Signal Suppression in LC-ESI-MS/MS from Concomitant Medications and Its Impact on Quantitative Studies: An Example Using Metformin and Glyburide
Abstract
1. Introduction
2. Results and Discussion
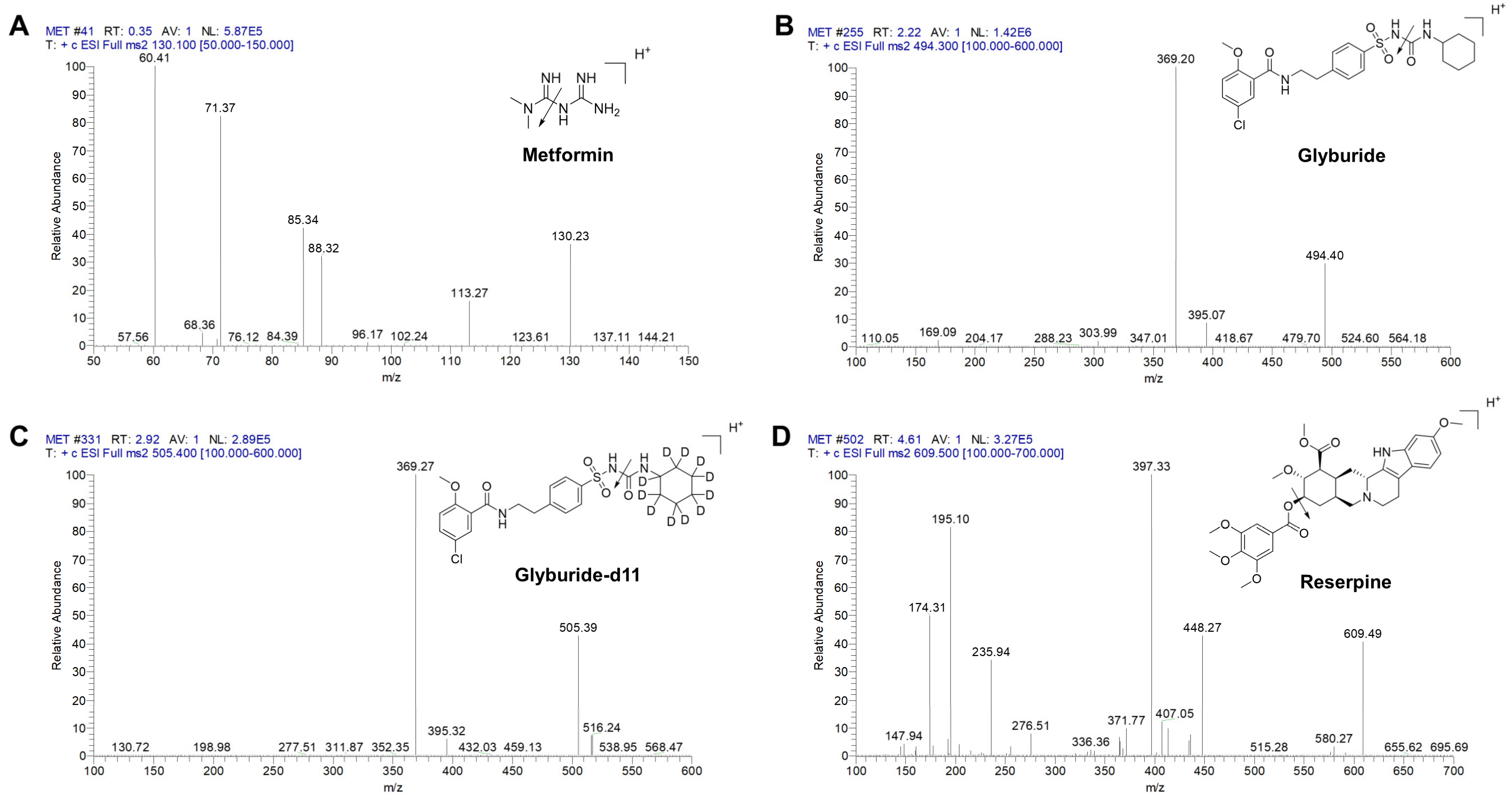
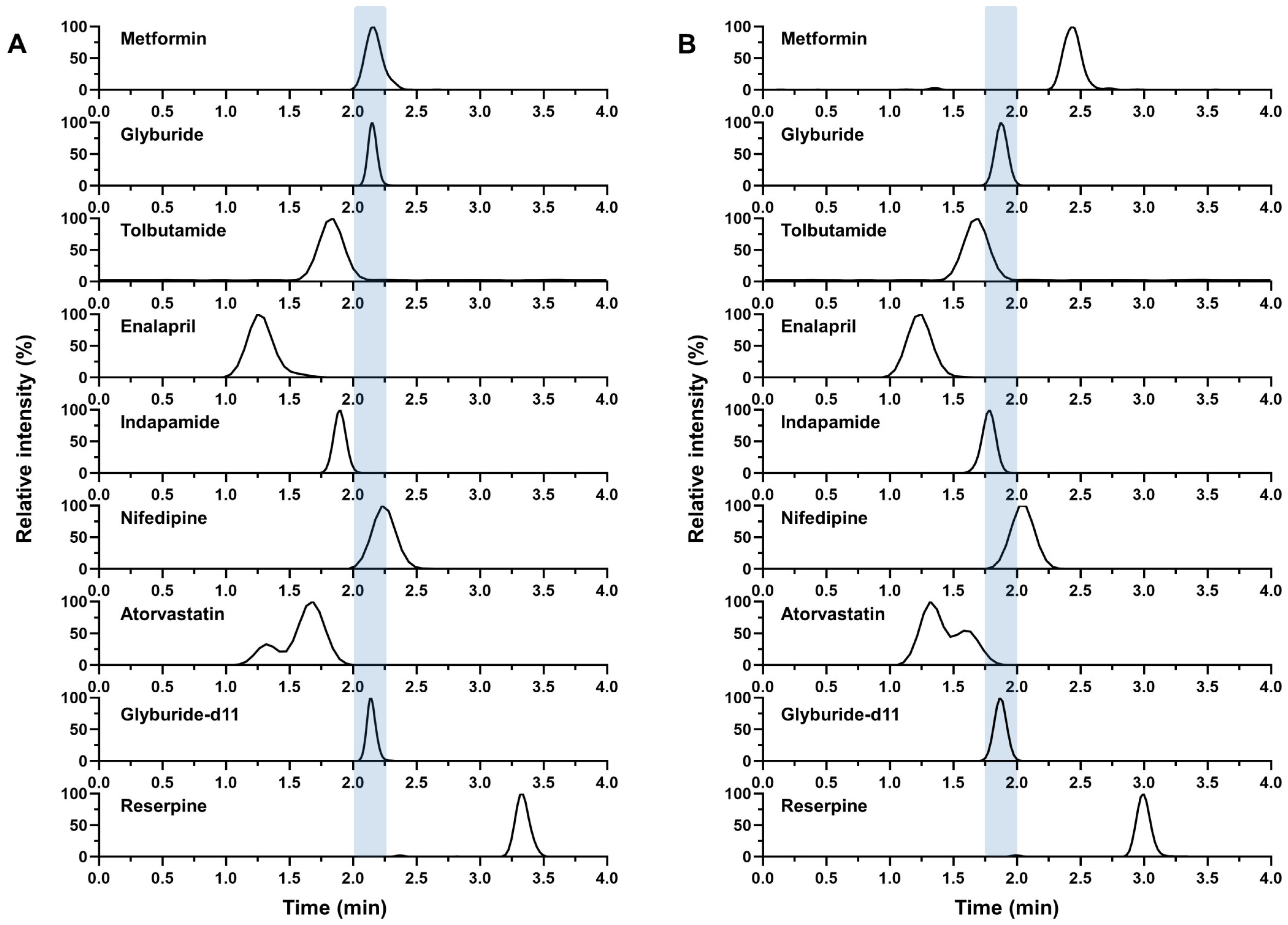
2.1. The Co-Eluting Chromatographic Method for Metformin and Glyburide
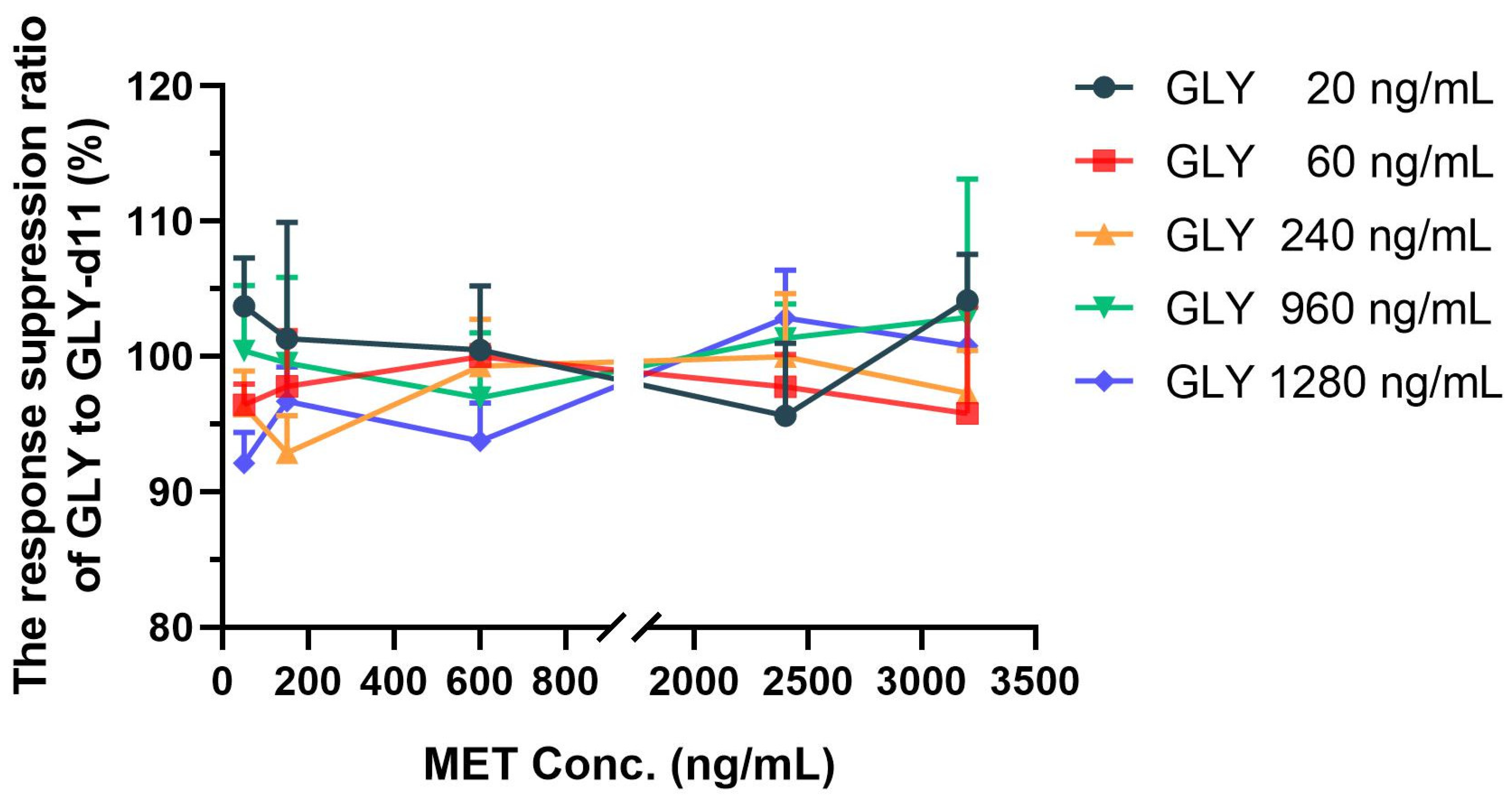
2.2. Signal Suppression between Metformin and Glyburide
2.3. Strategies to Solve Signal Suppression of Glyburide by Co-Eluting Metformin
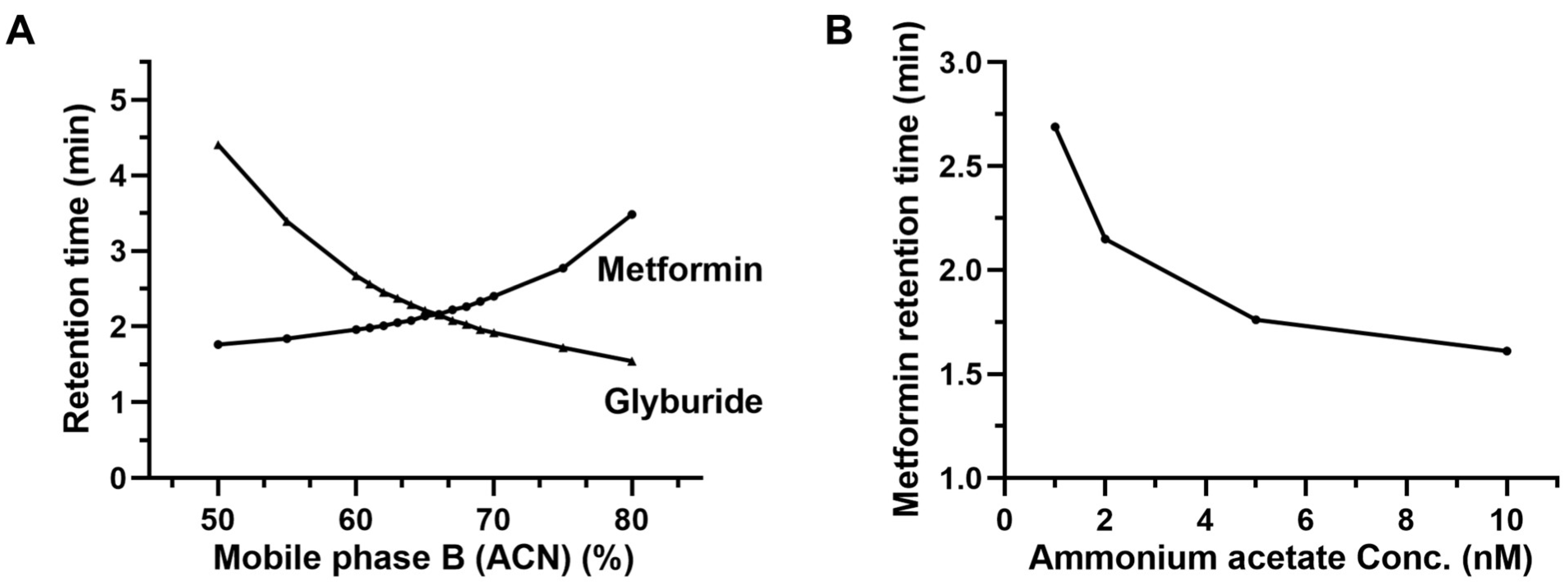
2.3.1. Chromatographic Separation
2.3.2. Sample Dilution
2.3.3. Correction of Stable-Isotope-Labeled Internal Standard
2.4. Method Validation of GLY
2.4.1. Linearity and LLOQs
2.4.2. Accuracy and Precision
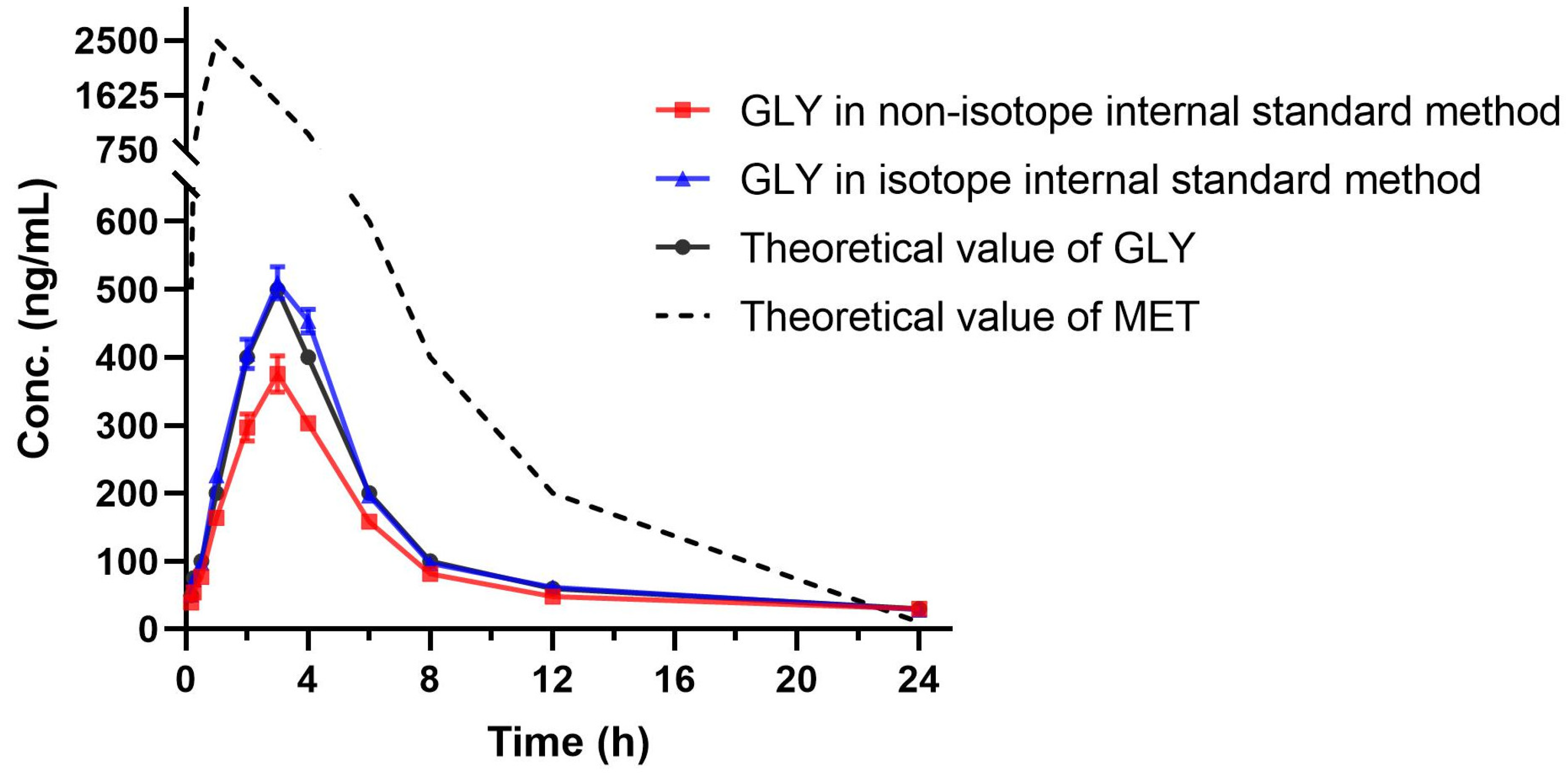
2.5. Pharmacokinetic Study of Simulated Sample
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Chromatographic and Mass Spectrometric Conditions
3.3. Stock Solutions, Calibration Standards and QC Samples Preparation
3.4. Sample Preparation
3.4.1. Signal Suppression Experiments at Five Concentration Levels
