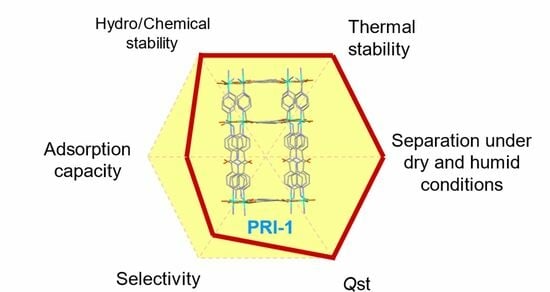
Exquisitely Constructing a Robust MOF with Dual Pore Sizes for Efficient CO2 Capture
Abstract
:1. Introduction
2. Results and Discussion
2.1. Sample Characterization
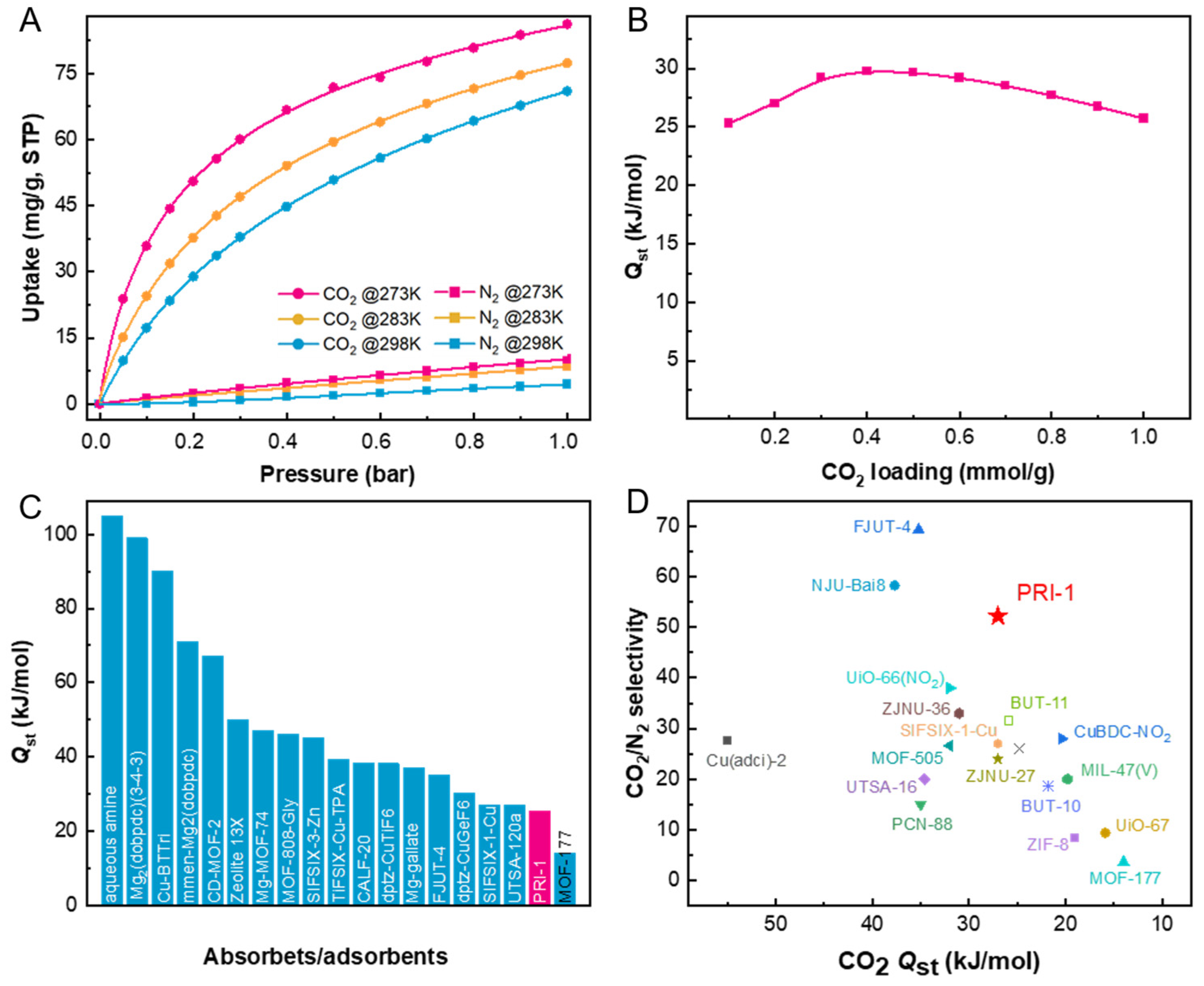
2.2. CO2 and N2 Isotherms, Adsorption Heat, and Selectivity of PRI-1
2.3. Adsorption Mechanism of CO2 and N2 on PRI-1
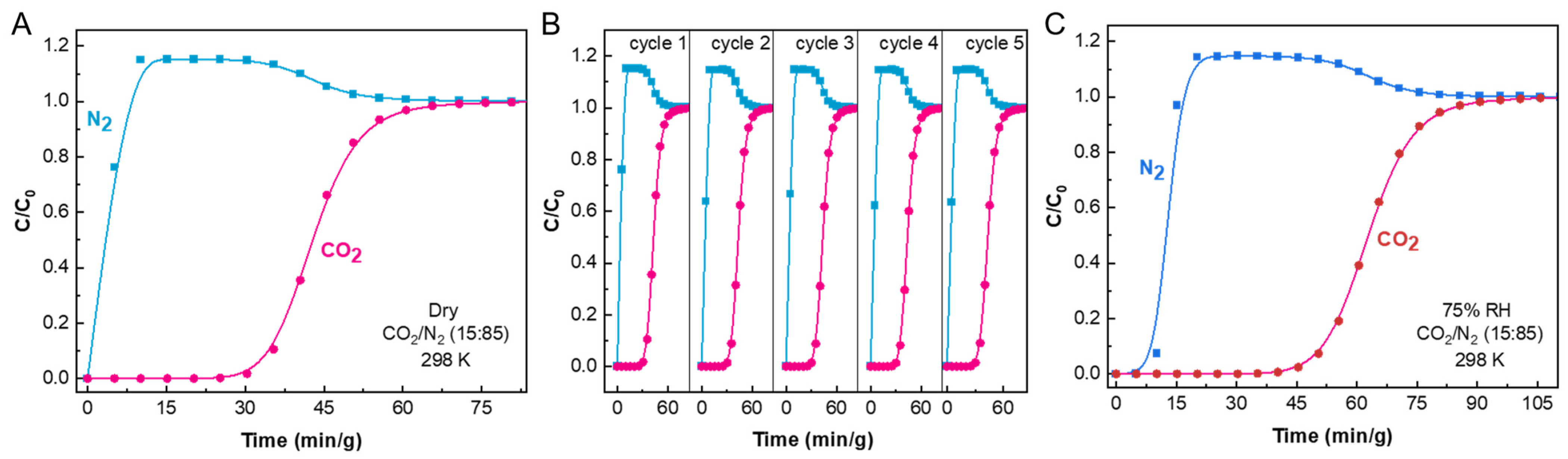
2.4. Breakthrough Curve of CO2/N2 Binary Mixtures
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Synthesis of PRI-1
4.3. Mother Liquor Circulation Synthesized Method
4.4. Characterization
4.5. Adsorption Isotherms Measurement
4.6. Solvent Stability Test
4.7. Fixed-Bed Breakthrough Experiments
4.8. Fitting with Dual-Site Langmuir-Freundlich Model
4.9. Ideal Adsorbed Solution Theory Calculations
4.10. Calculation of Isosteric Heat of Adsorption
4.11. Computational Method
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Xu, Y.; Ramanathan, V.; Victor, D.G. Global Warming Will Happen Faster than We Think. Nature 2018, 564, 30–32. [Google Scholar] [CrossRef]
- Ritchie, H.; Roser, M.; Rosado, P. CO₂ and Greenhouse Gas Emissions. OurWorldInData.org. 2020. Available online: https://ourworldindata.org/co2-and-greenhouse-gas-emissions (accessed on 15 August 2023).
