN during ball milling Ti in nitrogen. Appl. Phys. Lett. 1997, 70, 2380–2382. [Google Scholar] [CrossRef]
- Gotor, F.J.; Alcalá, M.D.; Real, C.; Criado, J.M. Combustion Synthesis of TiN Induced by High-energy Ball Milling of Ti Under Nitrogen Atmosphere. J. Mater. Res. 2011, 17, 1655–1663. [Google Scholar] [CrossRef]
- Liu, L.; Lu, L.; Chen, L.; Qin, Y.; Zhang, L.D. Solid-gas reactions driven by mechanical alloying of niobium and tantalum in nitrogen. Metall. Mater. Trans. A 1999, 30, 1097–1100. [Google Scholar] [CrossRef]
- Calka, A.; Williams, J.S.; Millet, P. Synthesis of silicon nitride by mechanical alloying. Scr. Metall. Mater. 1992, 27, 1853–1857. [Google Scholar] [CrossRef]
- Takacs, L.; Mandal, S.K. Preparation of some metal phosphides by ball milling. Mater. Sci. Eng. A 2001, 304–306, 429–433. [Google Scholar] [CrossRef]
- Chung, H.Y.; Weinberger, M.B.; Levine, J.B.; Cumberland, R.W.; Kavner, A.; Yang, J.M.; Tolbert, S.H.; Kaner, R.B. Synthesis of ultra-incompressible superhard rhenium diboride at ambient pressure. Science 2007, 316, 436–439. [Google Scholar] [CrossRef]
- Orlovskaya, N.; Xie, Z.; Klimov, M.; Heinrich, H.; Restrepo, D.; Blair, R.; Suryanarayana, C. Mechanochemical synthesis of ReB2 powder. J. Mater. Res. 2011, 26, 2772–2779. [Google Scholar] [CrossRef]
- Chen, Y.; Williams, J.S. Formation of metal hydrides by mechanical alloying. J. Alloys Compd. 1995, 217, 181–184. [Google Scholar] [CrossRef]
- Bobet, J. Study of Mg-M (M=Co, Ni and Fe) mixture elaborated by reactive mechanical alloying: Hydrogen sorption properties. Int. J. Hydrogen Energy 2001, 26, 493–501. [Google Scholar] [CrossRef]
- Bobet, J.L.; Even, C.; Nakamura, Y.; Akiba, E.; Darriet, B. Synthesis of magnesium and titanium hydride via reactive mechanical alloying. J. Alloys Compd. 2000, 298, 279–284. [Google Scholar] [CrossRef]
- Bouaricha, S.; Dodelet, J.P.; Guay, D.; Huot, J.; Schulz, R. Activation characteristics of graphite modified hydrogen absorbing materials. J. Alloys Compd. 2001, 325, 245–251. [Google Scholar] [CrossRef]
- Huot, J.; Tremblay, M.L.; Schulz, R. Synthesis of nanocrystalline hydrogen storage materials. J. Alloys Compd. 2003, 356–357, 603–607. [Google Scholar] [CrossRef]
- Gasgnier, M.; Szwarc, H.; Petit, A. Synthesis of ditin hexathiophosphate Sn2P2S6 by low-energy ball-milling and monomode microwave. Mater. Res. Bull. 2003, 38, 1681–1694. [Google Scholar] [CrossRef]
- Fuentes, A.F.; Takacs, L. Preparation of multicomponent oxides by mechanochemical methods. J. Mater. Sci. 2013, 48, 598–611. [Google Scholar] [CrossRef]
- Shen, J.; Blachnik, R. Mechanochemical syntheses of antimony selenide, tin selenides and two tin antimony selenides. Thermochim. Acta 2003, 399, 245–246. [Google Scholar] [CrossRef]
- Jena, A.K.; Kulkarni, A.; Miyasaka, T. Halide Perovskite Photovoltaics: Background, Status, and Future Prospects. Chem. Rev. 2019, 119, 3036–3103. [Google Scholar] [CrossRef]
- Li, J.; Duan, J.; Yang, X.; Duan, Y.; Yang, P.; Tang, Q. Review on recent progress of lead-free halide perovskites in optoelectronic applications. Nano Energy 2021, 80, 105526. [Google Scholar] [CrossRef]
- Palazon, F.; El Ajjouri, Y.; Bolink, H.J. Making by Grinding: Mechanochemistry Boosts the Development of Halide Perovskites and Other Multinary Metal Halides. Adv. Energy Mater. 2019, 10, 1902499. [Google Scholar] [CrossRef]
- Varghese, V.; Sharma, A.; Chattopadhyay, K. Reaction ball milling of systems involving ionic bonds. Mater. Sci. Eng. A 2001, 304–306, 434–437. [Google Scholar] [CrossRef]
- Thiessen, P.A.; Heinicke, G.; Schober, E. Zur tribochemischen Umsetzung von Gold und CO2 mit Hilfe radioaktiver Markierung. Z. Anorg. Allg. Chem. 1970, 377, 20–28. [Google Scholar] [CrossRef]
- Brody, E.; Millner, T. Kohlensäure-Kohlenoxydgleichgewicht über Kupfer. Z. Anorg. Allg. Chem. 1927, 164, 86–95. [Google Scholar] [CrossRef]
