Abstract
The heavy metal contamination of water systems has become a major environmental concern worldwide. Photocatalysis using metal-organic frameworks (MOFs) has emerged as a promising approach for heavy metal remediation, owing to the ability of MOFs to fully degrade contaminants through redox reactions that are driven by photogenerated charge carriers. This review provides a comprehensive analysis of recent developments in MOF-based photocatalysts for removing and decontaminating heavy metals from water. The tunable nature of MOFs allows the rational design of composition and features to enhance light harvesting, charge separation, pollutant absorptivity, and photocatalytic activities. Key strategies employed include metal coordination tuning, organic ligand functionalization, heteroatom doping, plasmonic nanoparticle incorporation, defect engineering, and morphology control. The mechanisms involved in the interactions between MOF photocatalysts and heavy metal contaminants are discussed, including light absorption, charge carrier separation, metal ion adsorption, and photocatalytic redox reactions. The review highlights diverse applications of MOF photocatalysts in treating heavy metals such as lead, mercury, chromium, cadmium, silver, arsenic, nickel, etc. in water remediation. Kinetic modeling provides vital insights into the complex interplay between coupled processes such as adsorption and photocatalytic degradation that influence treatment efficiency. Life cycle assessment (LCA) is also crucial for evaluating the sustainability of MOF-based technologies. By elucidating the latest advances, current challenges, and future opportunities, this review provides insights into the potential of MOF-based photocatalysts as a sustainable technology for addressing the critical issue of heavy metal pollution in water systems. Ongoing efforts are needed to address the issues of stability, recyclability, scalable synthesis, and practical reactor engineering.
1. Introduction
The heavy metal contamination of water systems has become a matter of major environmental concern worldwide. Rapid industrialization and inadequate wastewater management have led to the discharge of high levels of toxic heavy metals into water bodies, posing significant risks to ecosystems and human health [1]. Major heavy metal pollutants include arsenic, cadmium, chromium, mercury, and lead, which mainly originate from industrial activities, mining and ore processing, agricultural runoff, waste disposal and landfills, urban runoff, industrial effluents, power plants, vehicular emissions, construction activities, household products, and natural sources (Figure 1) [2,3].

Figure 1.
Heavy metal pollution sources.
Numerous toxic heavy metals are prevalent as contaminants within water systems, which is largely attributed to their extensive application across various industrial sectors. An illustrative example is chromium, which is widely employed in alloy manufacturing and tanning procedures, rendering it a predominant heavy metal pollutant within aquatic environments. Of particular concern is hexavalent chromium (Cr(VI)), which exhibits heightened solubility and toxicity in comparison to its trivalent counterpart (Cr(III)), consequently instigating mutagenic and carcinogenic repercussions [4]. Mercury, employed extensively in activities such as artisanal gold mining, dental amalgamations, and thermometer production, is often discharged into water bodies through industrial effluents. This elemental substance tends to accumulate along the aquatic food chain, consequently engendering implications for human health [5]. Cadmium, which is frequently utilized in metal plating and battery fabrication, is distinguished by its substantial toxicity and propensity for organismal accumulation [6]. Arsenic, originating from mining endeavors and pesticide application, inflicts contamination upon various drinking water sources, thereby imposing significant health hazards [7]. Lead, emitted from sources such as paint, batteries, and pipelines, emerges as a potent neurotoxin with the potential to undermine cognitive function and neural development [7]. Emanating from such industrial sources, the discharge of these hazardous heavy metals into water systems is manifestly associated with far-reaching ecological and health perils on a global scale. In light of these critical concerns, the imperative to advance efficacious remediation technologies emerges as pivotal in addressing the pervasive issue of heavy metal pollution and its profound ramifications for both ecosystems and human populations.
Such heavy metal pollution has led to contaminated drinking water sources, rendering them unsafe for human consumption. Therefore, there is an urgent need for efficient technologies that can remove toxic heavy metals from water according to stringent regulatory limits. Photocatalysis has emerged as a promising approach, owing to its ability to fully degrade heavy metal contaminants rather than just transferring them to a different phase. Metal-organic frameworks (MOFs), with their design flexibility, high surface area, and photocatalytic capabilities, have shown tremendous potential for heavy metal remediation through photocatalysis.
Conventional methods for removing heavy metals from wastewater include chemical precipitation, membrane filtration, ion exchange, and adsorption (Figure 2). However, these techniques have limitations such as incomplete metal removal, the generation of toxic sludge, high costs, and susceptibility to fouling (Table 1) [1].
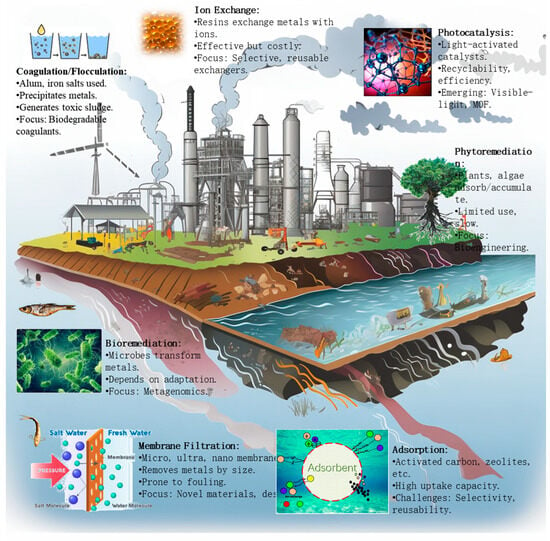
Figure 2.
Wastewater heavy metal removal methods.

Table 1.
Conventional and emerging technologies for heavy metal removal from wastewater.
Photocatalysis, on the other hand, offers a sustainable and efficient approach by utilizing solar energy to fully degrade heavy metal contaminants into less toxic forms. Upon photoirradiation, photocatalysts generate highly reactive radical species that can oxidize organic ligands and reduce toxic metals such as Cr(VI) and Hg(II) to their less soluble states [8]. Photocatalytic treatment enables the complete destruction of heavy metals rather than just transferring them to a different phase.
Moreover, photocatalysis can be performed under ambient conditions, which eliminates the need for chemical inputs and results in minimal sludge generation [9]. The oxidative power of photogenerated holes and the reducing capacity of electrons enables the degradation of a wide range of heavy metal species, as well as organic pollutants that may co-exist in wastewater. Photocatalysis also facilitates the recovery of valuable metals, thereby allowing wastewater to be used as a resource [10].
Given these advantages, photocatalytic treatment has garnered significant interest as a next-generation sustainable technology for heavy metal remediation. The development of highly efficient and stable photocatalysts is key to fully realizing the potential of photocatalysis for heavy metals control in water systems. Here is a brief introduction to MOFs and their potential as photocatalysts for heavy metal removal:
Metal-organic frameworks (MOFs) are an emerging class of porous materials that are constructed from metal ions/clusters coordinated to organic ligands. The modular nature of MOFs allows for the rational design of their chemical structure and properties [11]. MOFs possess ultrahigh porosity, large surface areas (of up to 7000 m2/g), and rich functionalities that can be incorporated via synthetic tuning [12].
These exceptional properties make MOFs highly promising photocatalysts for removing heavy metal contaminants from water. Their highly porous structure provides abundant active sites for adsorbing heavy metal ions, while the tunable organic ligands facilitate selective capture of target pollutants [13]. Band gaps and energy levels of MOFs can also be engineered to promote redox reactions for heavy metal degradation.
Furthermore, MOFs allow the incorporation of plasmonic nanoparticles that extend light absorption and enhance photocatalytic activities through hot electron injection [14]. The diverse metal clusters and organic linkers enable modulation of the MOFs’ electronic structure to suppress charge recombination and prolong charge carrier lifetimes. Overall, the structural and chemical versatility of MOFs creates ample opportunities for designing optimal photocatalysts for efficient heavy metal removal from water [15].
2. Design and Strategies
The tunable nature of MOFs provides ample opportunities to tailor their physical and chemical properties for efficient heavy metal removal. Through the judicious selection of the metal clusters and organic linkers, as well as post-synthetic modifications, MOFs can be rationally designed to optimize light harvesting, charge separation, adsorption capacities, and photocatalytic activities. Numerous structural engineering strategies have been employed, including metal coordination tuning, ligand functionalization, heteroatom doping, nanoscale architecture design, and defect incorporation, as shown in Figure 3. In this section, we highlight some of the most widely explored design strategies, demonstrating how the deliberate modulation of MOFs’ composition and features can enhance their photocatalytic performance for heavy metal control. A comprehensive understanding of structure–property relationships is key to unlocking the full potential of MOFs for sustainable water remediation through the continued development of high-efficiency photocatalytic systems. The various design strategies are briefly described in Table 2.
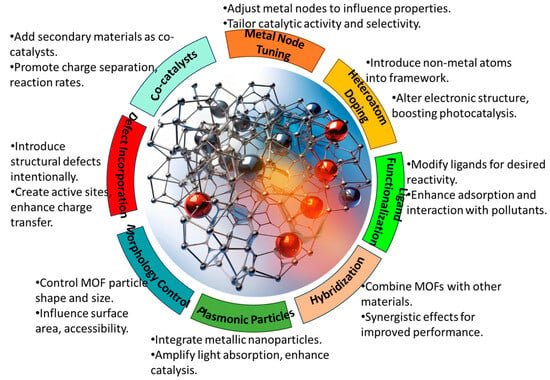
Figure 3.
The various strategies employed when designing MOF photocatalysts.

Table 2.
Design strategies employed to enhance MOFs for photocatalytic heavy metal remediation.
