Exploring the Mechanism of Chuanxiong Rhizoma against Thrombosis Based on Network Pharmacology, Molecular Docking and Experimental Verification
Abstract
:1. Introduction
2. Results
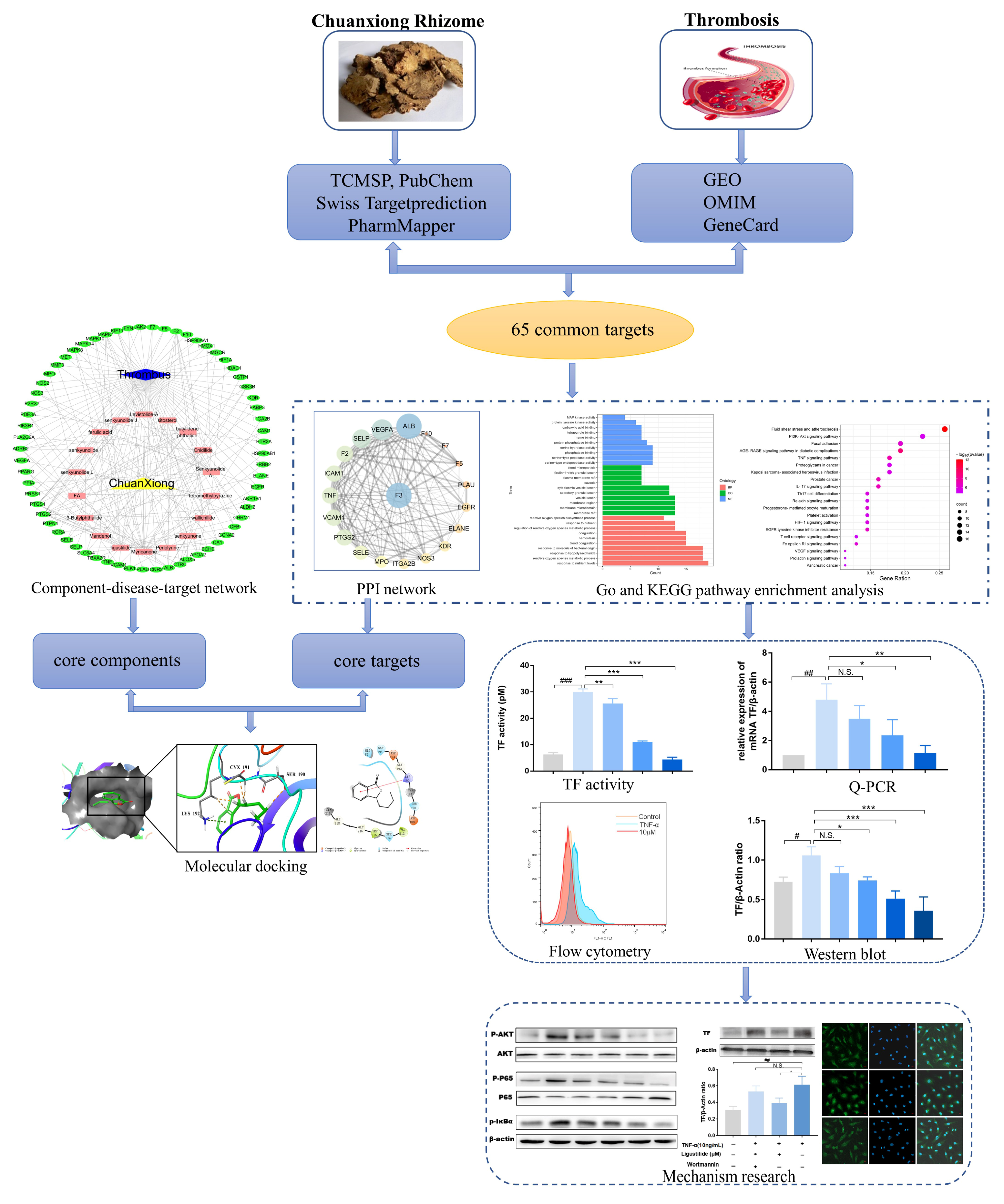
2.1. Analysis of Network Pharmacology of CX
2.1.1. Putative Active Components and Targets in Treating Thrombosis
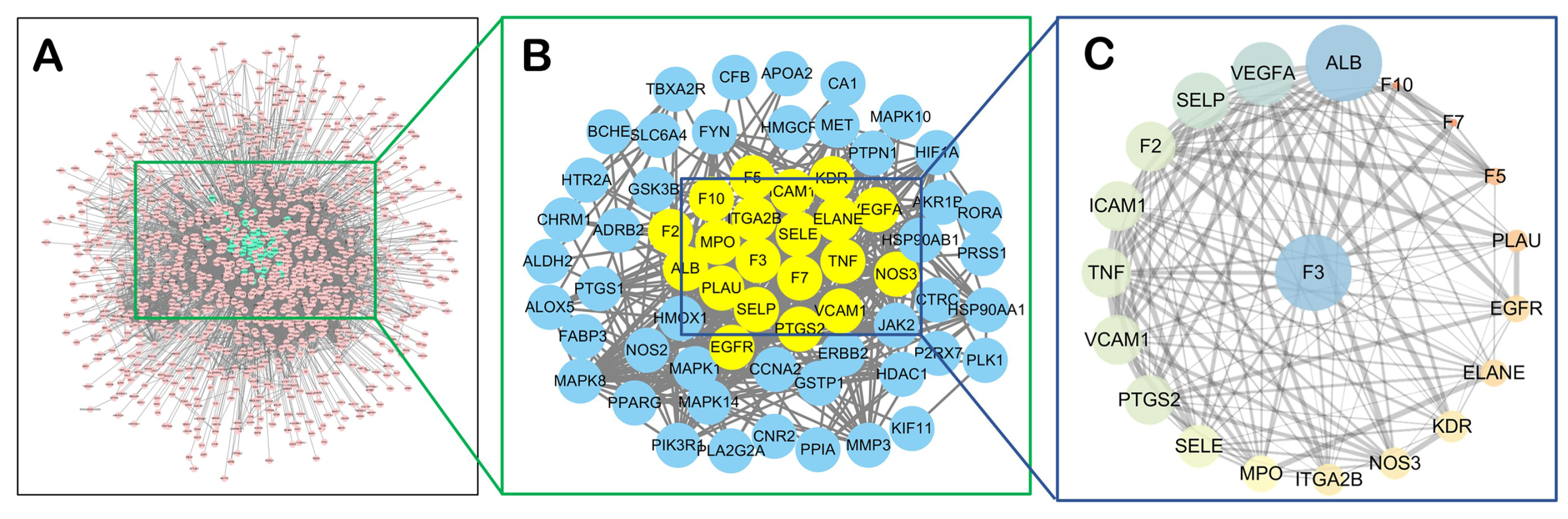
2.1.2. Network Analysis
2.1.3. Biological Function Enrichment Analysis
2.1.4. TF Might also Be a Central Target of CX Anti-Thrombosis
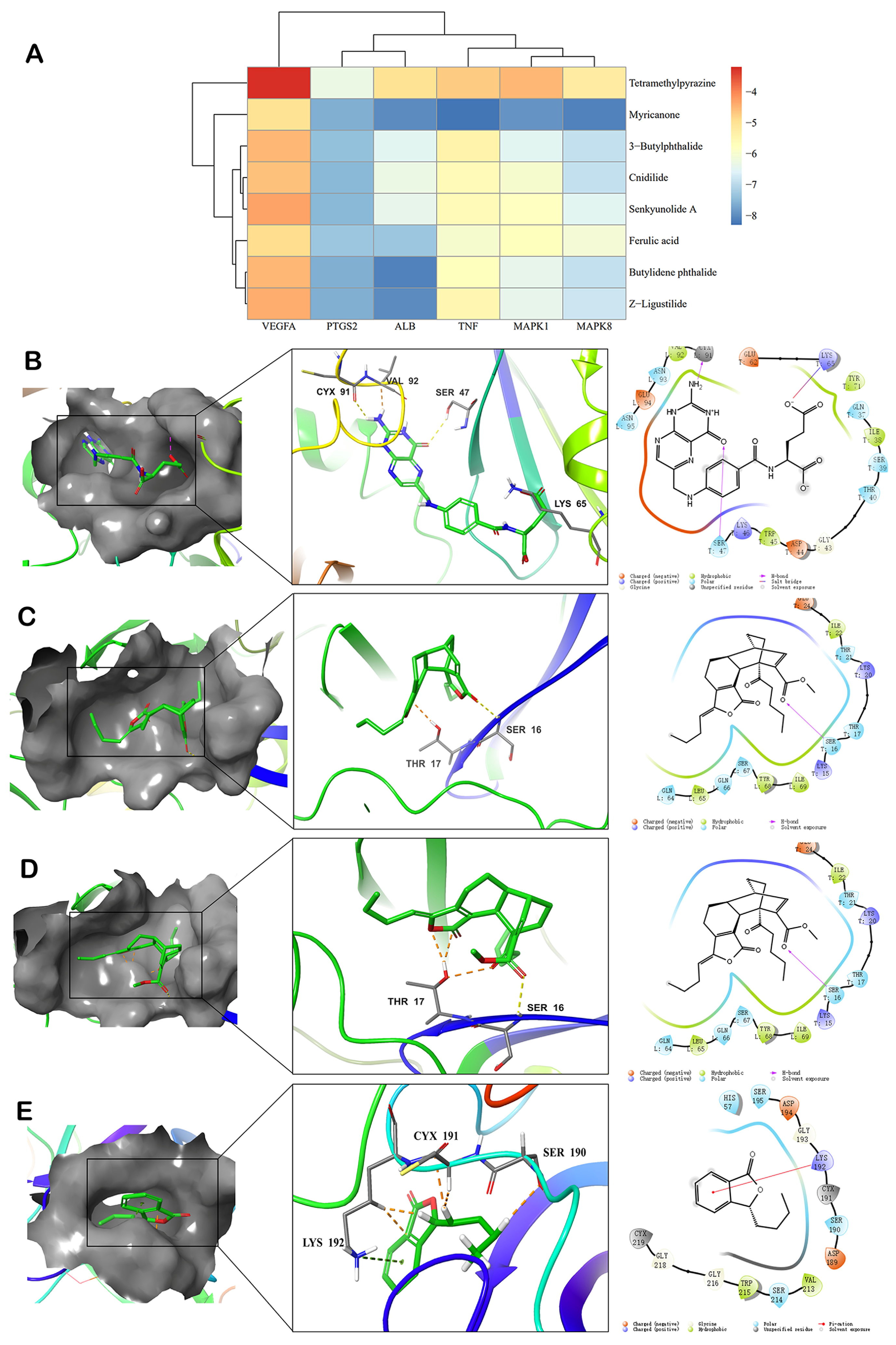
2.2. Components-Targets Molecular Docking
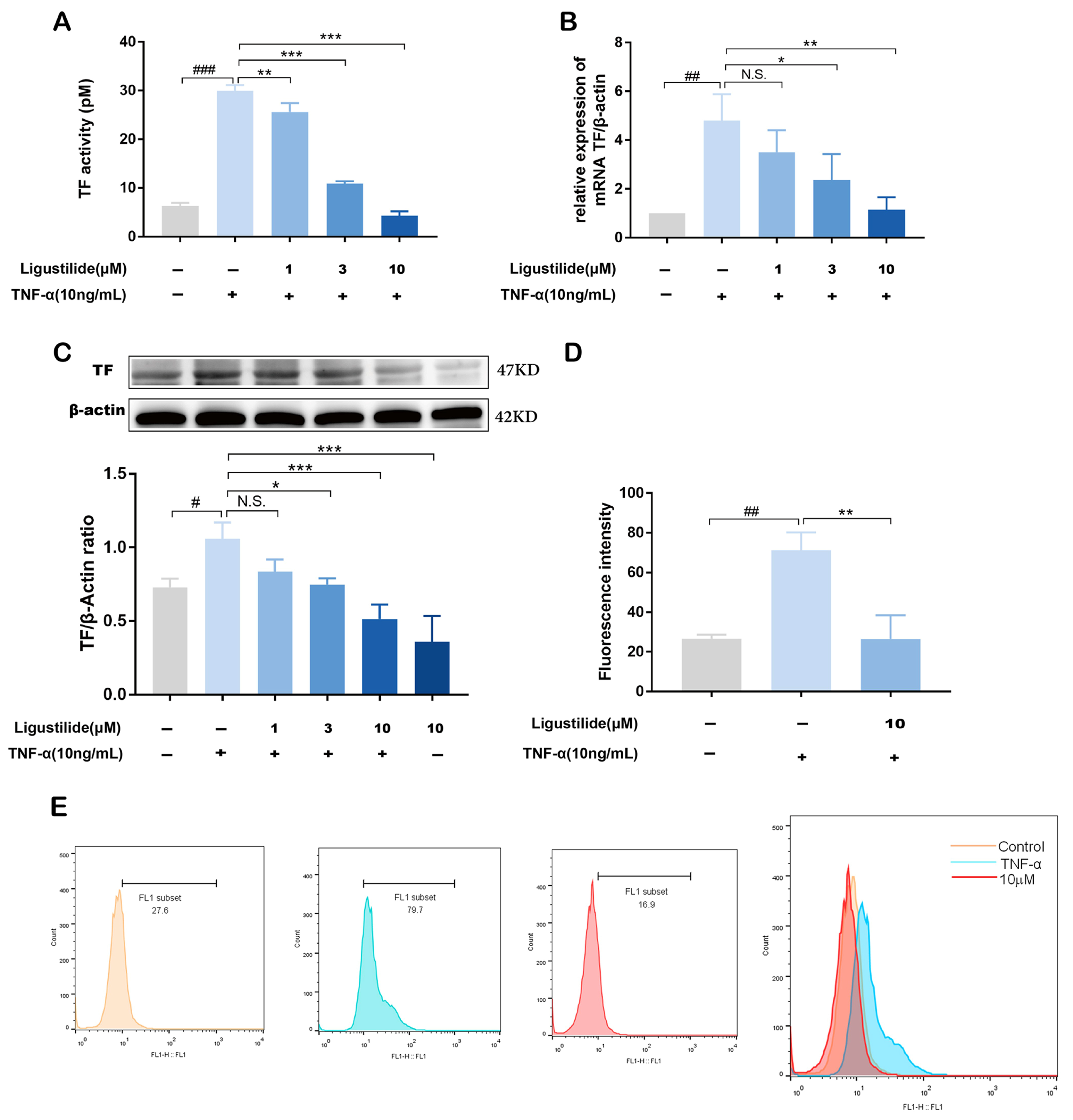
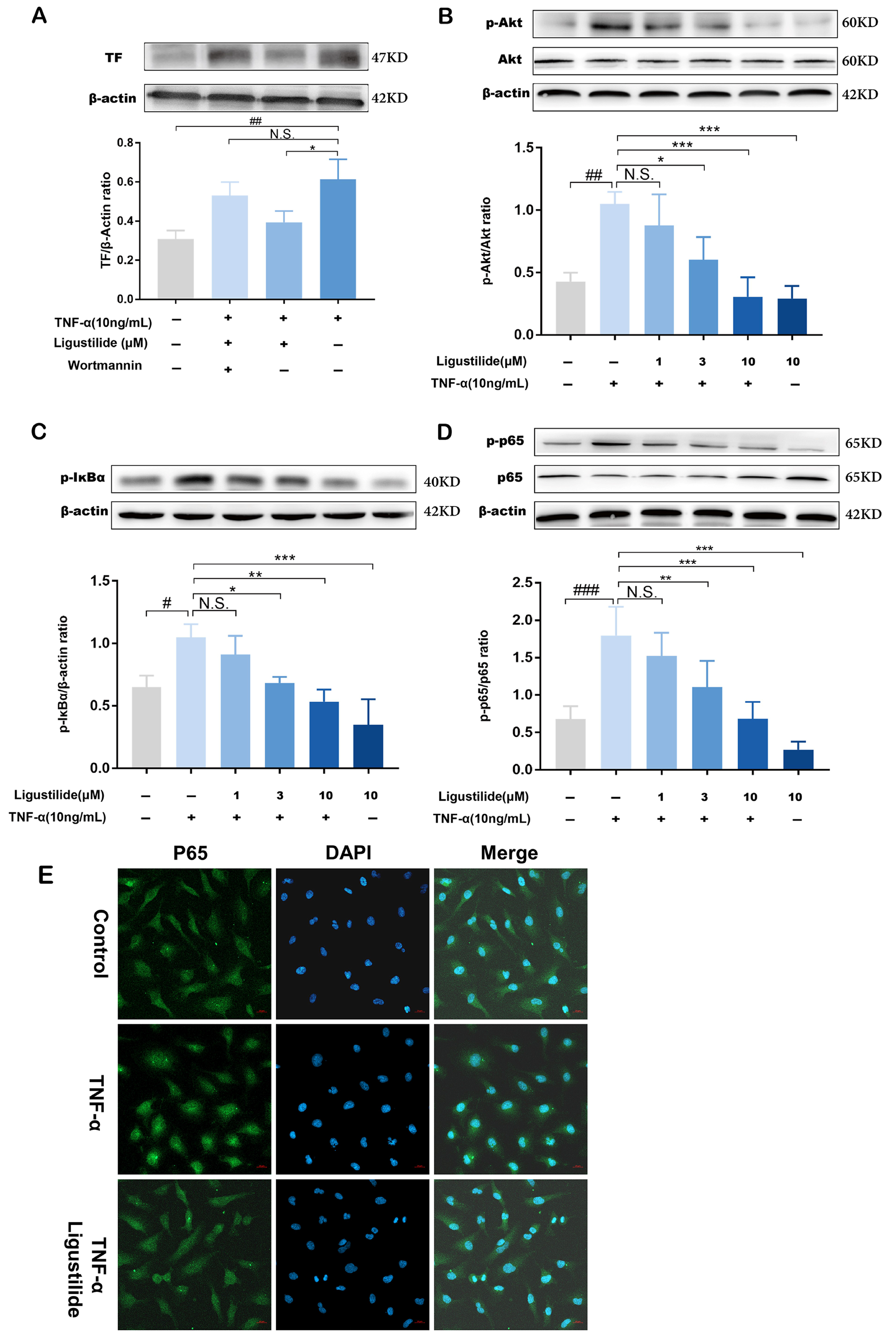
2.3. Representative Component of CX Suppresses TNF-α Induced TF Over-Expression
2.4. Ligustilide Modulates TF Expression via PI3K/Akt/NF-κB Signaling Pathways
3. Discussion
4. Materials and Methods
4.1. Materials and Reagents
4.2. Prediction of the Anti-Thrombosis Mechanism of CX Based on Network Pharmacology
4.2.1. The Compounds and Targets of CX when Treating Thrombosis
4.2.2. Network Construction
4.2.3. Biological Function Analysis
4.3. Molecular Docking
4.4. Cell Culture
4.5. Cell Viability Test
4.6. TF Procoagulant Activity
4.7. Flow Cytometry
4.8. Quantitative Real-Time PCR
4.9. Western Blot Assay
4.10. Cellular Immunofluorescence
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Mutch, N.J.; Walters, S.; Gardiner, E.E.; McCarty, O.J.T.; De Meyer, S.F.; Schroeder, V.; Meijers, J.C.M. Basic science research opportunities in thrombosis and hemostasis: Communication from the SSC of the ISTH. J. Thromb. Haemost. 2022, 20, 1496–1506. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, Z.; Ye, X.; Chen, Z.; Chen, Z.S. Mechanisms of thrombosis and research progress on targeted antithrombotic drugs. Drug Discov. Today 2021, 26, 2282–2302. [Google Scholar] [CrossRef]
