A Simple Schiff Base Probe for Quintuplicate-Metal Analytes with Four Emission-Wavelength Responses
Abstract
1. Introduction
2. Results
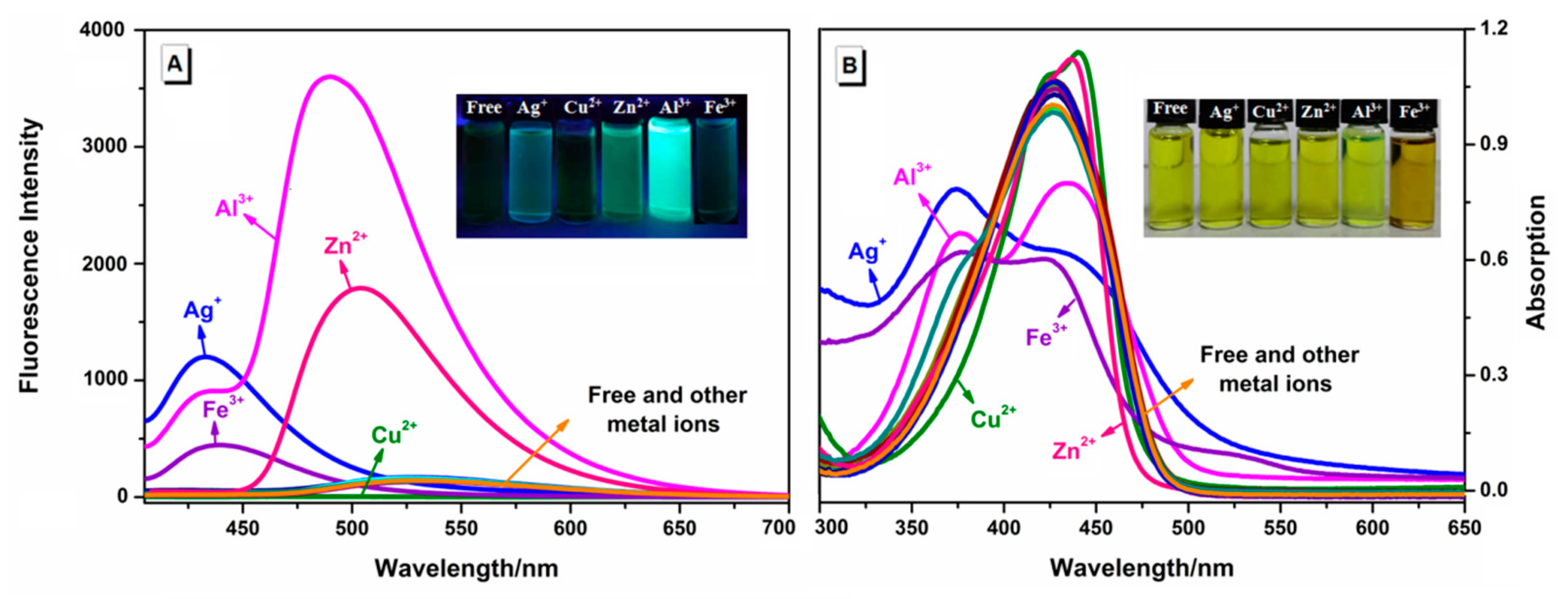
2.1. The Selectivity Ability
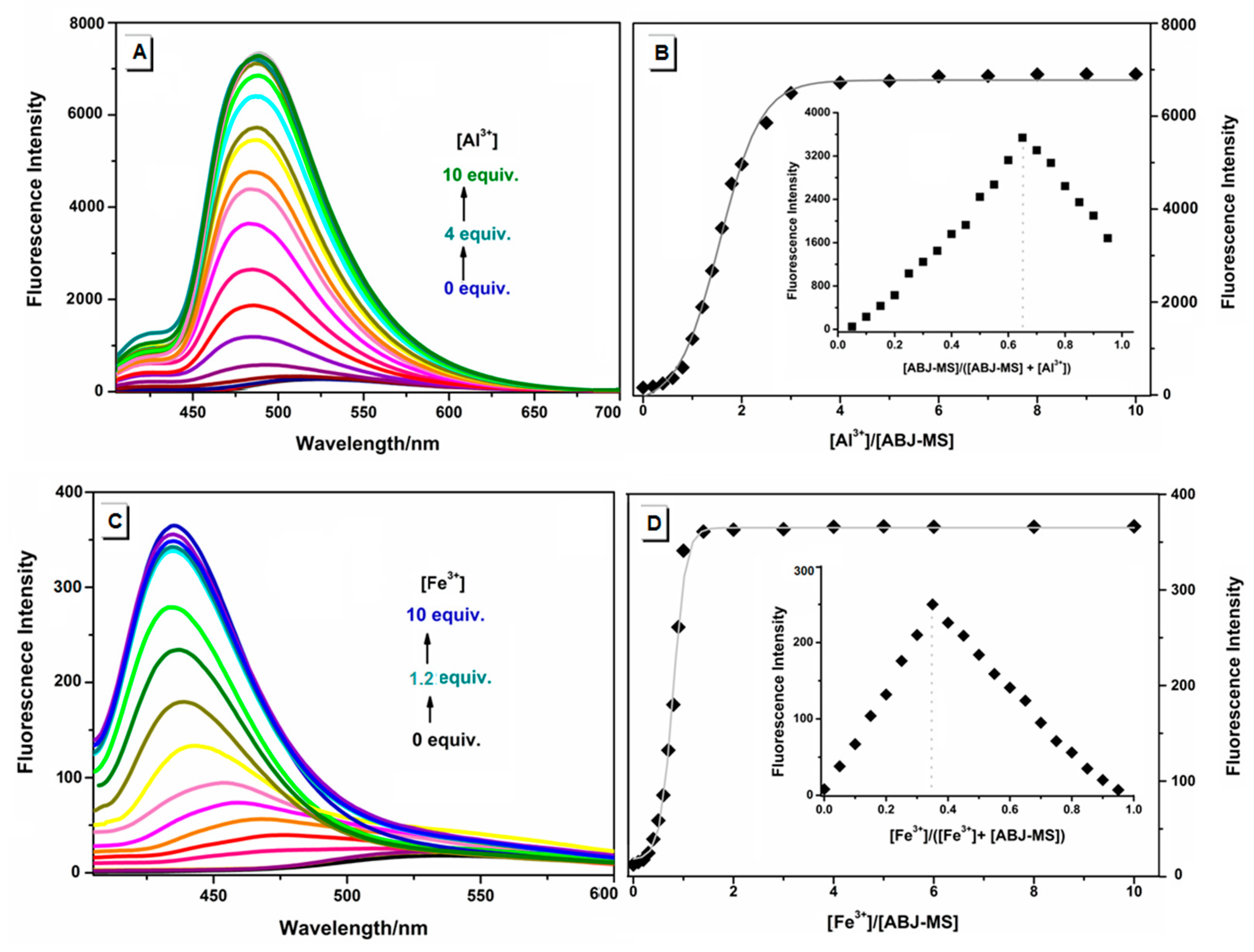
2.2. The Responsive Metal Sensing
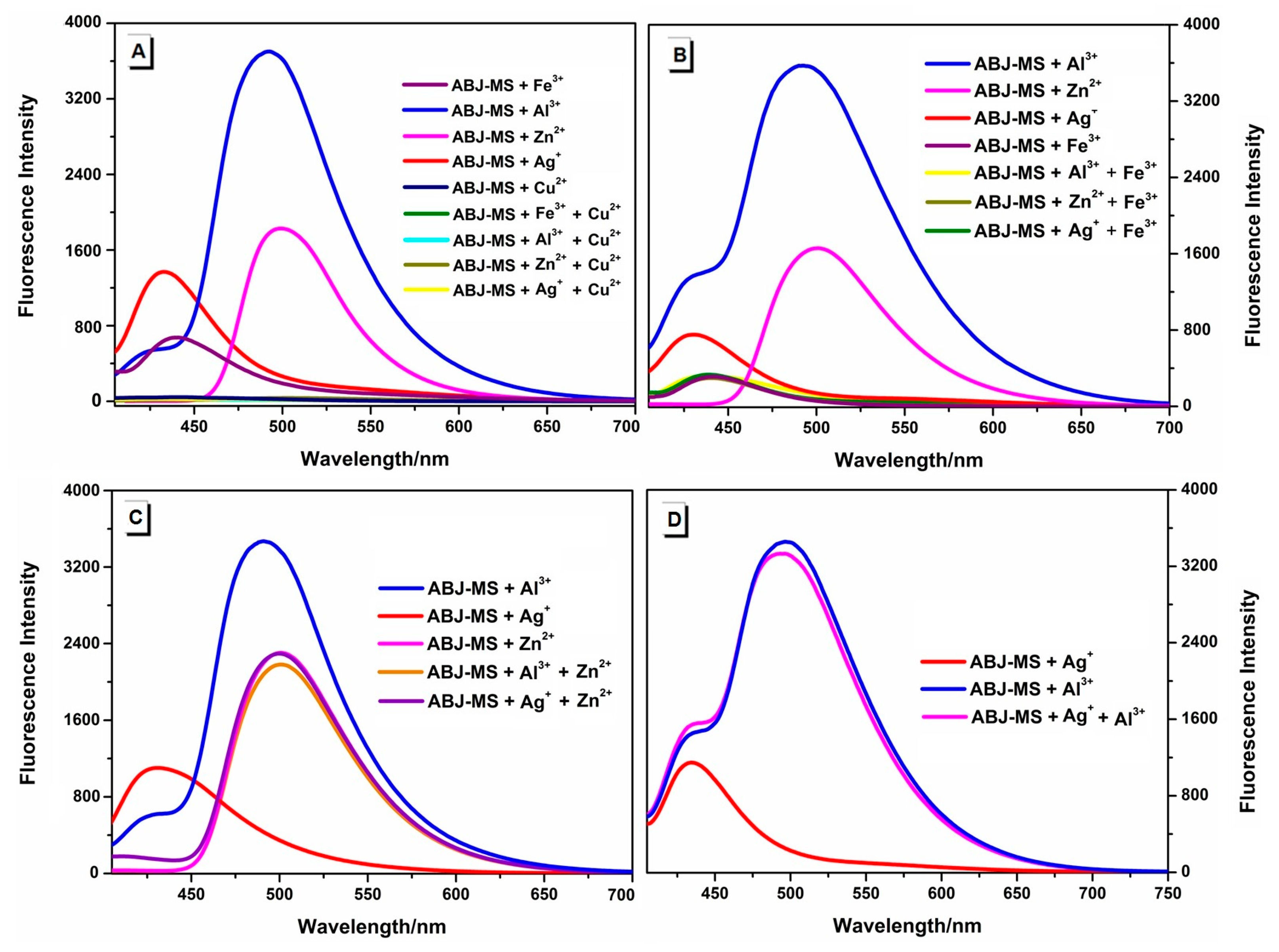
2.3. Competition Experiment
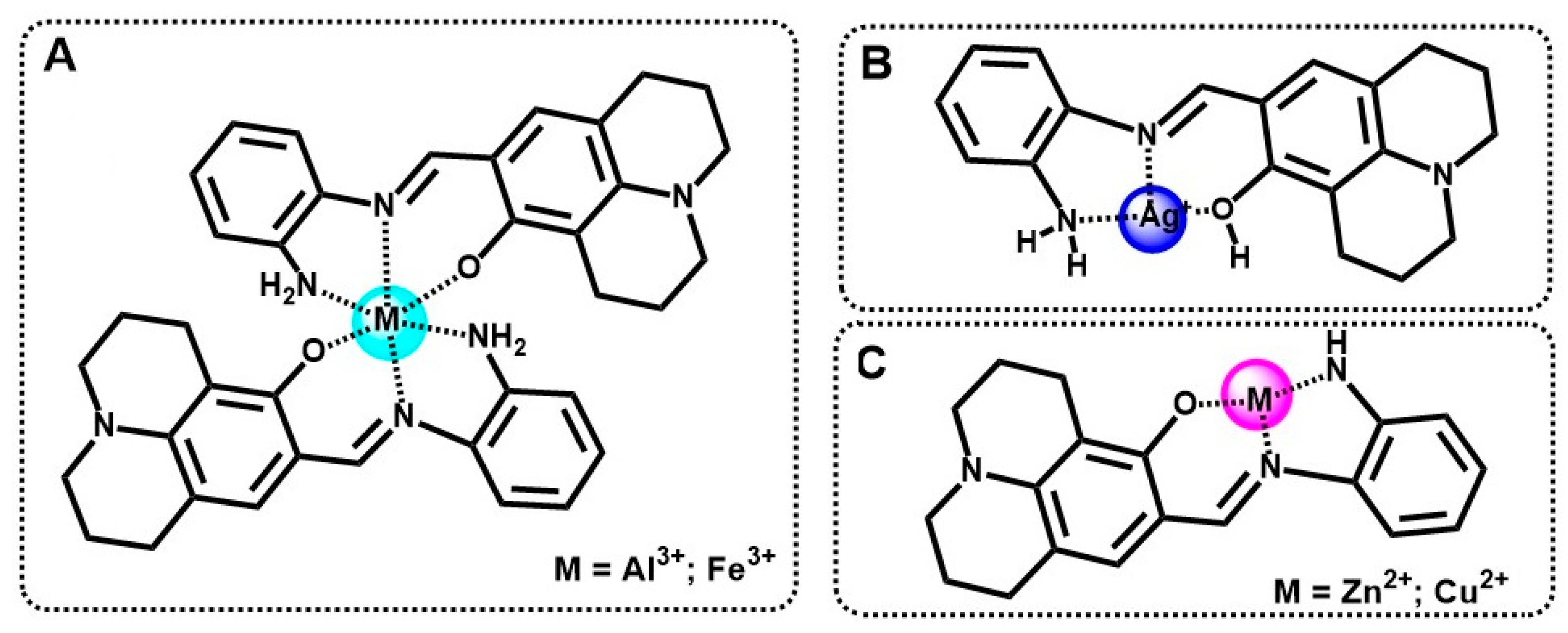
2.4. The Sensing Mechanism
2.5. Application of ABJ-MS in Zn2+/Al3+/Fe3+ Analysis in Water Samples
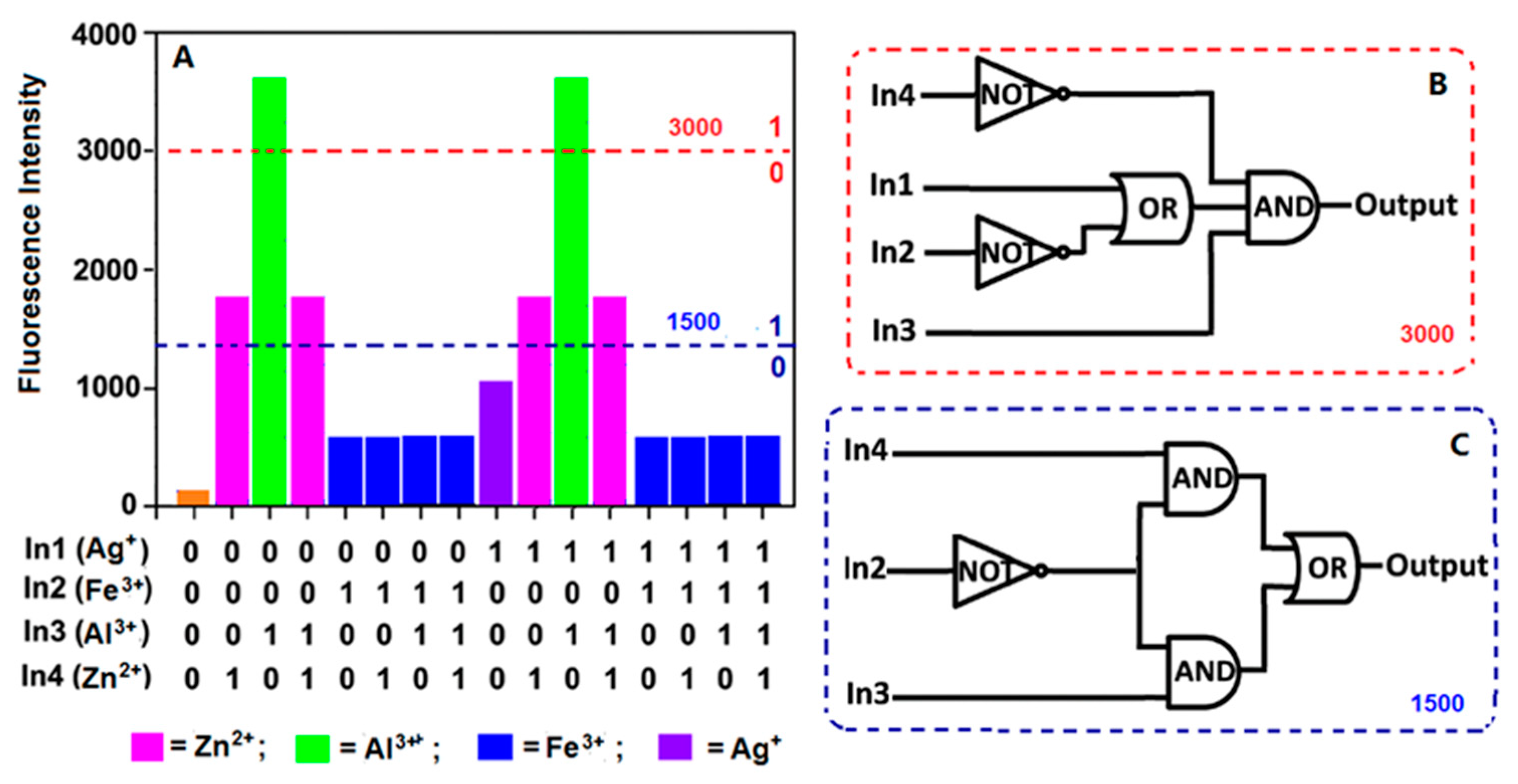
2.6. The Construction of the Logic Gate
3. Materials and Methods
3.1. Chemicals and Instruments
3.2. Preparation of Mono-Schiff Probe Consisting of o-Aminobenzene-Hydroxyjulolidine (ABJ-MS)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Zattaa, P.; Frank, A. Copper deficiency and neurological disorders in man and animals. Brain Res. Rev. 2007, 54, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, S.K.; Sharma, D.; Bera, R.K.; Crisponic, G.; Callan, J.F. Iron (III) selective molecular and supramolecular fluorescent probes. Chem. Soc. Rev. 2012, 41, 7195–7227. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.D.; Banasiewicz, M.; Wrzosek, A.; O’Mari, O.; Zochowska, M.; Vullev, V.I.; Jacquemin, D.; Szewczyk, A.; Gryko, D.T. A sensitive zinc probe operating via enhancement of excited-state intramolecular charge transfer. Org. Biomol. Chem. 2022, 20, 7439–7447. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Huang, Y.; Lu, A.; Wang, Z.; Li, H. A Highly Selective and Sensitive Sequential Recognition Probe Zn2+ and H2PO4− Based on Chiral Thiourea Schiff Base. Molecules 2023, 28, 4166. [Google Scholar] [CrossRef] [PubMed]
- Mohan, B.; Kunhumon, N.M.; Shanmugaraju, S. Fluorescence sensing and bioimaging of Cu(II) ions using amino-1,8-naphthalimide-based small-molecule chemosensors. Sens. Diagn. 2023. [Google Scholar] [CrossRef]
