All commercially sourced reagents were used as supplied unless otherwise stated. The 1H NMR and 13C NMR spectra were recorded on a Bruker Avance 400 spectrometer (400 MHz or 100 MHz, respectively). 1H NMR chemical shifts were reported in ppm (δ) relative to tetramethylsilane (TMS) with the solvent resonance employed as the internal standard (CDCl3, δ 7.26 ppm). 13C NMR chemical shifts were determined relative to the solvent: CDCl3 at δ 77.16 ppm. High-resolution mass spectroscopy data of the products were collected on a BioTOF Q instrument. Single-crystal (X-ray) diffraction measurements were conducted on Oxford Xcalibur E CCD X. Visualization on TLC was achieved by UV light (254 nm). Flash chromatography was performed on silica gel 200–300 mesh.
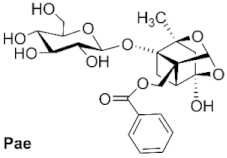
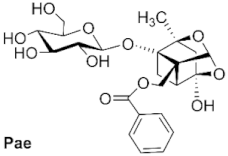
3.6.2. General Procedure for the Synthesis of Paeoniflorin Derivatives
A solution of paeoniflorin (0.5 mmol) in alcohol (12 mL) was treated with Sc(CF
3SO
3)
3 (0.5 mmol) and refluxed for 45 min. After completely consuming the substrates (monitored by TLC), the mixture was diluted with ethyl acetate and washed with saturated NaCl solution (×3). The organic layer was dried over Na
2SO
4 and concentrated under a vacuum. Then, the obtained mixture was acetylated for 1 h using an Ac
2O-Py combination (1:1, 6 mL) at 0 °C. The reaction solution was diluted with ethyl acetate and washed with 5% H
2SO
4 solution, followed by water and saturated NaHCO
3. The organic layer was dried over Na
2SO
4 and concentrated under a vacuum. The residue was purified by column chromatography to obtain Group I and Group II. Group I and Group II (0.09 mmol) were dissolved in CH
3OH (3 mL), and Et
3N (0.30 mL, 2.3 mmol) was added. After 24 h of reaction at room temperature and total consumption of substrates (monitored by TLC), the resulting mixture was diluted with CH
2Cl
2 and washed with saturated NaCl solution (×3). The organic layer was dried over Na
2SO
4 and concentrated under a vacuum. The residue was purified by column chromatography to obtain Group III, Group IV, Group V, and Group VI. Several compounds (compounds
1,
2,
3,
4,
6,
7,
8, and
10), which had been previously reported [
29,
33,
34], were confirmed using only
1H NMR spectra and compared with previous data. Detailed physicochemical properties of paeoniflorin derivatives (see the
Supplementary Materials for details):
2,3,4,6-Tetra-O-acetyl-4-O-methylpaeoniflorin (1): white solid, 127.2 mg (42.4%, petroleum: ethyl acetate, 4:1), Rf 0.38 (petroleum: ethyl acetate, 1:1), mp 119.0 °C, 1H NMR (400 MHz, Chloroform-d) δ 8.03 (d, J = 6.9 Hz 2H, H-2″, H-6″), 7.60 (t, J = 7.5 Hz, 1H, H-4″), 7.47 (t, J = 7.7 Hz, 2H, H-3″, H-5″), 5.46 (s, 1H, H-9), 5.13–4.96 (m, 3H, H-2′, H-3′, H-4′), 4.75 (d, J = 7.8 Hz, 1H, H-1′), 4.59 (d, J = 12.0 Hz, 1H, H-8), 4.47 (d, J = 12.0 Hz, 1H, H-8), 4.18 (dd, J = 12.2, 2.5 Hz, 1H, H-6′), 4.11 (dd, J = 12.2, 5.4 Hz, 1H, H-6′), 3.64–3.58 (m, 1H, H-5′), 3.41 (s, 3H, H-11), 2.77 (d, J = 6.9 Hz, 1H, H-5), 2.32 (dd, J = 10.7, 6.9 Hz, 1H, H-6), 2.10–1.93 (m, 14H, 4COCH3, H-3), 1.79 (d, J = 10.6 Hz, 1H, H-6), 1.36 (s, 3H, H-10). ESI-HRMS: m/z calcd for C32H38O15Na [M + Na]+ 685.2103, found 685.2111.
2,3,4,6-Tetra-O-acetyl-4-oxo-9-O-methylpaeoniflorin (2): white solid, 86.4 mg (28.8%, petroleum: ethyl acetate, 3:1), Rf 0.30 (petroleum: ethyl acetate, 1:1), mp 177.8 °C, 1H NMR (400 MHz, Chloroform-d) δ 8.01 (d, J = 7.0 Hz, 2H, H-2″, H-6″), 7.63–7.57 (m, 1H, H-4″), 7.46 (t, J = 7.7 Hz, 2H, H-3″, H-5″), 5.16 (t, J = 9.4 Hz, 1H, H-3′), 5.08–5.02 (m, 2H, H-2′, H-4′), 5.01 (s, 1H, H-9), 4.78 (d, J = 7.9 Hz, 1H, H-1′), 4.51 (d, J = 1.6 Hz, 2H, H-8), 4.19 (dd, J = 12.2, 2.6 Hz, 1H, H-6′), 4.14 (d, J = 5.5 Hz, 1H, H-6′), 3.68–3.62 (m, 1H, H-5′), 3.35 (s, 3H, H-11), 3.04 (d, J = 7.3 Hz, 1H, H-5), 2.71 (t, J = 9.3 Hz, 1H, H-6), 2.65 (d, J = 6.0 Hz, 2H, H-6, H-3), 2.09–1.96 (m, 13H, 4COCH3, H-3), 1.40 (s, 3H, H-10). ESI-HRMS: m/z calcd for C32H38O15Na [M + Na]+ 685.2103, found 685.2150.
4-O-methylpaeoniflorin (3): colorless syrup, 22.3 mg (44.5%, CH2Cl2: CH3OH, 22:1), Rf 0.36 (CH2Cl2: CH3OH, 7:1), 1H NMR (400 MHz, Chloroform-d) δ 7.93 (d, J = 7.2 Hz, 2H, H-2″, H-6″), 7.49 (t, J = 7.4 Hz, 1H, H-4″), 7.35 (t, J = 7.7 Hz, 2H, H-3″, H-5″), 5.49 (s, 1H, H-9), 4.70–4.61 (m, 2H, H-8, H-1′), 4.50 (d, J = 7.7 Hz, 1H, H-1′), 3.80 (s, 2H, H-6′), 3.59 (s, 2H, H-3′, H-4′), 3.42 (s, 1H, H-2′), 3.34 (s, 4H, H-5′, H-11), 2.68 (d, J = 6.6 Hz, 1H, H-6), 2.39 (d, J = 10.0 Hz, 1H, H-5), 1.95 (s, 2H, H-3), 1.78 (d, J = 10.7 Hz, 1H, H-6), 1.34 (s, 3H, H-10). ESI-HRMS: m/z calcd for C24H30O11Na [M + Na]+ 517.1681, found 517.1663.
4-Oxo-9-O-methylpaeoniflorin (4): colorless syrup, 29.7 mg (48%, CH2Cl2: CH3OH, 21:1), Rf 0.35 (CH2Cl2: CH3OH, 7:1), 1H NMR (400 MHz, Chloroform-d) δ 7.90 (d, J = 7.7 Hz, 2H, H-2″, H-6″), 7.51 (t, J = 7.4 Hz, 1H, H-4″), 7.35 (t, J = 7.7 Hz, 2H, H-3″, H-5″), 5.02 (s, 1H, H-9), 4.73 (d, J = 11.7 Hz, 1H, H-8), 4.56 (d, J = 12.8 Hz, 2H, H-8, H-1′), 3.84 (s, 2H, H-6′), 3.64 (s, 2H, H-3′, H-4′), 3.48 (s, 1H, H-2′), 3.35 (s, 1H, H-5′), 3.27 (s, 3H, H-11), 2.96 (d, J = 7.0 Hz, 1H, H-6), 2.80 (d, J = 10.6 Hz, 1H, H-5), 2.60 (q, J = 18.3 Hz, 2H, H-3), 2.00 (d, J = 10.9 Hz, 1H, H-6), 1.40 (s, 3H, H-10). ESI-HRMS: m/z calcd for C24H30O11Na [M + Na]+ 517.1681, found 517.1700.
4-Oxo-9-O-methyldebenzoylpaeoniflorin (5): white solid, 15.4 mg (31.5%, CH2Cl2: CH3OH, 16:1), Rf 0.08 (CH2Cl2:CH3OH, 7:1), mp 162.9 °C, 1H NMR (400 MHz, Acetone-d6) δ 5.10 (s, 1H, H-9), 4.76 (d, J = 7.7 Hz, 1H, H-8), 4.02 (d, J = 12.6 Hz, 1H, H-6′), 3.81 (dd, J = 11.7, 2.6 Hz, 1H, H-6′), 3.72 (d, J = 12.5 Hz, 1H, H-2′), 3.62 (dd, J = 10.2, 4.2 Hz, 2H, H-4′, H-5′), 3.48 (t, J = 8.6 Hz, 1H, H-3′), 3.39–3.31 (m, 2H, H-8, H-1′), 3.26 (s, 3H, H-11), 2.90 (dd, J = 11.1, 7.3 Hz, 1H, H-5), 2.75 (d, J = 17.9 Hz, 1H, H-6), 2.48 (d, J = 7.5 Hz, 1H, H-3), 2.36 (d, J = 17.3 Hz, 1H, H-3), 2.07 (d, J = 4.2 Hz, 1H, H-6), 1.35 (s, 3H, H-10). 13C NMR (101 MHz, Acetone-d6) δ 205.27 (C-4), 105.31 (C-9), 98.93 (C-1′), 88.26 (C-1), 87.13 (C-2), 78.22 (C-3′), 77.54 (C-5′), 74.59 (C-2′), 71.77 (C-11), 65.21 (C-7), 62.97 (C-6′), 60.19 (C-8), 55.48 (C-4′), 47.17 (C-3), 46.66 (C-5), 26.67 (C-6), 20.75 (C-10). ESI-HRMS: m/z calcd for C17H26O10Na [M + Na]+ 413.1419, found 413.1402.
2,3,4,6-Tetra-O-acetyl-4-O-ethylpaeoniflorin (6): white solid, 103.7 mg (35.5%, petroleum: ethyl acetate, 5:1), Rf 0.40 (petroleum: ethyl acetate, 1:1), mp 145.1 °C, 1H NMR (400 MHz, Chloroform-d) δ 8.03 (d, J = 7.1 Hz, 2H, H-2″, H-6″), 7.60 (t, J = 7.4 Hz, 1H, H-4″), 7.48 (t, J = 7.7 Hz, 2H, H-3″, H-5″), 5.44 (s, 1H, H-9), 5.12–4.95 (m, 3H, H-2′, H-3′, H-4′), 4.75 (d, J = 7.8 Hz, 1H, H-1′), 4.59 (d, J = 12.0 Hz, 1H, H-8), 4.47 (d, J = 12.0 Hz, 1H, H-8), 4.18 (dd, J = 12.2, 2.6 Hz, 1H, H-6′), 4.13–4.08 (m, 1H, H-6′), 3.69 (qd, J = 7.0, 2.7 Hz, 2H, H-11), 3.60 (ddd, J = 9.6, 5.3, 2.6 Hz, 1H, H-5′), 2.76 (d, J = 6.0 Hz, 1H, H-5), 2.32 (dd, J = 10.7, 6.9 Hz, 1H, H-6), 2.02 (dd, J = 21.9, 16.2 Hz, 14H, 4COCH3, H-3), 1.80 (d, J = 10.6 Hz, 1H, H-6), 1.35 (s, 3H, H-10), 1.21 (t, J = 7.0 Hz, 3H, H-12). ESI-HRMS: m/z calcd for C33H40O15Na [M + Na]+ 699.2260, found 699.2263.
2,3,4,6-Tetra-O-acetyl-4-oxo-9-O-ethylpaeoniflorin (7): white solid, 97.3 mg (33.4%, petroleum: ethyl acetate, 4:1), Rf 0.32 (petroleum: ethyl acetate, 1:1), mp 161.8 °C, 1H NMR (400 MHz, Chloroform-d) δ 8.01 (d, J = 6.9 Hz, 2H, H-2″, H-6″), 7.59 (t, J = 7.4 Hz, 1H, H-4″), 7.45 (t, J = 7.8 Hz, 2H, H-3″, H-5″), 5.17 (t, J = 9.4 Hz, 1H, H-3′), 5.11 (s, 1H, H-9), 5.08–5.00 (m, 2H, H-2′, H-4′), 4.78 (d, J = 7.8 Hz, 1H, H-1′), 4.57–4.47 (m, 2H, H-8), 4.19 (dd, J = 12.3, 2.6 Hz, 1H, H-6′), 4.12 (dd, J = 12.2, 5.5 Hz, 1H, H-6′), 3.73 (dd, J = 9.6, 7.1 Hz, 1H, H-11), 3.68–3.62 (m, 1H, H-5′), 3.51–3.41 (m, 1H, H-11), 3.04 (d, J = 7.3 Hz, 1H, H-5), 2.70 (dd, J = 11.1, 7.4 Hz, 1H, H-6), 2.65 (d, J = 4.1 Hz, 2H, H-3), 2.09–1.96 (m, 13H, 4COCH3, H-6), 1.39 (s, 3H, H-10), 1.10 (t, J = 7.0 Hz, 3H, H-12). ESI-HRMS: m/z calcd for C33H40O15Na [M + Na]+ 699.2260, found 699.2271.
