The Effect of Sample Pretreatment on the Anthocyanin Content in Czech Wild Elderberry (Sambucus nigra L.)
Abstract
:1. Introduction
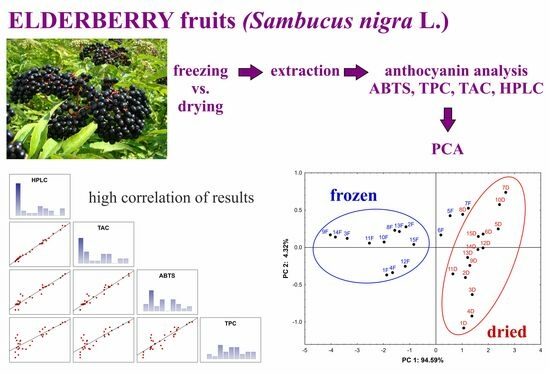
2. Results and Discussion
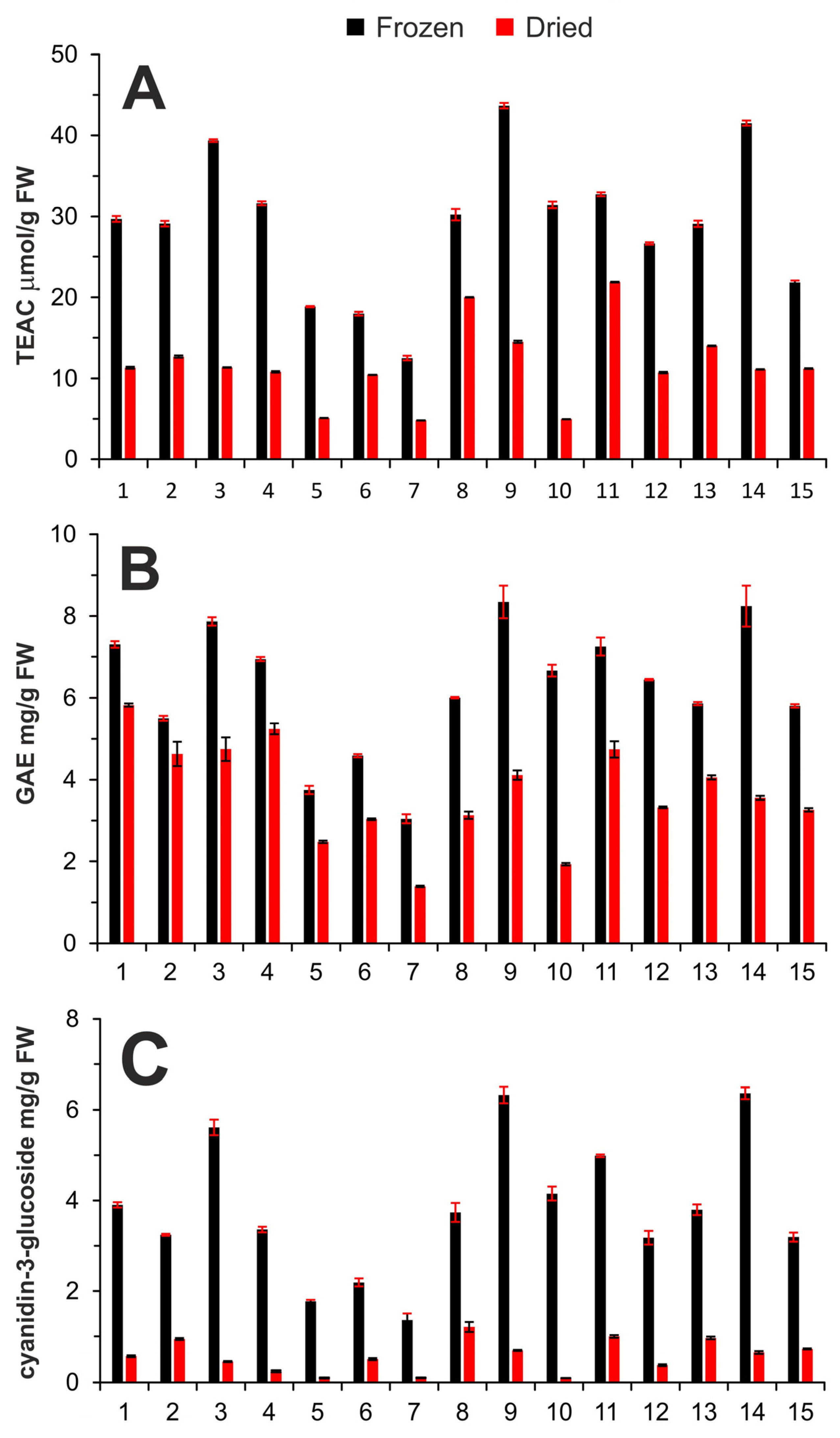
2.1. Determination of Antioxidant Capacity
2.2. Determination of the Total Phenolic Content
2.3. Determination of Total Anthocyanin Content
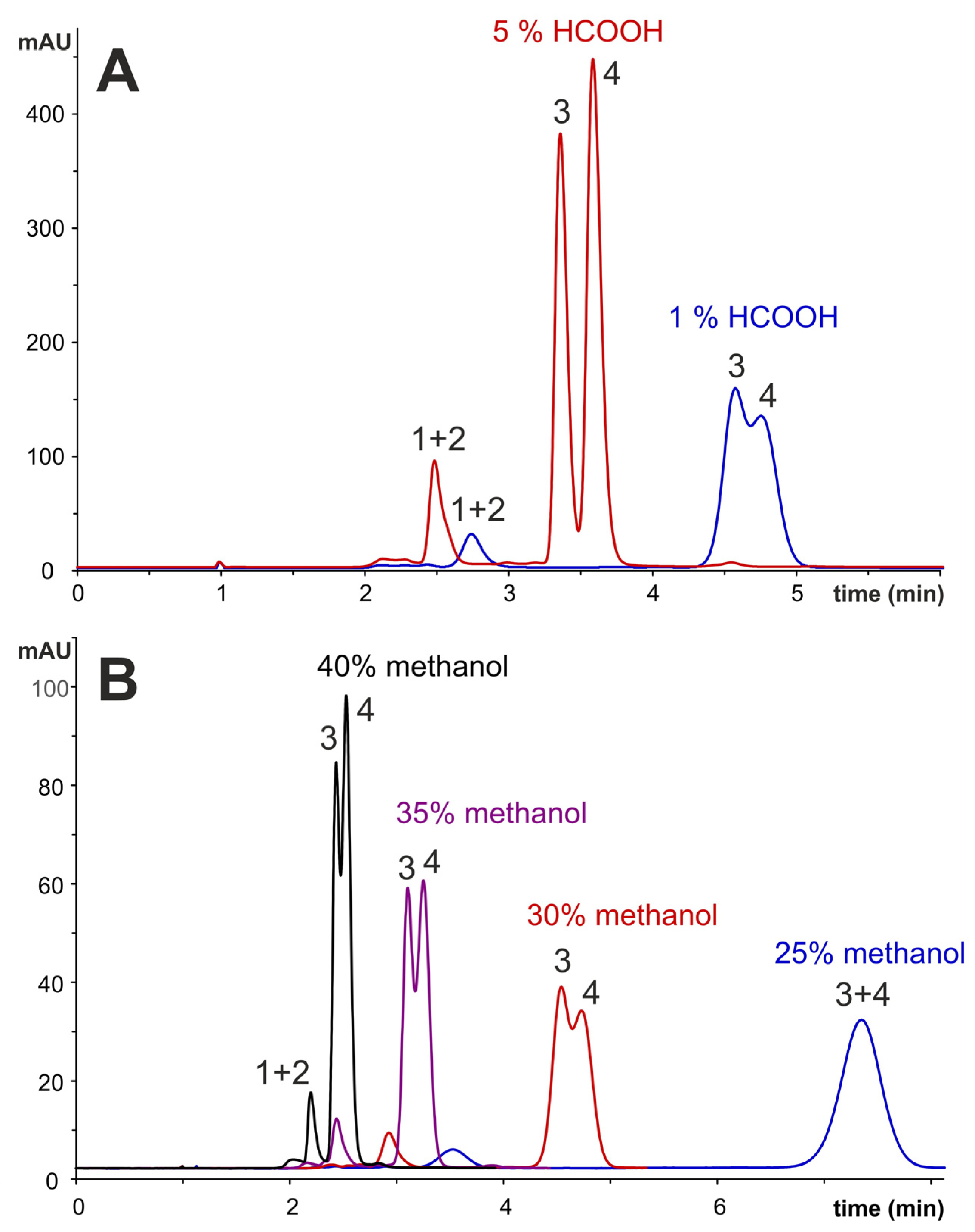
2.4. Qualitative and Quantitative HPLC Analysis of Anthocyanins
2.4.1. Optimization of HPLC Separation
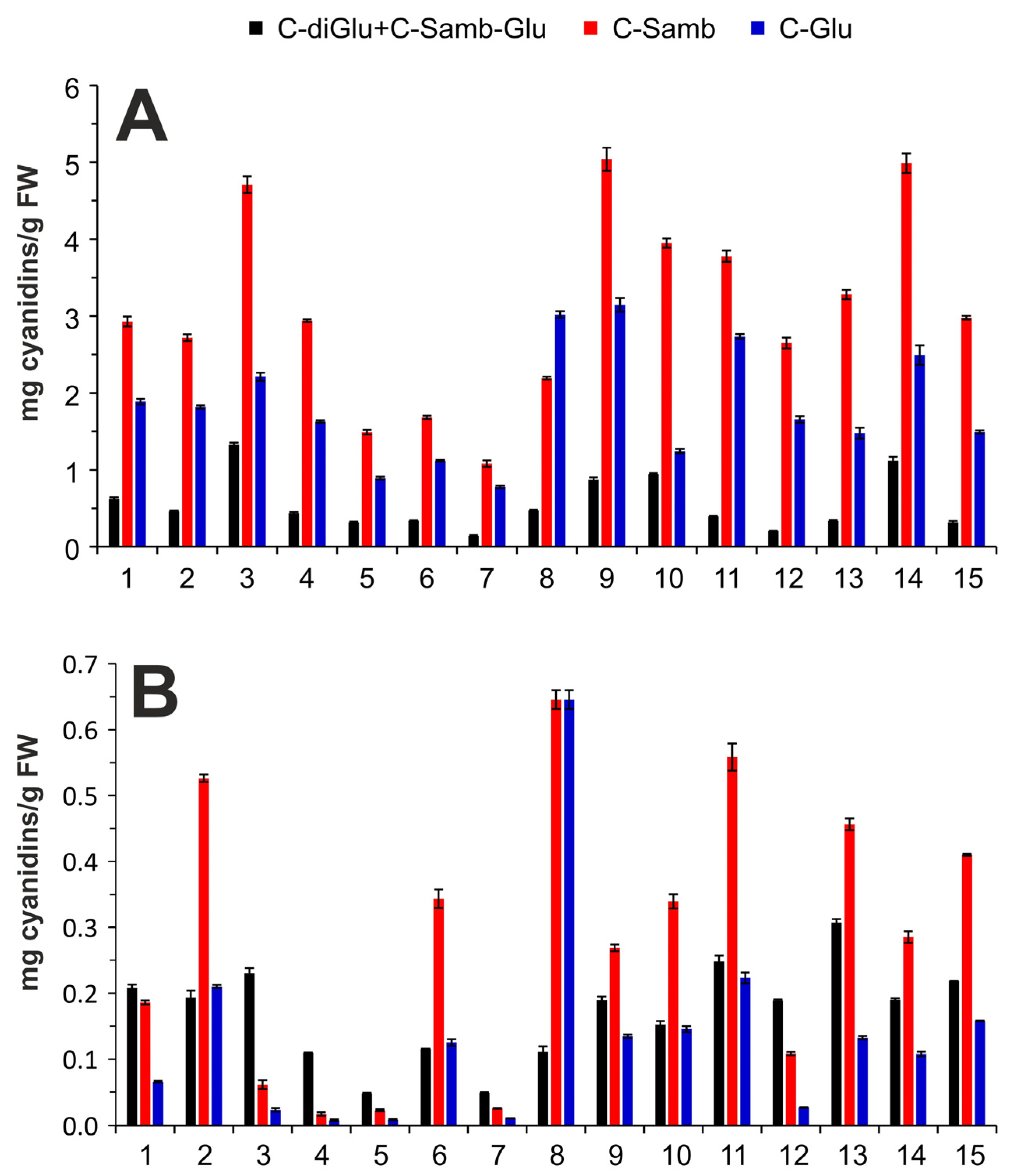
2.4.2. Identification and Quantification of Anthocyanins
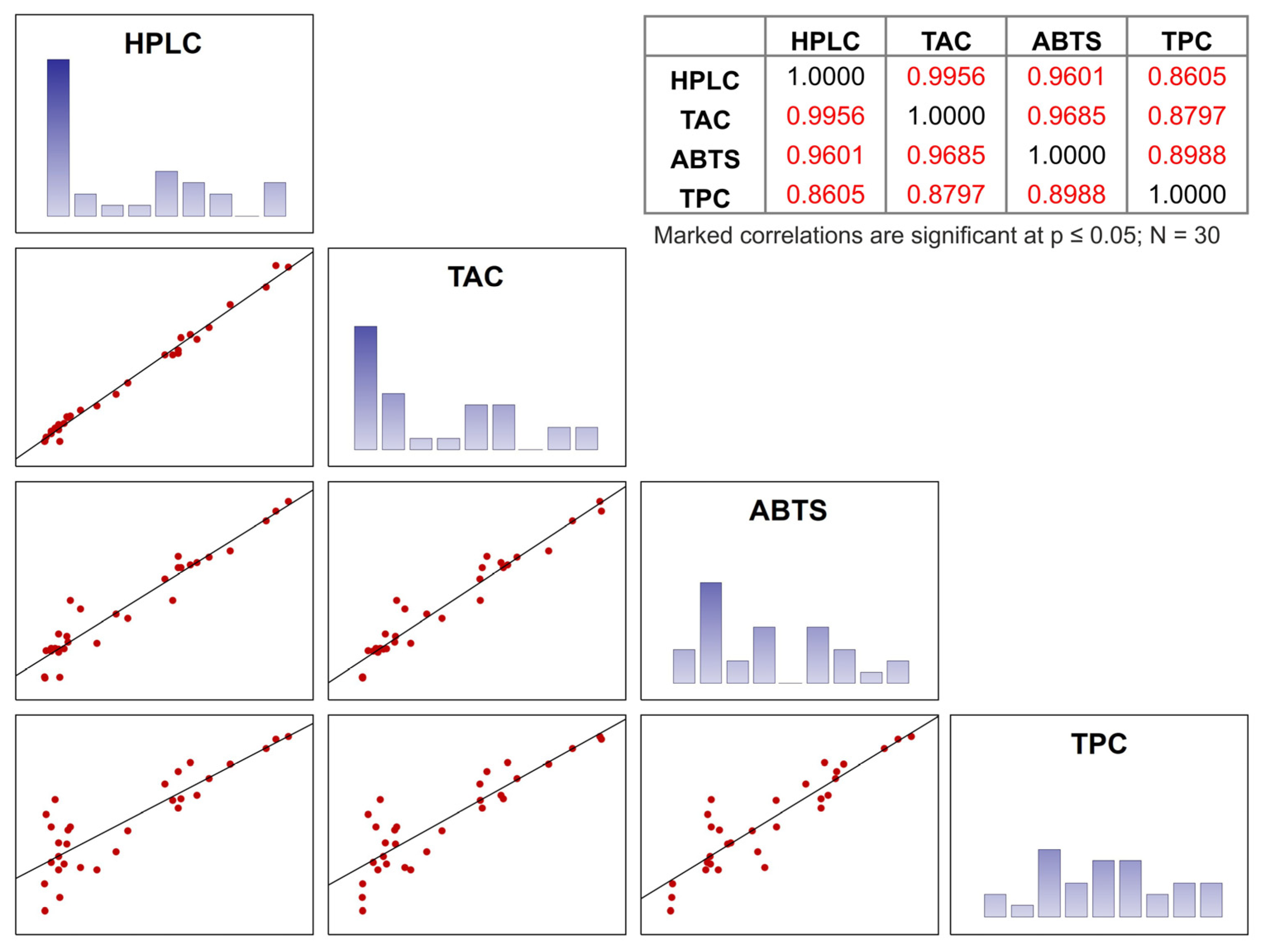
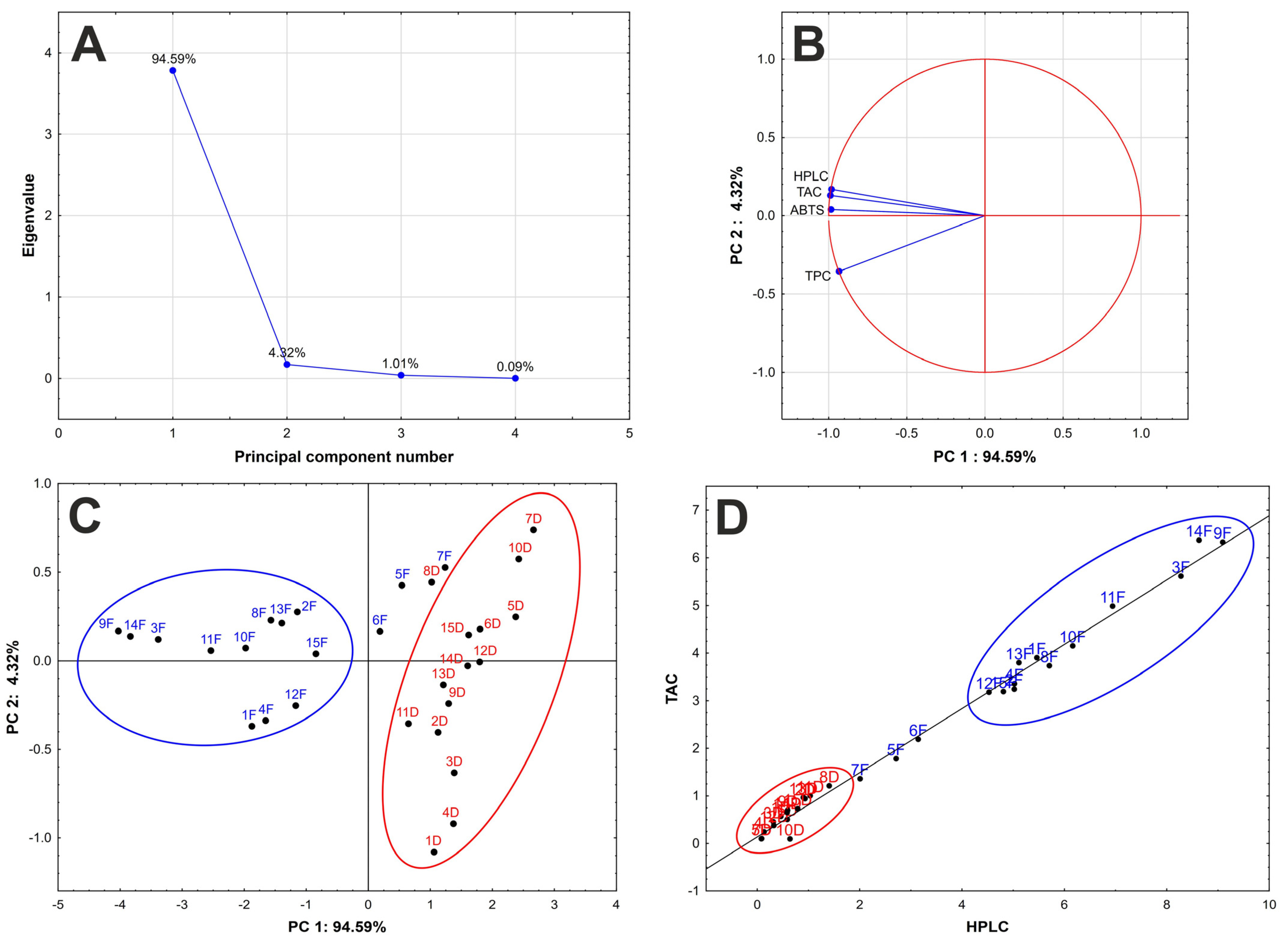
2.4.3. Data Evaluation Using Statistical Methods
3. Materials and Methods
3.1. Chemicals
3.2. Sample Preparation
3.3. HPLC Analysis
3.4. Spectrophotometric Analysis
3.5. Statistical Evaluation of Experimental Data
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Senica, M.; Stampar, F.; Veberic, R.; Mikulic-Petkovsek, M. Processed elderberry (Sambucus nigra L.) products: A beneficial or harmful food alternative? LWT-Food Sci. Technol. 2016, 72, 182–188. [Google Scholar] [CrossRef]
- Atkinson, M.D.; Atkinson, E. Sambucus nigra L. J. Ecol. 2002, 90, 895–923. [Google Scholar] [CrossRef]
