Lipase as a Chiral Selector Immobilised on Carboxylated Single-Walled Carbon Nanotubes and Encapsulated in the Organic Polymer Monolithic Capillary for Nano-High Performance Liquid Chromatography Enantioseparation of Racemic Pharmaceuticals
Abstract
:1. Introduction
2. Results and Discussion
2.1. Preparation and Characterisation of Functionalised Monolithic Capillaries
2.1.1. Polymer Monolith Backbone: Preparation
2.1.2. Characterisation of Functionalised Monolithic Capillaries
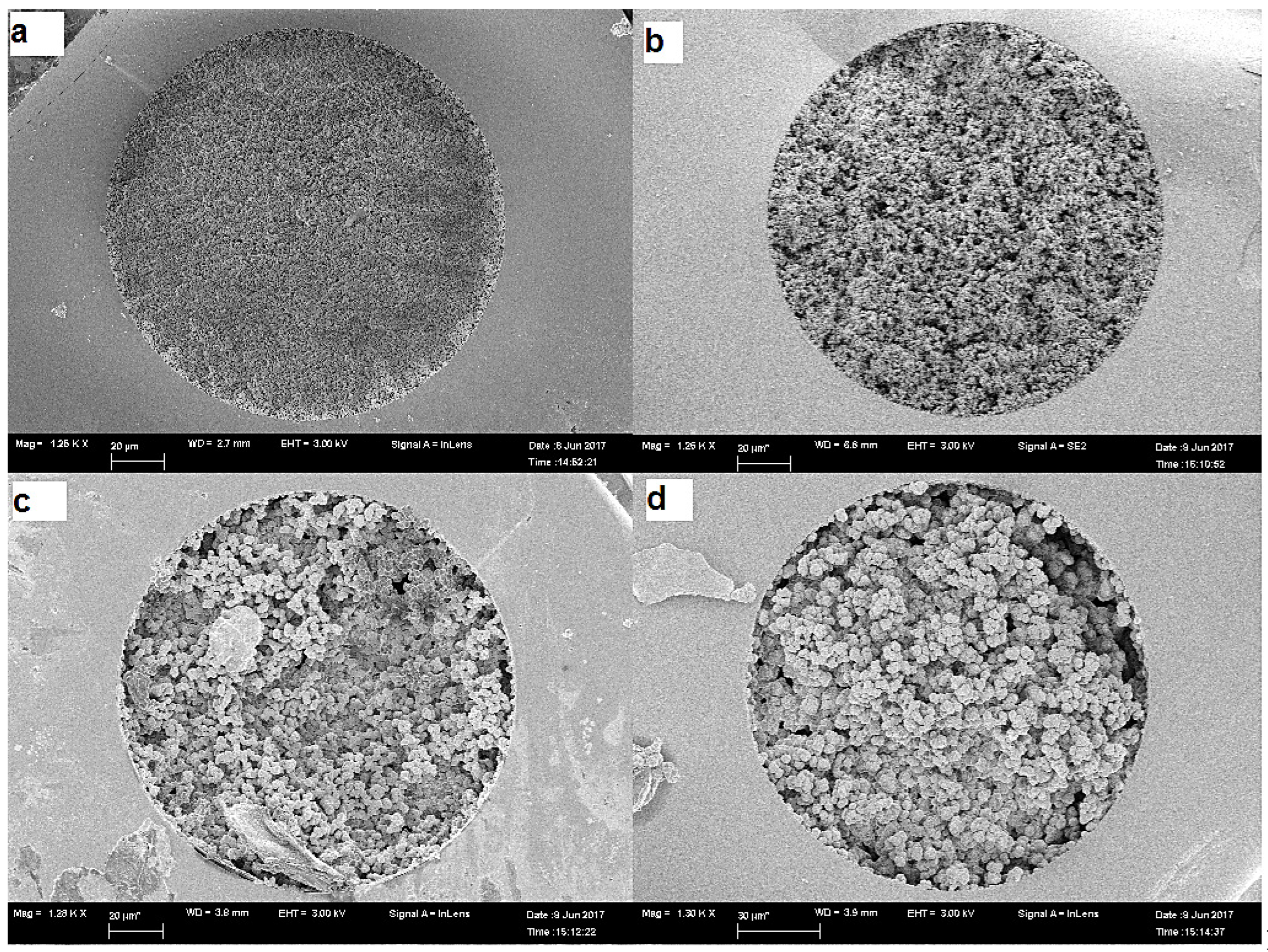
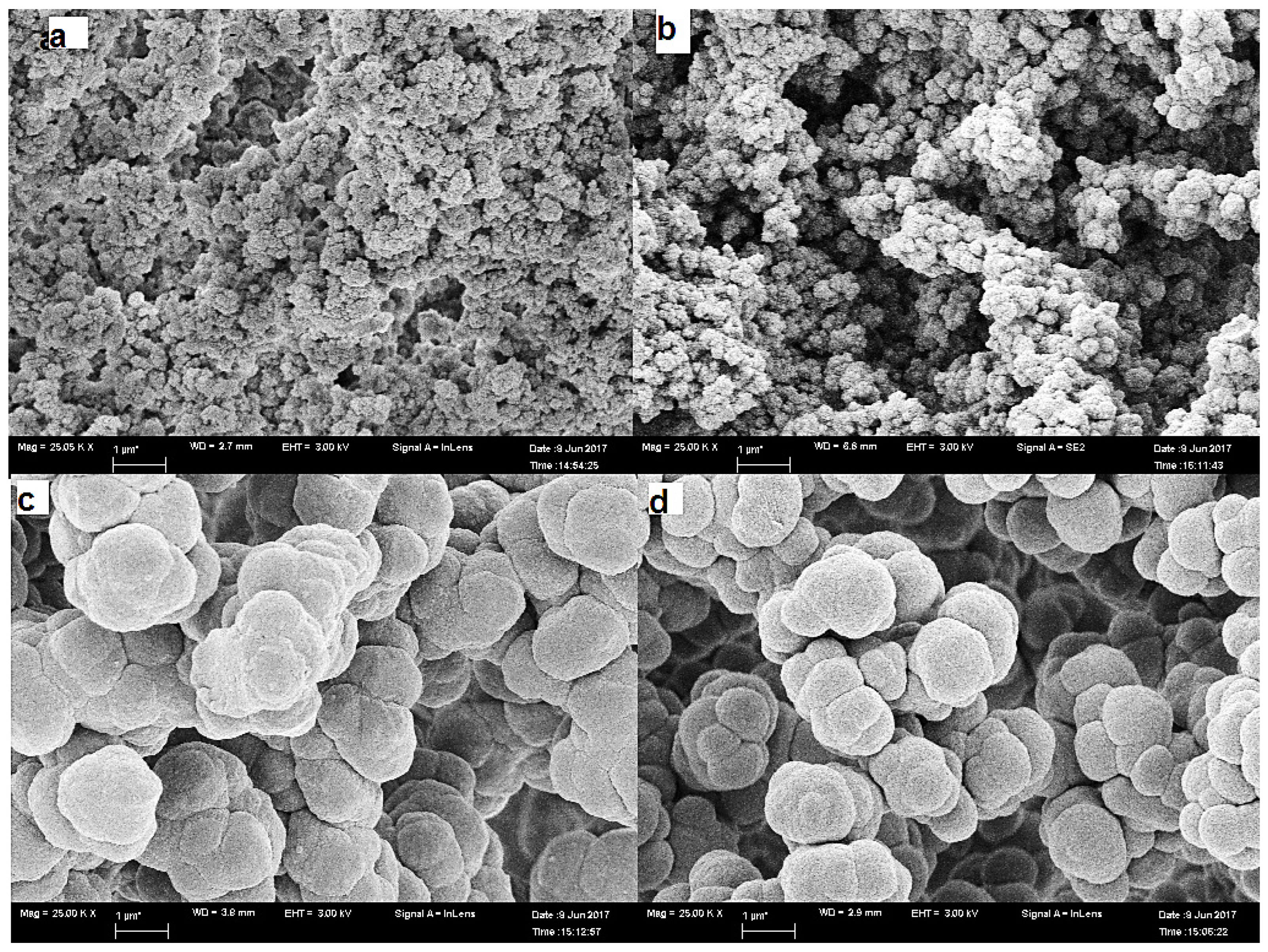
Microscopy
Total Porosity
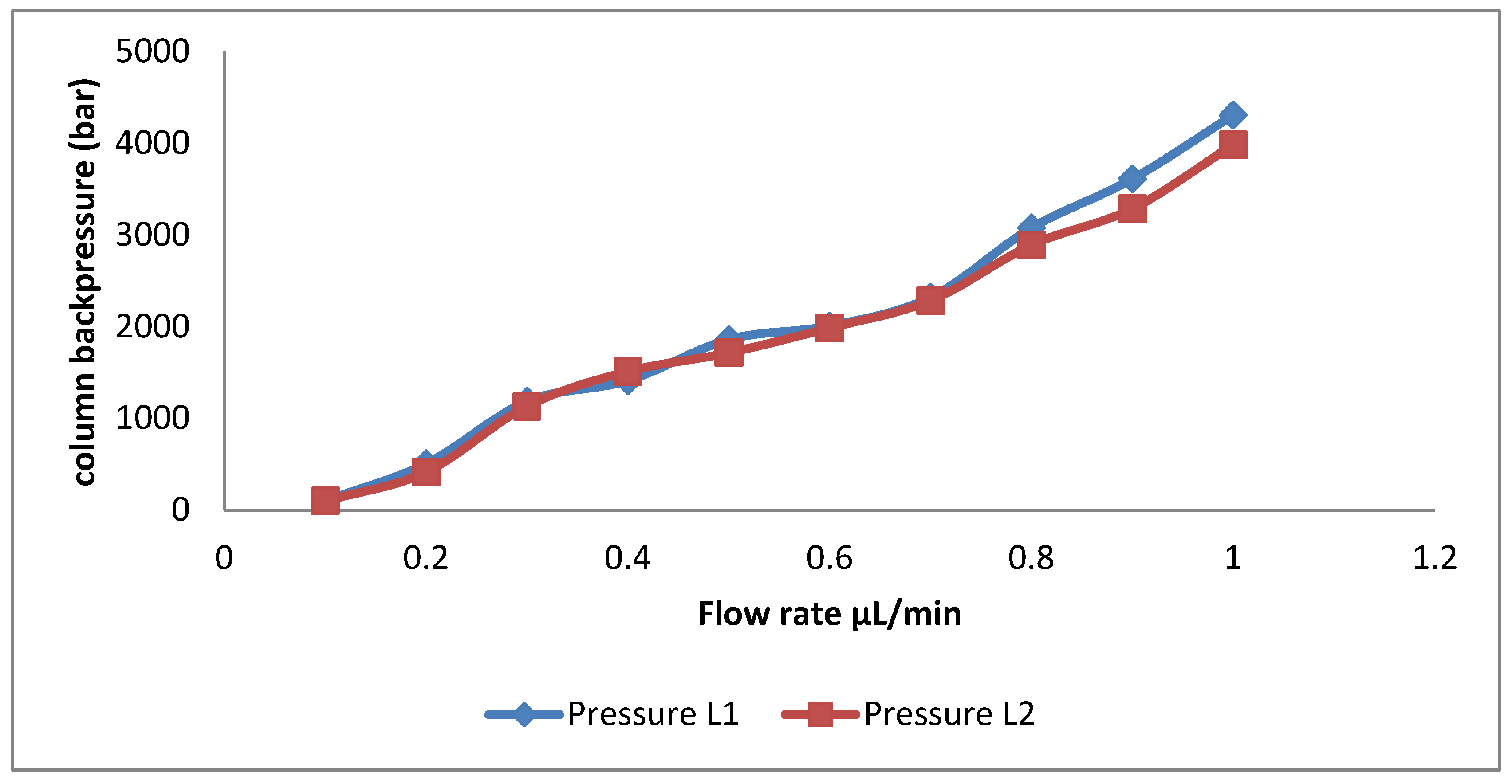
Mechanical Stability
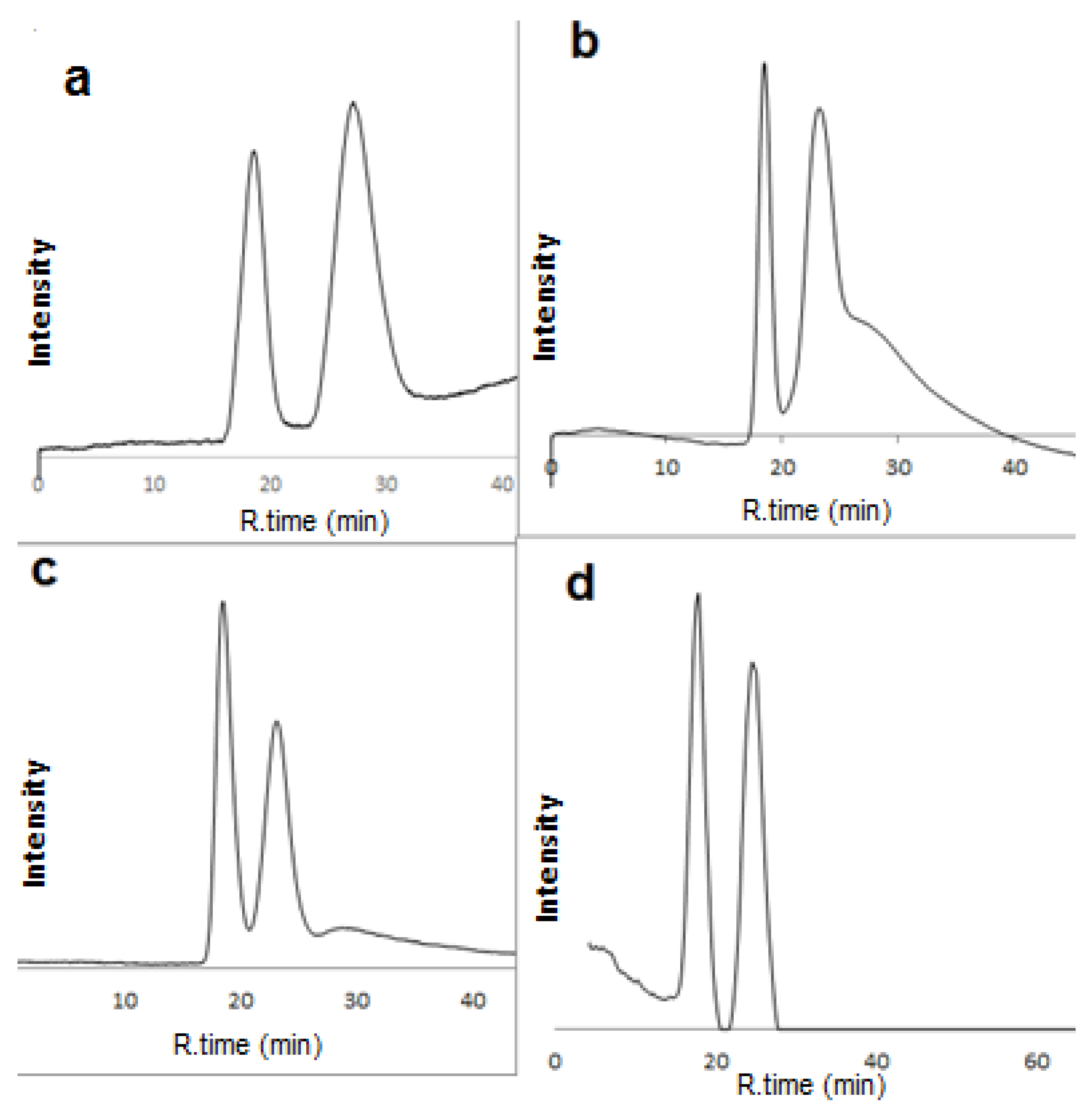
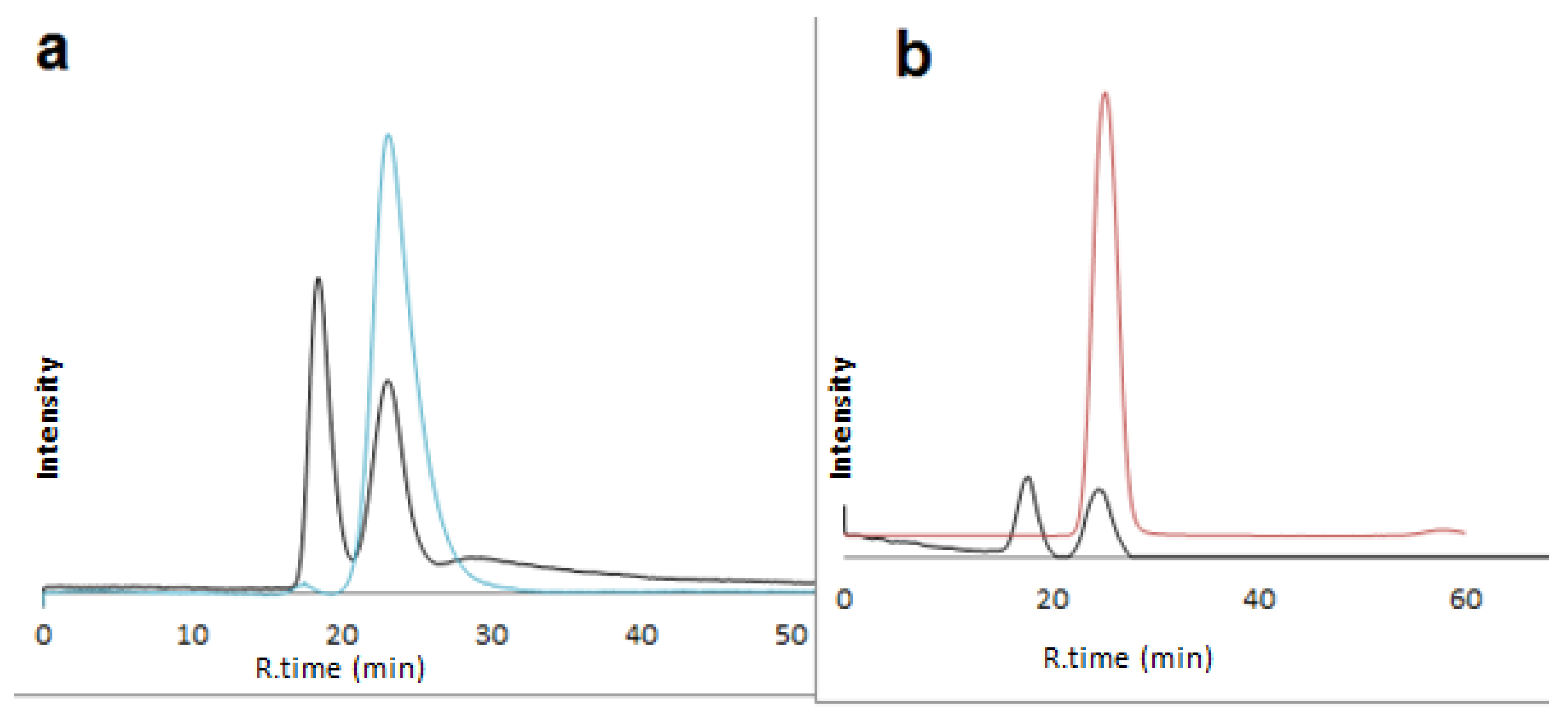
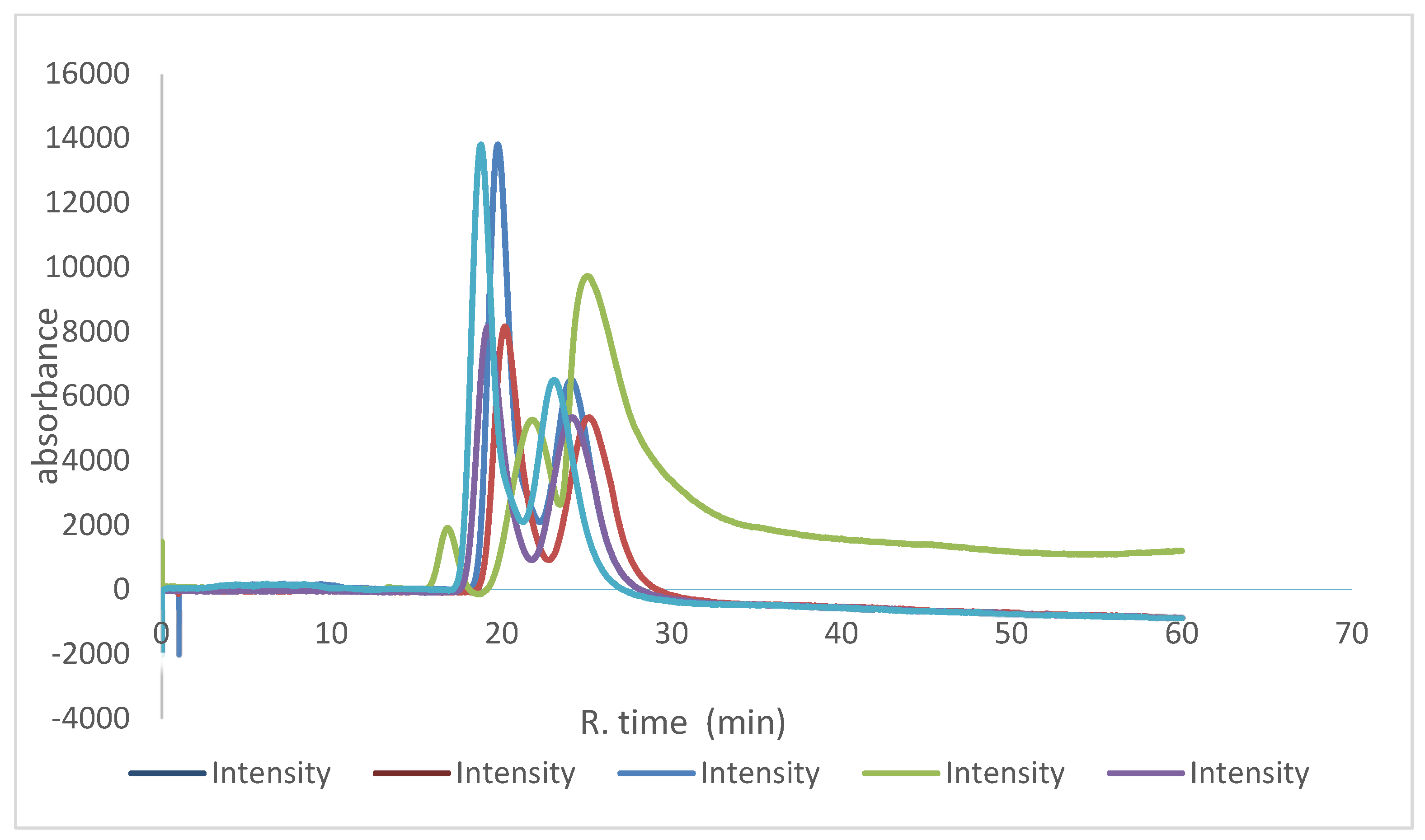
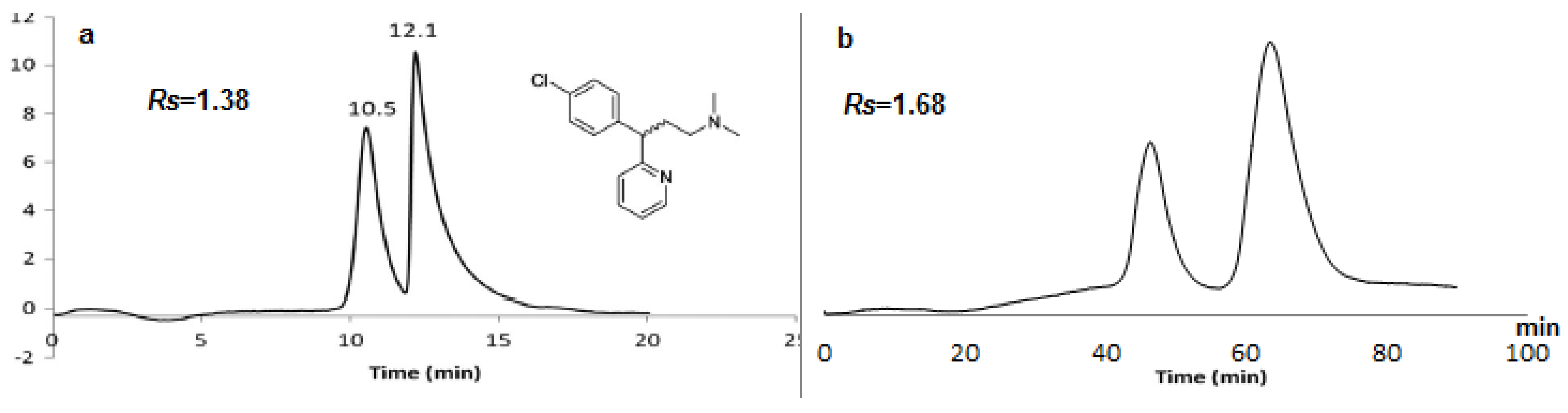
2.2. Enantioseparation of Different Classes of Pharmaceutical Racemates under Multimodal Elution
2.3. Discussion
3. Experimental Section
3.1. HPLC Conditions
3.1.1. Reagents
3.1.2. Instrumentation
3.2. Preparation of the Monolithic Columns
3.2.1. Preparation of the Carboxylated SWCNT (c-SWCNT)
3.2.2. Activation of the Fused Silica Capillaries
3.2.3. Preparation of the c-SWCNT Functionalised Monolithic Columns
3.3. Immobilisation of Lipase on the Surface of the Carboxylated Single-Walled Carbon Nanotubes Organic Monolithic Support
3.3.1. Scanning Electron Microscopy (SEM) and Total Porosity of the Prepared Monoliths
3.3.2. Partial Characterisation of the Prepared Monolith Quality through Determining Its Total Porosity (ƐT)
3.3.3. Composition of Stock Solutions and Sample Solutions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- US Food and Drug Administration. FDA’s Policy Statement for the Development of New Stereoisomeric Drugs. US Food and Drug Administration Regulatory Guidance. 1992. Available online: http://www.fda.gov/cder/guidance/stereo (accessed on 1 August 2022).
- Available online: https://www.ema.europa.eu/en/investigation-chiral-active-substances-human-scientific-guideline (accessed on 1 August 2022).
- Available online: https://www.tga.gov.au/resources/resource/guidance/biopharmaceutic-studies/154-medicines-require-biopharmaceutic-data (accessed on 1 August 2022).
