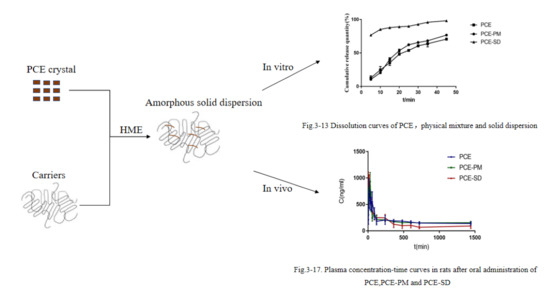
Preparation of Solid Dispersion of Polygonum Cuspidatum Extract by Hot Melt Extrusion to Enhance Oral Bioavailability of Resveratrol
Abstract
1. Introduction
2. Results
2.1. Preparation of Polygonum Cuspidatum Extract (PCE)
2.2. Results of Preliminary Qualitative Study on Main Attendant Substances in PCE
2.3. Univariate Analysis of Solid Dispersion of Polygonum Cuspidatum Extract
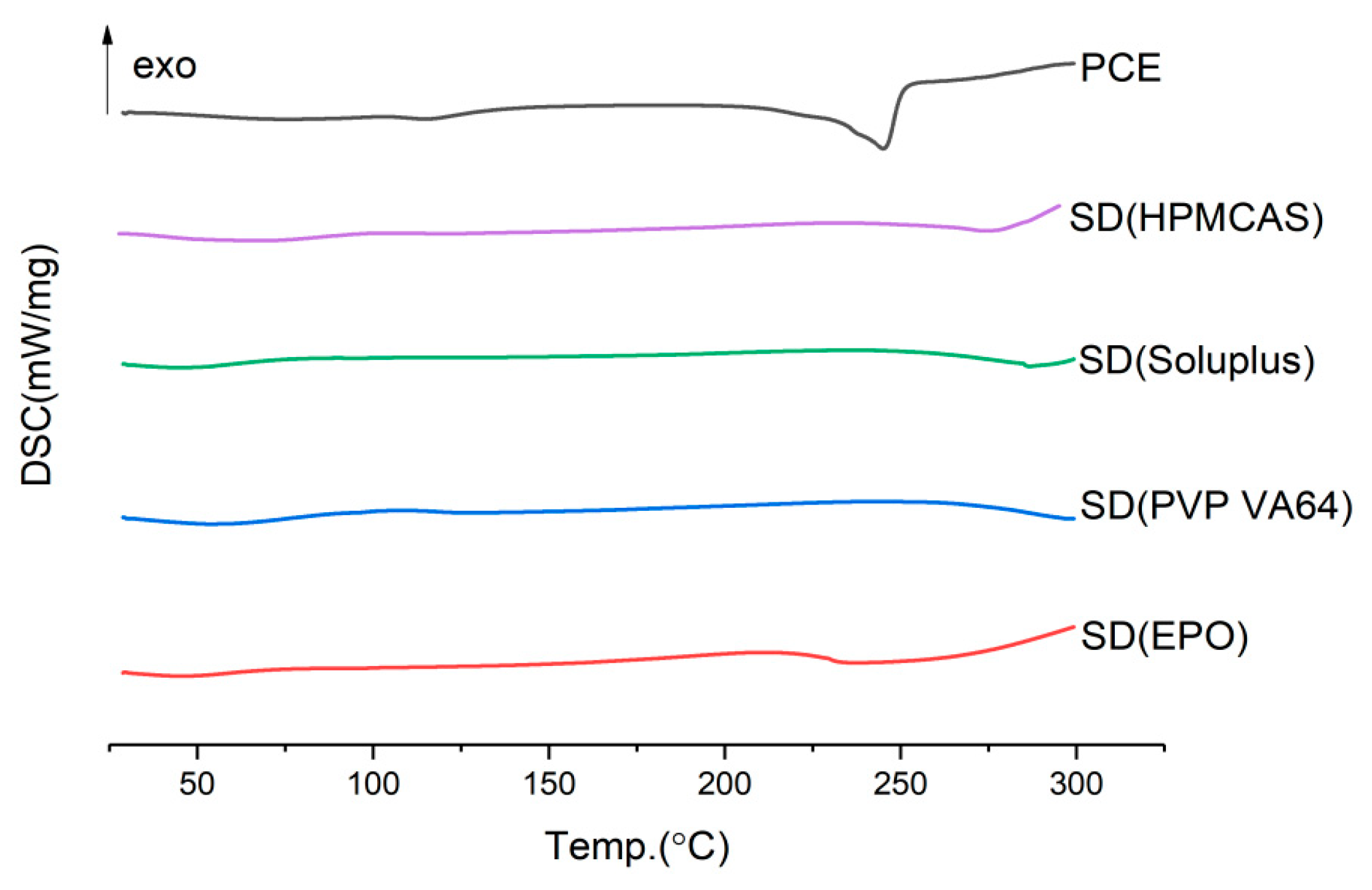
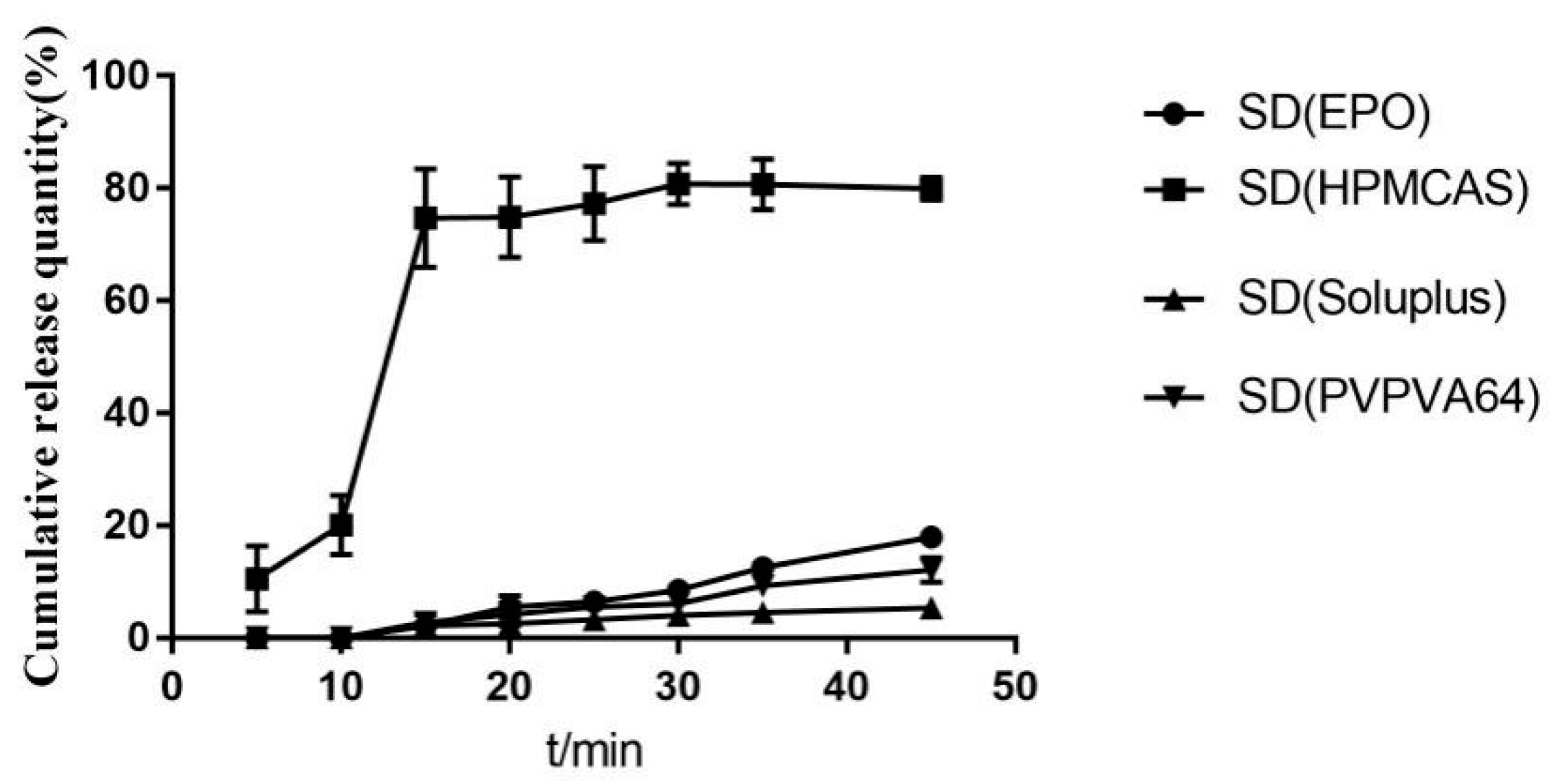
2.3.1. Type of Carrier
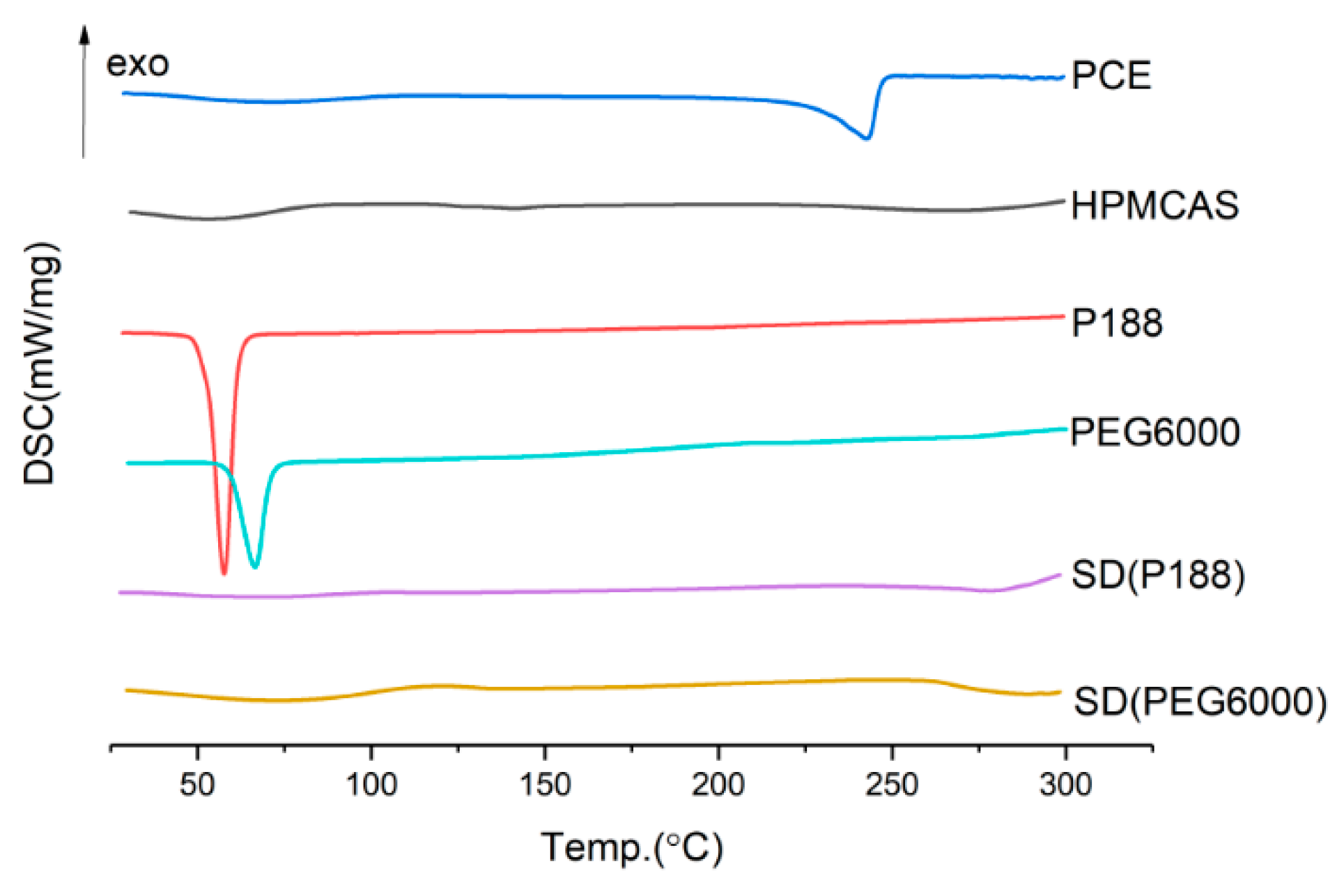
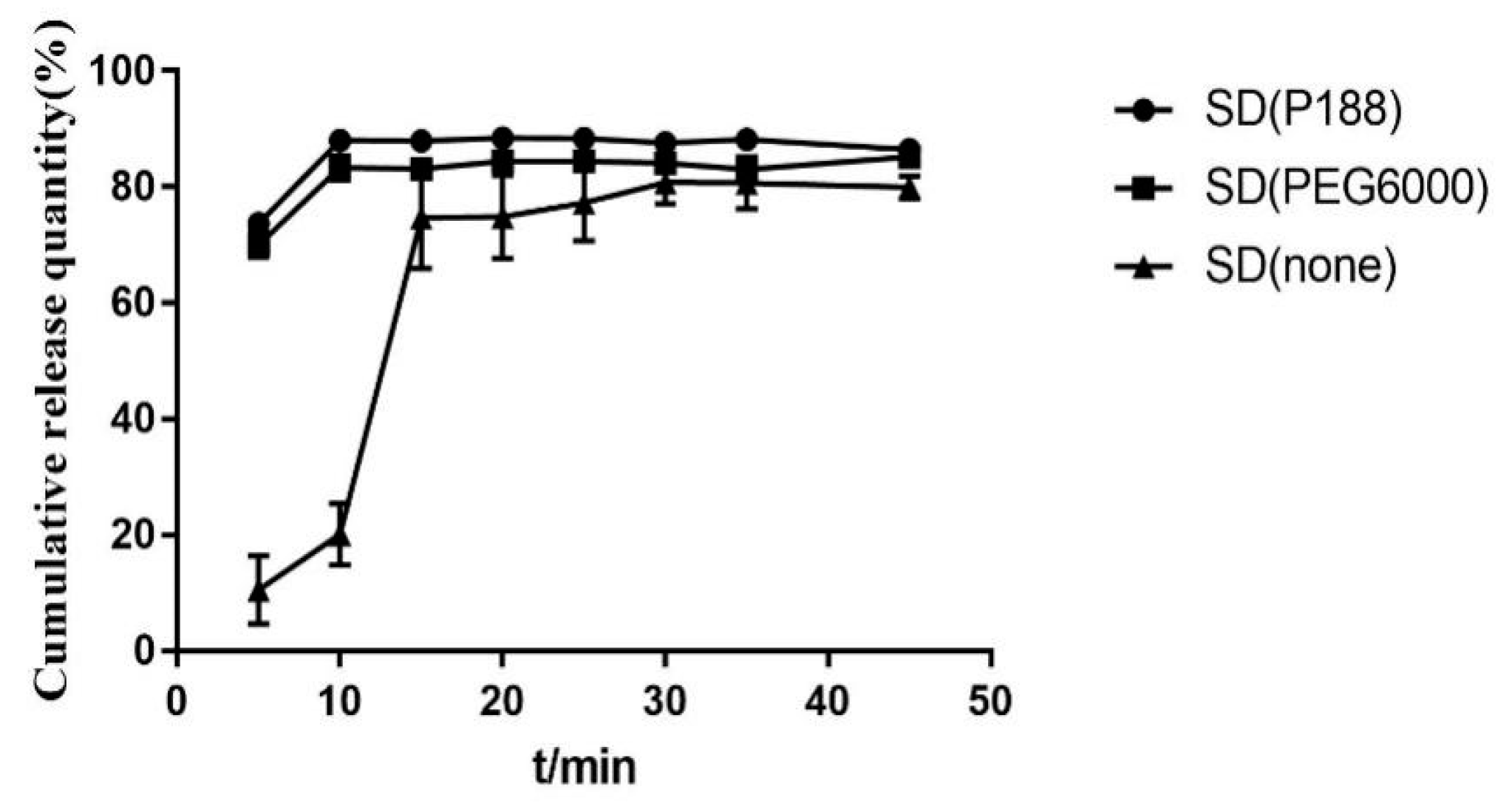
2.3.2. The Type and Dosage of Plasticizer
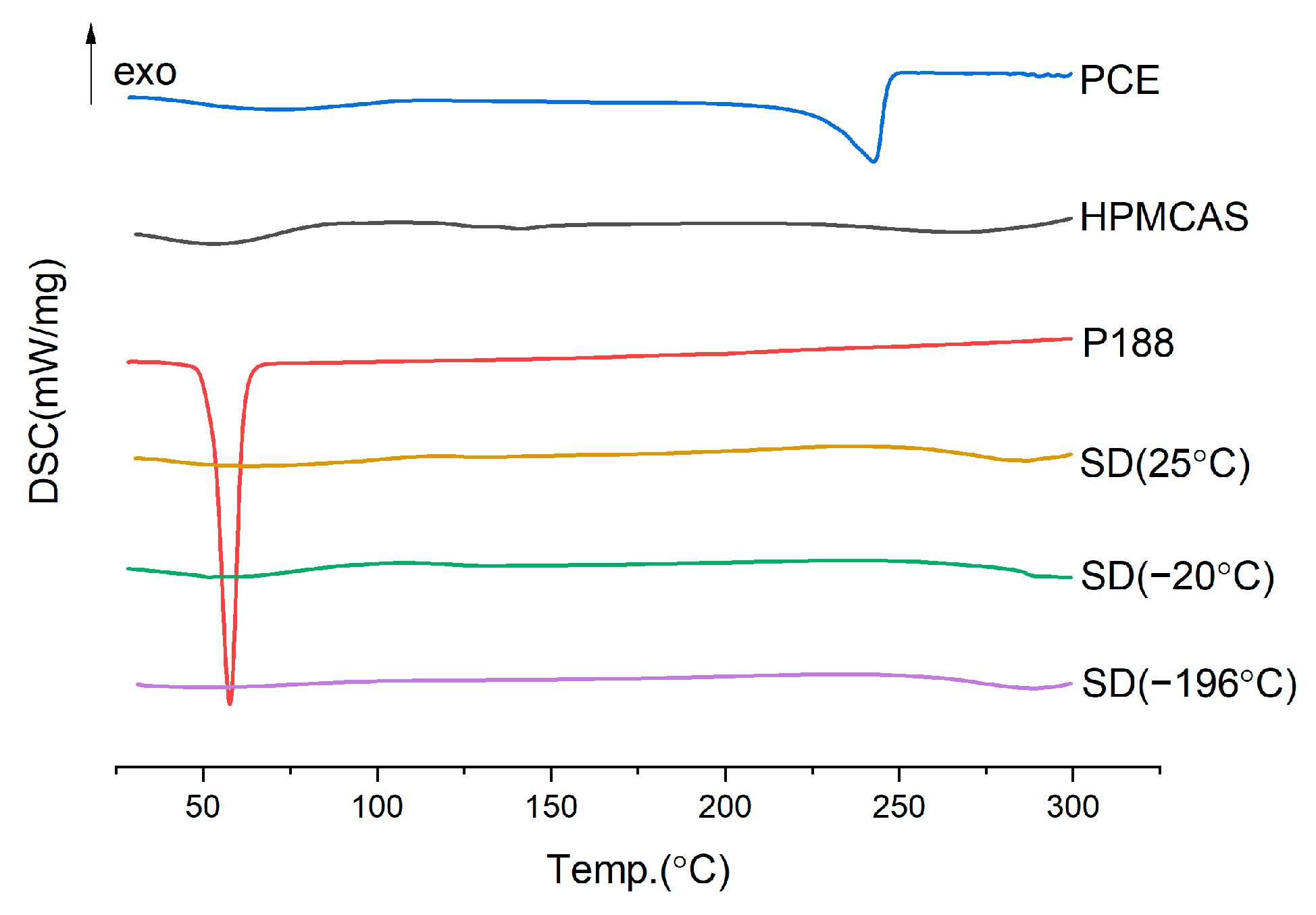
2.3.3. Cooling Temperature
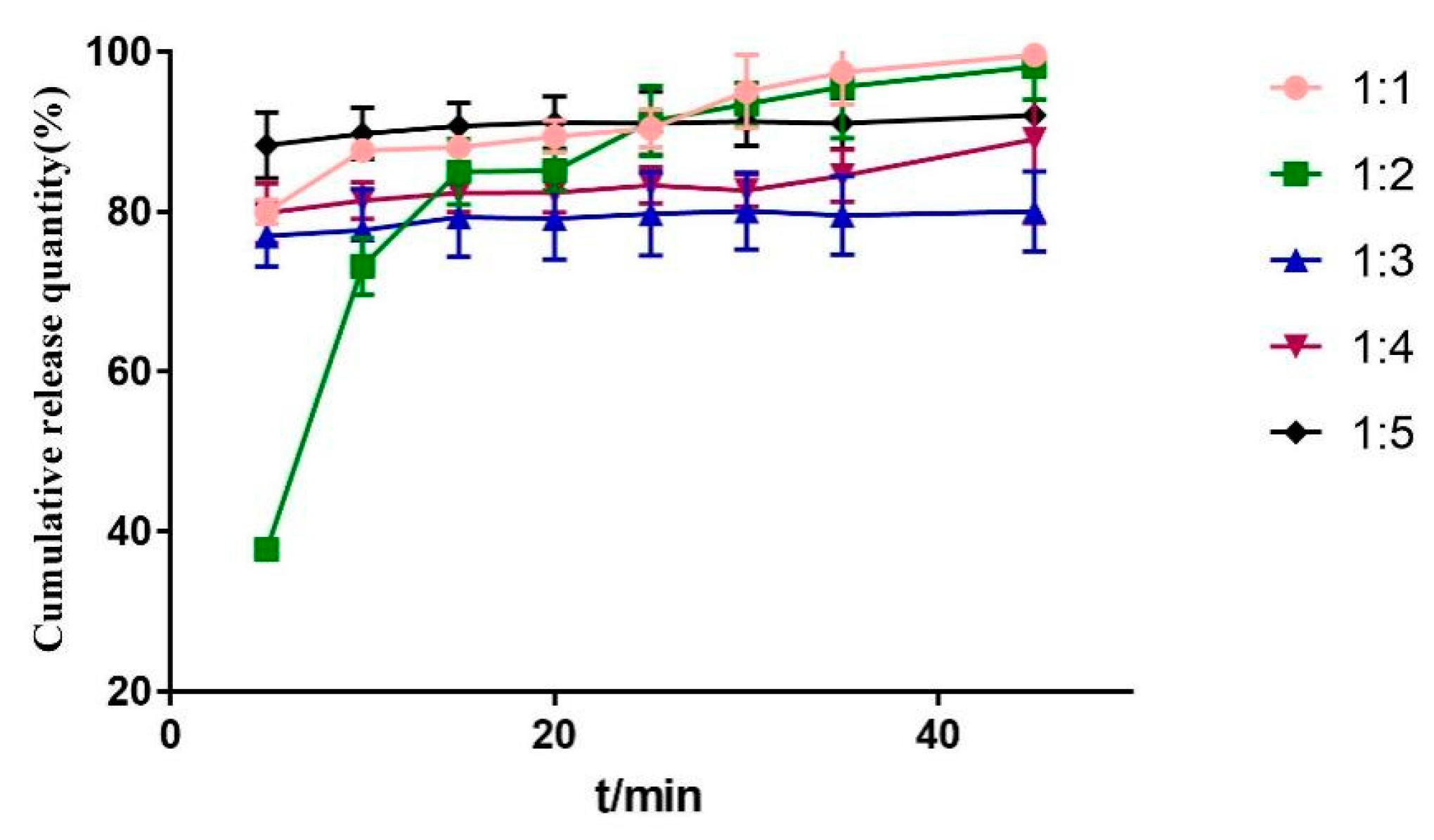
2.3.4. Mass Ratio
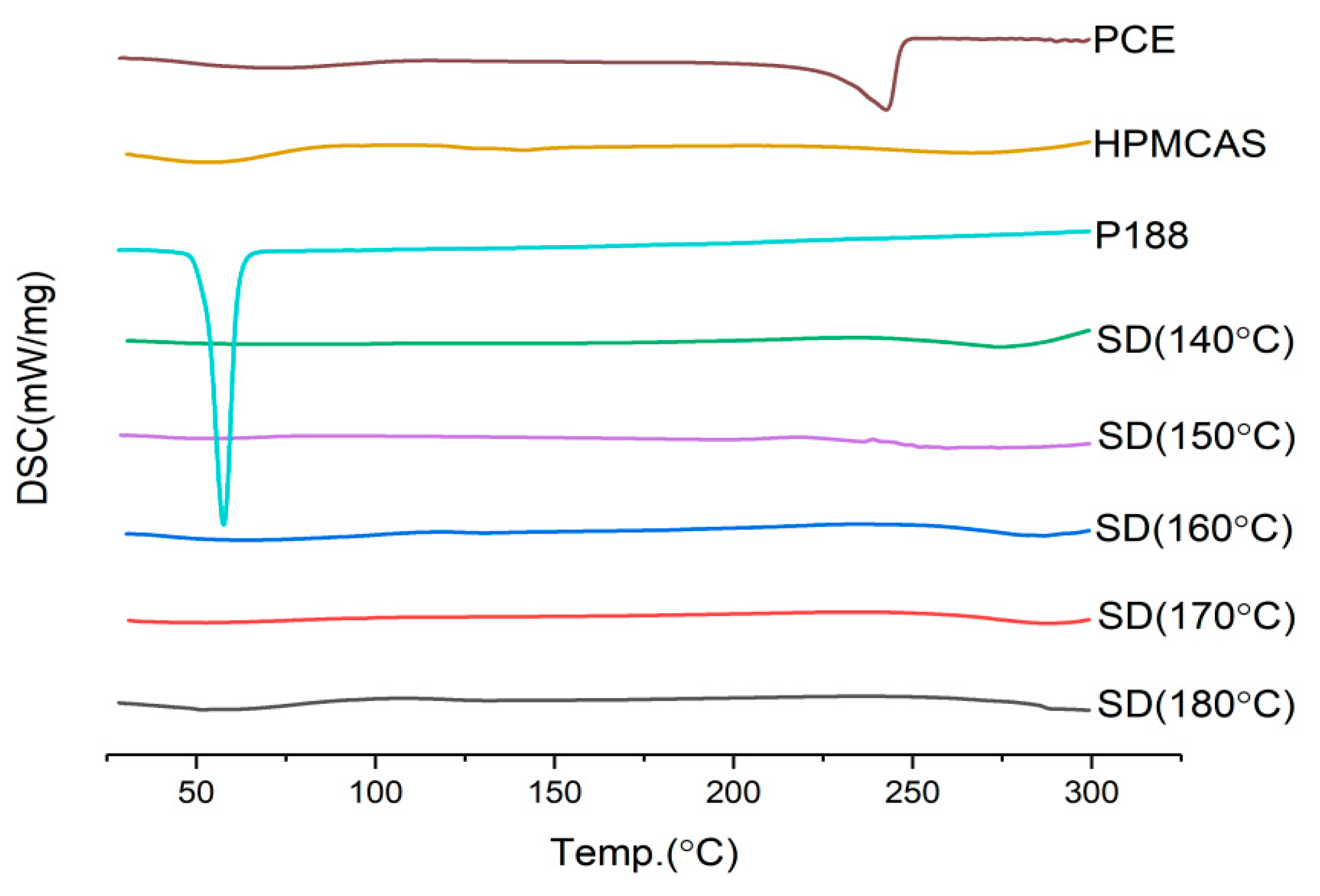
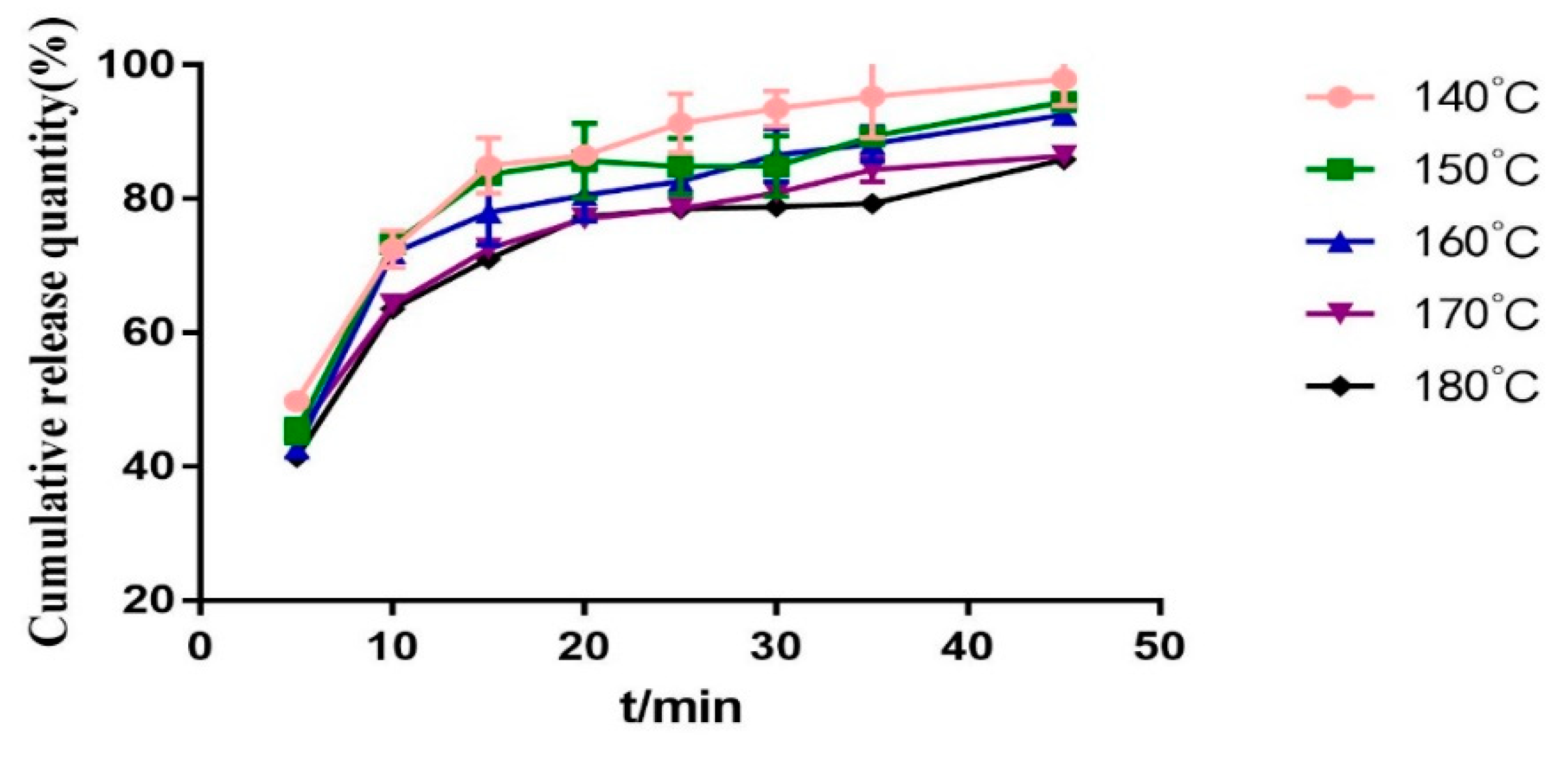
2.3.5. Screening Barrel Temperature
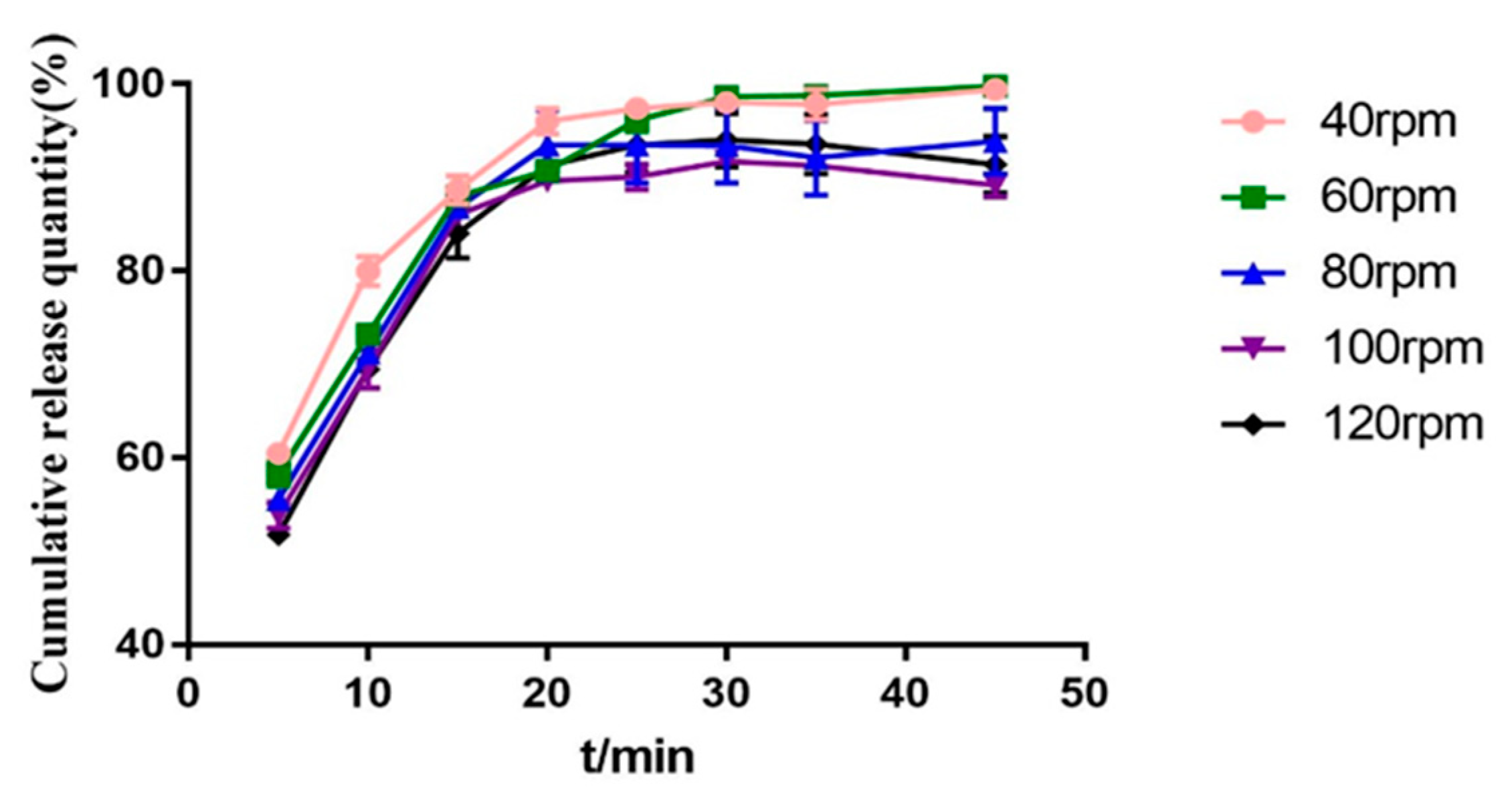
2.3.6. Screw Speed
2.4. Orthogonal Array Experimental Design
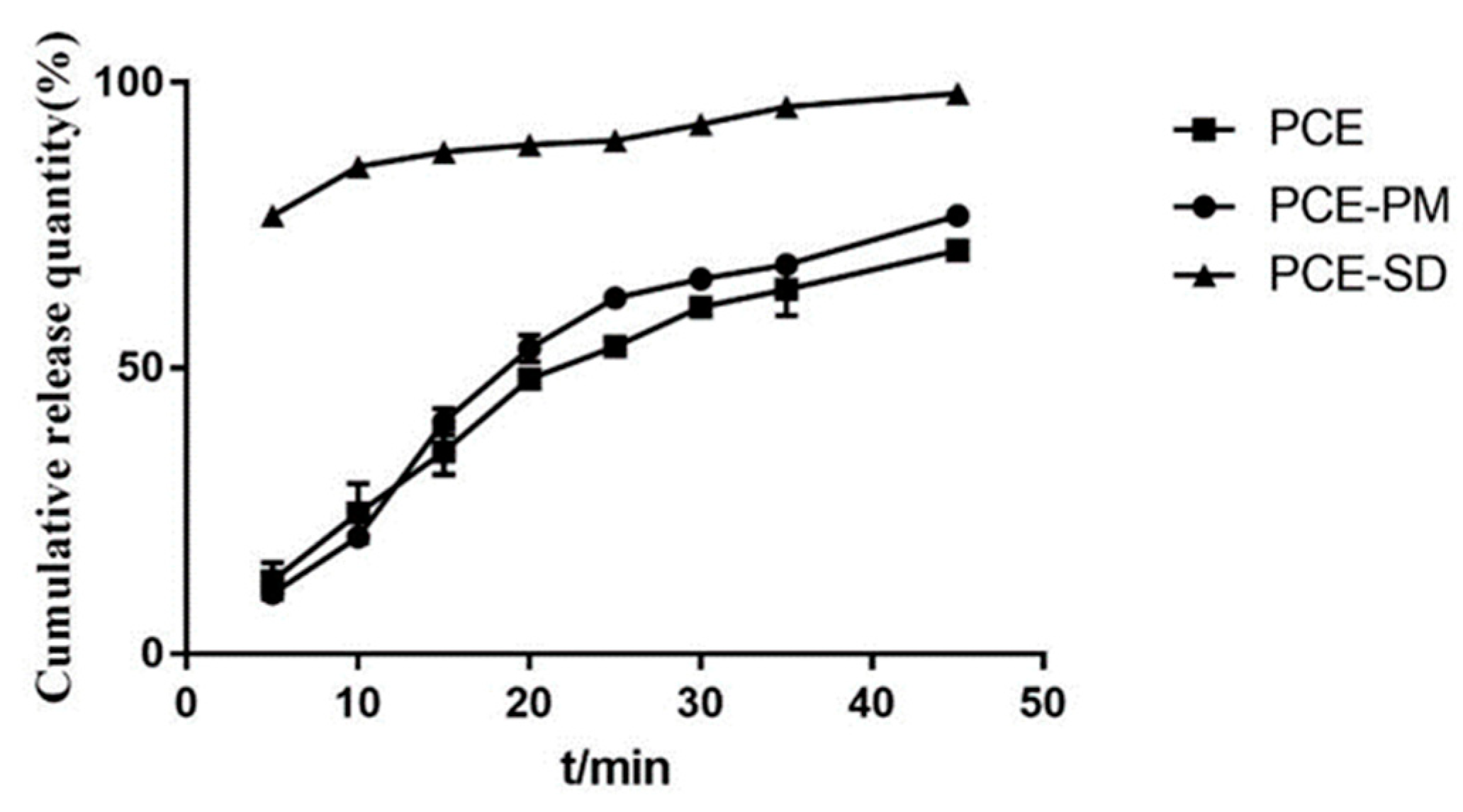
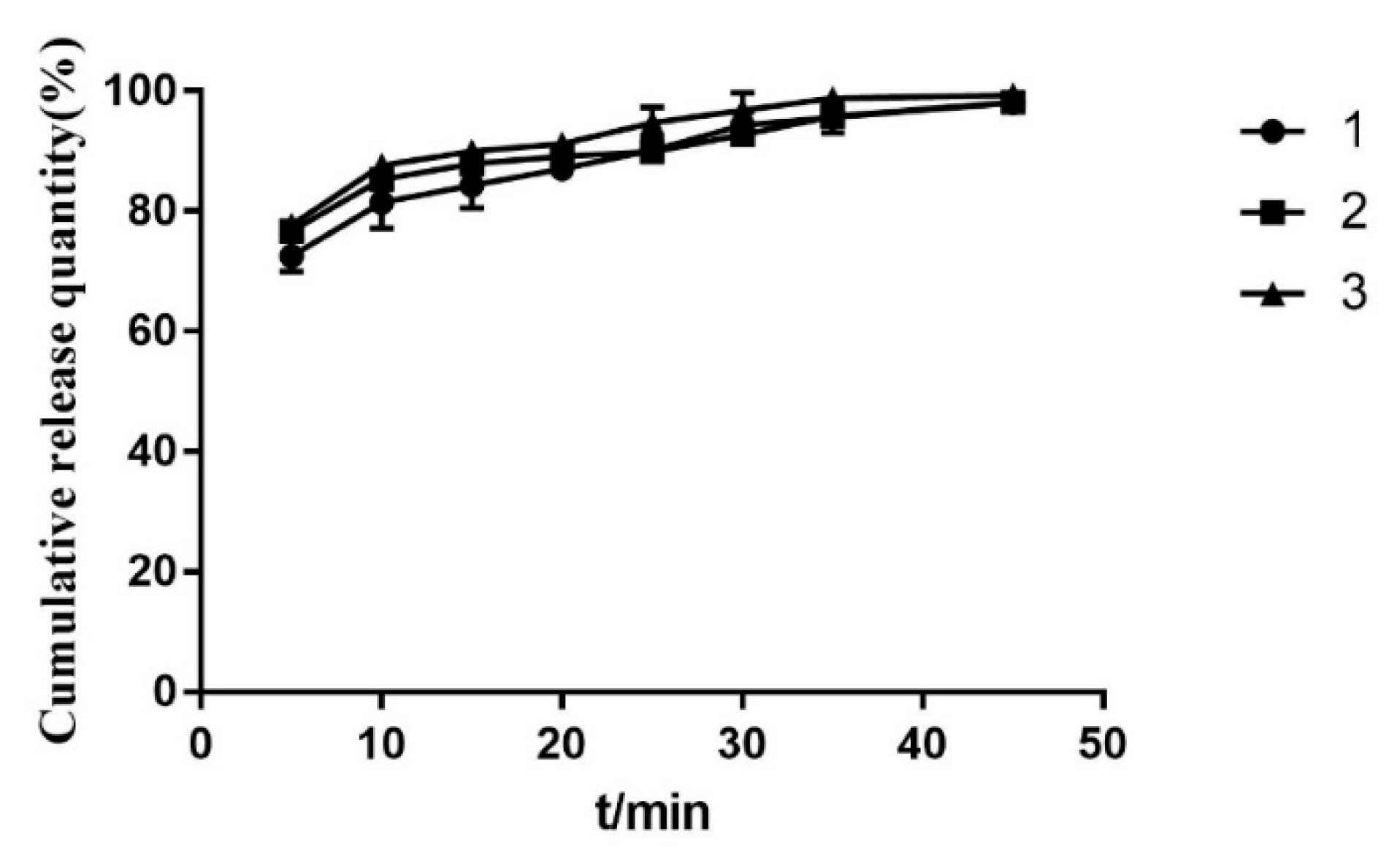
2.5. Optimal Prescription Process Validation
2.6. Characterization of the PCE-SD
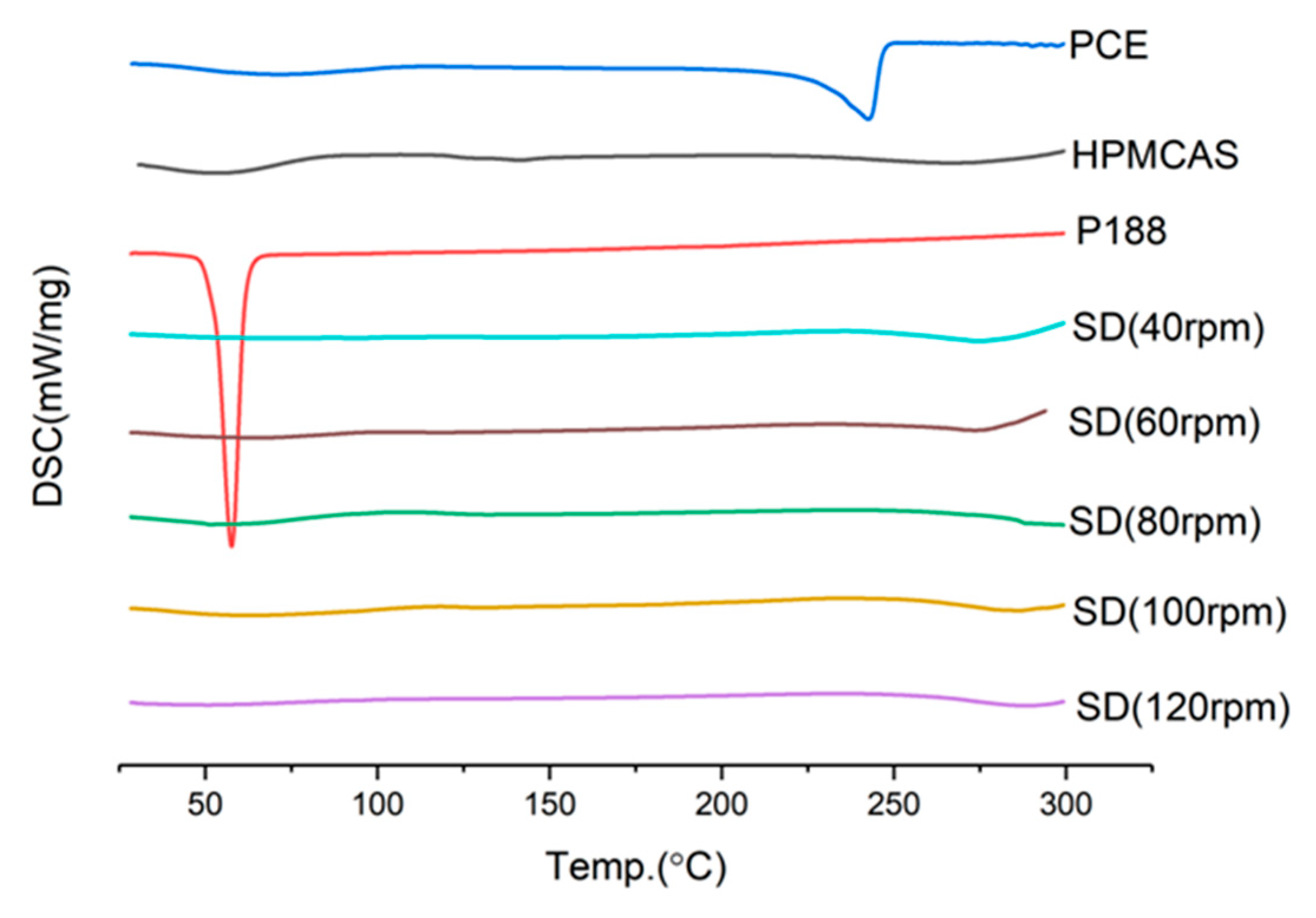
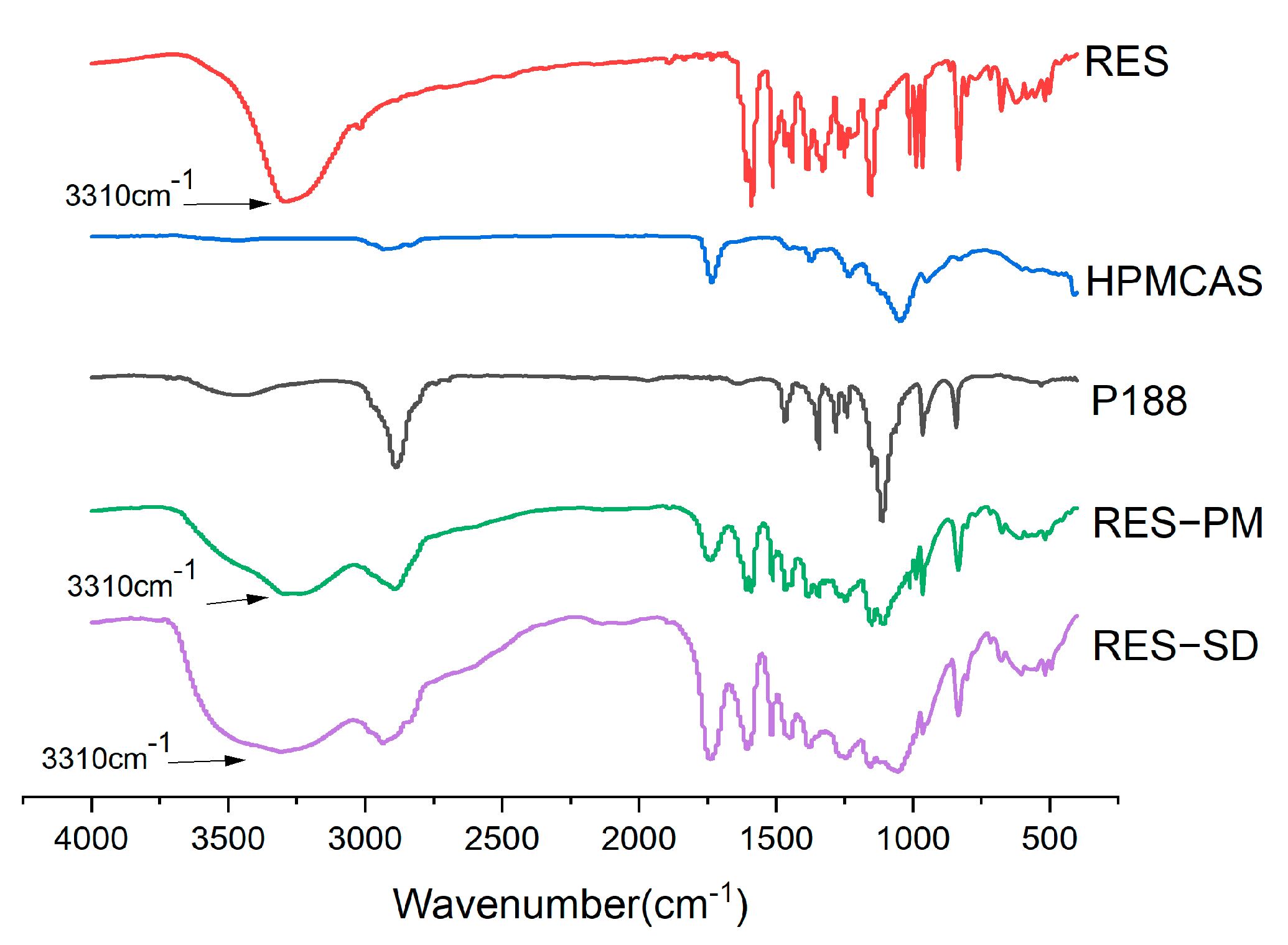
2.6.1. FTIR Assay
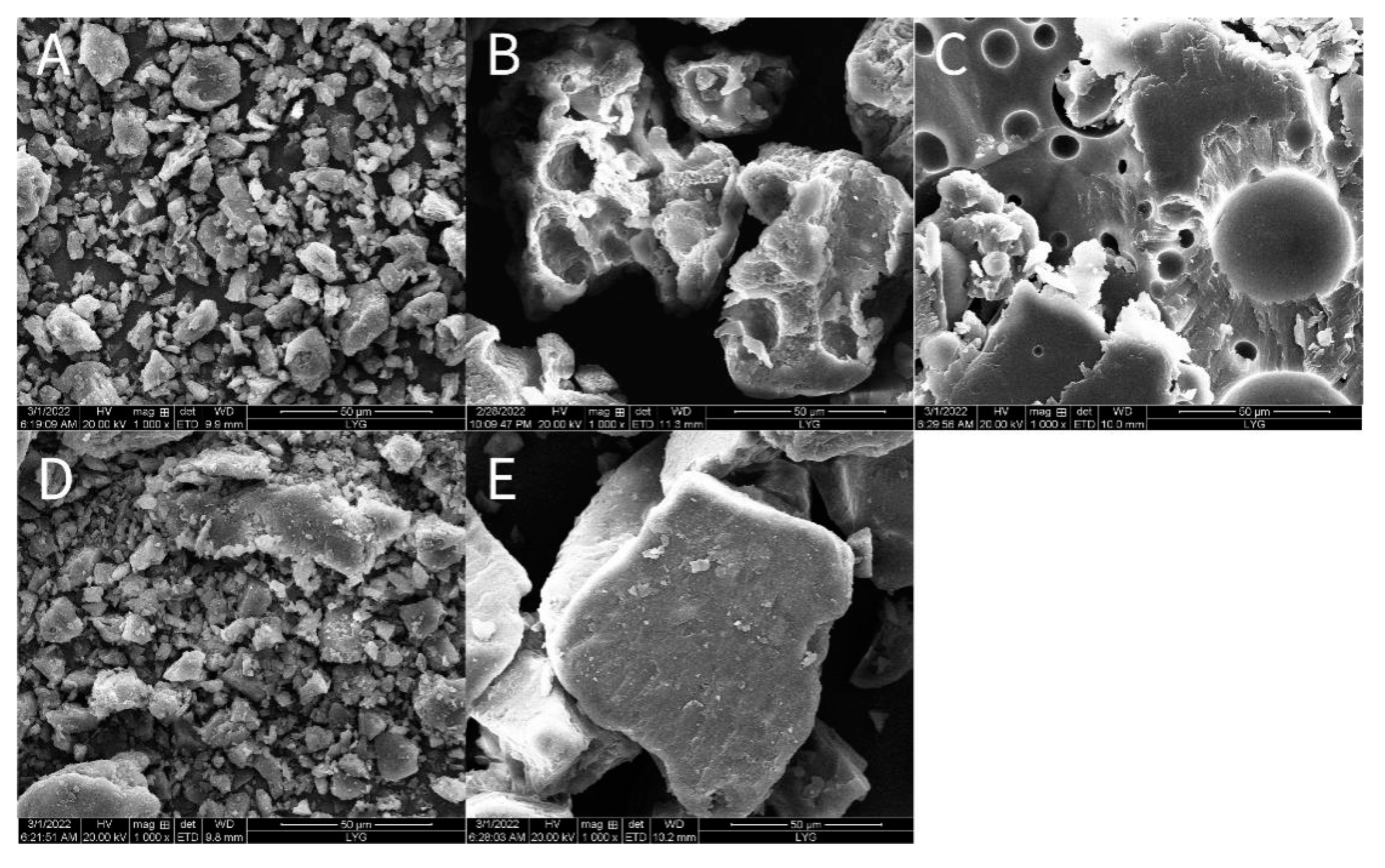
2.6.2. SEM
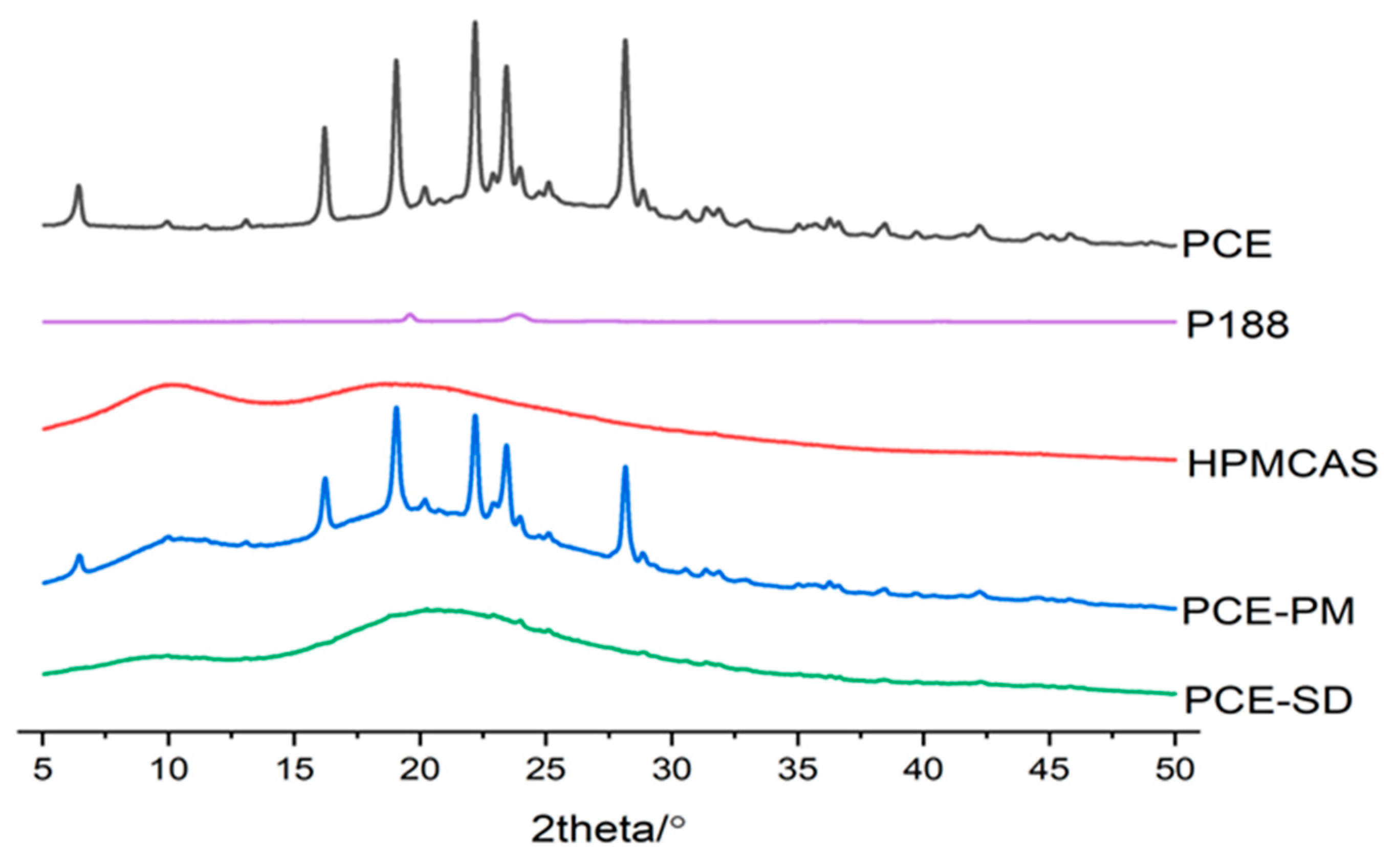
2.6.3. XRD
2.7. Saturated Solubility
2.8. Stability Experiment
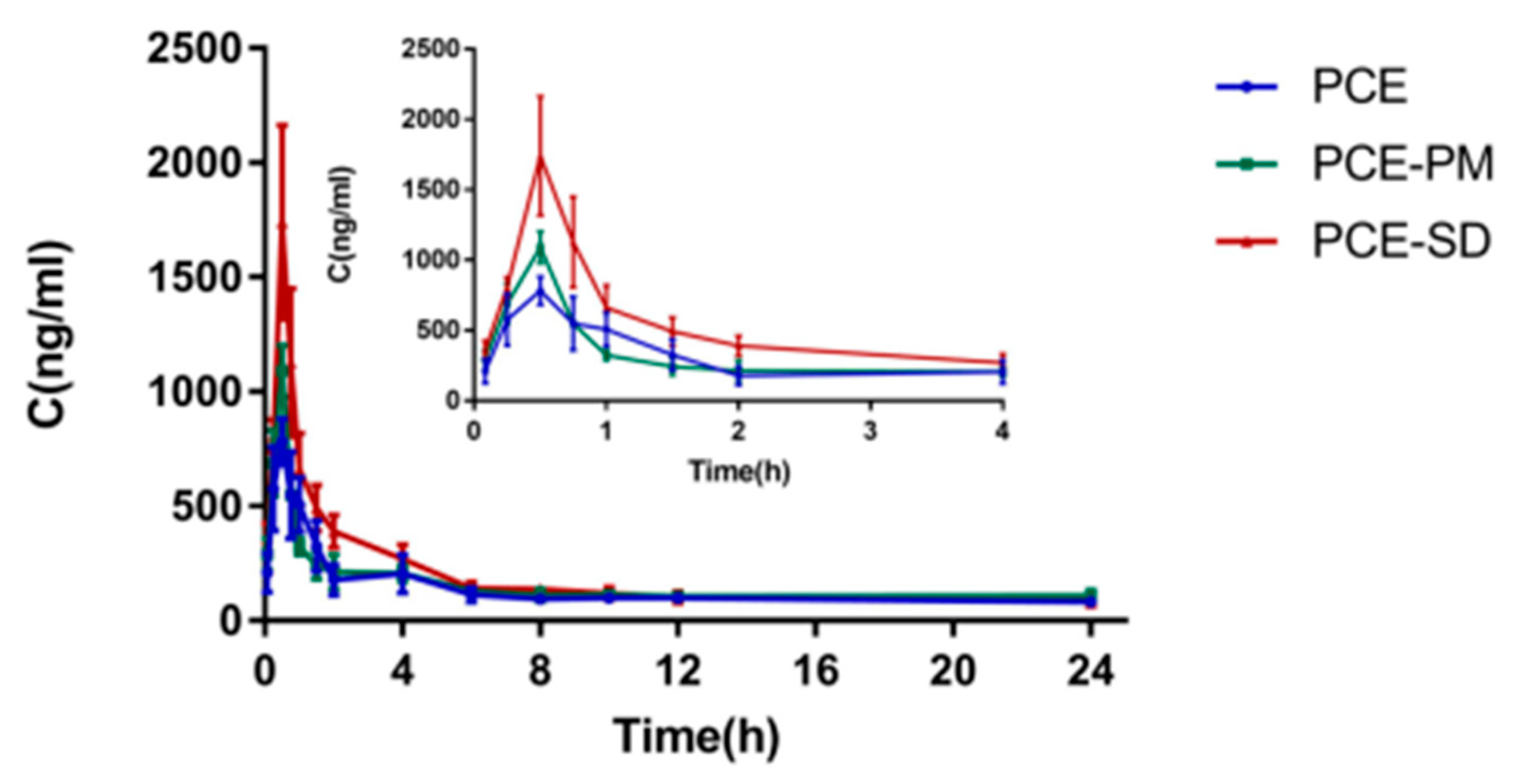
2.9. Pharmacokinetic Study
3. Materials and Methods
3.1. Materials
3.2. HPLC Analysis
3.3. Preparation of Polygonum Cuspidatum Extract (PCE)
3.4. Preliminary Qualitative Study on Main Attendant Substances in PCE
3.5. Preparation of PCE Solid Dispersions (PCE-SDs) and PCE Physical Mixtures (PCE-PMs)
3.5.1. Hot-Melt Extrusion (HME)
3.5.2. Physical Mixture
3.6. Evaluation Parameters
3.6.1. DSC (Differential Scanning Calorimetry) Study
3.6.2. In Vitro Dissolution Studies
3.7. Univariate Analysis of Solid Dispersion of Polygonum Cuspidatum Extract