- Abas, N.; Kalair, A.; Khan, N. Review of Fossil Fuels and Future Energy Technologies. Futures 2015, 69, 31–49. [Google Scholar] [CrossRef]
- Nguyen, T.T.T.; Lin, J.-B.; Shimizu, G.K.H.; Rajendran, A. Separation of CO2 and N2 on a Hydrophobic Metal Organic Framework CALF-20. Chem. Eng. J. 2022, 442, 136263. [Google Scholar] [CrossRef]
- Wang, M.; Lawal, A.; Stephenson, P.; Sidders, J.; Ramshaw, C. Post-Combustion CO2 Capture with Chemical Absorption: A State-of-the-Art Review. Chem. Eng. Res. Des. 2011, 89, 1609–1624. [Google Scholar] [CrossRef]
- Zhang, Z.; Borhani, T.N.; Olabi, A.G. Status and Perspective of CO2 Absorption Process. Energy 2020, 205, 118057. [Google Scholar] [CrossRef]
- Yu, C.-H.; Huang, C.-H.; Tan, C.-S. A Review of CO2 Capture by Absorption and Adsorption. Aerosol Air Qual. Res. 2012, 12, 745–769. [Google Scholar] [CrossRef]
- Al-Rowaili, F.N.; Zahid, U.; Onaizi, S.; Khaled, M.; Jamal, A.; AL-Mutairi, E.M. A Review for Metal-Organic Frameworks (MOFs) Utilization in Capture and Conversion of Carbon Dioxide into Valuable Products. J. CO2 Util. 2021, 53, 101715. [Google Scholar] [CrossRef]
- Ding, M.; Flaig, R.W.; Jiang, H.-L.; Yaghi, O.M. Carbon Capture and Conversion Using Metal-Organic Frameworks and MOF-Based Materials. Chem. Soc. Rev. 2019, 48, 2783–2828. [Google Scholar] [CrossRef]
- Kancharlapalli, S.; Snurr, R.Q. High-Throughput Screening of the CoRE-MOF-2019 Database for CO2 Capture from Wet Flue Gas: A Multi-Scale Modeling Strategy. ACS Appl. Mater. Interfaces 2023, 15, 28084–28092. [Google Scholar] [CrossRef]
- Zhang, Z.; Yao, Z.-Z.; Xiang, S.; Chen, B. Perspective of Microporous Metal-Organic Frameworks for CO2 Capture and Separation. Energy Environ. Sci. 2014, 7, 2868–2899. [Google Scholar] [CrossRef]
- Caskey, S.R.; Wong-Foy, A.G.; Matzger, A.J. Dramatic Tuning of Carbon Dioxide Uptake via Metal Substitution in a Coordination Polymer with Cylindrical Pores. J. Am. Chem. Soc. 2008, 130, 10870–10871. [Google Scholar] [CrossRef]
- Choe, J.H.; Kim, H.; Hong, C.S. MOF-74 Type Variants for CO2 Capture. Mater. Chem. Front. 2021, 5, 5172–5185. [Google Scholar] [CrossRef]
- Wu, D.; Zhang, P.-F.; Yang, G.-P.; Hou, L.; Zhang, W.-Y.; Han, Y.-F.; Liu, P.; Wang, Y.-Y. Supramolecular Control of MOF Pore Properties for the Tailored Guest Adsorption/Separation Applications. Coord. Chem. Rev. 2021, 434, 213709. [Google Scholar] [CrossRef]
- Guo, J.; Xue, X.; Yu, H.; Duan, Y.; Li, F.; Lian, Y.; Liu, Y.; Zhao, M. Metal–Organic Frameworks Based on Infinite Secondary Building Units: Recent Progress and Future Outlooks. J. Mater. Chem. A 2022, 10, 19320–19347. [Google Scholar] [CrossRef]
- Chen, C.-X.; Wei, Z.; Jiang, J.-J.; Fan, Y.-Z.; Zheng, S.-P.; Cao, C.-C.; Li, Y.-H.; Fenske, D.; Su, C.-Y. Precise Modulation of the Breathing Behavior and Pore Surface in Zr-MOFs by Reversible Post-Synthetic Variable-Spacer Installation to Fine-Tune the Expansion Magnitude and Sorption Properties. Angew. Chem. Int. Ed. 2016, 55, 9932–9936. [Google Scholar] [CrossRef] [PubMed]
- Yue, L.; Wang, X.; Lv, C.; Zhang, T.; Li, B.; Chen, D.-L.; He, Y. Substituent Engineering-Enabled Structural Rigidification and Performance Improvement for C2/CO2 Separation in Three Isoreticular Coordination Frameworks. Inorg. Chem. 2022, 61, 21076–21086. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Zhou, H.-F.; Hou, L.; Wang, Y.-Y. Functionalization of MOFs via a Mixed-Ligand Strategy: Enhanced CO2 Uptake by Pore Surface Modification. Dalton Trans. 2018, 47, 5298–5303. [Google Scholar] [CrossRef]