- Chankvetadze, B. Monolithic chiral stationary phases for liquid-phase enantioseparation techniques. J. Sep. Sci. 2010, 33, 305–314. [Google Scholar] [CrossRef]
- Saunders, K.C.; Ghanem, A.; Hon, W.B.; Hilder, E.F.; Haddad, P.R. Separation and sample pre-treatment in bioanalysis using monolithic phases: A review. Anal. Chim. Acta 2009, 652, 22–31. [Google Scholar] [CrossRef]
- Alhassen, H.; Antony, V.; Ghanem, A.; Yajadda, M.M.A.; Han, Z.J.; Ostrikov, K. Organic/Hybrid Nanoparticles and Single-Walled Carbon Nanotubes: Preparation Methods and Chiral Applications. Chirality 2014, 26, 683–691. [Google Scholar] [CrossRef]
- Healey, R.; Ghanem, A. An insight to chiral monolith for enantioselective nano and micro HPLC: Preparation and applications. Chirality 2013, 25, 314–323. [Google Scholar] [CrossRef]
- Rocco, A.; Maruška, A.; Fanali, S. Enantiomeric separations by means of nano-LC. J. Sep. Sci. 2013, 36, 421–444. [Google Scholar] [CrossRef]
- Chervet, J.; Ursem, M.; Salzmann, J. Instrumental requirements for nanoscale liquid chromatography. Anal. Chem. 1996, 68, 1507–1512. [Google Scholar] [CrossRef]
- Hjertén, S.; Mohammad, J.; Nakazato, K.i. Improvement in flow properties and pH stability of compressed, continuous polymer beds for high-performance liquid chromatography. J. Chromatogr. A 1993, 646, 121–128. [Google Scholar] [CrossRef]
- Tennikova, T.; Svec, F.; Belenkii, B. High-performance membrane chromatography. A novel method of protein separation. J. Liq. Chromatogr. 1990, 13, 63–70. [Google Scholar] [CrossRef]
- Tennikova, T.; Bleha, M.; Švec, F.; Almazova, T.; Belenkii, B. High-performance membrane chromatography of proteins, a novel method of protein separation. J. Chromatogr. A 1991, 555, 97–107. [Google Scholar] [CrossRef]
- Tanaka, M.; Yamazaki, H. Direct determination of pantoprazole enantiomers in human serum by reversed-phase high-performance liquid chromatography using a cellulose-based chiral stationary phase and column-switching system as a sample cleanup procedure. Anal. Chem. 1996, 68, 1513–1516. [Google Scholar] [CrossRef]