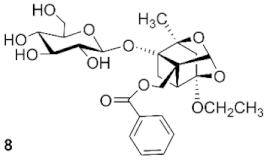
4-O-ethylpaeoniflorin (8): white solid, 14.5 mg (30.6%, CH2Cl2: CH3OH, 23:1), Rf 0.35 (CH2Cl2: CH3OH, 7:1), mp 123.6 °C, 1H NMR (400 MHz, Chloroform-d) δ 7.94 (d, J = 7.8 Hz, 2H, H-2″, H-6″), 7.50 (t, J = 7.4 Hz, 1H, H-4″), 7.36 (t, J = 7.6 Hz, 2H, H-3″, H-5″), 5.48 (s, 1H, H-9), 4.66 (q, J = 12.1 Hz, 2H, H-8, H-1′), 4.49 (d, J = 7.5 Hz, 1H, H-8), 3.81 (d, J = 15.0 Hz, 2H, H-6′), 3.60 (dt, J = 19.5, 9.5 Hz, 4H, H-3′, H-4′, H-11), 3.43–3.36 (m, 1H, H-2′), 3.28 (d, J = 8.6 Hz, 1H, H-5′), 2.67 (d, J = 6.5 Hz, 1H, H-6), 2.37 (dd, J = 11.0, 6.9 Hz, 1H, H-5), 1.97 (s, 2H, H-3), 1.79 (d, J = 10.7 Hz, 1H, H-6), 1.33 (s, 3H, H-10), 1.17 (t, J = 7.0 Hz, 3H, H-12). ESI-HRMS: m/z calcd for C25H32O11Na [M + Na]+ 531.1837, found 531.1871.
4-O-ethyldebenzoylpaeoniflorin (9): colorless syrup, 26 mg (61.5%, CH2Cl2: CH3OH, 15:1), Rf 0.07 (CH2Cl2: CH3OH, 7:1), 1H NMR (400 MHz, Acetone-d6) δ 5.21 (s, 1H, H-9), 4.65 (d, J = 7.7 Hz, 1H, H-8), 4.00 (d, J = 12.4 Hz, 1H, H-8), 3.90 (d, J = 12.3 Hz, 1H, H-1′), 3.81 (d, J = 11.5 Hz, 1H, H-6′), 3.67–3.58 (m, 3H, H-6′, H-11), 3.46 (t, J = 8.7 Hz, 1H, H-5′), 3.35–3.30 (m, 2H, H-3′, H-4′), 3.27–3.21 (m, 1H, H-2′), 2.49 (d, J = 8.6 Hz, 1H, H-6), 2.41 (dd, J = 10.6, 6.9 Hz, 1H, H-5), 1.98 (d, J = 17.3 Hz, 1H, H-3), 1.88–1.79 (m, 2H, H-6, H-3), 1.29 (s, 3H, H-10), 1.12 (t, J = 7.1 Hz, 3H, H-12). 13C NMR (101 MHz, Acetone-d6) δ 108.41 (C-4), 101.98 (C-9), 99.48 (C-1′), 88.96 (C-1), 86.10 (C-2), 78.14 (C-3′), 77.43 (C-5′), 74.77 (C-2′), 72.68 (C-11), 71.77 (C-4′), 62.99 (C-7), 59.51 (C-6′), 59.09 (C-8), 42.57 (C-3), 41.16 (C-5), 23.20 (C-12), 19.66 (C-6), 15.92 (C-10). ESI-HRMS: m/z calcd for C18H28O10Na [M + Na]+ 427.1575, found 427.1602.
4-Oxo-9-O-ethylpaeoniflorin (10): colorless syrup, 30 mg (26.8%, CH2Cl2: CH3OH, 22:1), Rf 0.34 (CH2Cl2: CH3OH, 7:1), 1H NMR (400 MHz, Chloroform-d) δ 7.90 (d, J = 7.4 Hz, 2H, H-2″, H-6″), 7.50 (t, J = 7.5 Hz, 1H, H-4″), 7.34 (t, J = 7.7 Hz, 2H, H-3″, H-5″), 5.12 (s, 1H, H-9), 4.74 (d, J = 11.3 Hz, 1H, H-8), 4.55 (d, J = 7.2 Hz, 2H, H-8, H-1′), 3.83 (s, 2H, H-6′), 3.64 (p, J = 9.7, 8.4 Hz, 3H, H-2′, H-3′, H-4′), 3.48 (s, 1H, H-5′), 3.39–3.31 (m, 2H, H-11), 2.96 (d, J = 7.1 Hz, 1H, H-6), 2.80 (d, J = 10.2 Hz, 1H, H-5), 2.60 (q, J = 18.2 Hz, 2H, H-3), 2.00 (d, J = 10.8 Hz, 1H, H-6), 1.39 (s, 3H, H-10), 1.02 (t, J = 7.0 Hz, 3H, H-12). ESI-HRMS: m/z calcd for C18H28O10Na [M + Na]+ 531.1837, found 531.1852.
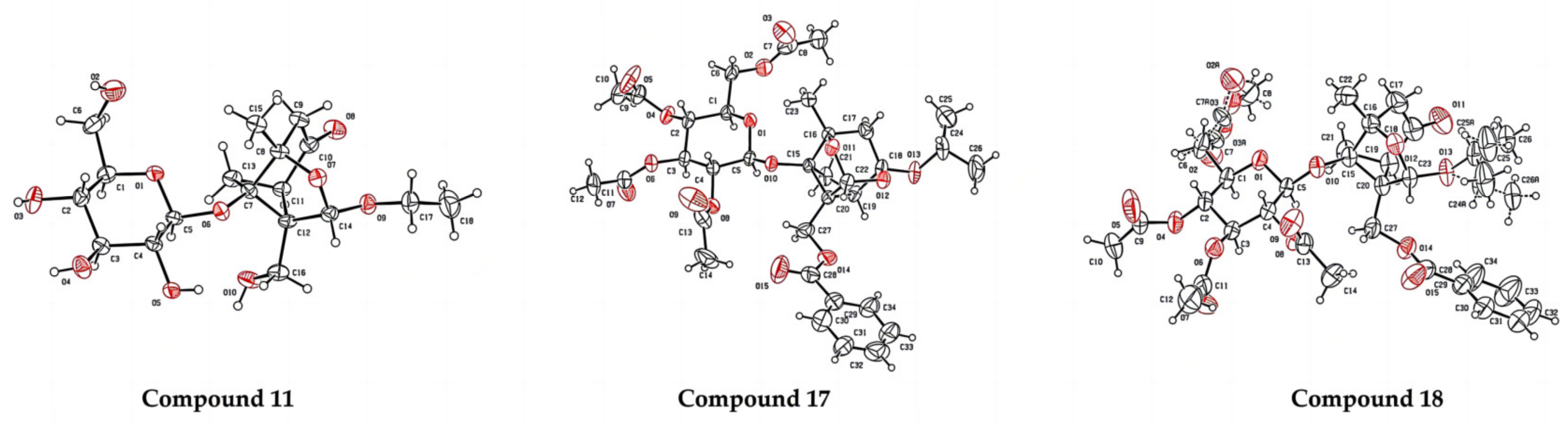
4-Oxo-9-O-ethyldebenzoylpaeoniflorin (11): white solid, 60 mg (67.5%, CH2Cl2: CH3OH, 16:1), mp 105.6 °C, Rf 0.06 (CH2Cl2: CH3OH, 7:1), 1H NMR (400 MHz, Acetone-d6) δ 5.20 (s, 1H, H-9), 4.76 (d, J = 7.7 Hz, 1H, H-8), 4.02 (dd, J = 12.5, 3.6 Hz, 1H, H-6′), 3.85–3.78 (m, 1H, H-11), 3.76–3.58 (m, 3H, H-6′, H-11, H-5′), 3.52–3.41 (m, 2H, H-3′, H-4′), 3.37–3.25 (m, 3H, H-2′, H-8, H-1′), 2.88 (d, J = 6.5 Hz, 1H, H-5), 2.74 (d, J = 17.9 Hz, 1H, H-6), 2.49 (d, J = 7.5 Hz, 1H, H-3), 2.38 (d, J = 17.9 Hz, 1H, H-3), 2.06 (d, J = 3.1 Hz, 1H, H-6), 1.34 (s, 3H, H-10), 1.06 (t, J = 7.1 Hz, 3H, H-12). 13C NMR (101 MHz, Acetone-d6) δ 205.35 (C-4), 103.66 (C-9), 98.89 (C-1′), 88.18 (C-1), 86.98 (C-2), 78.25 (C-3′), 77.50 (C-5′), 74.58 (C-2′), 71.79 (C-11), 65.24 (C-4′), 63.91 (C-7), 63.00 (C-8), 60.23 (C-6′), 49.48 (C-3), 47.23 (C-5), 26.63 (C-6), 20.77 (C-10), 15.09 (C-12). ESI-HRMS: m/z calcd for C18H28O10Na [M + Na]+ 427.1575, found 427.1612.
2,3,4,6-Tetra-O-acetyl-4-O-propylpaeoniflorin (12): white solid, 57.2 mg (30.7%, petroleum: ethyl acetate, 4:1), mp 143.1 °C, Rf 0.42 (petroleum: ethyl acetate, 1:1), 1H NMR (400 MHz, Chloroform-d) δ 8.03 (d, J = 7.0 Hz, 2H, H-2″, H-6″), 7.60 (t, J = 7.4 Hz, 1H, H-4″), 7.48 (t, J = 7.7 Hz, 2H, H-3″, H-5″), 5.44 (s, 1H, H-9), 5.11–4.95 (m, 3H, H-2′, H-3′, H-4′), 4.75 (d, J = 7.8 Hz, 1H, H-1′), 4.59 (d, J = 12.0 Hz, 1H, H-8), 4.48 (d, J = 12.0 Hz, 1H, H-8), 4.18 (dd, J = 12.2, 2.6 Hz, 1H, H-6′), 4.11 (dd, J = 12.2, 5.3 Hz, 1H, H-6′), 3.63–3.53 (m, 3H, H-11, H-5′), 2.77 (d, J = 6.8 Hz, 1H, H-5), 2.32 (dd, J = 10.7, 7.0 Hz, 1H, H-6), 2.02 (dd, J = 21.4, 16.8 Hz, 14H, 4COCH3, H-3), 1.80 (d, J = 10.7 Hz, 1H, H-6), 1.59 (q, J = 7.1 Hz, 2H, H-12), 1.35 (s, 3H, H-10), 0.90 (t, J = 7.4 Hz, 3H, H-13).13C NMR (101 MHz, Chloroform-d) δ 170.53, 170.31, 169.49, 169.40 (4CH3CO), 166.48 (C-7″), 133.54 (C-4″), 129.72 (C-2″, C-6″), 129.65 (C-1″), 128.73 (C-3″, C-5″), 107.62 (C-4), 101.16 (C-9), 96.41 (C-1′), 88.43 (C-1), 85.64 (C-2), 73.04 (C-3′), 71.81 (C-5′), 71.36 (C-2′), 69.83 (C-11), 68.47 (C-4′), 65.80 (C-7), 62.06 (C-6′), 60.25 (C-8), 41.60 (C-3), 40.90 (C-5), 23.21 (C-12), 22.31 (C-6), 20.82, 20.67, 20.65×2 (4CH3CO), 19.22 (C-10), 10.47 (C-13). ESI-HRMS: m/z calcd for C34H42O15Na [M + Na]+ 713.2416, found 713.2493.
