Ca2+-Sensitive Potassium Channels
Abstract
1. Introduction
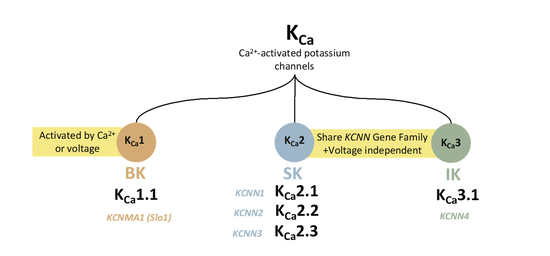
2. Ca2+-Activated KCa1.1 Channels
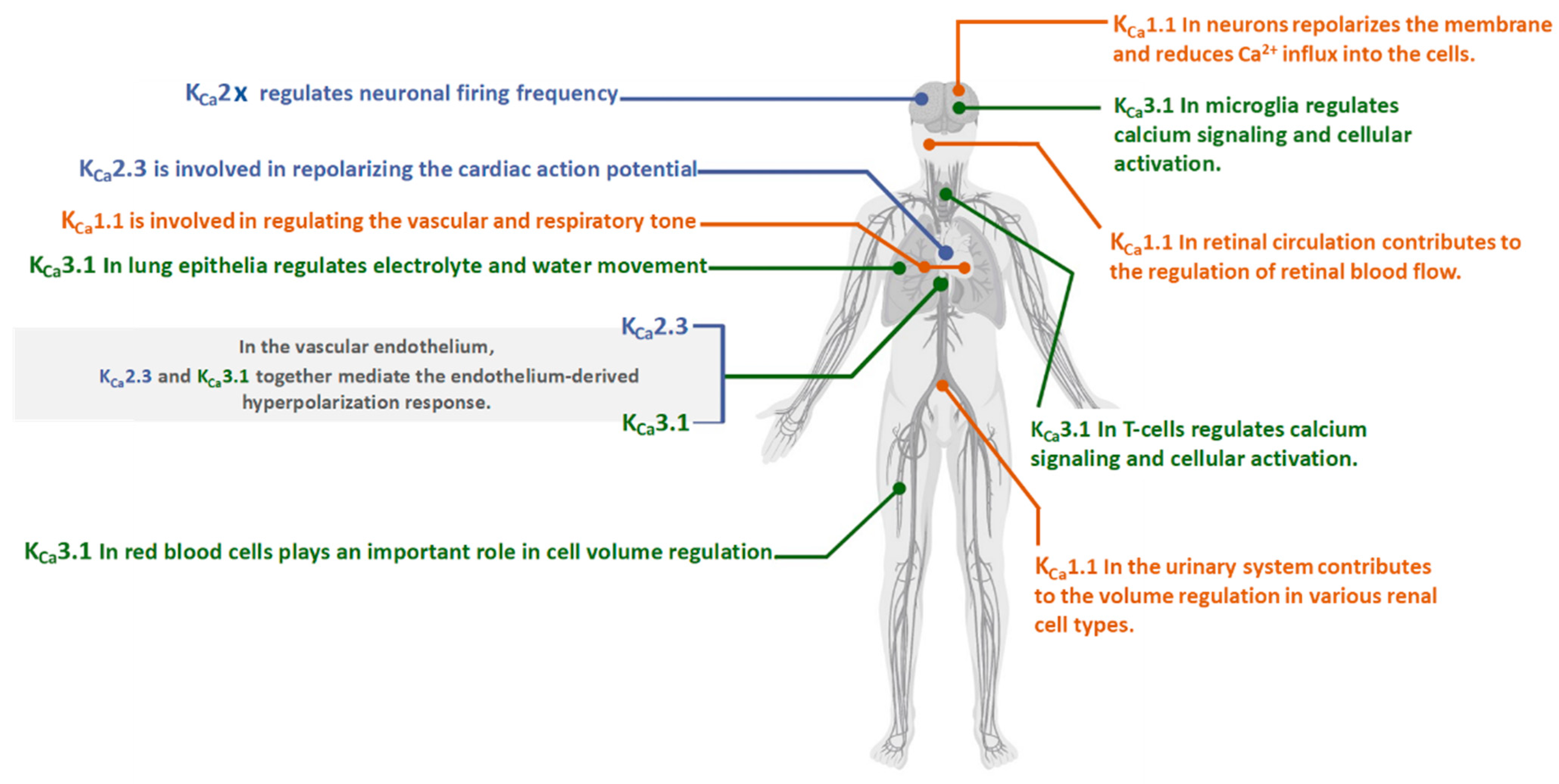
2.1. Expression and Physiology of KCa1.1 Channels
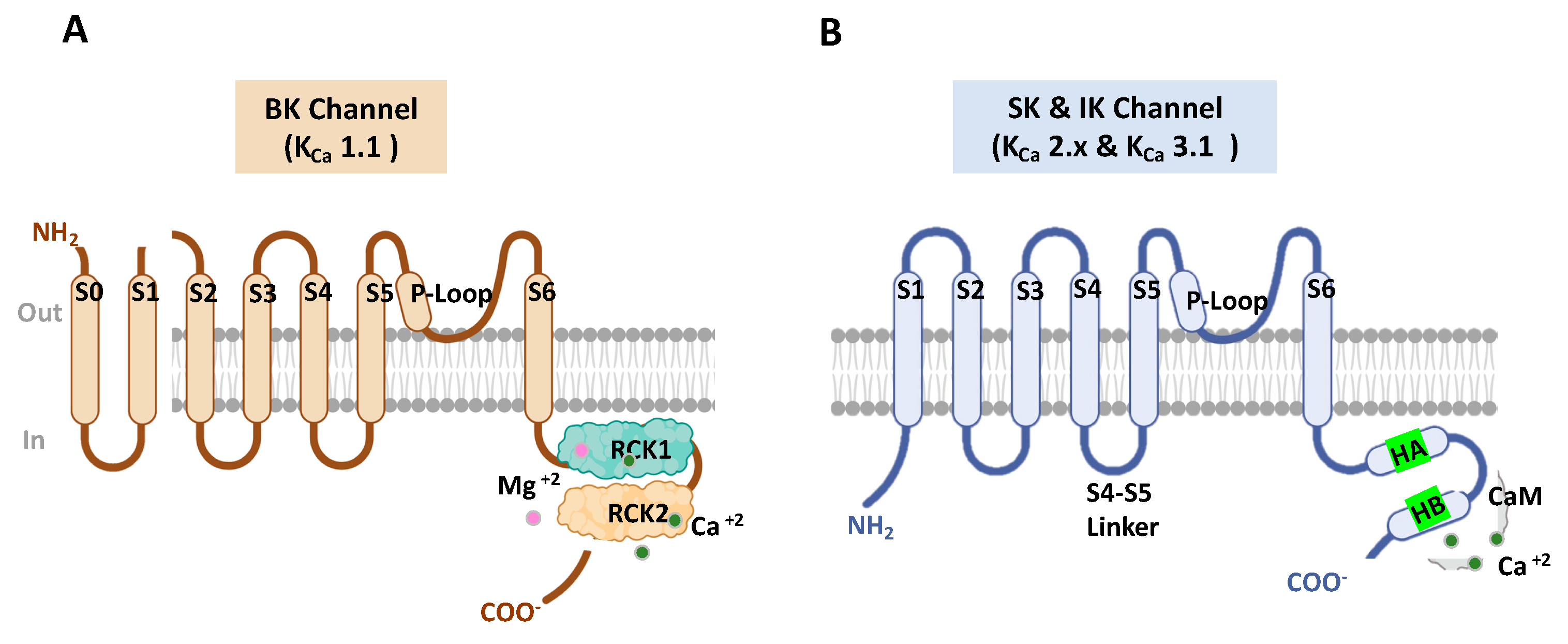
2.2. KCa1.1 Channels Structure
2.3. Channelopathies of KCa1.1 Channels
3. Ca2+-Activated KCa 2.x Channels
3.1. Expression and Physiology of KCa 2.x Channels
3.2. KCa 2.x Channels Structure
3.3. Channelopathies of KCa 2.x Channels
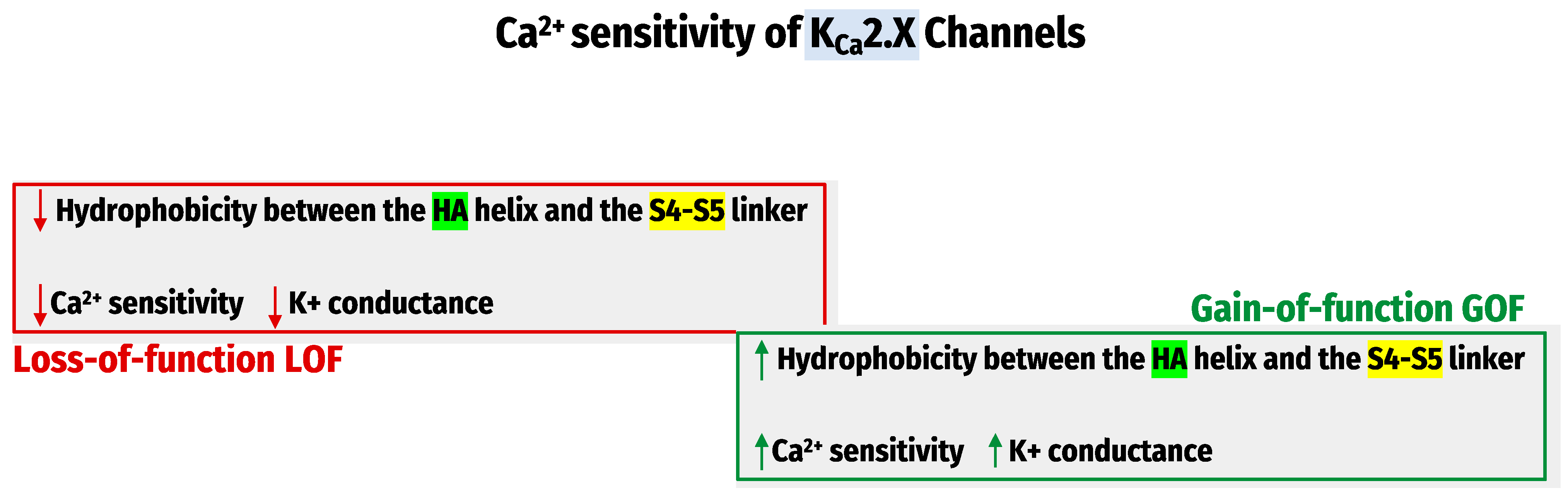
4. Ca2+- Sensitivity of KCa Channels
5. Summary