- Heinicke, G.; Sigrist, K. Über tribochemische Reaktionen einiger technisch wichtiger Metalle mit CO und CO2. Z. Chem. 1966, 6, 291–296. [Google Scholar] [CrossRef]
- Heinicke, G.; Sigrist, K. Zur Thermodynamik tribochemischer Reaktionen. Z. Chem. 1971, 11, 226–235. [Google Scholar] [CrossRef]
- Fiss, B.G.; Richard, A.J.; Douglas, G.; Kojic, M.; Friscic, T.; Moores, A. Mechanochemical methods for the transfer of electrons and exchange of ions: Inorganic reactivity from nanoparticles to organometallics. Chem. Soc. Rev. 2021, 50, 8279–8318. [Google Scholar] [CrossRef]
- Davison, N.; Quirk, J.A.; Tuna, F.; Collison, D.; McMullin, C.L.; Michaels, H.; Morritt, G.H.; Waddell, P.G.; Gould, J.A.; Freitag, M.; et al. A room-temperature-stable electride and its reactivity: Reductive benzene/pyridine couplings and solvent-free Birch reductions. Chem 2022, in press. [Google Scholar] [CrossRef]
- Fiore, C.; Sovic, I.; Lukin, S.; Halasz, I.; Martina, K.; Delogu, F.; Ricci, P.C.; Porcheddu, A.; Shemchuk, O.; Braga, D.; et al. Kabachnik–Fields Reaction by Mechanochemistry: New Horizons from Old Methods. ACS Sustain. Chem. Eng. 2020, 8, 18889–18902. [Google Scholar] [CrossRef]
- Rak, M.J.; Saadé, N.K.; Friščić, T.; Moores, A. Mechanosynthesis of ultra-small monodisperse amine-stabilized gold nanoparticles with controllable size. Green Chem. 2014, 16, 86–89. [Google Scholar] [CrossRef]
- Kovacheva, P.; Todorovsky, D.; Radev, D. Mechanochemistry of the 5f-elements compounds. 5. Influence of the reaction medium on the mechanochemically induced reduction of U3O8. J. Radioanal. Nucl. Chem. 2010, 287, 193–197. [Google Scholar] [CrossRef]
- Fulmer, D.A.; Shearouse, W.C.; Medonza, S.T.; Mack, J. Solvent-free Sonogashira coupling reaction via high speed ball milling. Green Chem. 2009, 11, 1821–1825. [Google Scholar] [CrossRef]
- Tan, D.; Strukil, V.; Mottillo, C.; Friscic, T. Mechanosynthesis of pharmaceutically relevant sulfonyl-(thio)ureas. Chem. Commun. 2014, 50, 5248–5250. [Google Scholar] [CrossRef]
- Vogt, C.G.; Gratz, S.; Lukin, S.; Halasz, I.; Etter, M.; Evans, J.D.; Borchardt, L. Direct Mechanocatalysis: Palladium as Milling Media and Catalyst in the Mechanochemical Suzuki Polymerization. Angew. Chem. Int. Ed. 2019, 58, 18942–18947. [Google Scholar] [CrossRef]
- Reguera, E.; Fernández-Bertrán, J.; Paneque, A.; Yee-Madeira, H. Mechanochemical Reaction Between the Probe and the Matrix: A Possible Source of Errors When IR Spectra of Alkali Acid Bifluorides Are Recorded in Alkali Halide Pressed Disks. Spectrosc. Lett. 2004, 37, 191–199. [Google Scholar] [CrossRef]
- Lukin, S.; Stolar, T.; Lončarić, I.; Milanović, I.; Biliškov, N.; di Michiel, M.; Friščić, T.; Halasz, I. Mechanochemical Metathesis between AgNO(3) and NaX (X = Cl, Br, I) and Ag(2)XNO(3) Double-Salt Formation. Inorg. Chem. 2020, 59, 12200–12208. [Google Scholar] [CrossRef]
- Fiss, B.G.; Vu, N.-N.; Douglas, G.; Do, T.-O.; Friščić, T.; Moores, A. Solvent-Free Mechanochemical Synthesis of Ultrasmall Nickel Phosphide Nanoparticles and Their Application as a Catalyst for the Hydrogen Evolution Reaction (HER). ACS Sustain. Chem. Eng. 2020, 8, 12014–12024. [Google Scholar] [CrossRef]
- Štefanić, G.; Musić, S.; Gajović, A. Structural and microstructural changes in monoclinic ZrO2 during the ball-milling with stainless steel assembly. Mater. Res. Bull. 2006, 41, 764–777. [Google Scholar] [CrossRef]
- Bailey, J.E.; Lewis, D.; Librant, Z.M.; Porter, L.J. Phase Transformations in Milled Zirconia. Trans. J. Br. Ceram. Soc. 1972, 71, 25–30. [Google Scholar]
- Štefanić, G.; Musić, S.; Gajović, A. A comparative study of the influence of milling media on the structural and microstructural changes in monoclinic ZrO2. J. Eur. Ceram. Soc. 2007, 27, 1001–1016. [Google Scholar] [CrossRef]