- Tang, X.; Liu, X.; Mikaye, M.S.; Zhao, H.; Zhang, Y. Traditional Chinese medicine in the treatment of high incidence diseases in cold areas: The thrombotic diseases. Frigid Zone Med. 2021, 1, 23–44. [Google Scholar] [CrossRef]
- Chang, J.C. Novel Classification of Thrombotic Disorders Based on Molecular Hemostasis and Thrombogenesis Producing Primary and Secondary Phenotypes of Thrombosis. Biomedicines 2022, 10, 2706. [Google Scholar] [CrossRef]
- D’Alessandro, E.; Posma, J.J.N.; Spronk, H.M.H.; Ten Cate, H. Tissue factor (:Factor VIIa) in the heart and vasculature: More than an envelope. Thromb. Res. 2018, 168, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Unruh, D.; Horbinski, C. Beyond thrombosis: The impact of tissue factor signaling in cancer. J. Hematol. Oncol. 2020, 13, 93. [Google Scholar] [CrossRef]
- Subramaniam, S.; Kothari, H.; Bosmann, M. Tissue factor in COVID-19-associated coagulopathy. Thromb. Res. 2022, 220, 35–47. [Google Scholar] [CrossRef]
- Grover, S.P.; Mackman, N. Tissue Factor: An Essential Mediator of Hemostasis and Trigger of Thrombosis. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 709–725. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.; Jeon, H.M.; Lee, J. Tissue factor links inflammation, thrombosis, and senescence in COVID-19. Sci. Rep. 2022, 12, 19842. [Google Scholar] [CrossRef] [PubMed]
- Eslamifar, Z.; Behzadifard, M.; Soleimani, M.; Behzadifard, S. Coagulation abnormalities in SARS-CoV-2 infection: Overexpression tissue factor. Thromb. J. 2020, 18, 38. [Google Scholar] [CrossRef]
- Sachetto, A.T.A.; Mackman, N. Tissue Factor and COVID-19: An Update. Curr. Drug Targets 2022, 23, 1573–1577. [Google Scholar] [CrossRef]
- Glunz, P.W.; Cheng, X.; Cheney, D.L.; Weigelt, C.A.; Wei, A.; Luettgen, J.M.; Wong, P.C.; Wexler, R.R.; Priestley, E.S. Design and synthesis of potent, selective phenylimidazole-based FVIIa inhibitors. Bioorg. Med. Chem. Lett. 2015, 25, 2169–2173. [Google Scholar] [CrossRef]