2,3,4,6-Tetra-O-acetyl-4-oxo-9-O-propylpaeoniflorin (13): colorless syrup, 73.4 mg (39.4%, petroleum: ethyl acetate, 2:1), Rf 0.34 (petroleum: ethyl acetate, 1:1), 1H NMR (400 MHz, Chloroform-d) δ 8.01 (d, J = 7.0 Hz, 2H, H-2″, H-6″), 7.59 (t, J = 7.5 Hz, 1H, H-4″), 7.45 (t, J = 7.8 Hz, 2H, H-3″, H-5″), 5.17 (t, J = 9.4 Hz, 1H, H-3′), 5.11 (s, 1H, H-9), 5.04 (dd, J = 16.9, 9.2 Hz, 2H, H-2′′, H-4′′), 4.78 (d, J = 7.9 Hz, 1H, H-1′), 4.56–4.47 (m, 2H, H-8), 4.19 (dd, J = 12.2, 2.5 Hz, 1H, H-6′), 4.13 (dd, J = 12.2, 5.5 Hz, 1H, H-6′), 3.64 (dt, J = 9.6, 6.7 Hz, 2H, H-11, H-5′), 3.35 (dt, J = 9.3, 6.4 Hz, 1H, H-11), 3.05 (d, J = 7.4 Hz, 1H, H-5), 2.73–2.67 (m, 1H, H-6), 2.65 (d, J = 4.4 Hz, 2H, H-3), 2.10–1.97 (m, 13H, 4COCH3, H-6), 1.49 (q, J = 6.9 Hz, 2H, H-12), 1.39 (s, 3H, H-10), 0.83 (t, J = 7.4 Hz, 3H, H-13).13C NMR (101 MHz, Chloroform-d) δ 204.89 (C-4), 170.48, 170.32, 169.51, 169.42 (4CH3CO), 166.46 (C-7″), 133.58 (C-4″), 129.78 (C-2″, C-6″), 129.51 (C-1″), 128.66 (C-3″, C-5″), 104.88 (C-9), 96.29 (C-1′), 87.93 (C-1), 85.78 (C-2), 72.98 (C-3′), 72.04 (C-5′), 71.51 (C-2′), 70.53 (C-11), 68.40 (C-4′), 63.16 (C-7), 62.41 (C-8), 62.08 (C-6′), 48.91 (C-3), 46.91 (C-5), 26.28 (C-6), 22.69 (C-12), 20.81, 20.73, 20.68×2 (4CH3CO), 20.47 (C-10), 10.72 (C-13). ESI-HRMS: m/z calcd for C34H42O15Na [M + Na]+ 713.2416, found 713.2420.
4-O-propylpaeoniflorin (14): colorless syrup, 25.3 mg (44.1%, CH2Cl2: CH3OH, 22:1), Rf 0.32 (CH2Cl2: CH3OH, 8:1), 1H NMR (400 MHz, Chloroform-d) δ 7.93 (d, J = 7.4 Hz, 2H, H-2″, H-6″), 7.49 (t, J = 7.4 Hz, 1H, H-4″), 7.34 (t, J = 7.7 Hz, 2H, H-3″, H-5″), 5.45 (s, 1H, H-9), 4.65 (q, J = 12.2 Hz, 2H, H-8, H-1′), 4.49 (d, J = 7.6 Hz, 1H, H-8), 3.78 (q, J = 11.8 Hz, 2H, H-6′), 3.57 (s, 2H, H-3′, H-4′), 3.50 (t, J = 5.5 Hz, 2H, H-11), 3.39 (s, 1H, H-2′), 3.27 (s, 1H, H-5′), 2.66 (d, J = 6.4 Hz, 1H, H-6), 2.36 (s, 1H, H-5), 1.95 (s, 2H, H-3), 1.78 (d, J = 10.6 Hz, 1H, H-6), 1.54 (q, J = 7.2 Hz, 2H, H-12), 1.32 (s, 3H, H-10), 0.86 (t, J = 7.4 Hz, 3H, H-13). 13C NMR (101 MHz, Chloroform-d) δ 167.13 (C-7″), 133.47 (C-4″), 129.85 (C-2″, C-6″), 129.61 (C-1″), 128.64 (C-3″, C-5″), 107.70 (C-4), 101.25 (C-9), 98.82 (C-1′), 88.41 (C-1), 85.72 (C-2), 76.22 (C-3′), 75.72 (C-5′), 73.61 (C-2′), 70.12 (C-11), 69.70 (C-4′), 65.55 (C-7), 61.61 (C-6′), 60.93 (C-8), 41.78 (C-3), 40.33 (C-5), 23.23 (C-12), 19.53 (C-6), 19.53 (C-10), 10.49 (C-13). ESI-HRMS: m/z calcd for C26H34O11Na [M + Na]+ 545.1994, found 545.2017.
4-Oxo-9-O-propylpaeoniflorin (15): white solid, 35.3 mg (30.7%, CH2Cl2: CH3OH = 22:1), mp 105.1 °C, Rf 0.36 (CH2Cl2: CH3OH, 7:1), 1H NMR (400 MHz, Chloroform-d) δ 7.90 (d, J = 7.1 Hz, 2H, H-2″, H-6″), 7.50 (t, J = 7.4 Hz, 1H, H-4″), 7.33 (t, J = 7.7 Hz, 2H, H-3″, H-5″), 5.10 (s, 1H, H-9), 4.75 (d, J = 11.7 Hz, 1H, H-8), 4.60–4.52 (m, 2H, H-8, H-1′), 3.84 (s, 2H, H-6′), 3.66 (s, 2H, H-3′, H-4′), 3.53 (m, 2H, H-11), 3.36 (d, J = 8.1 Hz, 1H, H-2′), 3.27–3.20 (m, 1H, H-5′), 2.97 (d, J = 7.1 Hz, 1H, H-6), 2.83–2.75 (m, 1H, H-5), 2.59 (q, J = 18.2 Hz, 2H, H-3), 1.99 (d, J = 10.9 Hz, 1H, H-6), 1.41 (d, J = 10.2 Hz, 5H, H-10, H-12), 0.77 (t, J = 7.4 Hz, 3H, H-13). 13C NMR (101 MHz, Chloroform-d) δ 205.24 (C-4), 167.28 (C-7″), 133.70 (C-4″), 129.81 (C-2″, C-6″), 129.33 (C-1″), 128.62 (C-3″, C-5″), 105.30 (C-9), 98.73 (C-1′), 87.97 (C-1), 85.82 (C-2),76.53 (C-3′), 75.89 (C-5′), 73.63 (C-2′), 70.42 (C-11), 69.52 (C-4′), 63.48 (C-7), 63.30 (C-8), 61.61 (C-6′), 48.85 (C-3), 46.98 (C-5), 26.59 (C-6), 22.69 (C-12), 20.72 (C-10), 10.73 (C-13). ESI-HRMS: m/z calcd for C26H34O11Na [M + Na]+ 545.1994, found 545.2075.
4-Oxo-9-O-propyldebenzoylpaeoniflorin (16): white solid, 46.7 mg (40.6%, CH2Cl2: CH3OH = 15:1), mp 133.3 °C, Rf 0.05 (CH2Cl2: CH3OH, 7:1), 1H NMR (400 MHz, Acetone-d6) δ 5.19 (s, 1H, H-9), 4.76 (d, J = 7.7 Hz, 1H, H-8), 4.02 (d, J = 12.6 Hz, 1H, H-6′), 3.81 (dd, J = 11.8, 2.6 Hz, 1H, H-11), 3.71 (d, J = 12.6 Hz, 1H, H-6′), 3.64–3.55 (m, 2H, H-5′, H-11), 3.48 (t, J = 8.7 Hz, 1H, H-3′), 3.40–3.32 (m, 3H, H-2′, H-4′, H-8), 3.27 (d, J = 9.0 Hz, 1H, H-1′), 2.90 (dd, J = 12.3, 7.6 Hz, 1H, H-5), 2.75 (d, J = 17.9 Hz, 1H, H-6), 2.49 (d, J = 7.4 Hz, 1H, H-3), 2.38 (d, J = 16.9 Hz, 1H, H-3), 2.09 (s, 1H, H-6), 1.47 (q, J = 6.9 Hz, 2H, H-12), 1.35 (s, 3H, H-10), 0.84 (t, J = 7.4 Hz, 3H, H-13). 13C NMR (101 MHz, Acetone-d6) δ 205.32 (C-4), 104.10 (C-9), 98.89 (C-1′), 88.21 (C-1), 86.96 (C-2), 78.17 (C-3′), 77.50 (C-5′), 74.53 (C-2′), 71.71 (C-11), 70.62 (C-4′), 65.34 (C-7), 62.92 (C-6′), 60.17 (C-8), 49.52 (C-3), 47.20 (C-5), 26.63 (C-6), 23.46 (C-12), 20.78 (C-10), 11.01 (C-13). ESI-HRMS: m/z calcd for C19H30O10Na [M + Na]+ 441,1732, found 441.1733.
2,3,4,6-Tetra-O-acetyl-4-O-iso-propylpaeoniflorin (17): white solid, 61mg (37.5%, petroleum: ethyl acetate = 4:1), mp 117.8 °C, Rf 0.40 (petroleum: ethyl acetate, 1:1), mp 113–115 °C, 1H NMR (400 MHz, Chloroform-d) δ 8.03 (d, J = 7.0 Hz, 2H, H-2″, H-6″), 7.60 (t, J = 7.4 Hz, 1H, H-4″), 7.48 (t, J = 7.7 Hz, 2H, H-3″, H-5″), 5.42 (s, 1H, H-9), 5.08–4.96 (m, 3H, H-2′, H-3′, H-4′), 4.74 (d, J = 7.7 Hz, 1H, H-1′), 4.59 (d, J = 11.9 Hz, 1H, H-8), 4.49 (d, J = 12.0 Hz, 1H, H-8), 4.17 (dd, J = 12.3, 2.7 Hz, 1H, H-6′), 4.11 (dd, J = 12.2, 5.6 Hz, 2H, H-6′, H-11), 3.62–3.56 (m, 1H, H-5′), 2.73 (d, J = 6.7 Hz, 1H, H-5), 2.32 (dd, J = 10.7, 7.0 Hz, 1H, H-6), 2.17 (d, J = 3.3 Hz, 1H, H-3), 2.07, 2.01, 1.97 (13H, 4COCH3, H-3), 1.80 (d, J = 10.6 Hz, 1H, H-6), 1.34 (s, 3H, H-10), 1.18 (dd, J = 6.2, 2.7 Hz, 6H, H-12, H-13). 13C NMR (101 MHz, Chloroform-d) δ 170.54, 170.32, 169.50, 169.40 (4CH3CO), 166.50 (C-7″), 133.55 (C-4″), 129.73 (C-2″, C-6″, C-1″), 128.76 (C-3″, C-5″), 107.81 (C-4), 101.15 (C-9), 96.44 (C-1′), 88.39 (C-1), 85.70 (C-2), 73.09 (C-3′), 71.83 (C-5′), 71.39 (C-2′), 69.57 (C-11), 68.51 (C-4′), 67.44 (C-7), 62.08 (C-6′), 60.34 (C-8), 42.14 (C-3), 41.88 (C-5), 24.26 (C-12), 24.13 (C-13), 22.40 (C-6), 20.83, 20.70, 20.68, 20.66 (4CH3CO), 19.26 (C-10). ESI-HRMS: m/z calcd for C34H42O15Na [M + Na]+ 713.2416, found 713.2490.
2,3,4,6-Tetra-O-acetyl-4-oxo-9-O-iso-propylpaeoniflorin (18): colorless syrup, 62 mg (38.1%, petroleum: ethyl acetate = 3:1), Rf 0.36 (petroleum: ethyl acetate, 1:1), mp 147–149 °C, 1H NMR (400 MHz, Chloroform-d) δ 8.01 (d, J = 7.0 Hz, 2H, H-2″, H-6″), 7.58 (t, J = 7.5 Hz, 1H, H-4″), 7.44 (t, J = 7.6 Hz, 2H, H-3″, H-5″), 5.20 (s, 1H, H-9), 5.15 (d, J = 9.4 Hz, 1H, H-2′), 5.07–4.99 (m, 2H, H-3′, H-4′), 4.77 (d, J = 7.9 Hz, 1H, H-1′), 4.51 (q, J = 11.8 Hz, 2H, H-8), 4.18 (dd, J = 12.2, 2.4 Hz, 1H, H-6′), 4.12 (dd, J = 12.2, 5.3 Hz, 1H, H-6′), 3.91 (p, J = 6.2 Hz, 1H, H-11), 3.68–3.62 (m, 1H, H-5′), 3.03 (d, J = 7.3 Hz, 1H, H-5), 2.67 (m, 3H, H-6, H-3), 2.08, 2.05, 2.02, 1.99 (13H, 4COCH3, H-3), 1.37 (s, 3H, H-10), 1.11 (d, J = 6.2 Hz, 3H, H-12), 1.00 (d, J = 6.1 Hz, 3H, H-13). 13C NMR (101 MHz, Chloroform-d) δ 13C NMR (101 MHz, Chloroform-d) δ 205.05 (C-4), 170.48, 170.33, 169.51, 169.43 (4CH3CO), 166.48 (C-7″), 133.58 (C-4″), 129.82 (C-2″, C-6″), 129.50 (C-1″), 128.65 (C-3″, C-5″), 102.42 (C-9), 96.29 (C-1′), 87.91 (C-1), 85.61 (C-2), 73.00 (C-3′), 72.04 (C-5′), 71.53 (C-2′), 70.13 (C-11), 68.42 (C-4′), 62.90 (C-7), 62.40 (C-8), 62.10 (C-6′), 49.01 (C-3), 46.97 (C-5), 26.26 (C-6), 22.54 (C-12), 21.32 (C-13), 20.82, 20.75, 20.69×2 (4CH3CO), 20.54 (C-10). ESI-HRMS: m/z calcd for C34H42O15Na [M + Na]+ 713.2416, found 713.2419.