Metal Coordination: The metal clusters/nodes (e.g., Zn, Cu, and Fe) in MOFs can be selected to provide specific affinities toward target heavy metal contaminants through coordinative interactions (Table 2). For example, MOFs with open Fe(III) sites exhibited the selective capture of As(III) ions via strong Lewis acid-base interactions [32]. Transition metals such as Cu, Ni, and Co are often incorporated as nodes in MOFs to provide the selective binding of heavy metal ions through coordinate covalent interactions. For instance, a Cu-based MOF (Cu-TCPP) displayed 95% removal efficiency for Hg(II) ions, which was attributed to the strong affinity between Hg(II) and Cu(I) sites [33].
Ligand Functionalization: Organic ligands in MOFs can be functionalized with groups including carboxylates, amines, thiols, sulfonates, etc., to introduce selective binding sites for heavy metals [13]. Ligands containing sulfur atoms have shown a particular affinity with toxic soft heavy metals such as Hg(II) and Cd(II) [34]. Thiol (-SH)-containing organic ligands are widely used to functionalize MOFs for the selective capture of soft heavy metals such as Hg(II), Cd(II), and As(III). For example, a cysteine-modified MOF showed the fast and efficient removal of Hg(II) through Hg-S bonding [35].
Heteroatom Doping: Incorporating heteroatoms such as N, S, and P into MOFs introduces mid-gap states that enhance visible light absorption. The heterojunctions also promote the separation of photogenerated charge carriers [36,37]. Doping MOFs with N atoms induces mid-gap states, boosts light absorption, and provides catalytic sites for metal reduction. N-doped MOFs exhibited excellent activity in terms of the photocatalytic reduction of Cr(VI) to the less toxic Cr(III) [38].
Plasmonic Metal Incorporation: Embedding plasmonic nanometals (e.g., Au and Ag) in MOFs enables hot electron injection upon plasmon resonance excitation, creating reactive species for photocatalysis [14]. Hybrid Ag/MOF photocatalysts utilize the localized surface plasmon resonance of Ag for the enhanced generation of electron-hole pairs that will drive heavy metal degradation [39].
Defect Engineering: Structural defects can be intentionally created in MOFs through techniques such as acid etching. These defect sites act as active centers for adsorbing heavy metal ions and in catalytic processes [40].
Morphology Control: Tailoring the MOF’s morphology, such as particle size, shape, and porosity enables the optimization of mass transfer, light absorption, and accessibility to active sites [41].
Band Structure Engineering: The electronic band structure of MOFs can be tuned by altering the metal clusters and organic linkers to optimize light harvesting and charge transfer properties. Strategies include incorporating redox-active components and narrowing the band gap [42].
Surface Functionalization: The MOF’s surface can be functionalized with ligands or nanomaterials to improve heavy metal adsorption. Surface modification also enhances dispersion in aqueous media and prevents aggregation [43].
Hybridization with Carbon Materials: Incorporating carbon materials such as graphene or CNTs within MOFs enhances their conductivity and charge mobility. These carbon hybrids exhibit synergistic effects for adsorption and photocatalysis [44].
Co-catalyst Loading: Depositing co-catalyst nanoparticles such as Pt and RuO2 provides active sites for surface redox reactions. This facilitates charge transfer to adsorbed substrates and improves photocatalytic activities [45].
Template Synthesis: Hard templates such as polystyrene beads or soft templates such as micelles can guide the growth of MOFs, allowing control over particle sizes and morphologies, which is ideal for photocatalysis [46].
Defect Engineering: Creating defects in a controlled manner modifies the electronic structure and produces active sites in MOFs. Common techniques include acid/base treatment, ion exchange, or harsh activation [47].
MOFs can be synthesized through various methods that allow sufficient control over the reaction conditions to produce materials with tailored properties. Table 3 summarizes some MOFs that have been synthesized via different methods and their photocatalytic performance regarding heavy metal remediation.

Table 3.
MOF synthesis methods, categorized by reaction conditions.
Solvothermal Synthesis: MOFs are crystallized from solutions containing metal salts and organic ligands at elevated temperatures (100–250 °C) and autogenous pressures in autoclaves or hydrothermal bombs. This enables better control over crystallization and yields a high-quality product.
Microwave-Assisted Synthesis: Microwave irradiation rapidly heats the MOF precursor solution to accelerate nucleation and growth. It enables the rapid synthesis of MOFs with small crystal sizes and high porosity.
Ultrasonication Synthesis: Ultrasonic waves provide localized heat and agitation, which can promote MOF crystallization. This results in small, uniform crystals with defects that enhance their photocatalytic activity.
Room Temperature Synthesis: MOF synthesis is achieved in ambient conditions through the slow diffusion of reagents. This eliminates the need for heating but yields slower crystallization.
The tunable nature of MOFs allows properties such as porosity, particle size, morphology, etc., to be optimized for heavy metal removal through the selection of appropriate synthetic techniques and the control of reaction parameters, including temperature, pressure, heating methods, time, pH, etc.
3. Mechanisms
The exceptional photocatalytic performance of MOFs in the context of heavy metal removal is enabled by various photo-induced mechanisms. Upon light irradiation, a series of complex processes occur, including light absorption, charge carrier generation, separation and migration, the adsorption of metal ions, and photocatalytic redox reactions [54,55] (Figure 4). A brief table of MOFs remove heavy metal mechanisms by means of photocatalytic processes, as shown in Table 4.
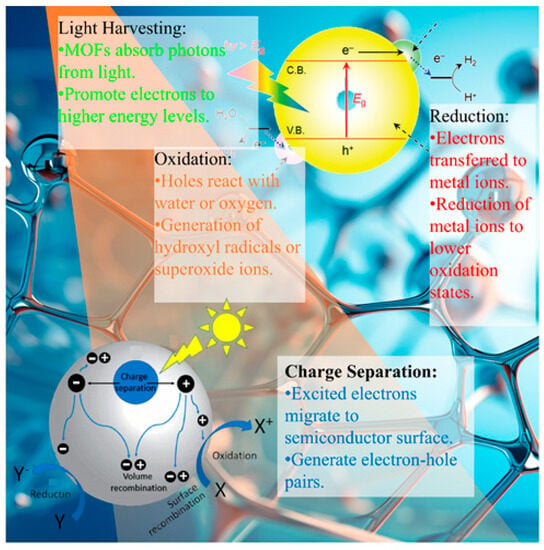
Figure 4.
Mechanisms involved in the MOF-based photocatalytic removal of heavy metals.

Table 4.
The mechanisms involved in the MOF-based photocatalytic removal of heavy metals.
3.1. Light Absorption
The irradiation of MOFs with light of the appropriate wavelength excites electrons from the valence band to the conduction band, generating electron-hole pairs [63]. Strategies such as bandgap engineering, heteroatom doping, and plasmonic metal incorporation help extend the light absorption into the visible range [64,65].
3.2. Charge Separation
The photogenerated electrons and holes must be separated before recombination, to enable redox reactions. In MOFs, heterojunctions between metal clusters, organic linkers, and integrated materials facilitate charge separation [66,67,68,69]. Type II heterojunctions are formed in MOFs that are hybridized with semiconductors such as TiO2, which favor the migration of electrons and holes to different materials [70,71]. Some metal clusters such as Zr6 act as electron sinks, which draw in excited electrons, thereby suppressing recombination [72,73]. Co-catalysts such as Pt nanoparticles provide a conduction band below that of MOFs, enabling charge transfer [74,75].
3.3. Heavy Metal Adsorption
The porous structure and functionalized sites on MOFs allow the adsorption of heavy metal ions through coordinative bonding, electrostatic interactions, π-complexation, etc. [76,77]. This draws the target pollutants closer to the photocatalytic active sites.
3.4. Reduction Mechanisms
Photogenerated electrons and superoxide radicals reduce toxic metals such as Hg(II) [78], Cr(VI) [79], and Cu(II) [80] into less soluble lower oxidation states for removal. Electron-rich Cu+ sites in Cu-containing MOFs drive the reduction of oxyanions such as Cr2O7− and SeO42− [81]. The superoxide radicals that are generated from O2 act as strong reducing agents [50].
3.5. Oxidation Mechanisms
Holes and powerful oxidants such as •OH and •O2 degrade the organic components of complexes, causing the precipitation or volatilization of metals [82]. Oxidation occurs through direct hole transfer or via •OH and •O2 generated from water and oxygen [83]. Singlet oxygen forms as a result of energy transfer from photoexcited MOFs to the adsorbed O2 [35].
3.6. Mineralization
Complete photocatalytic degradation into harmless ions, such as carbonates, water, and nitrates, enables sustainable remediation [84,85]. This requires sufficient oxidative strength to cleave all aliphatic and aromatic bonds [86]. Ion-exchange capacities help to remove inorganics such as NO3− and SO42− [87].
The tunable nature of MOFs provides opportunities to optimize the individual processes involved in photocatalytic mechanisms through the deliberate designing of composition and features. A comprehensive understanding of these mechanisms is key to engineering optimal MOF architectures for heavy metal remediation.
Computational modeling provides invaluable atomic-level insights into the mechanisms of MOF photocatalysis for heavy metal and organic contaminant removal. Techniques such as density functional theory (DFT) calculations can elucidate activation energies, reaction pathways, rate-determining steps, and excited state charge transfer dynamics, which are often difficult to probe experimentally. However, the accuracy of computational predictions depends strongly on the ability to appropriately represent the complex excited-state phenomena involved. Ongoing advancements in simulation methodology, the incorporation of van der Waals interactions, and transitions to explicit solvent modeling are still needed to improve accuracy. Additionally, calculated mechanisms require careful experimental validation via spectroscopic and kinetic techniques. When rigorously validated, the synergistic combination of computations and experiments serves as a powerful approach to unraveling photocatalytic mechanisms on a precise molecular scale.