- Rouault, T.A. The role of iron regulatory proteins in mammalian iron homeostasis and disease. Nat. Chem. Biol. 2006, 2, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Xu, Y.; Du, J.; Guan, Q.; Cai, X.; Li, F.; Wang, J.; Chen, W. Fluorescence Sensor based on Cascade Signal Amplification Strategy for Ultra-sensitive detection of Cu2+. Nanoscale 2023, 15, 1806–1812. [Google Scholar] [CrossRef]
- Mu, Y.Q.; Fan, H.H.; Li, M.Y.; Wang, R.J.; Chen, Z.; Fan, C.B.; Liu, G.; Pu, S.Z. Multiresponsive tetrarylethylene-based fluorescent dye with multicoloreded changes: AIEE properties, acidichromism, Al3+ recognition, and applications. J. Mater. Chem. B 2022, 10, 9235–9248. [Google Scholar] [CrossRef]
- Zhang, C.; Han, Z.; Wang, M.; Yang, Z.; He, W.; Ran, X. A New BODIPY-Derived Ratiometric Senor of Internal Charge Transfer (ICT) Effect: Colorimetric/Fluorometric Sensing for Ag+. Dalton Trans. 2018, 47, 2285–2291. [Google Scholar] [CrossRef]
- Lai, L.; Yan, F.; Chen, G.; Huang, Y.; Huang, L.; Li, D. Recent Progress on Fluorescent Probes in Heavy Metal Determinations for Food Safety: A Review. Molecules 2023, 28, 5689. [Google Scholar] [CrossRef]
- Prajapati, S.; Sinha, P.; Hindore, S.; Jana, S. Selective turn-on fluorescence sensing of Fe2+ in real water samples by chalcones. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2023, 287, 122107. [Google Scholar]
- Park, S.H.; Kwon, N.; Lee, J.H.; Yoon, J.; Shin, I. Synthetic ratiometric fluorescent probes for detection of ions. Chem. Soc. Rev. 2020, 49, 143–179. [Google Scholar] [CrossRef] [PubMed]
- Deng, F.; Liu, L.; Qiao, Q.L.; Huang, C.F.; Miao, L.; Xu, Z.C. A general strategy to develop cell membrane fluorescent probes with location- and target-specific fluorogenicities: A case of a Zn2+ probe with cellular selectivity. Chem. Commun. 2019, 55, 15045–15048. [Google Scholar] [CrossRef] [PubMed]
- Gond, S.; Yadav, P.; Singh, A.; Garai, S.; Shekher, A.; Guptab, S.C.; Singh, V.P. A colorimetric and ‘OFF–ON’ fluorometric chemosensor based on a rhodamine-pyrazole derivative for the detection of Al3+, Fe3+ and Cr3+ metal ions, and its intracellular application. Org. Biomol. Chem 2023, 21, 4482–4490. [Google Scholar] [CrossRef] [PubMed]
