4.2. Experimental Procedures and Characterization Data
4.2.1. General Procedure for Sulfonamide Reactions (General Procedure A)
A mixture of 2-bromobenzenethiol (1.0 g; 5.3 mmol), 30% H2O2 (1.5 mL, 8.0 mmol), and ZrCl4 (1.23 g, 5.3 mmol) were stirred in MeCN (15 mL) at 25 °C for 10 min. The reaction mixture was quenched by adding H2O (30 mL), and extracted with EtOAc (4 × 50 mL). The organic extract was dried with anhydrous Na2SO4 and the filtrate was evaporated under vacuum to afford 2 as red crystals (1.23 g, 92%).
To a stirred solution of 2-bromobenzenesulfonyl chloride (2, 3.91 mmol), the corresponding aniline (3.91 mmol), and anhydrous pyridine (1.0 mL, 11.7 mmol) in anhydrous dichloromethane (15 mL) was stirred under an argon atmosphere. The resulting mixture was stirred at room temperature for 14 h. After completion of the reaction (the progress of the reaction was monitored by Thin layer chromatography), the reaction mixture was diluted with washed with saturated NaHCO3 (20 mL). The reaction mixture was extracted with CH2Cl2 (3 × 30 mL). The combined organic extracts were washed with water (2 × 40 mL), separated, dried over anhydrous Na2SO4, then concentrated in vacuo to give a crude yellow oil. The crude product was purified by flash chromatography on silica gel.
4.2.2. General Procedure for Synthesis of Aldehyde Photoprecursors (General Procedure B)
The bromide (2.0 mmol, 1.0 equiv.), the corresponding boronic acid (3.0 mmol, 1.5 equiv.), PdCl2(PPh3)2 (0.07 mmol, 0.04 equiv.), and K2CO3 (8.0 mmol, 4.0 equiv.) are suspended in DMF:H2O (9:1) and heated to reflux in oil bath for 14 h. Upon completion of the reaction as indicated by 1H NMR, the mixture was diluted with EtOAc (30 mL) and washed with a saturated solution of NaHCO3 (2 × 40 mL). Combined organic phase was washed with H2O (20 mL) and dried over anhydrous Na2SO4. After concentration, a crude product was used for the next oxidation reaction. To a stirred solution of the corresponding alcohol (2.5 mmol, 1.0 equiv.) in CH2Cl2 (20 mL), was added MnO2 (12.5 mmol; 5.0 equiv.) at ambient temperature, and the resulting mixture was stirred at room temperature overnight. Upon completion (the progress of the reaction was monitored by 1H NMR), the solids were filtered off by passing the mixture through celite pad, and the pad was washed with additional 20 mL of CH2Cl2. After concentration, crude product was purified by flash chromatography on silica gel to give a desired photoprecursor.
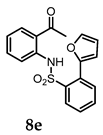
N-(2-Formylphenyl)-2-(furan-2-yl)benzenesulfonamide (8a). Following the general procedure B, compound 8a (126 mg, 79%) was obtained from the oxidation of 7a (160 mg) as a brown solid. 1H NMR (500 MHz, CDCl3): δ 11.08 (s, 1H), 9.81 (s, 1H), 8.32 (d, J = 8.0 Hz, 1H), 7.70 (d, J = 1.8 Hz, 1H), 7.60 (ddd, J = 8.7, 6.1, 4.3 Hz, 4H), 7.54–7.48 (m, 1H), 7.45 (t, J = 8.2 Hz, 1H), 7.12 (t, J = 7.5 Hz, 1H), 6.94 (d, J = 3.4 Hz, 1H), 6.59 (dd, J = 3.3, 1.8 Hz, 1H) ppm; 13C NMR (126 MHz, CDCl3): δ 194.11, 149.86, 143.75, 139.69, 136.08, 136.03, 135.65, 133.05, 131.24, 130.98, 130.09, 127.96, 122.24, 121.42, 116.29, 111.88, 111.82 ppm; HRMS (ESI) m/z: [M + Na]+ calcd for C17H13NO4SNa 350.0457; found: 350.0453.