4. Impact of Operating Parameters on Photocatalytic Efficiency
The photocatalytic degradation efficiency of heavy metals and organic pollutants can be significantly impacted by parameters such as pH, temperature, catalyst dosage, and initial contaminant concentration [88]. The pH affects photocatalytic reaction rates by influencing the surface charge of MOFs and the speciation of target pollutants, which, in turn, alters the adsorption capacities [89]. Elevated temperatures typically enhance reaction kinetics, as per the Arrhenius equation; however, excessive heating can impair adsorptivity. Increasing the photocatalyst dosage provides more active sites; however, excess amounts can reduce degradation efficiency due to factors such as light scattering. High initial contaminant concentrations can saturate the catalytic active sites and inhibit photocatalytic activity [90].
4.1. pH Effects
The pH influences the surface charge of the MOF photocatalyst and the speciation of target contaminants [91]. This, in turn, affects the adsorption capacities and photocatalytic reaction rates. For cationic heavy metals, a higher pH often impairs adsorption due to a reduced positive surface charge. However, an extremely low pH can also inhibit photocatalytic activity. The optimal pH needs to be determined for each MOF–contaminant system. pH influences kinetics by altering the adsorption equilibrium [76].
4.2. Temperature Effects
Photocatalytic reaction rates typically increase with temperature, as per the Arrhenius equation [92]. However, very high temperatures may also impair adsorption capacities. Temperature changes can also affect contaminant solubility and mass transport rates to the photocatalyst’s surface. Moderate temperatures of 20–60 °C are most suitable, although operation at ambient temperatures is preferred [93].
4.3. Dosage Effects
Increasing MOF photocatalyst dosages provide more active sites and can improve contaminant removal. However, excessive dosages beyond the optimum level can reduce degradation efficiency due to light scattering, particle aggregation, and a reduced surface area [94].
4.4. Initial Concentration Effects
Photocatalytic degradation rates are influenced by the initial contaminant concentration, due to kinetic and adsorption equilibria [95]. Very high concentrations can saturate the active sites and inhibit degradation. The optimization of initial concentrations is important [96].
5. Applications
5.1. Lead Removal
MIL-53(Al)-FA, a fumaric acid-modified MIL-53(Al) MOF, displayed an excellent Pb(II) adsorption capacity of 323.67 mg/g and rapid adsorption kinetics. It also exhibited the selective photoreduction of Pb(II) to Pb(0) under visible light irradiation [97].
CdS quantum dots, coupled with NH2-MIL-125(Ti) MOFs, showed synergy for enhanced Pb(II) removal. The optimal CdS/MOFs composite achieved 95.2% Pb(II) removal efficiency within 150 min under visible light [41].
Bio-MOFs-1, derived from biomass precursors, were effective for Pb(II) removal through combined adsorption and photocatalytic reduction mechanisms. A removal efficiency of 98.7% was reached in 120 min [26].
5.2. Mercury Remediation
Cysteine-functionalized MOFs (Cys-MIL-101) displayed excellent Hg(II) adsorption capacity (408 mg/g) and photocatalytic reduction performance, driven by Hg(II)-thiol coordination chemistry [98].
The Ag/ZIF-8 hybrid nanostructures exhibited synergistic effects between Ag nanoplasmonic transduction and ZIF-8 adsorption for the removal of Hg(II) ions from water. The Ag/ZIF-8 composites were magnetically separable for reuse [99,100].
5.3. Chromium Treatment
Azo-linked mesoporous MOFs showed excellent Cr(VI) removal capacity (501 mg/g) and photocatalytic reduction to less toxic Cr(III) species under solar light irradiation [101,102].
MIL-100(Fe) MOFs, modified with amine groups, were able to efficiently adsorb and photo-catalytically reduce Cr(VI) to Cr(III), which remained captured in the pores of the MOFs, preventing leaching [103,104,105].
Bio-MOFs-11, synthesized using fumaric acid and melamine precursors, rapidly reduced Cr(VI) within 30 min through a coupled adsorption–photocatalysis process [103].
6. Kinetic Modeling Research
Understanding reaction kinetics and developing appropriate mathematical models is critical for optimizing and implementing MOF-based photocatalytic systems for real-world water treatment applications. Kinetic investigations provide vital insights into the complex interplay between coupled processes such as mass transport, adsorption, charge transfer, and surface redox reactions that dictate treatment efficiency. These models allow the prediction of system performance under varied operating conditions and contaminant types. Robust kinetics and models enable rational MOF synthesis and reactor design for effective scaled-up photocatalytic treatment. Both experimental and simulation efforts to advance kinetics and modeling frameworks are, thus, integral to facilitating the translation of promising MOF photocatalysts into sustainable water purification technologies.
In this section, key aspects of kinetics and modeling that are applied to MOF photocatalysis for removing heavy metal ions, organic pollutants, and microbes from water are reviewed. The discussion illustrates the multifaceted reaction networks involved and highlights recent efforts to develop integrated kinetics models incorporating coupled adsorption, interfacial transfer, light absorption, contaminant degradation, and mass transport effects that influence the overall treatment rates and efficiencies.
6.1. Photomineralization Kinetics
The photocatalytic degradation of heavy metals and organic pollutants has been widely analyzed using the Langmuir–Hinshelwood models, which integrate reactant concentration and surface coverage effects [106]. Recent studies have developed more complex kinetic models to account for photonic efficiency, active sites, intermediates, and competitive adsorption between multiple contaminants [107].
Photomineralization kinetics directly influence disinfection rates because competition for active sites and surface intermediates can impede microbe inactivation. Particle transport also affects mineralization by altering the contaminant diffusion to catalytic sites.
6.2. Photo-Disinfection Kinetics
Empirical Chick–Watson, Hom, and other models are commonly applied to model the photocatalytic inactivation kinetics of microbes [108]. Mechanistic models based on Langmuir–Hinshelwood kinetics have also emerged to describe multi-step damage processes [109].
Photonic utilization efficiency is critical for photocatalytic disinfection. Transport effects that reduce light penetration into photocatalyst particles will, in turn, lower disinfection rates.
6.3. Particle Transport Effects
Mass transfer resistances and the particle diffusion effects strongly influence the observed photocatalytic reaction rates. Recent works have incorporated these factors into kinetic models using scaling relationships, along with particle size and reactor hydrodynamics [110,111,112].
The movement and aggregation of photocatalyst particles dictate the contaminant adsorption rates and light absorption, thereby affecting both photomineralization and disinfection kinetics (Table 5).

Table 5.
The kinetic models applied to analyze the MOF-based photocatalytic degradation of heavy metals and organic pollutants.
7. Life Cycle Assessment
Life cycle assessment (LCA) is an invaluable methodology by which to evaluate the sustainability of emerging technologies such as MOFs for heavy metal remediation. By examining the environmental impacts over the entire life cycle, from raw material extraction to synthesis, application, and end-of-life, LCA provides a comprehensive analysis of the technology’s green credentials.
Some key impact metrics that are relevant to the photocatalytic application of MOFs are included in Table 6 [15]:

Table 6.
Key impact metrics for a life cycle assessment of MOFs used in photocatalytic heavy metal remediation.
Embodied energy—energy utilized for materials synthesis and processing;
Global warming potential—greenhouse gas emissions across the life cycle;
Eutrophication potential—the impacts on aquatic ecosystems caused by discharges;
Human health criteria—exposure to hazardous substances.
Recent LCA studies on MOF synthesis have revealed high solvent usage, energy demands, and metal emissions as the current challenges [28]. However, photocatalysis with MOFs offers clear environmental advantages over conventional coagulation and ion exchange processes for heavy metal removal [1].
The circular economy potential of MOFs depends on the effective recycling of metals and ligands after water treatment [2], reducing the need for continuous virgin resource extraction. Advancements in more sustainable and greener MOF synthesis using biogenic or waste precursors are also promising [3].
Nevertheless, comprehensive LCA data that are directly relevant to MOFs for heavy metal photocatalysis are still scarce. Uncertainty in terms of long-term stability, reusability, and emissions during application warrants further research. Hybrid techno-economic analysis and LCA will also be crucial for scaling up production [4].
This LCA perspective aligns with the overall goal of this review in highlighting the potential of MOFs as photocatalysts for sustainable heavy metal remediation, while also elucidating the current knowledge gaps and future research needs. Further development of LCA methodologies tailored to MOFs can strengthen the environmental viability of this technology.
8. Challenges and Future Outlook
Despite the immense potential of MOFs as photocatalysts for heavy metal remediation, several challenges need to be addressed.
The limited stability and recyclability of some MOFs in aqueous media is a key challenge [41,119,120]. Developing MOFs with exceptional chemical, mechanical and thermal stability is required. Mass transfer limitations and diffusion barriers can reduce efficiency [43]. The limited stability and recyclability of some MOFs in aqueous media is a key challenge [121,122,123,124]. Developing MOFs with exceptional chemical, mechanical, and thermal stability is required. Preventing the leaching of metal ions and photocorrosion through structural modifications and composite formation is necessary [103,125]. Optimizing MOF pore sizes, particle sizes, and crystal morphologies is important. High material and synthesis costs could hinder large-scale adoption [46]. Exploring sustainable biosynthetic routes using inexpensive precursors is worthwhile. Performance should be assessed under real wastewater conditions, considering the effects of solution chemistry and interfering species [126]. Preventing the leaching of metal ions and photocorrosion through structural modifications and composite formation is necessary [127]. Scaling up fabrication while retaining control over morphology and properties is required [128]. Continuous flow and 3D printing synthesis methods are promising, but there is a lack of pilot-scale testing under solar illumination [129]. Finally, performance evaluation needs to shift from the laboratory to the real world.