- Lancaster, R.W.; Harris, L.D.; Pearson, D. Fifty-year old samples of progesterone demonstrate the complex role of synthetic impurities in stabilizing a metastable polymorph. CrystEngComm 2011, 13, 1775–1777. [Google Scholar] [CrossRef]
- In, S.; Orlov, A.; Garcia, F.; Tikhov, M.; Wright, D.S.; Lambert, R.M. Efficient visible light-active N-doped TiO2 photocatalysts by a reproducible and controllable synthetic route. Chem. Commun. 2006, 4236–4238. [Google Scholar] [CrossRef]
- Asahi, R.; Morikawa, T.; Ohwaki, T.; Aoki, K.; Taga, Y. Visible-light photocatalysis in nitrogen-doped titanium oxides. Science 2001, 293, 269–271. [Google Scholar] [CrossRef]
- Yin, S.; Zhang, Q.; Saito, F.; Sato, T. Preparation of Visible Light-Activated Titania Photocatalyst by Mechanochemical Method. Chem. Lett. 2003, 32, 358–359. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Moribe, S.; Ikoma, T.; Akiyama, K.; Zhang, Q.; Saito, F.; Tero-Kubota, S. Visible light induced paramagnetic sites in nitrogen-doped TiO2 prepared by a mechanochemical method. Mol. Phys. 2006, 104, 1733–1737. [Google Scholar] [CrossRef]
- Livraghi, S.; Chierotti, M.R.; Giamello, E.; Magnacca, G.; Paganini, M.C.; Cappelletti, G.; Bianchi, C.L. Nitrogen-Doped Titanium Dioxide Active in Photocatalytic Reactions with Visible Light: A Multi-Technique Characterization of Differently Prepared Materials. J. Phys. Chem. C 2008, 112, 17244–17252. [Google Scholar] [CrossRef]
- Yin, S.; Yamaki, H.; Zhang, Q.; Komatsu, M.; Wang, J.; Tang, Q.; Saito, F.; Sato, T. Mechanochemical synthesis of nitrogen-doped titania and its visible light induced NO destruction ability. Solid State Ion. 2004, 172, 205–209. [Google Scholar] [CrossRef]
- Yin, S.; Yamaki, H.; Komatsu, M.; Zhang, Q.; Wang, J.; Tang, Q.; Saito, F.; Sato, T. Synthesis of visible-light reactive TiO2−N photocatalyst by mechanochemical doping. Solid State Sci. 2005, 7, 1479–1485. [Google Scholar] [CrossRef]
- Yin, S.; Komatsu, M.; Zhang, Q.; Saito, F.; Sato, T. Synthesis of visible-light responsive nitrogen/carbon doped titania photocatalyst by mechanochemical doping. J. Mater. Sci. 2007, 42, 2399–2404. [Google Scholar] [CrossRef]
- Rattanakam, R.; Supothina, S. Visible-light-sensitive N-doped TiO2 photocatalysts prepared by a mechanochemical method: Effect of a nitrogen source. Res. Chem. Intermed. 2009, 35, 263–269. [Google Scholar] [CrossRef]
- Yang, L.C.; Qu, Q.T.; Shi, Y.; Wu, Y.P.; Van Ree, T. Materials for lithium-ion batteries by mechanochemical methods. In High-Energy Ball Milling; Woodhead Publishing: Sawston, UK, 2010; pp. 361–408. [Google Scholar]
- Janot, R.; Guerard, D. Ball-milling in liquid media Applications to the preparation of anodic materials for lithium-ion batteries. Prog. Mater Sci. 2005, 50, 1–92. [Google Scholar] [CrossRef]
- Friščić, T.; Trask, A.V.; Jones, W.; Motherwell, W.D. Screening for inclusion compounds and systematic construction of three-component solids by liquid-assisted grinding. Angew. Chem. Int. Ed. 2006, 45, 7546–7550. [Google Scholar] [CrossRef]
- Šepelák, V.; Bergmann, I.; Kipp, S.; Becker, K.D. Nanocrystalline Ferrites Prepared by Mechanical Activation and Mechanosynthesis. Z. Anorg. Allg. Chem. 2005, 631, 993–1003. [Google Scholar] [CrossRef]
- Tkáčová, K.; Šepelák, V.; Števulová, N.; Boldyrev, V.V. Structure–Reactivity Study of Mechanically Activated Zinc Ferrite. J. Solid State Chem. 1996, 123, 100–108. [Google Scholar] [CrossRef]
- Šepelák, V.; Tkáčová, K.; Boldyrev, V.V.; Wiβmann, S.; Becker, K.D. Mechanically induced cation redistribution in ZnFe2O4 and its thermal stability. Phys. B Condens. Matter 1997, 234–236, 617–619. [Google Scholar] [CrossRef]
- Šepelák, V.; Baabe, D.; Litterst, F.J.; Becker, K.D. Structural disorder in the high-energy milled magnesium ferrite. J. Appl. Phys. 2000, 88, 5884–5893. [Google Scholar] [CrossRef]