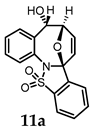
N-(2-Formylphenyl)-2-(thiophen-2-yl)benzenesulfonamide (8b). Following the general procedure B, compound 8b (190 mg, 83%) was obtained from the oxidation of 7b (230 mg) as a colorless red powder. 1H NMR (500 MHz, CDCl3): δ 10.32 (s, 1H), 9.66 (s, 1H), 8.42–8.36 (m, 1H), 7.56 (ddt, J = 10.2, 4.9, 2.0 Hz, 3H), 7.43 (d, J = 4.0 Hz, 1H), 7.41–7.33 (m, 2H), 7.28 (s, 1H), 7.27 (s, 1H), 7.16 (dd, J = 5.2, 3.5 Hz, 1H), 7.09 (t, J = 7.5 Hz, 1H). ppm; 13C NMR (126 MHz, CDCl3): δ 193.66, 139.35, 137.96, 137.76, 135.74, 135.45, 134.07, 133.72, 132.83, 130.83, 129.90, 128.22, 127.64, 127.12, 122.06, 121.11, 115.69 ppm; HRMS (ESI) m/z: [M + H]+ calcd for C17H14NO3S2 344.0409; found: 344.0416.
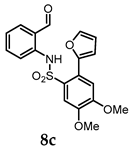
N-(2-Formylphenyl)-2-(furan-2-yl)-4,5-dimethoxybenzenesulfonamide (8c). Following the general procedure B, compound 8c (130 mg, 73%) was obtained from the oxidation of 7c (180 mg) as a white solid. 1H NMR (500 MHz, CDCl3): δ 10.90 (s, 1H), 9.77 (s, 1H), 7.79 (s, 1H), 7.58 (d, J = 8.4 Hz, 2H), 7.49 (d, J = 8.3 Hz, 1H), 7.43 (t, J = 7.9 Hz, 1H), 7.12 (t, J = 7.5 Hz, 1H), 7.01 (s, 1H), 6.92 (d, J = 3.4 Hz, 1H), 6.57 (dd, J = 3.5, 1.9 Hz, 1H), 4.02 (s, 3H), 3.93 (s, 3H) ppm; 13C NMR (126 MHz, CDCl3): 194.01, 152.12, 149.39, 148.05, 143.18, 139.70, 136.01, 135.55, 128.07, 124.08, 122.20, 121.36, 116.28, 113.65, 113.37, 111.84, 111.52, 56.51, 56.24 ppm; HRMS (ESI) m/z: [M + H]+ calcd for C19H18NO6S 388.0849; found: 388.0873.
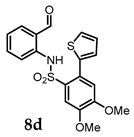
N-(2-Formylphenyl)-4,5-dimethoxy-2-(thiophen-2-yl)benzenesulfonamide (8d). Following the general procedure B, compound 8d (135 mg, 91%) was obtained from the oxidation of 7d (150 mg) as yellow solid. 1H NMR (500 MHz, CDCl3): δ 10.26 (s, 1H), 9.65 (s, 1H), 7.84 (s, 1H), 7.55 (d, J = 7.9 Hz, 1H), 7.42–7.32 (m, 3H), 7.25 (d, J = 8.5 Hz, 1H), 7.14–7.05 (m, 2H), 6.82 (s, 1H), 4.05 (s, 3H), 3.92–3.84 (m, 3H) ppm; 13C NMR (126 MHz, CDCl3): 193.63, 151.79, 148.26, 139.47, 137.82, 135.70, 135.43, 129.68, 127.54, 127.33, 126.75, 122.59, 121.94, 121.11, 116.04, 115.68, 113.29, 56.60, 56.25 ppm; HRMS (ESI) m/z: [M + Na]+ calcd for C19H17NO5S2Na 426.0440; found: 426.0441.