- Salvador, A.C.; Król, E.; Lemos, V.C.; Santos, S.A.O.; Bento, F.P.M.S.; Costa, C.P.; Almeida, A.; Szczepankiewicz, D.; Kulczyński, B.; Krejpcio, Z.; et al. Effect of Elderberry (Sambucus nigra L.) Extract Supplementation in STZ-Induced Diabetic Rats Fed with a High-Fat Diet. Int. J. Mol. Sci. 2017, 18, 13. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; He, X.-Q.; Wu, D.-T.; Li, H.-B.; Feng, Y.-B.; Zou, L.; Gan, R.-Y. Elderberry (Sambucus nigra L.): Bioactive Compounds, Health Functions, and Applications. J. Agric. Food Chem. 2022, 70, 4202–4220. [Google Scholar] [CrossRef] [PubMed]
- Veberic, R.; Jakopic, J.; Stampar, F.; Schmitzer, V. European elderberry (Sambucus nigra L.) rich in sugars, organic acids, anthocyanins and selected polyphenols. Food Chem. 2009, 114, 511–515. [Google Scholar] [CrossRef]
- Lee, J.; Finn, C.E. Anthocyanins and other polyphenolics in American elderberry (Sambucus canadensis) and European elderberry (S. nigra) cultivars. J. Sci. Food Agric. 2007, 87, 2665–2675. [Google Scholar] [CrossRef]
- Wu, X.; Gu, L.; Prior, R.L.; McKay, S. Characterization of anthocyanins and proanthocyanidins in some cultivars of Ribes, Aronia, and Sambucus and their antioxidant capacity. J. Agric. Food Chem. 2004, 52, 7846–7856. [Google Scholar] [CrossRef]
- Sidor, A.; Gramza-Michałowska, A. Advanced research on the antioxidant and health benefit of elderberry (Sambucus nigra) in food—A review. J. Funct. Foods 2015, 18, 941–958. [Google Scholar] [CrossRef]