Future prospects for advancing MOF photocatalysts include:
Developing novel visible-light responsive MOFs using conjugated ligands and doping [130]. Hybridizing with carbon materials such as graphene to enhance conductivity [131]. Incorporating plasmonic nanoparticles to facilitate hot electron transfer [132,133]. Exploring computational modeling to guide rational design [129,134,135]. Immobilizing MOFs on supports such as fibers and membranes for reuse [136,137]. Developing MOF-based composites and devices for practical applications [138,139].
9. Conclusions
Metal-organic frameworks (MOFs) have emerged as a promising class of porous materials for removing toxic heavy metals from contaminated water sources. While metal-organic frameworks present tremendous potential for efficient and sustainable heavy metal remediation, ongoing efforts are needed to address the issues of stability, recyclability, scalable synthesis, and practical reactor engineering. The tunable chemical structures and ultrahigh surface areas of MOFs allow heavy metal ions to be captured efficiently through size exclusion, adsorption, and photocatalysis. Key advances covered in this review include the tailoring of MOF composition using strategies such as metal node engineering, functionalized organic linkers, defect incorporation, and morphology control to optimize their adsorptive and redox properties.
Upon photoirradiation, MOFs generate reactive species, leading to photocatalytic oxidation and the reduction of heavy metals. The elucidation of mechanisms involving light harvesting, charge separation, contaminant adsorption, and interfacial redox reactions is crucial for designing optimal MOF photocatalysts. This review discussed the various applications of rationally designed MOFs for removing hazardous heavy metals including mercury, chromium, arsenic, lead, and cadmium from water through coupled adsorption-photocatalysis. While metal-organic frameworks present tremendous potential for efficient and sustainable heavy metal remediation, ongoing efforts are needed to address the issues of stability, recyclability, scalable synthesis, and practical reactor engineering. Hybridizing MOFs with plasmonic nanometals, carbon materials, and other photocatalysts could further enhance their visible light-harvesting capacity and charge separation. Their immobilization over supports improves their reusability and integration into continuous flow systems.
With increasing research advances in synthesis, characterization, and computational modeling, MOFs represent a versatile platform for developing next-generation photocatalytic technologies to address the significant global challenge of heavy metal pollution. Moving forward, pilot-scale testing under realistic conditions and life cycle assessments will be crucial to evaluate the promise of MOFs as green solutions for heavy metal removal and water purification.
Author Contributions
Q.M.: Writing—original draft. Y.L.: Conceptualization. B.X. and J.C.: Methodology, analysis, and experiment design. B.Y. and Y.T.: Investigation and writing—review and editing. Y.Z.: Supervision and resources. J.H.: Supervision. Q.W. (Qihong Wu) and Q.W. (Qingyuan Wang): Resources. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the Open Fund of Sichuan Provincial Engineering Research Center of City Solid Waste Energy and Building Materials Conversion & Utilization Technology (GF2022ZC012), and Key Laboratory of Coarse Cereal Processing, Ministry of Agriculture and Rural Affairs, Sichuan Engineering & Technology Research Center of Coarse Cereal Industrialization (2020CC020).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare that they have no known competing financial interest or personal relationship that could have appeared to influence the work reported in this paper.
Sample Availability
Not applicable.
Nomenclature
| C | Concentration of contaminant; mg/L |
| C0 | Initial concentration of contaminant; mg/L |
| k | Reaction rate constant; varies |
| kr | Intrinsic reaction rate constant; s−1 |
| K | Adsorption equilibrium constant; L/mg |
| t | Irradiation time; min |
| r | Reaction rate; mg/L∙min |
| θ | Surface coverage; dimensionless |
| q | Adsorbed amount; mg/g |
| Qt | Adsorbed amount at time t; mg/g |
| Qe | Adsorbed amount at equilibrium; mg/g |
| N | Number of viable microbes at time t |
| N0 | Initial number of viable microbes |
| F | Photon flux; mW/cm2 |
| D | Diffusion coefficient; m2/s |
| dp | Particle diameter; m |
References
- Fu, F.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, mechanism and health effects of some heavy metals. Interdiscip. Toxicol. 2014, 7, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Sharma, A.; Verma, R.K.; Chopade, R.L.; Pandit, P.P.; Nagar, V.; Aseri, V.; Choudhary, S.K.; Awasthi, G.; Awasthi, K.K. Heavy Metal Contamination of Water and Their Toxic Effect on Living Organisms. In The Toxicity of Environmental Pollutants; IntechOpen: London, UK, 2022. [Google Scholar] [CrossRef]
- Costa, M.; Klein, C.B. Toxicity and carcinogenicity of chromium compounds in humans. Crit. Rev. Toxicol. 2006, 36, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Feng, L.; Li, P.; Chen, X.; Jiang, J.; Zhang, S.; Zhang, C.; Zhang, A.; Chen, G.; Wang, H. Direct Z-scheme photocatalyst of hollow CoSx@CdS polyhedron constructed by ZIF-67-templated one-pot solvothermal route: A signal-on photoelectrochemical sensor for mercury (II). Chem. Eng. J. 2020, 395, 125072. [Google Scholar] [CrossRef]
- Kumar, V.; Wanchoo, R.K.; Toor, A.P. Sequential removal and recovery of cadmium ions (Cd2+) using photocatalysis and reduction crystallization from the aqueous phase. React. Chem. Eng. 2021, 6, 1677–1687. [Google Scholar] [CrossRef]
- Wang, Q.; Gao, Q.; Al-Enizi, A.M.; Nafady, A.; Ma, S. Recent advances in MOF-based photocatalysis: Environmental remediation under visible light. Inorg. Chem. Front. 2020, 7, 300–339. [Google Scholar] [CrossRef]
- Leary, R.; Westwood, A. Carbonaceous nanomaterials for the enhancement of TiO2 photocatalysis. Carbon 2011, 49, 741–772. [Google Scholar] [CrossRef]
- Chong, M.N.; Jin, B.; Chow, C.W.K.; Saint, C. Recent developments in photocatalytic water treatment technology: A review. Water Res. 2010, 44, 2997–3027. [Google Scholar] [CrossRef]
- Taghvaie Nakhjiri, A.; Sanaeepur, H.; Ebadi Amooghin, A.; Shirazi, M.M.A. Recovery of precious metals from industrial wastewater towards resource recovery and environmental sustainability: A critical review. Desalination 2022, 527, 115510. [Google Scholar] [CrossRef]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The chemistry and applications of metal-organic frameworks. Science 2013, 341, 1230444. [Google Scholar] [CrossRef]
- Li, J.-R.; Sculley, J.; Zhou, H.-C. Metal–Organic Frameworks for Separations. Chem. Rev. 2012, 112, 869–932. [Google Scholar] [CrossRef] [PubMed]
- Hasan, Z.; Jhung, S.H. Removal of hazardous organics from water using metal-organic frameworks (MOFs): Plausible mechanisms for selective adsorptions. J. Hazard. Mater. 2015, 283, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Lan, G.; Ni, K.; Lin, W. Nanoscale Metal-Organic Frameworks for Phototherapy of Cancer. Coord. Chem. Rev. 2019, 379, 65–81. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.-C.; Wang, J.; Guo, J.; Yan, M.-H.; Wang, J.; Srivastava, D.; Kumar, A.; Sakiyama, H.; Muddassir, M.; Pan, Y. A 3D rare cubane-like tetramer Cu(II)-based MOF with 4-fold dia topology as an efficient photocatalyst for dye degradation. Colloids Surf. A Physicochem. Eng. Asp. 2023, 656, 130475. [Google Scholar] [CrossRef]
- Chen, D.; Zhao, S.; Qu, Z.; Yan, N. Cu-BTC as a novel material for elemental mercury removal from sintering gas. Fuel 2018, 217, 297–305. [Google Scholar] [CrossRef]
