Adsorption of Basic Yellow 28 and Basic Blue 3 Dyes from Aqueous Solution Using Silybum Marianum Stem as a Low-Cost Adsorbent
Abstract
:1. Introduction
2. Results and Discussion
2.1. Elucidation of the SLM Stem
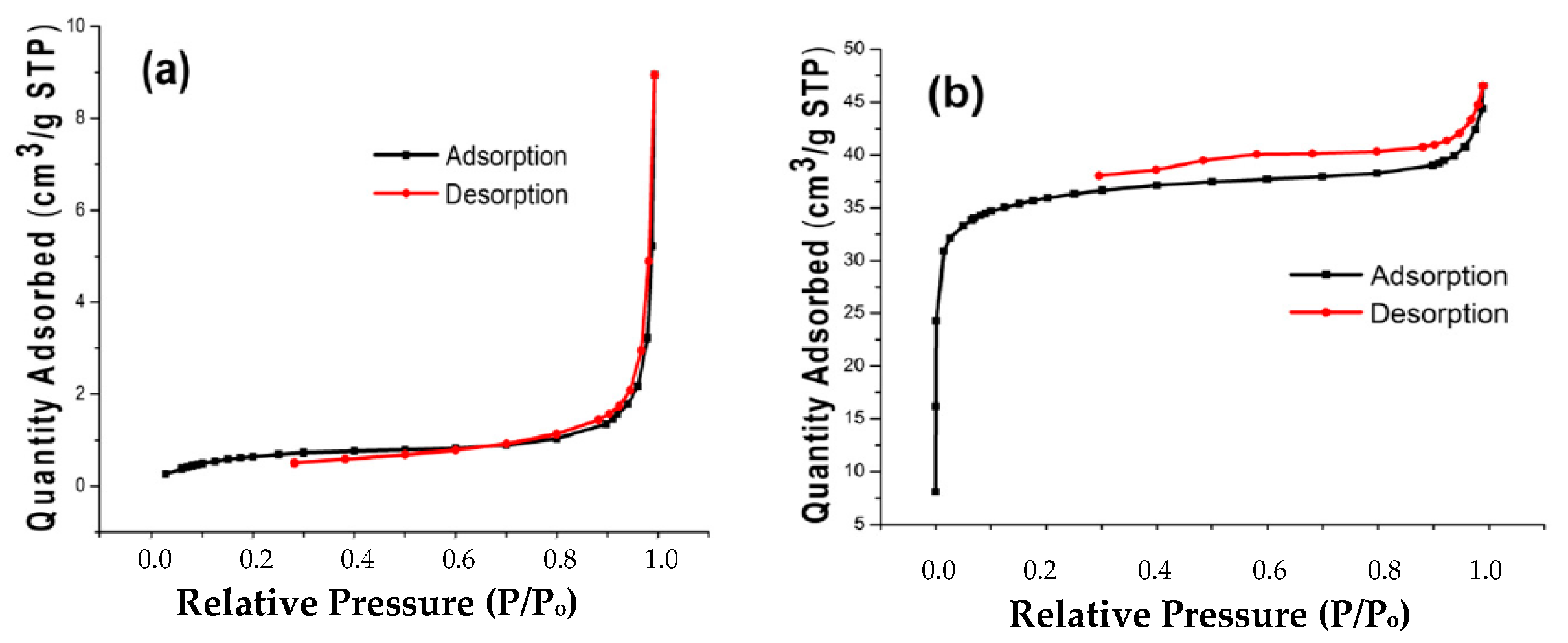
2.2. Characterization of the Adsorbent Obtained from SLM Stem
2.3. Determination of pH Zero-Point Charge
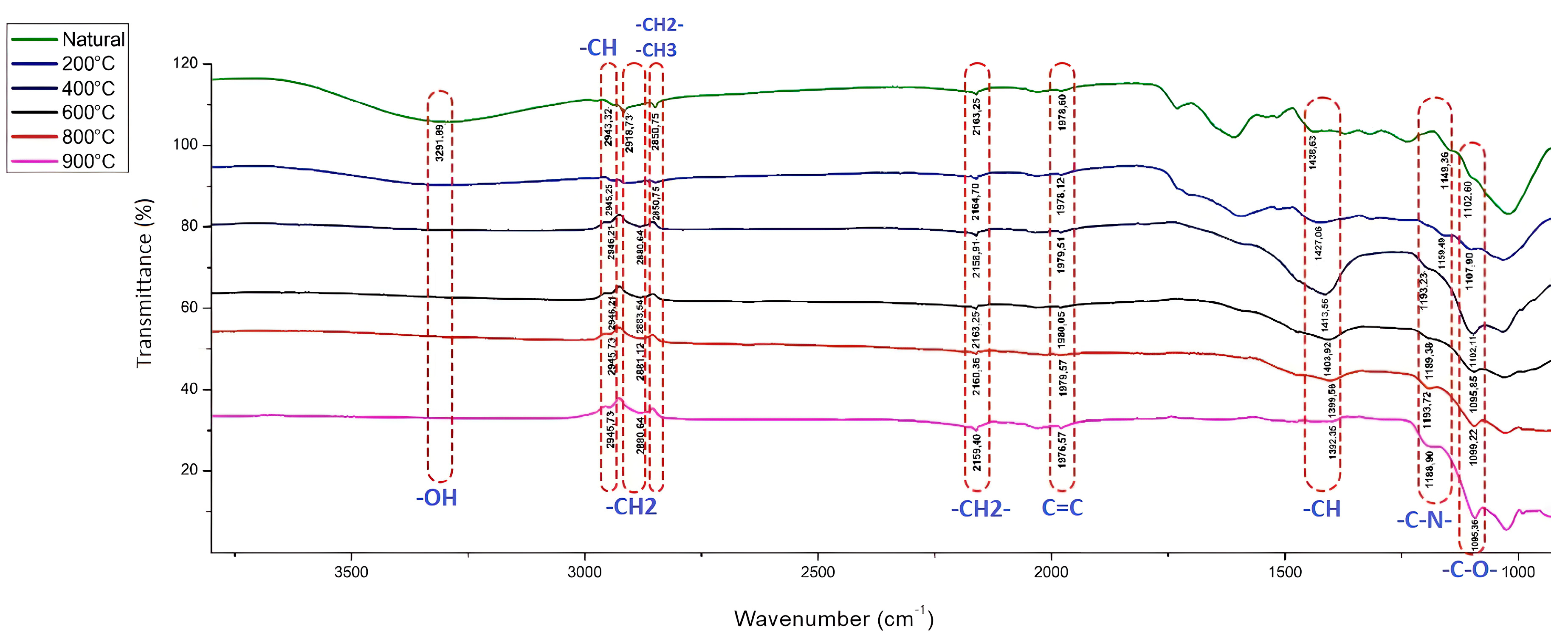
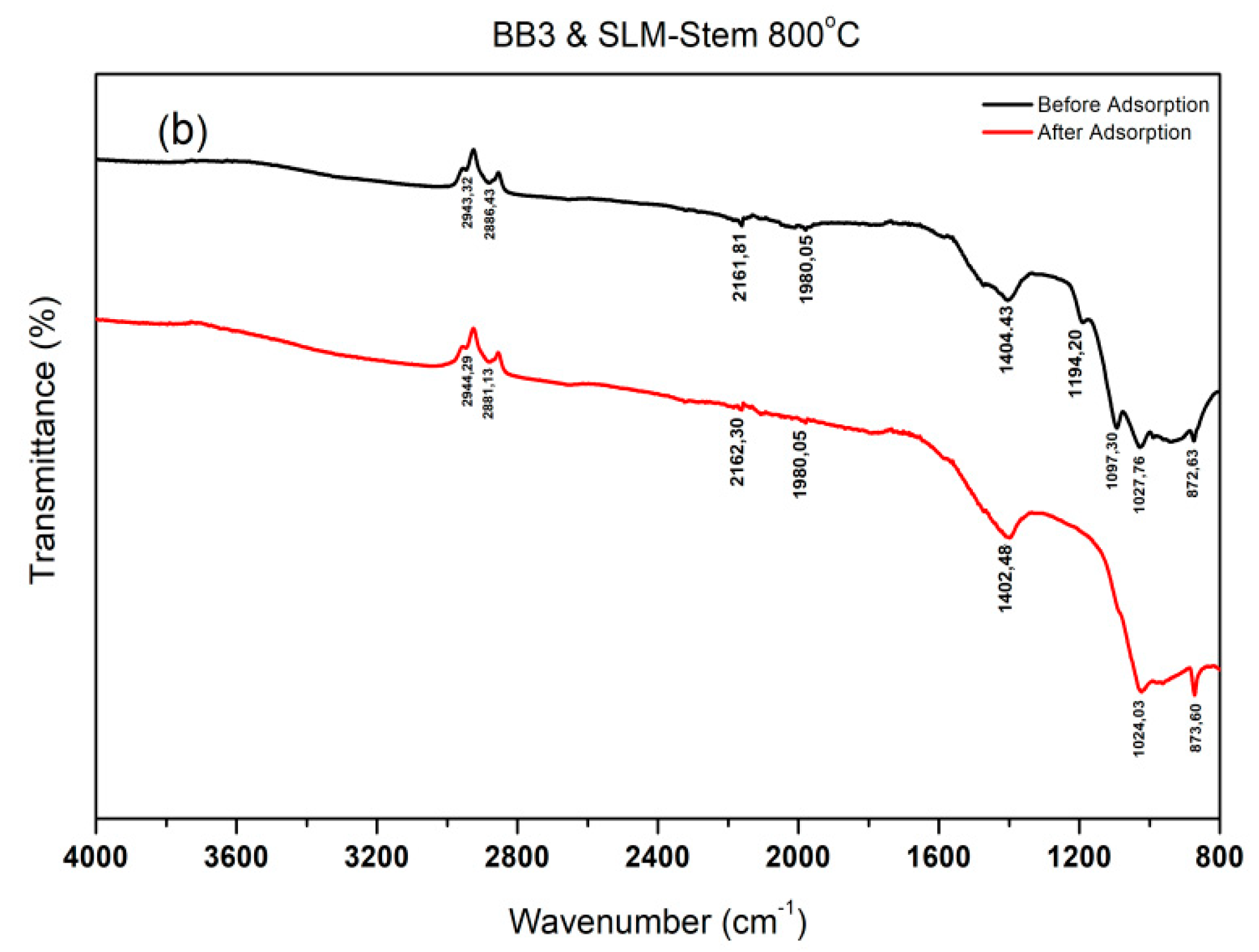
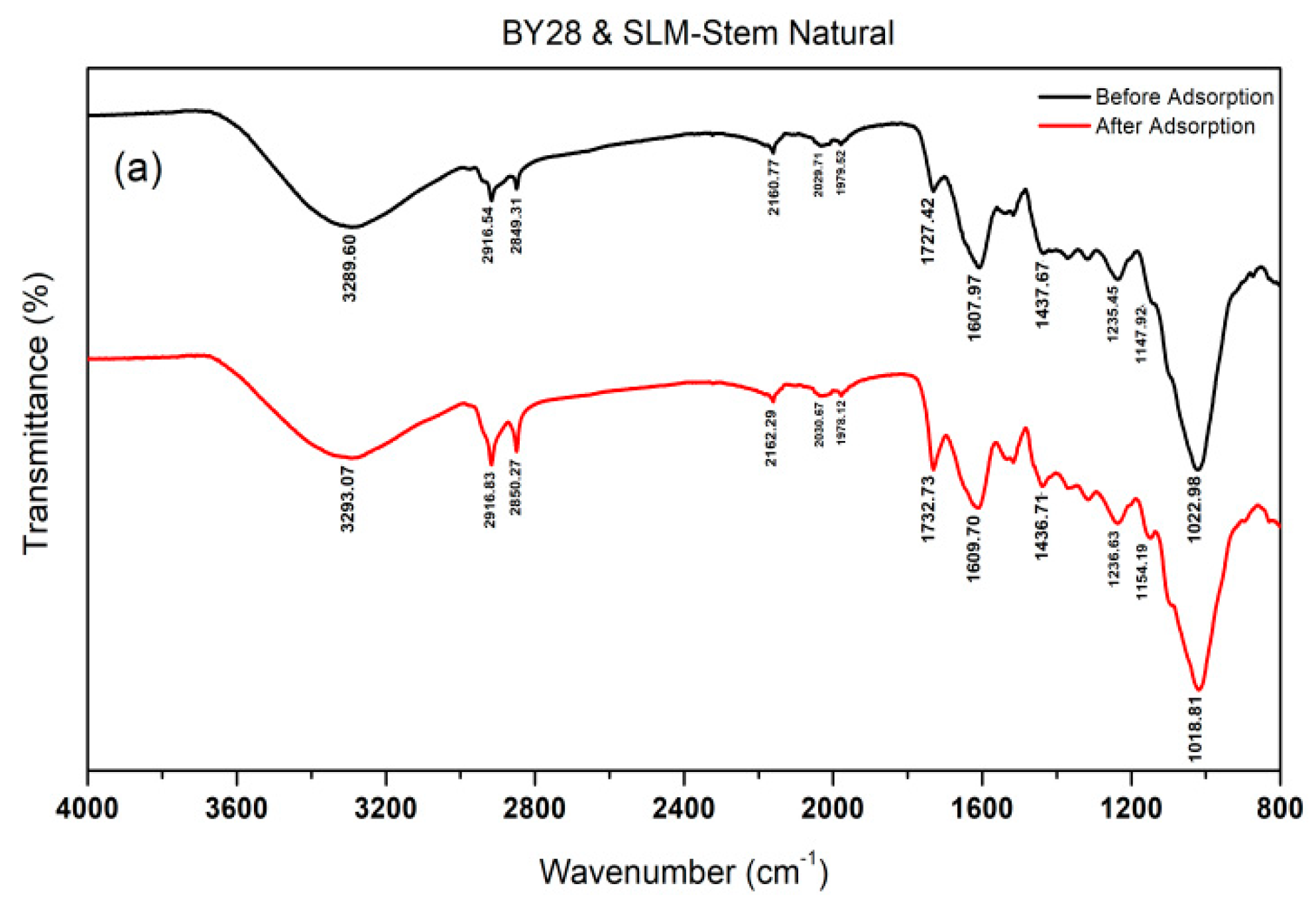
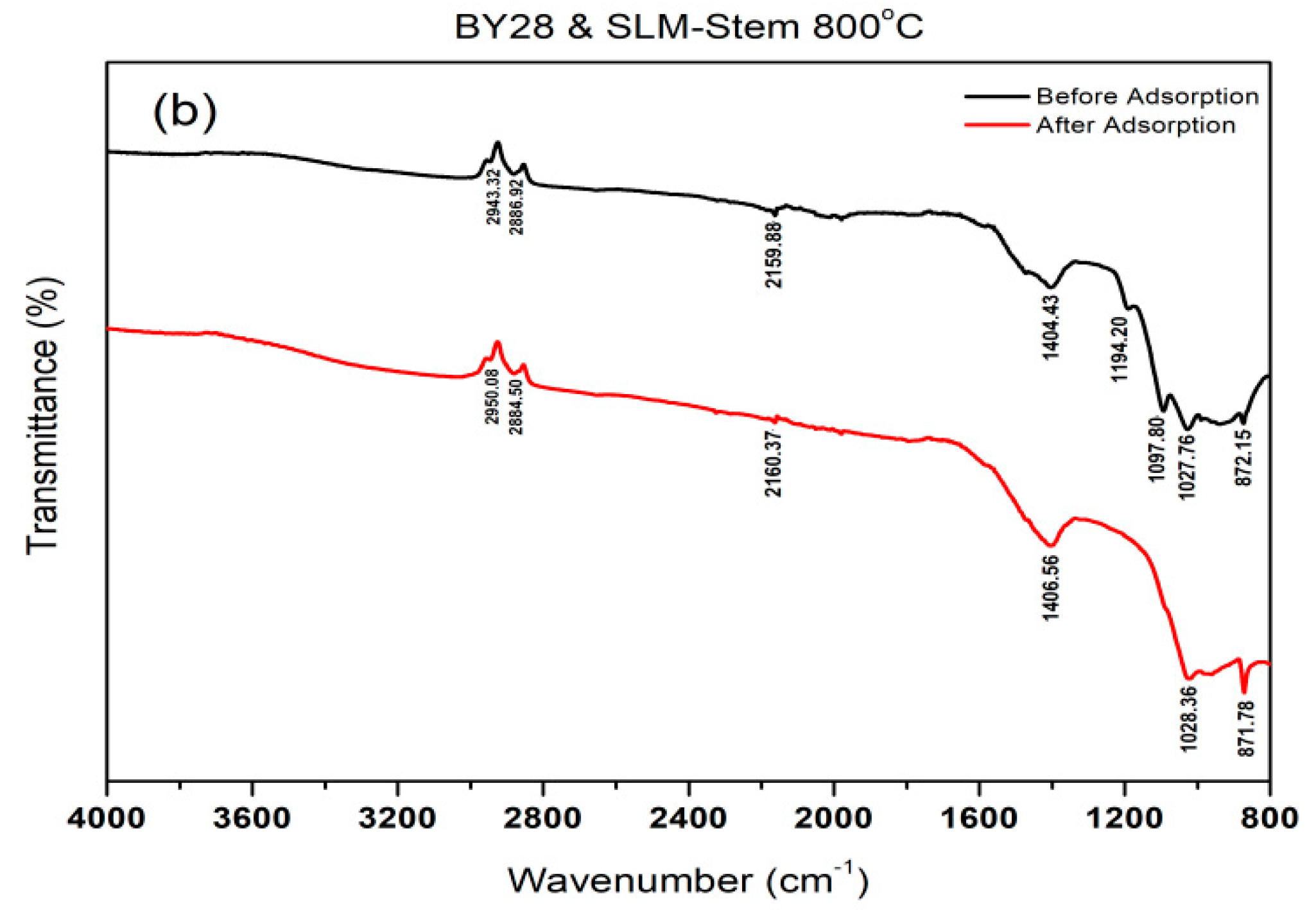
2.4. Determination of the Active Groups of the Adsorbent
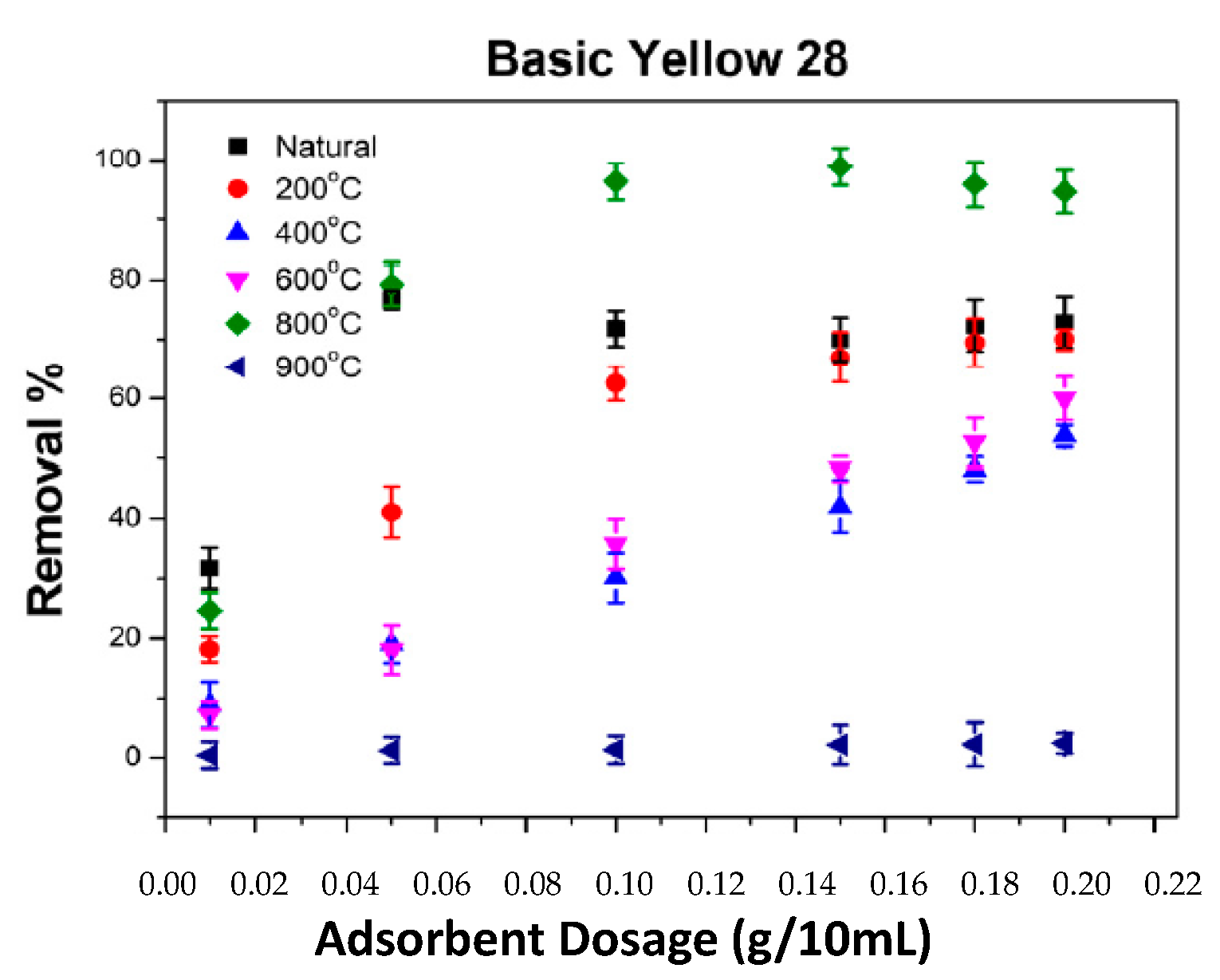
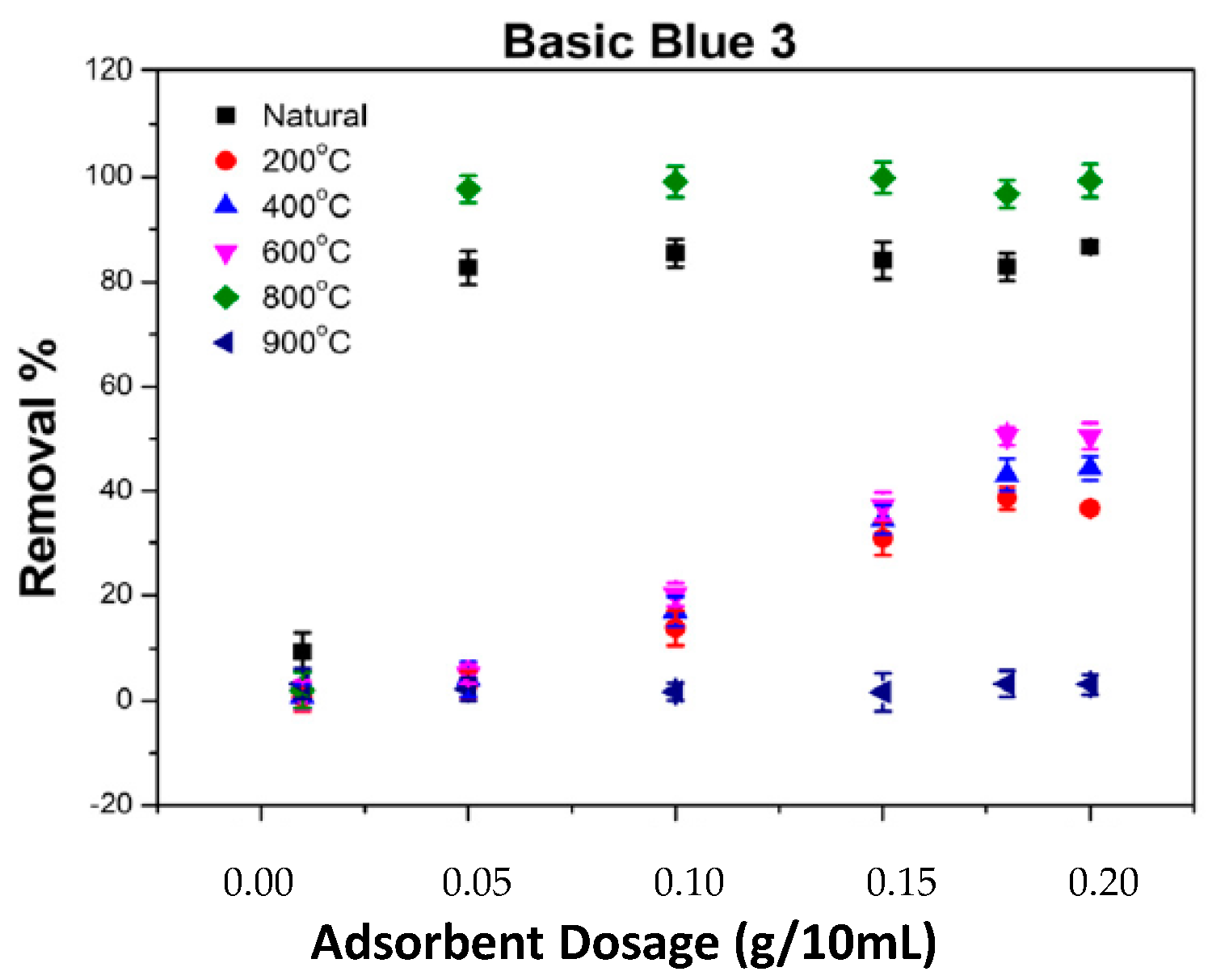
2.5. The Impact of Adsorbent Amount on the Adsorption of BB3 and BY28
2.6. The Effect of the Initial Solution Concentration on BB3 and BY28 Adsorption
2.7. Effect of Contact Time on BB3 and BY28 Adsorption
2.8. The Effect of Ambient Temperature on the Adsorption of BB3 and BY28
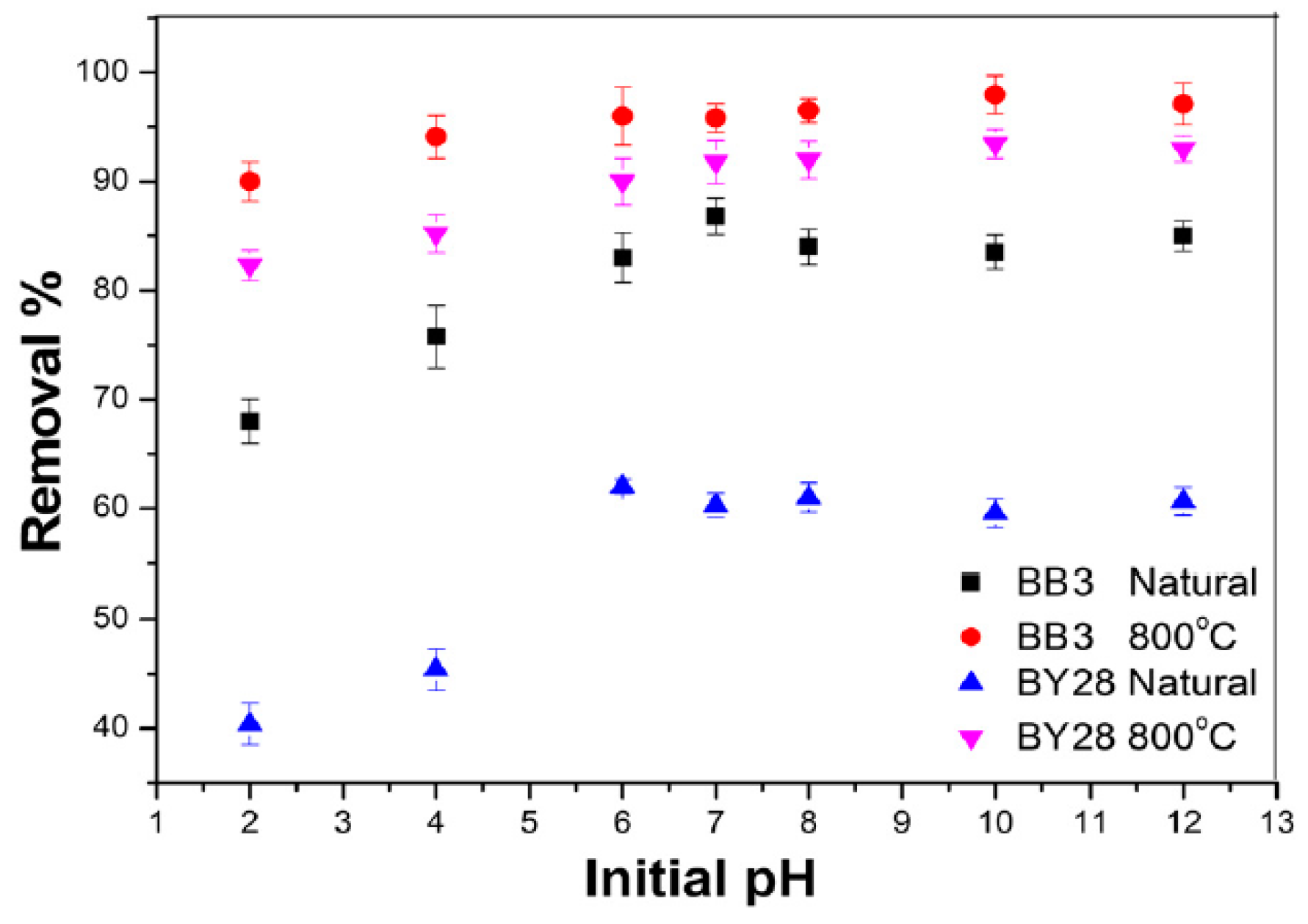
2.9. Effect of pH on BB3 and BY28 Adsorption
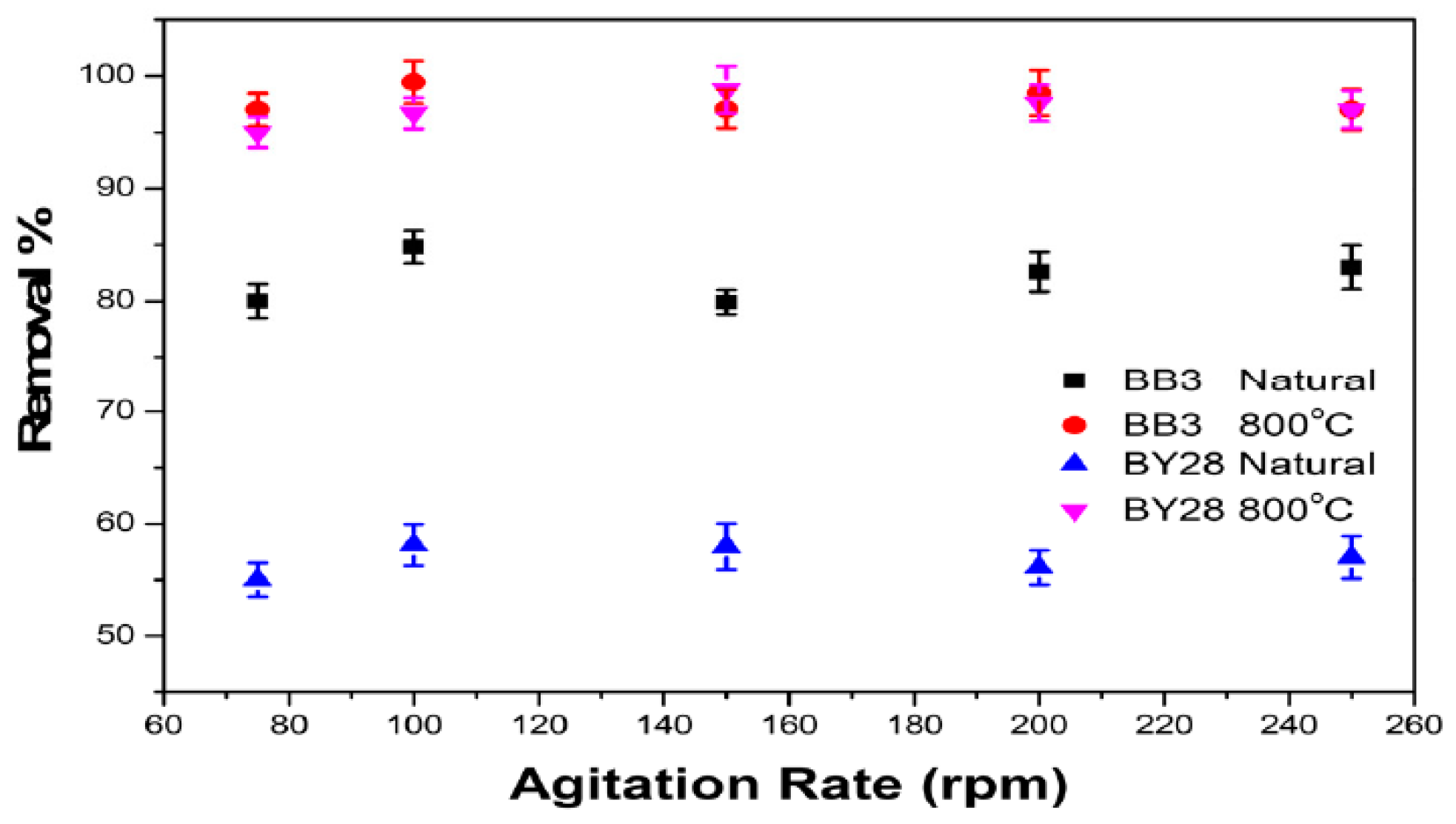
2.10. The Effect of Agitation Speed on BB3 and BY28 Adsorption
2.11. Real Wastewater Experiments
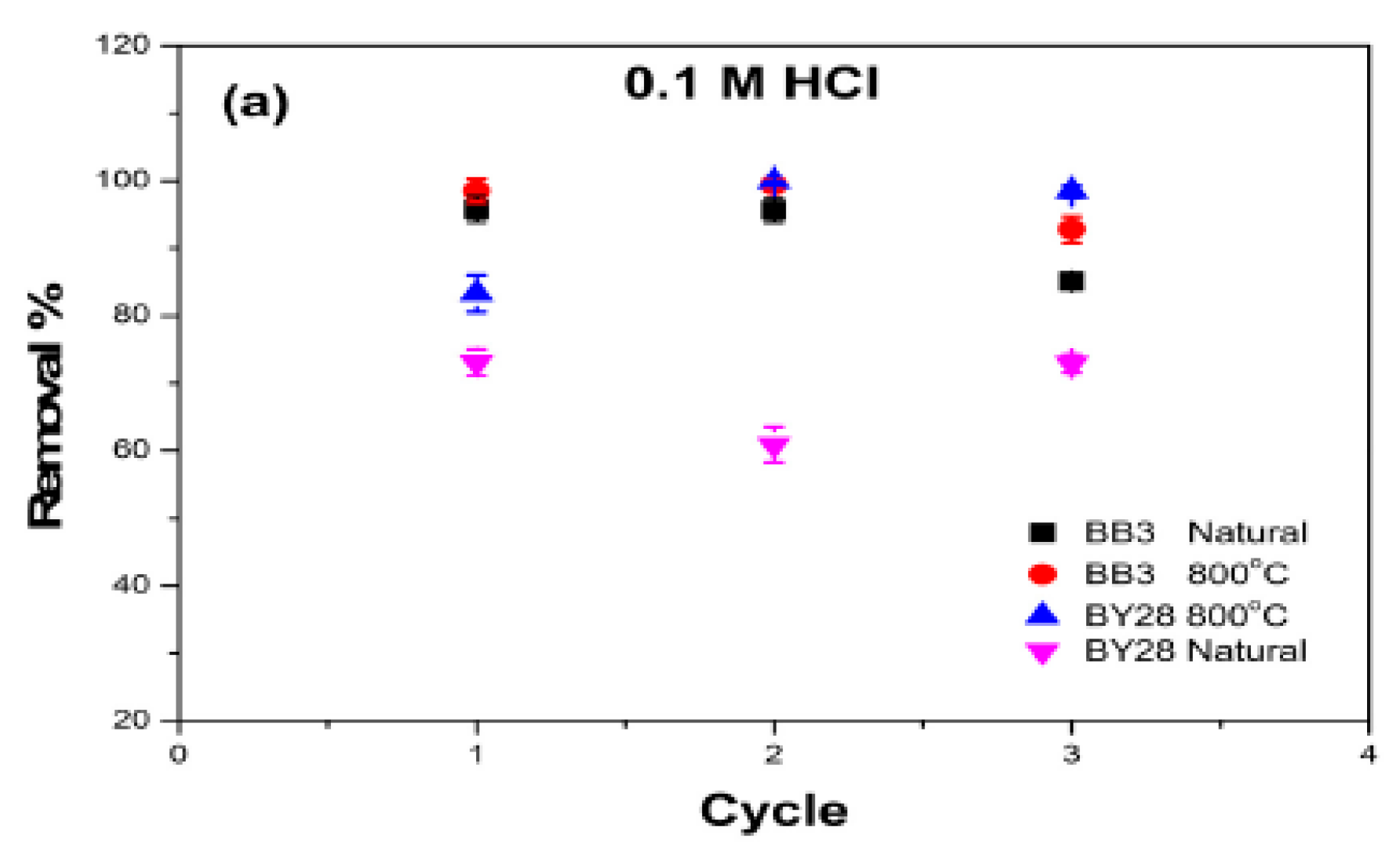
2.12. Reusability
2.13. Adsorption Isotherms
2.14. Comparative Research
3. Experimental
3.1. Materials and Methods
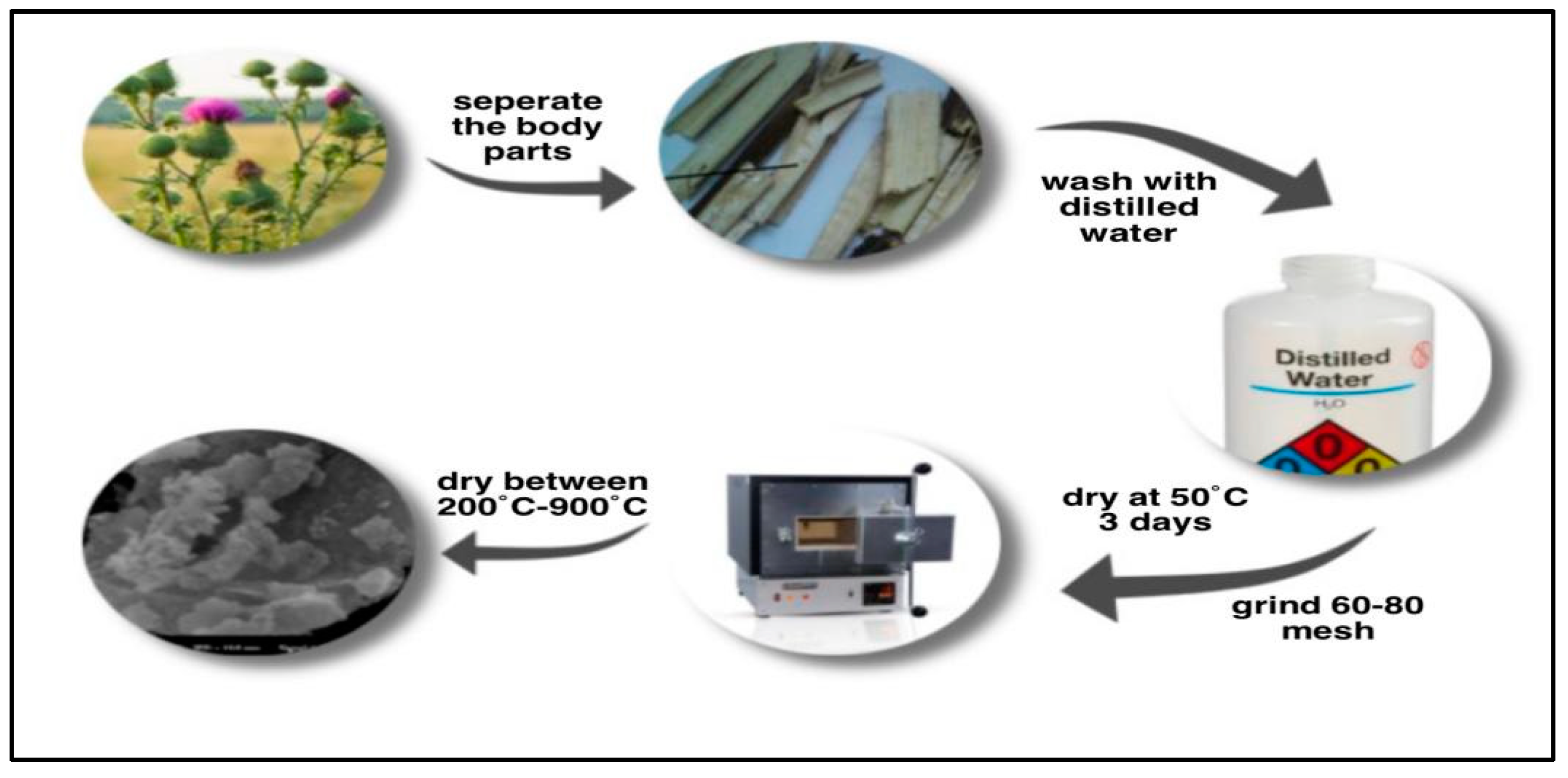
3.2. Preparation of Adsorbent
3.3. Batch Adsorption Experiments
3.4. Adsorption Isotherms
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hasani, N.; Selimi, T.; Mele, A.; Thaçi, V.; Halili, J.; Berisha, A.; Sadiku, M. Theoretical, Equilibrium, Kinetics and Thermodynamic Investigations of Methylene Blue Adsorption onto Lignite Coal. Molecules 2022, 27, 1856. [Google Scholar] [CrossRef] [PubMed]
- Renou, S.; Givaudan, J.; Poulain, S.; Dirassouyan, F.; Moulin, P. Landfill leachate treatment: Review and opportunity. J. Hazard. Mater. 2008, 150, 468–493. [Google Scholar] [CrossRef] [PubMed]
- Ojedabenitez, S.; Beraudlozano, J. The municipal solid waste cycle in Mexico: Final disposal. Resour. Conserv. Recycl. 2003, 39, 239–250. [Google Scholar] [CrossRef]