- Boranbayeva, T.; Karadeniz, F.; Yilmaz, E. Effect of Storage on Anthocyanin Degradation in Black Mulberry Juice and Concentrates. Food Bioproc. Tech. 2014, 7, 1894–1902. [Google Scholar] [CrossRef]
- Veberic, R.; Slatnar, A.; Bizjak, J.; Stampar, F.; Mikulic-Petkovsek, M. Anthocyanin composition of different wild and cultivated berry species. LWT-Food Sci. Technol. 2015, 60, 509–517. [Google Scholar] [CrossRef]
- Hubbermann, E.M.; Heins, A.; Stöckmann, H.; Schwarz, K. Influence of acids, salt, sugars and hydrocolloids on the colour stability of anthocyanin rich black currant and elderberry concentrates. Eur. Food Res. Technol. 2006, 223, 83–90. [Google Scholar] [CrossRef]
- Szalóki-Dorkó, L.; Stéger-Máté, M.; Abrankó, L. Effects of fruit juice concentrate production on individual anthocyanin species in elderberry. Int. J. Food Sci. Technol. 2016, 51, 641–648. [Google Scholar] [CrossRef]
- Bridle, P.; Timberlake, C.F. Anthocyanins as natural food colours—Selected aspects. Food Chem. 1997, 58, 103–109. [Google Scholar] [CrossRef]
- Cooper-Driver, G.A. Contributions of Jeffrey Harborne and co-workers to the study of anthocyanins. Phytochemistry 2001, 56, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Wrolstad, R.E.; Durst, R.W.; Lee, J. Tracking color and pigment changes in anthocyanin products. Trends Food Sci. Technol. 2005, 16, 423–428. [Google Scholar] [CrossRef]
