Corrosion Inhibition in CO2-Saturated Brine by Nd3+ Ions
Abstract
:1. Introduction
2. Results and Discussion
2.1. Potentiodynamic Polarization Curves
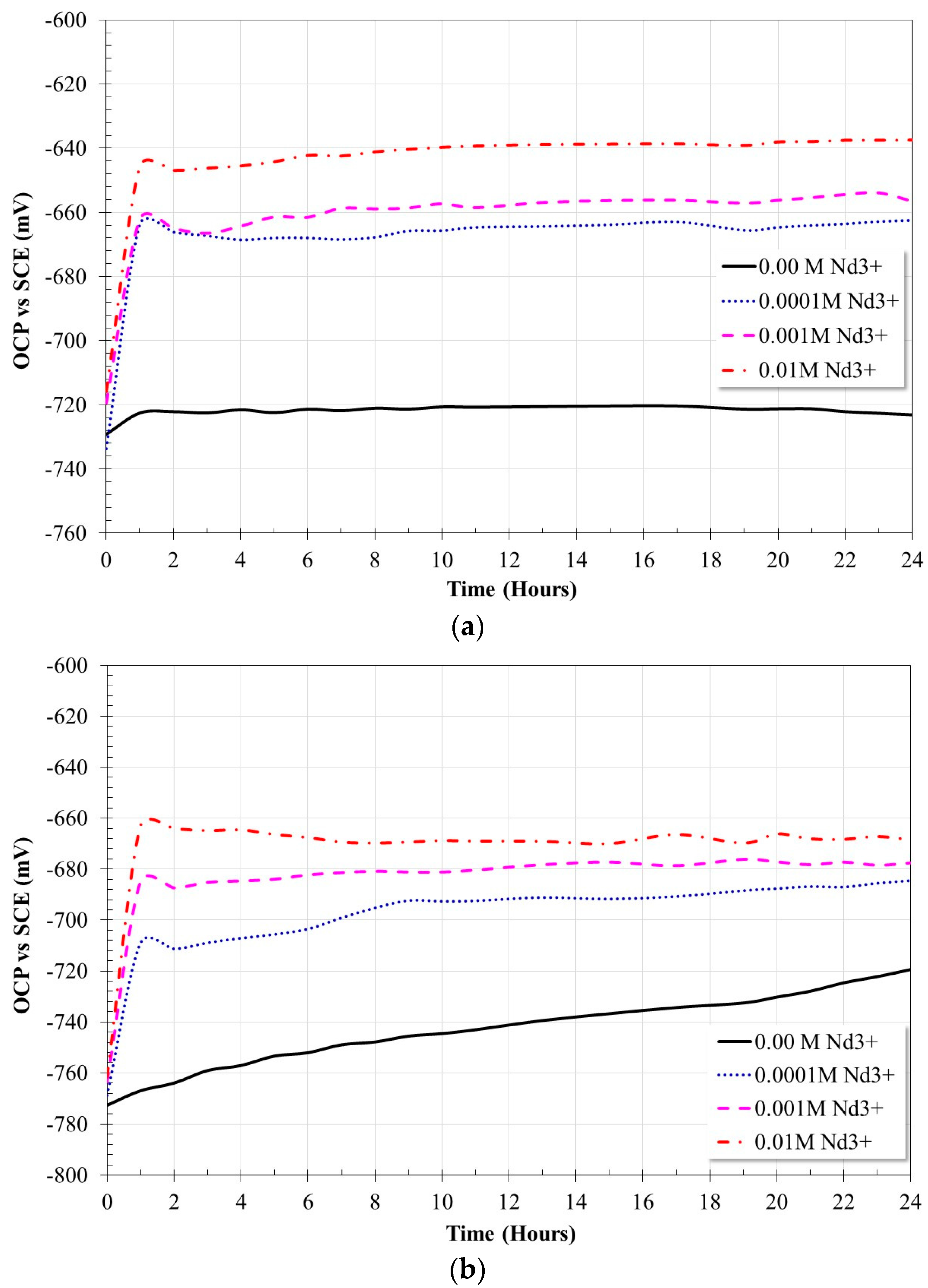
2.2. Open Circuit Potential Measurements
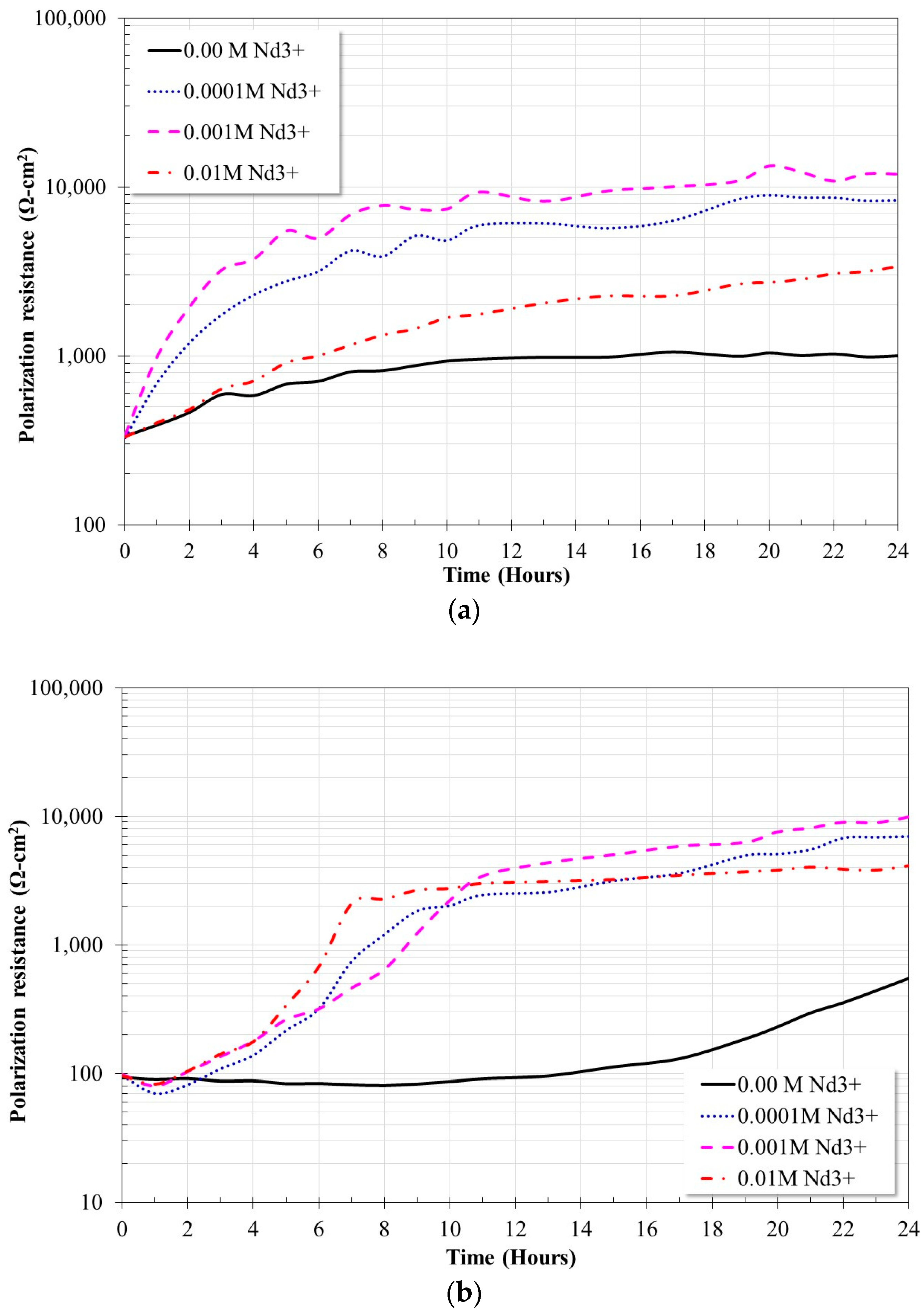
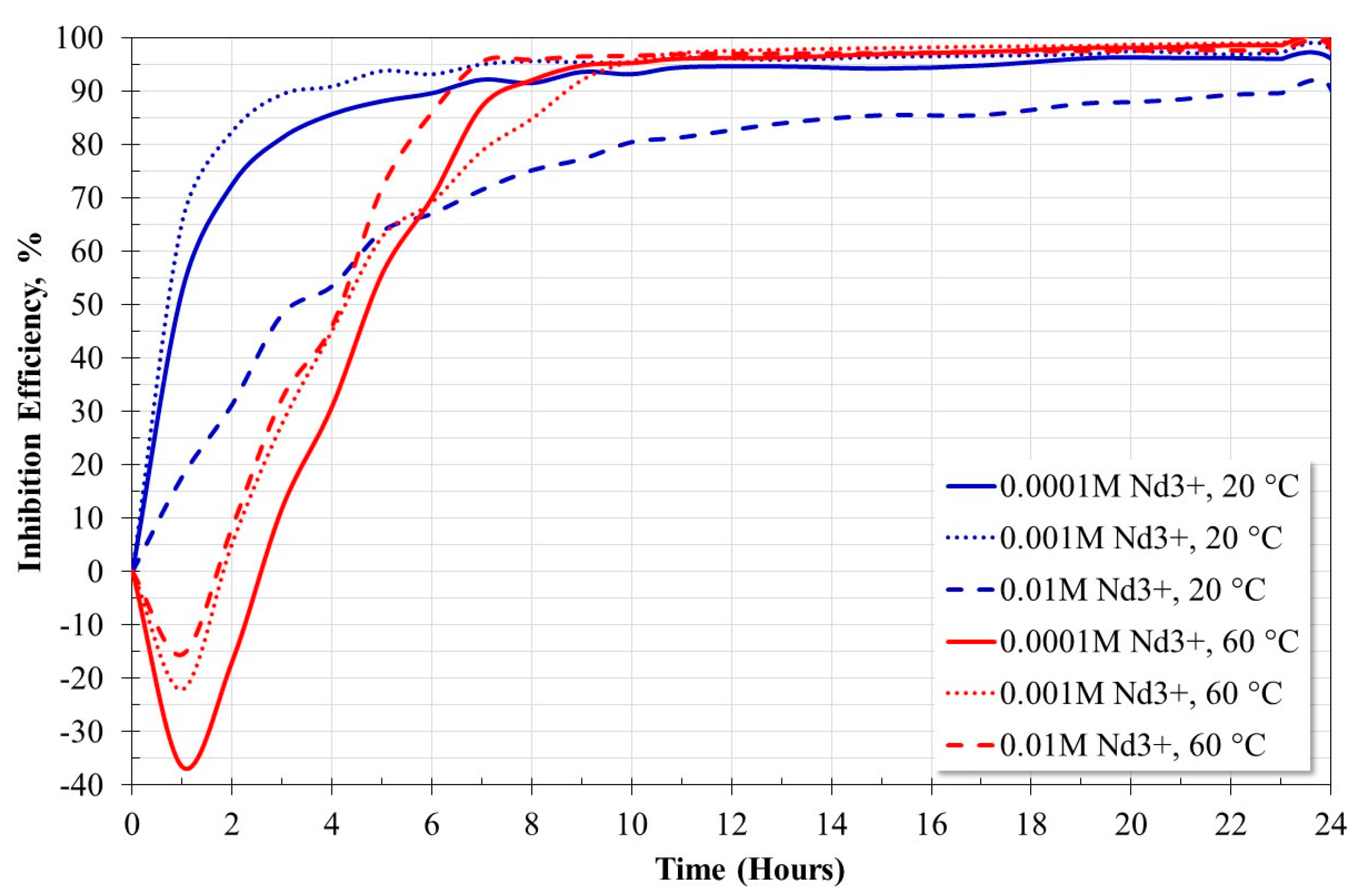
2.3. Linear Polarization Resistance Measurements
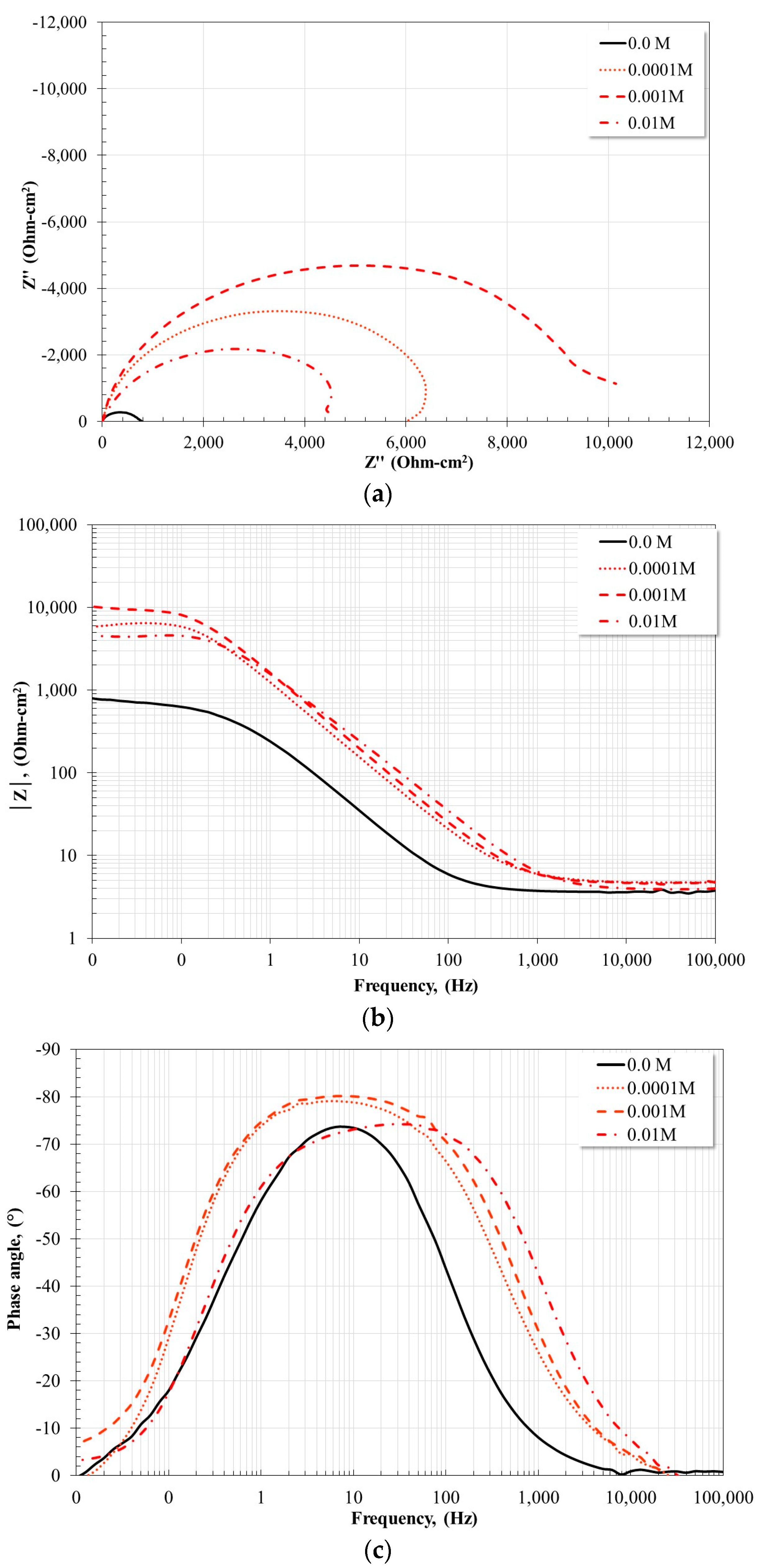
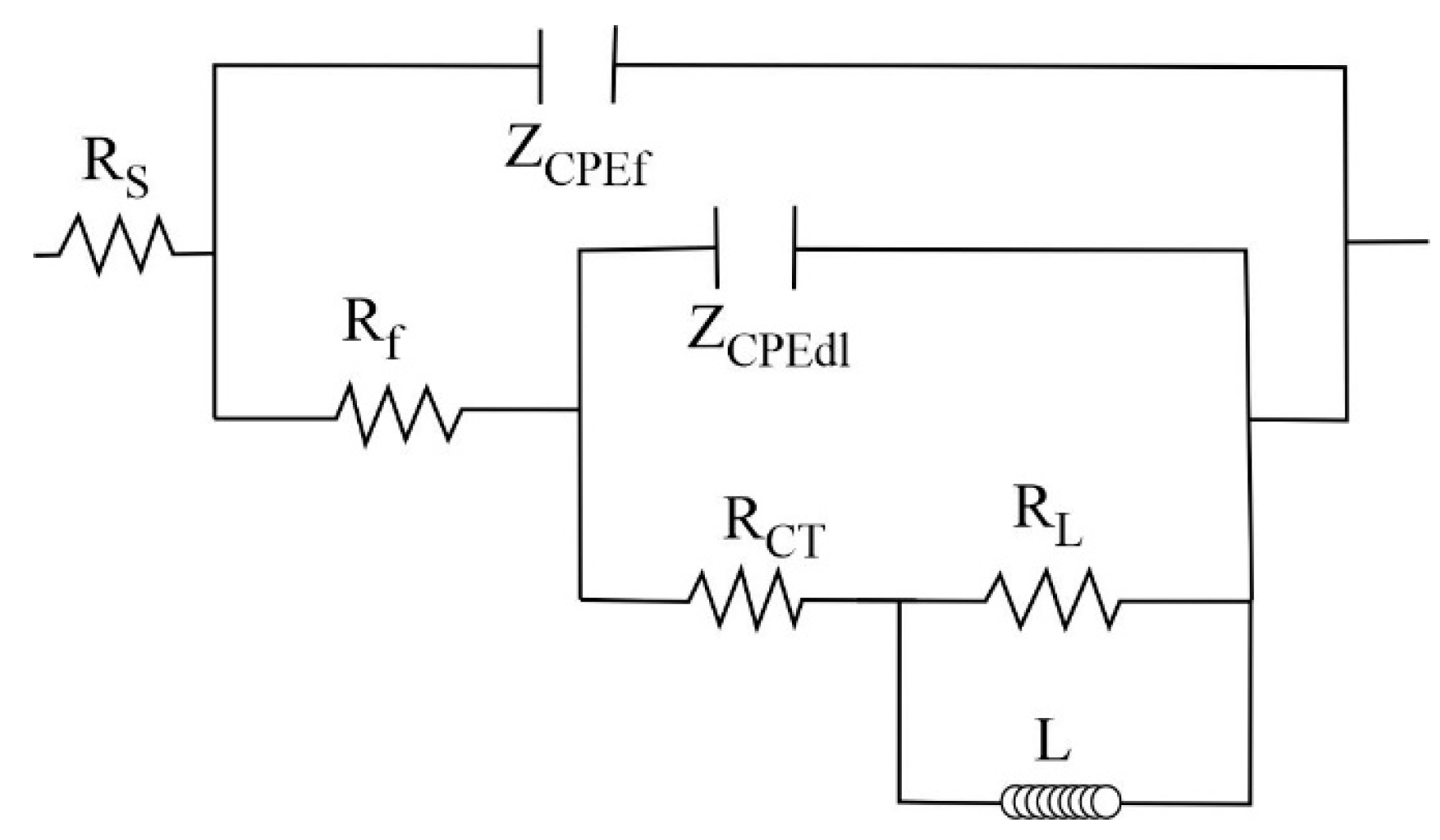
2.4. Electrochemical Impedance Spectroscopy Measurements
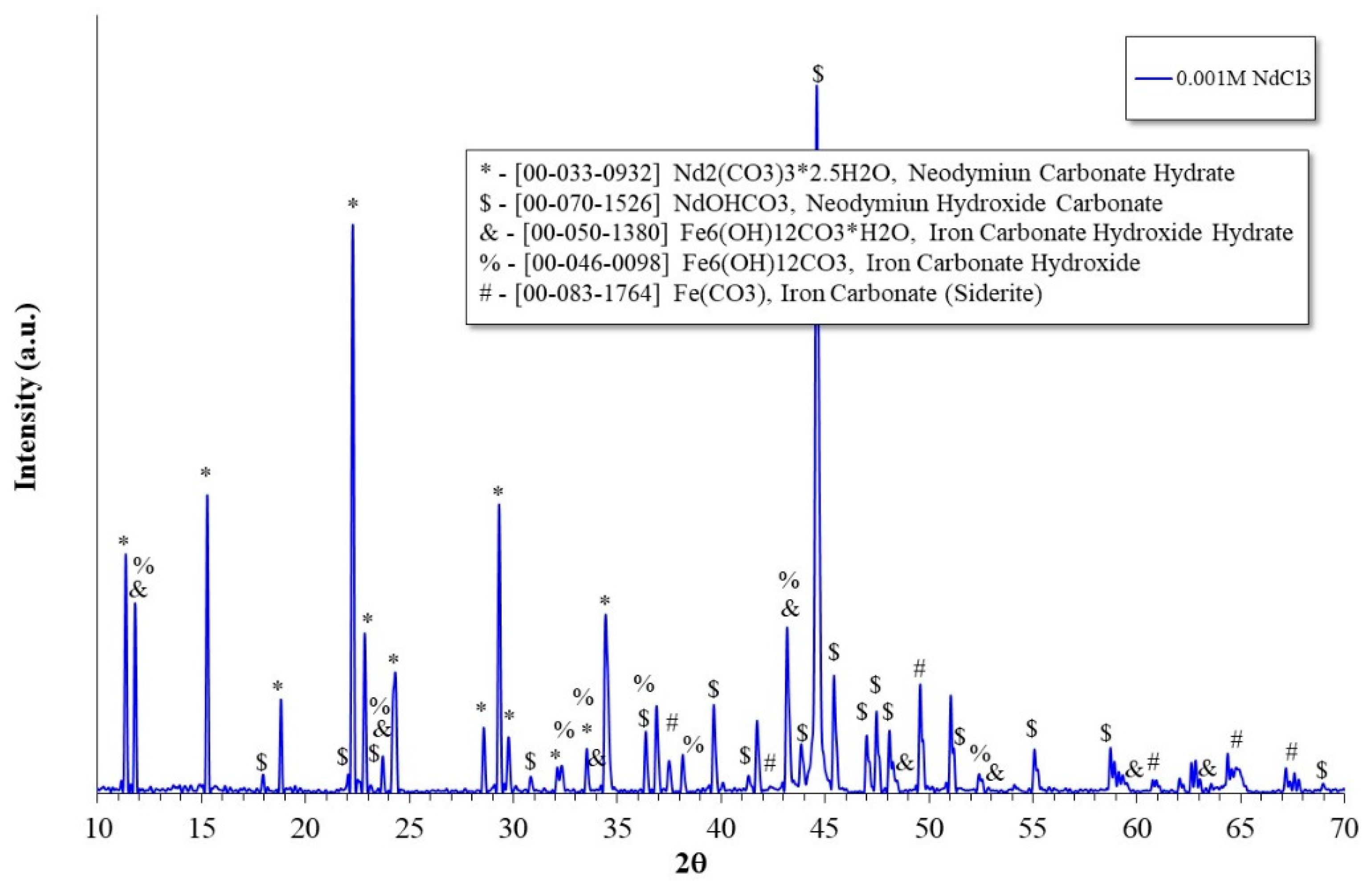
2.5. X-ray Diffraction Analysis
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Kahyarian, A.; Brown, B.; Nesic, S. The Unified Mechanism of Corrosion in Aqueous Weak Acids Solutions: A Review of the Recent Developments in Mechanistic Understandings of Mild Steel Corrosion in the Presence of Carboxylic Acids, Carbon Dioxide, and Hydrogen Sulfide. Corrosion 2020, 6, 268–278. [Google Scholar] [CrossRef]
- Kahyarian, A.; Nesic, S. On the mechanism of carbon dioxide corrosion of mild steel: Experimental investigation and mathematical modeling at elevated pressures and nonideal solutions. Corros. Sci. 2020, 173, 108719. [Google Scholar] [CrossRef]
- Wright, R.F.; Brand, E.R.; Ziomek-Moroz, M.; Tylczak, J.H.; Ohodnicki, P.R., Jr. Effect of HCO−3 on electrochemical kinetics of carbon steel corrosion in CO2-saturated brines. Electrochim. Acta 2018, 290, 626–638. [Google Scholar] [CrossRef]
