Effect of Storage Time and Bacterial Strain on the Quality of Probiotic Goat’s Milk Using Different Types and Doses of Collagens
Abstract
1. Introduction
2. Results and Discussion
2.1. Effect of Pasteurization on pH and Color of Milk with Collagen Addition
2.2. Acidity of Probiotic Goat’s Milk with Collagen
2.3. Syneresis of Probiotic Goat’s Milk with Collagen
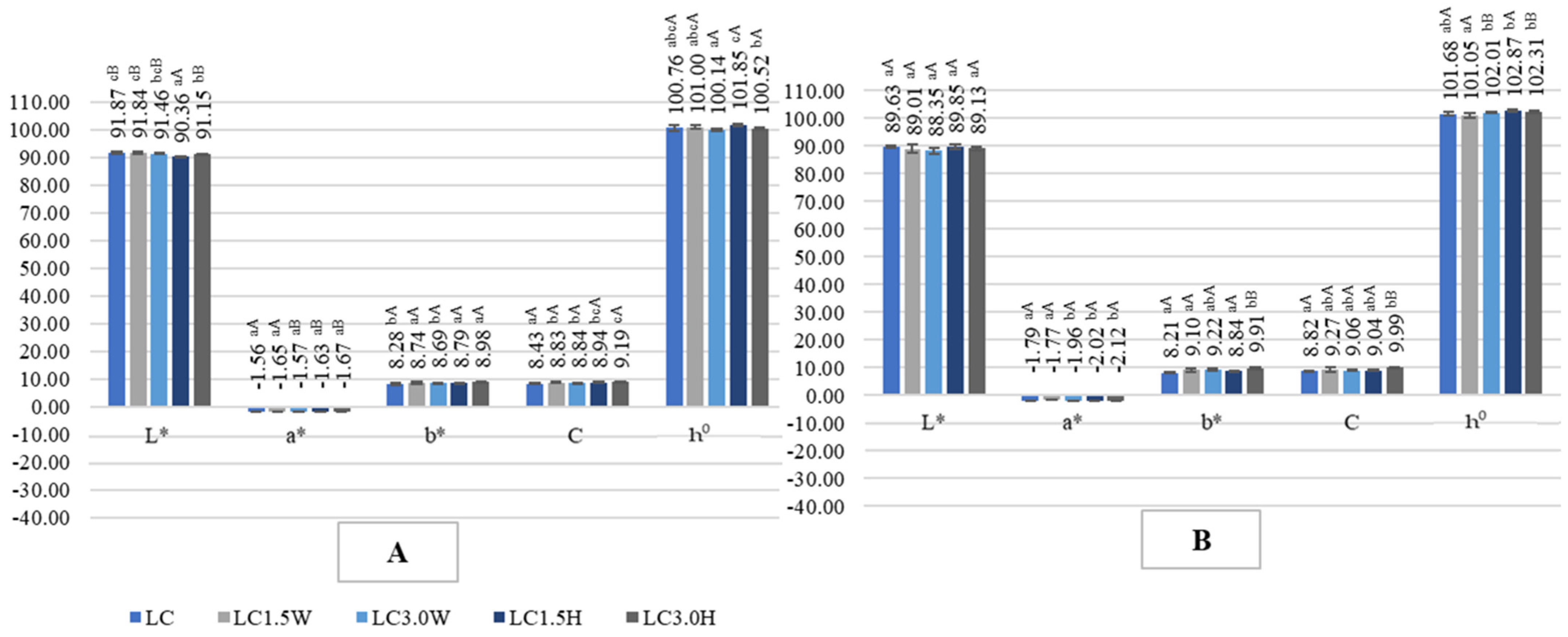
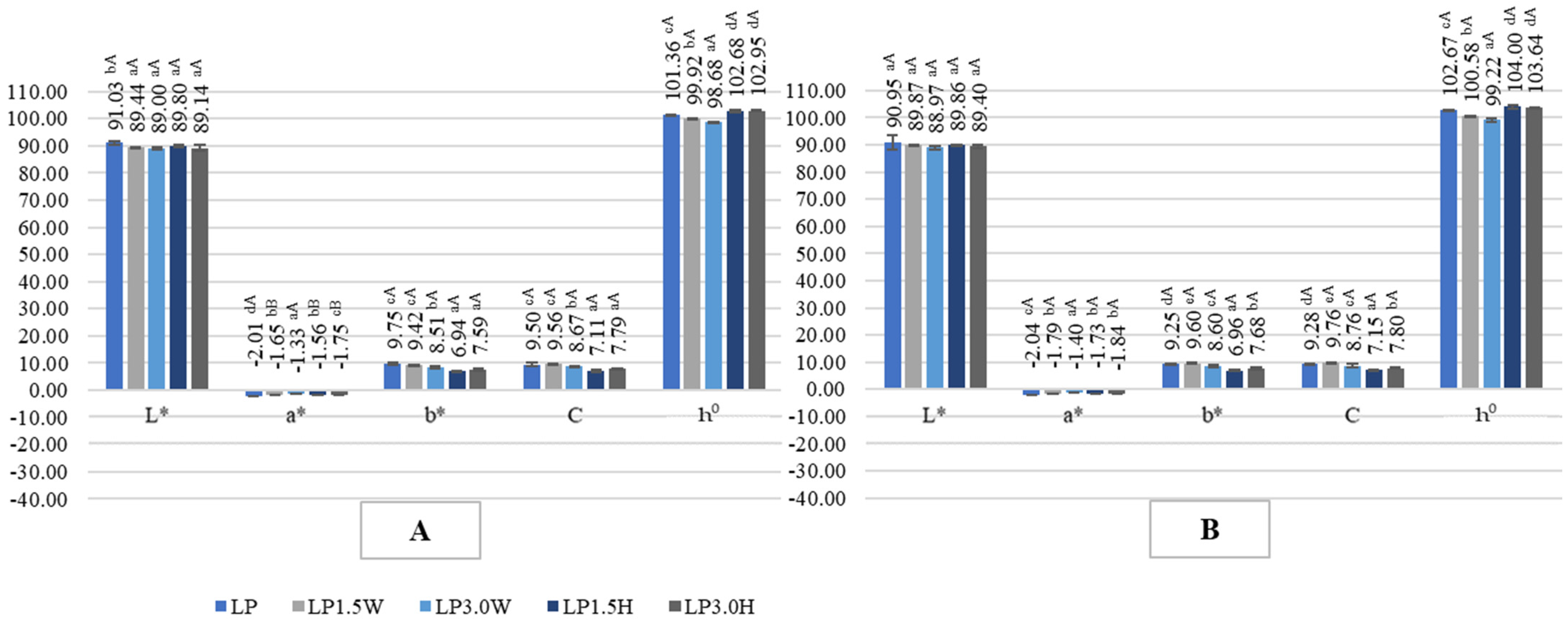
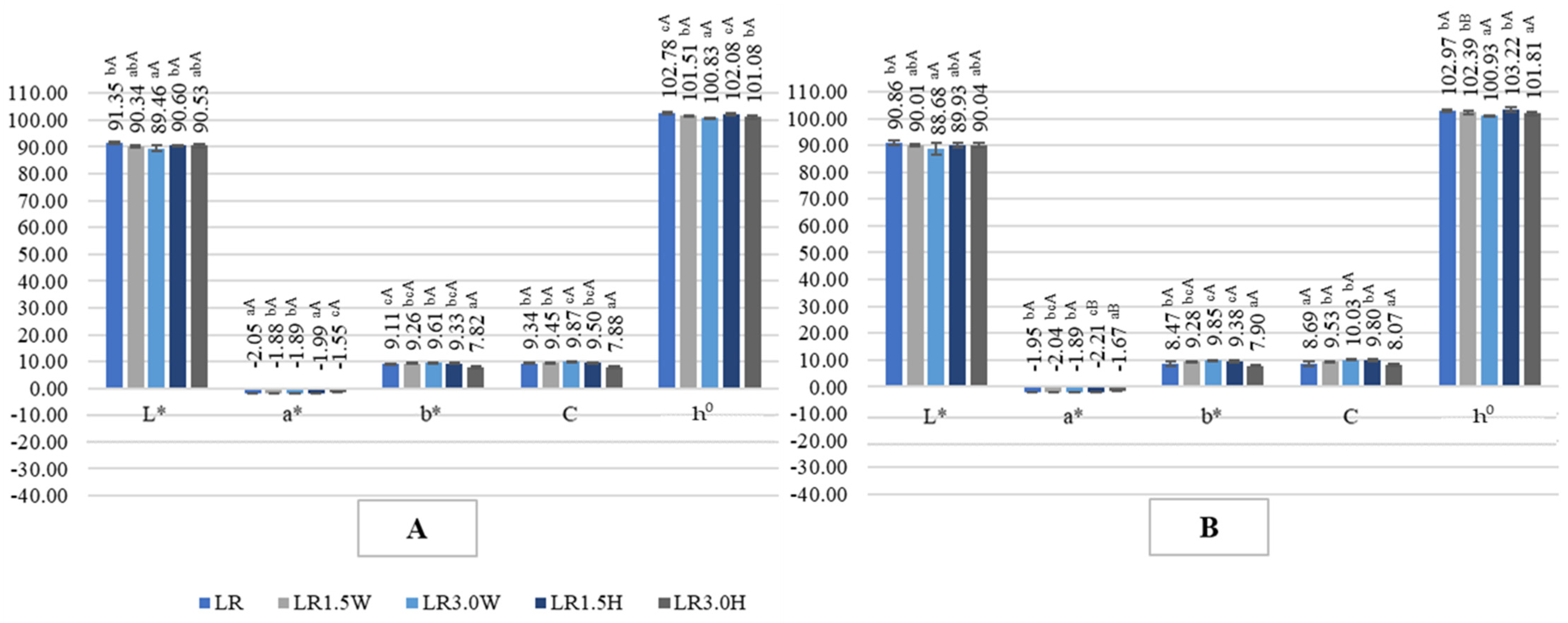
2.4. Color of Probiotic Goat’s Milk with Collagen
2.5. Texture Profile of Probiotic Goat’s Milk with Collagen
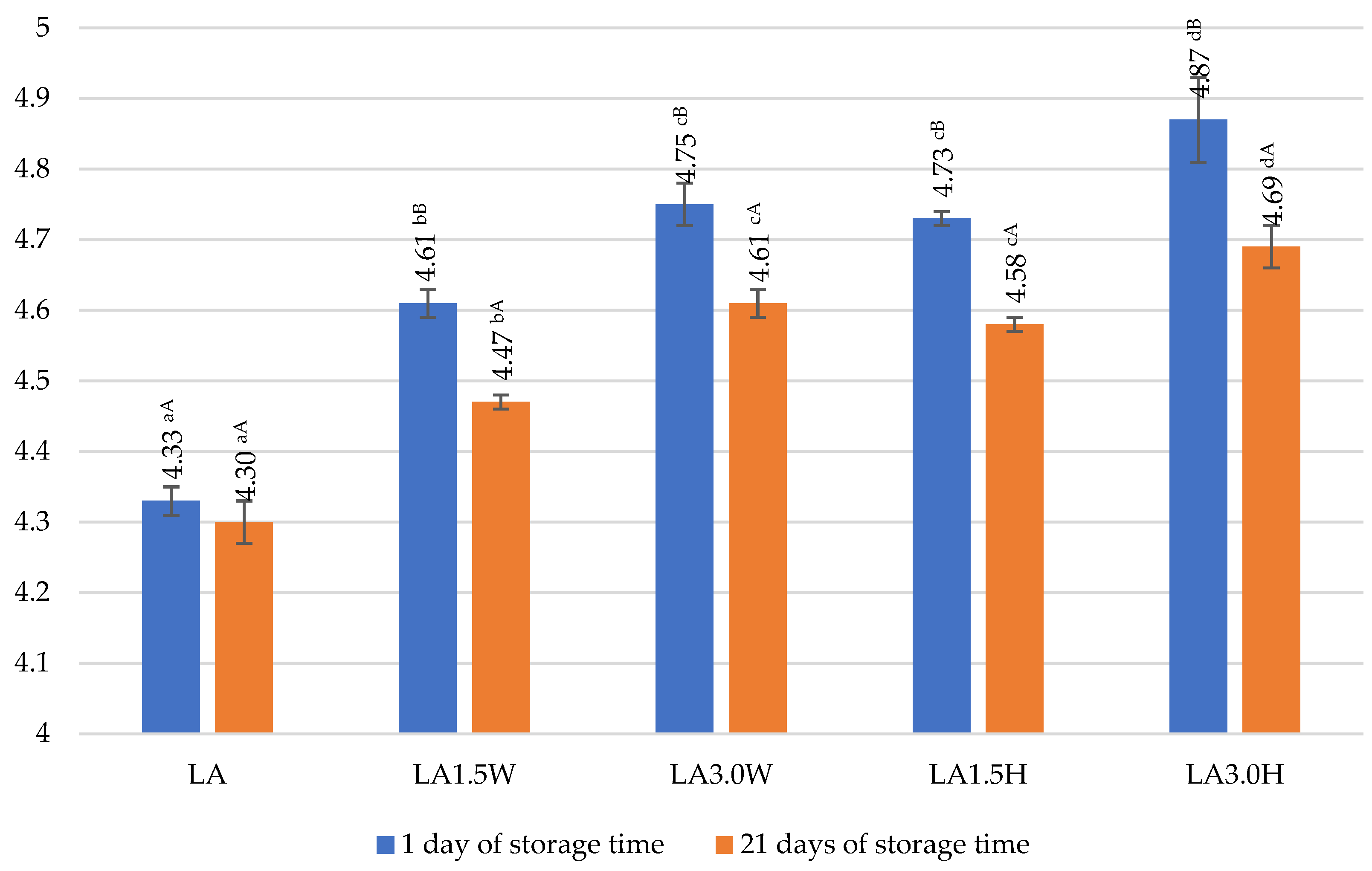
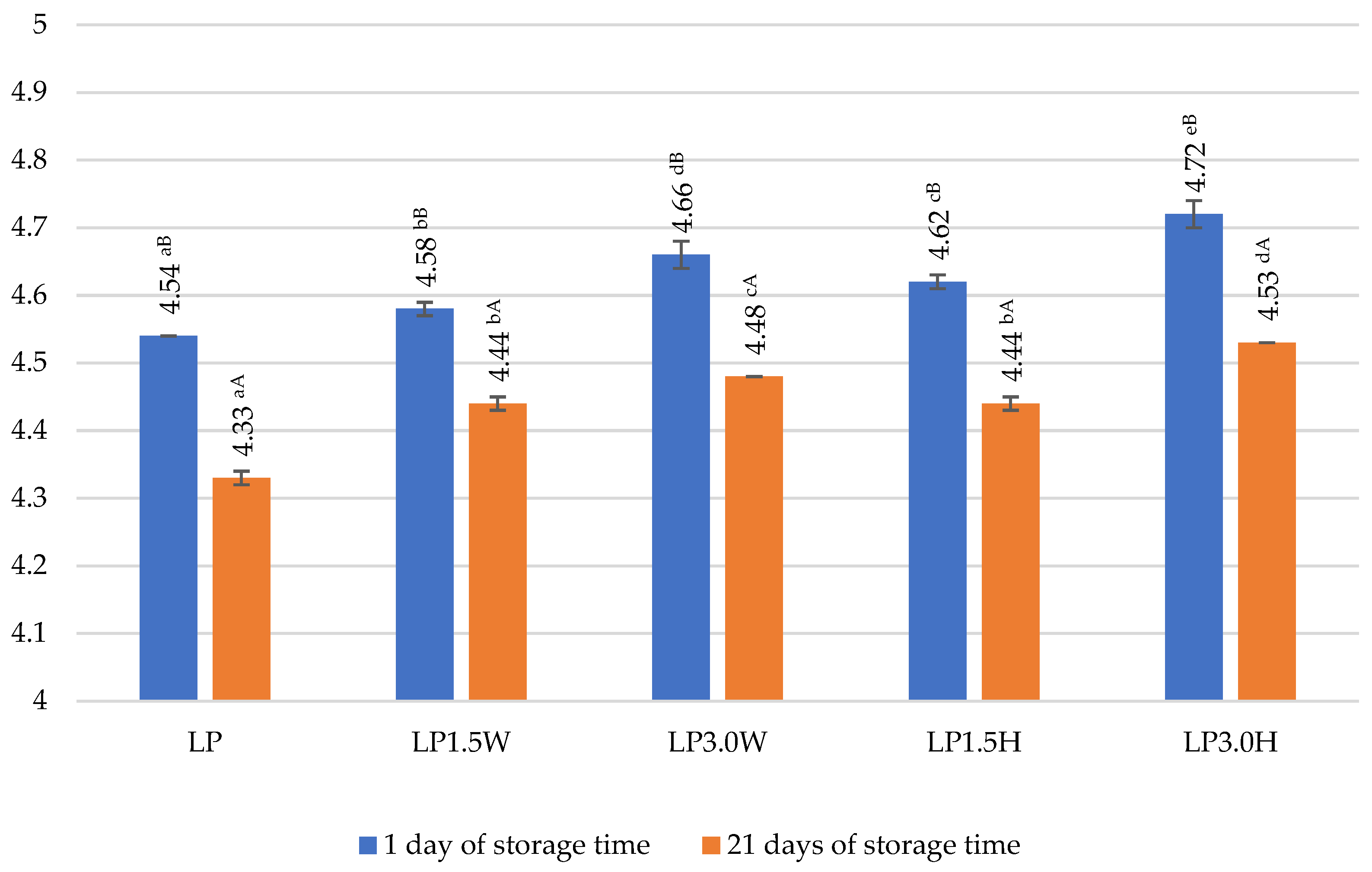
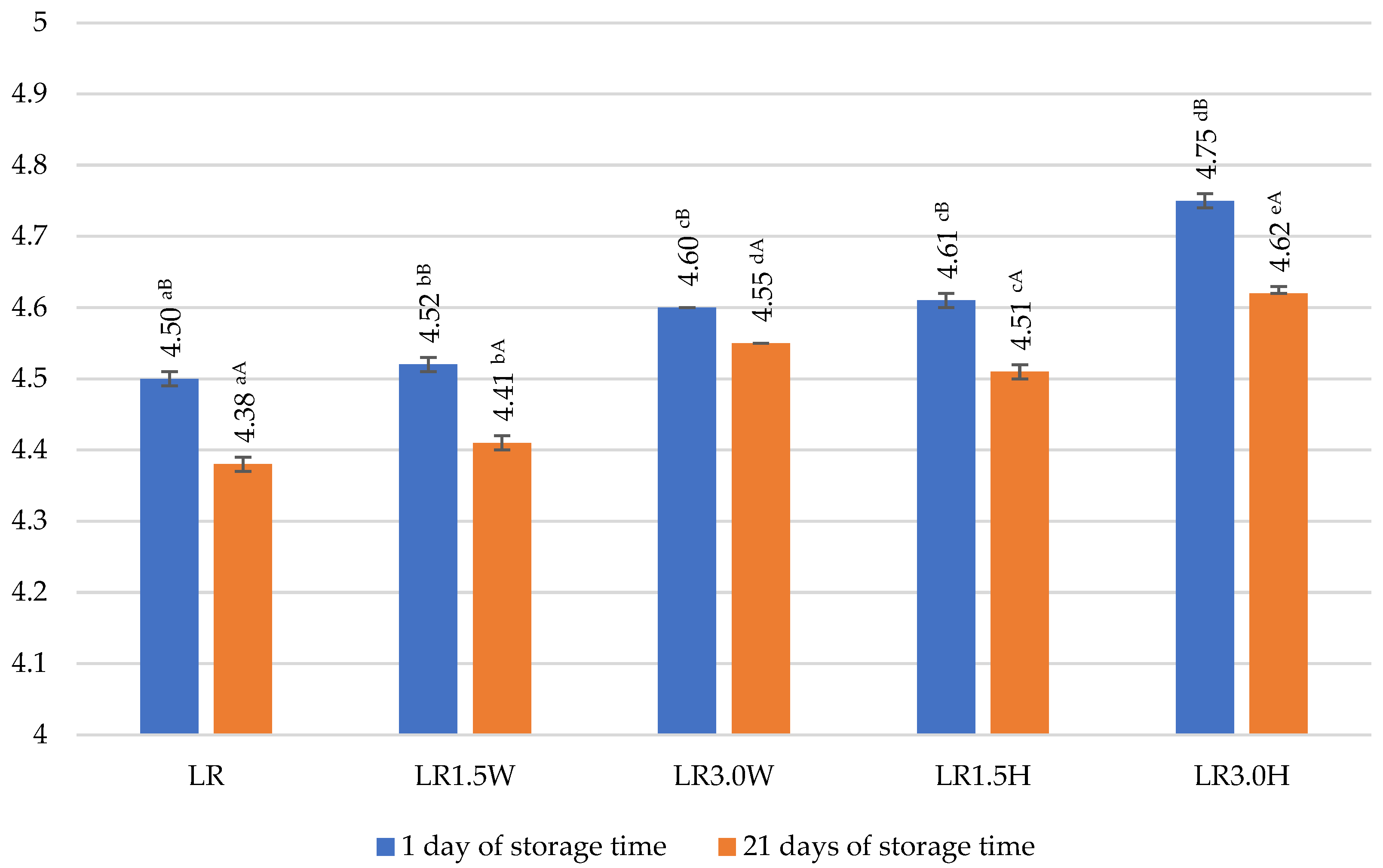
2.6. Viability of Probiotic Bacteria in Fermented Goat’s Milk with Collagen
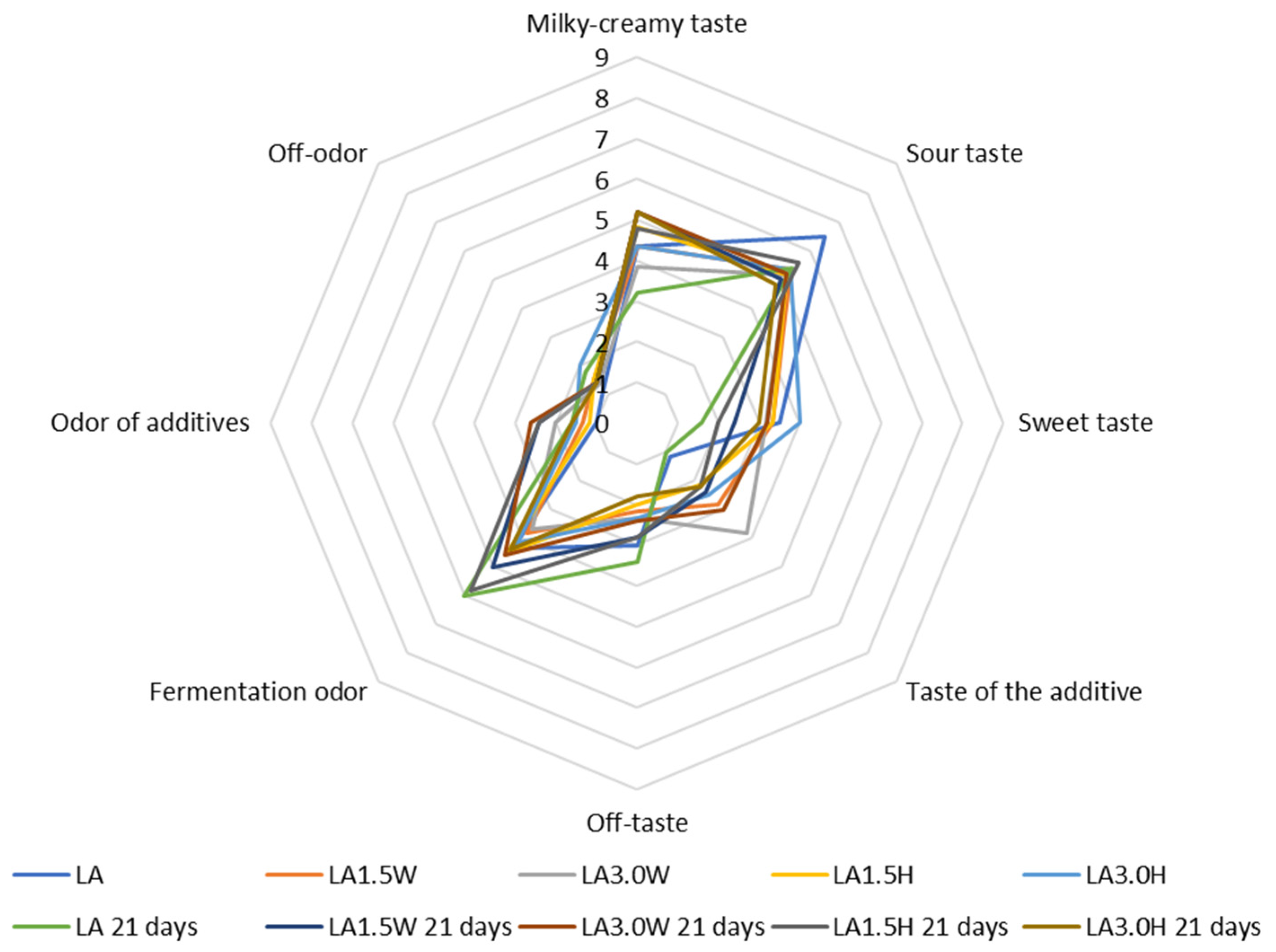
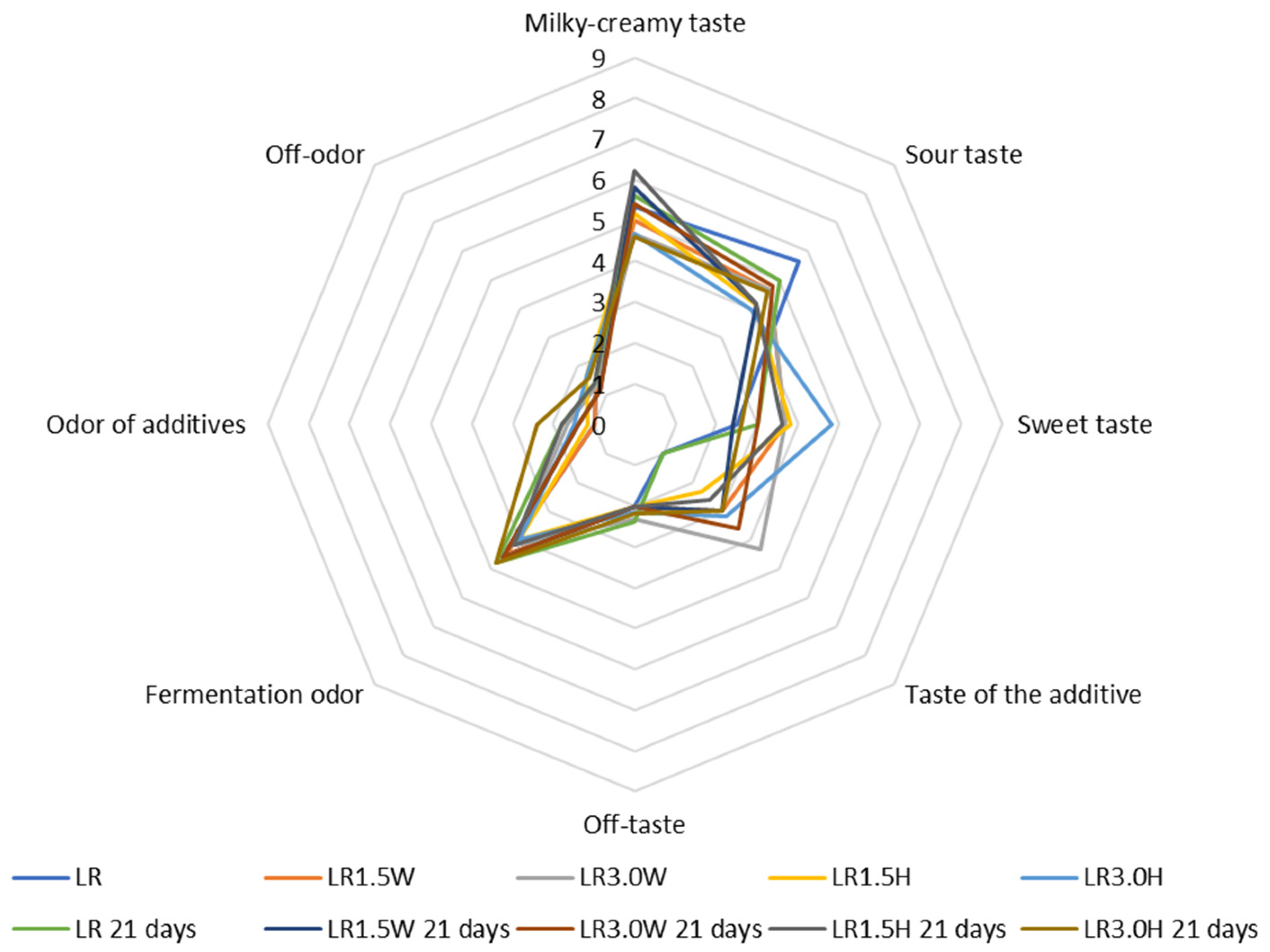
2.7. Organoleptic Evaluation of Probiotic Goat’s Milk with Collagen
3. Materials and Methods
3.1. Materials
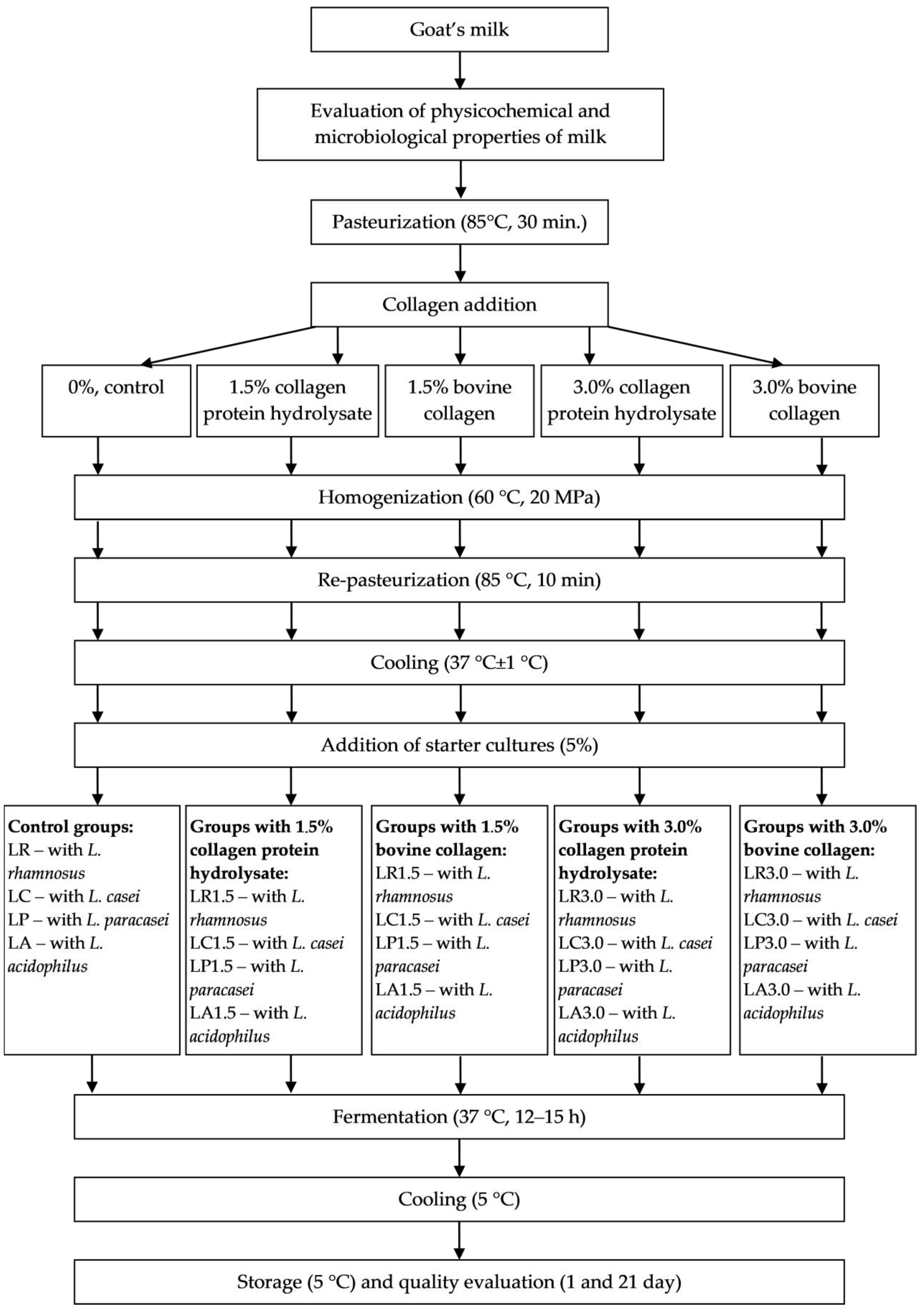
3.2. Fermented Milk Manufacture
3.3. Methods of Analyses
3.3.1. Determination of Freezing Point and Chemical Composition of Raw Goat’s Milk
3.3.2. Microbiological and Cytological Quality of Raw Goat’s Milk
3.3.3. Titratable Acidity Expressed as Lactic Acid Content and pH
3.3.4. Color Evaluation
3.3.5. Syneresis
3.3.6. Texture Profile Analysis
3.3.7. Microbiological Analysis
3.3.8. Organoleptic Evaluation
3.3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Szajnar, K.; Znamirowska, A.; Kuźniar, P. Sensory and textural properties of fermented milk with viability of Lactobacillus rhamnosus and Bifidobacterium animalis ssp. lactis Bb-12 and increased calcium concentration. Int. J. Food Prop. 2020, 23, 582–598. [Google Scholar] [CrossRef]
- Sun, Y.; Peng, C.; Wang, J.; Guo, S.; Sun, Z.H.; Zhang, H. Mesopic fermentation contributes more to the formation of important flavor compounds and increased growth of Lactobacillus casei Zhang than does high temperature during milk fermentation and storage. J. Dairy Sci. 2022, 105, 4857–4867. [Google Scholar] [CrossRef] [PubMed]