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nordin, B.E.C. Calcium in health and disease. Interrelat. Between Essent. Met. Ions Hum. Dis. 1997, 14, 81–137. [Google Scholar]
- Williams, R.J.P. Calcium: Outside/inside homeostasis and signalling. Biochim. Et Biophys. Acta BBA-Mol. Cell Res. 1998, 1448, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Clapham, D.E. Calcium signaling. Cell 2007, 131, 1047–1058. [Google Scholar] [PubMed]
- Carafoli, P.I.B.E.; Carafoli, E.; Klee, C.B. Calcium as a Cellular Regulator; Oxford University Press: Oxford, UK, 1999. [Google Scholar]
- Carafoli, E. Calcium–a universal carrier of biological signals. FEBS J. 2005, 272, 1073–1089. [Google Scholar] [CrossRef] [PubMed]
- Celio, M.R.; Pauls, T.L.; Schwaller, B. Guidebook to the Calcium-Binding Proteins; Sambrook & Tooze Publication at Oxford University Press: Oxford, UK; New York, NY, USA, 1996. [Google Scholar]
- Faber, E.S.L.; Sah, P. Calcium-Activated Potassium Channels: Multiple Contributions to Neuronal Function. Neuroscientist 2003, 9, 181–194. [Google Scholar] [CrossRef]
- Shah, K.R.; Guan, X.; Yan, J. Structural and Functional Coupling of Calcium-Activated BK Channels and Calcium-Permeable Channels Within Nanodomain Signaling Complexes. Frontiers in Physiology. 2022. Available online: https://www.frontiersin.org/articles/10.3389/fphys.2021.796540 (accessed on 16 August 2022).
