2-Alkyl-Substituted-4-Amino-Thieno[2,3-d]Pyrimidines: Anti-Proliferative Properties to In Vitro Breast Cancer Models
Abstract
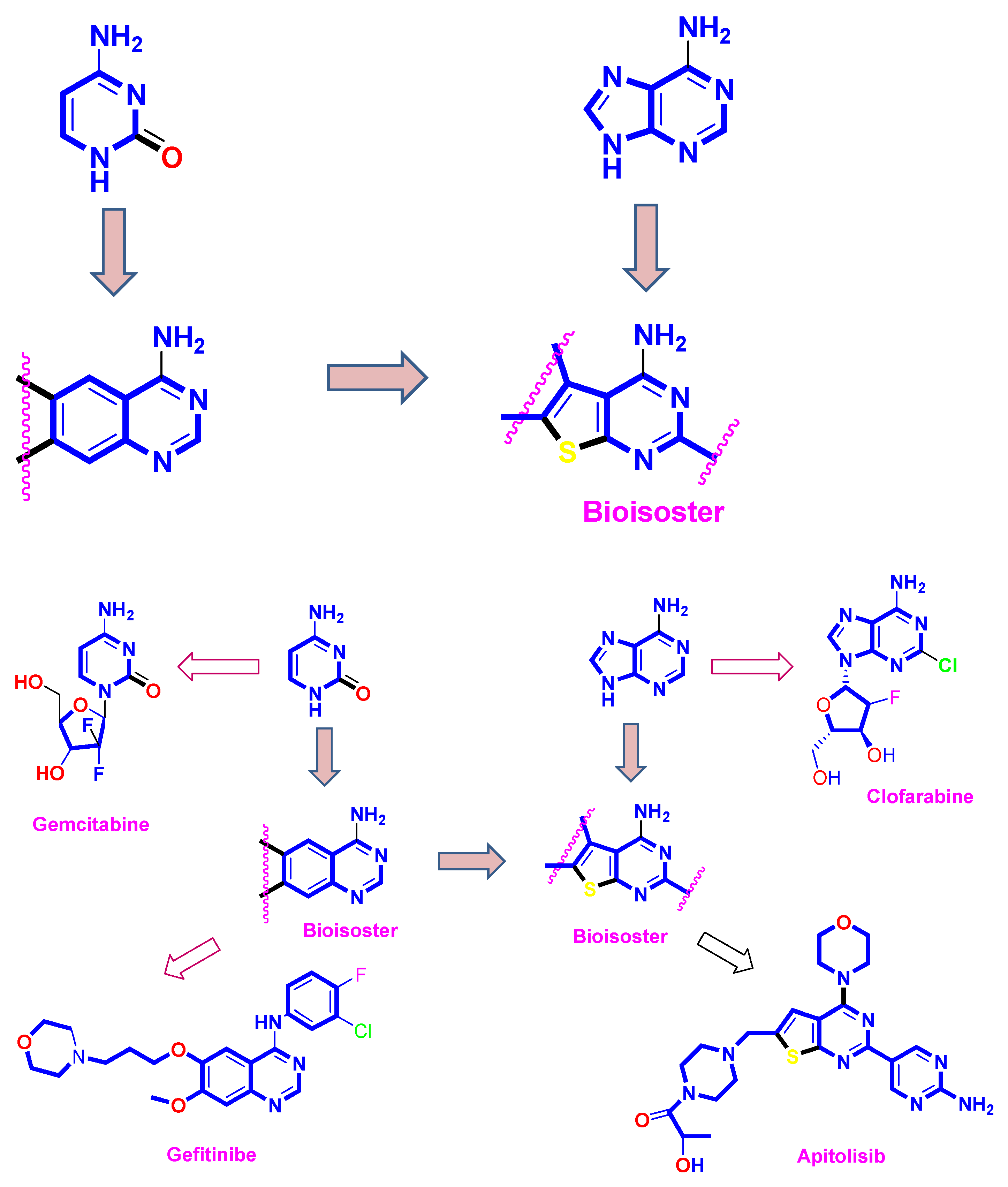
:1. Introduction
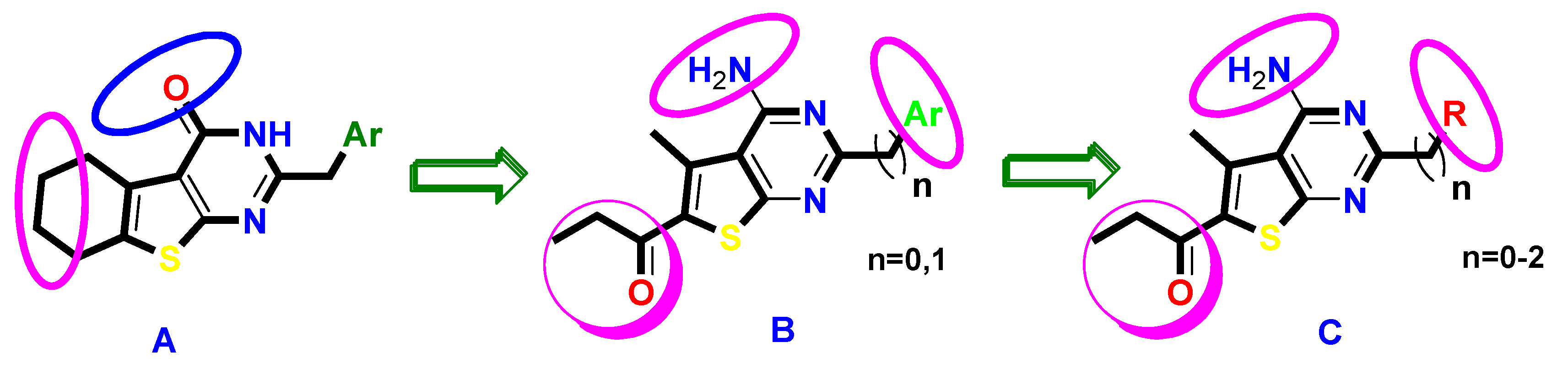
2. Results and Discussion
2.1. Chemistry
2.2. Biological Study
2.2.1. In Vitro Safety Test
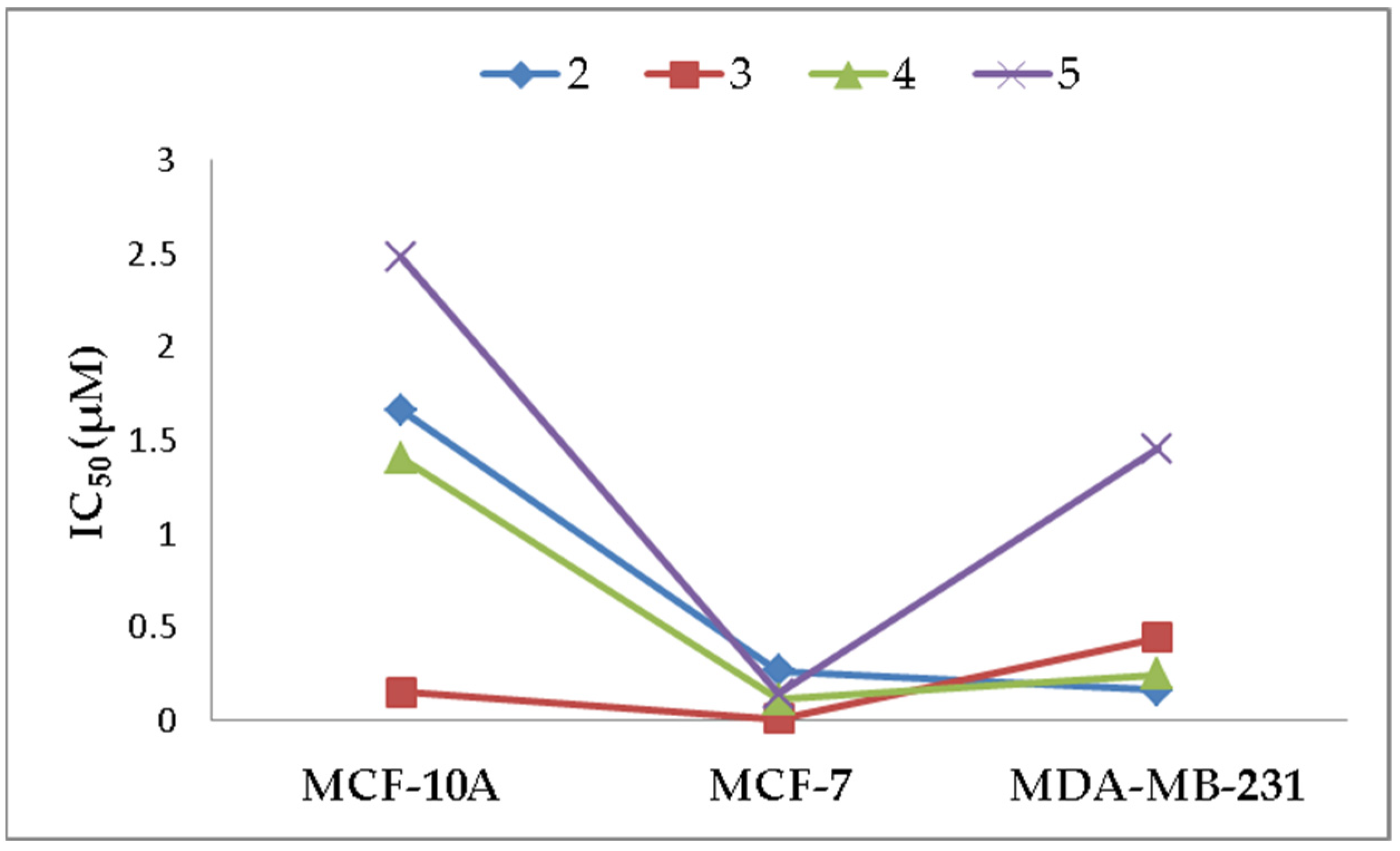
2.2.2. Anti-Proliferative Effects
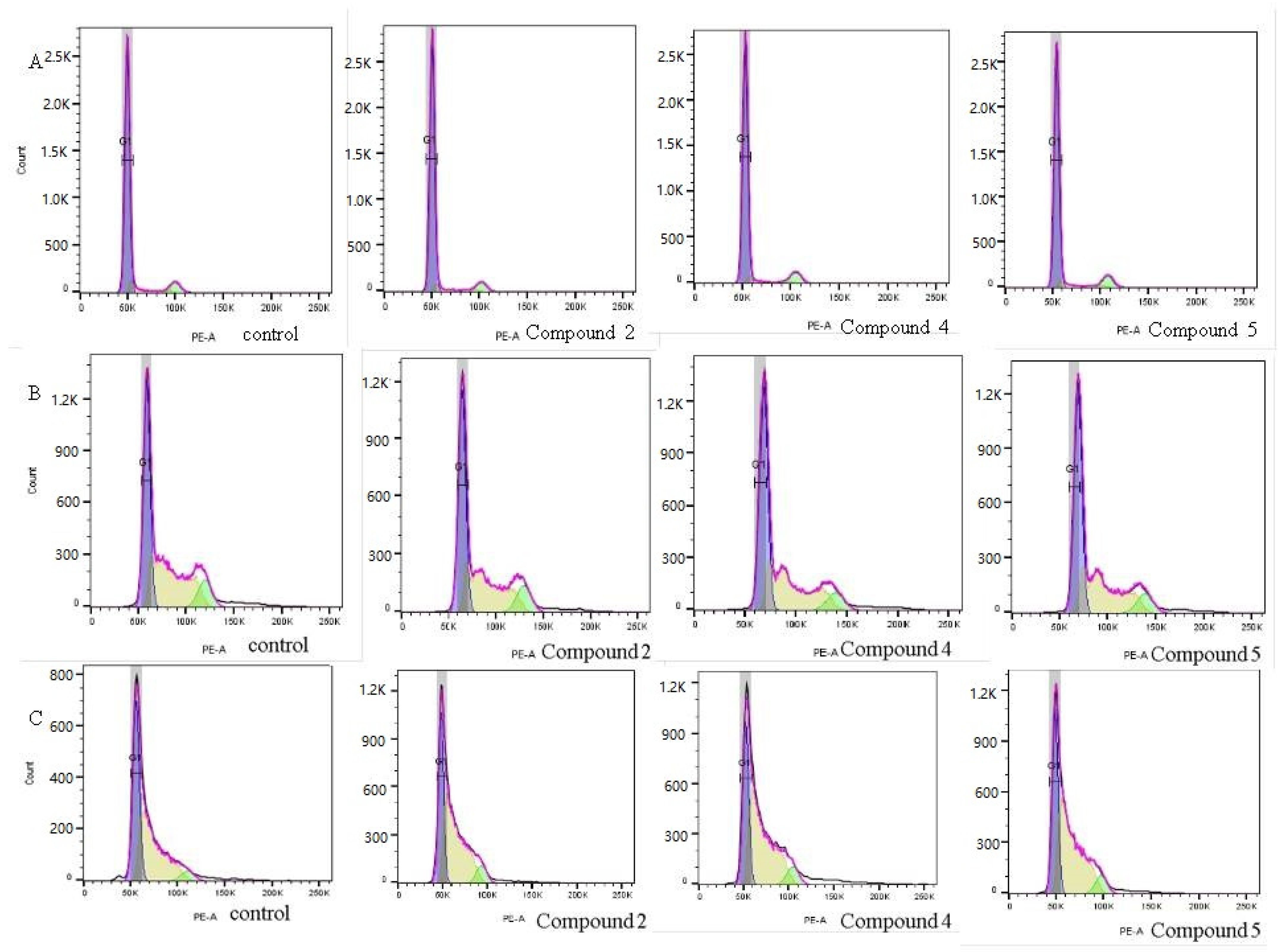
2.2.3. Cell Cycle Arrest
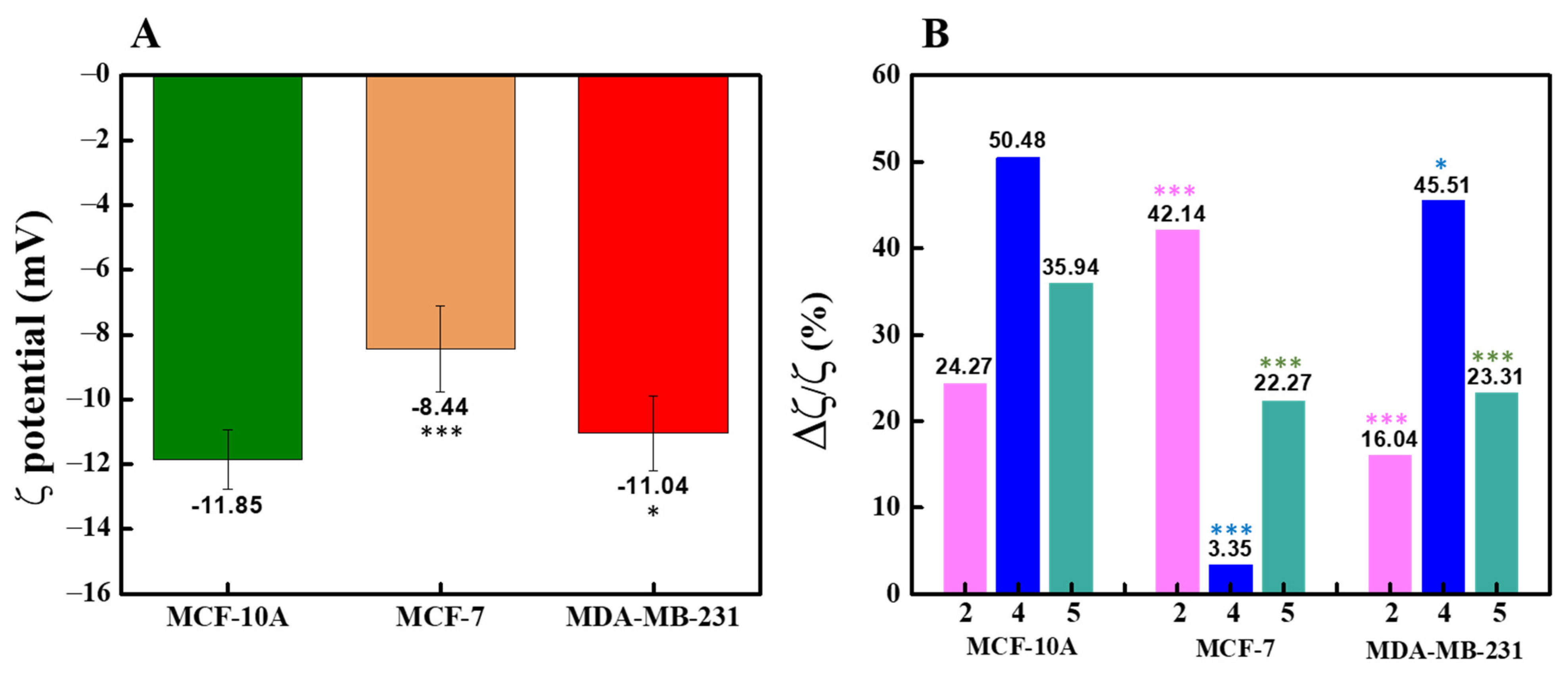