- Akdeniz, V.; Akalın, A.S. New approach for yoghurt and ice cream production: High-intensity ultrasound. Trends Food Sci. Technol. 2019, 86, 392–398. [Google Scholar] [CrossRef]
- Liszkowska, W.; Berlowska, J. Yeast fermentation at low temperatures: Adaptation to changing environmental conditions and formation of volatile compounds. Molecules 2021, 26, 1035. [Google Scholar] [CrossRef]
- Pawlos, M.; Znamirowska, A.; Kluz, M.; Szajnar, K.; Kowalczyk, M. Low-lactose fermented goat milks with Bifidobacterium animalis ssp. lactis BB-12. J. Microbiol. Biotechnol. Food Sci. 2020, 9, 751–755. [Google Scholar] [CrossRef]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimera, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef]
- Ranadheera, R.D.C.S.; Vidanarachchi, J.K.; Rocha, R.S.; Cruz, A.G.; Ajlouni, S. Probiotic Delivery through Fermentation: Dairy vs. Non-Dairy Beverages. Fermentation 2017, 3, 67. [Google Scholar] [CrossRef]
- Zommiti, M.; Feuilloley, M.G.J.; Connil, N. Update of Probiotics in Human World: A nonstop source of benefactions till the end 595 of time. Microorganisms 2020, 8, 1907. [Google Scholar] [CrossRef]
- Szopa, K.; Znamirowska-Piotrowska, A.; Szajnar, K.; Pawlos, M. Effect of Collagen Types, Bacterial Strains and Storage Duration on the Quality of Probiotic Fermented Sheep’s Milk. Molecules 2022, 27, 3028. [Google Scholar] [CrossRef]
- Znamirowska, A.; Szajnar, K.; Pawlos, M. Effect of Vitamin C Source on Its Stability during Storage and the Properties of Milk Fermented by Lactobacillus rhamnosus. Molecules 2021, 26, 6187. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Quinto, E.; Jiménez, P.; Caro, I.; Tejero, J.; Mateo, J.; Girbés, T. Probiotic Lactic Acid Bacteria: A Review. Food Nutr. Sci. 2014, 5, 1765–1775. [Google Scholar] [CrossRef]
- Szajnar, K.; Pawlos, M.; Znamirowska, A. The Effect of the Addition of Chokeberry Fiber on the Quality of Sheep’s Milk Fermented by Lactobacillus rhamnosus and Lact. acidophilus. Int. J. Food Sci. 2021, 2021, 7928745. [Google Scholar] [CrossRef] [PubMed]