- Hu, Y.; Jiang, Y.; Li, J.; Wang, L.; Steiner, M.; Neumann, R.F.; Luan, B.; Zhang, Y. New-Generation Anion-Pillared Metal–Organic Frameworks with Customized Cages for Highly Efficient CO2 Capture. Adv. Funct. Mater. 2023, 33, 2213915. [Google Scholar] [CrossRef]
- Kim, E.J.; Siegelman, R.L.; Jiang, H.Z.H.; Forse, A.C.; Lee, J.-H.; Martell, J.D.; Milner, P.J.; Falkowski, J.M.; Neaton, J.B.; Reimer, J.A.; et al. Cooperative Carbon Capture and Steam Regeneration with Tetraamine-Appended Metal–Organic Frameworks. Science 2020, 369, 392–396. [Google Scholar] [CrossRef]
- Liang, W.; Bhatt, P.M.; Shkurenko, A.; Adil, K.; Mouchaham, G.; Aggarwal, H.; Mallick, A.; Jamal, A.; Belmabkhout, Y.; Eddaoudi, M. A Tailor-Made Interpenetrated MOF with Exceptional Carbon-Capture Performance from Flue Gas. Chem 2019, 5, 950–963. [Google Scholar] [CrossRef]
- Hu, Z.; Faucher, S.; Zhuo, Y.; Sun, Y.; Wang, S.; Zhao, D. Combination of Optimization and Metalated-Ligand Exchange: An Effective Approach to Functionalize UiO-66(Zr) MOFs for CO2 Separation. Chem. Eur. J. 2015, 21, 17246–17255. [Google Scholar] [CrossRef]
- Walton, K.S.; Sholl, D.S. Predicting Multicomponent Adsorption: 50 Years of the Ideal Adsorbed Solution Theory. AIChE J. 2015, 61, 2757–2762. [Google Scholar] [CrossRef]
- Myers, A.L.; Prausnitz, J.M. Thermodynamics of Mixed-Gas Adsorption. AIChE J. 1965, 11, 121–127. [Google Scholar] [CrossRef]
- Luo, F.; Che, Y.; Zheng, J. Layer-Pillared Metal-Organic Framework Showing Two-Fold Interpenetration and Considerable Solvent-Accessible Channels. Microporous Mesoporous Mater. 2009, 117, 486–489. [Google Scholar] [CrossRef]
- Macrae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.A.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M.; et al. Mercury 4.0: From Visualization to Analysis, Design and Prediction. J. Appl. Crystallogr. 2020, 53, 226–235. [Google Scholar] [CrossRef]
- Chen, F.; Wang, J.; Guo, L.; Huang, X.; Zhang, Z.; Yang, Q.; Yang, Y.; Ren, Q.; Bao, Z. Carbon Dioxide Capture in Gallate-Based Metal-Organic Frameworks. Sep. Purif. Technol. 2022, 292, 121031. [Google Scholar] [CrossRef]
- Nugent, P.; Belmabkhout, Y.; Burd, S.D.; Cairns, A.J.; Luebke, R.; Forrest, K.; Pham, T.; Ma, S.; Space, B.; Wojtas, L.; et al. Porous Materials with Optimal Adsorption Thermodynamics and Kinetics for CO2 Separation. Nature 2013, 495, 80–84. [Google Scholar] [CrossRef]
- Zhang, L.; Lin, S.; Liu, Y.; Zeng, X.; You, J.; Xiao, T.; Feng, Y.; He, Z.; Chen, S.; Hua, N.; et al. Optimized Pore Nanospace through the Construction of a Cagelike Metal–Organic Framework for CO2/N2 Separation. Inorg. Chem. 2023, 62, 8058–8063. [Google Scholar] [CrossRef]
- Shen, S.; Xu, F.; Chen, X.; Miao, G.; Li, Z.; Zhou, X.; Wang, X. Facile Synthesis of Dptz-CuGeF6 at Room Temperature and Its Adsorption Performance for Separation of CO2, CH4 and N2. Sep. Purif. Technol. 2022, 302, 122054. [Google Scholar] [CrossRef]
- Parsaei, M.; Akhbari, K.; White, J. Modulating Carbon Dioxide Storage by Facile Synthesis of Nanoporous Pillared-Layered Metal–Organic Framework with Different Synthetic Routes. Inorg. Chem. 2022, 61, 3893–3902. [Google Scholar] [CrossRef]
- Lin, J.-B.; Nguyen, T.T.T.; Vaidhyanathan, R.; Burner, J.; Taylor, J.M.; Durekova, H.; Akhtar, F.; Mah, R.K.; Ghaffari-Nik, O.; Marx, S.; et al. A Scalable Metal-Organic Framework as a Durable Physisorbent for Carbon Dioxide Capture. Science 2021, 374, 1464–1469. [Google Scholar] [CrossRef] [PubMed]
- Demessence, A.; D’Alessandro, D.M.; Foo, M.L.; Long, J.R. Strong CO2 Binding in a Water-Stable, Triazolate-Bridged Metal-Organic Framework Functionalized with Ethylenediamine. J. Am. Chem. Soc. 2009, 131, 8784–8786. [Google Scholar] [CrossRef] [PubMed]