- Nagano, S.; Tamon, H.; Adzumi, T.; Nakagawa, K.; Suzuki, T. Activated carbon from municipal waste. Carbon 2000, 38, 915–920. [Google Scholar] [CrossRef]
- Montagnaro, F.; Santoro, L. Reuse of coal combustion ashes as dyes and heavy metal adsorbents: Effect of sieving and demineralization on waste properties and adsorption capacity. Chem. Eng. J. 2009, 150, 174–180. [Google Scholar] [CrossRef]
- Bello, O.S.; Adegoke, K.A.; Olaniyan, A.A.; Abdulazeez, H. Dye adsorption using biomass wastes and natural adsorbents: Overview and prospects. Desalination Water Treat. 2015, 53, 1292–1315. [Google Scholar] [CrossRef]
- Sivakumar, R.; Lee, N.Y. Adsorptive removal of organic pollutant methylene blue using polysaccharide-based composite hydrogels. Chemosphere 2022, 286, 131890. [Google Scholar] [CrossRef]
- Lofrano, G.; Meriç, S.; Zengin, G.E.; Orhon, D. Chemical and biological treatment technologies for leather tannery chemicals and wastewaters: A review. Sci. Total Environ. 2013, 461–462, 265–281. [Google Scholar] [CrossRef]
- Rojas, S.; Horcajada, P. Metal–Organic Frameworks for the Removal of Emerging Organic Contaminants in Water. Chem. Rev. 2020, 120, 8378–8415. [Google Scholar] [CrossRef]
- Fernández, C.; Larrechi, M.S.; Callao, M.P. An analytical overview of processes for removing organic dyes from wastewater effluents. TrAC Trends Anal. Chem. 2010, 29, 1202–1211. [Google Scholar] [CrossRef]
- Yao, Y.; Xu, F.; Chen, M.; Xu, Z.; Zhu, Z. Adsorption behavior of methylene blue on carbon nanotubes. Bioresour. Technol. 2010, 101, 3040–3046. [Google Scholar] [CrossRef] [PubMed]
- Fatima, H.; Kadi, S.; Lellou, S.; Marouf, R.; Benhebal, H. Synthesis, characterization and application of intercalated and tubular kaolinite to remove basic yellow 28. J. Iran. Chem. Soc. 2022, 19, 4687–4697. [Google Scholar] [CrossRef]
- Deniz, F.; Saygideger, S.D. Removal of a hazardous azo dye (Basic Red 46) from aqueous solution by princess tree leaf. Desalination 2011, 268, 6–11. [Google Scholar] [CrossRef]
- Zhao, H.; Zhong, H.; Jiang, Y.; Li, H.; Tang, P.; Li, D.; Feng, Y. Porous ZnCl2-Activated Carbon from Shaddock Peel: Methylene Blue Adsorption Behavior. Materials 2022, 15, 895. [Google Scholar] [CrossRef]
