Therapeutic Potential of Minor Cannabinoids in Dermatological Diseases—A Synthetic Review
Abstract
:1. Introduction
2. Methods of Obtaining Cannabinoids
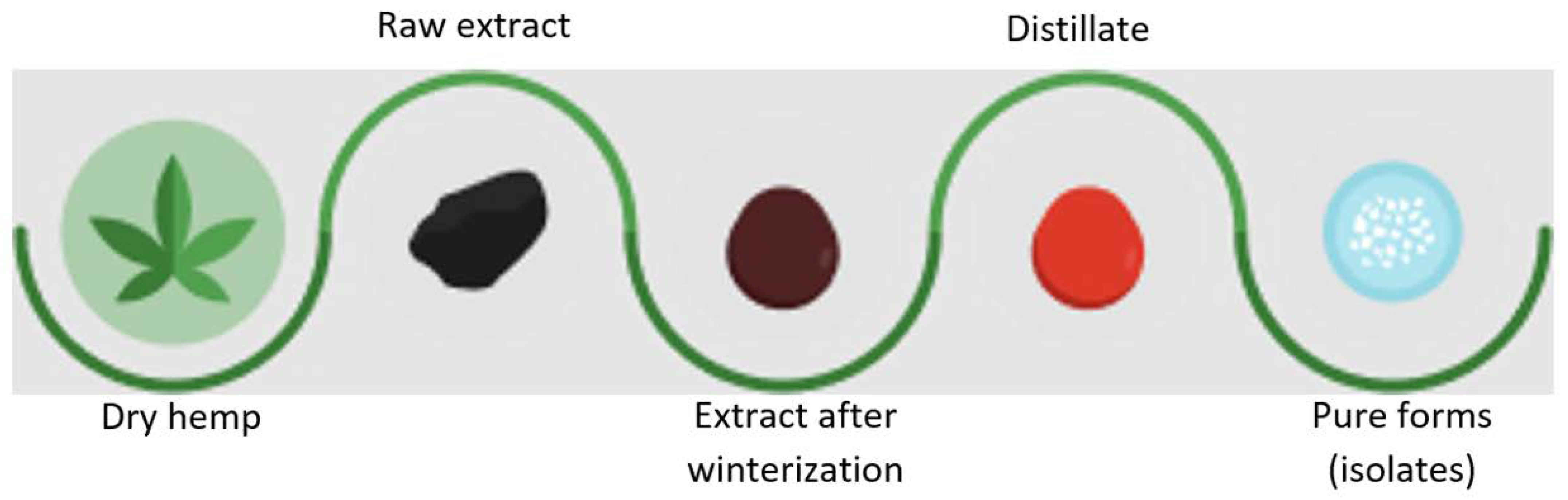
2.1. Conventional Extraction Methods
2.2. Chemical Synthesis
2.3. Biotechnological Synthesis
2.4. Enzymatic Synthesis
3. Mechanisms of Action of Cannabinoids on the Skin and Their Impact on Pathological Processes Occurring in the Skin
4. Cannabinoids in Dermatological Disorders
4.1. Cannabinoids in Psoriasis
4.2. Cannabinoids in Acne
4.3. Cannabinoids in Atopic Dermatitis (AD)
4.4. Cannabinoids in Allergic Contact Dermatitis (ACD)
4.5. Cannabinoids with Potential Anti-Inflammatory Properties
5. Limitations
6. Summary and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Li, W.; Thuan Lu, H.; Doblin, M.S.; Bacic, A.; Stevens, G.W.; Mumford, K.A. A novel efficient liquid–liquid solvent extraction process for cannabinoid mimic recovery. Sep. Purif. Technol. 2023, 309, 123011. [Google Scholar] [CrossRef]
- Mano-Sousa, B.J.; Alves, B.C.; Pedrosa, A.M.; Lima, P.L.; Pereira de Andrade, F.; Duarte-Almeida, J.M. Validation of analytical method of cannabinoids: Novel approach using turbo-extraction. Talanta 2023, 254, 124108. [Google Scholar] [CrossRef] [PubMed]
- Fiorito, S.; Epifano, F.; Palumbo, L.; Collevecchio, C.; Genovese, S. A subcritical butane-based extraction of non-psychoactive cannabinoids from hemp inflorescences. Ind. Crops Prod. 2022, 183, 114955. [Google Scholar] [CrossRef]
- Qamar, S.; Torres, Y.J.M.; Parekh, H.S.; Falconer, J.R. Extraction of medicinal cannabinoids through supercritical carbon dioxide technologies: A review. J. Chromatogr. B 2021, 1167, 122581. [Google Scholar] [CrossRef]
- Madia, V.M.; Di Santo, R.; Costi, R. Chapter 2-Medical cannabis and cannabinoids: How best to extract components from plant material. In Martin, Medicinal Usage of Cannabis and Cannabinoids; Preedy, V.R., Patel, V.B., Martin, C.R., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 15–23. [Google Scholar]
- Luo, J.; May, J.A. Enantioselective Total Synthesis of Cannabinoids via a Tandem Conjugate Addition/Enolate Alkylation Annulation with Ambiphilic Organoboronates. Org. Lett. 2023, 25, 708–713. [Google Scholar] [CrossRef]
- Kearney, S.E.; Gangano, A.J.; Barrus, D.G.; Rehrauer, K.J.; Reid, R.T.E.; Navaratne, P.V.; Tracy, E.K.; Roitberg, A.; Ghiviriga, I. Axially Chiral Cannabinoids: Design, Synthesis, and Cannabinoid Receptor Affinity. J. Am. Chem. Soc. 2023, 145, 13581–13591. [Google Scholar] [CrossRef]