3.8. Orthogonal Array Experimental Design to Obtainoptimal Prescription of PCE-SD
3.9. Optimal Prescription Process Validation
3.10. Characterization of the Extrudate
3.10.1. PXRD Study (Powder X-ray Diffraction)
3.10.2. FTIR Study
3.10.3. Scanning Electron Microscopy
3.10.4. Saturation Solubility
3.10.5. Stability Experiment
3.11. Pharmacokinetics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Poulsen, M.M.; Fjeldborg, K.; Ornstrup, M.J.; Kjær, T.N.; Nøhr, M.K.; Pedersen, S.B. Resveratrol and inflammation: Challenges in translating pre-clinical findings to improved patient outcomes. Biochim. Biophys. Acta 2015, 1852, 1124–1136. [Google Scholar] [CrossRef] [PubMed]
- Mu, Q.; Najafi, M. Resveratrol for targeting the tumor microenvironment and its interactions with cancer cells. Int. Immunopharmacol. 2021, 98, 107895. [Google Scholar] [CrossRef] [PubMed]
- Fonseca-Santos, B.; Chorilli, M. The uses of resveratrol for neurological diseases treatment and insights for nanotechnology based-drug delivery systems. Int. J. Pharm. 2020, 589, 119832. [Google Scholar] [CrossRef] [PubMed]
- Dyck, G.J.B.; Raj, P.; Zieroth, S.; Dyck, J.R.B.; Ezekowitz, J.A. The Effects of Resveratrol in Patients with Cardiovascular Disease and Heart Failure: A Narrative Review. Int. J. Mol. Sci. 2019, 20, 904. [Google Scholar] [CrossRef] [PubMed]
- Athar, M.; Back, J.H.; Tang, X.; Kim, K.H.; Kopelovich, L.; Bickers, D.R.; Kim, A.L. Resveratrol: A review of preclinical studies for human cancer prevention. Toxicol. Appl. Pharmacol. 2007, 224, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Gliemann, L.; Schmidt, J.F.; Olesen, J.; Biensø, R.S.; Peronard, S.L.; Grandjean, S.U.; Mortensen, S.; Nyberg, M.; Bangsbo, J.; Pilegaard, H.; et al. Resveratrol blunts the positive effects of exercise training on cardiovascular health in aged men. J. Physiol. 2013, 591, 5047–5059. [Google Scholar] [CrossRef]