- Aragaw, T.A.; Alene, A.N. A comparative study of acidic, basic, and reactive dyes adsorption from aqueous solution onto kaolin adsorbent: Effect of operating parameters, isotherms, kinetics, and thermodynamics. Emerg. Contam. 2022, 8, 59–74. [Google Scholar] [CrossRef]
- Duc, D.S.; Bin, N.; Trang, T.T.H. Adsorption of Basic Yellow 28 in Aqueous Solution by Activated Carbon. Asian J. Chem. 2013, 25, 2173–2176. [Google Scholar] [CrossRef]
- N’Diaye, A.D.; Kankou, M.S.A.; Hammouti, B.; Nandiyanto, A.B.D.; Al Husaeni, D.F. A review of biomaterial as an adsorbent: From the bibliometric literature review, the definition of dyes and adsorbent, the adsorption phenomena and isotherm models, factors affecting the adsorption process, to the use of typha species waste as adsorbent. Commun. Sci. Technol. 2022, 7, 140–153. [Google Scholar] [CrossRef]
- Shi, Y.; Chang, Q.; Zhang, T.; Song, G.; Sun, Y.; Ding, G. A review on selective dye adsorption by different mechanisms. J. Environ. Chem. Eng. 2022, 10, 108639. [Google Scholar] [CrossRef]
- Ceretta, M.B.; Vieira, Y.; Wolski, E.A.; Foletto, E.L.; Silvestri, S. Biological degradation coupled to photocatalysis by ZnO/polypyrrole composite for the treatment of real textile wastewater. J. Water Process. Eng. 2020, 35, 101230. [Google Scholar] [CrossRef]
- Azari, A.; Nabizadeh, R.; Nasseri, S.; Mahvi, A.H.; Mesdaghinia, A.R. Comprehensive systematic review and meta-analysis of dyes adsorption by carbon-based adsorbent materials: Classification and analysis of last decade studies. Chemosphere 2020, 250, 126238. [Google Scholar] [CrossRef]
- Mutunga, M.F.; Wanyonyi, W.C.; Ongera, G. Utilization of Macadamia seed husks as a low-cost sorbent for removing cationic dye (basic blue 3 dye) from aqueous solution. Environ. Chem. Ecotoxicol. 2020, 2, 194–200. [Google Scholar] [CrossRef]
- Wong, S.Y.; Tan, Y.P.; Abdullah, A.H.; Ong, S.T. Removal of basic blue three and reactive orange 16 by adsorption onto quartered sugar cane bagasse. Malays. J. Anal. Sci. 2009, 13, 185–193. [Google Scholar]
- Wawrzkiewicz, M. Removal of C.I. Basic Blue 3 dye by sorption onto cation exchange resin, functionalized and non-functionalized polymeric sorbents from aqueous solutions and wastewaters. Chem. Eng. J. 2013, 217, 414–425. [Google Scholar] [CrossRef]