- Currò, S.; De Marchi, M.; Claps, S.; Salzano, A.; De Palo, P.; Manuelian, C.L.; Neglia, G. Differences in the Detailed Milk Mineral Composition of Italian Local and Saanen Goat Breeds. Animals 2019, 9, 412. [Google Scholar] [CrossRef] [PubMed]
- Dimitrellou, D.; Salamoura, C.; Kontogianni, A.; Katsipi, D.; Kandylis, P.; Zakynthinos, G.; Varzakas, T. Effect of Milk Type on the Microbiological, Physicochemical and Sensory Characteristics of Probiotic Fermented Milk. Microorganisms 2019, 7, 274. [Google Scholar] [CrossRef] [PubMed]
- Znamirowska, A.; Szajnar, K.; Pawlos, M. Organic magnesium salts fortification in fermented goat’s milk. Int. J. Food Prop. 2019, 22, 1615–1625. [Google Scholar] [CrossRef]
- Kücükcetin, A.; Demir, M.; Asci, A.; Çomak, E. Graininess and roughness of stirred yoghurt made with goat’s, cow’s or a mixture of goat’s and cow’s milk. Small Rumin. Res. 2011, 96, 173–177. [Google Scholar] [CrossRef]
- Barac, M.; Pesic, M.; Zilic, S.; Smiljanic, M.; Sredovic Ignjatovic, I.; Vucic, T.; Kostic, A.; Milincic, D. The Influence of Milk Type on the Proteolysis and Antioxidant Capacity of White-Brined Cheese Manufactured from High-Heat-Treated Milk Pretreated with Chymosin. Foods. 2019, 8, 128. [Google Scholar] [CrossRef]
- Dzyuba, N.; Valevskaya, L.; Atanasova, V.; Sokolovskaya, A. Elaboration of the recipe of the fermented milk dessert for child food. Eureka Life Sci. 2017, 4, 3–9. [Google Scholar] [CrossRef]
- Ivanec, G.E.; Svetkina, E.A.; Potapov, A.N. Using of plant raw material for manufacture of aerated milk-based products. Food Process. Tech. Technol. 2012, 25, 1–7. [Google Scholar]
- Plekhanova, E.A.; Bannikova, A.V.; Ptichkina, N.M. Development of technology and compositions of milk desserts with dietary purposes. Food Process. Tech. Technol. 2013, 3, 53–57. [Google Scholar]
- Flechsenhar, K.; McAlindon, T. Change in Serum Biomarkers in Patients with Osteoarthritis treated with Collagen Hydrolysate: Results of a Prospective Randomized Study. J. Arthritis 2016, 5, 1–2. [Google Scholar] [CrossRef]