4.2.3. General Procedure for Synthesis of Keto Photoprecursors (General Procedure C)
The bromide (2.0 mmol, 1.0 equiv.), the corresponding boronic acid (3.0 mmol, 1.5 equiv.), PdCl2(PPh3)2 (0.07 mmol, 0.04 equiv.), and K2CO3 (8.0 mmol, 4.0 equiv.) are suspended in DMF:H2O (9:1) and heated to reflux in oil bath for 14 h. Upon completion of the reaction as indicated by 1H NMR, the mixture was diluted with EtOAc (30 mL) and washed with a saturated solution of NaHCO3 (2 × 40 mL). Combined organic phase was washed with H2O (20 mL) and dried over anhydrous Na2SO4. After concentration, the crude product was purified by flash chromatography on silica gel to give a desired keto photoprecursor.
2-(Furan-2-yl)-N-(8-oxo-5,6,7,8-tetrahydronaphthalen-1-yl)benzenesulfonamide (10c). Following the general procedure C, compound 10c (184 mg, 64%) was obtained from the suzuki coupling of 9b with 6a (300 mg) as white solid. 1H NMR (500 MHz, CDCl3): δ 12.16 (s, 1H), 8.31 (d, J = 8.0 Hz, 1H), 7.67–7.53 (m, 3H), 7.48 (t, J = 7.7 Hz, 1H), 7.33 (d, J = 8.4 Hz, 1H), 7.24 (t, J = 8.0 Hz, 1H), 6.98 (d, J = 3.4 Hz, 1H), 6.80 (d, J = 7.4 Hz, 1H), 6.60–6.55 (m, 1H), 2.90 (t, J = 6.1 Hz, 2H), 2.62 (t, J = 6.4 Hz, 2H), 2.04 (p, J = 6.3 Hz, 2H) ppm; 13C NMR (126 MHz, CDCl3): δ 201.78, 149.73, 146.35, 143.42, 140.74, 136.42, 134.46, 132.71, 131.06, 130.97, 130.07, 127.82, 122.30, 118.01, 114.63, 112.02, 111.86, 40.17, 30.76, 22.55 ppm; HRMS (ESI) m/z: [M + Na]+ calcd for C20H17NO4SNa 390.0770; found: 390.0758.
4.2.4. Irradiation of Photo Precursors (General Procedure D)