- Ali, F.; Ali, N.; Bibi, I.; Said, A.; Nawaz, S.; Ali, Z.; Salman, S.M.; Iqbal, H.M.; Bilal, M. Adsorption isotherm, kinetics and thermodynamic of acid blue and basic blue dyes onto activated charcoal. Case Stud. Chem. Environ. Eng. 2020, 2, 100040. [Google Scholar] [CrossRef]
- Naboulsi, A.; El Himri, M.; Gharibi, E.; El Haddad, M. Study of adsorption mechanism of Malachite Green (MG) and Basic Yellow 28 (BY28) onto smectite rich natural clays (Ghassoul) using DFT/B3LYP and DOE/FFD. Surf. Interfaces 2022, 33, 102227. [Google Scholar] [CrossRef]
- Yener, J.; Kopac, T.; Dogu, G.; Dogu, T. Adsorption of Basic Yellow 28 from aqueous solutions with clinoptilolite and amberlite. J. Colloid Interface Sci. 2006, 294, 255–264. [Google Scholar] [CrossRef]
- Roberts, H.; Palmeiro, B.S. Toxicology of Aquarium Fish. Vet. Clin. N. Am. Exot. Anim. Pract. 2008, 11, 359–374. [Google Scholar] [CrossRef]
- Kifuani, K.M.; Mayeko, A.K.K.; Vesituluta, P.N.; Lopaka, B.I.; Bakambo, G.E.; Mavinga, B.M.; Lunguya, J.M. Adsorption d’un colorant basique, Bleu de Méthylène, en solution aqueuse, sur un bioadsorbant issu de déchets agricoles de Cucumeropsis mannii Naudin. Int. J. Biol. Chem. Sci. 2018, 12, 558–575. [Google Scholar] [CrossRef]
- Kabdaşlı, I.; Arslan, T.; Ölmez-Hancı, T.; Arslan-Alaton, I.; Tünay, O. Complexing agent and heavy metal removals from metal plating effluent by electrocoagulation with stainless steel electrodes. J. Hazard. Mater. 2009, 165, 838–845. [Google Scholar] [CrossRef]
- Divyapriya, G.; Singh, S.; Martínez-Huitle, C.A.; Scaria, J.; Karim, A.V.; Nidheesh, P. Treatment of real wastewater by photoelectrochemical methods: An overview. Chemosphere 2021, 276, 130188. [Google Scholar] [CrossRef]
- Katheresan, V.; Kansedo, J.; Lau, S.Y. Efficiency of various recent wastewater dye removal methods: A review. J. Environ. Chem. Eng. 2018, 6, 4676–4697. [Google Scholar] [CrossRef]