- Porfírio, E.; Fanaro, G. Collagen supplementation as a complementary therapy for the prevention and treatment of osteoporosis and osteoarthritis: A systematic review. Rev. Bras. Geriatr. Gerontol. 2016, 19, 153–164. [Google Scholar] [CrossRef]
- Ranadheera, C.S.; Evans, C.A.; Baines, S.K.; Balthazar, C.F.; Cruz, A.G.; Esmerino, E.A.; Freitas, M.Q.; Pimentel, T.C.; Wittwer, A.E.; Naumovski, N.; et al. Probiotics in goat milk products: Delivery capacity and ability to improve sensory attributes. Compr. Rev. Food Sci. Food Saf. 2019, 18, 867–882. [Google Scholar] [CrossRef]
- Mituniewicz-Małek, A.; Ziarno, M.; Dmytrów, I. Zastosowanie zamrażalniczo utrwalonego mleka koziego do wyrobu potencjalnie probiotycznego napoju fermentowanego [Application of frozen goat’s milk to production of potentially probiotic fermented drink]. Żywność. Nauka. Technol. Jakość 2015, 6, 140–149. (In Polish) [Google Scholar] [CrossRef]
- Jia, R.; Chen, H.; Chen, H.; Ding, W. Effects of fermentation with Lactobacillus rhamnosus GG on product quality and fatty acids of goat milk yogurt. Int. J. Dairy Sci. 2016, 99, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Muelas, R.; de Olives, A.M.; Romero, G.; Diaz, J.R.; Sayas, M.E.; Sendra, E. Evaluation of individual lactic acid bacteria for the fermentation of goat milk: Quality parameters. LWT Food Sci. Technol. 2018, 98, 506–514. [Google Scholar] [CrossRef]
- Ranadheera, C.S.; Naumovski, N.; Ajlouni, S. Non-bovine milk products as emerging probiotic carriers: Recent developments and innovations. Curr. Opin. Food Sci. 2018, 22, 109–114. [Google Scholar] [CrossRef]
- Mituniewicz–Małek, A.; Zielińska, D.; Ziarno, M. Probiotic monocultures in fermented goat milk beverages—Sensory quality of final product. Int. J. Dairy Technol. 2019, 72, 240–247. [Google Scholar] [CrossRef]