- Chinnasamy, C.N.; Narayanasamy, A.; Ponpandian, N.; Joseyphus, R.J.; Chattopadhyay, K.; Shinoda, K.; Jeyadevan, B.; Tohji, K.; Nakatsuka, K.; Greneche, J.M. Ferrimagnetic ordering in nanostructured CdFe2O4 spinel. J. Appl. Phys. 2001, 90, 527–529. [Google Scholar] [CrossRef]
- Šepelák, V.; Baabe, D.; Mienert, D.; Schultze, D.; Krumeich, F.; Litterst, F.J.; Becker, K.D. Evolution of structure and magnetic properties with annealing temperature in nanoscale high-energy-milled nickel ferrite. J. Magn. Magn. Mater. 2003, 257, 377–386. [Google Scholar] [CrossRef]
- Šepelák, V.; Bergmann, I.; Indris, S.; Feldhoff, A.; Hahn, H.; Becker, K.D.; Grey, C.P.; Heitjans, P. High-resolution 27Al MAS NMR spectroscopic studies of the response of spinel aluminates to mechanical action. J. Mater. Chem. 2011, 21, 8332–8337. [Google Scholar] [CrossRef]
- Sepelak, V.; Begin-Colin, S.; Le Caer, G. Transformations in oxides induced by high-energy ball-milling. Dalton Trans. 2012, 41, 11927–11948. [Google Scholar] [CrossRef]
- Toolenaar, F.J.C.M. The formation of zinc ferrite. J. Mater. Sci. 1989, 24, 1089–1094. [Google Scholar] [CrossRef]
- Halikia, I.; Milona, E. Kinetic Study of the Solid State Reaction between Alpha-Fe2O3 and ZnO for Zinc Ferrite Formation. Can. Metall. Q. 1994, 33, 99–109. [Google Scholar] [CrossRef]
- Lefelshtel, N.; Nadiv, S.; Lin, I.J.; Zimmels, Y. Production of zinc ferrite in a mechano-chemical reaction by grinding in a ball mill. Powder Technol. 1978, 20, 211–217. [Google Scholar] [CrossRef]
- Kim, W.; Saito, F. Mechanochemical synthesis of zinc ferrite from zinc oxide and α-Fe2O3. Powder Technol. 2001, 114, 12–16. [Google Scholar] [CrossRef]
- Goya, G.F.; Rechenberg, H.R. Ionic disorder and Néel temperature in ZnFe2O4 nanoparticles. J. Magn. Magn. Mater. 1999, 196–197, 191–192. [Google Scholar] [CrossRef]
- Moustafa, S.F.; Morsi, M.B. The formation of Mg ferrite by mechanical alloying and sintering. Mater. Lett. 1998, 34, 241–247. [Google Scholar] [CrossRef]
- Jovalekić, Č.; Zdujić, M.; Radaković, A.; Mitrić, M. Mechanochemical synthesis of NiFe2O4 ferrite. Mater. Lett. 1995, 24, 365–368. [Google Scholar] [CrossRef]
- Šepelák, V.; Bergmann, I.; Feldhoff, A.; Heitjans, P.; Krumeich, F.; Menzel, D.; Litterst, F.J.; Campbell, S.J.; Becker, K.D. Nanocrystalline Nickel Ferrite, NiFe2O4: Mechanosynthesis, Nonequilibrium Cation Distribution, Canted Spin Arrangement, and Magnetic Behavior. J. Phys. Chem. C 2007, 111, 5026–5033. [Google Scholar] [CrossRef]
- Heitjans, P.; Masoud, M.; Feldhoff, A.; Wilkening, M. NMR and impedance studies of nanocrystalline and amorphous ion conductors: Lithium niobate as a model system. Faraday Discuss. 2007, 134, 67–82, discussion 103–118, 415–109. [Google Scholar] [CrossRef]
- Šepelák, V.; Becker, K.D.; Bergmann, I.; Suzuki, S.; Indris, S.; Feldhoff, A.; Heitjans, P.; Grey, C.P. A One-Step Mechanochemical Route to Core−Shell Ca2SnO4 Nanoparticles Followed by 119Sn MAS NMR and 119Sn Mössbauer Spectroscopy. Chem. Mater. 2009, 21, 2518–2524. [Google Scholar] [CrossRef]
- Düvel, A.; Wilkening, M.; Uecker, R.; Wegner, S.; Šepelák, V.; Heitjans, P. Mechanosynthesized nanocrystalline BaLiF(3): The impact of grain boundaries and structural disorder on ionic transport. Phys. Chem. Chem. Phys. 2010, 12, 11251–11262. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, K.L.; Menzel, D.; Feldhoff, A.; Kübel, C.; Bruns, M.; Paesano, A.; Düvel, A.; Wilkening, M.; Ghafari, M.; Hahn, H.; et al. Mechanosynthesized BiFeO3 Nanoparticles with Highly Reactive Surface and Enhanced Magnetization. J. Phys. Chem. C 2011, 115, 7209–7217. [Google Scholar] [CrossRef]