- McDonald, T.M.; Mason, J.A.; Kong, X.; Bloch, E.D.; Gygi, D.; Dani, A.; Crocellà, V.; Giordanino, F.; Odoh, S.O.; Drisdell, W.S.; et al. Cooperative Insertion of CO2 in Diamine-Appended Metal-Organic Frameworks. Nature 2015, 519, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, J.; Zhang, Z.; Yang, Q.; Yang, Y.; Su, B.; Bao, Z.; Ren, Q. Inverse Adsorption Separation of CO2/C2H2 Mixture in Cyclodextrin-Based Metal–Organic Frameworks. ACS Appl. Mater. Interfaces 2019, 11, 2543–2550. [Google Scholar] [CrossRef]
- Lyu, H.; Chen, O.I.-F.; Hanikel, N.; Hossain, M.I.; Flaig, R.W.; Pei, X.; Amin, A.; Doherty, M.D.; Impastato, R.K.; Glover, T.G.; et al. Carbon Dioxide Capture Chemistry of Amino Acid Functionalized Metal-Organic Frameworks in Humid Flue Gas. J. Am. Chem. Soc. 2022, 144, 2387–2396. [Google Scholar] [CrossRef]
- Choe, J.H.; Kim, H.; Kang, M.; Yun, H.; Kim, S.Y.; Lee, S.M.; Hong, C.S. Functionalization of Diamine-Appended MOF-Based Adsorbents by Ring Opening of Epoxide: Long-Term Stability and CO2 Recyclability under Humid Conditions. J. Am. Chem. Soc. 2022, 144, 10309–10319. [Google Scholar] [CrossRef]
- Zhang, L.; He, Z.; Liu, Y.; You, J.; Lin, L.; Jia, J.; Chen, S.; Hua, N.; Ma, L.-A.; Ye, X.; et al. A Robust Squarate-Cobalt Metal–Organic Framework for CO2/N2 Separation. ACS Appl. Mater. Interfaces 2023, 15, 30394–30401. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, Y.; Liu, P.; Wang, Y.; Yang, J.; Li, J.; Li, L. CO2 Capture from High-Humidity Flue Gas Using a Stable Metal-Organic Framework. Molecules 2022, 27, 5608. [Google Scholar] [CrossRef]
- Nuhnen, A.; Janiak, C. A Practical Guide to Calculate the Isosteric Heat/Enthalpy of Adsorption via Adsorption Isotherms in Metal–Organic Frameworks, MOFs. Dalton Trans. 2020, 49, 10295–10307. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficiency of Ab-Initio Total Energy Calculations for Metals and Semiconductors Using a Plane-Wave Basis Set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Kresse, G.; Joubert, D. From Ultrasoft Pseudopotentials to the Projector Augmented-Wave Method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Blöchl, P.E. Projector Augmented-Wave Method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef] [PubMed]
- Monkhorst, H.J.; Pack, J.D. Special Points for Brillouin-Zone Integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A Consistent and Accurate Ab Initio Parametrization of Density Functional Dispersion Correction (DFT-D) for the 94 Elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Bai, Y.; Wang, Z.; Gong, Q.; Li, M.; Bo, Y.; Xu, H.; Jiang, G.; Chi, K. Exquisitely Constructing a Robust MOF with Dual Pore Sizes for Efficient CO2 Capture. Molecules 2023, 28, 6276. https://doi.org/10.3390/molecules28176276
Li Y, Bai Y, Wang Z, Gong Q, Li M, Bo Y, Xu H, Jiang G, Chi K. Exquisitely Constructing a Robust MOF with Dual Pore Sizes for Efficient CO2 Capture. Molecules. 2023; 28(17):6276. https://doi.org/10.3390/molecules28176276
Chicago/Turabian StyleLi, Yanxi, Yuhua Bai, Zhuozheng Wang, Qihan Gong, Mengchen Li, Yawen Bo, Hua Xu, Guiyuan Jiang, and Kebin Chi. 2023. "Exquisitely Constructing a Robust MOF with Dual Pore Sizes for Efficient CO2 Capture" Molecules 28, no. 17: 6276. https://doi.org/10.3390/molecules28176276
APA StyleLi, Y., Bai, Y., Wang, Z., Gong, Q., Li, M., Bo, Y., Xu, H., Jiang, G., & Chi, K. (2023). Exquisitely Constructing a Robust MOF with Dual Pore Sizes for Efficient CO2 Capture. Molecules, 28(17), 6276. https://doi.org/10.3390/molecules28176276