A solution of photo precursor (0.30 mmol) in DMSO (80 mL, unless otherwise mentioned) was degassed by bubbling of nitrogen or argon for 45 min. The solution was irradiated with UV LED-based illuminator, seven 2.9 W (total power 20.3 W) @ 365 nm LED Engin chips. After completion of the reaction (progress of the reaction was monitored by 1H NMR), the solvent was removed under vacuum, and a residue was subjected to purification by flash chromatography on silica gel to obtain photoproducts with moderate to good yields.
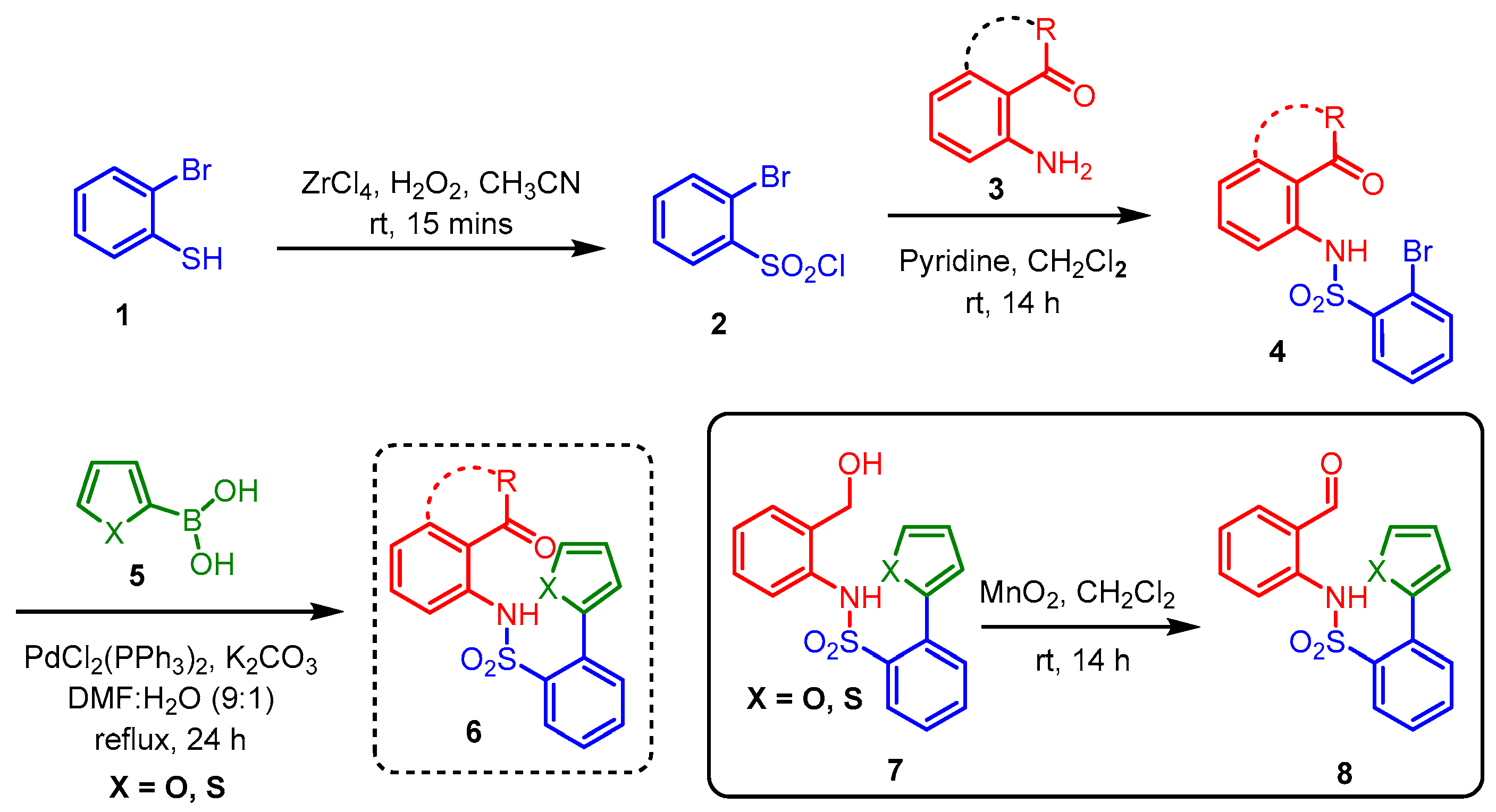
(5S,6S,8aS)-5-Hydroxy-5,6-dihydro-6,8a-epoxybenzo[g]benzo[4,5]isothiazolo[2,3-a]azocine 13,13-dioxide (11a). General procedure D was followed on 0.100 g (0.30 mmol) scale of photoprecursor 8a. After the photochemical reaction (irradiation time = 1 h), the crude product was purified by flash chromatography (SiO2, 0–40% ethyl acetate in hexanes) which afforded 87 mg (87%) of photoproduct (11a) as a white amorphous solid. 1H NMR (500 MHz, DMSO-d6) δ 8.08 (d, J = 7.6 Hz, 1H), 7.91–7.86 (m, 1H), 7.85–7.81 (m, 2H), 7.73 (dd, J = 5.9, 3.4 Hz, 1H), 7.65 (d, J = 7.4 Hz, 1H), 7.35 (dd, J = 6.1, 3.4 Hz, 2H), 6.79 (dd, J = 5.7, 1.8 Hz, 1H), 6.30 (d, J = 6.5 Hz, 1H), 6.12 (d, J = 5.7 Hz, 1H), 5.00 (s, 1H), 4.97 (dd, J = 6.5, 3.3 Hz, 1H) ppm; 13C NMR (126 MHz, DMSO-d6) δ 138.95, 137.44, 135.61, 135.13, 134.64, 132.30, 130.41, 129.03, 128.08, 126.95, 126.77, 126.26, 125.51, 121.37, 99.35, 84.95, 74.51 ppm; HRMS (ESI) m/z: [M + Na]+ calcd for C18H15NO4SNa 350.0457; found: 350.0455.