- Kolanowski, J.L.; Liu, F.; New, E.J. Fluorescent probes for the simultaneous detection of multiple analytes in biology. Chem. Soc. Rev. 2018, 47, 195–208. [Google Scholar] [CrossRef] [PubMed]
- Sedgwick, A.C.; Wu, L.; Han, H.H.; Bull, S.D.; He, X.P.; James, T.D.; Sessler, J.L.; Tang, B.Z.; Tian, H.; Yoon, J. Excited-state intramolecular proton-transfer (ESIPT) based fluorescence sensors and imaging agents. Chem. Soc. Rev. 2018, 47, 8842–8880. [Google Scholar]
- Zhao, J.; Lian, J.; Pan, W.; Du, J.; Liu, Z.; Zhao, L. A Bifunctional Fluorescence Probe Based on AIE-ICT Strategy for Visual Detection of Cu2+/Co2+ in Complex Matrix. Molecules 2023, 28, 2059. [Google Scholar] [CrossRef]
- He, L.; Dong, B.; Liu, Y.; Lin, W. Fluorescent chemosensors manipulated by dual/triple interplaying sensing mechanisms. Chem. Soc. Rev. 2016, 45, 6449–6461. [Google Scholar] [CrossRef]
- Wang, F.; Li, C.H.; Zhang, X.L.; Wang, A.L.; Zhou, L.W.; Jia, C.X.; Xu, J.; Chen, Y.T. A smart single molecular probe for Cu2+/Fe3+/Mg2+ by three-emission behaviors. Dyes Pigments 2019, 171, 107667. [Google Scholar] [CrossRef]
- Pundi, A.; Chang, C.J.; Chen, Y.S.; Chen, J.K.; Yeh, J.M.; Zhuang, C.S.; Lee, M.C. An aniline trimer-based multifunctional sensor for colorimetric Fe3+, Cu2+ and Ag+ detection, and its complex for fluorescent sensing of L-tryptophan. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 247, 119075. [Google Scholar] [CrossRef]
- Zhong, X.; Li, Z.K.; Shi, R.F.; Yan, L.; Zhu, Y.H.; Li, H. Schiff base-modified nanomaterials for ion detection: A review. ACS Appl. Nano Mater. 2022, 10, 13998–14020. [Google Scholar] [CrossRef]
- Wang, J.F.; Meng, Q.Y.; Yang, Y.Y.; Zhong, S.L.; Zhang, R.T.; Fang, Y.H.; Gao, Y.; Cui, X.J. Schiff Base Aggregation-Induced Emission Luminogens for Sensing Applications: A Review. ACS Sens. 2022, 7, 2521–2536. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.T.; Pan, H.H.; Wang, F.; Zhao, Y.Y.; Yin, H.Y.; Chen, Y.X.; Zhang, J.L.; Jiang, J.Z. An Ultrafast BODIPY Single Molecular Sensor for Multi-analytes (Acid/Base/Cu2+/Bi3+) with Different Sensing Mechanism. Dyes Pigments 2019, 165, 279–286. [Google Scholar] [CrossRef]