- Yuan, X.; Han, B.; Feng, Z.M.; Jiang, J.S.; Yang, Y.N.; Zhang, P.C. Chemical constituents of Ligusticum chuanxiong and their anti-inflammation and hepatoprotective activities. Bioorg. Chem. 2020, 101, 104016. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Fang, S.; Xiong, Z. Protective effect of polysaccharide from Ligusticum chuanxiong hort against H2O2-induced toxicity in zebrafish embryo. Carbohydr. Polym. 2019, 221, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhang, C.; Gao, F.; Fu, Q.; Fu, C.; He, Y.; Zhang, J. A systematic review on the rhizome of Ligusticum chuanxiong Hort. (Chuanxiong). Food Chem. Toxicol. 2018, 119, 309–325. [Google Scholar] [CrossRef]
- Shan, C.S.; Xu, Q.Q.; Shi, Y.H.; Wang, Y.; He, Z.X.; Zheng, G.Q. Chuanxiong Formulae for Migraine: A Systematic Review and Meta-Analysis of High-Quality Randomized Controlled Trials. Front. Pharmacol. 2018, 9, 589. [Google Scholar] [CrossRef] [PubMed]
- Ran, X.; Ma, L.; Peng, C.; Zhang, H.; Qin, L.P. Ligusticum chuanxiong Hort: A review of chemistry and pharmacology. Pharm. Biol. 2011, 49, 1180–1189. [Google Scholar] [CrossRef]
- Li, W.; Tang, Y.; Chen, Y.; Duan, J.A. Advances in the chemical analysis and biological activities of chuanxiong. Molecules 2012, 17, 10614–10651. [Google Scholar] [CrossRef]
- Yan, H.; Zhou, Y.; Tang, F.; Wang, C.; Wu, J.; Hu, C.; Xie, X.; Peng, C.; Tan, Y. A comprehensive investigation on the chemical diversity and efficacy of different parts of Ligusticum chuanxiong. Food Funct. 2022, 13, 1092–1107. [Google Scholar] [CrossRef]
- Huang, C.; Zhao, Y.; Huang, S.; Li, L.; Yuan, Z.; Xu, G. Screening of anti-thrombin active components from Ligusticum chuanxiong by affinity-ultrafiltration coupled with HPLC-Q-Orbitrap-MS(n). Phytochem. Anal. 2023, 34, 443–452. [Google Scholar] [CrossRef]