- Ren, B.; Kwah, M.X.-Y.; Liu, C.; Ma, Z.; Shanmugam, M.K.; Ding, L.; Xiang, X.; Ho, P.C.-L.; Wang, L.; Ong, P.S.; et al. Resveratrol for cancer therapy: Challenges and future perspectives. Cancer Lett. 2021, 515, 63–72. [Google Scholar] [CrossRef]
- La Porte, C.; Voduc, N.; Zhang, G.; Seguin, I.; Tardiff, D.; Singhal, N.; Cameron, D.W. Steady-State Pharmacokinetics and Tolerability of Trans-Resveratrol 2000 mg Twice Daily with Food, Quercetin and Alcohol (Ethanol) in Healthy Human Subjects. Clin. Pharmacokinet. 2010, 49, 449–454. [Google Scholar] [CrossRef]
- Boocock, D.J.; Faust, G.E.; Patel, K.R.; Schinas, A.M.; Brown, V.A.; Ducharme, M.P.; Booth, T.D.; Crowell, J.A.; Perloff, M.; Gescher, A.J.; et al. Phase I dose escalation pharmacokinetic study in healthy volunteers of resveratrol, a potential cancer chemopreventive agent. Cancer Epidemiol. Biomarkers Prev. 2007, 16, 1246–1252. [Google Scholar] [CrossRef]
- Pandita, D.; Kumar, S.; Poonia, N.; Lather, V. Solid lipid nanoparticles enhance oral bioavailability of resveratrol, a natural polyphenol. Food Res. Int. 2014, 62, 1165–1174. [Google Scholar] [CrossRef]
- Ting, Y.; Jiang, Y.; Ho, C.-T.; Huang, Q. Common delivery systems for enhancing in vivo bioavailability and biological efficacy of nutraceuticals. J. Funct. Foods 2014, 7, 112–128. [Google Scholar] [CrossRef]
- Yen, C.-C.; Chang, C.-W.; Hsu, M.-C.; Wu, Y.-T. Self-Nanoemulsifying Drug Delivery System for Resveratrol: Enhanced Oral Bioavailability and Reduced Physical Fatigue in Rats. Int. J. Mol. Sci. 2017, 18, 1853. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.-F.; Zhou, J.; Hu, X.; Cong, Z.-Q.; Liu, C.-Y.; Pan, R.-L.; Chang, Q.; Liu, X.-M.; Liao, Y.-H. Improving oral bioavailability of resveratrol by a UDP-glucuronosyltransferase inhibitory excipient-based self-microemulsion. Eur. J. Pharm. Sci. 2018, 114, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Calvo-Castro, L.A.; Schiborr, C.; David, F.; Ehrt, H.; Voggel, J.; Sus, N.; Behnam, D.; Bosy-Westphal, A.; Frank, J. The Oral Bioavailability of Trans -Resveratrol from a Grapevine-Shoot Extract in Healthy Humans is Significantly Increased by Micellar Solubilization. Mol. Nutr. Food Res. 2018, 62, e1701057. [Google Scholar] [CrossRef]