- Ishii, T.M.; Silvia, C.; Hirschberg, B. A human intermediate conductance calcium-activated potassium channel. Proc. Natl. Acad. Sci. USA 1997, 94, 11651–11656. [Google Scholar] [CrossRef]
- Cox, D.H. Ca(2+)-regulated ion channels. BMB Rep. 2011, 44, 635–646. [Google Scholar] [CrossRef]
- Wulff, H.; Köhler, R. Endothelial Small- and Intermediate-Conductance KCa Channels: An Update on Their Pharmacology and Usefulness as Cardiovascular Targets. J. Cardiovasc. Pharmacol. 2013, 61, 102–112. [Google Scholar] [CrossRef]
- Nam, Y.-W.; Cui, M.; El-Sayed, N.S. Subtype-selective positive modulation of KCa2 channels depends on the HA/HB helices. Br. J. Pharmacol. 2022, 179, 460–472. [Google Scholar] [CrossRef]
- Xia, X.M.; Fakler, B.; Rivard, A. Mechanism of calcium gating in small-conductance calcium-activated potassium channels. Nature 1998, 395, 503–507. [Google Scholar] [CrossRef]
- Wei, A.D.; Gutman, G.A.; Aldrich, R. International Union of Pharmacology. LII. Nomenclature and molecular relationships of calcium-activated potassium channels. Pharmacol. Rev. 2005, 57, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Fanger, C.M.; Ghanshani, S.; Logsdon, N.J. Calmodulin mediates calcium-dependent activation of the intermediate conductance KCa channel, IKCa1. J. Biol. Chem. 1999, 274, 5746–5754. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.-N. Large-conductance Ca2+- activated K+ channels:physiological role and pharmacology. Curr. Med. Chem. 2003, 10, 649–661. [Google Scholar] [CrossRef] [PubMed]
- Latorre, R.; Brauchi, S. Large conductance Ca2+-activated K+ (BK) channel: Activation by Ca2+ and voltage. Biol. Res. 2006, 39, 385–401. [Google Scholar] [CrossRef] [PubMed]
- Vetri, F.; Choudhury, M.S.R.; Pelligrino, D.A. BKCa channels as physiological regulators: A focused review. JRLCR 2014, 7, 3–13. [Google Scholar] [CrossRef]
- Gu, N.; Vervaeke, K.; Storm, J.F. BK potassium channels facilitate high-frequency firing and cause early spike frequency adaptation in rat CA1 hippocampal pyramidal cells. J. Physiol. 2007, 580, 859–882. [Google Scholar] [CrossRef] [PubMed]
- N’Gouemo, P. BKCa channel dysfunction in neurological diseases. Front. Physiol. 2014, 5, 373. [Google Scholar] [PubMed]
- Contet, C.; Goulding, S.P.; Kuljis, D.A. BK Channels in the Central Nervous System. Int. Rev. Neurobiol. 2016, 128, 281–342. [Google Scholar]
- Bond, C.T.; Herson, P.S.; Strassmaier, T. Small conductance Ca2+-activated K+ channel knock-out mice reveal the identity of calcium-dependent afterhyperpolarization currents. J. Neurosci. 2004, 24, 5301–5306. [Google Scholar] [CrossRef]
- Vetri, F.; Qi, M.; Xu, H. Impairment of neurovascular coupling in Type 1 Diabetes Mellitus in rats is prevented by pancreatic islet transplantation and reversed by a semi-selective PKC inhibitor. Brain Res. 2017, 1655, 48–54. [Google Scholar] [CrossRef]
- Nelson, M.T.; Cheng, H.; Rubart, M. Relaxation of Arterial Smooth Muscle by Calcium Sparks. Science 1995, 270, 633–637. [Google Scholar] [CrossRef] [PubMed]
- McGahon, M.K.; Dash, D.P.; Arora, A. Diabetes downregulates large-conductance Ca2+-activated potassium beta 1 channel subunit in retinal arteriolar smooth muscle. Circ. Res. 2007, 100, 703–711. [Google Scholar] [CrossRef] [PubMed]