- Huang, R.; He, L.; Zhang, T.; Li, D.; Tang, P.; Zhao, Y.; Feng, Y. Fabrication and Adsorption Behavior of Magnesium Silicate Hydrate Nanoparticles towards Methylene Blue. Nanomaterials 2018, 8, 271. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.M.; Carr, C.M. A critical review on recent advancements of the removal of reactive dyes from dyehouse effluent by ion-exchange adsorbents. Chemosphere 2018, 209, 201–219. [Google Scholar] [CrossRef]
- Nguyen, T.A.; Juang, R.-S. Treatment of waters and wastewaters containing sulfur dyes: A review. Chem. Eng. J. 2013, 219, 109–117. [Google Scholar] [CrossRef]
- Guo, Z.; Zhang, X.; Kang, Y.; Zhang, J. Biomass-Derived Carbon Sorbents for Cd(II) Removal: Activation and Adsorption Mechanism. ACS Sustain. Chem. Eng. 2017, 5, 4103–4109. [Google Scholar] [CrossRef]
- Sahraei, R.; Pour, Z.S.; Ghaemy, M. Novel magnetic bio-sorbent hydrogel beads based on modified gum tragacanth/graphene oxide: Removal of heavy metals and dyes from water. J. Clean. Prod. 2017, 142, 2973–2984. [Google Scholar] [CrossRef]
- Crini, G.; Gimbert, F.; Robert, C.; Martel, B.; Adam, O.; Morin-Crini, N.; De Giorgi, F.; Badot, P.-M. The removal of Basic Blue 3 from aqueous solutions by chitosan-based adsorbent: Batch studies. J. Hazard. Mater. 2008, 153, 96–106. [Google Scholar] [CrossRef]
- Haji, A.; Mehrizi, M.K.; Sarani, M. Surface modification of Polypropylene Nonwoven by plasma and β-Cyclodextrin: Optimization and cationic dye removal studies. Surf. Interfaces 2021, 25, 101278. [Google Scholar] [CrossRef]
- Soltan, F.K.M.; Hajiani, M.; Haji, A. Nylon-6/poly(propylene imine) dendrimer hybrid nanofibers: An effective adsorbent for the removal of anionic dyes. J. Text. Inst. 2020, 112, 444–454. [Google Scholar] [CrossRef]
- Ghasempour, H.; Zarekarizi, F.; Morsali, A. Acyl amide-functionalized and water-stable iron-based MOF for rapid and selective dye removal. CrystEngComm 2022, 24, 4074–4084. [Google Scholar] [CrossRef]
- Beydaghdari, M.; Saboor, F.H.; Babapoor, A.; Karve, V.V.; Asgari, M. Recent Advances in MOF-Based Adsorbents for Dye Removal from the Aquatic Environment. Energies 2022, 15, 2023. [Google Scholar] [CrossRef]
- Meshko, V.; Markovska, L.; Mincheva, M.; Rodrigues, A. Adsorption of basic dyes on granular acivated carbon and natural zeolite. Water Res. 2001, 35, 3357–3366. [Google Scholar] [CrossRef] [PubMed]