- Zhao, Q.; Yi, X.-H.; Wang, C.-C.; Wang, P.; Zheng, W. Photocatalytic Cr(VI) reduction over MIL-101(Fe)–NH2 immobilized on alumina substrate: From batch test to continuous operation. Chem. Eng. J. 2022, 429, 132497. [Google Scholar] [CrossRef]
- Tran, T.V.; Jalil, A.A.; Nguyen, D.T.C.; Alhassan, M.; Nabgan, W.; Cao, A.N.T.; Nguyen, T.M.; Vo, D.-V.N. A critical review on the synthesis of NH2-MIL-53(Al) based materials for detection and removal of hazardous pollutants. Environ. Res. 2023, 216, 114422. [Google Scholar] [CrossRef]
- Zadehahmadi, F.; Eden, N.T.; Mahdavi, H.; Konstas, K.; Mardel, J.I.; Shaibani, M.; Banerjee, P.C.; Hill, M.R. Removal of metals from water using MOF-based composite adsorbents. Environ. Sci. Water Res. Technol. 2023, 9, 1305–1330. [Google Scholar] [CrossRef]
- Ahmad, S.Z.N.; Salleh, W.N.W.; Yusof, N.; Yusop, M.Z.M.; Hamdan, R.; Ismail, A.F. Synthesis of zeolitic imidazolate framework-8 (ZIF-8) using different solvents for lead and cadmium adsorption. Appl. Nanosci. 2023, 13, 4005–4019. [Google Scholar] [CrossRef]
- Ma, X.; Li, Q.; Li, R.; Zhang, W.; Sun, X.; Li, J.; Shen, J.; Han, W. Removal performance and mechanisms of Pb(II) and Sb(V) from water by iron-doped phosphogypsum: Single and coexisting systems. Environ. Sci. Pollut. Res. 2022, 29, 87413–87425. [Google Scholar] [CrossRef]
- Wang, Z.; Lv, Z.; Guo, A.; Hu, G.; Liu, J.; Huang, J. Formaldehyde-modified NH2-UiO-66 for specific sensing and simultaneous removal of mercury ions. Sens. Actuators Rep. 2022, 4, 100120. [Google Scholar] [CrossRef]
- Jiang, D.; Xu, P.; Wang, H.; Zeng, G.; Huang, D.; Chen, M.; Lai, C.; Zhang, C.; Wan, J.; Xue, W. Strategies to improve metal organic frameworks photocatalyst’s performance for degradation of organic pollutants. Coord. Chem. Rev. 2018, 376, 449–466. [Google Scholar] [CrossRef]
- Zang, Y.; Li, L.; Xu, Y.; Zuo, Y.; Li, G. Hybridization of brookite TiO2 with g-C3N4: A visible-light-driven photocatalyst for As3+ oxidation, MO degradation and water splitting for hydrogen evolution. J. Mater. Chem. A 2014, 2, 15774–15780. [Google Scholar] [CrossRef]
- Li, J.; Sun, C.; Wen, C.; Wang, L.; Zhao, Y.; Li, W.; Chang, Z. A stable multifunctional Zn(II) based metal-organic framework for sensitive detection of Hg(II), Cr(VI), nitrobenzene and adsorption of methylene blue. J. Environ. Chem. Eng. 2022, 10, 107880. [Google Scholar] [CrossRef]
- Lu, G.; Li, S.; Guo, Z.; Farha, O.K.; Hauser, B.G.; Qi, X.; Wang, Y.; Wang, X.; Han, S.; Liu, X.; et al. Imparting functionality to a metal-organic framework material by controlled nanoparticle encapsulation. Nat. Chem. 2012, 4, 310–316. [Google Scholar] [CrossRef]
- Fu, Y.; Tan, M.; Guo, Z.; Hao, D.; Xu, Y.; Du, H.; Zhang, C.; Guo, J.; Li, Q.; Wang, Q. Fabrication of wide-spectra-responsive NA/NH2-MIL-125(Ti) with boosted activity for Cr(VI) reduction and antibacterial effects. Chem. Eng. J. 2023, 452, 139417. [Google Scholar] [CrossRef]
- Li, L.; Zou, J.; Han, Y.; Liao, Z.; Lu, P.; Nezamzadeh-Ejhieh, A.; Liu, J.; Peng, Y. Recent advances in Al(iii)/In(iii)-based MOFs for the detection of pollutants. New J. Chem. 2022, 46, 19577–19592. [Google Scholar] [CrossRef]
- Zhou, J.; Shen, Q.; Yang, J.; Tariq, M.; Sun, W.; Cao, L.; Yang, J. A novel nano-sized Co3O4@C catalyst derived from Co-MOF template for efficient Hg0 removal at low temperatures with outstanding SO2 resistance. Environ. Sci. Pollut. Res. 2021, 28, 65487–65498. [Google Scholar] [CrossRef]
- Joseph, L.; Saha, M.; Kim, S.; Jun, B.-M.; Heo, J.; Park, C.M.; Jang, M.; Flora, J.R.V.; Yoon, Y. Removal of Cu2+, Cd2+, and Pb2+ from aqueous solution by fabricated MIL-100(Fe) and MIL-101(Cr): Experimental and molecular modeling study. J. Environ. Chem. Eng. 2021, 9, 106663. [Google Scholar] [CrossRef]
- Tian, H.; Gu, Y.; Zhou, H.; Huang, Y.; Fang, Y.; Li, R.; Tang, C. BiOBr@UiO-66 photocatalysts with abundant activated sites for the enhanced photodegradation of rhodamine b under visible light irradiation. Mater. Sci. Eng. B 2021, 271, 115297. [Google Scholar] [CrossRef]
- Chen, J.; Abazari, R.; Adegoke, K.A.; Maxakato, N.W.; Bello, O.S.; Tahir, M.; Tasleem, S.; Sanati, S.; Kirillov, A.M.; Zhou, Y. Metal–organic frameworks and derived materials as photocatalysts for water splitting and carbon dioxide reduction. Coord. Chem. Rev. 2022, 469, 214664. [Google Scholar] [CrossRef]
- Vishnyakov, A.; Ravikovitch, P.I.; Neimark, A.V.; Bülow, M.; Wang, Q.M. Nanopore Structure and Sorption Properties of Cu–BTC Metal–Organic Framework. Nano Lett. 2003, 3, 713–718. [Google Scholar] [CrossRef]
- Mandal, S.; Natarajan, S.; Mani, P.; Pankajakshan, A. Post-Synthetic Modification of Metal–Organic Frameworks Toward Applications. Adv. Funct. Mater. 2020, 31, 2006291. [Google Scholar] [CrossRef]
- Li, Z.; Wang, L.; Qin, L.; Lai, C.; Wang, Z.; Zhou, M.; Xiao, L.; Liu, S.; Zhang, M. Recent advances in the application of water-stable metal-organic frameworks: Adsorption and photocatalytic reduction of heavy metal in water. Chemosphere 2021, 285, 131432. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Zhu, Y.; Jiang, H.; Shen, J.; Yang, X.; Zou, W.; Chen, J.; Li, C. Cobalt nanoparticles embedded in N-doped carbon as an efficient bifunctional electrocatalyst for oxygen reduction and evolution reactions. Nanoscale 2014, 6, 15080–15089. [Google Scholar] [CrossRef]
- Song, X.-L.; Chen, L.; Gao, L.-J.; Ren, J.-T.; Yuan, Z.-Y. Engineering g-C3N4 based materials for advanced photocatalysis: Recent advances. Green Energy Environ. 2022, in press. [CrossRef]
- Xia, Q.; Huang, B.; Yuan, X.; Wang, H.; Wu, Z.; Jiang, L.; Xiong, T.; Zhang, J.; Zeng, G.; Wang, H. Modified stannous sulfide nanoparticles with metal-organic framework: Toward efficient and enhanced photocatalytic reduction of chromium (VI) under visible light. J. Colloid Interface Sci. 2018, 530, 481–492. [Google Scholar] [CrossRef]
- Ma, L.; Falkowski, J.M.; Abney, C.; Lin, W. A series of isoreticular chiral metal-organic frameworks as a tunable platform for asymmetric catalysis. Nat. Chem. 2010, 2, 838–846. [Google Scholar] [CrossRef] [PubMed]
- Paramanik, L.; Subudhi, S.; Parida, K.M. Visible light active titanate perovskites: An overview on its synthesis, characterization and photocatalytic applications. Mater. Res. Bull. 2022, 155, 111965. [Google Scholar] [CrossRef]
- Zhao, M.; Ou, S.; Wu, C.D. Porous metal-organic frameworks for heterogeneous biomimetic catalysis. Acc. Chem. Res. 2014, 47, 1199–1207. [Google Scholar] [CrossRef] [PubMed]
- Butler, K.T.; Hendon, C.H.; Walsh, A. Electronic Chemical Potentials of Porous Metal–Organic Frameworks. J. Am. Chem. Soc. 2014, 136, 2703–2706. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Kazemian, H.; Rohani, S.; Huang, Y.; Song, Y. In situ high pressure study of ZIF-8 by FTIR spectroscopy. Chem. Commun. 2011, 47, 12694–12696. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Lin, W. Metal-organic frameworks for artificial photosynthesis and photocatalysis. Chem. Soc. Rev. 2014, 43, 5982–5993. [Google Scholar] [CrossRef]
- Wang, C.; Xie, Z.; de Krafft, K.E.; Lin, W. Doping metal-organic frameworks for water oxidation, carbon dioxide reduction, and organic photocatalysis. J. Am. Chem. Soc. 2011, 133, 13445–13454. [Google Scholar] [CrossRef]
- Ma, L.; Abney, C.; Lin, W. Enantioselective catalysis with homochiral metal-organic frameworks. Chem. Soc. Rev. 2009, 38, 1248–1256. [Google Scholar] [CrossRef] [PubMed]
- Xiang, W.; Zhang, Y.; Chen, Y.; Liu, C.-j.; Tu, X. Synthesis, characterization and application of defective metal–organic frameworks: Current status and perspectives. J. Mater. Chem. A 2020, 8, 21526–21546. [Google Scholar] [CrossRef]