- Hsin, K.Y.; Ghosh, S.; Kitano, H. Combining machine learning systems and multiple docking simulation packages to improve docking prediction reliability for network pharmacology. PLoS ONE 2013, 8, e83922. [Google Scholar] [CrossRef]
- Zhang, H.; Han, T.; Yu, C.H.; Jiang, Y.P.; Peng, C.; Ran, X.; Qin, L.P. Analysis of the chemical composition, acute toxicity and skin sensitivity of essential oil from rhizomes of Ligusticum chuanxiong. J. Ethnopharmacol. 2012, 144, 791–796. [Google Scholar] [CrossRef] [PubMed]
- Eto, M.; Kouroedov, A.; Cosentino, F.; Lüscher, T.F. Glycogen Synthase Kinase-3 Mediates Endothelial Cell Activation by Tumor Necrosis Factor-α. Circulation 2005, 112, 1316–1322. [Google Scholar] [CrossRef]
- Li, D.; Long, Y.; Yu, S.; Shi, A.; Wan, J.; Wen, J.; Li, X.; Liu, S.; Zhang, Y.; Li, N.; et al. Research Advances in Cardio-Cerebrovascular Diseases of Ligusticum chuanxiong Hort. Front. Pharmacol. 2021, 12, 832673. [Google Scholar] [CrossRef]
- Jiang, W.L.; Wang, C.Y.; Sun, F.; Zhang, T.P. Protective effect of ligusticum chuanxiong phthalides on focai cerebral ischemia in rats and its related mechanism of action. China J. Chin. Mater. Med. 2005, 30, 466–468. [Google Scholar]
- Zhang, M.; Li, P.; Zhang, S.; Zhang, X.; Wang, L.; Zhang, Y.; Li, X.; Liu, K. Study on the Mechanism of the Danggui-Chuanxiong Herb Pair on Treating Thrombus through Network Pharmacology and Zebrafish Models. ACS Omega 2021, 6, 14677–14691. [Google Scholar] [CrossRef]
- Lü, M.; Wang, T.Y.; Tian, X.X.; Shi, X.H.; Fan, G.W.; Zhang, Y.; Zhu, Y. Interaction of anti-thrombotic and anti-inflammatory activities of commonly used traditional Chinese medicine for promoting blood circulation and removing blood stasis revealed by network pharmacology analysis. Yao Xue Xue Bao 2015, 50, 1135–1141. [Google Scholar]
- Li, X.; Sim, M.M.S.; Wood, J.P. Recent Insights Into the Regulation of Coagulation and Thrombosis. Arterioscler. Thromb. Vasc. Biol. 2020, 40, e119–e125. [Google Scholar] [CrossRef] [PubMed]