- Wu, X.; Bopp, D.; Wallgren, J.; Dahlén, J.; Konradsson, P. Synthesis of nine potential synthetic cannabinoid metabolites with a 5F-4OH pentyl side chain from a scalable key intermediate. Synth. Commun. 2020, 51, 776–785. [Google Scholar] [CrossRef]
- Millimaci, A.; Trilles, R.; McNeely, J.; Brown, L.; Beeler, A.; Porco, J. Synthesis of Neocannabinoids Using Controlled Friedel-Crafts Reactions. ChemRxiv. Camb. Open Engag. 2023. [Google Scholar] [CrossRef]
- Chiurchiù, E.; Sampaolesi, S.; Allegrini, p.; Ciceri, D.; Ballini, R.; Palmieri, A. A Novel and Practical Continuous Flow Chemical Synthesis of Cannabidiol (CBD) and its CBDV and CBDB Analogues. Eur. J. Org. Chem. 2021, 8, 1286–1289. [Google Scholar] [CrossRef]
- Golliher, A.E.; Tenorio, A.J.; Dimauro, N.O.; Mairata, N.R.; Holguin, F.O.; Maio, W. Using (+)-carvone to access novel derivatives of (+)-ent-cannabidiol: The first asymmetric syntheses of (+)-ent-CBDP and (+)-ent-CBDV. Tetrahedron Lett. 2021, 67, 152891. [Google Scholar] [CrossRef]
- Yin, M.; Pan, G.; Tao, J.; Doblin, M.S.; Zeng, W.; Pan, L.; Zhao, L.; Li, Z.; Jiang, H.; Chang, L.; et al. Identification of MYB genes reveals their potential functions in cadmium stress response and the regulation of cannabinoid biosynthesis in hemp. Ind. Crops Prod. 2022, 180, 114607. [Google Scholar]
- Perez, E.; Fernandez, R.J.; Fitzgerald, C.; Rouzard, K.; Tamura, M.; Savile, C. In vitro and Clinical Evaluation of Cannabigerol (CBG) Produced via Yeast Biosynthesis: A Cannabinoid with a Broad Range of Anti-inflammatory and Skin Health Boosting Properties. Molecules 2022, 27, 491. [Google Scholar] [CrossRef]
- Degenhardt, F.; Stehle, F.; Kayser, O. Chapter 2—The Biosynthesis of Cannabinoids. In Handbook of Cannabis and Related Pathologies; Preedy, V.R., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 13–23. [Google Scholar]
- Luo, X.; Reiter, M.A.; d’Espaux, L.; Wong, J.; Denby, C.M.; Lechner, A.; Zhang, Y.; Grzybowski, A.T.; Harth, S.; Lin, W.; et al. Complete biosynthesis of cannabinoids and their unnatural analogues in yeast. Nature 2019, 567, 123–126. [Google Scholar] [PubMed]
- Carvalho, Â.; Hansen, E.H.; Kayser, O.; Carlsen, S.; Stehle, F. Designing microorganisms for heterologous biosynthesis of cannabinoids. FEMS Yeast Res. 2017, 17, fox037. [Google Scholar] [PubMed]