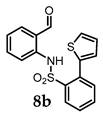
(4bR,7aS,8R)-8-Hydroxy-7a,8-dihydrobenzo[4,5]isothiazolo[2,3-a]thieno[2,3-b]quinoline 14,14-dioxide (11b). General procedure D was followed on 0.100 g (0.29 mmol) scale of photoprecursor 8b. After the photochemical reaction (irradiation time = 4 h), the crude product was purified by flash chromatography (SiO2, 0–60% ethyl acetate in hexanes) which afforded 81 mg (81%) of photoproduct (11b) as a brown solid. 1H NMR (500 MHz, CDCl3) δ 7.85 (dtd, J = 23.1, 16.9, 15.1, 7.9 Hz, 3H), 7.64 (s, 1H), 7.64–7.49 (m, 2H), 7.42 (q, J = 4.6 Hz, 2H), 6.24 (dd, J = 6.7, 2.7 Hz, 1H), 5.41 (dd, J = 6.7, 2.3 Hz, 1H), 5.05 (d, J = 5.7 Hz, 1H), 4.45 (dt, J = 5.7, 2.6 Hz, 1H), 2.40 (s, 1H; OH) ppm; 13C NMR (126 MHz, CDCl3) δ 140.12, 136.14, 134.27, 133.88, 130.91, 130.27, 128.28, 128.03, 127.28, 126.06, 125.53, 124.46, 121.36, 117.41, 82.49, 67.17, 66.48 ppm; HRMS (ESI) m/z: [M + Na]+ calcd for C17H13NO3S2Na 366.0229; found: 366.0242.
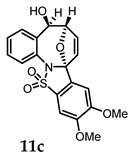
(5R,6R,8aR)-5-Hydroxy-10,11-dimethoxy-5,6-dihydro-6,8a-epoxybenzo[g]benzo[4,5]isothiazolo[2,3-a]azocine 13,13-dioxide (11c). General procedure D was followed on 0.100 g (0.26 mmol) scale of photoprecursor 8c. After the photochemical reaction (irradiation time = 2 h), the crude product was purified by flash chromatography (SiO2, 0–40% ethyl acetate in hexanes) which afforded 83 mg (83%) of photoproduct (11c) as a brown solid. 1H NMR (500 MHz, DMSO-d6) δ 8.08 (d, J = 7.6 Hz, 1H), 7.91–7.86 (m, 1H), 7.85–7.81 (m, 2H), 7.73 (dd, J = 5.9, 3.4 Hz, 1H), 7.65 (d, J = 7.4 Hz, 1H), 7.35 (dd, J = 6.1, 3.4 Hz, 2H), 6.79 (dd, J = 5.7, 1.8 Hz, 1H), 6.30 (d, J = 6.5 Hz, 1H), 6.12 (d, J = 5.7 Hz, 1H), 5.00 (s, 1H), 4.97 (dd, J = 6.5, 3.3 Hz, 1H) ppm; 13C NMR (126 MHz, DMSO-d6) δ 138.95, 137.44, 135.61, 135.13, 134.64, 132.30, 130.41, 129.03, 128.08, 126.95, 126.77, 126.26, 125.51, 121.37, 99.35, 84.95, 74.51 ppm; HRMS (ESI) m/z: [M + H]+ calcd for C19H18NO6S 388.0849; found: 388.0857.