- Grover, S.P.; Mackman, N. Tissue factor in atherosclerosis and atherothrombosis. Atherosclerosis 2020, 307, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Witkowski, M.; Landmesser, U.; Rauch, U. Tissue factor as a link between inflammation and coagulation. Trends Cardiovasc. Med. 2016, 26, 297–303. [Google Scholar] [CrossRef]
- Huang, J.; Lu, X.Q.; Zhang, C.; Lu, J.; Li, G.Y.; Lin, R.C.; Wang, J.H. Anti-inflammatory ligustilides from Ligusticum chuanxiong Hort. Fitoterapia 2013, 91, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhang, Y.; Wan, T.; Mei, Y.; Wang, Z.; Xue, J.; Luo, Y.; Li, M.; Fang, S.; Pan, H.; et al. Systems pharmacology approach uncovers Ligustilide attenuates experimental colitis in mice by inhibiting PPARγ-mediated inflammation pathways. Cell Biol. Toxicol. 2021, 37, 113–128. [Google Scholar] [CrossRef]
- Cao, Y.X.; Zhang, W.; He, J.Y.; He, L.C.; Xu, C.B. Ligustilide induces vasodilatation via inhibiting voltage dependent calcium channel and receptor-mediated Ca2+ influx and release. Vasc. Pharmacol. 2006, 45, 171–176. [Google Scholar] [CrossRef]
- Wang, C.; Liu, G.; Dou, G.; Yang, Y.; Chen, L.; Ma, H.; Jiang, Z.; Ma, H.; Li, C.; Li, L.; et al. Z-Ligustilide Selectively Targets AML by Restoring Nuclear Receptors Nur77 and NOR-1-mediated Apoptosis and Differentiation. Phytomedicine 2021, 82, 153448. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Liu, D.L.; Zeng, Q.K.; Shi, M.Q.; Zhao, L.X.; He, Q.; Kuang, X.; Du, J.R. The neuroprotective effects and probable mechanisms of Ligustilide and its degradative products on intracerebral hemorrhage in mice. Int. Immunopharmacol. 2018, 63, 43–57. [Google Scholar] [CrossRef]