- Šepelák, V.; Becker, S.M.; Bergmann, I.; Indris, S.; Scheuermann, M.; Feldhoff, A.; Kübel, C.; Bruns, M.; Stürzl, N.; Ulrich, A.S.; et al. Nonequilibrium structure of Zn2SnO4 spinel nanoparticles. J. Mater. Chem. 2012, 22, 3117–3126. [Google Scholar] [CrossRef]
- Stubičar, N.; Tonejc, A.; Stubičar, M. Microstructural evolution of some MgO–TiO2 and MgO–Al2O3 powder mixtures during high-energy ball milling and post-annealing studied by X-ray diffraction. J. Alloys Compd. 2004, 370, 296–301. [Google Scholar] [CrossRef]
- Ristic, M.M.; Obradovic, N.; Filipovic, S.; Bykov, A.I.; Vasil’kovskaya, M.A.; Klochkov, L.A.; Timofeeva, I.I. Formation of magnesium titanates. Powder Metall. Met. Ceram. 2009, 48, 371–374. [Google Scholar] [CrossRef]
- Kim, W.; Saito, F. Effect of grinding on synthesis of MgAl2O4 spinel from a powder mixture of Mg(OH)2 and Al(OH)3. Powder Technol. 2000, 113, 109–113. [Google Scholar] [CrossRef]
- Ding, J.; McCormick, P.G.; Street, R. Formation of spinel Mn-ferrite during mechanical alloying. J. Magn. Magn. Mater. 1997, 171, 309–314. [Google Scholar] [CrossRef]
- Ding, J.; Miao, W.F.; Pirault, E.; Street, R.; McCormick, P.G. Structural evolution of Fe + Fe2O3 during mechanical milling. J. Magn. Magn. Mater. 1998, 177–181, 933–934. [Google Scholar] [CrossRef]
- Ding, J.; McCormick, P.G.; Street, R. Magnetic properties of mechanically alloyed CoFe2O4. Solid State Commun. 1995, 95, 31–33. [Google Scholar] [CrossRef]
- Ding, J.; Reynolds, T.; Miao, W.F.; McCormick, P.G.; Street, R. High magnetic performance in mechanically alloyed Co-substituted Fe3O4. Appl. Phys. Lett. 1994, 65, 3135–3136. [Google Scholar] [CrossRef]
- Guigue-Millot, N.; Bégin-Colin, S.; Champion, Y.; Hÿtch, M.J.; Le Caër, G.; Perriat, P. Control of grain size and morphologies of nanograined ferrites by adaptation of the synthesis route: Mechanosynthesis and soft chemistry. J. Solid State Chem. 2003, 170, 30–38. [Google Scholar] [CrossRef]
- Kong, L.B.; Zhang, T.S.; Ma, J.; Boey, F. Progress in synthesis of ferroelectric ceramic materials via high-energy mechanochemical technique. Prog. Mater Sci. 2008, 53, 207–322. [Google Scholar] [CrossRef]
- Yip, T.W.; Cussen, E.J.; Wilson, C. Spontaneous formation of crystalline lithium molybdate from solid reagents at room temperature. Dalton Trans. 2010, 39, 411–417. [Google Scholar] [CrossRef]
- Yip, T.W.S.; Cussen, E.J.; MacLaren, D.A. Synthesis of HxLi1−xLaTiO4 from quantitative solid-state reactions at room temperature. Chem. Commun. 2010, 46, 698–700. [Google Scholar] [CrossRef]
- Karki, S.; Friščić, T.; Jones, W.; Motherwell, W.D. Screening for pharmaceutical cocrystal hydrates via neat and liquid-assisted grinding. Mol. Pharm. 2007, 4, 347–354. [Google Scholar] [CrossRef]
- Ay, A.N.; Zumreoglu-Karan, B.; Temel, A.; Rives, V. Bioinorganic magnetic core-shell nanocomposites carrying antiarthritic agents: Intercalation of ibuprofen and glucuronic acid into Mg-Al-layered double hydroxides supported on magnesium ferrite. Inorg. Chem. 2009, 48, 8871–8877. [Google Scholar] [CrossRef]
- Wang, M.; Cao, X.; Huang, Y.; Guo, C.; Huang, L.; Yin, S.; Sato, T. Solvent-free mechanochemical synthesis of well-dispersed single crystalline zinc hydroxystannate and their photocatalytic properties. CrystEngComm 2012, 14, 2950–2953. [Google Scholar] [CrossRef]
- Flanigen, E.M.; Broach, R.W.; Wilson, S.T. Introduction. In Zeolites in Industrial Separation and Catalysis; John Wiley & Sons: Hoboken, NJ, USA, 2010; pp. 1–26. [Google Scholar]
- Gordina, N.E.; Prokof’ev, V.Y.; Il’in, A.P. Synthesis of NaA Zeolite by Mechanochemical Methods. Russ. J. Appl. Chem. 2003, 76, 661–662. [Google Scholar] [CrossRef]
- Prokof’ev, V.Y.; Gordina, N.E.; Zhidkova, A.B. Investigation of mechanochemical synthesis of zeolite NaA made of metakaolin in the mills with shock-shear type of strain. Russ. J. Appl. Chem. 2012, 85, 1077–1082. [Google Scholar] [CrossRef]