- Sarı, A.; Tuzen, M. Biosorption of cadmium(II) from aqueous solution by red algae (Ceramium virgatum): Equilibrium, kinetic and thermodynamic studies. J. Hazard. Mater. 2008, 157, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Anayurt, R.A.; Sari, A.; Tuzen, M. Equilibrium, thermodynamic and kinetic studies on biosorption of Pb(II) and Cd(II) from aqueous solution by macrofungus (Lactarius scrobiculatus) biomass. Chem. Eng. J. 2009, 151, 255–261. [Google Scholar] [CrossRef]
- Sarı, A.; Tuzen, M. Biosorption of Pb(II) and Cd(II) from aqueous solution using green alga (Ulva lactuca) biomass. J. Hazard. Mater. 2008, 152, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Sarı, A.; Tuzen, M.; Uluözlü, Ö.D.; Soylak, M. Biosorption of Pb(II) and Ni(II) from aqueous solution by lichen (Cladonia furcata) biomass. Biochem. Eng. J. 2007, 37, 151–158. [Google Scholar] [CrossRef]
- Crini, G.; Peindy, H.N.; Gimbert, F.; Robert, C. Removal of C.I. Basic Green 4 (Malachite Green) from aqueous solutions by adsorption using cyclodextrin-based adsorbent: Kinetic and equilibrium studies. Sep. Purif. Technol. 2007, 53, 97–110. [Google Scholar] [CrossRef]
- Altınışık, A.; Gür, E.; Seki, Y. A natural sorbent, Luffa cylindrica for removing a model basic dye. J. Hazard. Mater. 2010, 179, 658–664. [Google Scholar] [CrossRef]
- El-Houseiny, W.; Khalil, A.A.; Abd-Elhakim, Y.M.; Arisha, A.H.; Moselhy, A.A.; Dahshan, H.; Saber, T.; Saber, T.M.; Ahmed, M.M. Alleviative effects of dietary Silybum marianum and co-enzyme Q10 on waterborne nickel-induced impaired growth, immunosuppression, tissue damage, immune-related genes dysregulation, and reduced resistance to Pseudomonas aeruginosa in Oreochromis niloticus. Aquac. Rep. 2022, 26, 101308. [Google Scholar] [CrossRef]
- Hackett, E.S.; Twedt, D.C.; Gustafson, D.L. Milk Thistle and Its Derivative Compounds: A Review of Opportunities for Treatment of Liver Disease. J. Vet. Intern. Med. 2013, 27, 10–16. [Google Scholar] [CrossRef]
- Ahmed, H.S.; Mohamed, W.R.; Moawad, A.S.; Owis, A.I.; Ahmed, R.R.; AbouZid, S.F. Cytotoxic, hepatoprotective and antioxidant activities of Silybum marianum variety albiflorum growing in Egypt. Nat. Prod. Res. 2020, 34, 3540–3544. [Google Scholar] [CrossRef] [PubMed]