- Kędzierska-Matysek, M.; Barłowska, J.; Litwińczuk, Z.; Koperska, N. Content of macro- and microelements in goat milk in relation to the lactation stage and region of production. J. Elem. 2015, 20, 107–114. [Google Scholar] [CrossRef]
- Park, Y.W.; Juárez, M.; Ramos, M.; Haenlein, G.F.W. Physico-Chemical Characteristics of Goat and Sheep Milk. Small Rumin. Res. 2007, 68, 88–113. [Google Scholar]
- Zamberlin, Š.; Antunac, N.; Havranek, J.; Smarzija, D. Mineral elements in milk and dairy products. Mljekarstvo 2012, 62, 111–125. [Google Scholar]
- Gurbanov, N.H.; Gadimova, N.S.; Gurbanova, R.I.; Akhundova, N.A.; Babashli, A.A. Substantiation and development of technology for a new assortment of combined sour-milk drinks based on bio modified bean raw materials. Food Sci. Technol. 2020, 40, 517–522. [Google Scholar] [CrossRef]
- Maximo, G.J.; Cunha, R.L. Mechanical properties of collagen fiber and powder gels. J. Texture Stud. 2010, 41, 842–862. [Google Scholar] [CrossRef]
- Moraes, M.C.; Cunha, R.L. Gelation property and water holding capacity of heat-treated collagen at different temperature and pH values. Food Res. Int. 2013, 50, 213–223. [Google Scholar] [CrossRef]
- Serhan, M.; Mattar, J.; Debs, L. Concentrated yogurt (Labneh) made of a mixture of goats’ and cows’ milk: Physicochemical, microbiological and sensory analysis. Small Rumin. Res. 2016, 138, 46–52. [Google Scholar] [CrossRef]
- Danków, R.; Pikul, J. Przydatnośc technologiczna mleka koziego do przetwórstwa. Nauka Przyr. Technmologie 2011, 5, 1–15. [Google Scholar]
- Pavlović, H.; Hardi, J.; Slačanac, V.; Halt, M.; Kocevski, D. Inhibitory Effect of Goat and Cow Milk Fermented by Bifidobacterium longum on Serratia marcescens and Campylobacter jejuni. Czech J. Food Sci. 2006, 24, 164–171. [Google Scholar] [CrossRef]
- Goto, H. Fermented Milk Containing Collagen and Method for Producing the Same. J. Patent 6194304 B2, 6 September 2017. Available online: https://worldwide.espacenet.com/patent/search/family/049160699/publication/JP6194304B2?q=pn%3DJP6194304B2 and https://patents.google.com/patent/JP6194304B2/en (accessed on 2 January 2022).