3.4.2. Analysis of Plasma Samples
3.4.3. Analysis of Simulated Pharmacokinetic Samples
3.5. Strategies to Solve Signal Suppression of Glyburide by Co-Eluting Metformin
3.5.1. Chromatographic Separation
3.5.2. Sample Dilution
3.5.3. Correction of Stable-Isotope-Labeled Internal Standard
3.6. Method Validation
3.7. Statistical Analysis and Software
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Wahab, M.F.; O’Haver, T.C.; Gritti, F.; Hellinghausen, G.; Armstrong, D.W. Increasing chromatographic resolution of analytical signals using derivative enhancement approach. Talanta 2019, 192, 492–499. [Google Scholar] [CrossRef]
- An, G.; Bach, T.; Abdallah, I.; Nalbant, D. Aspects of matrix and analyte effects in clinical pharmacokinetic sample analyses using LC-ESI/MS/MS—Two case examples. J. Pharm. Biomed. Anal. 2020, 183, 113135. [Google Scholar] [CrossRef] [PubMed]
- Jian, W.; Edom, R.W.; Xu, Y.; Gallagher, J.; Weng, N. Potential bias and mitigations when using stable isotope labeled parent drug as internal standard for LC-MS/MS quantitation of metabolites. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2010, 878, 3267–3276. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.R.; Foltz, R.L.; Meng, M.; Bennett, P. Ionization enhancement in atmospheric pressure chemical ionization and suppression in electrospray ionization between target drugs and stable-isotope-labeled internal standards in quantitative liquid chromatography/tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2003, 17, 2815–2821. [Google Scholar] [CrossRef] [PubMed]
- Remane, D.; Wissenbach, D.K.; Meyer, M.R.; Maurer, H.H. Systematic investigation of ion suppression and enhancement effects of fourteen stable-isotope-labeled internal standards by their native analogues using atmospheric-pressure chemical ionization and electrospray ionization and the relevance for multi-analyte liquid chromatographic/mass spectrometric procedures. Rapid Commun. Mass Spectrom. 2010, 24, 859–867. [Google Scholar] [PubMed]
- Konermann, L.; Ahadi, E.; Rodriguez, A.D.; Vahidi, S. Unraveling the mechanism of electrospray ionization. Anal. Chem. 2013, 85, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Cortese, M.; Gigliobianco, M.R.; Magnoni, F.; Censi, R.; Di Martino, P.D. Compensate for or minimize matrix effects? Strategies for overcoming matrix effects in liquid chromatography-mass spectrometry technique: A tutorial review. Molecules 2020, 25, 3047. [Google Scholar] [CrossRef]
- Nasiri, A.; Jahani, R.; Mokhtari, S.; Yazdanpanah, H.; Daraei, B.; Faizi, M.; Kobarfard, F. Overview, consequences, and strategies for overcoming matrix effects in LC-MS analysis: A critical review. Analyst 2021, 146, 6049–6063. [Google Scholar] [CrossRef]
- Kebarle, P. A brief overview of the present status of the mechanisms involved in electrospray mass spectrometry. J. Mass Spectrom. 2000, 35, 804–817. [Google Scholar] [CrossRef] [PubMed]
- Antignac, J.-P.; de Wasch, K.; Monteau, F.; De Brabander, H.; Andre, F.; Le Bizec, B. The ion suppression phenomenon in liquid chromatography–mass spectrometry and its consequences in the field of residue analysis. Anal. Chim. Acta 2005, 529, 129–136. [Google Scholar] [CrossRef]
- Furey, A.; Moriarty, M.; Bane, V.; Kinsella, B.; Lehane, M. Ion suppression; A critical review on causes, evaluation, prevention and applications. Talanta 2013, 115, 104–122. [Google Scholar] [CrossRef]
- Wang, S.; Cyronak, M.; Yang, E. Does a stable isotopically labeled internal standard always correct analyte response? A matrix effect study on a LC/MS/MS method for the determination of carvedilol enantiomers in human plasma. J. Pharm. Biomed. Anal. 2007, 43, 701–707. [Google Scholar] [CrossRef]
- Oldekop, M.L.; Herodes, K.; Rebane, R. Study of the matrix effects and sample dilution influence on the LC-ESI-MS/MS analysis using four derivatization reagents. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2014, 967, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Stahnke, H.; Kittlaus, S.; Kempe, G.; Alder, L. Reduction of matrix effects in liquid chromatography–electrospray ionization–mass spectrometry by dilution of the sample extracts: How much dilution is needed? Anal. Chem. 2012, 84, 1474–1482. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration. Bioanalytical Method Validation Guidance for Industry; Food and Drug Administration: Silver Spring, MD, USA, 2018.
- Raposo, F.; Barceló, D. Challenges and strategies of matrix effects using chromatography-mass spectrometry: An overview from research versus regulatory viewpoints. TrAC Trends Anal. Chem. 2021, 134, 116068. [Google Scholar] [CrossRef]
- de Zwart, M.; Lausecker, B.; Globig, S.; Neddermann, D.; Le Bras, B.; Guenzi, A.; White, S.; Scheel-Fjording, M.; Timmerman, P. Co-medication and interference testing in bioanalysis: A European Bioanalysis Forum recommendation. Bioanalysis 2016, 8, 2065–2070. [Google Scholar] [CrossRef] [PubMed]
- Annesley, T.M. Ion suppression in mass spectrometry. Clin. Chem. 2003, 49, 1041–1044. [Google Scholar] [CrossRef]
- Lau, H.S.; Florax, C.; Porsius, A.J.; De Boer, A. The completeness of medication histories in hospital medical records of patients admitted to general internal medicine wards. Br. J. Clin. Pharmacol. 2000, 49, 597–603. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. FDA-Approved Drugs: Abbreviated New Drug Application (ANDA) 076345. Available online: https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=076345 (accessed on 4 January 2023).