- Komatsu, H.; Citterio, D.; Fujiwara, Y.; Minamihashi, K.; Araki, Y.; Hagiwara, M.; Suzuki, K. Single molecular multianalyte probe: Jewel pendant ligand. Org. Lett. 2005, 7, 2857–2859. [Google Scholar] [CrossRef]
- Bozdemir, O.A.; Guliyev, R.; Buyukcakir, O.; Selcuk, S.; Kolemen, S.; Gulseren, G.; Nalbantoglu, T.; Boyaci, H.; Akkaya, E.U. Selective Manipulation of ICT and PET Processes in Styryl-Bodipy Derivatives: Applications in Molecular Logic and Fluorescence Sensing of Metal Ions. J. Am. Chem. Soc. 2010, 132, 8029–8036. [Google Scholar] [CrossRef]
- Chen, Y.T.; Wan, L.; Li, W.J.; Bian, Y.Z.; Jiang, J.Z. Rational design and synthesis for versatile FRET ratiometric probe for Hg2+ and Fe2+: A flexible 8-hydroxyquinoline-benzoate-linked BODIPY-porphyrin dyad. Org. Lett. 2011, 13, 5774–5777. [Google Scholar] [CrossRef]
- Ansari, S.N.; Saini, A.K.; Kumari, P.; Mobin, S.M. An imidazole derivative-based chemodosimeter for Zn2+ and Cu2+ ions through “ON-OFF-ON” switching with intracellular Zn2+ detection. Inorg. Chem. Front. 2019, 6, 736–745. [Google Scholar] [CrossRef]
- Wang, J.F.; Li, Y.B.; Patel, N.G.; Zhang, G.; Zhou, D.; Pang, Y. A single molecular probe for multi-analyte (Cr3+, Al3+ and Fe3+) detection in aqueous medium and its biological application. Chem. Commun. 2014, 50, 12258–12261. [Google Scholar] [CrossRef]
- Zhang, J.Z.; Zhao, Z.; Shang, H.; Liu, Q.S.; Liu, F. An easy-to-synthesize multi-photoresponse smart sensor for fast detecting Zn2+ and quantifying Fe3+ based on the enol/keto binding mode. New J. Chem. 2019, 43, 14179–14189. [Google Scholar] [CrossRef]
- Zhang, X.L.; Guo, X.H.; Yuan, H.H.; Jia, X.; Dai, B. One-pot synthesis of a natural phenol derived fluorescence sensor for Cu(II) and Hg(II) detection. Dyes Pigments 2018, 155, 100–106. [Google Scholar] [CrossRef]