2.2.4. ζ-Potential Measurement
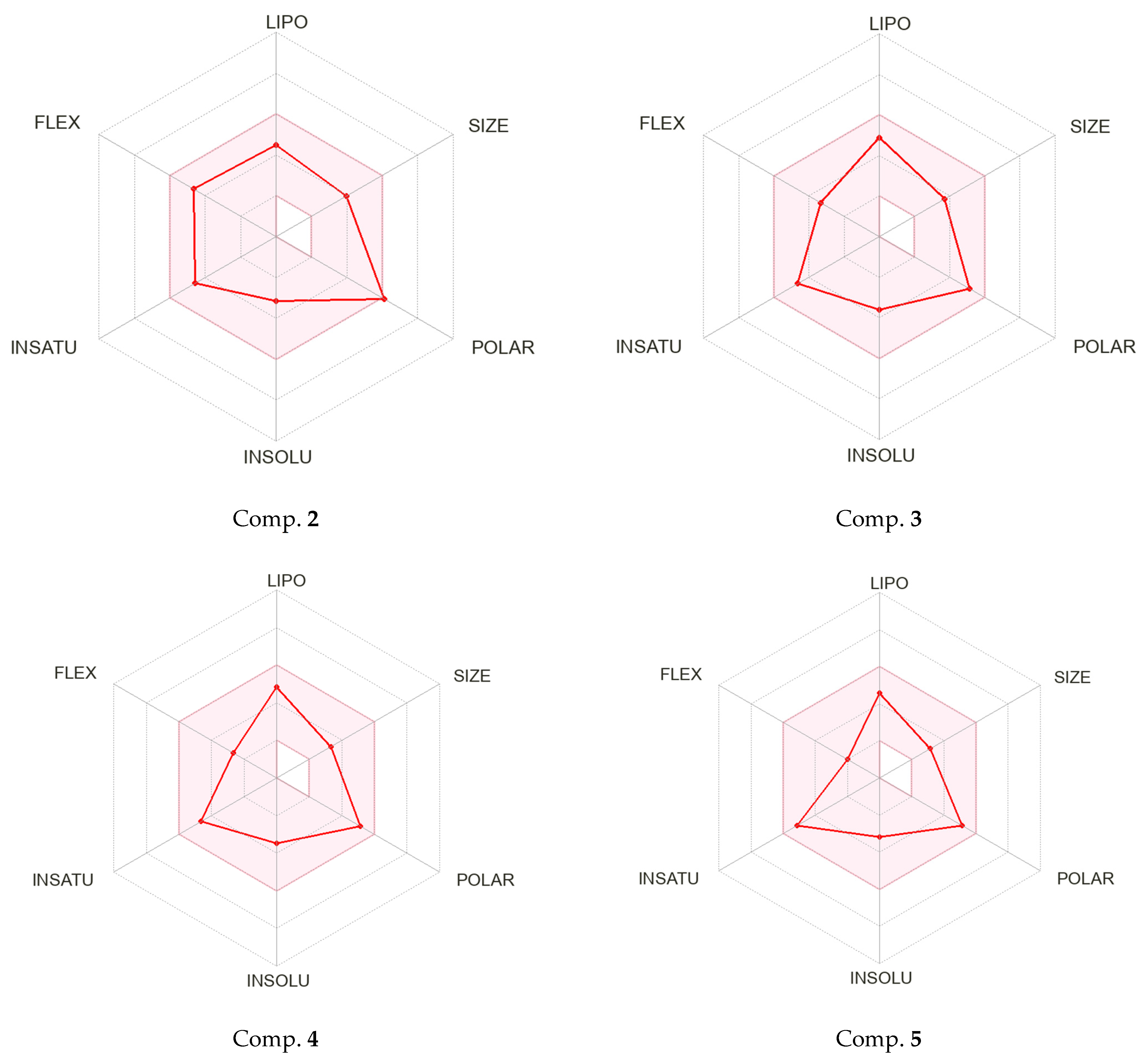
2.3. Physico-Chemical Properties and Drug-Likeness Analysis
3. Materials and Methods
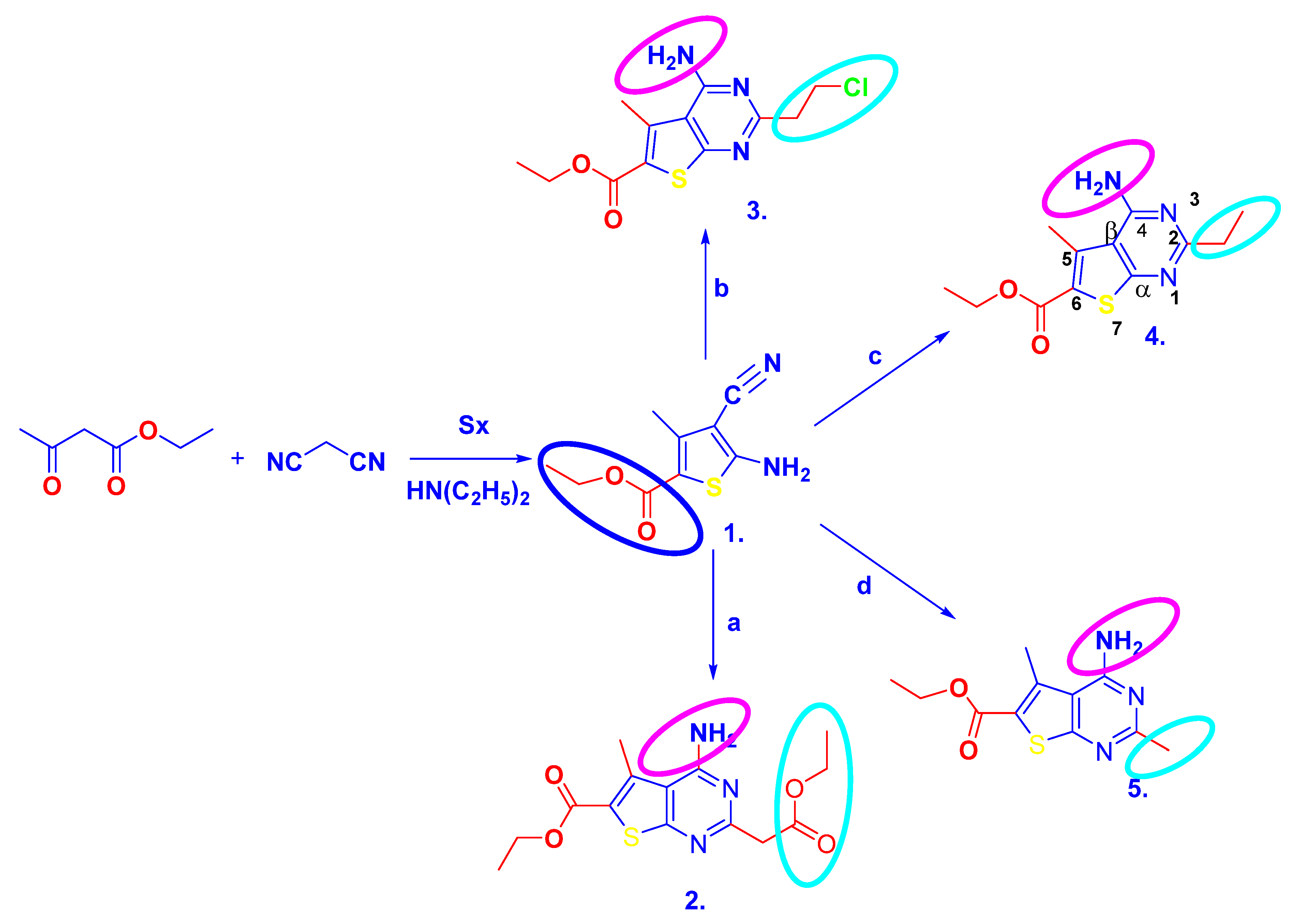
3.1. Synthesis
3.1.1. General Procedure for the Synthesis of Ethyl 2-Amino-3-Cyano-4-Methylthiophene-5-Carboxylate 1
3.1.2. General Procedure for the Synthesis of 2-Substituted 4-Aminothieno[2,3-d]Pyrimidines 2–5
Ethyl 4-Amino-2-(2-Ethoxy-2-Oxoethyl)-5-Methylthieno[2,3-d]Pyrimidine-6-Carboxylate (2)