- Herrera, G.M.; Heppner, T.J.; Nelson, M.T. Regulation of urinary bladder smooth muscle contractions by ryanodine receptors and BK and SK channels. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000, 279, R60–R68. [Google Scholar] [CrossRef] [PubMed]
- Stockand, J.D.; Sansom, S.C. Large Ca2+-activated K+ channels responsive to angiotensin II in cultured human mesangial cells. Am. J. Physiol. Ren. Physiol. 1994, 267, C1080–C1086. [Google Scholar] [CrossRef]
- Fallet, R.W.; Bast, J.P.; Fujiwara, K. Influence of Ca2+-activated K+ channels on rat renal arteriolar responses to depolarizing agonists. Am. J. Physiol. Renal. Physiol. 2001, 280, F583–F591. [Google Scholar] [CrossRef]
- Kim, E.Y.; Ridgway, L.D.; Zou, S. Alternatively spliced C-terminal domains regulate the surface expression of large conductance calcium-activated potassium (BKCa) channels. Neuroscience 2007, 146, 1652–1661. [Google Scholar] [CrossRef]
- Tseng-Crank, J.; Foster, C.D.; Krause, J.D. Cloning, expression, and distribution of functionally distinct Ca2+-activated K+ channel isoforms from human brain. Neuron 1994, 13, 1315–1330. [Google Scholar] [CrossRef]
- Chen, L.; Jeffries, O.; Rowe, I.C.M. Membrane Trafficking of Large Conductance Calcium-activated Potassium Channels Is Regulated by Alternative Splicing of a Transplantable, Acidic Trafficking Motif in the RCK1-RCK2 Linker *. J. Biol. Chem. 2010, 285, 23265–23275. [Google Scholar] [CrossRef]
- Dudem, S.; Large, R.J.; Kulkarni, S. LINGO1 is a regulatory subunit of large conductance, Ca2+-activated potassium channels. Proc. Natl. Acad. Sci. USA 2020, 117, 2194–2200. [Google Scholar] [CrossRef]
- Filosa, J.A.; Bonev, A.D.; Straub, S.V. Local potassium signaling couples neuronal activity to vasodilation in the brain. Nat. Neurosci. 2006, 9, 1397–1403. [Google Scholar] [CrossRef]
- Zarei, M.M.; Song, M.; Wilson, R.J. Endocytic trafficking signals in KCNMB2 regulate surface expression of a voltage and Ca2+-Activated K+ channel. Neuroscience 2007, 147, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Brenner, R.; Peréz, G.J.; Bonev, A.D. Vasoregulation by the β1 subunit of the calcium-activated potassium channel. Nature 2000, 407, 870–876. [Google Scholar] [CrossRef]
- McManus, O.B.; Helms, L.M.; Pallanck, L. Functional role of the beta subunit of high conductance calcium-activated potassium channels. Neuron 1995, 14, 645–650. [Google Scholar] [CrossRef] [PubMed]
- Tao, X.; Hite, R.K.; MacKinnon, R. Cryo-EM structure of the open high conductance Ca2+-activated K+ channel. Nature 2017, 541, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Hite, R.K.; Tao, X.; MacKinnon, R. Structural basis for gating the high-conductance Ca2+-activated K+ channel. Nature 2017, 541, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Bailey, C.S.; Moldenhauer, H.J.; Park, S.M. KCNMA1-linked channelopathy. J. Gen. Physiol. 2019, 151, 1173–1189. [Google Scholar] [CrossRef]
- Meredith, A.L. Genetic Methods for Studying Ion Channel Function in Physiology and Disease. In Handbook of Ion Channels; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Kim, J.-B. Channelopathies. Korean J. Pediatr. 2014, 57, 1–18. [Google Scholar] [CrossRef]
- Liu, Y.; Gutterman, D.D. The coronary circulation in diabetes: Influence of reactive oxygen species on K+ channel-mediated vasodilation. Vascul. Pharmacol. 2002, 38, 43–49. [Google Scholar] [CrossRef]