4-O-iso-propylpaeoniflorin (19): colorless syrup, 24.7 mg (52.5%, CH2Cl2: CH3OH, 23:1), Rf 0.39 (CH2Cl2: CH3OH, 7:1), 1H NMR (400 MHz, Chloroform-d) δ 7.94 (d, J = 6.9 Hz, 2H, H-2″, H-6″), 7.50 (t, J = 7.4 Hz, 1H, H-4″), 7.36 (t, J = 7.6 Hz, 2H, H-3″, H-5″), 5.46 (s, 1H, H-9), 4.70–4.60 (m, 2H, H-8, H-1′), 4.49 (d, J = 7.4 Hz, 1H, H-8), 4.05 (p, J = 6.1, 5.6 Hz, 1H, H-11), 3.79 (d, J = 9.3 Hz, 2H, H-6′), 3.54 (d, J = 10.6 Hz, 2H, H-3′, H-4′), 3.39 (s, 1H, H-2′), 3.26 (d, J = 8.6 Hz, 1H, H-5′), 2.64 (d, J = 6.6 Hz, 1H, H-6), 2.37 (dd, J = 10.9, 6.9 Hz, 1H, H-5), 1.96 (s, 2H, H-3), 1.79 (d, J = 10.7 Hz, 1H, H-6), 1.32 (s, 3H, H-10), 1.14 (d, J = 6.1 Hz, 6H, H-12, H-13). 13C NMR (101 MHz, Chloroform-d) δ 167.11 (C-7″), 133.45 (C-4″), 129.84 (C-2″, C-6″), 129.68 (C-1″), 128.63 (C-3″, C-5″), 107.87 (C-4), 101.19 (C-9), 98.91 (C-1′), 88.41 (C-1), 85.76 (C-2), 76.39 (C-3′), 75.73 (C-5′), 73.51 (C-2′), 69.84 (C-11), 69.53 (C-4′), 67.19 (C-7), 61.69 (C-6′), 60.92 (C-8), 42.34 (C-3), 41.21 (C-5), 24.25 (C-12), 24.13 (C-13), 22.80 (C-6), 19.55 (C-10). ESI-HRMS: m/z calcd for C26H34O11Na [M + Na]+ 545.1994, found 545.2083.
4-O-iso-propyldebenzoylpaeoniflorin (20): colorless syrup, 12.2 mg (32.4%, CH2Cl2: CH3OH, 16:1), Rf 0.08 (CH2Cl2: CH3OH, 7:1), 1H NMR (400 MHz, Acetone-d6) δ 5.20 (s, 1H, H-9), 4.65 (d, J = 7.7 Hz, 1H, H-8), 3.99 (d, J = 12.3 Hz, 1H, H-8), 3.88 (d, J = 12.4 Hz, 1H, H-1′), 3.81 (dd, J = 11.6, 2.1 Hz, 1H, H-6′), 3.61 (dd, J = 11.6, 5.3 Hz, 1H, H-11), 3.43 (t, J = 8.3 Hz, 1H, H-5′), 3.37–3.30 (m, 3H, H-3′, H-4′, H-6′), 3.25–3.20 (m, 1H, H-2′), 2.46–2.37 (m, 2H, H-5, H-6), 2.02 (d, J = 12.3 Hz, 1H, H-3), 1.87–1.79 (m, 2H, H-3, H-6), 1.28 (s, 3H, H-10), 1.11 (dd, J = 6.1, 1.6 Hz, 6H, H-12, H-13). 13C NMR (101 MHz, Acetone-d6) δ 108.58 (C-4), 101.96 (C-9), 99.47 (C-1′), 88.88 (C-1), 86.07 (C-2), 78.27 (C-3′), 77.46 (C-5′), 74.81 (C-2′), 72.43 (C-11), 71.82 (C-4′), 66.91 (C-7), 63.04 (C-6′), 59.12 (C-8), 43.07 (C-3), 41.91 (C-5), 24.55 (C-12), 24.39 (C-13), 23.16 (C-6), 19.65 (C-10). ESI-HRMS: m/z calcd for C19H30O10Na [M + Na]+ 441.1732, found 441.1787.
4-Oxo-9-O-iso-propylpaeoniflorin (21): colorless syrup, 18.3 mg (38.9%, CH2Cl2: CH3OH, 22:1), Rf 0.34 (CH2Cl2: CH3OH, 7:1), 1H NMR (400 MHz, Chloroform-d) δ 7.92 (d, J = 7.3 Hz, 2H, H-2″, H-6″), 7.50 (t, J = 7.5 Hz, 1H, H-4″), 7.34 (t, J = 7.7 Hz, 2H, H-3″, H-5″), 5.21 (s, 1H, H-9), 4.78 (d, J = 11.7 Hz, 1H, H-8), 4.58–4.51 (m, 2H, H-8, H-1′), 3.81 (dd, J = 11.2, 5.1 Hz, 3H, H-6′, H-11), 3.68–3.61 (m, 2H, H-3′, H-4′), 3.49 (s, 1H, H-2′), 3.35 (s, 1H, H-5′), 2.95 (d, J = 7.0 Hz, 1H, H-6), 2.78 (t, J = 9.3 Hz, 1H, H-5), 2.67–2.53 (m, 2H, H-3), 1.98 (d, J = 10.8 Hz, 1H, H-6), 1.38 (s, 3H, H-10), 1.05 (d, J = 6.2 Hz, 3H, H-12), 0.90 (d, J = 6.1 Hz, 3H, H-13).13C NMR (101 MHz, Chloroform-d) δ 205.42 (C-4), 167.31 (C-7″), 133.85 (C-4″), 129.89 (C-2″,C-6″), 129.33 (C-1″), 128.64 (C-3″, C-5″), 102.97 (C-9), 98.73 (C-1′), 87.86 (C-1), 85.67 (C-2),76.47 (C-3′), 75.93 (C-5′), 73.69 (C-2′), 70.22 (C-11), 69.72 (C-4′), 63.45 (C-7), 63.12(C-8), 61.67 (C-6′), 49.01 (C-3), 45.80 (C-5), 26.76 (C-6), 22.58 (C-12), 21.39 (C-13), 20.78 (C-10). ESI-HRMS: m/z calcd for C26H34O11Na [M + Na]+ 545.1994, found 545.2084.
4-Oxo-9-O-iso-propyldebenzoylpaeoniflorin (22): colorless syrup, 14.5 mg (38.5%, CH2Cl2: CH3OH, 15:1), Rf 0.04 (CH2Cl2: CH3OH, 7:1), 1H NMR (400 MHz, Acetone-d6) δ 5.32 (s, 1H, H-9), 4.76 (d, J = 7.7 Hz, 1H, H-8), 4.01 (d, J = 12.5 Hz, 1H, H-6′), 3.81 (dd, J = 11.7, 2.6 Hz, 1H, H-11), 3.70 (d, J = 12.5 Hz, 1H, H-6′), 3.62 (dd, J = 11.7, 5.6 Hz, 1H, H-5′), 3.48 (t, J = 8.6 Hz, 1H, H-3), 3.40–3.34 (m, 2H, H-2′, H-4′), 3.33–3.25 (m, 2H, H-8, H-1′), 2.89 (dd, J = 11.2, 7.4 Hz, 1H, H-5), 2.74 (d, J = 18.2 Hz, 1H, H-6), 2.47–2.38 (m, 2H, H-3), 2.06 (s, 1H, H-6), 1.34 (s, 3H, H-10), 1.05 (dd, J = 7.8, 6.2 Hz, 6H, H-12, H-13). 13C NMR (101 MHz, Acetone-d6) δ 205.48 (C-4), 101.61 (C-9), 98.87 (C-1′), 87.72 (C-1), 86.89 (C-2), 78.16 (C-3′), 77.48 (C-5′), 74.52 (C-2′), 71.70 (C-11), 69.93 (C-4′), 65.09 (C-7), 62.91 (C-6′), 60.07 (C-8), 49.59 (C-3), 47.24 (C-5), 26.69 (C-6), 22.91 (C-12), 21.64 (C-13), 20.83 (C-10). ESI-HRMS: m/z calcd for C19H30O10Na [M + Na]+ 441,1732, found 441.1783.
2,3,4,6-Tetra-O-acetyl-4-O-butylpaeoniflorin (23): white solid, 31.4 mg (17.7%, petroleum: ethyl acetate, 4:1), mp 108.4 °C, Rf 0.39 (petroleum: ethyl acetate, 1:1), 1H NMR (400 MHz, Chloroform-d) δ 8.03 (d, J = 7.1 Hz, 2H, H-2″, H-6″), 7.60 (d, J = 14.9 Hz, 1H, H-4″), 7.47 (t, J = 7.7 Hz, 2H, H-3″, H-5″), 5.44 (s, 1H, H-9), 5.12–4.96 (m, 3H, H-2′, H-3′, H-4′), 4.75 (d, J = 7.8 Hz, 1H, H-1′), 4.59 (d, J = 12.0 Hz, 1H, H-8), 4.48 (d, J = 12.0 Hz, 1H, H-8), 4.18 (dd, J = 12.2, 2.6 Hz, 1H, H-6′), 4.11 (dd, J = 12.2, 5.3 Hz, 1H, H-6′), 3.61 (tt, J = 5.3, 2.8 Hz, 3H, H-11, H-5′), 2.77 (d, J = 8.5 Hz, 1H, H-5), 2.32 (dd, J = 10.7, 6.9 Hz, 1H, H-6), 2.07, 2.03, 2.02, 1.98 (m, 14H, 4COCH3, H-3), 1.79 (d, J = 10.7 Hz, 1H, H-6), 1.58–1.50 (m, 2H, H-13), 1.35 (m, 3H, H-12, H-10), 0.90 (t, J = 7.4 Hz, 3H, H-14). 13C NMR (101 MHz, Chloroform-d) δ 170.58, 170.36, 169.53, 169.43 (4CH3CO), 166.52 (C-7″), 133.58 (C-4″), 129.75 (C-2″, C-6″), 129.67 (C-1″), 128.76 (C-3″, C-5″), 107.65 (C-4), 101.20 (C-9), 96.44 (C-1′), 88.45 (C-1), 85.67 (C-2), 73.08 (C-3′), 71.84 (C-5′), 71.39 (C-2′), 69.86 (C-11), 68.49 (C-4′), 64.03 (C-7), 62.09 (C-6′), 60.27 (C-8), 41.63 (C-3), 40.92 (C-5), 32.01 (C-12), 22.34 (C-6), 20.86×2, 20.72×2 (4CH3CO), 20.69 (C-10), 19.28 (C-13), 13.94 (C-14). ESI-HRMS: m/z calcd for C35H44O15Na [M + Na]+ 727.2573, found 727.2635.
2,3,4,6-Tetra-O-acetyl-4-oxo-9-O-butylpaeoniflorin (24): white solid, 72.6 mg (40.9%, petroleum: ethyl acetate, 3:1). Rf 0.35 (petroleum: ethyl acetate, 1:1), mp 119.7 °C, 1H NMR (400 MHz, Chloroform-d) δ 8.01 (d, J = 8.5 Hz, 2H, H-2″, H-6″), 7.59 (t, J = 7.4 Hz, 1H, H-4″), 7.45 (t, J = 7.7 Hz, 2H, H-3″, H-5″), 5.17 (t, J = 9.4 Hz, 1H, H-2′), 5.10 (s, 1H, H-9), 5.08–5.00 (m, 2H, H-3′, H-4′), 4.78 (d, J = 7.9 Hz, 1H, H-1′), 4.56–4.47 (m, 2H, H-8), 4.19 (dd, J = 12.2, 2.5 Hz, 1H, H-6′), 4.13 (dd, J = 12.2, 5.4 Hz, 1H, H-6′), 3.72–3.62 (m, 2H, H-11, H-5′), 3.37 (dt, J = 9.5, 6.4 Hz, 1H, H-11), 3.04 (d, J = 7.3 Hz, 1H, H-5), 2.73–2.67 (m, 1H, H-6), 2.65 (d, J = 5.7 Hz, 2H, H-3,H-6), 2.08, 2.05, 2.03, 2.00 (m, 13H, 4COCH3, H-3), 1.44 (dt, J = 8.8, 6.2 Hz, 2H, H-13), 1.39 (s, 3H, H-10), 1.26 (d, J = 7.8 Hz, 2H, H-12), 0.83 (t, J = 7.3 Hz, 3H, H-14).13C NMR (101 MHz, Chloroform-d) δ 204.90 (C-4), 170.52, 170.37, 169.54, 169.46 (4CH3CO), 166.50 (C-7″), 133.61(C-4″), 129.83 (C-2″, C-6″), 129.53 (C-1″), 128.68 (C-3″, C-5″), 104.94 (C-9), 96.32 (C-1′), 87.96 (C-1), 85.77 (C-2), 73.01 (C-3′), 72.08 (C-5′), 71.54 (C-2′), 68.64 (C-11), 68.41 (C-4′), 63.15 (C-7), 62.43 (C-8), 62.11 (C-6′), 48.94 (C-3), 46.92 (C-5), 31.48 (C-12), 26.31 (C-6), 20.85, 20.77, 20.72×2 (4CH3CO), 20.51 (C-10), 19.32 (C-13), 13.92 (C-14). ESI-HRMS: m/z calcd for C35H44O15Na [M + Na]+ 727.2573, found 727.2633.