- Huang, L.; Cao, H.; Ma, J.; Wang, X. Efficient removal of Pb(II) by UiO-66-NH2: A combined experimental and spectroscopic studies. Environ. Nanotechnol. Monit. Manag. 2022, 18, 100741. [Google Scholar] [CrossRef]
- Li, X.; Gao, X.; Ai, L.; Jiang, J. Mechanistic insight into the interaction and adsorption of Cr (VI) with zeolitic imidazolate framework-67 microcrystals from aqueous solution. Chem. Eng. J. 2015, 274, 238–246. [Google Scholar] [CrossRef]
- Cui, Y.; Xu, H.; Yue, Y.; Guo, Z.; Yu, J.; Chen, Z.; Gao, J.; Yang, Y.; Qian, G.; Chen, B. A luminescent mixed-lanthanide metal-organic framework thermometer. J. Am. Chem. Soc. 2012, 134, 3979–3982. [Google Scholar] [CrossRef]
- Nguyen, H.L. Metal–organic frameworks for photocatalytic water splitting. Sol. RRL 2021, 5, 2100198. [Google Scholar] [CrossRef]
- Gatou, M.-A.; Vagena, I.-A.; Lagopati, N.; Pippa, N.; Gazouli, M.; Pavlatou, E.A. Functional MOF-Based Materials for Environmental and Biomedical Applications: A Critical Review. Nanomaterials 2023, 13, 2224. [Google Scholar] [CrossRef] [PubMed]
- Mondloch, J.E.; Katz, M.J.; Planas, N.; Semrouni, D.; Gagliardi, L.; Hupp, J.T.; Farha, O.K. Are Zr6-based MOFs water stable? Linker hydrolysis vs. capillary-force-driven channel collapse. Chem. Commun. 2014, 50, 8944–8946. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Li, Z.; Zhu, J.; Zhou, Y.; Chen, L.; Mai, X.; Liufu, M.; Wu, Y.; Li, Y. Inverse and highly selective separation of CO2/C2H2 on a thulium–organic framework. J. Mater. Chem. A 2020, 8, 11933–11937. [Google Scholar] [CrossRef]
- Ke, F.; Pan, A.; Liu, J.; Liu, X.; Yuan, T.; Zhang, C.; Fu, G.; Peng, C.; Zhu, J.; Wan, X. Hierarchical camellia-like metal–organic frameworks via a bimetal competitive coordination combined with alkaline-assisted strategy for boosting selective fluoride removal from brick tea. J. Colloid Interface Sci. 2023, 642, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Pi, Y.; Wu, L.; Xia, Q.; Wu, J.; Li, Z.; Xiao, J. Facilitation of the visible light-induced Fenton-like excitation of H2O2 via heterojunction of g-C3N4/NH2-Iron terephthalate metal-organic framework for MB degradation. Appl. Catal. B Environ. 2017, 202, 653–663. [Google Scholar] [CrossRef]
- Zheng, M.; Xu, L.; Chen, C.; Labiadh, L.; Yuan, B.; Fu, M.-L. MOFs and GO-based composites as deliberated materials for the adsorption of various water contaminants. Sep. Purif. Technol. 2022, 294, 121187. [Google Scholar] [CrossRef]
- Xia, Q.; Wang, H.; Huang, B.; Yuan, X.; Zhang, J.; Zhang, J.; Jiang, L.; Xiong, T.; Zeng, G. State-of-the-Art Advances and Challenges of Iron-Based Metal Organic Frameworks from Attractive Features, Synthesis to Multifunctional Applications. Small 2019, 15, e1803088. [Google Scholar] [CrossRef]
- Li, X.; Wang, B.; Cao, Y.; Zhao, S.; Wang, H.; Feng, X.; Zhou, J.; Ma, X. Water contaminant elimination based on metal–organic frameworks and perspective on their industrial applications. ACS Sustain. Chem. Eng. 2019, 7, 4548–4563. [Google Scholar] [CrossRef]
- Du, X.; Zhou, M. Strategies to enhance catalytic performance of metal–organic frameworks in sulfate radical-based advanced oxidation processes for organic pollutants removal. Chem. Eng. J. 2021, 403, 126346. [Google Scholar] [CrossRef]
- Tan, X.; Wang, S.; Han, N. Metal organic frameworks derived functional materials for energy and environment related sustainable applications. Chemosphere 2022, 313, 137330. [Google Scholar] [CrossRef]
- Li, R.; Wang, Z.; Yuan, Z.; Van Horne, C.; Freger, V.; Lin, M.; Cai, R.; Chen, J. A comprehensive review on water stable metal-organic frameworks for large-scale preparation and applications in water quality management based on surveys made since 2015. Crit. Rev. Environ. Sci. Technol. 2022, 52, 4038–4071. [Google Scholar] [CrossRef]
- Zhuang, Z.; Liu, D. Conductive MOFs with Photophysical Properties: Applications and Thin-Film Fabrication. Nano-Micro Lett. 2020, 12, 132. [Google Scholar] [CrossRef] [PubMed]
- Ning, J.; Mu, C.; Guo, X.; Yang, R.; Jonathan, R.; Jiao, W.; Wu, X.; Jian, X. Efficient defect engineering and in-situ carbon doping in ultra-fine TiO2 with enhanced visible-light-response photocatalytic performance. J. Alloys Compd. 2022, 901, 163490. [Google Scholar] [CrossRef]
- Yu, X.; Su, H.; Zou, J.; Liu, Q.; Wang, L.; Tang, H. Doping-induced metal–N active sites and bandgap engineering in graphitic carbon nitride for enhancing photocatalytic H2 evolution performance. Chin. J. Catal. 2022, 43, 421–432. [Google Scholar] [CrossRef]
- Chatterjee, A.; Wang, L.; Van Der Voort, P. Metal–organic frameworks in photocatalytic Z-scheme heterojunctions: An emerging technology. Chem. Commun. 2023, 59, 3627–3654. [Google Scholar] [CrossRef]
- Deng, X.; Long, R.; Gao, C.; Xiong, Y. Metal–organic frameworks for artificial photosynthesis via photoelectrochemical route. Curr. Opin. Electrochem. 2019, 17, 114–120. [Google Scholar] [CrossRef]
- Luo, T.; Gilmanova, L.; Kaskel, S. Advances of MOFs and COFs for photocatalytic CO2 reduction, H2 evolution and organic redox transformations. Coord. Chem. Rev. 2023, 490, 215210. [Google Scholar] [CrossRef]
- Khan, M.M.; Rahman, A.; Matussin, S.N. Recent Progress of Metal-Organic Frameworks and Metal-Organic Frameworks-Based Heterostructures as Photocatalysts. Nanomaterials 2022, 12, 2820. [Google Scholar] [CrossRef]
- Li, K.; Teng, C.; Wang, S.; Min, Q. Recent Advances in TiO2-Based Heterojunctions for Photocatalytic CO2 Reduction With Water Oxidation: A Review. Front. Chem. 2021, 9, 637501. [Google Scholar] [CrossRef]
- Wang, C.-C.; Wang, X.; Liu, W. The synthesis strategies and photocatalytic performances of TiO2/MOFs composites: A state-of-the-art review. Chem. Eng. J. 2020, 391, 123601. [Google Scholar] [CrossRef]
- Kidanemariam, A.; Lee, J.; Park, J. Recent Innovation of Metal-Organic Frameworks for Carbon Dioxide Photocatalytic Reduction. Polymers 2019, 11, 2090. [Google Scholar] [CrossRef] [PubMed]
- Gibbons, B.; Cai, M.; Morris, A.J. A Potential Roadmap to Integrated Metal Organic Framework Artificial Photosynthetic Arrays. J. Am. Chem. Soc. 2022, 144, 17723–17736. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Wang, J.; Ouyang, Y.; Li, D.; Xiao, Y.; Zhang, Q.; Huang, S. Multiple roles of 2D conductive metal-organic framework enable noble metal-free photocatalytic hydrogen evolution. Appl. Surf. Sci. 2023, 622, 156853. [Google Scholar] [CrossRef]
- Ejsmont, A.; Jankowska, A.; Goscianska, J. Insight into the Photocatalytic Activity of Cobalt-Based Metal–Organic Frameworks and Their Composites. Catalysts 2022, 12, 110. [Google Scholar] [CrossRef]
- Wang, C.; Liu, X.; Yang, T.; Sridhar, D.; Algadi, H.; Bin Xu, B.; El-Bahy, Z.M.; Li, H.; Ma, Y.; Li, T.; et al. An overview of metal-organic frameworks and their magnetic composites for the removal of pollutants. Sep. Purif. Technol. 2023, 320, 124144. [Google Scholar] [CrossRef]
- Mohan, B.; Kumar, S.; Bhatti, V.; Kumar, A.; Kumar, K.; Modi, K.; Jiao, T.; Chen, Q. Analogize of metal-organic frameworks (MOFs) adsorbents functional sites for Hg2+ ions removal. Sep. Purif. Technol. 2022, 297, 121471. [Google Scholar] [CrossRef]
- Chen, D.; Ray, A.K. Removal of toxic metal ions from wastewater by semiconductor photocatalysis. Chem. Eng. Sci. 2001, 56, 1561–1570. [Google Scholar] [CrossRef]
- Jafarzadeh, M. Recent Progress in the Development of MOF-Based Photocatalysts for the Photoreduction of Cr(VI). ACS Appl. Mater. Interfaces 2022, 14, 24993–25024. [Google Scholar] [CrossRef]
- Qian, Y.; Ma, D.; Zhong, J. Metal-Organic Frameworks With Variable Valence Metal-Photoactive Components: Emerging Platform for Volatile Organic Compounds Photocatalytic Degradation. Front. Chem. 2021, 9, 749839. [Google Scholar] [CrossRef]