Ethyl 4-Amino-2-(2-Chloroethyl)-5-Methylthieno[2,3-d] Pyrimidine-6-Carboxylate (3)
Ethyl 4-Amino-2-Ethyl-5-Methylthieno[2,3-d]Pyrimidine-6-Carboxylate (4)
Ethyl 4-Amino-2,5-Dimethyl-Thieno[2,3-d]Pyrimidine-6-Carboxylate (5)
3.2. Biological Assay
3.2.1. Chemicals
3.2.2. Cell Lines
3.2.3. Cytotoxicity and Phototoxicity Testing
3.2.4. In Vitro Anti-Proliferative Activity
3.2.5. Cell Cycle Analysis by Propidium Iodide (PI) Staining
3.2.6. ζ-Potential Measurement
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Bray, F.; Laversanne, M.; Weiderpass, E.; Soerjomataram, I. The ever-increasing importance of cancer as a leading cause of premature death worldwide. Cancer 2021, 127, 3029–3030. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Global Health Estimates 2020: Deaths by Cause, Age, Sex, by Country and by Region, 2000–2019; WHO: Geneva, Switzerland, 2020; Available online: https://www.who.int/data/gho/data/themes/mortality-and-global-health-estimates/ghe-leading-causes-of-death (accessed on 1 January 2023).