- Wiecha, J.; Schläger, B.; Voisard, R. Ca(2+)-activated K+ channels in human smooth muscle cells of coronary atherosclerotic plaques and coronary media segments. Basic Res. Cardiol. 1997, 92, 233–239. [Google Scholar] [CrossRef]
- Yang, Y.; Li, P.-Y.; Cheng, J. Function of BKCa channels is reduced in human vascular smooth muscle cells from Han Chinese patients with hypertension. Hypertension 2013, 61, 519–525. [Google Scholar] [CrossRef]
- Kim, N.; Chung, J.; Kim, E. Changes in the Ca2+-activated K+ channels of the coronary artery during left ventricular hypertrophy. Circ. Res. 2003, 93, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Meredith, A.L.; Thorneloe, K.S.; Werner, M.E. Overactive Bladder and Incontinence in the Absence of the BK Large Conductance Ca2+-activated K+ Channel *. J. Biol. Chem. 2004, 279, 36746–36752. [Google Scholar] [CrossRef] [PubMed]
- Rüttiger, L.; Sausbier, M.; Zimmermann, U. Deletion of the Ca2+-activated potassium (BK) α-subunit but not the BKβ1-subunit leads to progressive hearing loss. Proc. Natl. Acad. Sci. USA 2004, 101, 12922–12927. [Google Scholar] [CrossRef] [PubMed]
- Werner, M.E.; Zvara, P.; Meredith, A.L. Erectile dysfunction in mice lacking the large-conductance calcium-activated potassium (BK) channel. J. Physiol. 2005, 567, 545–556. [Google Scholar] [CrossRef] [PubMed]
- Tighilet, B.; Bourdet, A.; Péricat, D. SK Channels Modulation Accelerates Equilibrium Recovery in Unilateral Vestibular Neurectomized Rats. Pharmaceuticals 2021, 14, 1226. [Google Scholar] [CrossRef]
- Subtype-Selective Positive Modulation of KCa2.3 Channels Increases Cilia Length | ACS Chemical Biology. Available online: https://pubs.acs.org/doi/10.1021/acschembio.2c004692344–2354 (accessed on 12 January 2023).
- Wang, J.; Morishima, S.; Okada, Y. IK channels are involved in the regulatory volume decrease in human epithelial cells. Am. J. Physiol. Cell Physiol. 2003, 284, C77–C84. [Google Scholar] [CrossRef] [PubMed]
- Womack, M.D.; Khodakhah, K. Somatic and Dendritic Small-Conductance Calcium-Activated Potassium Channels Regulate the Output of Cerebellar Purkinje Neurons. J. Neurosci. 2003, 23, 2600–2607. [Google Scholar] [CrossRef]
- Cui, M.; Qin, G.; Yu, K. Targeting the Small- and Intermediate-Conductance Ca2+-Activated Potassium Channels: The Drug-Binding Pocket at the Channel/Calmodulin Interface. NSG 2014, 22, 65–78. [Google Scholar]
- Dolga, A.M.; de Andrade, A.; Meissner, L. Subcellular expression and neuroprotective effects of SK channels in human dopaminergic neurons. Cell Death Dis. 2014, 5, e999. [Google Scholar] [CrossRef]
- Sankaranarayanan, A.; Raman, G.; Busch, C. Naphtho [1,2-d]thiazol-2-ylamine (SKA-31), a new activator of KCa2 and KCa3.1 potassium channels, potentiates the endothelium-derived hyperpolarizing factor response and lowers blood pressure. Mol. Pharmacol. 2009, 75, 281–295. [Google Scholar] [CrossRef]
- McNeish, A.J.; Dora, K.A.; Garland, C.J. Possible role for K+ in endothelium-derived hyperpolarizing factor-linked dilatation in rat middle cerebral artery. Stroke 2005, 36, 1526–1532. [Google Scholar] [CrossRef]
- Pharmacology of Small- and Intermediate-Conductance Calcium-Activated Potassium Channels. Available online: https://www.annualreviews.org/doi/epdf/10.1146/annurev-pharmtox-010919-023420 (accessed on 24 July 2022).