- Garzón, G.A.; Wrolstad, R.E. The stability of pelargonidin-based anthocyanins at varying water activity. Food Chem. 2001, 75, 185–196. [Google Scholar] [CrossRef]
- Casati, C.B.; Baeza, R.; Samchez, V.; Catalano, A.; López, P.; Zamora, M.C. Thermal degradation kinetics of monomeric anthocyanins, colour changes and storage effect in elderberry juices. J. Berry Res. 2015, 5, 29–39. [Google Scholar] [CrossRef]
- Garzón, G.A.; Wrolstad, R.E. Comparision of the stability of pelargonidin-based anthocyanins in strawberry juice and concentrate. J. Food Sci. 2002, 67, 1288–1299. [Google Scholar] [CrossRef]
- Wang, W.D.; Xu, S.Y. Degradation kinetics of anthocyanins in blackberry juice and concentrate. J. Food Eng. 2007, 82, 271–275. [Google Scholar] [CrossRef]
- Jiménez, N.; Bohuon, P.; Lima, J.; Dornier, M.; Vaillant, F.; Pérez, A.M. Kinetics of anthocyanin degradation and browning in reconstituted blackberry juice treated at high temperatures (100–180 °C). J. Agric. Food Chem. 2010, 58, 2314–2322. [Google Scholar] [CrossRef]
- Weber, F.; Larsen, L.R. Influence of fruit juice processing on anthocyanin stability. Food Res. Int. 2017, 100, 354–365. [Google Scholar] [CrossRef] [PubMed]