4-O-butylpaeoniflorin (25): colorless syrup, 21.7 mg (44.9%, CH2Cl2: CH3OH, 22:1), Rf 0.34 (CH2Cl2: CH3OH, 7:1). 1H NMR (400 MHz, Chloroform-d) δ 7.92 (d, J = 7.2 Hz, 2H, H-2″, H-6″), 7.48 (t, J = 7.5 Hz, 1H, H-4″), 7.34 (t, J = 7.6 Hz, 2H, H-3″, H-5″), 5.46 (s, 1H, H-9), 4.66 (t, J = 8.5 Hz, 2H, H-8, H-1′), 4.50 (d, J = 7.2 Hz, 1H, H-8), 3.79 (d, J = 10.4 Hz, 2H, H-6′), 3.62–3.47 (m, 4H, H-3′, H-4′, H-11), 3.41 (s, 1H, H-2′), 3.29 (s, 1H, H-5′), 2.66 (d, J = 6.3 Hz, 1H, H-6), 2.38 (d, J = 10.0 Hz, 1H, H-5), 1.94 (s, 2H, H-3), 1.78 (d, J = 10.4 Hz, 1H, H-6), 1.50 (p, J = 6.9 Hz, 2H, H-12), 1.36–1.27 (m, 5H, H-10, H-13), 0.87 (t, J = 7.3 Hz, 3H, H-14). 13C NMR (101 MHz, Chloroform-d) δ 167.12 (C-7″), 133.45 (C-4″), 129.85 (C-2″, C-6″), 129.64 (C-1″), 128.62 (C-3″, C-5″), 107.72 (C-4), 101.25 (C-9), 98.90 (C-1′), 88.48 (C-1), 85.73 (C-2), 76.38 (C-3′), 75.75 (C-5′), 73.57 (C-2′), 70.10 (C-11), 69.53 (C-4′), 63.74 (C-7), 61.67 (C-6′), 60.94 (C-8), 41.81 (C-3), 40.27 (C-5), 32.01 (C-12), 29.84 (C-6), 19.55 (C-10), 19.26 (C-13), 13.94 (C-14). ESI-HRMS: m/z calcd for C27H36O11Na [M + Na]+ 559.2150, found 559.2115.
4-Oxo-9-O-butylpaeoniflorin (26): colorless syrup, 25.1 mg (42.5%, CH2Cl2: CH3OH, 23:1), Rf 0.35 (CH2Cl2: CH3OH, 7:1), 1H NMR (400 MHz, Chloroform-d) δ 7.90 (d, J = 7.2 Hz, 2H, H-2″, H-6″), 7.50 (t, J = 7.5 Hz, 1H, H-4″), 7.33 (t, J = 7.7 Hz, 2H, H-3″, H-5″), 5.09 (s, 1H, H-9), 4.75 (d, J = 11.7 Hz, 1H, H-8), 4.59–4.51 (m, 2H, H-8, H-1′), 3.84 (s, 2H, H-6′), 3.66 (d, J = 6.9 Hz, 2H, H-11), 3.58 (t, J = 7.9 Hz, 1H, H-3′), 3.50 (s, 1H, H-4′), 3.38–3.34 (m, 1H, H-2′), 3.25 (q, J = 7.3, 6.5 Hz, 1H, H-5′), 2.97 (d, J = 7.1 Hz, 1H, H-6), 2.79 (d, J = 9.6 Hz, 1H, H-5), 2.67–2.49 (m, 2H, H-3), 1.99 (d, J = 10.8 Hz, 1H, H-6), 1.36 (d, J = 20.3 Hz, 5H, H-10, H-12), 1.18 (q, J = 7.5 Hz, 2H, H-13), 0.77 (t, J = 7.3 Hz, 3H, H-14). 13C NMR (101 MHz, Chloroform-d) δ 205.17 (C-4), 167.30 (C-7″), 133.71 (C-4″), 129.85 (C-2″, C-6″), 129.37 (C-1″), 128.63 (C-3″, C-5″), 105.35 (C-9), 98.74 (C-1′), 87.98 (C-1), 85.82 (C-2), 76.55 (C-3′), 75.92 (C-5′), 73.69 (C-2′), 69.62 (C-11), 68.52 (C-4′), 63.49 (C-7), 63.30 (C-8), 61.64 (C-6′), 48.87 (C-3), 46.99 (C-5), 31.48 (C-12), 26.62 (C-6), 20.74 (C-10), 19.29 (C-13), 13.90 (C-14). ESI-HRMS: m/z calcd for C27H36O11Na [M + Na]+ 559.2150, found 559.2146.
4-Oxo-9-O-butyldebenzoylpaeoniflorin (27): white solid, 17.8 mg (37.4%, CH2Cl2: CH3OH, 15:1), Rf 0.06 (CH2Cl2: CH3OH, 7:1), mp 125.9 °C, 1H NMR (400 MHz, Acetone-d6) δ 5.18 (s, 1H, H-9), 4.76 (d, J = 7.7 Hz, 1H, H-8), 4.02 (d, J = 12.5 Hz, 1H, H-6′), 3.81 (dd, J = 11.7, 2.6 Hz, 1H, H-11), 3.72 (d, J = 12.6 Hz, 1H, H-6′), 3.66–3.59 (m, 2H, H-11, H-5′), 3.49–3.44 (m, 1H, H-3′), 3.39–3.33 (m, 2H, H-2′, H-4′), 3.32–3.25 (m, 2H, H-8,H-1′), 2.89 (dd, J = 11.1, 7.6 Hz, 1H, H-5), 2.74 (d, J = 16.7 Hz, 1H, H-6), 2.49 (d, J = 7.4 Hz, 1H, H-3), 2.38 (d, J = 17.9 Hz, 1H, H-3), 2.09 (s, 1H, H-6), 1.47–1.39 (m, 2H, H-12), 1.34 (s, 3H, H-10), 1.33–1.28 (m, 2H, H-13), 0.87 (t, J = 7.3 Hz, 3H, H-14). 13C NMR (101 MHz, Acetone-d6) δ 205.30 (C-4), 104.11 (C-9), 98.88 (C-1′), 88.19 (C-1), 86.95 (C-2), 78.17 (C-3′), 77.49 (C-5′), 74.50 (C-2′), 71.72 (C-11), 68.59 (C-4′), 65.33 (C-7), 62.93 (C-6′), 60.19 (C-8), 49.52 (C-3), 47.19 (C-5), 32.32 (C-12), 26.65 (C-6), 20.77 (C-10), 19.82 (C-13), 14.10 (C-14). ESI-HRMS: m/z calcd for C20H32O10Na [M + Na]+ 455.1888, found 455.1990.
2,3,4,6-Tetra-O-acetyl-4-O-sec-butylpaeoniflorin (28): white solid, 68 mg (37.0%, petroleum: ethyl acetate, 4:1), mp 110.9 °C, Rf 0.40 (petroleum: ethyl acetate, 1:1), 1H NMR (400 MHz, Chloroform-d) δ 8.03 (d, J = 8.1 Hz, 2H, H-2″, H-6″), 7.60 (t, J = 7.4 Hz, 1H, H-4″), 7.48 (t, J = 7.7 Hz, 2H, H-3″, H-5″), 5.42 (s, 1H, H-9), 5.11–4.95 (m, 3H, H-2′, H-3′, H-4′), 4.74 (d, J = 7.7 Hz, 1H, H-1′), 4.59 (d, J = 11.9 Hz, 1H, H-8), 4.49 (d, J = 11.9 Hz, 1H, H-8), 4.19–4.08 (m, 2H, H-6′), 3.85 (dq, J = 12.1, 6.1 Hz, 1H, H-11), 3.59 (dq, J = 8.1, 5.2, 4.2 Hz, 1H, H-5′), 2.73 (t, J = 5.9 Hz, 1H, H-5), 2.32 (dt, J = 12.0, 6.4 Hz, 1H, H-6), 2.07, 2.02, 2.01, 1.97 (14H, 4COCH3, H-3), 1.80 (dd, J = 10.6, 4.0 Hz, 1H, H-6), 1.57–1.48 (m, 1H, H-12), 1.43 (dt, J = 14.4, 7.3 Hz, 1H, H-12), 1.34 (s, 3H, H-10), 1.19–1.14 (m, 3H, H-13), 0.86 (q, J = 7.1 Hz, 3H, H-14). 13C NMR (101 MHz, Chloroform-d) δ 170.56, 170.34, 169.52, 169.42 (4CH3CO), 166.51 (C-7″), 133.57 (C-4″), 129.74 (C-2″, C-6″), 128.77 (C-1″, C-3″, C-5″), 107.91, 107.82 (C-4), 101.24, 101.08 (C-9), 96.48 (C-1′), 88.42 (C-1), 85.71 (C-2), 73.11 (C-3′), 72.42, 72.27 (C-11), 71.86 (C-5′), 71.43, 71.41 (C-2′), 69.67, 69.50 (C-7), 68.55 (C-4′), 62.14 (C-6′), 60.37 (C-8), 42.14 (C-3), 41.48 (C-5), 30.70, 30.65 (C-12), 22.42 (C-6), 21.84, 21.67 (C-13), 20.83, 20.72, 20.69, 20.68 (4CH3CO), 19.29 (C-10), 10.23, 10.04 (C-14). ESI-HRMS: m/z calcd for C35H44O15Na [M + Na]+ 727.2573, found 727.2539.
2,3,4,6-Tetra-O-acetyl-4-oxo-9-O-sec-butylpaeoniflorin (29): white solid, 50.7 mg (27.6%, petroleum: ethyl acetate = 3:1). mp 123.7 °C, Rf 0.37 (petroleum: ethyl acetate, 1:1), 1H NMR (400 MHz, Chloroform-d) δ 8.01 (d, J = 8.0 Hz, 2H, H-2″, H-6″), 7.58 (t, J = 7.4 Hz, 1H, H-4″), 7.44 (t, J = 7.6 Hz, 2H, H-3″, H-5″), 5.21 (d, J = 10.7 Hz, 1H, H-9), 5.16 (dt, J = 9.4, 4.7 Hz, 1H, H-2′), 5.03 (qd, J = 9.4, 2.1 Hz, 2H, H-3′, H-4′), 4.77 (d, J = 7.8 Hz, 1H, H-1′), 4.55–4.46 (m, 2H, H-8), 4.20–4.09 (m, 2H, H-6′), 3.69 (dq, J = 23.0, 7.1, 6.6 Hz, 2H, H-11, H-5′), 3.03 (d, J = 7.0 Hz, 1H, H-5), 2.68 (d, J = 12.3 Hz, 3H, H-3, H-6), 2.08, 2.07, 2.05, 2.02, 1.99 (m, 13H, 4COCH3, H-3), 1.56–1.40 (m, 2H, H-12), 1.37 (s, 3H, H-10), 1.11 (d, J = 6.2 Hz, 1H, H-13), 0.97 (d, J = 6.1 Hz, 2H, H-13), 0.83 (t, J = 7.5 Hz, 2H, H-14), 0.72 (t, J = 7.4 Hz, 1H, H-14). 13C NMR (101 MHz, Chloroform-d) δ 205.16, 205.08 (C-4), 170.50, 170.34, 169.52, 169.45 (4CH3CO), 166.53, 166.49 (C-7″), 133.60 (C-4″), 129.84 (C-2″, C-6″), 129.50 (C-1″), 128.66 (C-3″, C-5″), 103.70, 102.24 (C-9), 96.31 (C-1′), 88.06, 87.94 (C-1), 85.64, 85.59 (C-2), 76.19, 74.89 (C-11), 73.03 (C-2′), 72.06 (C-5′), 71.55 (C-3′), 68.45 (C-4′), 63.00, 62.93 (C-7), 62.47, 62.45 (C-8), 62.13(C-6′), 49.06, 49.03 (C-6), 46.93 (C-5), 29.38, 28.58 (C-12), 26.41, 26.31 (C-3), 20.83, 20.76, 20.74, 20.69 (4CH3CO), 20.58 (C-10), 19.77, 18.04 (C-13), 9.52, 9.34 (C-14). ESI-HRMS: m/z calcd for C35H44O15Na [M + Na]+ 727.2573, found 727.2544.