- Prokof’ev, V.Y.; Gordina, N.E.; Zhidkova, A.B.; Efremov, A.M. Mechanochemical synthesis of granulated LTA zeolite from metakaolin. J. Mater. Sci. 2012, 47, 5385–5392. [Google Scholar] [CrossRef]
- Ren, L.; Wu, Q.; Yang, C.; Zhu, L.; Li, C.; Zhang, P.; Zhang, H.; Meng, X.; Xiao, F.-S. Solvent-Free Synthesis of Zeolites from Solid Raw Materials. J. Am. Chem. Soc. 2012, 134, 15173–15176. [Google Scholar] [CrossRef]
- Rainer, D.N.; Morris, R.E. New avenues for mechanochemistry in zeolite science. Dalton Trans. 2021, 50, 8995–9009. [Google Scholar] [CrossRef] [PubMed]
- Štefanić, G.; Krehula, S.; Štefanić, I. The high impact of a milling atmosphere on steel contamination. Chem. Commun. 2013, 49, 9245–9247. [Google Scholar] [CrossRef]
- Huskić, I.; Lennox, C.B.; Friščić, T. Accelerated ageing reactions: Towards simpler, solvent-free, low energy chemistry. Green Chem. 2020, 22, 5881–5901. [Google Scholar] [CrossRef]
- Abe, O.; Sano, T. Mechanochemical reduction of manganese dioxide by grinding in organic vapors. J. Ceram. Soc. Jpn. 2009, 117, 999–1003. [Google Scholar] [CrossRef]
- Julien, P.A.; Friščić, T. Methods for Monitoring Milling Reactions and Mechanistic Studies of Mechanochemistry: A Primer. Cryst. Growth Des. 2022, 22, 5726–5754. [Google Scholar] [CrossRef]
- Volkov, V.V.; Myakishev, K.G. Mechanochemical reactions in the chemistry of boranes. Inorg. Chim. Acta 1999, 289, 51–57. [Google Scholar] [CrossRef]
- Schlesinger, H.I.; Brown, H.C.; Finholt, A.E. The Preparation of Sodium Borohydride by the High Temperature Reaction of Sodium Hydride with Borate Esters. J. Am. Chem. Soc. 1953, 75, 205–209. [Google Scholar] [CrossRef]
- Jeon, E.; Cho, Y. Mechanochemical synthesis and thermal decomposition of zinc borohydride. J. Alloys Compd. 2006, 422, 273–275. [Google Scholar] [CrossRef]
- Gennari, F.C.; Fernández Albanesi, L.; Rios, I.J. Synthesis and thermal stability of Zr(BH4)4 and Zr(BD4)4 produced by mechanochemical processing. Inorg. Chim. Acta 2009, 362, 3731–3737. [Google Scholar] [CrossRef]
- Balema, V.P.; Pecharsky, V.K.; Dennis, K.W. Solid state phase transformations in LiAlH4 during high-energy ball-milling. J. Alloys Compd. 2000, 313, 69–74. [Google Scholar] [CrossRef]
- Andreasen, A.; Vegge, T.; Pedersen, A.S. Dehydrogenation kinetics of as-received and ball-milled LiAlH4. J. Solid State Chem. 2005, 178, 3672–3678. [Google Scholar] [CrossRef]
- Balde, C.P.; Hereijgers, B.P.; Bitter, J.H.; de Jong, K.P. Sodium alanate nanoparticles—Linking size to hydrogen storage properties. J. Am. Chem. Soc. 2008, 130, 6761–6765. [Google Scholar] [CrossRef]
- Ares, J.R.; Aguey-Zinsou, K.F.; Porcu, M.; Sykes, J.M.; Dornheim, M.; Klassen, T.; Bormann, R. Thermal and mechanically activated decomposition of LiAlH4. Mater. Res. Bull. 2008, 43, 1263–1275. [Google Scholar] [CrossRef]
- Balema, V.P.; Dennis, K.W.; Pecharsky, V.K. Rapid solid-state transformation of tetrahedral [AlH4]− into octahedral [AlH6]3− in lithium aluminohydride. Chem. Commun. 2000, 1665–1666. [Google Scholar] [CrossRef]
- Balema, V.P.; Wiench, J.W.; Dennis, K.W.; Pruski, M.; Pecharsky, V.K. Titanium catalyzed solid-state transformations in LiAlH4 during high-energy ball-milling. J. Alloys Compd. 2001, 329, 108–114. [Google Scholar] [CrossRef]
- Aresfernandez, J.; Agueyzinsou, F.; Elsaesser, M.; Ma, X.; Dornheim, M.; Klassen, T.; Bormann, R. Mechanical and thermal decomposition of LiAlH4 with metal halides. Int. J. Hydrog. Energy 2007, 32, 1033–1040. [Google Scholar] [CrossRef]
- Bogdanović, B.; Felderhoff, M.; Pommerin, A.; Schüth, F.; Spielkamp, N. Advanced Hydrogen-Storage Materials Based on Sc-, Ce-, and Pr-Doped NaAlH4. Adv. Mater. 2006, 18, 1198–1201. [Google Scholar] [CrossRef]