- Edwards, A.; Osborne, C.; Webster, S.; Klenerman, D.; Joseph, M.; Ostovar, P.; Doyle, M. Mechanistic studies of the corrosion inhibitor oleic imidazoline. Corros. Sci. 1994, 36, 315–325. [Google Scholar] [CrossRef]
- Zhang, G.; Chen, C.; Lu, M.; Chai, C.; Wu, Y. Evaluation of inhibition efficiency of an imidazoline derivative in CO2-containing aqueous solution. Mater. Chem. Phys. 2007, 105, 331–340. [Google Scholar] [CrossRef]
- El-Lateef, H.M.A.; El-Beltagi, H.S.; Mohamed, M.E.M.; Kandeel, M.; Bakir, E.; Toghan, A.; Shalabi, K.; Tantawy, A.H.; Khalaf, M.M. Novel Natural Surfactant-Based Fatty Acids and Their Corrosion-Inhibitive Characteristics for Carbon Steel-Induced Sweet Corrosion: Detailed Practical and Computational Explorations. Front. Mater. 2022, 9, 843438. [Google Scholar] [CrossRef]
- Shahzad, K.; Sliem, M.H.; Shakoor, R.A.; Radwan, A.B.; Kahraman, R.; Umer, M.A.; Manzoor, U.; Abdullah, A.M. Electrochemical and thermodynamic study on the corrosion performance of API X120 steel in 3.5% NaCl solution. Sci. Rep. 2020, 10, 4314. [Google Scholar] [CrossRef] [PubMed]
- Sk, M.H.; Abdullah, A.M.; Ko, M.; Ingham, B.; Laycock, N.; Arul, R.; Williams, D.E. Local supersaturation and the growth of protective scales during CO2 corrosion of steel: Effect of pH and solution flow. Corr. Sci. 2017, 126, 26–36. [Google Scholar] [CrossRef]
- Barker, R.; Burkle, D.; Charpentier, T.; Thompson, H.; Neville, A. A review of iron carbonate (FeCO3) formation in the oil and gas industry. Corros. Sci. 2018, 142, 312–341. [Google Scholar] [CrossRef]