- Nassir, A.M.; Shahzad, N.; Ibrahim, I.A.A.; Ahmad, I.; Md, S.; Ain, M.R. Resveratrol-loaded PLGA nanoparticles mediated programmed cell death in prostate cancer cells. Saudi Pharm. J. 2018, 26, 876–885. [Google Scholar] [CrossRef]
- Spogli, R.; Bastianini, M.; Ragonese, F.; Iannitti, R.G.; Monarca, L.; Bastioli, F.; Nakashidze, I.; Brecchia, G.; Menchetti, L.; Codini, M.; et al. Solid Dispersion of Resveratrol Supported on Magnesium DiHydroxide (Resv@MDH) Microparticles Improves Oral Bioavailability. Nutrients 2018, 10, 1925. [Google Scholar] [CrossRef]
- Singh, S.K.; Makadia, V.; Sharma, S.; Rashid, M.; Shahi, S.; Mishra, P.R.; Wahajuddin, M.; Gayen, J.R. Preparation and in-vitro/in-vivo characterization of trans-resveratrol nanocrystals for oral administration. Drug Deliv. Transl. Res. 2017, 7, 395–407. [Google Scholar] [CrossRef]
- Shareena, G.; Kumar, D. Traversing through half a century research timeline on Ginkgo biloba, in transforming a botanical rarity into an active functional food ingredient. Biomed. Pharmacother. 2022, 153, 113299. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, M.; Yu, H.; Yuan, G.; Luo, L.; Xu, X.; Xu, Y.; Sui, X.; Leung, E.L.-H.; Wu, Q. The Role and Mechanisms of Action of Natural Compounds in the Prevention and Treatment of Cancer and Cancer Metastasis. Front. Biosci. 2022, 27, 192. [Google Scholar] [CrossRef]
- Guo, L.-W.; Pan, L.-M.; Zhu, H.-X. Characteristics of biopharmaceutics and significance of preparation in attendants of Chinese herbal extracts. Chin. Tradit. Herb. Drugs 2007, 38, 1281–1286. [Google Scholar]