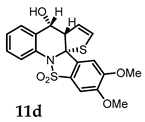
(4bR,7aS,8R)-8-Hydroxy-2,3-dimethoxy-7a,8-dihydrobenzo[4,5]isothiazolo[2,3-a]thieno[2,3-b]quinoline 14,14-dioxide (11d). General procedure D was followed on 0.100 g (0.24 mmol) scale of photoprecursor 8d. After the photochemical reaction (irradiation time = 8 h), the crude product was purified by flash chromatography (SiO2, 0–60% ethyl acetate in hexanes) which afforded 78 mg (78%) of photoproduct (11d) as a white amorphous solid. 1H NMR (500 MHz, CDCl3) δ 7.55 (dd, J = 6.4, 2.7 Hz, 1H), 7.50 (dd, J = 5.8, 3.0 Hz, 1H), 7.41 (dd, J = 6.2, 2.8 Hz, 2H), 7.24 (s, 1H), 7.13 (s, 1H), 6.23 (dd, J = 6.7, 2.8 Hz, 1H), 5.39 (dd, J = 6.6, 2.2 Hz, 1H), 5.04 (d, J = 5.7 Hz, 1H), 4.39 (dt, J = 5.5, 2.6 Hz, 1H), 4.03 (s, 3H), 4.00 (s, 3H) ppm; 13C NMR (126 MHz, CDCl3) δ 154.15, 151.26, 136.48, 132.70, 131.22, 128.24, 128.09, 127.28, 125.94, 125.88, 124.39, 117.56, 106.66, 102.21, 82.58, 67.11, 66.24, 56.66, 56.59 ppm; HRMS (ESI) m/z: [M + Na]+ calcd for C19H17NO5S2Na 426.0440; found: 426.0442.
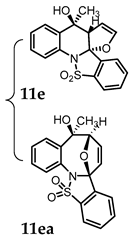
(4bR,7aS,8S)-8-Hydroxy-8-methyl-7a,8-dihydrobenzo[4,5]isothiazolo[2,3-a]furo[2,3-b]quinoline 14,14-dioxide (11e). General procedure D was followed on 0.100 g (0.29 mmol) scale of photoprecursor 10a. After the photochemical reaction (irradiation time = 5 h), the crude product was purified by flash chromatography (SiO2, 0–40% ethyl acetate in hexanes) which afforded 79 mg (79%) of photoproduct (11e) and 14 mg (14%) of photoproduct (11ea) as a white solid. 1H NMR (500 MHz, CDCl3) δ 7.90 (d, J = 7.9 Hz, 1H), 7.81 (t, J = 7.8 Hz, 1H), 7.69 (q, J = 8.3, 7.8 Hz, 3H), 7.49 (q, J = 7.8 Hz, 2H), 7.38 (t, J = 7.5 Hz, 1H), 6.47–6.42 (m, 1H), 4.83 (s, 1H), 4.20 (s, 1H), 2.99 (s, 1H; OH), 1.75 (s, 3H) ppm; 13C NMR (126 MHz, CDCl3) δ 147.12, 138.79, 135.71, 134.40, 133.14, 131.81, 131.15, 129.94, 127.79, 125.90, 125.71, 124.51, 121.55, 100.29, 97.35 70.39, 63.44, 24.59 ppm; HRMS (ESI) m/z: [M + Na]+ calcd for C18H15NO4SNa 364.0614; found: 364.0599.
(5R,6S,8aS)-5-Hydroxy-5-methyl-5,6-dihydro-6,8a-epoxybenzo[g]benzo[4,5]isothiazolo[2,3-a]azocine 13,13-dioxide (11ea). 1H NMR (500 MHz, CDCl3) δ 7.96 (d, J = 7.3 Hz, 1H), 7.75 (dt, J = 13.0, 7.8 Hz, 3H), 7.64 (d, J = 7.8 Hz, 1H), 7.59 (d, J = 7.2 Hz, 1H), 7.37 (dt, J = 18.7, 7.4 Hz, 2H), 6.62 (d, J = 5.5 Hz, 1H), 5.88 (d, J = 5.8 Hz, 1H), 4.91 (s, 1H), 3.62 (s, 1H; OH), 1.81 (s, 3H) ppm; 13C NMR (126 MHz, CDCl3) δ 138.62, 138.33, 135.98, 135.24, 133.54, 131.35, 130.13, 129.23, 129.02, 128.20, 127.74, 125.47, 124.35, 121.36, 99.40, 89.60, 78.06, 24.62 ppm; HRMS (ESI) m/z: [M + Na]+ calcd for C18H15NO4SNa 364.0614; found: 364.0593.