- Znamirowska, A.; Szajnar, K.; Pawlos, M. Probiotic Fermented Milk with Collagen. Dairy 2020, 1, 126–134. [Google Scholar] [CrossRef]
- Kavaz, A.; Bakirci, I. Influence of inulin and demineralised whey powder addition on the organic acid profiles of probiotic yoghurts. Int. J. Dairy Technol. 2014, 67, 577–583. [Google Scholar] [CrossRef]
- Shori, A.B.; Baba, A.S.; Chuah, P.F. The effects of fish collagen on the proteolysis of milk proteins, ACE inhibitory activity and sensory evaluation of plain- and Allium sativum-yogurt. J. Taiwan Inst. Chem. Eng. 2013, 44, 701–706. [Google Scholar] [CrossRef]
- Shori, A.B.; Yong, Y.S.; Baba, A.S. Effects of herbal yogurt with fish collagen on bioactive peptides with angiotensin-I converting enzyme inhibitory activity. Ciênc. Tecnol. Aliment. 2020, 41, 902–907. [Google Scholar] [CrossRef]
- Gomes, J.J.L.; Duarte, A.M.; Batista, A.S.M.; De Figueirêdo, R.M.F.; De Sousa, E.P.; De Souza, E.L.; do Egypto, R.D.C.R. Physicochemical and sensory properties of fermented dairy beverages made with goat’s milk, cow’s milk and a mixture of the two milks. LWT 2013, 54, 18–24. [Google Scholar] [CrossRef]
- Khorshidi, M.; Heshmati, A.; Taheri, M.; Karami, M.; Mahjub, R. Effect of whey protein- and xanthan-based coating on the viability of microencapsulated Lactobacillus acidophilus and physiochemical, textural, and sensorial properties of yogurt. Food Sci. Nutr. 2021, 9, 3942–3953. [Google Scholar] [CrossRef]
- Dmytrów, I. Wpływ probiotycznych bakterii kwasu mlekowego na stabilność przechowalniczą kwasowych serów twarogowych [Effect of lactic acid probiotic bacteria on storage stability of acid curd cheeses (tvarog)]. Żywność. Nauka. Technol. Jakość 2015, 5, 49–60. (In Polish) [Google Scholar] [CrossRef]
- Gerhardt, Â.; Monteiro, B.W.; Gennari, A.; Lehn, D.N.; De Souza, C.F.V. Características físico-químicas e sensoriais de bebidas lácteas fermentadas utilizando soro de ricota e colágeno hidrolisado. Physicochemical and sensory characteristics of fermented dairy drink using ricotta cheese whey and hydrolyzed collagen. Rev. Inst. Laticinios Candido Tostes 2013, 68, 41–50. [Google Scholar]
- Miocinovic, J.; Miloradovic, Z.; Josipovic, M.; Nedeljkovic, A.; Radovanovic, M.; Pudja, P. Rheological and textural properties of goat and cow milk set type yoghurts. Int. Dairy J. 2016, 58, 43–45. [Google Scholar] [CrossRef]
- Ingham, B.; Smialowska, A.; Kirby, N.N.; Wang, C.; Carr, A.J. A structural comparison of casein micelles in cow, goat and sheep milk using X-ray scattering. Soft Matter 2018, 14, 3336–3343. [Google Scholar] [CrossRef]
- Nguyen, H.T.L.; Afsar, S.; Day, L. Differences in the microstructure and rheological properties of low-fat yoghurts from goat, sheep and cow milk. Food Res. Int. 2018, 108, 423–429. [Google Scholar] [CrossRef]
- Wang, Y.; Eastwood, B.; Yang, Z.; de Campo, L.; Knott, R.; Prosser, C.; Carpenter, E.; Hemar, Y. Rheological and structural characterization of acidified skim milks and infant formulae made from cow and goat milk. Food Hydrocoll. 2019, 96, 161–170. [Google Scholar] [CrossRef]
- Prosser, C.G. Compositional and functional characteristics of goat milk and relevance as a base for infant formula. J. Food Sci. 2021, 86, 257–265. [Google Scholar] [CrossRef]
- Da Mata Rigoto, J.; Ribeiro, T.H.S.; Stevanato, N.; Sampaio, A.R.; Ruiz, S.P.; Bolanho, B.C. Effect of açaí pulp, cheese whey, and hydrolysate collagen on the characteristics of dairy beverages containing probiotic bacteria. J. Food Process. Eng. 2019, 42, 12953. [Google Scholar] [CrossRef]
- Lasik, A.; Pikul, J. Production of fermented goat beverage using a mixed starter culture of lactic acid bacteria and yeasts. Eng. Life Sci. 2012, 12, 486–493. [Google Scholar] [CrossRef]
- Pawlos, M.; Znamirowska, A.; Szajnar, K. Effect of Calcium Compound Type and Dosage on the Properties of Acid Rennet Goat’s Milk Gels. Molecules 2021, 26, 5563. [Google Scholar] [CrossRef] [PubMed]
- Widodo, W.; Taufiq, T.T.; Anindita, N.S. Fermented goat milk and cow milk produced by different starters of lactic acid bacteria: Quality studies. J. Agric. Sci. Technol. A 2013, 3, 904–911. [Google Scholar]
- Gursel, A.; Gursoy, A.; Anli, E.A.K.; Budak, S.O.; Aydemir, S.; Durlu-Ozkaya, F. Role of milk protein–based products in some quality attributes of goat milk yogurt. J. Dairy Sci. 2016, 99, 2694–2703. [Google Scholar] [CrossRef]
- Pal, M.; Dudhrejiya, T.P.; Pinto, S.; Brahamani, D.; Vijayageetha, V.; Reddy, Y.K.; Kate, P. Goat milk products and their significance. Beverage Food World 2017, 44, 21–25. [Google Scholar]
- Prasanna, P.H.P.; Grandison, S.; Charalampopoulos, D. Effect of Dairy—Based Protein Sources and Temperature on Growth, Acidification and Exopolysaccharide Production of Bifidobacterium Strains in Skim Milk. Food Res. Int. 2012, 47, 6–12. [Google Scholar] [CrossRef]
- Karam, M.C.; Gaiani, C.; Hosri, C.; Burgain, J.; Scher, J. Effect of Dairy Powders Fortification on Yogurt Textural and Sensorial Properties: A Review. J. Dairy Res. 2013, 80, 400–409. [Google Scholar] [CrossRef]
- Temerbayeva, M.; Rebezov, M.; Okuskhanova, E.; Zinina, O.; Gorelik, O.; Vagapova, O.; Beginer, T.; Gritsenko, S.; Serikova, A.; Yessimbekov, Z. Development of Yoghurt from Combination of Goat and Cow Milk. Annu. Res. Rev. Biol. 2018, 23, 1–7. [Google Scholar] [CrossRef]
- Andic, S.; Boran, G.; Tuncturk, Y. Effects of carboxyl methyl cellulose and edible cow gelatin on physico-chemical, textural and sensory properties of yoghurt. Int. J. Agric. Biol. 2013, 15, 245–251. [Google Scholar]
- Akalın, A.S.; Unal, G.; Dinkci, N.; Hayaloglu, A.A. Microstructural, textural, and sensory characteristics of probiotic yogurts fortified with sodium calcium caseinate or whey protein concentrate. J. Dairy Sci. 2012, 95, 3617–3628. [Google Scholar] [CrossRef] [PubMed]
- Yerlikaya, O.; Ender, G.; Torunoglu, F.A.; Akbulut, N. Production of probiotic milk drink containing Lactobacillus acidophilus, Bifidobacterium animalis subsp. lactis and Lactobacillus casei. Agrofood Ind. 2013, 24, 49–52. [Google Scholar]
- Luo, Y.; Liu, X.; Pang, Z. Tribo-rheological properties of acid milk gels with different types of gelatin: Effect of concentration. J. Dairy Sci. 2019, 102, 7849–7862. [Google Scholar] [CrossRef]
- Pang, Z.; Deeth, H.; Yang, H.; Prakash, S.; Bansal, N. Evaluation of tilapia skin gelatin as a mammalian gelatin replacer in acid milk gels and low-fat stirred yogurt. J. Dairy Sci. 2017, 100, 3436–3447. [Google Scholar] [CrossRef]
- Mituniewicz-Małek, A.; Dmytrów, I.; Balejko, J.; Ziarno, M. Komercyjne kultury probiotyczne Lactobacillus sp. (Lb. paracasei, Lb. casei i Lb. acidophilus) w napojach fermentowanych z mleka koziego [Commercial probiotic Lactobacillus ssp. cultures (Lb. paracasei, Lb. casei and Lb. acidophilus) in fermented drinks made from goat’s milk]. Żywność. Nauka. Technol. Jakość 2013, 3, 99–110. (In Polish) [Google Scholar]
- Minervini, F.; Bilancia, M.T.; Siragusa, S.; Gobbetti, M.; Caponio, F. Fermented goats’ milk produced with selected multiple starters as a potentially functional food. Food Microbiol. 2009, 26, 559–564. [Google Scholar] [CrossRef]
- Kim, S.-I.; Kim, J.W.; Kim, K.T.; Kang, C.H. Survivability of Collagen-Peptide Microencapsulated Lactic Acid Bacteria during Storage and Simulated Gastrointestinal Conditions. Fermentation 2021, 7, 177. [Google Scholar] [CrossRef]
- Zhang, Y.; Meng, L.; Ai, M.; Qiao, Y.; Liu, G.; Fan, X.; Lv, X.; Feng, Z. Nutrient requirements of Lactobacillus casei Shirota and their application in fermented milk. LWT—Food Sci. Technol. 2020, 118, 108735. [Google Scholar] [CrossRef]
- Morita, H.; Toh, H.; Oshima, K.; Murakami, M.; Taylor, T.D.; Igimi, S.; Hattori, M. Complete genome sequence of the probiotic Lactobacillus rhamnosus ATCC 53103. J. Bacteriol. 2009, 191, 7630–7631. [Google Scholar] [CrossRef]
- Sun, J.H.; Chen, H.Y.; Qiao, Y.L.; Liu, G.F.; Leng, C.; Zhang, Y.J. The nutrient requirements of Lactobacillus rhamnosus GG and their application to fermented milk. J. Dairy Sci. 2019, 102, 5971–5978. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Li, S.; Liu, G.; Fan, X.; Qiao, Y.; Zhang, A.; Lin, Y.; Zhao, X.; Huang, K.; Feng, Z. The nutrient requirements of Lactobacillus acidophilus LA-5 and their application to fermented milk. J Dairy Sci. 2021, 104, 138–150. [Google Scholar] [CrossRef] [PubMed]
- Minelli, E.B.; Benini, A. Relationship between number of bacteria and their probiotic effects. Microb. Ecol. Health Dis. 2008, 20, 180–183. [Google Scholar] [CrossRef]
- Granato, D.; Branco, G.F.; Cruz, A.G.; Faria, J.A.F.; Shah, N.P. Probiotic dairy products as functional foods. Compr. Rev. Food Sci. Food Saf. 2010, 9, 455–470. [Google Scholar] [CrossRef] [PubMed]
- Paskaš, S.; Miŏcinovíc, J.; Lopičíc-Vasíc, T.; Mugoša, I.; Pajíc, M.; Becskei, Z. Consumer attitudes towards goat milk and goat milkproducts in Vojvodina. Mljekarstvo 2020, 70, 171–183. [Google Scholar] [CrossRef]
- Voloshyna, I.M.; Soloshenko, K.I.; Lych, I.V.; Shkotova, L.V. Practical use of goat milk and colostrum. Biotechnol. Acta 2021, 14, 38–48. [Google Scholar] [CrossRef]
- Gao, J.; Li, X.; Zhang, G.; Sadiq, F.A.; Simal-Gandara, J.; Xiao, J.; Sang, Y. Probiotics in the dairy industry-Advances and opportunities. Compr. Rev. Food Sci. Food Saf. 2021, 20, 3937–3982. [Google Scholar] [CrossRef]
- Aryana, K.J.; Olson, D.W. A 100-Year Review: Yogurt and other cultured dairy products. J. Dairy Sci. 2017, 100, 9987–10013. [Google Scholar] [CrossRef]
- Ramasubramanian, L.; Webb, R.; Arcy, R.B.; Deeth, H. Characteristic of calcium-milk coagulum. J. Food Eng. 2013, 114, 147–152. [Google Scholar] [CrossRef]
- Commission Regulation (EC) No 1662/2006 of 6 November 2006 amending Regulation (EC) No 853/2004 of the European Parliament and of the Council laying down specific hygiene rules for food of animal origin (Text with EEA relevance). Off. J. Eur. Union 2006, L320, 1–10. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=CELEX:32006R1662&from=EN (accessed on 20 June 2022).