- Krüger, S.; Mirgos, M.; Morlock, G.E. Effect-directed analysis of fresh and dried elderberry (Sambucus nigra L.) via hyphenated planar chromatography. J. Chromatogr. A 2015, 1426, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Mikulic-Petkovsek, M.; Ivancic, A.; Schmitzer, V.; Veberic, R.; Stampar, F. Comparison of major taste compounds and antioxidative properties of fruits and flowers of different Sambucus species and interspecific hybrids. Food Chem. 2016, 200, 134–140. [Google Scholar] [CrossRef]
- Silva, P.; Ferreira, S.; Nunes, F.M. Elderberry (Sambucus nigra L.) by-products a source of anthocyanins and antioxidant polyphenols. Ind. Crops Prod. 2017, 95, 227–234. [Google Scholar] [CrossRef]
- Mlynarczyk, K.; Walkowiak-Tomczak, D.; Staniek, H.; Kidoń, M.; Łysiak, G.P. The content of selected minerals, bioactive compounds, and the antioxidant properties of the flowers and fruit of selected cultivars and wildly growing plants of Sambucus nigra L. Molecules 2020, 25, 876. [Google Scholar] [CrossRef] [PubMed]
- Caruso, M.C.; Galgano, F.; Tolve, R.; Pecora, M.; Tedesco, I.; Favati, F.; Condelli, N. Nutraceutical properties of wild berry fruits from Southern Italy. J. Berry Res. 2016, 6, 321–332. [Google Scholar] [CrossRef]
- Ferreira, S.S.; Silva, P.; Silva, A.M.; Nunes, F.M. Effect of harvesting year and elderberry cultivar on the chemical composition and potential bioactivity: A three-year study. Food Chem. 2020, 302, 125366. [Google Scholar] [CrossRef]