- Allachi, H.; Chaouket, F.; Draoui, K. Corrosion inhibition of AA6060 aluminium alloy by lanthanide salts in chloride solution. J. Alloys Compd. 2009, 475, 300–303. [Google Scholar] [CrossRef]
- Bernal, S.; Botana, F.J.; Calvino, J.J.; Marcos, M.; Perez-Omil, J.A.; Vidal, H. Lanthanide salts as alternative corrosion inhibitors. J. Alloys Compd. 1995, 225, 638–641. [Google Scholar] [CrossRef]
- Davó, B.; De Damborenea, J.J. Use of rare earth salts as electrochemical corrosion inhibitors for an Al–Li–Cu (8090) alloy in 3.56% NaCl. Electrochim. Acta 2004, 49, 4957–4965. [Google Scholar] [CrossRef]
- Allachi, H.; Chaouket, F.; Draoui, K. Protection against corrosion in marine environments of AA6060 aluminium alloy by cerium chlorides. J. Alloys Compd. 2010, 491, 223–229. [Google Scholar] [CrossRef]
- Mohammedi, D.; Ismail, F.; Rehamnia, R.; Bensalem, R.; Savadogo, O. Corrosion behaviour of steel in the presence of rare earth salts: Synergistic effect. Corros. Eng. Sci. Technol. 2015, 50, 633–638. [Google Scholar] [CrossRef]
- Arenas, M.A.; Conde, A.; de Damborenea, J.J. Cerium: A suitable green corrosion inhibitor for Tinplate. Corros. Sci. 2002, 44, 511–520. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhuang, J.; Yu, Y.; Zeng, X. Research on anti-corrosion property of rare earth inhibitor for X70 steel. J. Rare Earths 2013, 31, 734–740. [Google Scholar] [CrossRef]
- Vazquez-Ramirez, R.; Salinas-Solano, G.; Porcayo-Calderon, J.; Gonzalez-Rodriguez, J.G. Use of (Nd, Pr) Sulfates Obtained from Electronic Scrap as Corrosion Inhibitors for a C-Mn Steel in 3.5 NaCl. Int. J. Corros. Scale Inhib. 2021, 10, 602–617. [Google Scholar] [CrossRef]