- Mao, Z.; Tian, L.; Liu, J.; Wu, Q.; Wang, N.; Wang, G.; Wang, Y.; Seto, S. Ligustilide ameliorates hippocampal neuronal injury after cerebral ischemia reperfusion through activating PINK1/Parkin-dependent mitophagy. Phytomedicine 2022, 101, 154111. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, X.; Ji, S.; Cui, X.; Li, M. Screening of Bioactive Ingredients in Ligusticum Chuanxiong Hort for Protection against Myocardial Ischemia. Cell. Physiol. Biochem. 2016, 40, 770–780. [Google Scholar] [CrossRef]
- Himber, J.; Wohlgensinger, C.; Roux, S.; Damico, L.A.; Fallon, J.T.; Kirchhofer, D.; Nemerson, Y.; Riederer, M.A. Inhibition of tissue factor limits the growth of venous thrombus in the rabbit. J. Thromb. Haemost. 2003, 1, 889–895. [Google Scholar] [CrossRef]
- Cirillo, P.; Conte, S.; Cimmino, G.; Pellegrino, G.; Ziviello, F.; Barra, G.; Sasso, F.C.; Borgia, F.; De Palma, R.; Trimarco, B. Nobiletin inhibits oxidized-LDL mediated expression of Tissue Factor in human endothelial cells through inhibition of NF-kappaB. Biochem. Pharmacol. 2017, 128, 26–33. [Google Scholar] [CrossRef]
- Zheng, B.; Qi, J.; Yang, Y.; Li, L.; Liu, Y.; Han, X.; Qu, W.; Chu, L. Mechanisms of cinnamic aldehyde against myocardial ischemia/hypoxia injury in vivo and in vitro: Involvement of regulating PI3K/AKT signaling pathway. Biomed. Pharmacother. 2022, 147, 112674. [Google Scholar] [CrossRef]
- Abulizi, A.; Simayi, J.; Nuermaimaiti, M.; Han, M.; Hailati, S.; Talihati, Z.; Maihemuti, N.; Nuer, M.; Khan, N.; Abudurousuli, K.; et al. Quince extract resists atherosclerosis in rats by down-regulating the EGFR/PI3K/Akt/GSK-3beta pathway. Biomed. Pharmacother. 2023, 160, 114330. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.F.; Liu, F.; Qiao, M.M.; Shu, H.Z.; Li, X.C.; Peng, C.; Xiong, L. Vasorelaxant effect of curcubisabolanin A isolated from Curcuma longa through the PI3K/Akt/eNOS signaling pathway. J. Ethnopharmacol. 2022, 294, 115332. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Xie, J.; Qu, B. Apolipoprotein L Domain Containing 1 Inhibits Tissue Factor to Impede Thrombus Formation in a Rat Model of Deep Vein Thrombosis via Activating PI3K/Akt Pathway. Ann. Vasc. Surg. 2023, 89, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Dong, R.; Chen, W.; Feng, W.; Xia, C.; Hu, D.; Zhang, Y.; Yang, Y.; Wang, D.W.; Xu, X.; Tu, L. Exogenous Bradykinin Inhibits Tissue Factor Induction and Deep Vein Thrombosis via Activating the eNOS/Phosphoinositide 3-Kinase/Akt Signaling Pathway. Cell. Physiol. Biochem. 2015, 37, 1592–1606. [Google Scholar] [CrossRef]
- He, X.; Zeng, Y.; Jiang, W. Eleven isoquinoline alkaloids on inhibiting tissue factor activity: Structure-activity relationships and molecular docking. Z. Naturforsch. C J. Biosci. 2021, 76, 11–19. [Google Scholar] [CrossRef]


| MOL | Compound Name | OB% a | DL b | Structure |
|---|---|---|---|---|
| 001494 | Mandenol | 42 | 0.19 | 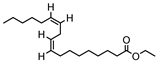 |
| 0021135 | Myricanone | 40.6 | 0.51 | 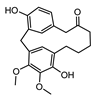 |
| 002140 | Perlolyrine | 65.95 | 0.27 |  |
| 002151 | senkyunone | 47.66 | 0.24 | 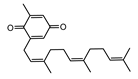 |
| 002157 | wallichilide | 42.31 | 0.71 | 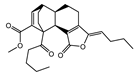 |
| 000359 | sitosterol | 36.91 | 0.75 | 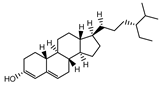 |
| 000433 | Folic acid | 68.96 | 0.71 |  |
| 002122 | (Z)-Ligustilide | 53.72 | 0.07 | 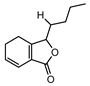 |
| 000360 | Ferulic acid | 39.56 | 0.06 |  |
| 002208 | Senkyunolide A | 26.56 | 0.07 | 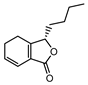 |
| 002202 | tetramethylpyrazine | 20.01 | 0.03 |  |
| 002127 | Cnidilide | 77.55 | 0.07 | 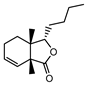 |
| 002111 | Butylidene phthalide | 42.44 | 0.07 | 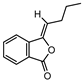 |
| 002143 | senkyunolide I | 46.8 | 0.08 | 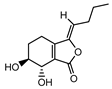 |
| 002200 | Levistolide-A | 9.96 | 0.82 |  |