- Sobczyk, L.; Chudoba, D.; Tolstoy, P.M.; Filarowski, A. Some brief notes on theoretical and experimental investigations of intramolecular hydrogen bonding. Molecules 2016, 21, 1657. [Google Scholar] [CrossRef] [PubMed]
- Jezierska, A.; Tolstoy, P.M.; Panek, J.J.; Filarowski, A. Intramolecular hydrogen bonds in selected aromatic compounds: Recent developments. Catalysts 2019, 9, 909. [Google Scholar] [CrossRef]
- Ortiz-Sánchez, J.M.; Gelabert, R.; Moreno, M.; Lluch, J.M. Electronic-structure and quantum dynamical study of the photochromism of the aromatic Schiff base salicylideneaniline. J. Chem. Phys. 2008, 129, 214308–214314. [Google Scholar] [CrossRef]
- Liu, H.; Liu, T.; Li, J.; Zhang, Y.; Li, J.; Song, J.; Qu, J.; Wong, W. A simple Schiff base as dual-responsive fluorescent sensor for bioimaging recognition of Zn2+ and Al3+ in living cells. J. Mater. Chem. B 2018, 6, 5435–5442. [Google Scholar] [CrossRef]
- Mathivanan, M.; Tharmalingam, B.; Lin, C.H.; Pandiyan, B.V.; Thiagarajan, V.; Murugesapandian, B. ESIPT-active multi-color aggregation-induced emission features of triphenylamine–salicylaldehyde-based unsymmetrical azine family. Cryst. Eng. Comm. 2020, 22, 213–228. [Google Scholar] [CrossRef]
- Liu, S.D.; Zhang, L.W.; Zan, W.Y.; Yao, X.J.; Yang, Y.; Liu, X. A novel HBT-based Schiff base for colorimetric detection of aluminum: Synthesis, characterization, spectral and DFT computational studies. Sens. Actuators B 2014, 192, 386–392. [Google Scholar] [CrossRef]
- Kumar, V.; Kumar, A.; Diwan, U.; Ramesh, S.; Srivastava, S.K.; Upadhyay, K.K. Salicylideneimines as efficient dual channel emissive probes for Al3+: Harnessing ESIPT and ICT processes. Sens. Actuators B 2015, 207, 650–657. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, Y.T.; Jiang, J.Z. Solid state fluorescent functionalized-triphenylamine Bodipy detector for HCl vapor with high stability and absolute fluorescent quantum yield. Dyes Pigments 2016, 124, 110–119. [Google Scholar] [CrossRef]