- Nam, N.D.; Somers, A.; Mathesh, M.; Seter, M.; Hinton, B.; Forsyth, M.; Tan, M.Y.J. The behaviour of praseodymium 4-hydroxycinnamate as an inhibitor for carbon dioxide corrosion and oxygen corrosion of steel in NaCl solutions. Corros. Sci. 2014, 80, 128–138. [Google Scholar] [CrossRef]
- Porcayo-Calderon, J.; Canto, J.; Martinez-de-la-Escalera, L.M.; Neri, A. Sweet Corrosion Inhibition by CO2 Capture. Molecules 2022, 27, 5209. [Google Scholar] [CrossRef]
- Dastgheib, A.; Attar, M.M.; Zarebidaki, A. Evaluation of Corrosion Inhibition of Mild Steel in 3.5 wt% NaCl Solution by Cerium Nitrate. Met. Mater. Int. 2020, 26, 1634–1642. [Google Scholar] [CrossRef]
- Chen, X.-B.; Cain, T.; Scully, J.R.; Birbilis, N. Experimental Survey of Corrosion Potentials for Rare Earth Metals Ce, Er, Gd, La, and Nd as a Function of pH and Chloride Concentration. Corrosion 2013, 70, 323–328. [Google Scholar] [CrossRef]
- Gobara, M.; Baraka, A.; Akid, R.; Zorainy, M. Corrosion protection mechanism of Ce4+/organic inhibitor for AA2024 in 3.5% NaCl. RSC Adv. 2020, 10, 2227–2240. [Google Scholar] [CrossRef]
- Bethencourt, M.; Botana, F.J.; Calvino, J.J.; Marcos, M.; Rodriguez-Chacon, M.A. Lanthanide compounds as environmentally-friendly corrosion inhibitors of aluminium alloys: A review. Corros. Sci. 1998, 40, 1803–1819. [Google Scholar] [CrossRef]
- El Miligy, A.A.; Geana, D.; Lorenz, W.J. A theoretical treatment of the kinetics of iron dissolution and passivation. Electrochim. Acta 1975, 20, 273–281. [Google Scholar] [CrossRef]