- Csorba, V.; Tóth, M.; László, A.M.; Kardos, L.; Kovács, S. Cultivar and year effects on the chemical composition of elderberry (Sambucus nigra L.) fruits. Not. Bot. Horti Agrobot. Cluj-Napoca 2020, 48, 770–782. [Google Scholar] [CrossRef]
- Giusti, M.M.; Wrolstad, R.E. Characterization and measurement of anthocyanins by UV-visible spectroscopy. In Current Protocols in Food Analytical Chemistry; Giusti, M.M., Wrolstad, R.E., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2001. [Google Scholar]
- Caruso, M.C.; Galgano, F.; Pecora, M.; Tolve, R.; Verrastro, M.; Favati, F. Improvement of Analytical Methods for the Determination of Polyphenolic Bioactive Compounds in Berry Fruits. J. Chem. 2015, 2015, 384051. [Google Scholar] [CrossRef]
- Garcia-Herrera, P.; Pérez-Rodríguez, M.-L.; Aguilera-Delgado, T.; Labari-Reyes, M.-J.; Olmedilla-Alonso, B.; Camara, M.; de Pascual-Teresa, S. Anthocyanin profile of red fruits and black carrot juices, purees and concentrates by HPLC-DAD-ESI/MS-QTOF. Int. J. Food Sci. Technol. 2016, 51, 2290–2300. [Google Scholar] [CrossRef]
- Mikulic-Petkovsek, M.; Schmitzer, V.; Slatnar, A.; Todorovic, B.; Veberic, R.; Stampar, F.; Ivancic, A. Investigation of anthocyanin profile of four elderberry species and interspecific hybrids. J. Agric. Food. Chem. 2014, 62, 5573–5580. [Google Scholar] [CrossRef] [PubMed]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef] [PubMed]