- Qin, F.; Xie, C.; Yu, Z.; Kong, L.; Ye, M.; Zou, H. Monolithic enantiomer-selective stationary phases for capillary electrochromatography. J. Sep. Sci. 2006, 29, 1332–1343. [Google Scholar] [CrossRef]
- Guiochon, G. Monolithic columns in high-performance liquid chromatography. J. Chromatogr. A 2007, 1168, 101–168. [Google Scholar] [CrossRef]
- Svec, F. Recent developments in the field of monolithic stationary phases for capillary electrochromatography. J. Sep. Sci. 2005, 28, 729–745. [Google Scholar] [CrossRef]
- Svec, F.; Tennikova, T.B.; Deyl, Z. Monolithic materials: Preparation, Properties and Applications; Elsevier: Amsterdam, The Netherlands, 2003; Volume 67. [Google Scholar]
- Fouad, A.; Ibrahim, D.; Adly, F.G.; Ghanem, A. An insight into chiral monolithic stationary phases for enantioselective high-performance liquid chromatography applications. J. Sep. Sci. 2019, 42, 2303–2340. [Google Scholar] [CrossRef]
- Bragg, W.; Shamsi, S.A. A novel positively charged achiral co-monomer for β-cyclodextrin monolithic stationary phase: Improved chiral separation of acidic compounds using capillary electrochromatography coupled to mass spectrometry. J. Chromatogr. A 2012, 1267, 144–155. [Google Scholar] [CrossRef]
- Ghanem, A.; Adly, F.G.; Sokerik, Y.; Antwi, N.Y.; Shenashen, M.A.; El-Safty, S.A. Trimethyl-β-cyclodextrin-encapsulated monolithic capillary columns: Preparation, characterization and chiral nano-LC application. Talanta 2017, 169, 239–248. [Google Scholar] [CrossRef]
- Sebaee, M.M.M. A Novel Polymer-Based Monolithic Capillary with Sulphated Β-Cyclodextrin Chiral for the Enantioselective Pharmaceutical Analysis by Nano-HPLC. Sch. Res. Libr. 2018, 10, 63–80. [Google Scholar]
- Fouad, A.; Marzouk, A.A.; Ibrahim, S.M.; El-Adl, S.M.; Ghanem, A. Functionalized polymer monoliths with carbamylated amylose for the enantioselective reversed phase nano-liquid chromatographic separation of a set of racemic pharmaceuticals. J. Chromatogr. A 2017, 1515, 91–99. [Google Scholar] [CrossRef]
- Fouad, A.; Shaykoon, M.S.A.; Ibrahim, S.M.; El-Adl, S.M.; Ghanem, A. Colistin Sulfate Chiral Stationary Phase for the Enantioselective Separation of Pharmaceuticals Using Organic Polymer Monolithic Capillary Chromatography. Molecules 2019, 24, 833. [Google Scholar] [CrossRef]