- Riggs, O.L., Jr. Corrosion Inhibition, 2nd ed.; Nathan, C.C., Ed.; National Association of Corrosion Engineers (NACE): Houston, TX, USA, 1973. [Google Scholar]
- Amin, M.A.; Ahmed, M.A.; Arida, H.A.; Kandemirli, F.; Saracoglu, M.; Arslan, T.; Basaran, M.A. Monitoring corrosion and corrosion control of iron in HCl by non-ionic surfactants of the TRITON-X series—Part III. Immersion time effects and theoretical studies. Corros. Sci. 2011, 53, 1895–1909. [Google Scholar] [CrossRef]
- Al-Otaibi, M.S.; Al-Mayouf, A.M.; Khan, M.; Mousa, A.A.; Al-Mazroa, S.A.; Alkhathlan, H.Z. Corrosion inhibitory action of some plant extracts on the corrosion of mild steel in acidic media. Arab. J. Chem. 2014, 7, 340–346. [Google Scholar] [CrossRef]
- Hurtado, M.R.F.; Sumodjo, P.T.A.; Benedetti, A.V. Electrochemical studies with a Cu-5wt.%Ni alloy in 0.5 M H2SO4. Electrochim. Acta 2003, 48, 2791–2798. [Google Scholar] [CrossRef]
- Prabhu, D.; Rao, P. Garcinia indica as an environmentally safe corrosion inhibitor for aluminium in 0.5M phosphoric acid. Int. J. Corros. 2013, 2013, 945143. [Google Scholar] [CrossRef]
- Porcayo-Calderon, J.; Rodriguez-Diaz, R.A.; Porcayo-Palafox, E.; Colin, J.; Molina-Ocampo, A.; Martinez-Gomez, L. Effect of Cu addition on the electrochemical corrosion performance of Ni3Al in 1.0 M H2SO4. Adv. Mater. Sci. Eng. 2015, 2015, 209286. [Google Scholar] [CrossRef]