- Dumur, F.; Contal, E.; Wantz, G.; Gigmes, D. Photoluminescence of zinc complexes: Easily tunable optical properties by variation of the bridge between the imido groups of schiff base ligands. Eur. J. Inorg. Chem. 2014, 2014, 4186–4198. [Google Scholar] [CrossRef]
- Chen, Y.T.; Zhao, L.Y.; Jiang, J.Z. The naphthoate-modifying Cu2+-detective Bodipy sensors with the fluorescent ON-OFF performance unaffected by molecular configuration. Spectrochim. Acta. Part A 2017, 175, 269–275. [Google Scholar] [CrossRef]
- Zhou, X.F.; Su, F.Y.; Tian, Y.Q.; Youngbull, C.; Johnson, R.H.; Meldrum, D.R. A New Highly Selective Fluorescent K+ Sensor. J. Am. Chem. Soc. 2011, 133, 18530–18533. [Google Scholar] [CrossRef]
- Jung, H.S.; Park, M.; Han, D.Y.; Kim, E.; Lee, C.; Ham, S.; Kim, J.S. Cu2+ Ion-Induced Self-Assembly of Pyrenylquinoline with a Pyrenyl Excimer Formation. Org. Lett. 2009, 11, 3378–3391. [Google Scholar] [CrossRef]
- Huo, Y.P.; Wang, S.Y.; Lu, T.H.; Pan, C.Q.; Lu, Y.J.; Yang, X.H.; Hua, D.P.; Hua, S. Highly selective and sensitive colorimetric chemosensors for Hg2+ based on novel diaminomaleonitrile derivatives. RSC Adv. 2016, 6, 5503–5511. [Google Scholar] [CrossRef]
- Nandre, J.P.; Patil, S.R.; Sahoo, S.K.; Pradeep, C.P.; Churakov, A.; Yu, F.; Chen, L.; Redshaw, C.; Patil, A.A.; Patil, U.D. A chemosensor for micro- to nano-molar detection of Ag+ and Hg2+ ions in pure aqueous media and its applications in cell imaging. Dalton Trans. 2017, 46, 14201–14209. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, X.; Wang, K.; Zhang, X. A Benzo-15-crown-5-modifying ratiometric-absorption and fluorescent OFF-ON chemoprobe for Cu2+. Spectrochim. Acta. A 2016, 161, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Toullec, J.; Dubois, J.E. Methylidyne-methylidyne-d and water-water-d2 isotope effects on the forward and reverse rates of keto-enol tautomerization of acetone in acidic media. J. Am. Chem. Soc. 1974, 96, 3524–3532. [Google Scholar] [CrossRef]