- Ratu, R.N.; Usturoi, M.G.; Avarvarei, B.V. Quality of Raw Cow Milk Utilised in Cheese Processing. Sci. Pap. Anim. Sci. Ser. Lucr. Stiintifice Ser. Zooteh. 2015, 63, 128–130. [Google Scholar]
- Jemaa, M.B.; Falleh, H.; Neves, M.A.; Isoda, H.; Nakajima, M.; Ksouri, R. Quality preservation of deliberately contaminated milk using thyme free and nanoemulsified essential oils. Food Chem. 2017, 217, 726–734. [Google Scholar] [CrossRef] [PubMed]
- Szajnar, K.; Znamirowska, A.; Kalicka, D.; Kuźniar, P. Fortification of yoghurts with various magnesium compounds. J. Elem. 2017, 22, 559–568. [Google Scholar] [CrossRef]
- Kowalczyk, M.; Znamirowska, A.; Buniowska, M. Probiotic Sheep Milk Ice Cream with Inulin and Apple Fiber. Foods 2021, 10, 678. [Google Scholar] [CrossRef] [PubMed]
- Lima, K.G.D.; Kruger, M.F.; Behrens, J.; Destro, M.T.; Landgraf, M.; Franco, B.D.G.M. Evaluation of culture media for enumeration of Lactobacillus acidophilus, Lactobacillus casei and Bifidobacterium animalis in the presence of Lactobacillus delbrueckii subsp. bulgaricus and Streptococcus thermophilus. LWT Food Sci. Technol. 2009, 42, 491–495. [Google Scholar] [CrossRef]
- Santillan-Urquiza, E.; Mendez-Rojas, M.; Valez-Ruiz, J.F. Fortification of yogurt with nano and micro sized calcium, iron and zinc, effect on the physicochemical and rheological properties. LWT Food Sci. Technol. 2017, 80, 462–469. [Google Scholar] [CrossRef]
- PN-ISO 22935-2:2013-07; In Milk and Milk Products—Sensory Analysis—Part. 2: Recommended Methods for Sensory Evaluation. Polish Committee for Standardization: Warsaw, Poland, 2013. (In Polish)




| Properties | Mean ± SD 1 | |
|---|---|---|
| Total solids, g 100 g−1 | 11.45 ± 1.46 | |
| Protein, g 100 g−1 | 2.64 ± 0.27 | |
| Fat, g 100 g−1 | 3.43 ± 0.66 | |
| Lactose, g 100 g−1 | 4.35 ± 0.52 | |
| Density, g mL−1 | 1.027 ± 0.003 | |
| Freezing point, °C | −0.603 ± 0.06 | |
| pH | 6.88 ± 0.03 | |
| Color | L* | 89.88 ± 1.05 |
| a* | −1.95 ± 0.22 | |
| b* | 8.13 ± 1.01 | |
| C | 8.21 ± 1.03 | |
| h0 | 97.87 ± 1.61 | |
| TBC 2, log CFU mL−1 | 6.56 ± 0.21 | |
| SCC 3, log cells mL−1 | 5.77 ± 0.41 | |
| Properties | C | L1.5W | L3.0W | L1.5H | L3.0H | |
|---|---|---|---|---|---|---|
| pH | 6.89 d ± 0.07 | 6.64 b ± 0.11 | 6.51 a ± 0.10 | 6.77 c ± 0.04 | 6.67 b ± 0.04 | |
| Color | L* | 89.49 a ± 0.85 | 87.32 a ± 2.03 | 87.90 a ± 1.66 | 89.15 a ± 0.17 | 88.07 a ± 1.60 |
| a* | −1.94 a ± 0.22 | −1.84 a ± 0.03 | −1.61 a ± 0.31 | −1.52 a ± 0.47 | −1.65 a ± 0.24 | |
| b* | 8.79 a ± 0.29 | 8.76 a ± 0.42 | 7.60 a ± 1.31 | 7.43 a ± 1.63 | 7.25 a ± 1.24 | |
| C | 9.01 a ± 0.33 | 8.90 a ± 0.40 | 7.77 a ± 1.33 | 7.59 a ± 1.69 | 7.42 a ± 1.27 | |
| h0 | 102.43 a ± 1.00 | 101.96 a ± 0.42 | 101.89 a ± 1.28 | 101.38 a ± 1.62 | 102.82 a ± 0.81 | |
| Properties | Storage Time (Days) | LC | LC1.5W | LC3.0W | LC1.5H | LC3.0H |
|---|---|---|---|---|---|---|
| Lactic acid, g L−1 | 1 | 0.99 aA ± 0.00 | 1.03 bA ± 0.00 | 1.14 dA ± 0.00 | 1.03 bA ± 0.00 | 1.06 cA ± 0.01 |
| 21 | 1.22 aB ± 0.01 | 1.26 bB ± 0.01 | 1.34 dB ± 0.01 | 1.30 cB ± 0.01 | 1.35 dB ± 0.01 | |
| Syneresis, % | 1 | 46.99 aA ± 2.06 | 50.28 aB ± 1.93 | 48.45 aB ± 2.37 | 48.00 aB ± 1.94 | 46.20 aB ± 2.41 |
| 21 | 47.09 abA ± 0.50 | 46.32 abA ± 1.18 | 45.30 bA ± 0.99 | 45.54 abA ± 0.69 | 43.31 aA ± 0.29 | |
| Hardness, N | 1 | 0.59 abA ± 0.04 | 0.55 aA ± 0.01 | 0.54 aA ± 0.02 | 0.60 abA ± 0.05 | 0.63 bA ± 0.02 |
| 21 | 0.57 aA ± 0.03 | 0.57 aA ± 0.02 | 0.61 abA ± 0.11 | 0.60 abA ± 0.03 | 0.65 bA ± 0.01 | |
| Cohesiveness | 1 | 0.67 aA ± 0.03 | 0.66 aA ± 0.03 | 0.69 aA ± 0.04 | 0.69 aA ± 0.01 | 0.65 aA ± 0.01 |
| 21 | 0.68 aA ± 0.03 | 0.65 aA ± 0.06 | 0.72 bA ± 0.02 | 0.67 abA ± 0.04 | 0.68 abA ± 0.03 | |
| Springiness, mm | 1 | 13.62 aA ± 0.26 | 13.55 aA ± 0.52 | 13.15 aA ± 0.50 | 13.45 aA ± 0.26 | 13.50 aA ± 0.33 |
| 21 | 13.53 aA ± 0.52 | 13.35 aA ± 0.80 | 13.88 aA ± 0.18 | 13.49 aA ± 0.60 | 13.80 aA ± 0.23 |
| Properties | Storage Time (Days) | LA | LA1.5W | LA3.0W | LA1.5H | LA3.0 H |
|---|---|---|---|---|---|---|
| Lactic acid, g L−1 | 1 | 1.05 cA ± 0.01 | 1.05 cA ± 0.00 | 1.07 dA ± 0.01 | 1.02 bB ± 0.01 | 0.99 aA ± 0.01 |
| 21 | 1.08 bB ± 0.01 | 1.11 cB ± 0.01 | 1.09 bB ± 0.00 | 0.97 aA ± 0.00 | 0.99 aA ± 0.00 | |
| Syneresis, % | 1 | 56.55 bcA ± 1.54 | 51.97 aB ± 0.74 | 59.55 cB ± 1.45 | 58.82 cA ± 2.74 | 54.13 abA ± 0.81 |
| 21 | 58.01 cA ± 0.66 | 47.46 aA ± 0.57 | 55.46 bA ± 0.63 | 59.34 cA ± 0.98 | 55.45 bA ± 1.37 | |
| Hardness, N | 1 | 0.43 aA ± 0.02 | 0.45 aA ± 0.02 | 0.45 aA ± 0.01 | 0.44 aA ± 0.02 | 0.45 aA ± 0.04 |
| 21 | 0.43 aA ± 0.02 | 0.45 aA ± 0.02 | 0.45 aA ± 0.02 | 0.46 aA ± 0.02 | 0.46 aA ± 0.01 | |
| Cohesiveness | 1 | 0.76 aA ± 0.01 | 0.68 aA ± 0.02 | 0.72 aA ± 0.07 | 0.60 aA ± 0.23 | 0.72 aA ± 0.25 |
| 21 | 0.76 aA ± 0.02 | 0.66 aA ± 0.05 | 0.68 aA ± 0.03 | 0.67 aA ± 0.04 | 0.76 aA ± 0.04 | |
| Springiness, mm | 1 | 13.59 aA ± 0.68 | 13.82 aA ± 0.51 | 13.85 aA ± 1.31 | 13.70 aA ± 1.00 | 13.83 aA ± 0.19 |
| 21 | 13.69 aA ± 0.32 | 13.49 aA ± 1.05 | 13.27 aA ± 0.73 | 13.74 aA ± 0.27 | 13.74 aA ± 0.61 |
| Properties | Storage Time (Days) | LP | LP1.5W | LP3.0W | LP1.5H | LP3.0H |