- Howarth, A.J.; Liu, Y.; Hupp, J.T.; Farha, O.K. Metal–organic frameworks for applications in remediation of oxyanion/cation-contaminated water. CrystEngComm 2015, 17, 7245–7253. [Google Scholar] [CrossRef]
- Rao, R.; Ma, S.; Gao, B.; Bi, F.; Chen, Y.; Yang, Y.; Liu, N.; Wu, M.; Zhang, X. Recent advances of metal-organic framework-based and derivative materials in the heterogeneous catalytic removal of volatile organic compounds. J. Colloid Interface Sci. 2023, 636, 55–72. [Google Scholar] [CrossRef]
- Subudhi, S.; Tripathy, S.P.; Parida, K. Metal oxide integrated metal organic frameworks (MO@MOF): Rational design, fabrication strategy, characterization and emerging photocatalytic applications. Inorg. Chem. Front. 2021, 8, 1619–1636. [Google Scholar] [CrossRef]
- Serrà, A.; Philippe, L.; Perreault, F.; Garcia-Segura, S. Photocatalytic treatment of natural waters. Reality or hype? The case of cyanotoxins remediation. Water Res. 2021, 188, 116543. [Google Scholar] [CrossRef] [PubMed]
- Jun, B.-M.; Elanchezhiyan, S.S.; Yoon, Y.; Wang, D.; Kim, S.; Muthu Prabhu, S.; Park, C.M. Accelerated photocatalytic degradation of organic pollutants over carbonate-rich lanthanum-substituted zinc spinel ferrite assembled reduced graphene oxide by ultraviolet (UV)-activated persulfate. Chem. Eng. J. 2020, 393, 124733. [Google Scholar] [CrossRef]
- Singh, S.; Kumar, P.; Patidar, R.; Srivastava, V.C.; Lo, S.-L.; Štangar, U.L. Catalytic oxidation of Bisphenol A with Co3+ rich spinel Co3O4: Performance evaluation with peroxymonosulfate activation and mineralization mechanism. J. Environ. Chem. Eng. 2023, 11, 110023. [Google Scholar] [CrossRef]
- Zereini, F.; Wiseman, C.L.S.; Poprizki, J.; Albers, P.; Schneider, W.; Leopold, K. Assessing the potential of inorganic anions (Cl−, NO3−, SO42− and PO43−) to increase the bioaccessibility of emitted palladium in the environment: Experimental studies with soils and a Pd model substance. Environ. Pollut. 2017, 220, 1050–1058. [Google Scholar] [CrossRef] [PubMed]
- Hasanpour, M.; Hatami, M. Photocatalytic performance of aerogels for organic dyes removal from wastewaters: Review study. J. Mol. Liq. 2020, 309, 113094. [Google Scholar] [CrossRef]
- Wang, D.; Li, Y.; Jiang, Y.; Cai, X.; Yao, X. Perspectives on surface chemistry of nanostructured catalysts for heterogeneous advanced oxidation processes. Environ. Funct. Mater. 2022, 1, 182–186. [Google Scholar] [CrossRef]
- Abdollahzadeh, S.; Sayadi, M.H.; Shekari, H. Synthesis of biodegradable antibacterial nanocomposite (metal–organic frameworks supported by chitosan and graphene oxide) with high stability and photocatalytic activities. Inorg. Chem. Commun. 2023, 156, 111302. [Google Scholar] [CrossRef]
- Ashraf, G.A.; Rasool, R.T.; Al-Sulaimi, S.; Rasool, R.U.; Hassan, N.; Ajmal, Z.; Mahmood, Q.; Khan, A.; Xiao, C.; Jie, W. Construction of type-II scheme SnO@HfC photocatalyst for bisphenol A degradation via peroxymonosulfate activation; DFT and self-cleaning analysis. Chemosphere 2023, 341, 140095. [Google Scholar] [CrossRef]
- Ma, L.; Lu, M.; Li, K.; Zhang, S.; Liu, H.; Huang, Y.; Xing, Z.; Wu, Z.; Ye, X. Photocatalytic degradation of octadecylamine and 4-dodecylmorpholine over titanium based photocatalyst: Activity and mechanism insights. Chem. Eng. J. 2023, 472, 144782. [Google Scholar] [CrossRef]
- Rodríguez-Chueca, J.; Carbajo, J.; García-Muñoz, P. Intensification of Photo-Assisted Advanced Oxidation Processes for Water Treatment: A Critical Review. Catalysts 2023, 13, 401. [Google Scholar] [CrossRef]
- Fattahi, M.; Niazi, Z.; Esmaeili, F.; Mohammadi, A.A.; Shams, M.; Nguyen Le, B. Boosting the adsorptive and photocatalytic performance of MIL-101(Fe) against methylene blue dye through a thermal post-synthesis modification. Sci. Rep. 2023, 13, 14502. [Google Scholar] [CrossRef]
- Xu, P.; Ding, C.; Li, Z.; Yu, R.; Cui, H.; Gao, S. Photocatalytic degradation of air pollutant by modified nano titanium oxide (TiO2) in a fluidized bed photoreactor: Optimizing and kinetic modeling. Chemosphere 2023, 319, 137995. [Google Scholar] [CrossRef] [PubMed]
- Akbari, M.Z.; Xu, Y.; Liang, C.; Lu, Z.; Siyuan, S.; Peng, L. Synthesis of ZnO@VC for Enhancement of Synergic Photocatalysis Degradation of SMX: Toxicity Assessment, Kinetics and Transformation Pathway Determination. Chem. Eng. Process.-Process Intensif. 2023, 47, 1199–1207. [Google Scholar] [CrossRef]
- Wen, M.; Li, G.; Liu, H.; Chen, J.; An, T.; Yamashita, H. Metal–organic framework-based nanomaterials for adsorption and photocatalytic degradation of gaseous pollutants: Recent progress and challenges. Environ. Sci. Nano 2019, 6, 1006–1025. [Google Scholar] [CrossRef]
- Kim, Y.; Lam, I.T.Y.; Choi, S.-J.; Lu, D. Functionalized Metal–Organic Frameworks for Heavy Metal Ion Removal from Water. Nanoscale 2023, 15, 10189–10205. [Google Scholar] [CrossRef]
- Ahmad, K.; Ashfaq, M.; Shah, S.S.A.; Hussain, E.; Naseem, H.A.; Parveen, S.; Ayub, A. Effect of metal atom in zeolitic imidazolate frameworks (ZIF-8 & 67) for removal of Pb2+ & Hg2+ from water. Food Chem. Toxicol. 2021, 149, 112008. [Google Scholar] [CrossRef]
- Yin, L.L.; Kong, X.Y.; Zhang, Y.; Ji, Y.Q. Facile synthesis of the magnetic metal organic framework Fe3O4@ UiO-66-NH2 for separation of strontium. Biomed. Environ. Sci. 2018, 31, 483–488. [Google Scholar] [CrossRef] [PubMed]
- Cherifi, Y.; Barras, A.; Addad, A.; Ouddane, B.; Roussel, P.; Chaouchi, A.; Szunerits, S.; Boukherroub, R. Simultaneous photocatalytic Cr (VI) reduction and phenol degradation over copper sulphide-reduced graphene oxide nanocomposite under visible light irradiation: Performance and reaction mechanism. Chemosphere 2021, 268, 128798. [Google Scholar] [CrossRef]
- Sun, H.; Wang, L.; Liu, Y.; Cheng, Z.; Zhao, Y.; Guo, H.; Qu, G.; Wang, T.; Yin, X. Photocatalytic reduction of Cr (VI) via surface modified g-C3N4 by acid-base regulation. J. Environ. Manag. 2022, 324, 116431. [Google Scholar] [CrossRef] [PubMed]
- Joseph, J.; Iftekhar, S.; Srivastava, V.; Fallah, Z.; Zare, E.N.; Sillanpää, M. Iron-based metal-organic framework: Synthesis, structure and current technologies for water reclamation with deep insight into framework integrity. Chemosphere 2021, 284, 131171. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Wen, J.; Zeng, G.; Jia, F.; Zhang, S.; Peng, Z.; Zhang, H. Effect of mineralizing agents on the adsorption performance of metal–organic framework MIL-100 (Fe) towards chromium (VI). Chem. Eng. J. 2018, 337, 532–540. [Google Scholar] [CrossRef]
- García, A.; Rodríguez, B.; Rosales, M.; Quintero, Y.M.; Saiz, P.G.; Reizabal, A.; Wuttke, S.; Celaya-Azcoaga, L.; Valverde, A.; Fernández de Luis, R. A State-of-the-Art of Metal-Organic Frameworks for Chromium Photoreduction vs. Photocatalytic Water Remediation. Nanomaterials 2022, 12, 4263. [Google Scholar] [CrossRef]
- Hossein Panahi, A.; Al-Musawi, T.J.; Masihpour, M.; Fard, S.F.; Nasseh, N. Photocatalytic Degradation of Humic Acid Using Bentonite@Fe3O4@ZnO Magnetic Nanocomposite: An Investigation of the Characterization of the Photocatalyst, Degradation Pathway, and Modeling by Solver Plugin. Water 2023, 15, 2931. [Google Scholar] [CrossRef]
- Tulebekov, Y.; Orazov, Z.; Satybaldiyev, B.; Snow, D.D.; Schneider, R.; Uralbekov, B. Reaction Steps in Heterogeneous Photocatalytic Oxidation of Toluene in Gas Phase—A Review. Molecules 2023, 28, 6451. [Google Scholar] [CrossRef]
- Chowdhury, P.; Hashim, N.; Ray, A.K. Photocatalytic Degradation of Organic Pollutants and Airborne Pathogen in Air. In Photocatalysis for Environmental Remediation and Energy Production: Recent Advances and Applications; Garg, S., Chandra, A., Eds.; Springer International Publishing: Cham, Switzerland, 2023; pp. 211–234. [Google Scholar]