| 011770 | senkyunolide J | 42.34 | 0.1 | 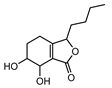 |
| 011771 | senkyunolide L | 10.68 | 0.09 | 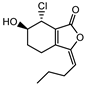 |
| NA | 3-butylphthalide | NA | NA | 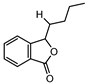 |
| Target | Compound Name | Vina Core |
|---|---|---|
| 4YLQ | Mandenol | −6.20 |
| Myricanone | −7.50 | |
| Perlolyrine | −7.60 | |
| Senkyunone | −7.20 | |
| Wallichilide | −7.90 | |
| Sitosterol | −7.40 | |
| Folic Acid | −8.40 | |
| (Z)-Ligustilide | −6.60 | |
| Ferulic acid | −7.20 | |
| Senkyunolide A | −6.60 | |
| tetramethylpyrazinte | −5.70 | |
| Cnidilide | −6.30 | |
| Butylidene phthalide | −7.00 | |
| Senkyunolide I | −7.10 | |
| Levistolide A | −9.20 | |
| Senkyunolide J | −6.30 | |
| Senkyunolide L | −5.80 | |
| 3-butylphthalide | −6.50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, S.; He, X.; Pan, S.; Jiang, W. Exploring the Mechanism of Chuanxiong Rhizoma against Thrombosis Based on Network Pharmacology, Molecular Docking and Experimental Verification. Molecules 2023, 28, 6702. https://doi.org/10.3390/molecules28186702
He S, He X, Pan S, Jiang W. Exploring the Mechanism of Chuanxiong Rhizoma against Thrombosis Based on Network Pharmacology, Molecular Docking and Experimental Verification. Molecules. 2023; 28(18):6702. https://doi.org/10.3390/molecules28186702
Chicago/Turabian StyleHe, Shasha, Xuhua He, Shujuan Pan, and Wenwen Jiang. 2023. "Exploring the Mechanism of Chuanxiong Rhizoma against Thrombosis Based on Network Pharmacology, Molecular Docking and Experimental Verification" Molecules 28, no. 18: 6702. https://doi.org/10.3390/molecules28186702
APA StyleHe, S., He, X., Pan, S., & Jiang, W. (2023). Exploring the Mechanism of Chuanxiong Rhizoma against Thrombosis Based on Network Pharmacology, Molecular Docking and Experimental Verification. Molecules, 28(18), 6702. https://doi.org/10.3390/molecules28186702