4-O-sec-butylpaeoniflorin (30): white solid, 31.8 mg (49.4%, CH2Cl2: CH3OH, 23:1), Rf 0.36 (CH2Cl2: CH3OH, 7:1), mp 117.2 °C, 1H NMR (400 MHz, Chloroform-d) δ 7.93 (d, J = 8.5 Hz, 2H, H-2″, H-6″), 7.48 (t, J = 7.5 Hz, 1H, H-4″), 7.34 (t, J = 6.9 Hz, 2H, H-3″, H-5″), 5.45 (s, 1H, H-9), 4.65 (q, J = 12.1 Hz, 2H, H-8, H-1′), 4.49 (d, J = 7.5 Hz, 1H, H-8), 3.85–3.73 (m, 3H, H-6′, H-11), 3.61 (m, 2H, H-3′, H-4′), 3.46–3.38 (m, 1H, H-2′), 3.28 (d, J = 8.2 Hz, 1H, H-5′), 2.63 (d, J = 6.4 Hz, 1H, H-6), 2.35 (t, J = 7.9 Hz, 1H, H-5), 2.01–1.88 (m, 2H, H-3), 1.76 (d, J = 10.7 Hz, 1H, H-6), 1.53–1.37 (m, 2H, H-12), 1.31 (s, 3H, H-10), 1.11 (d, J = 6.1 Hz, 3H, H-13), 0.82 (q, J = 5.7 Hz, 3H, H-14). 13C NMR (101 MHz, Chloroform-d) δ 167.07 (C-7″), 133.42 (C-4″), 129.82 (C-2″, C-6″), 129.69 (C-1″), 128.61 (C-3″, C-5″), 107.92, 107.88 (C-4), 101.23, 101.14 (C-9), 98.89 (C-1′), 88.40, 88.37 (C-1), 85.78, 85.74 (C-2), 76.33 (C-3′), 75.73 (C-5′), 73.58 (C-2′), 72.09, 72.03 (C-11), 69.96, 69.80 (C-7), 69.65 (C-4′), 61.68 (C-6′), 60.93 (C-8), 42.64, 42.31 (C-3), 41.33, 40.89 (C-5), 30.67, 30.63 (C-12), 22.75 (C-6), 21.83, 21.57 (C-13), 19.53 (C-10), 10.19, 10.03 (C-14). ESI-HRMS: m/z calcd for C27H36O11Na [M + Na]+ 559.2150, found 559.2239.
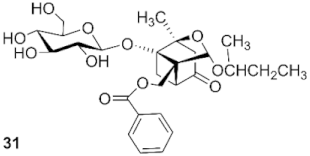
4-O-sec-butyldebenzoylpaeoniflorin (31): colorless syrup, 20.4 mg (47.2%, CH2Cl2: CH3OH, 16:1), Rf 0.06 (CH2Cl2: CH3OH, 7:1), 1H NMR (400 MHz, Acetone-d6) δ 5.20 (d, J = 3.1 Hz, 1H, H-9), 4.66 (d, J = 7.7 Hz, 1H, H-8), 3.99 (d, J = 12.4 Hz, 1H, H-8), 3.89 (d, J = 8.3 Hz, 1H,H-1′), 3.80 (d, J = 11.5 Hz, 1H, H-6′), 3.62 (dd, J = 12.1, 4.3 Hz, 1H, H-6′), 3.52 (d, J = 7.2 Hz, 1H, H-11), 3.45 (d, J = 8.4 Hz, 1H, H-5′), 3.33 (d, J = 5.3 Hz, 2H, H-3′, H-4′), 3.24 (t, J = 8.3 Hz, 1H, H-2′), 2.51–2.37 (m, 2H, H-6, H-5), 2.08 (d, J = 5.0 Hz, 2H, H-3), 1.82 (d, J = 10.3 Hz, 1H, H-6), 1.43 (m, 2H, H-12), 1.29 (s, 3H, H-10), 1.10 (dd, J = 6.1, 2.9 Hz, 3H, H-13), 0.85 (td, J = 7.5, 3.2 Hz, 3H, H-14). 13C NMR (101 MHz, Acetone-d6) δ 128.71, 108.66 (C-4), 102.05, 101.80 (C-9), 99.49 (C-1′), 88.94 (C-1), 86.08 (C-2), 78.08 (C-3′), 77.44 (C-5′), 74.71 (C-2′), 72.41, 72.33 (C-11), 71.80, 71.72, 71.63 (C-7), 62.95 (C-4′), 59.15 (C-6′), 59.04 (C-8), 43.46, 43.05 (C-3), 42.29, 41.48 (C-5), 31.33, 31.25 (C-12), 23.18 (C-6), 22.19, 22.02 (C-13), 19.69 (C-10), 10.29, 10.14 (C-14). ESI-HRMS: m/z calcd for C20H32O10Na [M + Na]+ 455.47, found 455.19.
4-oxo-9-O-sec-butylpaeoniflorin (32): colorless syrup, 27.5 mg (46.6%, CH2Cl2: CH3OH, 22:1), Rf 0.37 (CH2Cl2: CH3OH, 7:1), 1H NMR (400 MHz, Chloroform-d) δ 7.92 (d, J = 8.0 Hz, 2H, H-2″, H-6″), 7.50 (t, J = 7.5 Hz, 1H, H-4″), 7.34 (t, J = 7.7 Hz, 2H, H-3″, H-5″), 5.22 (d, J = 13.5 Hz, 1H, H-9), 4.77 (t, J = 12.3 Hz, 1H, H-8), 4.56 (d, J = 8.0 Hz, 2H, H-8, H-1′), 3.85 (t, J = 13.4 Hz, 2H, H-6′), 3.64 (m, 3H, H-3′, H-4′, H-11), 3.49 (s, 1H, H-2′), 3.36 (s, 1H, H-5′), 2.96 (d, J = 7.1 Hz, 1H, H-6), 2.79 (t,J = 9.4 Hz, 1H, H-5), 2.68–2.55 (m, 2H, H-3), 1.99 (d, J = 11.8 Hz, 1H, H-6), 1.47–1.32 (m, 5H, H-10, H-12), 1.06 (d, J = 6.2 Hz, 1H, H-13), 0.86 (d, J = 6.1 Hz, 2H, H-13), 0.78 (t, J = 7.4 Hz, 2H, H-14), 0.63 (t, J = 7.4 Hz, 1H, H-14). 13C NMR (101 MHz, Chloroform-d) δ 205.47 (C-4), 167.39 (C-7″), 133.74 (C-4″), 129.91 (C-2″, C-6″), 129.31 (C-1″), 128.63 (C-3″, C-5″), 104.19, 102.66 (C-9), 98.72 (C-1′), 88.01, 87.94 (C-1), 85.66, 85.57 (C-2), 76.50, 76.17 (C-11), 75.93 (C-3′), 74.75 (C-5′), 73.67 (C-2′), 69.70 (C-4′), 63.15(C-7), 63.11 (C-8), 61.68 (C-6′), 48.97 (C-6), 46.99 (C-5), 29.83, 29.33 (C-12), 28.59 (C-3), 20.80 (C-10), 19.74, 17.95 (C-13), 9.43, 9.23 (C-14). ESI-HRMS: m/z calcd for C27H36O11Na [M + Na]+ 558.2150, found 559.2246.
4-oxo-9-O-sec-butyldebenzoylpaeoniflorin (33): white solid, 17.5 mg (36.8%, CH2Cl2: CH3OH, 15:1), mp 98.2 °C, Rf 0.05 (CH2Cl2: CH3OH, 7:1), 1H NMR (400 MHz, Acetone-d6) δ 5.33 (d, J = 16.3 Hz, 1H, H-9), 4.76 (d, J = 9.5 Hz, 1H, H-8), 4.02 (dd, J = 12.5, 5.9 Hz, 1H, H-6′), 3.81 (d, J = 9.3 Hz, 1H, H-11), 3.73–3.59 (m, 3H, H-6′, H-5′, H-4′), 3.46 (t, J = 8.8 Hz, 1H, H-3′), 3.32 (m, 3H, H-2′, H-1′, H-8), 2.89 (t, J = 11.0 Hz, 1H, H-5), 2.74 (dd, J = 17.7, 3.2 Hz, 1H, H-6), 2.43 (dd, J = 17.4, 4.3 Hz, 2H, H-3), 2.08 (d, J = 3.9 Hz, 1H, H-6), 1.41 (m, 2H, H-12), 1.34 (s, 3H, H-10), 1.05 (dd, J = 21.8, 6.2 Hz, 3H, H-13), 0.83 (q, J = 7.7 Hz, 3H, H-14). 13C NMR (101 MHz, Acetone-d6) δ 205.62, 205.44 (C-4), 103.33, 101.47 (C-9), 98.88 (C-1′), 88.20, 87.95 (C-1), 86.89 (C-2), 78.22, 77.50 (C-11), 76.37 (C-3′), 75.06 (C-5′), 74.53 (C-2′), 71.74 (C-4′), 65.32, 65.15 (C-7), 62.96 (C-6′), 60.23, 60.04 (C-8), 59.64 (C-3), 49.68, 49.62 (C-5), 47.34, 47.23 (C-12), 26.81, 26.71 (C-6), 20.87 (C-10), 20.56, 18.80 (C-13), 10.01, 9.64 (C-14). ESI-HRMS: m/z calcd for C20H32O10Na [M + Na]+ 455.1888, found 455.1910.
2,3,4,6-Tetra-O-acetyl-4-O-benzylpaeoniflorin (34): white solid, 25.2 mg (22.9%, petroleum: ethyl acetate, 6:1) Rf 0.40 (petroleum: ethyl acetate, 1.5:1), mp 172.4 °C, 1H NMR (400 MHz, Chloroform-d) δ 8.04 (d, J = 7.0 Hz, 2H, H-2″, H-6″), 7.61 (t, J = 7.4 Hz, 1H, H-4″), 7.49 (d, J = 7.9 Hz, 2H, H-3″, H-5″), 7.34–7.26 (m, 5H, H-2″′, H-3″′, H-4″′, H-5′″, H-6″′), 5.50 (s, 1H, H-9), 5.12–4.97 (m, 3H, H-2′, H-3′, H-4′), 4.75 (d, J = 7.8 Hz, 1H, H-1′), 4.71 (s, 2H, H-11), 4.61 (d, J = 12.0 Hz, 1H, H-8), 4.50 (d, J = 11.9 Hz, 1H, H-8), 4.18 (dd, J = 12.2, 2.6 Hz, 1H, H-6′), 4.11 (dd, J = 12.2, 5.4 Hz, 1H, H-6′), 3.64–3.58 (m, 1H, H-5′), 2.85 (d, J = 6.8 Hz, 1H, H-5), 2.33 (dd, J = 10.8, 6.9 Hz, 1H, H-6), 2.16 (d, J = 10.6 Hz, 1H, H-3), 2.08–1.97 (m, 13H, 4COCH3, H-3), 1.82 (d, J = 10.6 Hz, 1H, H-6), 1.37 (s, 3H, H-10). 13C NMR (101 MHz, Chloroform-d) δ 170.56, 170.35, 169.53, 169.44 (4CH3CO), 166.53 (C-7″), 137.81 (C-1″′), 133.61 (C-4″), 129.77 (C-2″, C-6″), 129.67 (C-1″), 128.80 (C-3″, C-5″), 128.61 (C-3″′, C-5″′), 127.93 (C-4″′), 127.73 (C-2″′, C-6″′), 107.83 (C-4), 101.31 (C-9), 96.49 (C-1′), 88.46 (C-1), 85.77 (C-2), 73.09 (C-3′), 71.90 (C-5′), 71.43 (C-2′), 70.01 (C-7), 68.53 (C-4′), 66.20 (C-11), 62.12 (C-6′), 60.22 (C-8), 41.93 (C-3), 40.98 (C-5), 22.36 (C-6), 20.87, 20.72, 20.70×2 (4CH3CO), 19.26 (C-10). ESI-HRMS: m/z calcd for C38H42O15Na [M + Na]+ 761.2416, found 761.2439.
2,3,4,6-Tetra-O-acetyl-4-oxo-9-O-benzylpaeoniflorin (35): white solid, 55.7 mg (53.4%, petroleum: ethyl acetate, 4:1). Rf 0.30 (petroleum: ethyl acetate, 1.5:1), mp 140.4 °C, 1H NMR (400 MHz, Chloroform-d) δ 7.94 (d, J = 7.8 Hz, 2H, H-2″, H-6″), 7.58 (t, J = 7.5 Hz, 1H, H-4″), 7.38 (t, J = 7.7 Hz, 2H, H-3″, H-5″), 7.23 (m, 5H, H-2″′, H-3″′, H-4″′, H-5′″, H-6″′), 5.25–5.16 (m, 2H, H-9, H-2′), 5.06 (q, J = 9.0, 8.2 Hz, 2H, H-3′, H-4′), 4.83–4.75 (m, 2H, H-1′, H-8), 4.56–4.45 (m, 3H, H-8, H-11), 4.23–4.12 (m, 2H, H-6′), 3.71–3.64 (m, 1H, H-5′), 3.11 (d, J = 7.4 Hz, 1H, H-5), 2.72 (s, 2H, H-6, H-3), 2.10–1.97 (m, 14H, 4COCH3, H-6, H-3), 1.44 (s, 3H, H-10). 13C NMR (101 MHz, Chloroform-d) δ 204.87 (C-4), 170.52, 170.36, 169.54, 169.48 (4CH3CO), 166.50 (C-7″), 137.04 (C-1″′), 133.56 (C-4″), 129.86 (C-1″, C-2″, C-6″), 128.66 (C-3″, C-5″), 128.43 (C-3″′, C-5″′), 127.77 (C-2″′, C-4″′, C-6″′), 104.13 (C-9), 96.33 (C-1′), 88.06 (C-1), 86.22 (C-2), 73.01 (C-2′), 72.11 (C-5′), 71.54 (C-3′), 70.26 (C-8), 68.44 (C-4′), 63.14 (C-7), 62.38 (C-11), 62.12 (C-6′), 48.97 (C-5), 46.96 (C-3), 26.31 (C-6), 20.83, 20.76, 20.71×2 (4CH3CO), 20.53 (C-10). ESI-HRMS: m/z calcd for C38H42O15Na [M + Na]+ 761.2416, found 761.2444.