- Amin, M.A.; Khaled, K.F.; Mohsen, Q.; Arida, H.A. A study of the inhibition of iron corrosion in HCl solutions by some amino acids. Corros. Sci. 2010, 52, 1684–1695. [Google Scholar] [CrossRef]
- Bouamra, B.; Djellab, M.; Bentrah, H.; Chala, A.; Kherief, S.; Taoui, H. Ghars date extract/iodide ions system as an eco-friendly corrosion inhibitor for API 5L X70 steel in sulfuric acid. J. Chin. Chem. Soc. 2023, 1–12. [Google Scholar] [CrossRef]
- Yasakau, K.A.; Zheludkevich, M.L.; Lamaka, S.V.; Ferreira, M.G.S. Mechanism of Corrosion Inhibition of AA2024 by Rare-Earth Compounds. J. Phys. Chem. B 2006, 110, 5515–5528. [Google Scholar] [CrossRef] [PubMed]
- Matter, E.A.; Kozhukharov, S.; Machkova, M.; Kozhukharov, V. Comparison between the inhibition efficiencies of Ce(III) and Ce(IV) ammonium nitrates against corrosion of AA2024 aluminum alloy in solutions of low chloride concentration. Corros. Sci. 2012, 62, 22–33. [Google Scholar] [CrossRef]
- Hu, T.; Shi, H.; Wei, T.; Liu, F.; Fan, S.; Han, E.-H. Cerium tartrate as a corrosion inhibitor for AA 2024-T3. Corros. Sci. 2015, 95, 152–161. [Google Scholar] [CrossRef]
- Zhang, L.J.; Zhang, Z.; Zhang, J.Q.; Cao, C.N. The study of the La(NO3)3 inhibition on X70 pipeline steel in 3.0(wt.%) NaCl solution. Corros. Mater. 2005, 56, 630–635. [Google Scholar] [CrossRef]
- Hassas, B.V.; Rezaee, M.; Pisupati, S.V. Precipitation of rare earth elements from acid mine drainage by CO2 mineralization process. J. Chem. Eng. 2020, 399, 125716. [Google Scholar] [CrossRef]
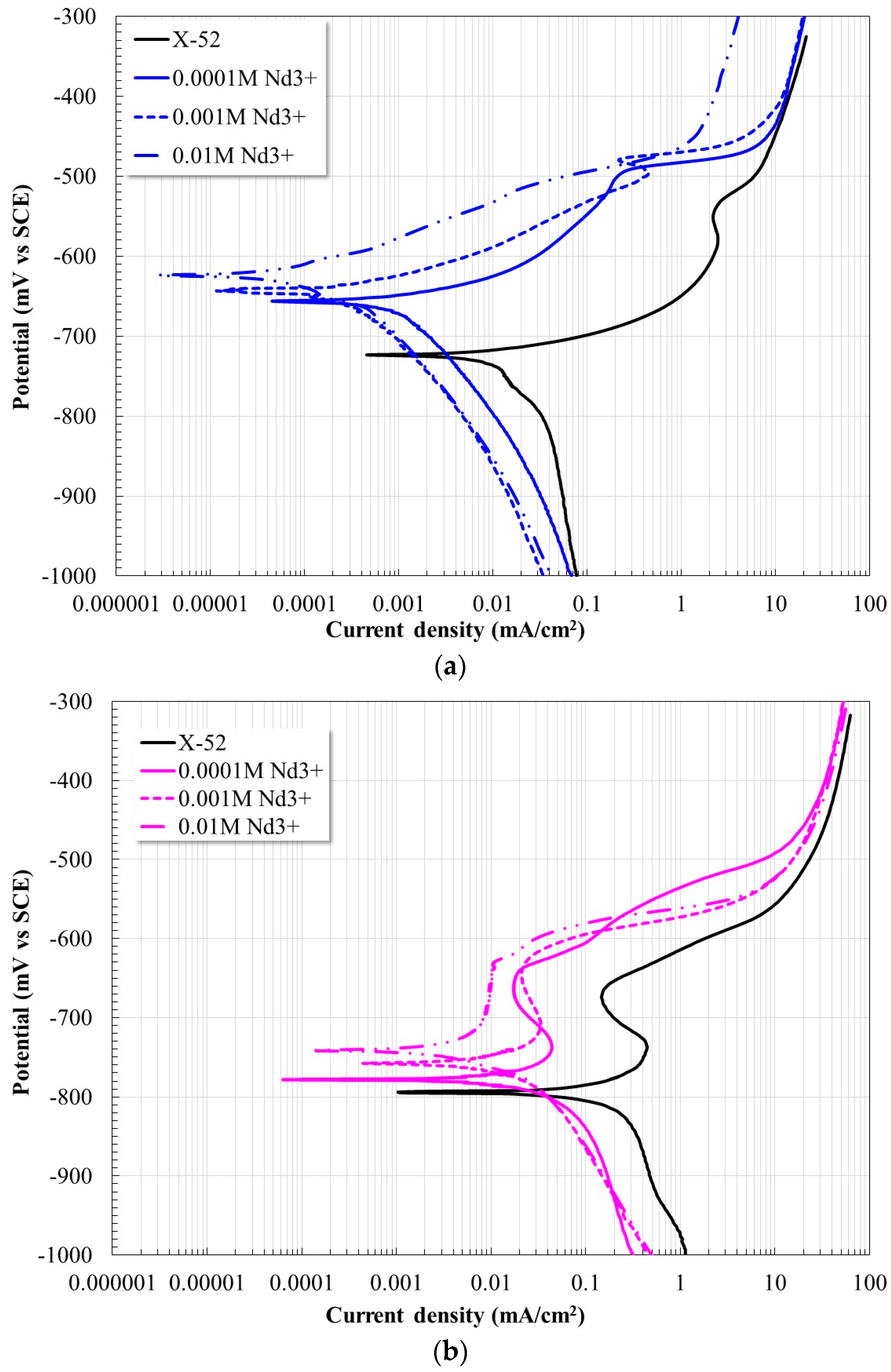
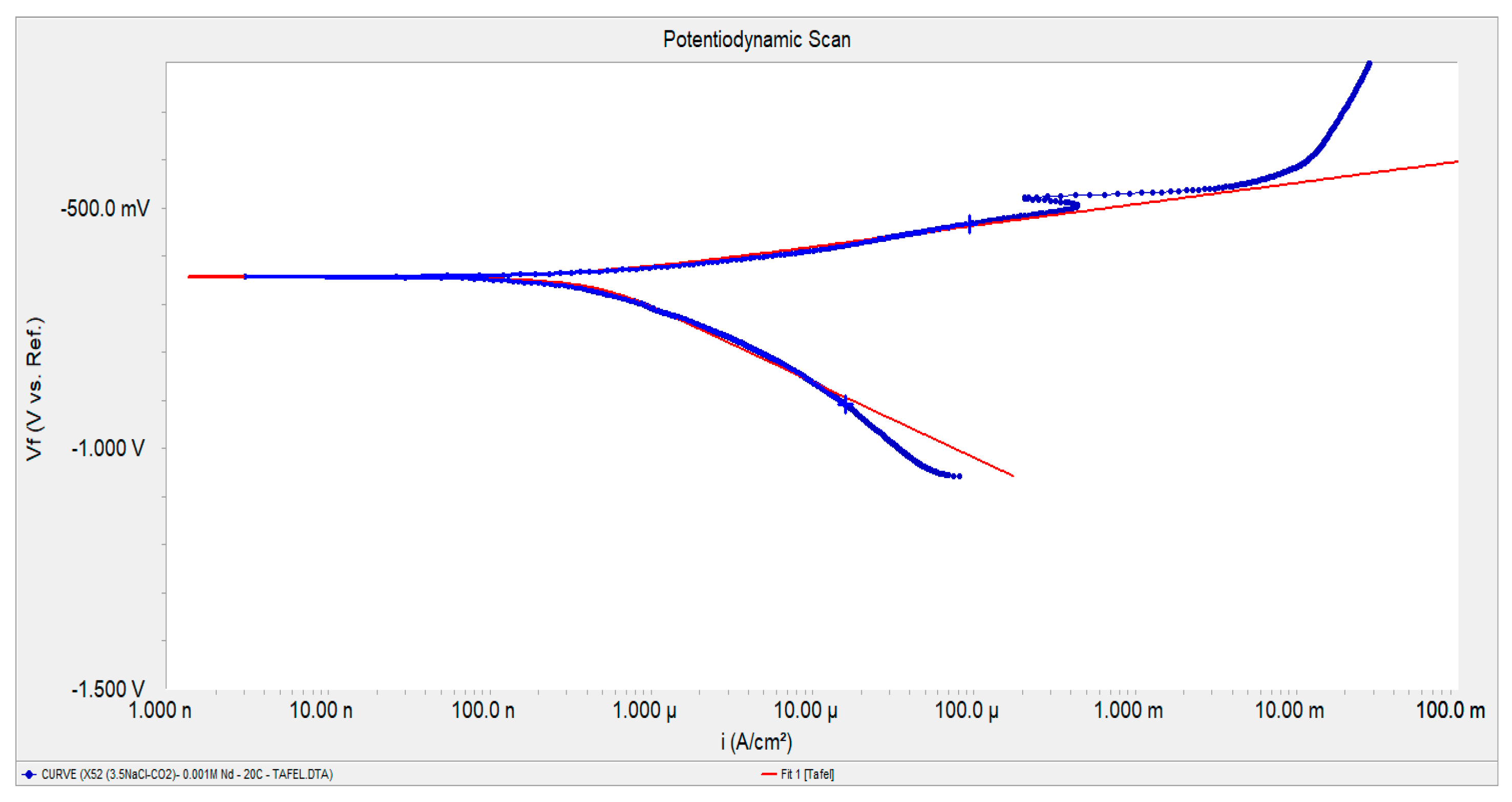