- Yoo, P.; Liao, P. Multiple redox mechanisms for water-gas shift reaction on Fe3O4 (111) surface: A density functional theory and mean-field microkinetic modeling study. Appl. Surf. Sci. 2023, 630, 157501. [Google Scholar] [CrossRef]
- Binjhade, R.; Mondal, R.; Mondal, S. Continuous photocatalytic reactor: Critical review on the design and performance. J. Environ. Chem. Eng. 2022, 10, 107746. [Google Scholar] [CrossRef]
- Rasul, M.G.; Ahmed, S.; Sattar, M.A.; Jahirul, M.I. Modelling and analysis of hydrodynamics and flow phenomena of fluid with formic acid as pollutant in the reactive area of a flat plate photocatalytic reactor with top and bottom turbulence promote. Chem. Eng. J. 2023, 466, 142760. [Google Scholar] [CrossRef]
- Rivera De la Rosa, J.; González-Casamachin, D.A.; Lucio-Ortiz, C.J.; De Haro-Del Río, D.A. Chapter 2-Kinetic and mass-transfer analyses in continuous photocatalytic reactors. In Nanostructured Photocatalysts; Nguyen, V.-H., Vo, D.-V.N., Nanda, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 35–62. [Google Scholar]
- Largitte, L.; Pasquier, R. A review of the kinetics adsorption models and their application to the adsorption of lead by an activated carbon. Chem. Eng. Res. Des. 2016, 109, 495–504. [Google Scholar] [CrossRef]
- Fan, G.; Zheng, X.; Luo, J.; Peng, H.; Lin, H.; Bao, M.; Hong, L.; Zhou, J. Rapid synthesis of Ag/AgCl@ZIF-8 as a highly efficient photocatalyst for degradation of acetaminophen under visible light. Chem. Eng. J. 2018, 351, 782–790. [Google Scholar] [CrossRef]
- Wang, H.; Wang, S.; Wang, S.; Tang, J.; Chen, Y.; Zhang, L. Adenosine-functionalized UiO-66-NH2 to efficiently remove Pb(II) and Cr(VI) from aqueous solution: Thermodynamics, kinetics and isothermal adsorption. J. Hazard. Mater. 2022, 425, 127771. [Google Scholar] [CrossRef]
- Jin, Y.; Li, Y.; Du, Q.; Chen, B.; Chen, K.; Zhang, Y.; Wang, M.; Sun, Y.; Zhao, S.; Jing, Z.; et al. Efficient adsorption of Congo red by MIL-53(Fe)/chitosan composite hydrogel spheres. Microporous Mesoporous Mater. 2023, 348, 112404. [Google Scholar] [CrossRef]
- Huang, X.; Huang, L.; Babu Arulmani, S.R.; Yan, J.; Li, Q.; Tang, J.; Wan, K.; Zhang, H.; Xiao, T.; Shao, M. Research progress of metal organic frameworks and their derivatives for adsorption of anions in water: A review. Environ. Res. 2022, 204, 112381. [Google Scholar] [CrossRef]
- Che, J.; Chen, K.; Song, J.; Tu, Y.; Reymick, O.O.; Chen, X.; Tao, N. Fabrication of γ-cyclodextrin-Based metal-organic frameworks as a carrier of cinnamaldehyde and its application in fresh-cut cantaloupes. Curr. Res. Food Sci. 2022, 5, 2114–2124. [Google Scholar] [CrossRef]
- Li, B.; Wen, H.M.; Zhou, W.; Chen, B. Porous Metal-Organic Frameworks for Gas Storage and Separation: What, How, and Why? J. Phys. Chem. Lett. 2014, 5, 3468–3479. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, L.; Lin, R.-B.; Zhou, W.; Zhang, Z.; Xiang, S.; Chen, B. Porous metal-organic frameworks for gas storage and separation: Status and challenges. EnergyChem 2019, 1, 100006. [Google Scholar] [CrossRef]
- Zheng, M.; Chen, J.; Zhang, L.; Cheng, Y.; Lu, C.; Liu, Y.; Singh, A.; Trivedi, M.; Kumar, A.; Liu, J. Metal organic frameworks as efficient adsorbents for drugs from wastewater. Mater. Today Commun. 2022, 31, 103514. [Google Scholar] [CrossRef]
- Sahoo, R.; Mondal, S.; Chand, S.; Manna, A.K.; Das, M.C. A Water-Stable Cationic SIFSIX MOF for Luminescent Probing of Cr2O72− via Single-Crystal to Single-Crystal Transformation. Small 2023, 2304581. [Google Scholar] [CrossRef]
- Pramanik, B.; Sahoo, R.; Das, M.C. pH-stable MOFs: Design principles and applications. Coord. Chem. Rev. 2023, 493, 215301. [Google Scholar] [CrossRef]
- Zhao, W.; Shen, Q.; Nan, T.; Zhou, M.; Xia, Y.; Hu, G.; Zheng, Q.; Wu, Y.; Bian, T.; Wei, T.; et al. Cobalt-based catalysts for heterogeneous peroxymonosulfate (PMS) activation in degradation of organic contaminants: Recent advances and perspectives. J. Alloys Compd. 2023, 958, 170370. [Google Scholar] [CrossRef]
- Hao, J.; Mao, W.; Ye, G.; Xia, Y.; Wei, C.; Zeng, L.; Zhou, J. Tin–chromium bimetallic metal–organic framework MIL-101 (Cr, Sn) as a catalyst for glucose conversion into HMF. Biomass Bioenergy 2022, 159, 106395. [Google Scholar] [CrossRef]
- Al-Nuaim, M.A.; Alwasiti, A.A.; Shnain, Z.Y. The photocatalytic process in the treatment of polluted water. Chem. Pap. 2023, 77, 677–701. [Google Scholar] [CrossRef]
- Bai, Y.; Liu, C.; Shan, Y.; Chen, T.; Zhao, Y.; Yu, C.; Pang, H. Metal-organic frameworks nanocomposites with different dimensionalities for energy conversion and storage. Adv. Energy Mater. 2022, 12, 2100346. [Google Scholar] [CrossRef]
- Ma, Q.; Zhang, T.; Wang, B. Shaping of metal-organic frameworks, a critical step toward industrial applications. Matter 2022, 5, 1070–1091. [Google Scholar] [CrossRef]
- Naghdi, S.; Shahrestani, M.M.; Zendehbad, M.; Djahaniani, H.; Kazemian, H.; Eder, D. Recent advances in application of metal-organic frameworks (MOFs) as adsorbent and catalyst in removal of persistent organic pollutants (POPs). J. Hazard. Mater. 2023, 442, 130127. [Google Scholar] [CrossRef]
- Mukherjee, D.; Van der Bruggen, B.; Mandal, B. Advancements in visible light responsive MOF composites for photocatalytic decontamination of textile wastewater: A review. Chemosphere 2022, 295, 133835. [Google Scholar] [CrossRef] [PubMed]
- Jayaramulu, K.; Mukherjee, S.; Morales, D.M.; Dubal, D.P.; Nanjundan, A.K.; Schneemann, A.; Masa, J.; Kment, S.; Schuhmann, W.; Otyepka, M.; et al. Graphene-Based Metal–Organic Framework Hybrids for Applications in Catalysis, Environmental, and Energy Technologies. Chem. Rev. 2022, 122, 17241–17338. [Google Scholar] [CrossRef]
- Koh, C.S.L.; Sim, H.Y.F.; Leong, S.X.; Boong, S.K.; Chong, C.; Ling, X.Y. Plasmonic Nanoparticle-Metal–Organic Framework (NP–MOF) Nanohybrid Platforms for Emerging Plasmonic Applications. ACS Mater. Lett. 2021, 3, 557–573. [Google Scholar] [CrossRef]
- Ly, N.H.; Vasseghian, Y.; Joo, S.-W. Plasmonic photocatalysts for enhanced solar hydrogen production: A comprehensive review. Fuel 2023, 344, 128087. [Google Scholar] [CrossRef]
- Gao, M.; Liu, G.; Gao, Y.; Chen, G.; Huang, X.; Xu, X.; Wang, J.; Yang, X.; Xu, D. Recent advances in metal-organic frameworks/membranes for adsorption and removal of metal ions. TrAC Trends Anal. Chem. 2021, 137, 116226. [Google Scholar] [CrossRef]
- Bernales, V.; Ortuño, M.A.; Truhlar, D.G.; Cramer, C.J.; Gagliardi, L. Computational Design of Functionalized Metal–Organic Framework Nodes for Catalysis. ACS Cent. Sci. 2018, 4, 5–19. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Duan, C.; Liu, C.; Zhang, Y.; Meng, X.; Dai, L.; Wang, W.; Yu, H.; Ni, Y. A self-cleaning and photocatalytic cellulose-fiber- supported “Ag@AgCl@MOF- cloth’’ membrane for complex wastewater remediation. Carbohydr. Polym. 2020, 247, 116691. [Google Scholar] [CrossRef]
- Kang, M.; Yu, S.H.; Baek, K.-Y.; Sung, M.M.; Cho, S. MIL-101-NH2 (Fe)-Coated Nylon Microfibers for Immobilized Photocatalysts in RhB and Cr (VI) Removal. ACS Omega 2023, 8, 15298–15305. [Google Scholar] [CrossRef] [PubMed]
- Lv, S.-W.; Cong, Y.; Chen, X.; Wang, W.; Che, L. Developing fine-tuned metal–organic frameworks for photocatalytic treatment of wastewater: A review. Chem. Eng. J. 2022, 433, 133605. [Google Scholar] [CrossRef]
- Younis, S.A.; Kwon, E.E.; Qasim, M.; Kim, K.-H.; Kim, T.; Kukkar, D.; Dou, X.; Ali, I. Metal-organic framework as a photocatalyst: Progress in modulation strategies and environmental/energy applications. Prog. Energy Combust. Sci. 2020, 81, 100870. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).