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.V.; Oesterreich, S.; Davidson, N.E. MCF-7 cells—Changing the course of breast cancer research and care for 45 years. J. Natl. Cancer Inst. 2015, 107, djv073. [Google Scholar] [CrossRef] [PubMed]
- Comşa, Ş.; Cîmpean, A.M.; Raica, M. The Story of MCF-7 Breast Cancer Cell Line: 40 years of Experience in Research. Anti-Cancer Res. 2015, 35, 3147–3154. [Google Scholar]
- Huang, Z.; Yu, P.; Tang, J. Characterization of Triple-Negative Breast Cancer MDA-MB-231 Cell Spheroid Model. OncoTargets Ther. 2020, 13, 5395–5405. [Google Scholar] [CrossRef] [PubMed]
- Collignon, J.; Lousberg, L.; Schroeder, H.; Jerusalem, G. Triple-negative breast cancer: Treatment challenges and solutions. Breast Cancer Targets Ther. 2016, 20, 93–107. [Google Scholar]
- Shu, S.K.; Lin, C.Y.; He, H.S. Response and resistance to BET bromodomain inhibitors in triple-negative breast cancer. Nature 2016, 529, 413–417. [Google Scholar] [CrossRef]
- Mezni, E.; Vicier, C.; Guerin, M.; Sabatier, R.; Bertucci, F.; Gonçalves, A. New Therapeutics in HER2-Positive Advanced Breast Cancer: Towards a Change in Clinical Practices? Cancers 2020, 12, 1573. [Google Scholar] [CrossRef]
- Taghour, M.S.; Elkady, H.; Eldehna, W.M.; El-Deeb, N.; Kenawy, A.M.; Elkaeed, E.B.; Alsfouk, B.A.; Alesawy, M.S.; Husein, D.Z.; Metwaly, A.M.; et al. Design, synthesis, anti-proliferative evaluation, docking, and MD simulations studies of new thiazolidine-2,4-diones targeting VEGFR-2 and apoptosis pathway. PLoS ONE 2022, 17, e0272362. [Google Scholar] [CrossRef]
- Kotb, A.R.; Bakhotmah, D.A.; Abdallah, A.E.; Elkady, H.; Taghour, M.S.; Eissa, I.H.; El-Zahabi, M.A. Design, synthesis, and biological evaluation of novel bioactive thalidomide analogs as anticancer immunomodulatory agents. RSC Adv. 2022, 12, 33525–33539. [Google Scholar] [CrossRef]
- Barreca, M.; Ingarra, A.M.; Raimondi, M.V.; Spanò, V.; Piccionello, A.P.; De Franco, M.; Menilli, L.; Gandin, V.; Miolo, G.; Barraja, P.; et al. New tricyclic systems as photosensitizers towards triple negative breast cancer cells. Arch. Pharm. Res. 2022, 45, 806–821. [Google Scholar] [CrossRef] [PubMed]
- Ali, E.M.H.; Abdel-Maksoud, M.S.; Oh, C.H. Thieno[2,3-d]pyrimidine as a promising scaffold in medicinal chemistry: Recent advances. Bioorganic Med. Chem. 2019, 27, 1159–1194. [Google Scholar] [CrossRef]
- Elmongy, E.I.; Attallah, N.G.M.; Altwaijry, N.; AlKahtani, M.M.; Henidi, H.A. Design and Synthesis of New Thiophene/Thieno[2,3-d]pyrimidines along with Their Cytotoxic Biological Evaluation as Tyrosine Kinase Inhibitors in Addition to Their Apoptotic and Autophagic Induction. Molecules 2021, 27, 123. [Google Scholar] [CrossRef]
- Schmittel, S.M.; Reck, A.M. First-line treatment of EGFR-mutated nonsmall cell lung cancer: Critical review on study methodology. Eur. Respir. Rev. 2014, 23, 92–105. [Google Scholar] [CrossRef]
- Safwat, G.M.; Hassanin, K.M.A.; Mohammed, E.T.; Ahmed, E.K.; Abdel Rheim, M.R.; Ameen, M.A.; Abdel-Aziz, M.; Gouda, A.M.; Peluso, I.; Almeer, R.; et al. Synthesis, Anticancer Assessment, and Molecular Docking of Novel Chalcone-Thienopyrimidine Derivatives in HepG2 and MCF-7 Cell Lines. Oxid. Med. Cell. Longev. 2021, 2021, 4759821. [Google Scholar] [CrossRef] [PubMed]
- Mishra, R.; Patel, H.; Alanazi, S.; Kilroy, M.K.; Garrett, J.T. PI3K Inhibitors in Cancer: Clinical Implications and Adverse Effects. Int. J. Mol. Sci. 2021, 22, 3464. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Liu, F.; Hou, X.; Cao, J.; Dai, X.; Yu, J.; Huang, G. Synthesis of Novel Analogs of Thieno[2,3-d] Pyrimidin-4(3H)-ones as Selective Inhibitors of Cancer Cell Growth. Biomolecules 2019, 9, 631. [Google Scholar] [CrossRef]
- Landsburg, D.J.; Barta, S.K.; Ramchandren, R.; Batlevi, C.; Iyer, S.; Kelly, K.; Micallef, I.N.; Smith, S.M.; Stevens, D.A.; Alvarez, M.; et al. Fimepinostat (CUDC-907) in patients with relapsed/refractory diffuse large B cell and high-grade B-cell lymphoma: Report of a phase 2 trial and exploratory biomarker analyses. Br. J. Haematol. 2021, 195, 201–209. [Google Scholar] [CrossRef]
- Song, X.-J.; Yang, P.; Gao, H.; Wang, Y.; Dong, X.-G.; Tan, X.-H. Facile synthesis and antitumor activity of novel 2-trifluoromethylthieno [2,3-d]pyrimidine derivatives. Chin. Chem. Lett. 2014, 25, 1006–1010. [Google Scholar] [CrossRef]
- Yong, J.; Lu, C.; Wu, X. Synthesis and Preliminarily Cytotoxicity to A549, HCT116 and MCF-7 Cell Lines of Thieno[2,3-d]pyrimidine Derivatives Containing Isoxazole Moiety. Lett. Drug Des. Discov. 2018, 15, 463–474. [Google Scholar] [CrossRef]
- Saddik, A.A.; El-Dean, A.M.; El-Sokary, G.H.; Hassan, K.M.; Abbady, M.S.; Ismail, I.A.; Saberb, S.H. Synthesis and Cytotoxicity of Some Thieno[2,3-d]pyrimidine Derivatives. J. Chin. Chem. Soc. 2017, 64, 87–93. [Google Scholar] [CrossRef]
- Kandeel, M.; Abdelhameid, M.K.; Eman, K.; Labib, M.B. Synthesis of some novel thieno[3,2-d]pyrimidines as potential cytotoxic small molecules against breast cancer. Chem. Pharm. Bull. 2013, 61, 637–647. [Google Scholar] [CrossRef] [PubMed]
- Shyyka, O.; Pokhodylo, N.; Finiuk, N.; Matiychuk, V.; Stoika, R.; Obushak, M. Anticancer Activity Evaluation of New Thieno[2,3-d]pyrimidin-4(3H)-ones and Thieno[3,2-d]pyrimidin-4(3H)-one Derivatives. Sci. Pharm. 2018, 86, 28. [Google Scholar] [CrossRef] [PubMed]
- Adly, M.E.; Gedawy, E.M.; El-Malah, A.A.; El-Telbany, F.A. Synthesis of Novel Thieno[2,3-d]pyrimidine Derivatives and Evaluation of Their Cytotoxicity and EGFR Inhibitory Activity. Anti-Cancer Agents Med. Chem. 2018, 18, 747–756. [Google Scholar] [CrossRef] [PubMed]
- Islam, F.; Doshi, A.; Robles, A.J.; Quadery, T.M.; Zhang, X.; Zhou, X.; Hamel, E.; Mooberry, S.L.; Gangjee, A. Design, Synthesis, and Biological Evaluation of 5,6,7,8-Tetrahydrobenzo[4,5]thieno[2,3-d]pyrimidines as Microtubule Targeting Agents. Molecules 2022, 27, 321. [Google Scholar] [CrossRef]