- Fouad, A.; Marzouk, A.A.; Shaykoon, M.S.A.; Ibrahim, S.M.; El-Adl, S.M.; Ghanem, A. Daptomycin: A Novel Macrocyclic Antibiotic as a Chiral Selector in an Organic Polymer Monolithic Capillary for the Enantioselective Analysis of a set of Pharmaceuticals. Molecules 2021, 26, 3527. [Google Scholar] [CrossRef]
- Ghanem, A.; Marzouk, A.A.; Sobhy, M.; Fouad, A. A Polymer-based Monolithic Capillary Column with Polymyxin-B Chiral Selector for the Enantioselective Nano-High Performance Liquid Chromatographic Pharmaceutical Analysis. J. Chromatogr. A 2022, 1662, 462714. [Google Scholar] [CrossRef]
- Fouad, A.; El-Sayed, D.H.; Salman, B.E.; Bakr, H.H.; Adel, S.E.; Alzarak, T.M.; Mahmoud, A. Macrocyclic Antibiotics as Effective Chiral Selectors in Liquid Chromatography for Enantiomeric Separation of Pharmaceutical Compounds: A Review. Crit. Rev. Anal. Chem. 2023, 1–19. [Google Scholar] [CrossRef]
- Wang, X.; Li, Q.; Xie, J.; Jin, Z.; Wang, J.; Li, Y.; Jiang, K.; Fan, S. Fabrication of ultralong and electrically uniform single-walled carbon nanotubes on clean substrates. Nano Lett. 2009, 9, 3137–3141. [Google Scholar] [CrossRef]
- Iijima, S.; Ichihashi, T. Single-shell carbon nanotubes of 1-nm diameter. Nature 1993, 363, 603–605. [Google Scholar] [CrossRef]
- Navarro-Pascual-Ahuir, M.; Lucena, R.; Cárdenas, S.; Ramis-Ramos, G.; Valcárcel, M.; Herrero-Martínez, J.M. UV-polymerized butyl methacrylate monoliths with embedded carboxylic single-walled carbon nanotubes for CEC applications. Anal. Bioanal. Chem. 2014, 406, 6329–6336. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, Z.; Liao, Y.; Liu, H. Applications of nanomaterials in liquid chromatography: Opportunities for separation with high efficiency and selectivity. J. Sep. Sci. 2006, 29, 1872–1878. [Google Scholar] [CrossRef]
- Luong, J.H.; Bouvrette, P.; Liu, Y.; Yang, D.-Q.; Sacher, E. Electrophoretic separation of aniline derivatives using fused silica capillaries coated with acid treated single-walled carbon nanotubes. J. Chromatogr. A 2005, 1074, 187–194. [Google Scholar] [CrossRef]