(4bR,7aS,8R)-8-Hydroxy-8-methyl-7a,8-dihydrobenzo[4,5]isothiazolo[2,3-a]thieno[2,3-b]quinoline 14,14-dioxide (11f). General procedure D was followed on 0.100 g (0.28 mmol) scale of photoprecursor 10b. After the photochemical reaction (irradiation time = 3 h), the crude product was purified by flash chromatography (SiO2, 0–60% ethyl acetate in hexanes) which afforded 78 mg (78%) of photoproduct (11f) as a white solid. 1H NMR (500 MHz, CDCl3) δ 7.82–7.74 (m, 3H), 7.61 (dd, J = 13.3, 7.4 Hz, 2H), 7.47 (d, J = 7.5 Hz, 2H), 7.36 (t, J = 7.7 Hz, 1H), 6.22 (dd, J = 6.9, 3.1 Hz, 1H), 5.13 (d, J = 6.7 Hz, 1H), 4.36 (s, 1H), 1.80 (s, 3H) ppm; 13C NMR (126 MHz, CDCl3) δ 142.08, 136.61, 134.03, 132.53, 132.21, 130.03, 129.97, 127.84, 127.16, 126.31, 125.75, 125.62, 121.48, 118.29, 82.62, 71.21, 71.02, 25.61 ppm; HRMS (ESI) m/z: [M + Na]+ calcd for C18H15NO3S2Na 380.0385; found: 380.0392.
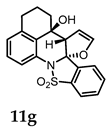
(3aR,15aS,15bS)-15a-Hydroxy-14,15,15a,15b-tetrahydro-13H-benzo[de]benzo[4,5]isothiazolo[2,3-a]furo[2,3-b]quinoline 8,8-dioxide (11g). General procedure D was followed on 0.100 g (0.27 mmol) scale of photoprecursor 10c. After the photochemical reaction (irradiation time = 2 h), the crude product was purified by flash chromatography (SiO2, 0–50% ethyl acetate in hexanes) which afforded 91 mg (91%) of photoproduct (11g) as a pale-yellow solid. 1H NMR (500 MHz, CDCl3) δ 7.89 (d, J = 7.8 Hz, 1H), 7.81 (t, J = 7.6 Hz, 1H), 7.75–7.66 (m, 2H), 7.50 (d, J = 7.7 Hz, 1H), 7.35 (t, J = 7.8 Hz, 1H), 7.16 (d, J = 7.8 Hz, 1H), 6.47 (t, J = 3.0 Hz, 1H), 4.87 (t, J = 2.7 Hz, 1H), 4.20 (t, J = 2.6 Hz, 1H), 3.03 (s, 1H; OH), 2.87–2.81 (m, 1H), 2.75 (ddd, J = 16.9, 12.1, 5.2 Hz, 1H), 2.10–2.00 (m, 2H), 1.91–1.85 (m, 1H), 1.84–1.76 (m, 1H) ppm; 13C NMR (126 MHz, CDCl3) δ 146.94, 139.24, 138.93, 134.35, 133.08, 131.69, 131.07, 130.77, 129.11, 128.63, 124.54, 123.22, 121.55, 100.52, 97.43, 68.61, 61.98, 34.59, 29.54, 18.15 ppm; HRMS (ESI) m/z: [M + Na]+ calcd for C20H17NO4SNa 390.0770; found: 390.0780.
4.2.5. Postphotochemical Modifications (General Procedure E)
Typically, 1 equiv. photoproduct and 1 equiv. 1,3-dicarbonyl compound were dissolved in 0.7 mL dry acetonitrile. To this solution, 0.08 equiv. L-proline and 1.3 equiv. 37% aqueous formaldehyde solution were added. The reaction was stirred at ambient temperature until complete consumption of the photoproduct, as determined by 1H NMR analysis. The reaction was diluted with water and extracted with EtOAc. The organic layer was separated, dried over Na2SO4, and concentrated under vacuum. The mixture was then purified by flash chromatography.