- Sharaky, M.; Kamel, M.; Aziz, M.A.; Omran, M.; Rageh, M.M.; Abouzid, K.A.M.; Shouman, S.A. Design, synthesis and biological evaluation of a new thieno[2,3-d]pyrimidine-based urea derivative with potential antitumor activity against tamoxifen sensitive and resistant breast cancer cell lines. J. Enzym. Inhib. Med. Chem. 2020, 35, 1641–1656. [Google Scholar] [CrossRef]
- Mahapatra, A.; Prasad, T.; Sharma, T. Pyrimidine: A review on anticancer activity with key emphasis on SAR. Future J. Pharm. Sci. 2021, 7, 123. [Google Scholar] [CrossRef]
- Ayana, R.; Vijayakumar, B.; Athulya, P.; Anjana, V.S.; Vismaya, K.V.; Swarnalatha, G.A. Short Review on Heterocyclic Compounds Showing Anti-Breast Cancer Activity. J. Sci. Res. 2021, 13, 1075–1098. [Google Scholar] [CrossRef]
- Dimov, S.; Mavrova, A.T.; Yancheva, D.; Nikolova, B.; Tsoneva, I. Thieno[2,3-d]pyrimidin-4(3H)-one Derivatives of Benzimidazole as Potential Anti-Breast Cancer (MDA-MB-231, MCF-7) Agents. Anti-Cancer Agents Med. Chem. 2021, 21, 1441–1450. [Google Scholar] [CrossRef]
- Mavrova, A.T.; Dimov, S.; Yancheva, D.; Rangelov, M.; Wesselinova, D.; Naydenova, E. New C2- and N3-Modified Thieno[2,3-d]Pyrimidine Conjugates with Cytotoxicity in the Nanomolar Range. Anti-Cancer Agents Med. Chem. 2022, 22, 1201–1212. [Google Scholar] [CrossRef]
- Mavrova, A.; Dimov, S.; Sulikovska, I.; Yancheva, D.; Iliev, I.; Tsoneva, I.; Staneva, G.; Nikolova, B. Design, Cytotoxicity and Antiproliferative Activity of 4-Amino-5-methyl-thieno[2,3-d]pyrimidine-6-carboxylates against MFC-7 and MDA-MB-231 Breast Cancer Cell Lines. Molecules 2022, 27, 3314. [Google Scholar] [CrossRef] [PubMed]
- Gewald, K. Heterocyclen aus CH-aciden Nitrilen, VII. 2-Amino-thiophene aus α-Oxo-mercaptanen und methylenaktiven Nitrilen. Chem. Berichte 1965, 98, 3571–3577. [Google Scholar] [CrossRef]
- Sabnis, R.W. The Gewald Synthesis; Sulfur Reports; Taylor & Francis: Abingdon, UK, 1994; Volume 16, pp. 1–17. [Google Scholar]
- Puterová, Z.; Krutošíková, A.; Végh, D. Gewald reaction: Synthesis, properties and applications of substituted 2-aminothiophenes. Arkivoc 2010, 1, 209–246. [Google Scholar] [CrossRef]
- Le, W.; Chen, B.; Cui, Z.; Liu, Z.; Shi, D. Detection of cancer cells based on glycolytic-regulated surface electrical charges. J. Biophys. Rep. 2019, 5, 10–18. [Google Scholar] [CrossRef]
- Baca, J.A.M.; Ortega, A.O.; Jiménez, A.A.; Principal, S.G. Cells electric charge analyses define specific properties for cancer cells activity. Bioelectrochemistry 2022, 144, 108028. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Le, W.; Wang, Y.; Li, Z.; Wang, D.; Ren, L.; Lin, L.; Cui, S.; Hu, J.J.; Hu, Y.; et al. Targeting Negative Surface Charges of Cancer Cells by Multifunctional Nanoprobes. Theranostics 2016, 6, 1887–1898. [Google Scholar] [CrossRef] [PubMed]
- Henslee, E.A.; Sano, M.B.; Rojas, A.D.; Schmelz, E.M.; Davalos, R.V. Selective concentration of human cancer cells using contactless dielectrophoresis. Electrophoresis 2011, 32, 2523–2529. [Google Scholar] [CrossRef]
- Dobrzynska, I.; Skrzydlewska, E.; Figaszewski, Z.A. Changes in electric properties of human breast cancer cells. J. Membr. Biol. 2013, 246, 161–166. [Google Scholar] [CrossRef]
- Sommi, P.; Vitali, A.; Coniglio, S.; Callegari, D.; Barbieri, S.; Falqui, A.C.A.; Vigano, L.; Vigani, B.; Ferrari, F.; Anselmi-Tamburini, U. Microvilli Adhesion: An Alternative Route for Nanoparticle Cell Internalization. ACS Nano 2021, 15, 15803–15814. [Google Scholar] [CrossRef]
- Szlasa, W.; Zendran, I.; Zalesicska, A.; Tarek, M.; Kulbacka, J. Lipid composition of the cancer cell membrane. J. Bioenerg. Biomembr. 2020, 52, 321–342. [Google Scholar] [CrossRef]
- Tzoneva, R.; Stoyanova, T.; Petrich, A.; Popova, D.; Uzunova, V.; Momchilova, A.; Chiantia, S. Effect of Erufosine on Membrane Lipid Order in Breast Cancer Cell Models. Biomolecules 2020, 10, 802. [Google Scholar] [CrossRef]
- Molinspiration Cheminformatics. Molinspiration Property Engine v2013.09. Available online: https://www.molinspiration.com (accessed on 23 January 2023).
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeabilityin drug discovery and development settings. Adv. Drug Deliv. Rev. 1997, 23, 3–25. [Google Scholar] [CrossRef]
- Hopkins, A.L.; Keserü, G.M.; Leeson, P.D.; Rees, D.C.; Reynolds, C.H. The role of ligand efficiency metrics in drug discovery. Nat. Rev. Drug Discov. 2014, 13, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Polanski, J.; Tkocz, A.; Kucia, U. Beware of ligand efficiency (LE): Understanding LE data in modeling structure-activity and structure-economy relationships. J. Cheminformatics 2017, 9, 49. [Google Scholar] [CrossRef] [PubMed]
- Kenny, P.W.; Leitão, A.; Montanari, C.A. Ligand efficiency metrics considered harmful. J. Comput. Aided Mol. Des. 2014, 28, 699–710. [Google Scholar] [CrossRef]
- Kenny, P.W. The nature of ligand efficiency. J. Cheminformatics 2019, 11, 8. [Google Scholar] [CrossRef]
- Shultz, M.D. The thermodynamic basis for the use of lipophilic efficiency (LipE) in enthalpic optimizations. Bioorganic Med. Chem. Lett. 2013, 23, 5992–6000. [Google Scholar] [CrossRef]
- Chen, H.; Engkvist, O.; Kogej, T. Compound properties and their influence on drug quality. In The Practice of Medicinal Chemistry, 4th ed.; Wermuth, C., Aldous, D., Raboisson, P., Rognan, D., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 379–393. [Google Scholar]
- Murray, C.W.; Erlanson, D.A.; Hopkins, A.L.; Keserü, G.M.; Leeson, P.D.; Rees, D.C.; Reynolds, C.H.; Richmond, N.J. Validity of ligand efciency metrics. ACS. Med. Chem. Lett. 2014, 5, 616–618. [Google Scholar] [CrossRef]
- SwissADME. Available online: https://www.swissadme.ch (accessed on 24 April 2023).