- Mistri, H.N.; Jangid, A.G.; Shrivastav, P.S. Liquid chromatography tandem mass spectrometry method for simultaneous determination of antidiabetic drugs metformin and glyburide in human plasma. J. Pharm. Biomed. Anal. 2007, 45, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Georgita, C.; Albu, F.; David, V.; Medvedovici, A. Simultaneous assay of metformin and glibenclamide in human plasma based on extraction-less sample preparation procedure and LC/(APCI)MS. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2007, 854, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Enke, C.G. A predictive model for matrix and analyte effects in electrospray ionization of singly-charged ionic analytes. Anal. Chem. 1997, 69, 4885–4893. [Google Scholar] [CrossRef] [PubMed]
- Amad, M.H.; Cech, N.B.; Jackson, G.S.; Enke, C.G. Importance of gas-phase proton affinities in determining the electrospray ionization response for analytes and solvents. J. Mass Spectrom. 2000, 35, 784–789. [Google Scholar] [CrossRef] [PubMed]
- Bruins, C.H.; Jeronimus-Stratingh, C.M.; Ensing, K.; van Dongen, W.D.; de Jong, G.J. On-line coupling of solid-phase extraction with mass spectrometry for the analysis of biological samples. I. Determination of clenbuterol in urine. J. Chromatogr. A 1999, 863, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Sojo, L.E.; Lum, G.; Chee, P. Internal standard signal suppression by co-eluting analyte in isotope dilution LC-ESI-MS. Analyst 2003, 128, 51–54. [Google Scholar] [CrossRef]
- Jiang, S.; Zhao, W.; Chen, Y.; Zhong, Z.; Zhang, M.; Li, F.; Xu, P.; Zhao, K.; Li, Y.; Liu, L.; et al. Paroxetine decreased plasma exposure of glyburide partly via inhibiting intestinal absorption in rats. Drug Metab. Pharmacokinet. 2015, 30, 240–246. [Google Scholar] [CrossRef]
- Gai, S.; Huang, A.; Feng, T.; Gou, N.; Wang, X.; Lu, C.; Tang, H.; Xu, D.; Zhang, B.; Wang, L. LC-MS/MS method for simultaneous determination of rivaroxaban and metformin in rat plasma: Application to pharmacokinetic interaction study. Bioanalysis 2019, 11, 2269–2281. [Google Scholar] [CrossRef]
- Li, N.; Deng, Y.; Wang, D.; Qiao, Y.; Li, F. Determination of glibenclamide and puerarin in rat plasma by UPLC-MS/MS: Application to their pharmacokinetic interaction study. Talanta 2013, 104, 109–115. [Google Scholar] [CrossRef]
- Maqbool, M.; Cooper, M.E.; Jandeleit-Dahm, K.A.M. Cardiovascular disease and diabetic kidney disease. Semin. Nephrol. 2018, 38, 217–232. [Google Scholar] [CrossRef]
| MET Conc. (ng/mL) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| LLOQ (50) | LQC (150) | MQC (600) | HQC (2400) | ULOQ (3200) | LLOQ (50) | LQC (150) | MQC (600) | HQC (2400) | ULOQ (3200) | ||
| GLY Conc. (ng/mL) | Degree of Signal Suppression of GLY by MET (%) | Degree of Signal Suppression of MET by GLY (%) | |||||||||
| Nominal concentration | LLOQ (20) | 87 | 88 | 74 | 72 | 66 | 93 | 99 | 92 | 111 | 113 |
| LQC (60) | 89 | 86 | 74 | 73 | 72 | 94 | 90 | 91 | 101 | 100 | |
| MQC (240) | 93 | 91 | 80 | 77 | 71 | 106 | 105 | 103 | 95 | 100 | |
| HQC (960) | 87 | 90 | 81 | 71 | 69 | 106 | 95 | 108 | 88 | 110 | |
| ULOQ (1280) | 92 | 90 | 79 | 74 | 73 | 113 | 98 | 106 | 89 | 111 | |