- Sombra, L.; Moliner-Martínez, Y.; Cárdenas, S.; Valcárcel, M. Carboxylic multi-walled carbon nanotubes as immobilized stationary phase in capillary electrochromatography. Electrophoresis 2008, 29, 3850–3857. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.L.; Lin, Y.C. The role of methacrylate polymerized as porous-layered and nanoparticle-bound phases for open-tubular capillary electrochromatography: Substitution of a charged monomer for a bulk monomer. Electrophoresis 2010, 31, 3949–3958. [Google Scholar] [CrossRef]
- Pauwels, J.; Schepdael, A. Carbon nanotubes in capillary electrophoresis, capillary electrochromatography and microchip electrophoresis. Open Chem. 2012, 10, 785–801. [Google Scholar] [CrossRef]
- Suárez, B.; Simonet, B.M.; Cárdenas, S.; Valcarcel, M. Surfactant-coated single-walled carbon nanotubes as a novel pseudostationary phase in capillary EKC. Electrophoresis 2007, 28, 1714–1722. [Google Scholar] [CrossRef]
- Moliner-Martínez, Y.; Cárdenas, S.; Valcárcel, M. Evaluation of carbon nanostructures as chiral selectors for direct enantiomeric separation of ephedrines by EKC. Electrophoresis 2007, 28, 2573–2579. [Google Scholar] [CrossRef] [PubMed]
- Moliner-Martínez, Y.; Cárdenas, S.; Simonet, B.M.; Valcárcel, M. Recent developments in capillary EKC based on carbon nanoparticles. Electrophoresis 2009, 30, 169–175. [Google Scholar] [CrossRef]
- Valcárcel, M.; Cárdenas, S.; Simonet, B.M.; Moliner-Martínez, Y.; Lucena, R. Carbon nanostructures as sorbent materials in analytical processes. TrAC Trends Anal. Chem. 2008, 27, 34–43. [Google Scholar] [CrossRef]
- Li, Y.; Chen, Y.; Xiang, R.; Ciuparu, D.; Pfefferle, L.D.; Horváth, C.; Wilkins, J.A. Incorporation of single-wall carbon nanotubes into an organic polymer monolithic stationary phase for μ-HPLC and capillary electrochromatography. Anal. Chem. 2005, 77, 1398–1406. [Google Scholar] [CrossRef] [PubMed]
- Chambers, S.D.; Svec, F.; Fréchet, J.M. Incorporation of carbon nanotubes in porous polymer monolithic capillary columns to enhance the chromatographic separation of small molecules. J. Chromatogr. A 2011, 1218, 2546–2552. [Google Scholar] [CrossRef]
- Aqel, A.; Yusuf, K.; Al-Othman, Z.A.; Badjah-Hadj-Ahmed, A.Y.; Alwarthan, A.A. Effect of multi-walled carbon nanotubes incorporation into benzyl methacrylate monolithic columns in capillary liquid chromatography. Analyst 2012, 137, 4309–4317. [Google Scholar] [CrossRef]