- Nora, G.-I.; Venkatasubramanian, R.; Strindberg, S.; Siqueira-Jørgensen, S.D.; Pagano, L.; Romanski, F.S.; Swarnakar, N.K.; Rades, T.; Müllertz, A. Combining lipid based drug delivery and amorphous solid dispersions for improved oral drug absorption of a poorly water-soluble drug. J. Control. Release 2022, 349, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Alshetaili, A.; Almutairy, B.; Alshehri, S.; Repka, M. Development and Characterization of Sustained-Released Donepezil Hydrochloride Solid Dispersions Using Hot Melt Extrusion Technology. Pharmaceutics 2021, 13, 213. [Google Scholar] [CrossRef] [PubMed]
- Qi, Q.; Taylor, L.S. Improved dissolution of an enteric polymer and its amorphous solid dispersions by polymer salt formation. Int. J. Pharm. 2022, 622, 121886. [Google Scholar] [CrossRef] [PubMed]
- Alshehri, S.; Imam, S.S.; Hussain, A.; Altamimi, M.A.; Alruwaili, N.K.; Alotaibi, F.; Alanazi, A.; Shakeel, F. Potential of solid dispersions to enhance solubility, bioavailability, and therapeutic efficacy of poorly water-soluble drugs: Newer formulation techniques, current marketed scenario and patents. Drug Deliv. 2020, 27, 1625–1643. [Google Scholar] [CrossRef]
- Virro, J.J.; Besinque, K.; Carney, C.E.; Gross, D.; Bernick, B.; Mirkin, S. Long-Lasting, Patient-Controlled, Procedure-Free Contraception: A Review of Annovera with a Pharmacist Perspective. Pharmacy 2020, 8, 156. [Google Scholar] [CrossRef]
- Granados, P.A.; Pinho, L.A.; Sa-Barreto, L.L.; Gratieri, T.; Gelfuso, G.M.; Cunha-Filho, M. Application of hot-melt extrusion in the complexation of naringenin with cyclodextrin using hydrophilic polymers. Adv. Powder Technol. 2021, 33, 103380. [Google Scholar] [CrossRef]
- Jonas, M.M.; Rhee, S.; Kelly, D.A.; Del Valle-Segarra, A.; Feiterna-Sperling, C.; Gilmour, S.; Gonzalez-Peralta, R.P.; Hierro, L.; Leung, D.H.; Ling, S.C.; et al. Pharmacokinetics, Safety, and Efficacy of Glecaprevir/Pibrentasvir in Children With Chronic HCV: Part 2 of the DORA Study. Hepatology 2021, 74, 19–27. [Google Scholar] [CrossRef]