- Delaney, J.S. ESOL: Estimating Aqueous Solubility Directly from Molecular Structure. J. Chem. Inf. Comput. Sci. 2004, 44, 1000–1005. [Google Scholar] [CrossRef]
- Ali, J.; Camilleri, P.; Brown, M.B.; Hutt, A.J.; Kirton, S.B. Revisiting the general solubility equation: In silico prediction of aqueous solubility incorporating the effect of topographical polar surface area. J. Chem. Inf. Model. 2012, 52, 420–428. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. Swiss ADME: A free web tool to evaluate pharmacokinetics, druglikenes and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef] [PubMed]
- Ghose, A.K.; Viswanadhan, V.N.; Wendoloski, J.J. A knowledge-based approach in designing combinatorial or medicinal chemistry libraries for drug discovery. 1. A qualitative and quantitative characterization of known drug databases. J. Comb. Chem. 1999, 1, 55–68. [Google Scholar] [CrossRef]
- Egan, W.J.; Merz, K.M., Jr.; Baldwin, J.J. Prediction of Drug Absorption Using Multivariate Statistics. J. Med. Chem. 2000, 43, 3867–3877. [Google Scholar] [CrossRef] [PubMed]
- Veber, D.F.; Johnson, S.R.; Cheng, H.Y.; Smith, B.R.; Ward, K.W.; Kopple, K.D. Molecular properties that influence the oral bioavailability of drug candidates. J. Med. Chem. 2002, 45, 2615–2623. [Google Scholar] [CrossRef] [PubMed]
- Muegge, I.; Heald, S.; Brittelli, D. Simple Selection Criteria for Drug-like Chemical Matter. J. Med. Chem. 2001, 44, 1841–1846. [Google Scholar] [CrossRef] [PubMed]
- Baell, J.B.; Nissink, J.W.M. Seven Year Itch: Pan-Assay Interference Compounds (PAINS) in 2017-Utility and Limitations. ACS Chem. Biol. 2018, 13, 36–44. [Google Scholar] [CrossRef]
- Gfeller, D.; Michielin, D.; Zoete, V. Shaping the interaction landscape of bioactive molecules. Bioinformatics 2013, 29, 3073–3079. [Google Scholar] [CrossRef]
- Halle, W. The Registry of Cytotoxicity: Toxicity testing in cell cultures to predict acute toxicity (LD50) and to reduce testing in animals. Altern. Lab. Anim. 2003, 31, 189–198. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
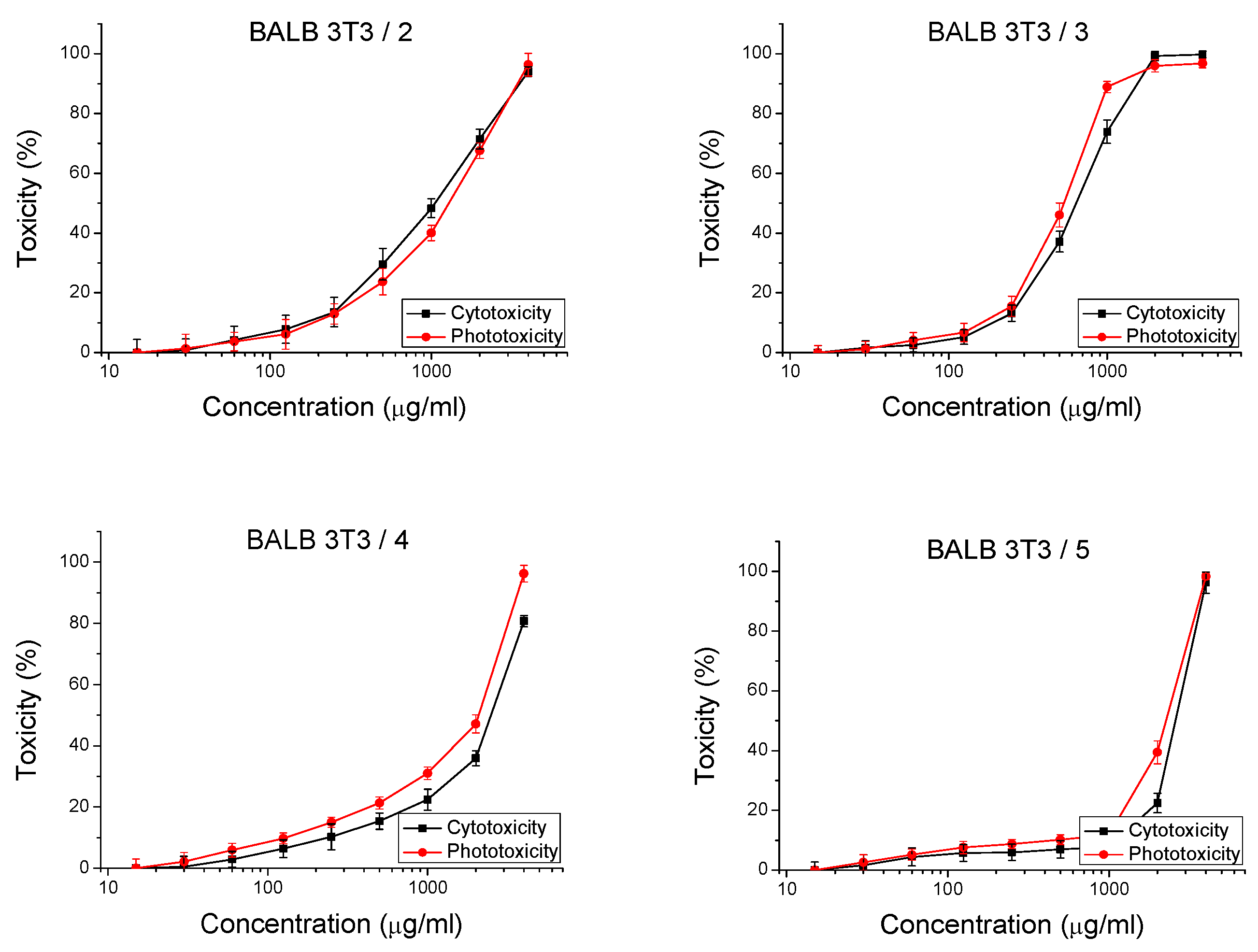
| Cell Line | Compounds | IC50 of Mean ± SD (µg/mL) (IC50 µM) | PIF * | |
|---|---|---|---|---|
| Cytotoxicity | Phototoxicity | |||
| BALB 3T3 | 2 | 1736 ± 75.5 (5.4) | 1081 ± 116.8 (3.3) | 1.3 |
| 3 | 674.5 ± 31.8 (2.2) | 545.1 ± 40.1 (1.9) | 1.2 | |
| 4 | 1678 ± 70.4 (6.4) | 2113 ± 121.5 (8.0) | 1.2 | |
| 5 | 1539 ± 61.5 (5.4) | 2354 ± 114.3 (9.4) | 1.2 | |
| Compounds | IC50 of Mean ± SD (g/mL) (IC50 μM) | ||
|---|---|---|---|
| Anti-Proliferative Activity | |||
| MCF-10A | MCF-7 | MDA-MB-231 | |
| 2 | 503.4 ± 16.7 (1.7) | 83.09 ± 7.3 (0.3) | 52.6 ± 3.2 (0.17) |
| 3 | 45.3 ± 3.5 (0.1) | 13.4 ± 0.8 (0.005) | 130.8 ± 3.0 (0.4) |
| 4 | 367 ± 17.8 (1.4) | 28.89 ± 2.1 (0.1) | 62.9 ± 5.7 (0.2) |
| 5 | 618.3 ± 29.7 (2.5) | 35.5 ± 1.5 (0.1) | 365.4 ± 26.6 (1.5) |
| Compounds | SI* | |
|---|---|---|
| MCF-7 | MDA-MB-231 | |
| 2 | 6.1 | 9.6 |
| 3 | 3.4 | 0.3 |
| 4 | 12.7 | 5.8 |
| 5 | 17.4 | 1.7 |
| Cell Line | %G1 | %S | %G2 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C* | 2 | 4 | 5 | C* | 2 | 4 | 5 | C* | 2 | 4 | 5 | |
| MCF-10A | 81.5 100% | 85.9 +5.439% | 85.5 +4.9% | 86 +5.52% | 9.20 100% | 7.328 −20.8% | 6.53 −29.0% | 7.92 −13.9% | 7.0 100% | 6.55 −6.42% | 8.108 +15.4% | 8.72 +24.657% |
| MCF-7 | 40.4 100% | 44.9 +11.1% | 48.5 +20% | 48.7 +20.54% | 43.1 100% | 39.8 −7.65% | 39.5 −8.35% | 38.7 −10.2% | 9.93 100% | 10.7 +7.75% | 7.769 −22.55% | 8.40 −15.7% |
| MDA- MB-231 | 41.4 100% | 34.1 −17.63% | 29.2 −29.46% | 33.1 −20% | 46.6 100% | 53.6 +15% | 53.3 +14.4% | 53.8 +15.45% | 4.438 100% | 6.73 +53.65% | 6.35 +44.9% | 6.439 +45.9% |
| MCF-7 | MDA-MB-231 Test | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | IC50 (µM)/ (pCI50) | LE kcal/mol | LLE | IC50 (µM)/ (pCI50) | LE kcal/mol | LLE | cLogP * | MW | TPSA | NHA | NHD | nrot |
| 2 | 0.26 (6.59) | 0.43 | 4.46 | 0.16 (6.79) | 0.42 | 4.66 | 2.13 | 323.37 | 104.42 | 7 | 2 | 7 |