- Zimmermann, H.E. Kinetic protonation of enols, enolates, and analogs. The stereochemistry of ketonization. Acc. Chem. Res. 1987, 20, 263–268. [Google Scholar] [CrossRef]
- Ziółek, M.; Burdziński, G.; Filipczak, K.; Karolczak, J.; Maciejewski, A. Spectroscopic and photophysical studies of the hydroquinone family of photochromic Schiff bases analyzed over a 17-orders-of-magnitude time scale. Phys. Chem. Chem. Phys. 2008, 10, 1304–1318. [Google Scholar] [CrossRef]
- Zhu, M.L.; Xing, P.P.; Zhou, Y.B.; Gong, L.; Zhang, J.J.; Qi, D.D.; Bian, Y.Z.; Du, H.W.; Jiang, J.Z. Lysosome-targeting ratiometric fluorescent pH probes based on long-wavelength BODIPY. J. Mater. Chem. B 2018, 6, 4422–4426. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, V.; Upadhyay, K.K. An Al3+ and H2PO4−/HSO4− selective conformational arrest and bail to a pyrimidine-naphthalene anchored molecular switch. Analyst 2013, 138, 1891–1897. [Google Scholar] [CrossRef]
- Wang, W.; Wen, Q.; Zhang, Y.; Fei, X.; Li, Y.; Yang, Q.; Xu, X. Simple naphthalimide-based fluorescent sensor for highly sensitive and selective detection of Cd2+ and Cu2+ in aqueous solution and living cells. Dalton Trans. 2013, 42, 1827–1833. [Google Scholar] [CrossRef]
- Zhu, M.; Yuan, M.; Liu, X.; Xu, J.L.; Lv, J.; Huang, C.S.; Liu, H.B.; Li, Y.L.; Wang, S.; Zhu, D.B. Visible near-infrared chemosensor for mercury ion. Org. Lett. 2008, 10, 1481–1484. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Pang, Y. A simple sensitive ESIPT on-off fluorescent sensor for selective detection of Al3+ in water. RSC Adv. 2014, 4, 5845–5848. [Google Scholar] [CrossRef] [PubMed]
- Broring, M.; Kruger, R.; Link, S.; Kleeberg, C.; Kohler, S.; Xie, X.; Ventura, B.; Flamigni, L. Bis(BF2)-2,2’-bidipyrrins (BisBODIPYs): Highly fluorescent BODIPY dimers with large stokes shifts. Chem. Eur. J. 2008, 14, 2976–2983. [Google Scholar] [PubMed]