|---|---|---|---|---|---|---|
| Lactic acid, g L−1 | 1 | 0.89 aA ± 0.00 | 0.98 bA ± 0.01 | 0.98 bA ± 0.00 | 0.89 aA ± 0.00 | 0.97 bA ± 0.01 |
| 21 | 0.98 cB ± 0.03 | 1.07 bB ± 0.01 | 1.12 aB ± 0.01 | 1.01 bcB ± 0.00 | 1.09 aB ± 0.01 | |
| Syneresis, % | 1 | 45.60 aA ± 1.65 | 45.57 aA ± 0.48 | 51.74 bA ± 1.78 | 57.25 cA ± 1.48 | 54.33 bcA ± 0.92 |
| 21 | 45.52 aA ± 1.91 | 44.94 aA ± 1.10 | 49.65 bA ± 1.25 | 57.21 dA ± 0.01 | 55.05 cA ± 1.39 | |
| Hardness, N | 1 | 0.52 aA ± 0.02 | 0.53 aA ± 0.03 | 0.52 aA ± 0.04 | 0.55 aA ± 0.03 | 0.59 bA ± 0.04 |
| 21 | 0.53 aA ± 0.02 | 0.53 aA ± 0.01 | 0.56 aA ± 0.01 | 0.55 aA ± 0.02 | 0.59 bA ± 0.02 | |
| Cohesiveness | 1 | 0.58 aA ± 0.06 | 0.56 aA ± 0.02 | 0.68 bA ± 0.02 | 0.62 abA ± 0.03 | 0.67 bA ± 0.02 |
| 21 | 0.63 bA ± 0.01 | 0.58 aA ± 0.02 | 0.70 cA ± 0.01 | 0.60 aA ± 0.04 | 0.64 bA ± 0.04 | |
| Springiness, mm | 1 | 13.27 aA ± 0.37 | 13.61 aA ± 0.17 | 13.41 aA ± 0.23 | 13.21 aA ± 0.55 | 13.41 aA ± 0.22 |
| 21 | 13.78 aA ± 0.21 | 13.56 aA ± 0.52 | 13.50 aA ± 0.26 | 13.34 aA ± 0.49 | 13.48 aA ± 0.13 |
| Properties | Storage Time (Days) | LR | LR1.5W | LR3.0W | LR1.5H | LR3.0H |
|---|---|---|---|---|---|---|
| Lactic acid, g L−1 | 1 | 0.90 aA ± 0.00 | 1.05 dA ± 0.01 | 1.05 dA ± 0.00 | 0.94 bA ± 0.01 | 0.98 cA ± 0.01 |
| 21 | 0.94 aB ± 0.00 | 1.11 dB ± 0.01 | 1.11 dB ± 0.01 | 1.02 bB ± 0.01 | 1.06 cB ± 0.01 | |
| Syneresis, % | 1 | 52.26 bA ± 0.92 | 47.26 aA ± 0.19 | 47.45 aA ± 0.89 | 49.68 aA ± 1.49 | 52.06 bA ± 0.45 |
| 21 | 55.18 bB ± 0.95 | 50.33 aB ± 0.75 | 51.98 aB ± 0.63 | 53.57 bB ± 0.61 | 53.89 bB ± 0.36 | |
| Hardness, N | 1 | 0.61 aA ± 0.02 | 0.62 aA ± 0.01 | 0.62 aA ± 0.02 | 0.64 aA ± 0.06 | 0.68 bA ± 0.07 |
| 21 | 0.63 aA ± 0.03 | 0.62 aA ± 0.03 | 0.59 aA ± 0.01 | 0.60 aA ± 0.03 | 0.64 aA ± 0.02 | |
| Cohesiveness | 1 | 0.67 aA ± 0.01 | 0.66 aB ± 0.01 | 0.68 aA ± 0.02 | 0.65 aA ± 0.02 | 0.70 aA ± 0.07 |
| 21 | 0.65 bA ± 0.01 | 0.61 aA ± 0.02 | 0.66 bcA ± 0.01 | 0.63 aA ± 0.03 | 0.69 cA ± 0.02 | |
| Springiness, mm | 1 | 13.54 aA ± 0.30 | 13.69 aA ± 0.32 | 13.58 aA ± 0.33 | 13.41 aA ± 0.22 | 14.27 aA ± 0.49 |
| 21 | 13.62 aA ± 0.20 | 13.34 aA ± 0.19 | 13.63 aA ± 0.40 | 13.61 aA ± 0.43 | 13.72 aA ± 0.29 |
| Fermented Milk Group | Storage Time (Days) | The Survival Rate of Probiotic Bacteria (%) | |
|---|---|---|---|
| 1 | 21 | ||
| LR | 8.73 aA ± 0.43 | 8.67 aA ± 0.29 | 99.31 |
| LR1.5W | 9.31 bA ± 0.72 | 8.82 aA ± 0.35 | 94.75 |
| LR3.0W | 9.28 bA ± 0.04 | 8.64 aA ± 0.19 | 93.10 |
| LR1.5H | 8.90 aA ± 0.40 | 8.88 aA ± 0.71 | 99.78 |
| LR3.0H | 9.17 abA ± 0.11 | 8.66 aA ± 0.07 | 94.44 |
| LP | 8.93 aA ± 0.36 | 8.83 aA ± 0.11 | 98.88 |
| LP1.5W | 9.15 aA ± 0.66 | 8.85 aA ± 0.22 | 96.72 |
| LP3.0W | 8.99 aA ± 0.71 | 8.93 aA ± 0.29 | 99.33 |
| LP1.5H | 8.97 aA ± 0.45 | 8.85 aA ± 0.20 | 98.66 |
| LP3.0H | 9.18 aA ± 0.12 | 8.93 aA ± 0.65 | 97.28 |
| LA | 8.97 aB ± 0.18 | 8.24 aA ± 0.32 | 91.86 |
| LA1.5W | 8.89 aA ± 0.38 | 8.58 bA ± 0.11 | 96.51 |
| LA3.0W | 8.83 aA ± 0.50 | 8.68 abA ± 0.74 | 98.30 |
| LA1.5H | 8.96 aA ± 0.22 | 8.41 abA ± 0.86 | 93.86 |
| LA3.0H | 8.91 aA ± 0.30 | 8.63 bA ± 0.17 | 96.86 |
| LC | 9.24 aB ± 0.29 | 8.29 aA ± 0.12 | 89.72 |
| LC1.5W | 9.29 aB ± 0.13 | 8.36 aA ± 0.58 | 89.99 |
| LC3.0W | 9.05 aB ± 0.65 | 8.54 abA ± 0.12 | 94.37 |
| LC1.5H | 8.98 aB ± 0.12 | 8.68 bA ± 0.11 | 96.66 |
| LC3.0H | 9.01 aB ± 0.30 | 9.34 bA ± 0.27 | 103.66 |
| Attribute | Definition |
|---|---|
| Milky-creamy taste | taste stimulated by milk powder |
| Sour taste | taste stimulated by lactic acid |
| Taste of additives | taste stimulated by added collagen depending on the collagen type |
| Sweet taste | taste stimulated by sucrose |
| Off-taste | the occurrence of an atypical taste similar to meat broth |
| Fermentation odor | the intensity of odor associated with sour milk, i.e., lactic acid |
| Odor of additives | odor characteristic stimulated by added collagen depending on the collagen type |
| Off-odor | the occurrence of an atypical odor similar to meat broth |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szopa, K.; Pawlos, M.; Znamirowska-Piotrowska, A. Effect of Storage Time and Bacterial Strain on the Quality of Probiotic Goat’s Milk Using Different Types and Doses of Collagens. Molecules 2023, 28, 657. https://doi.org/10.3390/molecules28020657
Szopa K, Pawlos M, Znamirowska-Piotrowska A. Effect of Storage Time and Bacterial Strain on the Quality of Probiotic Goat’s Milk Using Different Types and Doses of Collagens. Molecules. 2023; 28(2):657. https://doi.org/10.3390/molecules28020657
Chicago/Turabian StyleSzopa, Kamil, Małgorzata Pawlos, and Agata Znamirowska-Piotrowska. 2023. "Effect of Storage Time and Bacterial Strain on the Quality of Probiotic Goat’s Milk Using Different Types and Doses of Collagens" Molecules 28, no. 2: 657. https://doi.org/10.3390/molecules28020657
APA StyleSzopa, K., Pawlos, M., & Znamirowska-Piotrowska, A. (2023). Effect of Storage Time and Bacterial Strain on the Quality of Probiotic Goat’s Milk Using Different Types and Doses of Collagens. Molecules, 28(2), 657. https://doi.org/10.3390/molecules28020657