| 3 | 0.045 (7.35) | 0.51 | 4.69 | 0.44 (6.36) | 0.46 | 3.70 | 2.66 | 299.78 | 78.11 | 5 | 2 | 5 |
| 4 | 0.11 (6.96) | 0.52 | 4.01 | 0.24 (6.62) | 0.50 | 3.67 | 2.95 | 265.33 | 78.11 | 5 | 2 | 4 |
| 5 | 0.14 (6.85) | 0.55 | 4.43 | 1.45 (5.84) | 0.47 | 3.42 | 2.42 | 251.31 | 76.11 | 5 | 2 | 3 |
| Compound 2 | Compound 3 | ||
|---|---|---|---|
| Log S (ESOL) | −3.15 | Log S (ESOL) | −3.62 |
| Solubility | 2.30 × 10−1 mg/mL; 7.13 × 10−4 mol/L | Solubility | 7.27 × 10−2 mg/mL; 2.42 × 10−4 mol/L |
| Class | Soluble | Class | Soluble |
| Log S (Ali) | −4.74 | Log S (Ali) | −4.91 |
| Solubility | 5.83 × 10−3 mg/mL; 1.80 × 10−5 mol/L | Solubility | 3.71 × 10−3 mg/mL; 1.24 × 10−5 mol/L |
| Class | Moderately soluble | Class | Moderately soluble |
| Log S (SILICOS-IT) | −3.97 | Log S (SILICOS-IT) | −4.52 |
| Solubility | 3.46 × 10−2 mg/mL; 1.07 × 10−4 mol/L | Solubility | 9.01 × 10−3 mg/mL; 3.01 × 10−5 mol/L |
| Class | Soluble | Class | Moderately soluble |
| Compound 4 | Compound 5 | ||
| Log S (ESOL) | −3.46 | Log S (ESOL) | −3.18 |
| Solubility | 9.29 × 10−2 mg/mL; 3.50 × 10−4 mol/L | Solubility | 1.66 × 10−1 mg/mL; 6.62 × 10−4 mol/L |
| Class | Soluble | Class | Soluble |
| Log S (Ali) | −4.86 | Log S (Ali) | −4.40 |
| Solubility | 3.70 × 10−3 mg/mL; 1.39 × 10−5 mol/L | Solubility | 1.00 × 10−2 mg/mL; 3.99 × 10−5 mol/L |
| Class | Moderately soluble | Class | Moderately soluble |
| Log S (SILICOS-IT) | −3.90 | Log S (SILICOS-IT) | −3.50 |
| Solubility | 3.33 × 10−2 mg/mL; 1.26 × 10−4 mol/L | Solubility | 7.95 × 10−2 mg/mL; 3.16 × 10−4 mol/L |
| Class | Soluble | Class | Soluble |
| No. | GI Absorption | BBB Permeant | Pg Substrate | CYP Substrate/Inhibitors | LogKp [cm/s] | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1A2 | 2C19 | 2C9 | 2C6 | 2C4 | ||||||
| 2 | High | No | No | Yes | Yes | No | No | No | −6.63 | |
| 3 | High | No | No | Yes | Yes | Yes | No | No | −5.99 | |
| 4 | High | No | No | Yes | Yes | Yes | No | No | −5.82 | |
| 5 | High | No | No | Yes | Yes | Yes | No | No | −6.04 | |
| Drug-likeness | ||||||||||
| Compounds | 2 | 3 | 4 | 5 | ||||||
| Lipinski | Yes; 0 violation | Yes; 0 violation | Yes; 0 violation | Yes; 0 violation | ||||||
| Ghose | Yes | Yes | Yes | Yes | ||||||
| Egan * | No; 1 violation: TPSA > 131.6 | Yes | Yes | Yes | ||||||
| Veber | Yes | Yes | Yes | Yes | ||||||
| Muegge | Yes | Yes | Yes | Yes | ||||||
| Bioavailability Score | 0.55 | 0.55 | 0.55 | 0.55 | ||||||
| Medicinal Chemistry | ||||||||||
| PAINS | 0 alert | 0 alert | 0 alert | 0 alert | ||||||
| Brenk | 1 alert: more than 2 ester groups | 1 alert: alkyl halide | 0 alert | 0 alert | ||||||
| Leadlikeness | Yes | Yes | Yes | Yes | ||||||
| Synthetic accessibility | 3.22 | 3.02 | 2.99 | 2.87 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iliev, I.; Mavrova, A.; Yancheva, D.; Dimov, S.; Staneva, G.; Nesheva, A.; Tsoneva, I.; Nikolova, B. 2-Alkyl-Substituted-4-Amino-Thieno[2,3-d]Pyrimidines: Anti-Proliferative Properties to In Vitro Breast Cancer Models. Molecules 2023, 28, 6347. https://doi.org/10.3390/molecules28176347
Iliev I, Mavrova A, Yancheva D, Dimov S, Staneva G, Nesheva A, Tsoneva I, Nikolova B. 2-Alkyl-Substituted-4-Amino-Thieno[2,3-d]Pyrimidines: Anti-Proliferative Properties to In Vitro Breast Cancer Models. Molecules. 2023; 28(17):6347. https://doi.org/10.3390/molecules28176347
Chicago/Turabian StyleIliev, Ivan, Anelia Mavrova, Denitsa Yancheva, Stefan Dimov, Galya Staneva, Alexandrina Nesheva, Iana Tsoneva, and Biliana Nikolova. 2023. "2-Alkyl-Substituted-4-Amino-Thieno[2,3-d]Pyrimidines: Anti-Proliferative Properties to In Vitro Breast Cancer Models" Molecules 28, no. 17: 6347. https://doi.org/10.3390/molecules28176347
APA StyleIliev, I., Mavrova, A., Yancheva, D., Dimov, S., Staneva, G., Nesheva, A., Tsoneva, I., & Nikolova, B. (2023). 2-Alkyl-Substituted-4-Amino-Thieno[2,3-d]Pyrimidines: Anti-Proliferative Properties to In Vitro Breast Cancer Models. Molecules, 28(17), 6347. https://doi.org/10.3390/molecules28176347