- Fichtner, M.; Fuhr, O.; Kircher, O.; Rothe, J. Small Ti clusters for catalysis of hydrogen exchange in NaAlH4. Nanotechnology 2003, 14, 778–785. [Google Scholar] [CrossRef]
- Brinks, H.W.; Hauback, B.C.; Srinivasan, S.S.; Jensen, C.M. Synchrotron X-ray studies of Al(1-y)Ti(y) formation and re-hydriding inhibition in Ti-enhanced NaAlH4. J. Phys. Chem. B 2005, 109, 15780–15785. [Google Scholar] [CrossRef]
- Kim, Y.; Lee, E.-K.; Shim, J.-H.; Cho, Y.W.; Yoon, K.B. Mechanochemical synthesis and thermal decomposition of Mg(AlH4)2. J. Alloys Compd. 2006, 422, 283–287. [Google Scholar] [CrossRef]
- Varin, R.A.; Chiu, C.; Czujko, T.; Wronski, Z. Mechano-chemical activation synthesis (MCAS) of nanocrystalline magnesium alanate hydride [Mg(AlH4)2] and its hydrogen desorption properties. J. Alloys Compd. 2007, 439, 302–311. [Google Scholar] [CrossRef]
- Brinks, H.W.; Hauback, B.C.; Jensen, C.M.; Zidan, R. Synthesis and crystal structure of Na2LiAlD6. J. Alloys Compd. 2005, 392, 27–30. [Google Scholar] [CrossRef]
- Severa, G.; Rönnebro, E.; Jensen, C.M. Direct hydrogenation of magnesium boride to magnesium borohydride: Demonstration of >11 weight percent reversible hydrogenstorage. Chem. Commun. 2010, 46, 421–423. [Google Scholar] [CrossRef]
- Gupta, S.; Hlova, I.Z.; Kobayashi, T.; Denys, R.V.; Chen, F.; Zavaliy, I.Y.; Pruski, M.; Pecharsky, V.K. Facile synthesis and regeneration of Mg(BH4)2 by high energy reactive ball milling of MgB2. Chem. Commun. 2013, 49, 828–830. [Google Scholar] [CrossRef]
- Li, B.; Kaye, S.S.; Riley, C.; Greenberg, D.; Galang, D.; Bailey, M.S. Hydrogen storage materials discovery via high throughput ball milling and gas sorption. ACS Comb. Sci. 2012, 14, 352–358. [Google Scholar] [CrossRef] [PubMed]
- Ravnsbaek, D.B.; Sorensen, L.H.; Filinchuk, Y.; Besenbacher, F.; Jensen, T.R. Screening of metal borohydrides by mechanochemistry and diffraction. Angew. Chem. Int. Ed. 2012, 51, 3582–3586. [Google Scholar] [CrossRef]
- Uzarevic, K.; Halasz, I.; Friscic, T. Real-Time and In Situ Monitoring of Mechanochemical Reactions: A New Playground for All Chemists. J. Phys. Chem. Lett. 2015, 6, 4129–4140. [Google Scholar] [CrossRef] [PubMed]
- Michalchuk, A.A.L.; Emmerling, F. Time-Resolved In Situ Monitoring of Mechanochemical Reactions. Angew. Chem. Int. Ed. 2022, 61, e202117270. [Google Scholar] [CrossRef]
- Biliškov, N.; Borgschulte, A.; Užarević, K.; Halasz, I.; Lukin, S.; Milošević, S.; Milanović, I.; Novaković, J.G. In-Situ and Real-time Monitoring of Mechanochemical Preparation of Li2Mg(NH2BH3)4 and Na2Mg(NH2BH3)4 and Their Thermal Dehydrogenation. Chem. Eur. J. 2017, 23, 16274–16282. [Google Scholar] [CrossRef] [PubMed]
- Castilla-Martinez, C.A.; Moury, R.; Demirci, U.B. Amidoboranes and hydrazinidoboranes: State of the art, potential for hydrogen storage, and other prospects. Int. J. Hydrog. Energy 2020, 45, 30731–30755. [Google Scholar] [CrossRef]
- Petersen, H.; Weidenthaler, C. A review of recent developments for the in situ/operando characterization of nanoporous materials. Inorg. Chem. Front. 2022, 9, 4244–4271. [Google Scholar] [CrossRef]
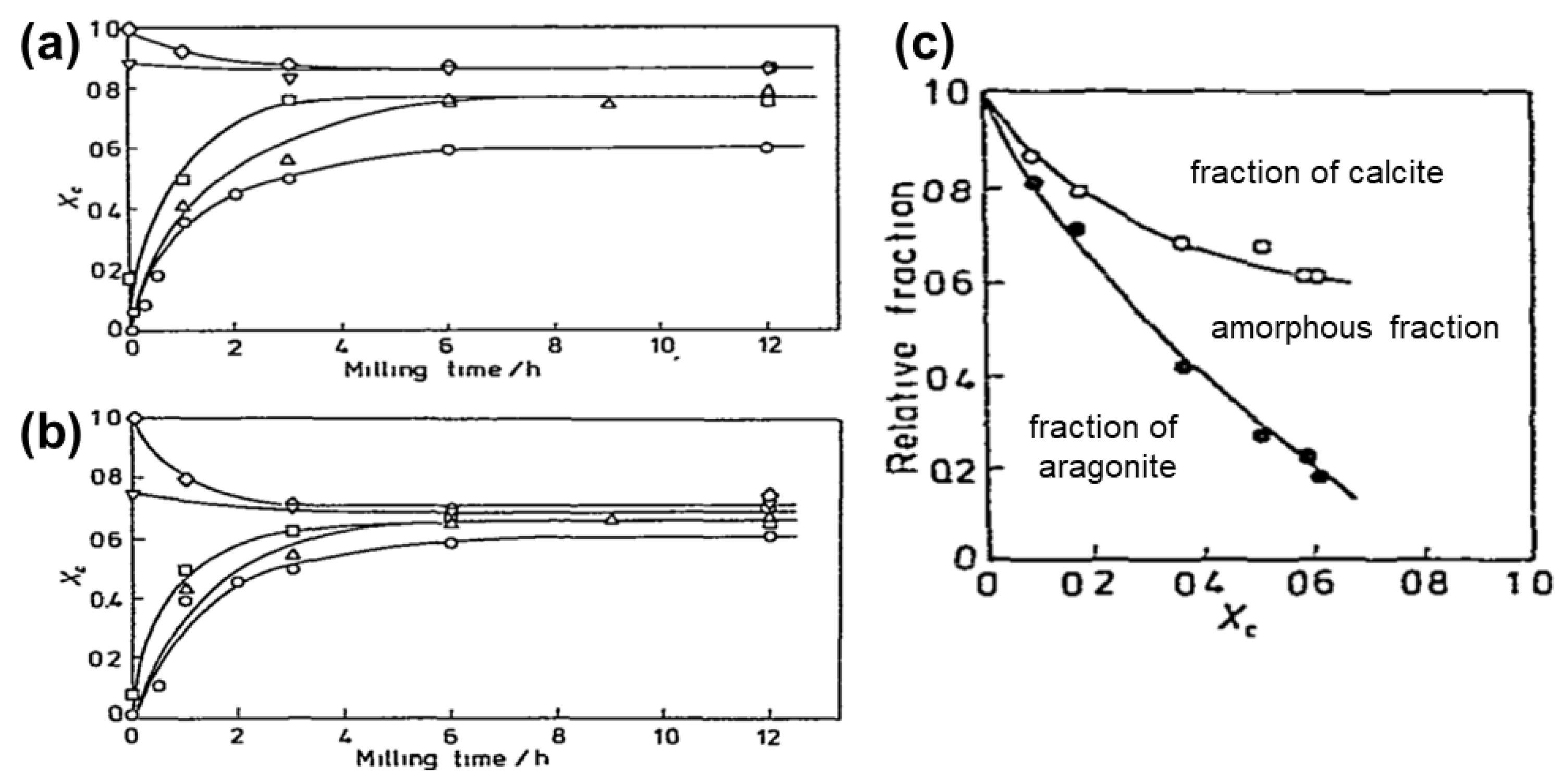
- Iguchi, Y.; Senna, M. Mechanochemical polymorphic transformation and its stationary state between aragonite and calcite I. Effects of preliminary annealing. Powder Technol. 1985, 43, 155–162. [Google Scholar] [CrossRef]
- Schrader, R.; Hoffmann, B. ber die Mechanische Aktivierung von Calciumcarbonat. Z. Anorg. Allg. Chem. 1969, 369, 41–47. [Google Scholar] [CrossRef]
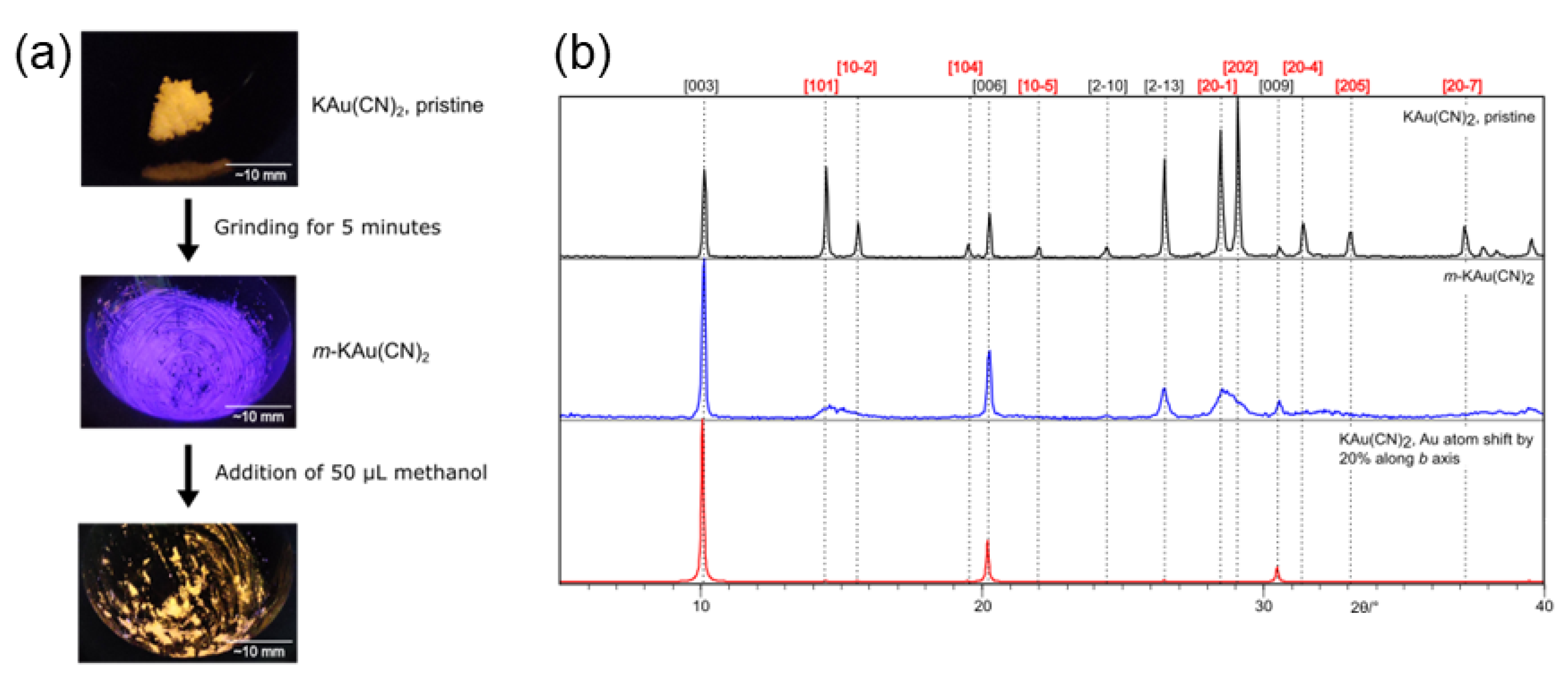
- Vainauskas, J.; Topic, F.; Arhangelskis, M.; Titi, H.M.; Friscic, T. Polymorphs and solid solutions: Materials with new luminescent properties obtained through mechanochemical transformation of dicyanoaurate(I) salts. Faraday Discuss. 2023, 241, 425–447. [Google Scholar] [CrossRef]