- Šilarová, P.; Česlová, L.; Meloun, M. Fast gradient HPLC/MS separation of phenolics in green tea to monitor their degradation. Food Chem. 2017, 237, 471–480. [Google Scholar] [CrossRef]
- Česlová, L.; Klikarová, J.; Šalomounová, T. The content and profile of biologically active compounds present in individual parts of nasturtium (Tropaeolum majus L.): Comprehensive study. Eur. Food Res. Technol. 2023, 249, 413–428. [Google Scholar] [CrossRef]
| Sample | City | Region | Coordinates | Altitude | Pretreatment | Dry Matter (%) |
|---|---|---|---|---|---|---|
| 1 | Nové Město nad Metují | HK | 50.331 N, 16.155 E | 320 m | D, F | 18.1 |
| 2 | Nové Město nad Metují | HK | 50.361 N, 16.149 E | 320 m | D, F | 21.6 |
| 3 | Kutná Hora | CB | 49.968 N, 15.287 E | 260 m | D, F | 19.0 |
| 4 | Nové Město nad Metují | HK | 50.336 N, 16.145 E | 320 m | D, F | 20.8 |
| 5 | Blansko | SM | 49.377 N, 16.569 E | 400 m | D, F | 15.3 |
| 6 | Náchod | HK | 50.406 N, 16.118 E | 390 m | D, F | 18.7 |
| 7 | Náchod | HK | 50.419 N, 16.199 E | 390 m | D, F | 14.6 |
| 8 | Jaroměř | HK | 50.351 N, 15.853 E | 300 m | D, F | 13.8 |
| 9 | Hradec Králové | HK | 50.217 N, 15.727 E | 290 m | D, F | 28.0 |
| 10 | Chlumec nad Cidlinou | HK | 50.150 N, 15.571 E | 230 m | D, F | 14.7 |
| 11 | Nové Město nad Metují | HK | 50.312 N, 16.134 E | 300 m | D, F | 22.0 |