(5aR,17aS,17bS)-17a-Hydroxy-2-methylene-1,2,4a,16,17,17a,17b,17c-octahydro-3H,15H-benzo[de]benzo[4,5]isothiazolo[2,3-a]pyrano[3′,2′:4,5]furo[2,3-b]quinolin-3-one 10,10-dioxide (12). General procedure E was followed using 50 mg (11g; 0.13 mmol), 20 mg of Meldrum’s acid (0.13 mmol), 3.0 mg L-proline (0.04 mmol), and 0.03 mL formaldehyde solution (37% w/w) in water (0.11 mmol) to generate the title compound 12 (33 mg, 54%). 1H NMR (500 MHz, CDCl3) δ 7.84 (d, J = 7.9 Hz, 1H), 7.79 (t, J = 7.7 Hz, 1H), 7.66 (t, J = 7.6 Hz, 1H), 7.61 (d, J = 7.9 Hz, 1H), 7.48 (d, J = 7.8 Hz, 1H), 7.42 (t, J = 7.8 Hz, 1H), 7.21 (d, J = 7.7 Hz, 1H), 6.54 (s, 1H), 5.84 (d, J = 5.7 Hz, 1H), 5.77 (s, 1H), 3.32 (d, J = 10.5 Hz, 1H), 3.02 (s, 1H; OH), 2.95–2.85 (m, 2H), 2.85–2.74 (m, 2H), 2.40 (dtd, J = 11.1, 5.7, 3.0 Hz, 1H), 2.04–1.93 (m, 2H), 1.92–1.84 (m, 1H), 1.72 (td, J = 13.1, 2.8 Hz, 1H) ppm; 13C NMR (126 MHz, CDCl3) δ 164.41, 139.52, 138.85, 134.83, 132.54, 131.58, 131.38, 131.04, 130.04, 129.90, 129.22, 129.06, 124.83, 124.01, 121.49, 103.54, 98.87, 69.47, 58.18, 40.54, 34.87, 31.24, 29.51, 17.86 ppm.
(5aR,17aS,17bS)-17a-Hydroxy-2-methylene-1,2,4a,16,17,17a,17b,17c-octahydro-3H,15H-benzo[de]benzo[4,5]isothiazolo[2,3-a]pyrano[3′,2′:4,5]furo[2,3-b]quinolin-3-one 10,10-dioxide (13). General procedure E was followed using 50 mg (11g; 0.13 mmol), 22 mg of 1,3-dimethylbarbituric acid (0.13 mmol), 3.0 mg L-proline (0.04 mmol), and 0.03 mL formaldehyde solution (37% w/w) in water (0.11 mmol) to generate the title compound 13 (32 mg, 43%). 1H NMR (500 MHz, CDCl3) δ 7.88 (d, J = 7.7 Hz, 1H), 7.76 (t, J = 7.6 Hz, 1H), 7.69 (t, J = 7.6 Hz, 1H), 7.50 (d, J = 7.8 Hz, 1H), 7.45–7.38 (m, 2H), 7.21 (d, J = 7.8 Hz, 1H), 5.77 (d, J = 4.8 Hz, 1H), 3.46 (s, 3H), 3.42 (s, 3H), 3.27 (d, J = 11.2 Hz, 1H), 3.02 (d, J = 17.1 Hz, 1H), 2.88 (d, J = 16.5 Hz, 1H), 2.77–2.69 (m, 2H), 2.47–2.39 (m, 1H), 1.99 (d, J = 11.0 Hz, 1H), 1.92 (s, 3H) ppm; 13C NMR (126 MHz, CDCl3) δ 162.59, 153.72, 151.03, 139.83, 138.91, 134.19, 133.10, 131.13, 131.00, 129.82, 129.51, 128.84, 124.10, 123.05, 121.77, 103.55, 99.41, 83.96, 69.70, 57.53, 39.90, 35.40, 29.68, 28.80, 28.17, 19.99, 18.03 ppm.