- Mei, Y.-Z.; Liu, R.-X.; Wang, D.-P.; Wang, X.; Dai, C.-C. Biocatalysis and biotransformation of resveratrol in microorganisms. Biotechnol. Lett. 2014, 37, 9–18. [Google Scholar] [CrossRef]
- Yun-Ting, Z.; Xiao, H.; Yun-Zhong, C.; Jun-de, L.I.; Kun, Y.U. Chemical constituents and their biosynthesis mechanisms of Polygonum cuspidatum. Zhongguo Zhong Yao Za Zhi 2020, 45, 4364–4372. [Google Scholar]
- Singh, S.M.; Panda, A.K. Solubilization and refolding of bacterial inclusion body proteins. J. Biosci. Bioeng. 2005, 99, 303–310. [Google Scholar] [CrossRef]
- Chu, K.K.W.; Chow, A.H. Impact of carbohydrate constituents on moisture sorption of herbal extracts. Pharm. Res. 2000, 17, 1133–1137. [Google Scholar] [CrossRef] [PubMed]
- Spanopoulos, I.; Hadar, I.; Ke, W.; Tu, Q.; Chen, M.; Tsai, H.; He, Y.; Shekhawat, G.; Dravid, V.P.; Wasielewski, M.R.; et al. Uniaxial Expansion of the 2D Ruddlesden–Popper Perovskite Family for Improved Environmental Stability. J. Am. Chem. Soc. 2019, 141, 5518–5534. [Google Scholar] [CrossRef] [PubMed]


| Inspection Items | Identification Reagent or Reaction Name | Experimental Result |
|---|---|---|
| Amino acid | Ninhydrin Test | (−) |
| Polypeptide, Protein | Biuret Reaction | (−) |
| Reducing sugars, Polysaccharides and Plycosides | Molisch Reaction | (+) |
| Fehling’s Solution | (+) | |
| Saponins | Foam Stability Test | (−) |
| Tannin | Liquor Ferri Trichloridi | (+) |
| Sodium Chloride Gelatin Solution | (+) | |
| Organic acid | pH Test Strips | (+) |
| Bromocresol Green Test Solution | (+) | |
| Alkaloid | Tetrapotassium Heptaiodobismuthate | (−) |
| Flavone | HCI-Mg Reaction Colorimetry | (−) |
| Anthraquinone | 1% Sodium Hydroxide Solution | (+) |
| Run | Factors | Responses | ||
|---|---|---|---|---|
| Barrel Temperature (°C) | Screw Speed (rpm) | Mass Ratio | ||
| 1 | 140 | 40 | 1:1 | 90.15 |
| 2 | 140 | 60 | 1:2 | 92.26 |
| 3 | 140 | 80 | 1:3 | 93.18 |
| 4 | 150 | 40 | 1:2 | 90.87 |
| 5 | 150 | 60 | 1:3 | 85.79 |
| 6 | 150 | 80 | 1:1 | 82.08 |
| 7 | 160 | 40 | 1:3 | 90.69 |