- Chai, X.; Fu, Y.X.; James, T.D.; Zhang, J.; He, X.P.; Tian, H. Photochromism and molecular logic gate operation of a water-compatible bis-glycosyl diarylethene. Chem. Commun. 2017, 53, 9494–9497. [Google Scholar] [CrossRef]
- Singh, A.K.; Yadav, P.K.; Kumari, N.; Nagarajan, R.; Mishra, L. A light/pH/multiple ion-driven smart switchable module for computing sequential logic operations via a resettable dual-optical readout. J. Mater. Chem. C 2015, 3, 12123–12129. [Google Scholar] [CrossRef]



| Sample | Zn2+ added (μM) | Zn2+ found(μM) | Recovery (%) | R.S.D. a (%) |
| River water | 0.00 | 0.00 | ||
| 10.00 | 10.06 | 101 | 0.6 | |
| Artificial water b | 0.00 | 10.05 | 101 | 0.5 |
| 10.00 | 20.28 | 102 | 1.4 | |
| Sample | Al3+ added (μM) | Al3+ found (μM) | Recovery (%) | R.S.D. a (%) |
| River water | 0.00 | 0.00 | ||
| 10.00 | 10.08 | 101 | 0.8 | |
| Artificial water c | 0.00 | 10.05 | 101 | 0.5 |
| 10.00 | 20.30 | 102 | 1.5 | |
| Sample | Fe3+ added (μM) | Fe3+ found (μM) | Recovery (%) | R.S.D. a (%) |
| River water | 0.00 | 0.00 | ||
| 10.00 | 10.08 | 101 | 0.8 | |
| Artificial water d | 0.00 | 10.05 | 101 | 0.5 |
| 10.00 | 20.30 | 102 | 1.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Wang, K.; Sun, Y. A Simple Schiff Base Probe for Quintuplicate-Metal Analytes with Four Emission-Wavelength Responses. Molecules 2023, 28, 6400. https://doi.org/10.3390/molecules28176400
Zhang J, Wang K, Sun Y. A Simple Schiff Base Probe for Quintuplicate-Metal Analytes with Four Emission-Wavelength Responses. Molecules. 2023; 28(17):6400. https://doi.org/10.3390/molecules28176400
Chicago/Turabian StyleZhang, Jingzhe, Kaili Wang, and Yilu Sun. 2023. "A Simple Schiff Base Probe for Quintuplicate-Metal Analytes with Four Emission-Wavelength Responses" Molecules 28, no. 17: 6400. https://doi.org/10.3390/molecules28176400
APA StyleZhang, J., Wang, K., & Sun, Y. (2023). A Simple Schiff Base Probe for Quintuplicate-Metal Analytes with Four Emission-Wavelength Responses. Molecules, 28(17), 6400. https://doi.org/10.3390/molecules28176400