- Wulff, H.; Zhorov, B.S. K + Channel Modulators for the Treatment of Neurological Disorders and Autoimmune Diseases. Chem. Rev. 2008, 108, 1744–1773. [Google Scholar] [CrossRef] [PubMed]
- Crotti, L.; Odening, K.E.; Sanguinetti, M.C. Heritable arrhythmias associated with abnormal function of cardiac potassium channels. Cardiovasc. Res. 2020, 116, 1542–1556. [Google Scholar] [CrossRef] [PubMed]
- Nam, Y.-W.; Downey, M.; Rahman, M.A. Channelopathy of small- and intermediate-conductance Ca2+-activated K+ channels. Acta Pharmacol. Sin. 2022, 17, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Gripp, K.W.; Smithson, S.F.; Scurr, I.J. Syndromic disorders caused by gain-of-function variants in KCNH1, KCNK4, and KCNN3—A subgroup of K+ channelopathies. Eur. J. Hum. Genet. 2021, 29, 1384–1395. [Google Scholar] [CrossRef]
- Orfali, R.; Nam, Y.-W.; Nguyen, H.M. Channelopathy-causing mutations in the S45A/S45B and HA/HB helices of KCa2.3 and KCa3.1 channels alter their apparent Ca2+ sensitivity. Cell Calcium. 2022, 102, 102538. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, M.; Ryba, L.; Křepelová, A. Zimmermann-Laband syndrome in monozygotic twins with a mild neurobehavioral phenotype lacking gingival overgrowth-A case report of a novel KCNN3 gene variant. Am. J. Med. Genet. A. 2022, 188, 1083–1087. [Google Scholar] [CrossRef]
- Bauer, C.K.; Schneeberger, P.E.; Kortüm, F. Gain-of-Function Mutations in KCNN3 Encoding the Small-Conductance Ca2+-Activated K+ Channel SK3 Cause Zimmermann-Laband Syndrome. Am. J. Hum. Genet. 2019, 104, 1139–1157. [Google Scholar] [CrossRef]
- Koot, B.G.P.; Alders, M.; Verheij, J. A de novo mutation in KCNN3 associated with autosomal dominant idiopathic non-cirrhotic portal hypertension. J. Hepatol. 2016, 64, 974–977. [Google Scholar] [CrossRef]
- A CAG repeat polymorphism of KCNN3 predicts SK3 channel function and cognitive performance in schizophrenia. EMBO Mol. Med. 2011, 3, 309–319. [CrossRef]
- Miller, M.J.; Rauer, H.; Tomita, H. Nuclear Localization and Dominant-negative Suppression by a Mutant SKCa3 N-terminal Channel Fragment Identified in a Patient with Schizophrenia*. J. Biol. Chem. 2001, 276, 27753–27756. [Google Scholar] [CrossRef]
- Glogowska, E.; Lezon-Geyda, K.; Maksimova, Y. Mutations in the Gardos channel (KCNN4) are associated with hereditary xerocytosis. Blood 2015, 126, 1281–1284. [Google Scholar] [CrossRef] [PubMed]
- Fermo, E.; Monedero-Alonso, D.; Petkova-Kirova, P. Gardos channelopathy: Functional analysis of a novel KCNN4 variant. Blood Adv. 2020, 4, 6336–6341. [Google Scholar] [CrossRef] [PubMed]
- Small-Conductance, Calcium-Activated Potassium Channels from Mammalian Brain | Science. Available online: https://www.science.org/doi/10.1126/science.273.5282.1709?keytype2=tf_ipsecsha&ijkey=85150f659083d52f2496eacd09e26e41880530f4 (accessed on 2 September 2022).
- Yan, J.; Aldrich, R.W. LRRC26 auxiliary protein allows BK channel activation at resting voltage without calcium. Nature 2010, 466, 513–516. [Google Scholar] [CrossRef] [PubMed]
- Sancho, M.; Kyle, B.D. The Large-Conductance, Calcium-Activated Potassium Channel: A Big Key Regulator of Cell Physiology. Frontiers in Physiology. 2021. Available online: https://www.frontiersin.org/articles/10.3389/fphys.2021.750615 (accessed on 27 August 2022).