| 8 | 160 | 60 | 1:1 | 81.55 |
| 9 | 160 | 80 | 1:2 | 87.88 |
| K1 | 91.863 | 90.570 | 84.593 | |
| K2 | 86.247 | 86.533 | 90.337 | |
| K3 | 86.707 | 87.713 | 89.887 | |
| Rj | 5.616 | 4.037 | 5.744 | |
| PCE | RES | PCE-SD | |
|---|---|---|---|
| Saturated solubility (μg/mL) | 46.75 ± 0.47% | 44.34 ± 0.87% | 130.06 ± 0.12% |
| PCE | PCE-SD | ||
|---|---|---|---|
| moisture absorption/% | 0d | 0 | 0 |
| 5d | 8.87 | 7.02 | |
| 10d | 5.27 | 5.59 | |
| content/% | 0d | 50.24 | 99.83 |
| 5d | 44.83 | 97.29 | |
| 10d | 39.88 | 92.36 | |
| PCE | PCE-SD | ||
|---|---|---|---|
| moisture absorption/% | 0d | 0 | 0 |
| 5d | 4.93 | 4.45 | |
| 10d | 3.27 | 3.07 | |
| content/% | 0d | 50.17 | 99.36 |
| 5d | 50.13 | 100.15 | |
| 10d | 50.27 | 99.48 | |
| PCE | PCE-SD | ||
|---|---|---|---|
| content/% | 0d | 50.24 | 100.05 |
| 5d | 44.19 | 98.05 | |
| 10d | 36.36 | 99.52 | |
| PCE | PCE-SD | ||
|---|---|---|---|
| content/% | 0d | 50.35 | 99.92 |
| 5d | 50.04 | 99.83 | |
| 10d | 50.28 | 100.25 | |
| PCE | PCE-SD | ||
|---|---|---|---|
| content/% | 30d | 50.19 | 99.80 |
| 60d | 51.13 | 98.05 | |
| 90d | 50.44 | 99.59 | |
| Parameters | PCE | PCE-PM | PCE-SD |
|---|---|---|---|
| AUC0-t (μg/L·min) | 111,471.22 ± 11.4 | 146,598.478 ± 5.6 | 160,458.968 ± 15.7 |
| AUC0-∞ (μg/L·min) | 496,970.649 ± 36.3 | 548,742.124 ± 59.1 | 283,435.733 ± 39.7 |
| t1/2 z (min) | 375.809 ± 44.8 | 226.132 ± 70.2 | 252.522 ± 79.1 |
| Tmax (min) | 21 ± 39.1 | 21.22 ± 11.0 | 19 ± 60.9 |
| Cmax (μg/L) | 761.161 ± 25.8 | 831.46 ± 18.7 | 946.048 ± 8.1 |
| No. | Carrier | Plasticizer | Cooling Temperature | Mass Ratio | Barrel Temperature (°C) | Screw Speed (rpm) |
|---|---|---|---|---|---|---|
| 1 | EPO | none | −20 °C | 1:4 | 180 | 80 |
| 2 | PVP VA64 | none | −20 °C | 1:4 | 180 | 80 |
| 3 | Soluplus | none | −20 °C | 1:4 | 180 | 80 |
| 4 | HPMCAS | none | −20 °C | 1:4 | 180 | 80 |
| 5 | HPMCAS | P188 | −20 °C | 1:4 | 180 | 80 |
| 6 | HPMCAS | PEG6000 | −20 °C | 1:4 | 180 | 80 |