- Friscic, T.; Halasz, I.; Beldon, P.J.; Belenguer, A.M.; Adams, F.; Kimber, S.A.; Honkimaki, V.; Dinnebier, R.E. Real-time and in situ monitoring of mechanochemical milling reactions. Nat. Chem. 2013, 5, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Gracin, D.; Strukil, V.; Friscic, T.; Halasz, I.; Uzarevic, K. Laboratory real-time and in situ monitoring of mechanochemical milling reactions by Raman spectroscopy. Angew. Chem. Int. Ed. 2014, 53, 6193–6197. [Google Scholar] [CrossRef] [PubMed]
- Batzdorf, L.; Fischer, F.; Wilke, M.; Wenzel, K.J.; Emmerling, F. Direct in situ investigation of milling reactions using combined X-ray diffraction and Raman spectroscopy. Angew. Chem. Int. Ed. 2015, 54, 1799–1802. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, P.F.M.; Michalchuk, A.A.L.; Buzanich, A.G.; Bienert, R.; Torresi, R.M.; Camargo, P.H.C.; Emmerling, F. Tandem X-ray absorption spectroscopy and scattering for in situ time-resolved monitoring of gold nanoparticle mechanosynthesis. Chem. Commun. 2020, 56, 10329–10332. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, M.; Richard, A.J.; Valdez, J.; Fiss, B.G.; Titi, H.M.; Provatas, N.; Friscic, T.; Moores, A. Direct observation by high resolution transmission electron microscopy of gold(III) particle transformation during aging reduction reaction. Faraday Discuss. 2023, 241, 278–288. [Google Scholar] [CrossRef]
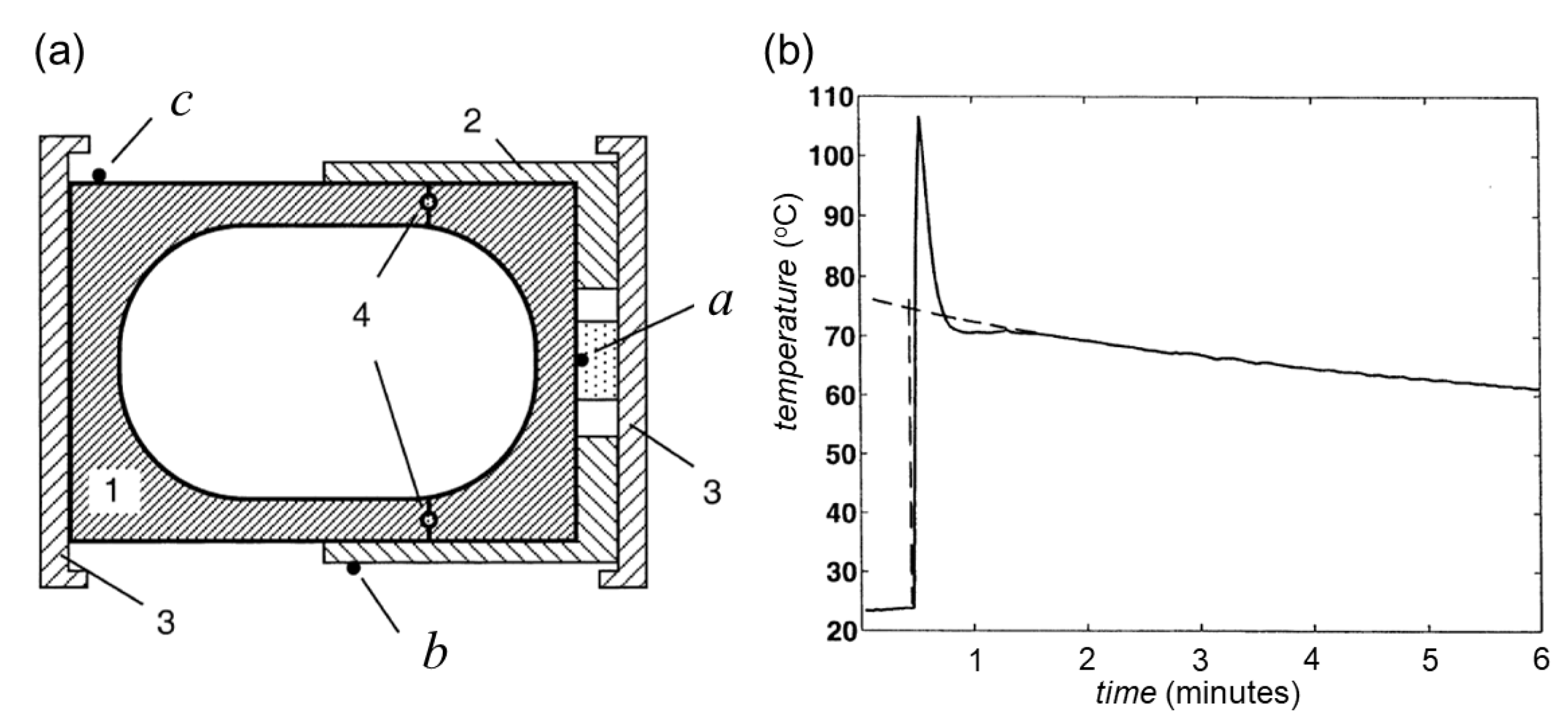
- Užarević, K.; Ferdelji, N.; Mrla, T.; Julien, P.A.; Halasz, B.; Friscic, T.; Halasz, I. Enthalpy vs. friction: Heat flow modelling of unexpected temperature profiles in mechanochemistry of metal-organic frameworks. Chem. Sci. 2018, 9, 2525–2532. [Google Scholar] [CrossRef]
- Mottillo, C.; Friscic, T. Carbon dioxide sensitivity of zeolitic imidazolate frameworks. Angew. Chem. Int. Ed. 2014, 53, 7471–7474. [Google Scholar] [CrossRef]
- Martinez, V.; Stolar, T.; Karadeniz, B.; Brekalo, I.; Užarević, K. Advancing mechanochemical synthesis by combining milling with different energy sources. Nat. Rev. Chem. 2023, 7, 51–65. [Google Scholar] [CrossRef]
- Sokolov, A.N.; Bucar, D.K.; Baltrusaitis, J.; Gu, S.X.; MacGillivray, L.R. Supramolecular catalysis in the organic solid state through dry grinding. Angew. Chem. Int. Ed. 2010, 49, 4273–4277. [Google Scholar] [CrossRef]
- Teoh, Y.; Ayoub, G.; Huskic, I.; Titi, H.M.; Nickels, C.W.; Herrmann, B.; Friščić, T. SpeedMixing: Rapid Tribochemical Synthesis and Discovery of Pharmaceutical Cocrystals without Milling or Grinding Media. Angew. Chem. Int. Ed. 2022, 61, e202206293. [Google Scholar] [CrossRef] [PubMed]
- Titi, H.M.; Do, J.L.; Howarth, A.J.; Nagapudi, K.; Friščić, T. Simple, scalable mechanosynthesis of metal-organic frameworks using liquid-assisted resonant acoustic mixing (LA-RAM). Chem. Sci. 2020, 11, 7578–7584. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Auvray, T.; Friščić, T. Shaking Things from the Ground-Up: A Systematic Overview of the Mechanochemistry of Hard and High-Melting Inorganic Materials. Molecules 2023, 28, 897. https://doi.org/10.3390/molecules28020897
Auvray T, Friščić T. Shaking Things from the Ground-Up: A Systematic Overview of the Mechanochemistry of Hard and High-Melting Inorganic Materials. Molecules. 2023; 28(2):897. https://doi.org/10.3390/molecules28020897
Chicago/Turabian StyleAuvray, Thomas, and Tomislav Friščić. 2023. "Shaking Things from the Ground-Up: A Systematic Overview of the Mechanochemistry of Hard and High-Melting Inorganic Materials" Molecules 28, no. 2: 897. https://doi.org/10.3390/molecules28020897
APA StyleAuvray, T., & Friščić, T. (2023). Shaking Things from the Ground-Up: A Systematic Overview of the Mechanochemistry of Hard and High-Melting Inorganic Materials. Molecules, 28(2), 897. https://doi.org/10.3390/molecules28020897