- Schoffers, E.; Golebiowski, A.; Johnson, C.R. Enantioselective synthesis through enzymatic asymmetrization. Tetrahedron 1996, 52, 3769–3826. [Google Scholar] [CrossRef]
- Calleri, E.; Temporini, C.; Furlanetto, S.; Loiodice, F.; Fracchiolla, G.; Massolini, G. Lipases for biocatalysis: Development of a chromatographic bioreactor. J. Pharm. Biomed. Anal. 2003, 32, 715–724. [Google Scholar] [CrossRef]
- He, Z.; Lv, C.; Fan, X.; Zhou, Z. A novel lipase-based stationary phase in liquid chromatography. Anal. Chim. Acta 2011, 689, 143–148. [Google Scholar] [CrossRef]
- Bertucci, C.; Petri, A.; Felix, G.; Perini, B.; Salvadori, P. Lipase-based HPLC stationary phase: Enantioselective synthesis of 2-substituted 1,3-propanediol monoacetates. Tetrahedron Asymmetry 1999, 10, 4455–4462. [Google Scholar] [CrossRef]
- Millot, M. Separation of drug enantiomers by liquid chromatography and capillary electrophoresis, using immobilized proteins as chiral selectors. J. Chromatogr. B 2003, 797, 131–159. [Google Scholar] [CrossRef]
- Ahmed, M.; Ghanem, A. Enantioselective Nano Liquid Chromatographic Separation of Racemic Pharmaceuticals: A Facile One-Pot In Situ Preparation of Lipase-Based Polymer Monoliths in Capillary Format. Chirality 2014, 26, 754–763. [Google Scholar] [CrossRef] [PubMed]
- Urban, J.; Svec, F.; Fréchet, J.M. A monolithic lipase reactor for biodiesel production by transesterification of triacylglycerides into fatty acid methyl esters. Biotechnol. Bioeng. 2012, 109, 371–380. [Google Scholar] [CrossRef]
- Viklund, C.; Svec, F.; Frechet, J.M.; Irgum, K. Monolithic,“molded”, porous materials with high flow characteristics for separations, catalysis, or solid-phase chemistry: Control of porous properties during polymerization. Chem. Mater. 1996, 8, 744–750. [Google Scholar] [CrossRef]
- Ahmed, M.; Ghanem, A. Chiral β-cyclodextrin functionalized polymer monolith for the direct enantioselective reversed phase nano liquid chromatographic separation of racemic pharmaceuticals. J. Chromatogr. A 2014, 1345, 115–127. [Google Scholar] [CrossRef]