| 7 | HPMCAS | P188 | 25 °C | 1:4 | 180 | 80 |
| 8 | HPMCAS | P188 | −20 °C | 1:4 | 180 | 80 |
| 9 | HPMCAS | P188 | −196 °C | 1:4 | 180 | 80 |
| 10 | HPMCAS | P188 | −20 °C | 1:1 | 180 | 80 |
| 11 | HPMCAS | P188 | −20 °C | 1:2 | 180 | 80 |
| 12 | HPMCAS | P188 | −20 °C | 1:3 | 180 | 80 |
| 13 | HPMCAS | P188 | −20 °C | 1:4 | 180 | 80 |
| 14 | HPMCAS | P188 | −20 °C | 1:5 | 180 | 80 |
| 15 | HPMCAS | P188 | −20 °C | 1:2 | 140 | 80 |
| 16 | HPMCAS | P188 | −20 °C | 1:2 | 150 | 80 |
| 17 | HPMCAS | P188 | −20 °C | 1:2 | 160 | 80 |
| 18 | HPMCAS | P188 | −20 °C | 1:2 | 170 | 40 |
| 19 | HPMCAS | P188 | −20 °C | 1:2 | 180 | 60 |
| 20 | HPMCAS | P188 | −20 °C | 1:2 | 140 | 40 |
| 21 | HPMCAS | P188 | −20 °C | 1:2 | 140 | 60 |
| 22 | HPMCAS | P188 | −20 °C | 1:2 | 140 | 80 |
| 23 | HPMCAS | P188 | −20 °C | 1:2 | 140 | 100 |
| 24 | HPMCAS | P188 | −20 °C | 1:2 | 140 | 120 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, W.; Wu, J.; Gao, M.; Zhang, X.; Zhu, W. Preparation of Solid Dispersion of Polygonum Cuspidatum Extract by Hot Melt Extrusion to Enhance Oral Bioavailability of Resveratrol. Molecules 2023, 28, 737. https://doi.org/10.3390/molecules28020737
Fan W, Wu J, Gao M, Zhang X, Zhu W. Preparation of Solid Dispersion of Polygonum Cuspidatum Extract by Hot Melt Extrusion to Enhance Oral Bioavailability of Resveratrol. Molecules. 2023; 28(2):737. https://doi.org/10.3390/molecules28020737
Chicago/Turabian StyleFan, Wenling, Jiali Wu, Meiqi Gao, Xiaotong Zhang, and Wenjing Zhu. 2023. "Preparation of Solid Dispersion of Polygonum Cuspidatum Extract by Hot Melt Extrusion to Enhance Oral Bioavailability of Resveratrol" Molecules 28, no. 2: 737. https://doi.org/10.3390/molecules28020737
APA StyleFan, W., Wu, J., Gao, M., Zhang, X., & Zhu, W. (2023). Preparation of Solid Dispersion of Polygonum Cuspidatum Extract by Hot Melt Extrusion to Enhance Oral Bioavailability of Resveratrol. Molecules, 28(2), 737. https://doi.org/10.3390/molecules28020737