- Li, Q.; Li, Y.; Wei, H. Molecular determinants of Ca2+ sensitivity at the intersubunit interface of the BK channel gating ring. Sci. Rep. 2018, 8, 509. [Google Scholar] [CrossRef]
- Wang, L.; Sigworth, F.J. Structure of the BK potassium channel in a lipid membrane from electron cryomicroscopy. Nature 2009, 461, 292–295. [Google Scholar] [CrossRef]
- Nam, Y.-W.; Cui, M.; Orfali, R. Hydrophobic interactions between the HA helix and S4-S5 linker modulate apparent Ca2+ sensitivity of SK2 channels. Acta Physiol. 2021, 231, e13552. [Google Scholar] [CrossRef]
- Kuramoto, T.; Yokoe, M.; Kunisawa, N. Tremor dominant Kyoto (Trdk) rats carry a missense mutation in the gene encoding the SK2 subunit of small-conductance Ca2+-activated K+ channel. Brain Res. 2017, 1676, 38–45. [Google Scholar] [CrossRef]
- Mochel, F.; Rastetter, A.; Ceulemans, B. Variants in the SK2 channel gene (KCNN2) lead to dominant neurodevelopmental movement disorders. Brain 2020, 143, 3564–3573. [Google Scholar] [CrossRef]
- Castori, M.; Valiante, M.; Pascolini, G.; Leuzzi, V.; Pizzuti, A.; Grammatico, P. Clinical and genetic study of two patients with Zimmermann-Laband syndrome and literature review. Eur. J. Med. Genet. 2013, 56, 570–576. [Google Scholar] [CrossRef]
- Mutations in KCNH1 and ATP6V1B2 cause Zimmermann-Laband syndrome. Nature Genetics. 2015, 47, 661–667. [CrossRef] [PubMed]
- Kmeid, M.; Liu, H.; Ballentine, S.; Lee, H. Idiopathic Non-Cirrhotic Portal Hypertension and Porto-Sinusoidal Vascular Disease: Review of Current Data. Gastroenterol Res. 2021, 14, 49–65. [Google Scholar] [CrossRef] [PubMed]
- Hoffmanz, J.F.; Joiner, W.; Nehrke, K. The hSK4 (KCNN4) isoform is the Ca2+-activated K+ channel (Gardos channel) in human red blood cells. Proc. Natl. Acad. Sci. USA 2003, 100, 7366–7371. [Google Scholar] [CrossRef] [PubMed]
| KCa1.1 | KCa2.x | KCa3.1 | |
|---|---|---|---|
| Expression | Neurons [7,18,19] vascular, respiratory, endocrine, retinal circulation [26], glomerular mesangial cells [27], and podocytes [29]. | Neurons, heart, and vascular endothelium [57]. | Microglia, lung epithelia, GI epithelia, T cells, and red blood cells [57]. |
| Mechanism | Activated by both Ca2+ and Voltage [18]. | Activated by low intracellular Ca2+ concentrations (Voltage-independent). | |
| Ca2+ binding sites | Two intracellular Ca2+-sensing RCK domains (RCK1 and RCK2) [16]. | Ca2+- binding (CaM) [10,70]. | |
| Ca2+ sensitivity Regulation | The inter-subunit assembly interface contains molecular determinants of Ca2+- sensitivity in KCa1.1 channels [73]. | Hydrophobic interactions between the HA helix and S4-S5 linker regulate the KCa2.x/KCa3.1 channel’s apparent Ca2+ sensitivity [75]. | |
| Channelopathies | Neurological disorders, diabetes [42], atherosclerosis [43], hypertension [44], cardiac hypertrophy [45], paroxysmal nonkinesigenic dyskinesia (PNKD), ataxia, hearing loss [47], urinary incontinence and overactive bladder [46]. | Cerebellar ataxia and tremor are associated with LOF KCa2.2 mutations [59]. The GOF mutations of KCa2.3 are linked with (ZLS) [60,62,63,64] and (INCPH) [60,62,65], and schizophrenia [66]. | The GOF mutations of KCa3.1 are linked with a subset of (HX) [68]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orfali, R.; Albanyan, N. Ca2+-Sensitive Potassium Channels. Molecules 2023, 28, 885. https://doi.org/10.3390/molecules28020885
Orfali R, Albanyan N. Ca2+-Sensitive Potassium Channels. Molecules. 2023; 28(2):885. https://doi.org/10.3390/molecules28020885
Chicago/Turabian StyleOrfali, Razan, and Nora Albanyan. 2023. "Ca2+-Sensitive Potassium Channels" Molecules 28, no. 2: 885. https://doi.org/10.3390/molecules28020885
APA StyleOrfali, R., & Albanyan, N. (2023). Ca2+-Sensitive Potassium Channels. Molecules, 28(2), 885. https://doi.org/10.3390/molecules28020885