| 12 | Chlumec nad Cidlinou | HK | 50.112 N, 15.484 E | 220 m | D, F | 21.4 |
| 13 | Nové Město nad Metují | HK | 50.330 N, 16.147 E | 320 m | D, F | 22.5 |
| 14 | Nové Město nad Metují | HK | 50.321 N, 16.124 E | 320 m | D, F | 17.8 |
| 15 | Náchod | HK | 50.417 N, 16.151 E | 380 m | D, F | 19.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Česlová, L.; Kalendová, P.; Dubnová, L.; Pernica, M.; Fischer, J. The Effect of Sample Pretreatment on the Anthocyanin Content in Czech Wild Elderberry (Sambucus nigra L.). Molecules 2023, 28, 6690. https://doi.org/10.3390/molecules28186690
Česlová L, Kalendová P, Dubnová L, Pernica M, Fischer J. The Effect of Sample Pretreatment on the Anthocyanin Content in Czech Wild Elderberry (Sambucus nigra L.). Molecules. 2023; 28(18):6690. https://doi.org/10.3390/molecules28186690
Chicago/Turabian StyleČeslová, Lenka, Petra Kalendová, Lucie Dubnová, Marek Pernica, and Jan Fischer. 2023. "The Effect of Sample Pretreatment on the Anthocyanin Content in Czech Wild Elderberry (Sambucus nigra L.)" Molecules 28, no. 18: 6690. https://doi.org/10.3390/molecules28186690
APA StyleČeslová, L., Kalendová, P., Dubnová, L., Pernica, M., & Fischer, J. (2023). The Effect of Sample Pretreatment on the Anthocyanin Content in Czech Wild Elderberry (Sambucus nigra L.). Molecules, 28(18), 6690. https://doi.org/10.3390/molecules28186690