- Urban, J.; Jandera, P. Recent advances in the design of organic polymer monoliths for reversed-phase and hydrophilic interaction chromatography separations of small molecules. Anal. Bioanal. Chem. 2013, 405, 2123–2131. [Google Scholar] [CrossRef]
- Ahmed, M.; Yajadda, M.M.A.; Han, Z.J.; Su, D.; Wang, G.; Ostrikov, K.K.; Ghanem, A. Single-walled carbon nanotube-based polymer monoliths for the enantioselective nano-liquid chromatographic separation of racemic pharmaceuticals. J. Chromatogr. A 2014, 1360, 100–109. [Google Scholar] [CrossRef]
- Aral, H.; Çelik, K.S.; Aral, T.; Topal, G. Preparation of a novel ionic hybrid stationary phase by non-covalent functionalization of single-walled carbon nanotubes with amino-derivatized silica gel for fast HPLC separation of aromatic compounds. Talanta 2016, 149, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Fouad, A.; Maher, W.; Ghanem, A. Non-commercial Polysaccharides-based Chiral Selectors in Enantioselective Chromatography. Recent Adv. Anal. Tech. 2019, 3, 228–262. [Google Scholar]
- Fouad, A.; Ghanem, A. Immobilized Chiral Selectors on Monolithic High-Performance Liquid Chromatography Columns. In Advances in Chromatography; Taylor & Francis Group: Abingdon, UK, 2017; pp. 111–167. [Google Scholar]

| Column | ƐT % |
|---|---|
| L2 | 28.4 ± (2.09) |
| L1 | 26.7 ± (1.9) |
| Column | Nitrogen (% w/w) |
|---|---|
| G | 1.8 |
| L1 | 2.25 |
| L2 | 2.41 |
| Phase | Capillary | Mobile Phase | Drug | Rt1 (min) | Rt2 (min) | Separation Factor (α) | Resolution (Rs) |
|---|---|---|---|---|---|---|---|
| Reversed phase | L2 | Methanol:water 10:90 | Miconazole | 21 | 28 | 1.4 | 1.4 |
| L1 | Aminoglutathemide | 1.1 | 1.2 | ||||
| L2 | o-methoxy mandilic acid | 23 | 37 | 1.3 | 1.9 | ||
| L1 | Methanol:water 5:95 | Flavanone | 17 | 23 | 1.4 | 1.6 | |
| L2 | Methanol:water 20:80%, | Clopidogril | 16 | 22 | 1.4 | 1.6 | |
| L1 | propafenone | 24 | 30 | 1.2 | 1.2 | ||
| L2 | Nomifensine | 17 | 22 | 1.2 | 1.5 | ||
| L2 | Chlorphinarmine | 46 | 62 | 1.4 | 1.7 | ||
| L2 | Methanol:water 80:20%, | Ifosfamid | 18 | 23 | 1.3 | 2 | |
| L1 | Ampicilline | 18 | 24 | 1.3 | 1.3 | ||
| L2 | phenylalanine | 19 | 25 | 1.3 | 1.8 | ||