4-O-benzyldebenzoylpaeoniflorin (36): white solid, 15.3 mg (32.8%, CH2Cl2: CH3OH, 22:1), Rf 0.08 (CH2Cl2: CH3OH, 7:1), mp 214.7 °C, 1H NMR (400 MHz, Acetone-d6) δ 7.39–7.23 (m, 5H, H-2″′, H-3″′, H-4″′, H-5″′, H-6″′), 5.28 (s, 1H, H-9), 4.73–4.64 (m, 3H, H-8, H-1′), 4.03 (d, J = 12.3 Hz, 1H, H-11), 3.93 (d, J = 12.3 Hz, 1H, H-11), 3.82 (d, J = 11.7 Hz, 1H, H-6′), 3.63 (dd, J = 12.0, 4.2 Hz, 1H, H-6′), 3.46 (t, J = 8.4 Hz, 1H, H-3′), 3.34 (d, J = 4.7 Hz, 2H, H-4′, H-5′), 3.24 (t, J = 9.2 Hz, 1H, H-2′), 2.61 (d, J = 8.6 Hz, 1H, H-6), 2.44 (dd, J = 10.7, 6.9 Hz, 1H, H-5), 2.13 (d, J = 12.2 Hz, 1H, H-3), 1.95 (d, J = 12.2 Hz, 1H, H-3), 1.87 (d, J = 10.7 Hz, 1H, H-6), 1.32 (s, 3H, H-10). 13C NMR (101 MHz, Acetone-d6) δ 139.66 (C-1″′), 129.03 (C-3″′, C-5″′), 128.23 (C-2″′, C-6″′), 128.13 (C-4″′), 108.66 (C-4), 102.08 (C-9), 99.47 (C-1′), 88.96 (C-1), 86.21 (C-2), 78.18 (C-3′), 77.44 (C-5′), 74.80 (C-2′), 72.84 (C-7), 71.78 (C-4′), 66.02 (C-11), 62.99 (C-6′), 59.07 (C-8), 42.82 (C-3), 41.07 (C-5), 23.25 (C-6), 19.64 (C-10). ESI-HRMS: m/z calcd for C23H30O10Na [M + Na]+ 489.1732, found 489.1752.
4-Oxo-9-O-benzylpaeoniflorin (37): colorless syrup, 29.0 mg (46.2%, CH2Cl2: CH3OH, 23:1), Rf 0.35 (CH2Cl2: CH3OH, 7:1), 1H NMR (400 MHz, Chloroform-d) δ 7.76 (d, J = 7.7 Hz, 2H, H-2″, H-6″), 7.40 (t, J = 7.5 Hz, 1H, H-4″), 7.13 (m, 7H, H-3″, H-5″, H-2″′, H-3″′, H-4″′, H-5″′, H-6″′), 5.20 (s, 1H, H-9), 4.69 (dd, J = 12.1, 6.8 Hz, 2H, H-11), 4.55 (d, J = 11.2 Hz, 2H, H-8, H-1′), 4.33 (d, J = 12.1 Hz, 1H, H-8), 3.83 (s, 2H, H-6′), 3.66 (s, 2H, H-3′, H-4′), 3.49 (s, 1H, H-2′), 3.38 (s, 1H, H-5′), 3.00 (d, J = 6.8 Hz, 1H, H-6), 2.81 (s, 1H, H-5), 2.72–2.55 (q, J = 22.3 Hz, 2H, H-3), 2.02 (d, J = 10.6 Hz, 1H, H-6), 1.41 (s, 3H, H-10). 13C NMR (101 MHz, Chloroform-d) δ 205.40 (C-4), 167.35 (C-7′′), 137.17 (C-1″′), 133.62 (C-4″), 129.84 (C-2″′, C-6″′), 129.16 (C-4″′), 128.59 (C-3″′, C-5″′), 128.32 (C-3″, C-5″), 127.56 (C-1″), 127.42 (C-2″, 6″), 104.95 (C-9), 98.69 (C-1′), 87.98 (C-1), 86.33 (C-2), 76.49 (C-2′), 75.92 (C-5′), 73.72 (C-3′), 70.34 (C-8), 69.71 (C-4′), 63.57 (C-7), 63.27 (C-11), 61.59 (C-6′), 48.90 (C-5), 47.06 (C-3), 26.62 (C-6), 20.72 (C-10). ESI-HRMS: m/z calcd for C30H34O11Na [M + Na]+ 593.1994, found 593.2039.
4-Oxo-9-O-benzyldebenzoylpaeoniflorin (38): white solid, 18.8 mg (36.6%, CH2Cl2: CH3OH, 16:1), Rf 0.08 (CH2Cl2: CH3OH, 7:1), mp 169.4 °C, 1H NMR (400 MHz, Acetone-d6) δ 7.51–7.37 (m, 5H, H-2″′, H-3″′, H-4″′, H-5″′, H-6″′), 5.50 (s, 1H, H-9), 4.64 (d, J = 12.2 Hz, 1H, H-8), 4.20 (d, J = 12.5 Hz, 1H, H-6′), 3.99 (dd, J = 11.7, 2.6 Hz, 1H, H-11), 3.91 (d, J = 12.5 Hz, 1H, H-6′), 3.79 (dd, J = 11.7, 5.6 Hz, 1H, H-11), 3.70–3.62 (m, 2H, H-4′, H-5′), 3.50 (m, 4H, H-2′, H-3′, H-8, H-1′), 3.10 (dd, J = 11.1, 7.5 Hz, 1H, H-5), 2.97 (d, J = 18.0 Hz, 1H, H-6), 2.73 (d, J = 7.4 Hz, 1H, H-3), 2.64 (d, J = 18.0 Hz, 1H, H-6), 2.27 (d, J = 11.0 Hz, 1H, H-3), 1.55 (s, 3H, H-10). 13C NMR (101 MHz, Acetone-d6) δ 205.58 (C-4), 139.16 (C-1″′), 128.92 (C-3″′, C-5″′), 128.25 (C-2″′, C-6″′), 128.02 (C-4″′), 103.88 (C-9), 98.94 (C-1′), 88.32 (C-1), 87.42 (C-2), 78.24 (C-2′), 77.53 (C-5′), 74.58 (C-3′), 71.80 (C-8), 70.63 (C-4′), 65.46 (C-7), 63.01 (C-11), 60.18 (C-6′), 49.59 (C-5), 47.29 (C-3), 26.74 (C-6), 20.78 (C-10). ESI-HRMS: m/z calcd for C23H30O10Na [M + Na]+ 489.1732, found 489.1763.
2,3,4,6-Tetra-O-acetyl-4-O-ethylbenzenepaeoniflorin (39): white solid, 32.8 mg (30.7%, petroleum: ethyl acetate, 6:1), Rf 0.42 (petroleum: ethyl acetate, 1.5:1), mp 192.3 °C, 1H NMR (400 MHz, Chloroform-d) δ 8.02 (d, J = 8.5 Hz, 2H, H-2″, H-6″), 7.60 (t, J = 7.5 Hz, 1H, H-4″), 7.46 (t, J = 7.8 Hz, 2H, H-3″, H-5″), 7.26–7.16 (m, 5H, H-2″′, H-3″′, H-4″′, H-5′″, H-6″′), 5.43 (s, 1H, H-9), 5.11–4.96 (m, 3H, H-2′, H-3′, H-4′), 4.73 (d, J = 7.8 Hz, 1H, H-1′), 4.58 (d, J = 12.0 Hz, 1H, H-8), 4.47 (d, J = 12.0 Hz, 1H, H-8), 4.16 (d, J = 2.6 Hz, 1H, H-6′), 4.11 (dd, J = 12.2, 5.3 Hz, 1H, H-6′), 3.83 (t, J = 6.8 Hz, 2H, H-11), 3.63–3.57 (m, 1H, H-5′), 2.88 (t, J = 7.7 Hz, 2H, H-12), 2.72 (d, J = 8.6 Hz, 1H, H-5), 2.28 (dd, J = 10.8, 7.0 Hz, 1H, H-6), 2.06, 2.03, 2.02, 1.98 (m, 13H, 4COCH3, H-3), 1.93 (d, J = 12.5 Hz, 1H, H-3), 1.75 (d, J = 10.7 Hz, 1H, H-6), 1.33 (s, 3H, H-10). 13C NMR (101 MHz, Chloroform-d) δ 170.54, 170.33, 169.51, 169.41 (4CH3CO), 166.49 (C-7″), 138.50 (C-1″′), 133.57 (C-4″), 129.74 (C-2″, C-6″), 129.66 (C-1″), 129.06 (C-3″, C-5″), 128.76 (C-3″′, C-5″′), 128.48 (C-2″′, C-6″′), 126.45 (C-4″′), 107.67 (C-4), 101.21 (C-9), 96.45 (C-1′), 88.44 (C-1), 85.65 (C-2), 73.07 (C-3′), 71.87 (C-5′), 71.41 (C-2′), 69.87 (C-7), 68.52 (C-4′), 64.97 (C-11), 62.10 (C-6′), 60.22 (C-8), 41.66 (C-3), 40.75 (C-5), 36.58 (C-12), 22.31 (C-6), 20.85, 20.70, 20.68×2 (4CH3CO), 19.21 (C-10). ESI-HRMS: m/z calcd for C39H44O15Na [M + Na]+ 775.2573, found 775.2615.
2,3,4,6-Tetra-O-acetyl-4-oxo-9-O-ethylbenzenepaeoniflorin (40): colorless syrup, 50.8 mg (47.6%, petroleum: ethyl acetate, 4:1), Rf 0.34 (petroleum: ethyl acetate, 1.5:1), 1H NMR (400 MHz, Chloroform-d) δ 7.96 (d, J = 6.9 Hz, 2H, H-2″, H-6″), 7.62–7.55 (m, 1H, H-4″), 7.43 (t, J = 7.8 Hz, 2H, H-3″, H-5″), 7.25–7.18 (m, 2H, H-2″′, H-3″′), 7.18–7.10 (m, 3H, H-4″′, H-5′″, H-6″′), 5.16 (t, J = 9.4 Hz, 1H, H-2′), 5.10 (s, 1H, H-9), 5.07–4.99 (m, 2H, H-3′, H-4′), 4.77 (d, J = 7.8 Hz, 1H, H-1′), 4.53–4.44 (m, 2H, H-8), 4.19 (dd, J = 12.2, 2.6 Hz, 1H, H-6′), 4.12 (dd, J = 12.3, 5.5 Hz, 1H, H-6′), 3.89 (t, J = 7.2 Hz, 1H, H-11), 3.67–3.57 (m, 2H, H-11, H-5′), 3.06 (d, J = 7.4 Hz, 1H, H-5), 2.78 (t, J = 7.4 Hz, 2H, H-12), 2.71 (dd, J = 10.8, 7.5 Hz, 1H, H-6), 2.63 (d, J = 14.7 Hz, 1H, H-3), 2.05, 2.02, 1.99 (m, 14H, 4COCH3, H-6, H-3), 1.38 (s, 3H, H-10). 13C NMR (101 MHz, Chloroform-d) δ 204.94 (C-4), 170.49, 170.33, 169.52, 169.44 (4CH3CO), 166.47 (C-7″), 138.50 (C-1″′), 133.60 (C-4″), 129.78 (C-2″, C-6″), 129.49 (C-1″), 129.03 (C-3″, C-5″), 128.75 (C-3″′, C-5″′), 128.43(C-2″′, C-6″′), 126.36 (C-4″′), 104.77 (C-9), 96.32 (C-1′), 87.96 (C-1), 86.02 (C-2), 73.01 (C-2′), 72.08 (C-5′), 71.53 (C-3′), 69.58 (C-8), 68.43 (C-4′), 63.07 (C-7), 62.34 (C-11), 62.10 (C-6′), 48.86 (C-5), 46.98 (C-3), 35.89 (C-12), 26.34 (C-6), 20.82, 20.72, 20.69×2 (4CH3CO), 20.48 (C-10). ESI-HRMS: m/z calcd for C39H44O15Na [M + Na]+ 775.2573, found 775.2655.