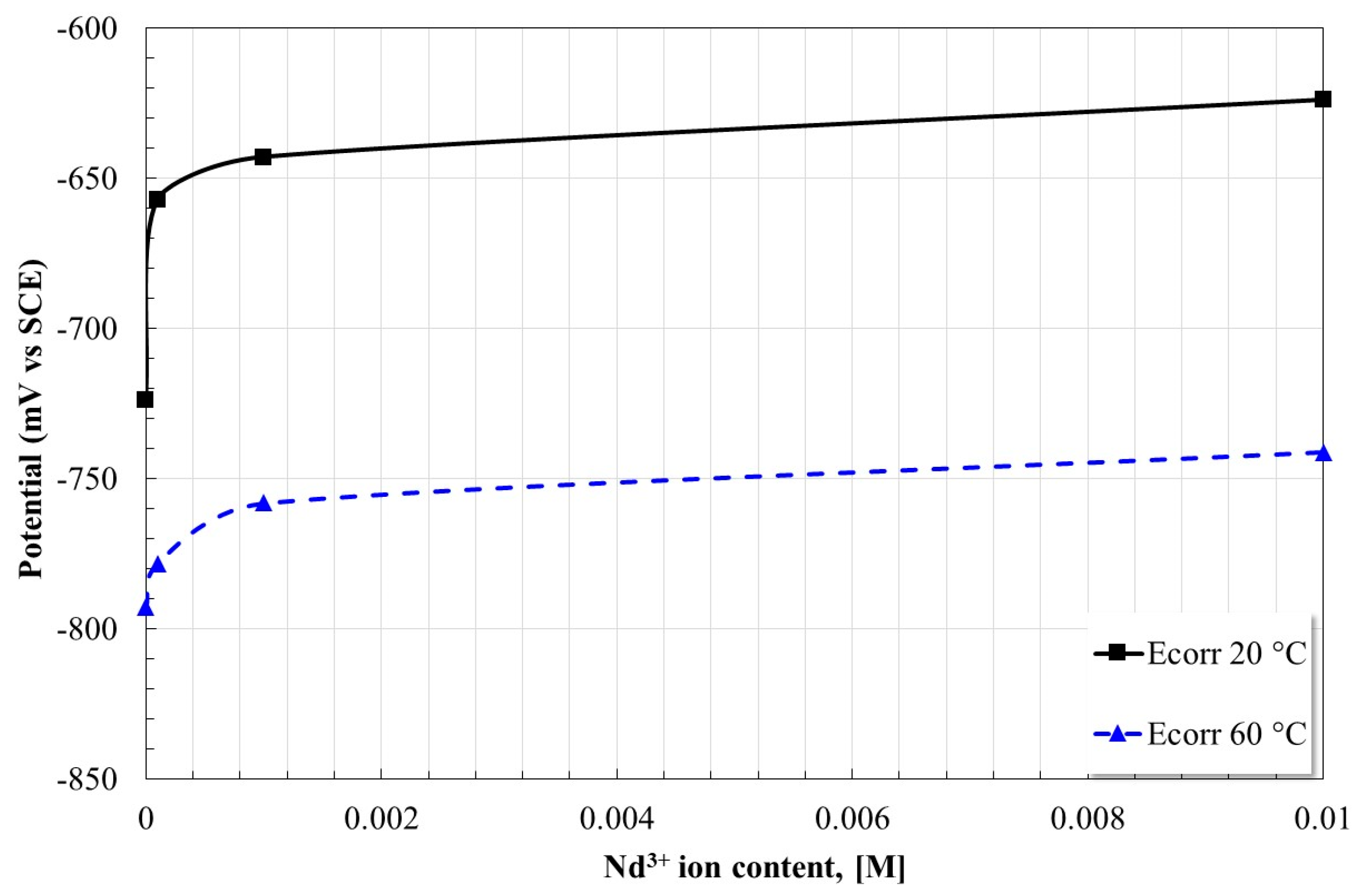
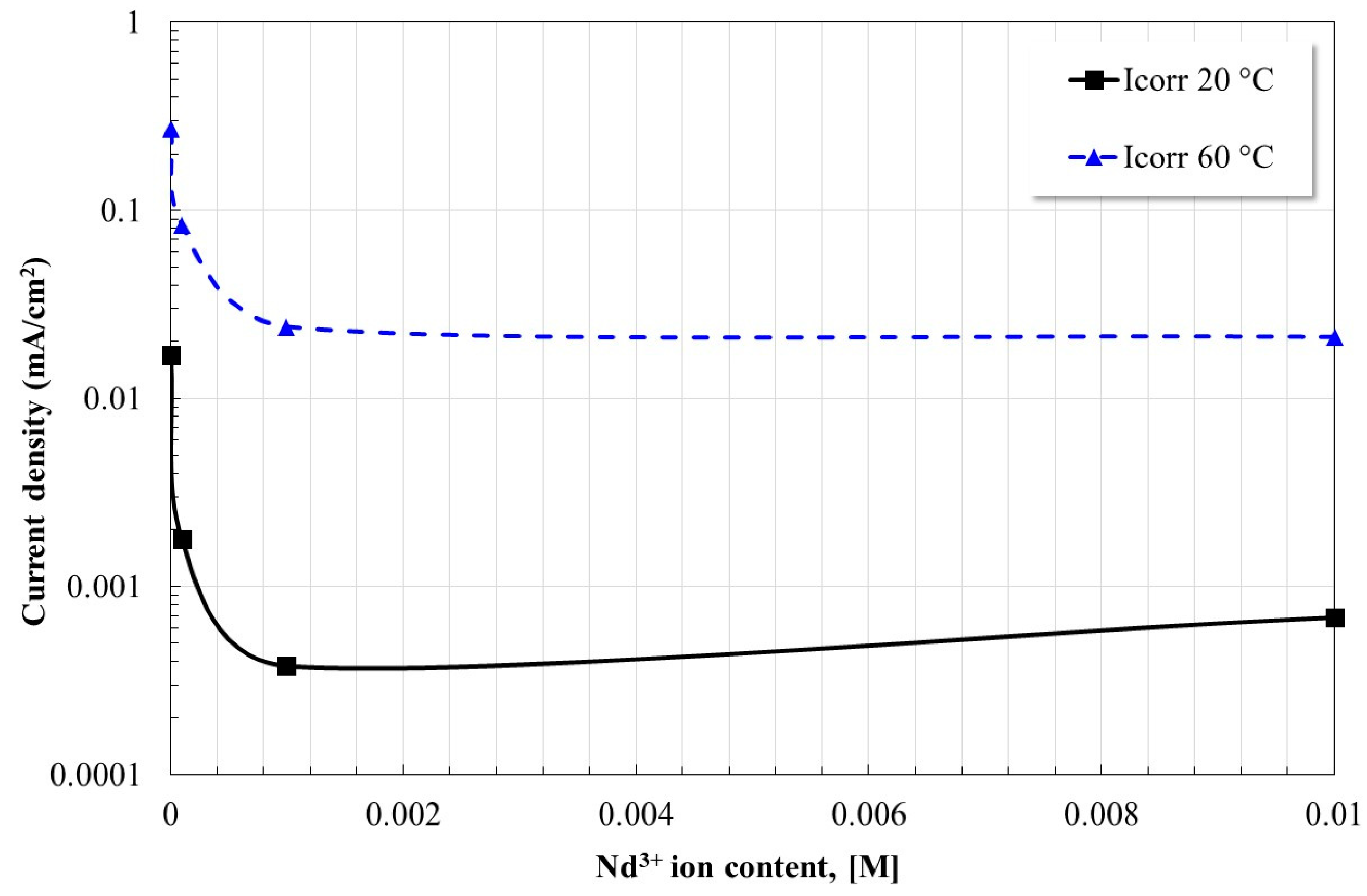

| [Nd3+] Molar Concentration | Ecorr [mV] | βa [mV/Dec] | βc [mV/Dec] | Icorr [µA/cm2] | Inhibition Efficiency, [%] |
|---|---|---|---|---|---|
| 0.0 | −724 | 35 | 347 | 17 | --- |
| 0.0001 | −657 | 57 | 201 | 1.78 | 89.5 |
| 0.001 | −643 | 44 | 162 | 0.379 | 97.8 |
| 0.01 | −624 | 55 | 122 | 0.688 | 95.9 |
| [Nd3+] Molar Concentration | Ecorr [mV] | βa [mV/Dec] | βc [mV/Dec] | Icorr [µA/cm2] | Inhibition Efficiency, [%] |
|---|---|---|---|---|---|
| 0.0 | −793 | 46 | 421 | 270 | --- |
| 0.0001 | −778 | 39 | 397 | 83 | 69.3 |
| 0.001 | −758 | 38 | 183 | 24 | 91.1 |
| 0.01 | −741 | 34 | 196 | 21 | 92.2 |
| NdCl3 (M) | 20 °C Rp (Ω·cm2) | 20 °C Inhibition Efficiency (%) | 60 °C Rp (Ω·cm2) | 60 °C Inhibition Efficiency (%) |
|---|---|---|---|---|
| 0.0001 | 8549.80 | 96.16 | 372.60 | 98.45 |
| 0.001 | 11,994.00 | 97.13 | 6255.60 | 98.86 |
| 0.01 | 3044.00 | 89.06 | 3926.20 | 97.56 |
| NdCl3 (M) | Rf (Ω·cm2) | Y0f (Ω−1·cm−2·sn) | n | RCT (Ω·cm2) | Y0dl (Ω−1·cm−2·sn) | ndl | RL (Ω·cm2) | L (H·cm2) | ΣR (Ω·cm2) |
|---|---|---|---|---|---|---|---|---|---|
| 0 | 6.4 | 9.1836 × 10−5 | 0.92 | 770.3 | 1.7251 × 10−4 | 0.90 | 478 | 1614 | 1254.7 |
| 0.0001 | 463 | 3.3588 × 10−5 | 0.89 | 7753 | 6.8142 × 10−6 | 0.97 | 5728 | 10,079 | 13,944 |
| 0.001 | 261 | 2.6998 × 10−5 | 0.87 | 10977 | 7.9978 × 10−6 | 0.95 | 9127 | 23638 | 20,365 |
| 0.01 | 9.7 | 4.7457 × 10−5 | 0.94 | 2492 | 6.6498 × 10−6 | 0.90 | 2003 | 6126 | 4504.7 |