| L1 | Arternol | 18 | 23 | 1.3 | 1.4 | ||
| L2 | hexaconazole | 18 | 27 | 1.4 | 1.6 | ||
| L1 | Tocainide | 19 | 23 | 1.3 | 1 | ||
| L2 | diniconazole | 18 | 24 | 1.4 | 1.8 | ||
| L1 | Propranolol | 19 | 24 | 1.3 | 1.3 | ||
| L2 | Normatenphrine | 18 | 24 | 1.4 | 1.9 | ||
| L2 | Alprenolol | 19 | 24 | 1.3 | 1.6 | ||
| L1 | 4-hydroxy mandlic acid | 22 | 32 | 2 | 1.9 | ||
| L2 | Methanol:water 50:50% | Ibuprofen | 17 | 25 | 1.4 | 1.4 | |
| L2 | Glutamic acid | 18 | 22 | 1.4 | 1.6 | ||
| L2 | Atenolol | 38 | 47 | 1.4 | 2.3 | ||
| L1 | Methanol 100% | Fluribiprofen | 16 | 23 | 1.2 | 1.3 | |
| L1 | Metoprolol | 21 | 28 | 1.3 | 1.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fouad, A.; Adly, F.G.; Soltan, M.K.; Ghanem, A. Lipase as a Chiral Selector Immobilised on Carboxylated Single-Walled Carbon Nanotubes and Encapsulated in the Organic Polymer Monolithic Capillary for Nano-High Performance Liquid Chromatography Enantioseparation of Racemic Pharmaceuticals. Molecules 2023, 28, 6663. https://doi.org/10.3390/molecules28186663
Fouad A, Adly FG, Soltan MK, Ghanem A. Lipase as a Chiral Selector Immobilised on Carboxylated Single-Walled Carbon Nanotubes and Encapsulated in the Organic Polymer Monolithic Capillary for Nano-High Performance Liquid Chromatography Enantioseparation of Racemic Pharmaceuticals. Molecules. 2023; 28(18):6663. https://doi.org/10.3390/molecules28186663
Chicago/Turabian StyleFouad, Ali, Frady G. Adly, Moustafa K. Soltan, and Ashraf Ghanem. 2023. "Lipase as a Chiral Selector Immobilised on Carboxylated Single-Walled Carbon Nanotubes and Encapsulated in the Organic Polymer Monolithic Capillary for Nano-High Performance Liquid Chromatography Enantioseparation of Racemic Pharmaceuticals" Molecules 28, no. 18: 6663. https://doi.org/10.3390/molecules28186663
APA StyleFouad, A., Adly, F. G., Soltan, M. K., & Ghanem, A. (2023). Lipase as a Chiral Selector Immobilised on Carboxylated Single-Walled Carbon Nanotubes and Encapsulated in the Organic Polymer Monolithic Capillary for Nano-High Performance Liquid Chromatography Enantioseparation of Racemic Pharmaceuticals. Molecules, 28(18), 6663. https://doi.org/10.3390/molecules28186663