4-O-ethylbenzenepaeoniflorin (41): colorless syrup, 23.6 mg (44.9%, CH2Cl2: CH3OH, 23:1), Rf 0.36 (CH2Cl2: CH3OH, 7:1), 1H NMR (400 MHz, Chloroform-d) δ 7.89 (d, J = 7.7 Hz, 2H, H-2″, H-6″), 7.44 (t, J = 7.5 Hz, 1H, H-4″), 7.29 (d, J = 7.7 Hz, 2H, H-3″, H-5″), 7.25–7.11 (m, 5H, H-2″′, H-3″′, H-4″′, H-5″′, H-6″′), 5.46 (s, 1H, H-9), 4.63 (q, J = 12.1 Hz, 2H, H-8, H-1′), 4.48 (d, J = 7.3 Hz, 1H, H-8), 3.83–3.71 (m, 4H, H-6′, H-11), 3.57 (s, 2H, H-3′, H-4′), 3.39 (s, 1H, H-2′), 3.28 (s, 1H, H-5′), 2.84 (t, J = 7.4 Hz, 2H, H-12), 2.64 (d, J = 6.5 Hz, 1H, H-6), 2.38–2.30 (t, J = 11.5 Hz 1H, H-5), 1.92 (s, 2H, H-3), 1.75 (d, J = 10.6 Hz, 1H, H-6), 1.31 (s, 3H, H-10). 13C NMR (101 MHz, Chloroform-d) δ 167.10 (C-7′′), 138.50 (C-1″′), 133.48 (C-4″), 129.81 (C-2″, C-6″), 129.58 (C-1″), 129.05 (C-3″, C-5″), 128.64 (C-3″′, C-5″′), 128.49 (C-2″′, C-6″′), 126.43 (C-4″′), 107.75 (C-4), 101.28 (C-9), 98.92 (C-1′), 88.48 (C-1), 85.71 (C-2), 76.41 (C-3′), 75.72 (C-5′), 73.52 (C-2′), 70.08 (C-7), 69.52 (C-4′), 64.80 (C-11), 61.65 (C-6′), 60.84 (C-8), 41.79 (C-3), 40.24 (C-5), 36.57 (C-12), 22.76 (C-6), 19.51 (C-10). ESI-HRMS: m/z calcd for C31H36O11Na [M + Na]+ 607.2150, found 607.2206.
4-O-ethylbenzenedebenzoylpaeoniflorin (42): white solid, 14.7 mg (34.0%, CH2Cl2: CH3OH, 16:1), Rf 0.07 (CH2Cl2: CH3OH, 7:1), mp 168.7 °C, 1H NMR (400 MHz, Acetone-d6) δ 7.30–7.16 (m, 5H, H-2″′, H-3″′, H-4″′, H-5″′, H-6″′), 5.22 (s, 1H, H-9), 4.65 (d, J = 7.7 Hz, 1H, H-8), 3.99 (dd, J = 12.4, 4.0 Hz, 1H, H-11), 3.89 (dd, J = 12.3, 6.4 Hz, 1H, H-11), 3.84–3.76 (m, 3H, H-8, H-1′, H-6′), 3.62 (d, J = 10.9 Hz, 1H, H-6′), 3.46–3.42 (m, 1H, H-3′), 3.35–3.30 (m, 2H, H-4′, H-5′), 3.23 (t, J = 7.4 Hz, 1H, H-2′), 2.83 (t, J = 7.2 Hz, 2H, H-12), 2.50 (d, J = 8.7 Hz, 1H, H-6), 2.39 (dd, J = 10.7, 6.9 Hz, 1H, H-5), 2.01 (d, J = 12.2 Hz, 1H, H-3), 1.85 (dd, J = 12.3, 1.9 Hz, 1H, H-3), 1.79 (d, J = 10.6 Hz, 1H, H-6), 1.28 (s, 3H, H-10). 13C NMR (101 MHz, Acetone-d6) δ 139.88 (C-1″′), 129.79 (C-3″′, C-5″′), 129.07 (C-2″′, C-6″′), 126.94 (C-4″′), 108.45 (C-4), 101.98 (C-9), 99.44 (C-1′), 88.90 (C-1), 86.09 (C-2), 78.16 (C-3′), 77.43 (C-5′), 74.41 (C-2′), 72.69 (C-7), 71.76 (C-4′), 64.97 (C-11), 62.98 (C-6′), 58.46 (C-8), 42.56 (C-3), 40.93 (C-5), 37.17 (C-12), 23.18 (C-6), 19.61 (C-10). ESI-HRMS: m/z calcd for C24H32O10Na [M + Na]+ 503.51, found 503.19.
4-Oxo-9-O-ethylbenzenepaeoniflorin (43): white solid, 30.5 mg (47.4%, CH2Cl2: CH3OH, 22:1), Rf 0.37 (CH2Cl2: CH3OH, 7:1), mp 171.8 °C, 1H NMR (400 MHz, Chloroform-d) δ 7.82 (d, J = 7.8 Hz, 2H, H-2″, H-6″), 7.45 (t, J = 7.5 Hz, 1H, H-4″), 7.27 (d, J = 15.3 Hz, 2H, H-3″, H-5″), 7.20–7.02 (m, 5H, H-2″′, H-3″′, H-4″′, H-5″′, H-6″′), 5.10 (s, 1H, H-9), 4.71 (d, J = 11.7 Hz, 1H, H-8), 4.52 (d, J = 11.0 Hz, 2H, H-8, H-1′), 3.86–3.75 (m, 3H, H-6′, H-11), 3.65 (s, 2H, H-3′, H-4′), 3.50–3.43 (m, 2H, H-2′, H-11), 3.35 (s, 1H, H-5′), 2.97 (d, J = 6.9 Hz, 1H, H-6), 2.78 (t, J = 9.0 Hz, 1H, H-5), 2.69 (t, J = 7.4 Hz, 2H H-12), 2.66–2.46 (q, J = 27.9 Hz, 2H, H-3), 1.97 (d, J = 10.9 Hz, 1H, H-6), 1.36 (s, 3H, H-10). 13C NMR (101 MHz, Chloroform-d) δ 205.36 (C-4), 167.30 (C-7′′), 138.48 (C-1″′), 133.67 (C-4″), 129.78 (C-2″, C-6″), 129.29 (C-1″), 129.00 (C-3″, C-5″), 128.70 (C-3″′, C-5″′), 128.41 (C-2″′, C-6″′), 126.34 (C-4″′), 105.11 (C-9), 98.71 (C-1′), 88.00 (C-1), 86.00 (C-2), 76.51 (C-2′), 75.91 (C-5′), 73.65 (C-3′), 69.58 (C-8), 69.47 (C-4′), 63.45 (C-7), 63.13 (C-11), 61.58 (C-6′), 48.80 (C-5), 47.01 (C-3), 46.12, 35.84 (C-12), 26.60 (C-6), 20.68 (C-10). ESI-HRMS: m/z calcd for C31H36O11Na [M + Na]+ 607.2150, found 607.2201.
4-Oxo-9-O-ethylbenzenedebenzoylpaeoniflorin (44): colorless syrup, 17.4 mg (32.9%, CH2Cl2: CH3OH, 16:1), Rf 0.06 (CH2Cl2: CH3OH, 7:1), 1H NMR (400 MHz, Acetone-d6) δ 7.36–7.12 (m, 5H, H-2″′, H-3″′, H-4″′, H-5″′, H-6″′), 5.22 (s, 1H, H-9), 4.78 (d, J = 7.7 Hz, 1H, H-8), 4.01 (d, J = 12.5 Hz, 1H, H-6′), 3.89–3.81 (m, 2H, H-11, H-6′), 3.71–3.57 (m, 3H, H-11, H-4′, H-5′), 3.50–3.46 (m, 1H, H-3′), 3.40–3.27 (m, 3H, H-8, H-1′, H-2′), 2.92 (dd, J = 11.1, 7.6 Hz, 1H, H-5), 2.78 (q, J = 7.4 Hz, 3H, H-6, H-12), 2.53 (d, J = 7.5 Hz, 1H, H-3), 2.42 (d, J = 17.9 Hz, 1H, H-3), 2.09 (d, J = 9.6 Hz, 1H, H-6), 1.36 (s, 3H, H-10). 13C NMR (101 MHz, Acetone-d6) δ 205.45 (C-4), 140.02 (C-1″′), 129.89 (C-3″′, C-5″′), 129.01 (C-2″′, C-6″′), 126.86 (C-4″′), 103.96 (C-9), 98.89 (C-1′), 88.17 (C-1), 87.16 (C-2), 78.27 (C-2′), 77.53 (C-5′), 74.56 (C-3′), 71.78 (C-8), 69.85 (C-4′), 65.30 (C-7), 63.00 (C-11), 60.16 (C-6′), 49.46 (C-5), 47.30 (C-3), 36.48 (C-12), 26.65 (C-6), 20.75 (C-10). ESI-HRMS: m/z calcd for C24H32O10Na [M + Na]+ 503.1888, found 503.1914.
4-O-t-butylbenzenepaeoniflorin (45): white solid, 24.1 mg (14.5%, CH2Cl2: CH3OH, 25:1), Rf 0.46 (CH2Cl2: CH3OH, 7:1), mp 81.2 °C, 1H NMR (400 MHz, Chloroform-d) δ 7.97 (d, J = 7.1 Hz, 2H, H-2″, H-6″), 7.51 (t, J = 7.6 Hz, 1H, H-4″), 7.37 (t, J = 7.7 Hz, 2H, H-3″, H-5″), 5.51 (s, 1H, H-9), 4.73 (d, J = 12.0 Hz, 1H, H-8), 4.61 (d, J = 12.3 Hz, 1H, H-8), 4.43 (d, J = 7.5 Hz, 1H, H-1′), 3.62–3.31 (m, 6H, H-6′, H-5′, H-4′, H-3′, H-2′), 2.59 (d, J = 6.3 Hz, 1H, H-6), 2.38–2.31 (m, 1H, H-5), 2.09 (d, J = 12.4 Hz, 1H, H-3), 1.88 (dd, J = 22.4, 11.5 Hz, 2H, H-3, H-6), 1.33 (s, 3H, H-12), 1.25 (s, 3H, H-14), 1.14 (d, J = 9.9 Hz, 6H, H-10, H-13). 13C NMR (101 MHz, Chloroform-d) δ 167.14 (C-7″), 133.60 (C-4″), 129.90 (C-2″, C-6″), 129.54 (C-1″), 128.70 (C-3″, C-5″), 105.27 (C-4), 100.90 (C-9), 98.52 (C-1′), 88.38 (C-1), 86.00 (C-2), 76.45 (C-3′), 74.11 (C-5′), 73.65 (C-2′), 73.39 (C-4′), 72.33 (C-8), 70.43 (C-11), 62.85 (C-7), 60.45 (C-6′), 42.95 (C-6), 29.71 (C-12, C-3), 27.43 (C-10, C-13), 22.70 (C-5), 19.27 (C-14). ESI-HRMS: m/z calcd for C27H36O11Na [M + Na]+ 559.2150, found 559.2203.
4-Oxo-9-O-t-butylpaeoniflorin (46): colorless syrup, 21.3 mg (12.8%, CH2Cl2: CH3OH, 21:1), Rf 0.26 (CH2Cl2: CH3OH, 7:1), 1H NMR (400 MHz, Chloroform-d) δ 7.93 (d, J = 7.3 Hz, 2H, H-2″, H-6″), 7.51 (t, J = 7.4 Hz, 1H, H-4″), 7.36 (t, J = 7.7 Hz, 2H, H-3″, H-5″), 5.39 (s, 1H, H-9), 4.80 (d, J = 11.8 Hz, 1H, H-8), 4.59–4.49 (m, 2H, H-8, H-1′), 3.84 (s, 2H, H-6′), 3.65 (s, 2H, H-3′, H-4′), 3.48 (s, 1H, H-2′), 3.36 (s, 1H, H-5′), 2.95 (d, J = 7.1 Hz, 1H, H-6), 2.81–2.74 (m, 1H, H-5), 2.63 (s, 2H, H-3), 1.98 (d, J = 10.9 Hz, 1H, H-6), 1.38 (s, 3H, H-14), 1.25 (s, 2H, H-12), 1.07 (s, 7H, H-10, H-13, H-12). 13C NMR (101 MHz, Chloroform-d) δ 205.88 (C-4), 167.23 (C-7″), 133.71 (C-4″), 129.87 (C-2″, C-6″), 129.36 (C-1″), 128.67 (C-3″, C-5″), 99.98 (C-9),98.67 (C-1′), 87.58 (C-1), 85.43 (C-2), 76.50 (C-2′), 75.91 (C-5′), 73.72 (C-3′), 69.70 (C-4′), 63.50 (C-8), 63.44 (C-7), 61.63 (C-6′), 49.11 (C-3), 46.86 (C-6), 29.84 (C-12), 28.77 (C-10, C-13), 28.56 (C-5), 20.92 (C-14). ESI-HRMS: m/z calcd for C27H36O11Na [M + Na]+ 559.2150, found 559.2217.