| 10-fold dilution for MET and GLY | LLOQ (20) | 106 | 91 | 101 | 76 | 73 | 89 | 111 | 107 | 114 | 85 |
| LQC (60) | 102 | 106 | 103 | 82 | 81 | 109 | 105 | 91 | 115 | 87 | |
| MQC (240) | 105 | 93 | 105 | 88 | 89 | 90 | 103 | 97 | 102 | 90 | |
| HQC (960) | 96 | 95 | 96 | 92 | 89 | 95 | 111 | 99 | 95 | 94 | |
| ULOQ (1280) | 89 | 92 | 92 | 88 | 93 | 93 | 110 | 106 | 96 | 104 | |
| 20-fold dilution for MET and GLY | LLOQ (20) | 107 | 109 | 91 | 71 | 75 | 91 | 110 | 95 | 93 | 100 |
| LQC (60) | 114 | 101 | 102 | 82 | 92 | 110 | 95 | 92 | 86 | 105 | |
| MQC (240) | 102 | 97 | 99 | 96 | 87 | 99 | 107 | 89 | 93 | 99 | |
| HQC (960) | 87 | 101 | 108 | 92 | 91 | 106 | 114 | 98 | 92 | 94 | |
| ULOQ (1280) | 89 | 106 | 87 | 90 | 88 | 99 | 113 | 89 | 86 | 94 | |
| Plasma samples for MET and GLY | LLOQ (20) | 88 | 82 | 76 | 79 | 77 | 94 | 100 | 96 | 97 | 104 |
| LQC (60) | 92 | 82 | 85 | 84 | 81 | 92 | 107 | 107 | 102 | 106 | |
| MQC (240) | 90 | 87 | 83 | 80 | 81 | 104 | 106 | 88 | 91 | 110 | |
| HQC (960) | 94 | 90 | 93 | 83 | 85 | 100 | 96 | 98 | 94 | 106 | |
| ULOQ (1280) | 103 | 97 | 93 | 95 | 88 | 91 | 97 | 91 | 91 | 101 | |
| Intar Run (n = 6) | Inter Run (n = 18) | ||||||
|---|---|---|---|---|---|---|---|
| Nominal Conc. (ng/mL) | Calculated Conc. (ng/mL) | Accuracy (%) | CV (%) | Calculated Conc. (ng/mL) | Accuracy (%) | CV (%) | |
| Non-isotope internal standard method | 20 | 20.8 ± 2.6 | 3.8 | 12.7 | 20.2 ± 0.9 | 0.8 | 4.3 |
| 60 | 63.5 ± 7.5 | 5.9 | 11.7 | 59.4 ± 4.7 | −1.1 | 7.8 | |
| 240 | 234.4 ± 19.3 | −2.3 | 8.3 | 235.6 ± 18.5 | −1.8 | 7.9 | |
| 960 | 904.0 ± 75.8 | −5.8 | 8.4 | 981.3 ± 86.1 | 2.2 | 8.8 | |
| Isotope internal standard method | 20 | 21.8 ± 1.4 | 9.0 | 6.3 | 20.4 ± 1.2 | 2.0 | 6.0 |
| 60 | 61.3 ± 1.8 | 2.2 | 3.0 | 60.4 ± 1.0 | 0.7 | 1.7 | |
| 240 | 244.7 ± 10.3 | 2.0 | 4.2 | 241.7 ± 5.1 | 0.7 | 2.1 | |
| 960 | 932.5 ± 35.5 | −2.9 | 3.8 | 943.0 ± 9.3 | −1.8 | 1.0 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Jiang, F.; Lu, Z.; Zhang, C.; Liu, P.; Huang, M.; Zhong, G. Signal Suppression in LC-ESI-MS/MS from Concomitant Medications and Its Impact on Quantitative Studies: An Example Using Metformin and Glyburide. Molecules 2023, 28, 746. https://doi.org/10.3390/molecules28020746
Liu J, Jiang F, Lu Z, Zhang C, Liu P, Huang M, Zhong G. Signal Suppression in LC-ESI-MS/MS from Concomitant Medications and Its Impact on Quantitative Studies: An Example Using Metformin and Glyburide. Molecules. 2023; 28(2):746. https://doi.org/10.3390/molecules28020746
Chicago/Turabian StyleLiu, Jingyu, Fulin Jiang, Zihan Lu, Chang Zhang, Peiqing Liu, Min Huang, and Guoping Zhong. 2023. "Signal Suppression in LC-ESI-MS/MS from Concomitant Medications and Its Impact on Quantitative Studies: An Example Using Metformin and Glyburide" Molecules 28, no. 2: 746. https://doi.org/10.3390/molecules28020746
APA StyleLiu, J., Jiang, F., Lu, Z., Zhang, C., Liu, P., Huang, M., & Zhong, G. (2023). Signal Suppression in LC-ESI-MS/MS from Concomitant Medications and Its Impact on Quantitative Studies: An Example Using Metformin and Glyburide. Molecules, 28(2), 746. https://doi.org/10.3390/molecules28020746