- El Sherif, F.; Khattab, S.; Ibrahim, A.K.; Ahmed, S.A. Improved silymarin content in elicited multiple shoot cultures of Silybum marianum L. Physiol. Mol. Biol. Plants 2013, 19, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Rakhmanberdyeva, R.; Zhauynbayeva, K.; Senchenkova, S.; Shashkov, A.; Bobakulov, K. Structure of arabinogalactan and pectin from the Silybum marianum. Carbohydr. Res. 2019, 485, 107797. [Google Scholar] [CrossRef] [PubMed]
- Lukic, I.; Milovanovic, S.; Pantic, M.; Srbljak, I.; Djuric, A.; Tadic, V.; Tyśkiewicz, K. Separation of high-value extracts from Silybum marianum seeds: Influence of extraction technique and storage on composition and bioactivity. LWT 2022, 160, 113319. [Google Scholar] [CrossRef]
- Ben Rahal, N.; Barba, F.J.; Barth, D.; Chevalot, I. Supercritical CO2 extraction of oil, fatty acids and flavonolignans from milk thistle seeds: Evaluation of their antioxidant and cytotoxic activities in Caco-2 cells. Food Chem. Toxicol. 2015, 83, 275–282. [Google Scholar] [CrossRef]
- Elateeq, A.A.; Sun, Y.; Nxumalo, W.; Gabr, A.M. Biotechnological production of silymarin in Silybum marianum L.: A review. Biocatal. Agric. Biotechnol. 2020, 29, 101775. [Google Scholar] [CrossRef]
- Zhang, Z.-S.; Wang, S.; Liu, H.; Li, B.-Z.; Che, L. Constituents and thermal properties of milk thistle seed oils extracted with three methods. Lwt 2020, 126, 109282. [Google Scholar] [CrossRef]
- Chronakis, I.S.; Triantafyllou, A.Ö.; Öste, R. Solid-state characteristics and redispersible properties of powders formed by spray-drying and freeze-drying cereal dispersions of varying (1 3,1 4)-b-glucan content. J. Cereal Sci. 2004, 40, 183–193. [Google Scholar] [CrossRef]
- Türk, G.; Tacer-Caba, Z.; Çakmak, B.; Özpınar, H. Evaluation of Turkish olive oil quality: Some quality characteristics and Turkish food codex. Int. J. Food Eng. Res. 2016, 2, 1–17. [Google Scholar]
- FAO, Food and Nutrition Paper 77, ISSN 0254-4725, Report of A Technical Workshop, Rome, 3–6 December 2002. Available online: https://www.fao.org/3/Y5022E/Y5022E00.htm (accessed on 1 July 2023).