| NdCl3 (M) | Rf (Ω·cm2) | Y0f (Ω−1·cm−2·sn) | n | RCT (Ω·cm2) | Yodl (Ω−1·cm−2·sn) | ndl | RL (Ω·cm2) | L (H·cm2) | ΣR (Ω·cm2) |
|---|---|---|---|---|---|---|---|---|---|
| 0 | 0.1 | 43364 × 10−4 | 0.78 | 664.1 | 3.326 × 10−4 | 0.97 | 68.41 | 3200 | 732.61 |
| 0.0001 | 18.42 | 1.0078 × 10−4 | 0.93 | 5966 | 4.4948 × 10−5 | 0.88 | 1616 | 6047 | 7600.42 |
| 0.001 | 15.41 | 4.872 × 10−5 | 0.99 | 10188 | 7.1392 × 10−5 | 0.82 | 3390 | 2313 | 13,593.41 |
| 0.01 | 63.91 | 4.7547 × 10−5 | 0.96 | 4664 | 1.2279 × 10−4 | 0.84 | 1058 | 172.06 | 5785.91 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Canto, J.; Rodríguez-Díaz, R.A.; Martinez-de-la-Escalera, L.M.; Neri, A.; Porcayo-Calderon, J. Corrosion Inhibition in CO2-Saturated Brine by Nd3+ Ions. Molecules 2023, 28, 6593. https://doi.org/10.3390/molecules28186593
Canto J, Rodríguez-Díaz RA, Martinez-de-la-Escalera LM, Neri A, Porcayo-Calderon J. Corrosion Inhibition in CO2-Saturated Brine by Nd3+ Ions. Molecules. 2023; 28(18):6593. https://doi.org/10.3390/molecules28186593
Chicago/Turabian StyleCanto, Jorge, Roberto Ademar Rodríguez-Díaz, Lorenzo Martinez Martinez-de-la-Escalera, Adrian Neri, and Jesus Porcayo-Calderon. 2023. "Corrosion Inhibition in CO2-Saturated Brine by Nd3+ Ions" Molecules 28, no. 18: 6593. https://doi.org/10.3390/molecules28186593
APA StyleCanto, J., Rodríguez-Díaz, R. A., Martinez-de-la-Escalera, L. M., Neri, A., & Porcayo-Calderon, J. (2023). Corrosion Inhibition in CO2-Saturated Brine by Nd3+ Ions. Molecules, 28(18), 6593. https://doi.org/10.3390/molecules28186593