- Liu, K.S. Selected factors affecting crude oil analysis of distillers dried grains with solubles (DDGS) compared with milled corn. Cereal Chem. 2010, 87, 243–249. [Google Scholar] [CrossRef]
- ISO 5516; Fruits, Vegetables, and Derived Products. Decom Position of Organic Matter Before Analysis. Ashing Methods. International Organization for Standardization: Geneva, Switzerland, 1978. Available online: http://lib3.dss.go.th/fulltext/scan_ebook/aoac_1993_v76_n2.pdf (accessed on 1 July 2023).
- ISO 1026:1982; Determination of Dry Matter Content by Drying Under Reduced Pressure and of Water Content by Azeotropic Distillation. The International Organization for Standardization: Geneva, Switzerland, 1982. Available online: https://www.iso.org/obp/ui/en/#iso:std:iso:1026:ed-1:v1:en (accessed on 1 July 2023).
- Yu, S.; Bo, J.; Fengli, L.; Jiegang, L. Structure and fractal characteristic of micro- and meso-pores in low, middle-rank tectonic deformed coals by CO2 and N2 adsorption. Microporous Mesoporous Mater. 2017, 253, 191–202. [Google Scholar] [CrossRef]
- Wang, Z.; Jiang, X.; Pan, M.; Shi, Y. Nano-Scale Pore Structure and Its Multi-Fractal Characteristics of Tight Sandstone by N2 Adsorption/Desorption Analyses: A Case Study of Shihezi Formation from the Sulige Gas Filed, Ordos Basin, China. Minerals 2020, 10, 377. [Google Scholar] [CrossRef]
- Kousha, M.; Daneshvar, E.; Sohrabi, M.S.; Jokar, M.; Bhatnagar, A. Adsorption of acid orange II dye by raw and chemically modified brown macroalga Stoechospermum marginatum. Chem. Eng. J. 2012, 192, 67–76. [Google Scholar] [CrossRef]
- Kassimi, A.E.; Haddad, M.E.; Laamari, R.; Himri, M.E.; Achour, Y.; Yazid, H. Use of Natural Safiot Clay for the Removal of chemical substances from aqueous solutions by adsorption: A combined experimental and theoretical study. Mineralogy 2022. [Google Scholar] [CrossRef]
- Boehm, H.P. Some aspects of the surface chemistry of carbon blacks and other carbons. Carbon 1994, 32, 759–769. [Google Scholar] [CrossRef]
- Djelloul, C.; Hasseine, A. Ultrasound-assisted removal of methylene blue from aqueous solution by milk thistle seed. Desalination Water Treat. 2013, 51, 5805–5812. [Google Scholar] [CrossRef]
- Djelloula, C.; Hasseineb, A.; Hamdaoui, O. Adsorption of cationic dye from aqueous solution by milk thistle seeds: Isotherm, kinetic and thermodynamic studies. Desalınatıon Water Treat. 2017, 78, 313–320. [Google Scholar] [CrossRef]
- Omer, A.M.; Elgarhy, G.S.; El-Subruiti, G.M.; Khalifa, R.E.; Eltaweil, A.S. Fabrication of novel iminodiacetic acid-functionalized carboxymethyl cellulose microbeads for efficient removal of cationic crystal violet dye from aqueous solutions. Int. J. Biol. Macromol. 2020, 148, 1072–1083. [Google Scholar] [CrossRef]
- Ayub, A.; Raza, Z.A.; Majeed, M.I.; Tariq, M.R.; Irfan, A. Development of sustainable magnetic chitosan biosorbent beads for kinetic remediation of arsenic contaminated water. Int. J. Biol. Macromol. 2020, 163, 603–617. [Google Scholar] [CrossRef]










| Name of Analysis | Results | Applied Methods |
|---|---|---|
| Total protein | 13.9 g/100 g ± 1.72 | NMKL 6 [58] |
| Amount of polyunsaturated fatty acids | 38.88% ± 0.08 | Turkish food codex. [59] |
| Saturated fatty acids | 25.87% ± 0.10 | Turkish food codex. [59] |
| Monounsaturated fatty acids | 16.49% ± 0.04 | Turkish food codex. [59] |
| Trans fatty acids | 18.76% ± 0.02 | Turkish food codex. [59] |
| Carbohydrate content | 68.93 g/100 g ± 0.13 | FAO [60] |
| Total amount of fat | 1.17% ± 0.08 | AOCS (Am 5-04) [61] |
| Ash content | 9.36% ± 0.03 | ISO 5516 [62] |
| Humidity analysis | 6.64% ± 0.02 | ISO 1026 [63] |
| Name of Analysis | SLM Stem-Natural | SLM Stem-800 °C |
|---|---|---|
| Total basic sites (meq g−1) | 0.61 ± 0.03 | 0.35 ± 0.01 |
| Total acidic sites (meq g−1) | 0.02 ± 0.01 | 0.001 ± 0.0002 |
| Langmuir | BB3 onto SLM Stem-800 °C | BB3 onto SLM Stem-Natural | BY28 onto SLM Stem-800 °C | BY28 onto SLM Stem-Natural |
|---|---|---|---|---|
| KL (mg/L) | 0.0811 | 0.0496 | 0.0129 | 0.0536 |
| qmax (mg/g) | 36.8053 | 13.9645 | 271.7391 | 36.5497 |
| r2 | 0.9978 | 0.9720 | 0.9987 | 0.9897 |
| Freundlich | ||||
| Kf (mg/L) | 3.1314 | 0.7335 | 3.4516 | 2.2981 |
| 1/n | 0.7289 | 0.7835 | 0.9743 | 0.7291 |
| r2 | 0.9813 | 0.8593 | 0.9861 | 0.9863 |
| Adsorbent | Adsorption Capacity (mg/g) | Adsorption Model | References | |
|---|---|---|---|---|
| Intercalated and tubular kaolinite | - | 142.26 | Freundlich | [12] |
| Kaolin | - | 5.71 | Langmuir | [15] |
| Macadamia seed husks | 1.40 | - | Langmuir-Freundlich | [21] |
| Powder activated charcoal (PAC) | 151.30 | - | Langmuir-Freundlich | [24] |
| Smectite rich natural clays (Ghassoul) | - | 384.60 | Langmuir | [25] |
| Chitosan-based adsorbent | 166.50 | - | Langmuir | [37] |
| Silybum marianum (SLM) Stem-Natural | 13.96 | 36.54 | Langmuir | [This study] |
| Silybum marianum (SLM) Stem-800 °C | 36.80 | 271.73 | Langmuir | [This study] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Börklü Budak, T. Adsorption of Basic Yellow 28 and Basic Blue 3 Dyes from Aqueous Solution Using Silybum Marianum Stem as a Low-Cost Adsorbent. Molecules 2023, 28, 6639. https://doi.org/10.3390/molecules28186639
Börklü Budak T. Adsorption of Basic Yellow 28 and Basic Blue 3 Dyes from Aqueous Solution Using Silybum Marianum Stem as a Low-Cost Adsorbent. Molecules. 2023; 28(18):6639. https://doi.org/10.3390/molecules28186639
Chicago/Turabian StyleBörklü Budak, Türkan. 2023. "Adsorption of Basic Yellow 28 and Basic Blue 3 Dyes from Aqueous Solution Using Silybum Marianum Stem as a Low-Cost Adsorbent" Molecules 28, no. 18: 6639. https://doi.org/10.3390/molecules28186639
APA StyleBörklü Budak, T. (2023). Adsorption of Basic Yellow 28 and Basic Blue 3 Dyes from Aqueous Solution Using Silybum Marianum Stem as a Low-Cost Adsorbent. Molecules, 28(18), 6639. https://doi.org/10.3390/molecules28186639