- Darigh, F.; Iranbakhsh, A.; Oraghi Ardebili, Z.; Ebadi, M. Non-thermal plasma improved callogenesis performance and elicited the production of cannabinoids by modifying DNA methylome, expression of WRKY1 and ERF1B transcription factors, and expression of genes that contributed to the biosynthesis of cannabinoids. Protoplasma 2023, 260, 159–170. [Google Scholar] [CrossRef]
- Singh, A.; Bilichak, A.; Kovalchuk, I. The genetics of Cannabis—Genomic variations of key synthases and their effect on cannabinoid content. Genome 2020, 64, 490–501. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.S.; Zhang, N.N.; Guo, T.T.; Sheen, L.T.; Ho, C.T.; Bai, N.S. The impact of phyto- and endo-cannabinoids on central nervous system diseases: A review. J. Tradit. Complement. Med. 2023, 13, 30–38. [Google Scholar] [CrossRef]
- Tóth, K.F.; Ádám, D.; Bíró, T.; Oláh, A. Cannabinoid Signaling in the Skin: Therapeutic Potential of the “C(ut)annabinoid” System. Molecules 2019, 24, 918. [Google Scholar]
- Busquets-García, A.; Bolaños, J.P.; Marsicano, G. Metabolic Messengers: Endocannabinoids. Nat Metab. 2022, 4, 848–855. [Google Scholar]
- Alizamini, M.M.; Li, Y.; Zhang, J.J.; Liang, J.; Haghparast, A. Endocannabinoids and addiction memory: Relevance to methamphetamine/morphine abuse. World J. Biol. Psychiatry 2022, 23, 743–763. [Google Scholar] [CrossRef]
- Navarro, G.; Varani, K.; Lillo, A.; Vincenzi, F.; Rivas-Santisteban, R.; Raïch, I.; Reyes-Resina, I.; Ferreiro-Vera, C.; Borea, P.A.; Sánchez de Medina, V.; et al. Pharmacological data of cannabidiol- and cannabigerol-type phytocannabinoids acting on cannabinoid CB1, CB2 and CB1/CB2 heteromer receptors. Pharmacol. Res. 2020, 159, 104940. [Google Scholar] [CrossRef] [PubMed]
- Jarocka-Karpowicz, I.; Biernacki, M.; Wroński, A.; Gęgotek, A.; Skrzydlewska, E. Cannabidiol Effects on Phospholipid Metabolism in Keratinocytes from Patients with Psoriasis Vulgaris. Biomolecules 2020, 10, 367. [Google Scholar] [CrossRef] [PubMed]
- Han, K.H.; Lim, S.; Ryu, J.; Lee, C.W.; Kim, Y.; Kang, J.H.; Kang, S.S.; Ahn, Y.K.; Park, C.S.; Kim, J.J. CB1 and CB2 cannabinoid receptors differentially regulate the production of reactive oxygen species by macrophages. Cardiovasc. Res. 2009, 84, 378–386. [Google Scholar] [CrossRef]
- Slominski, A.T.; Slominski, R.M.; Raman, C.; Chen, J.Y.; Athar, M.; Elmets, C. Neuroendocrine signaling in the skin with a special focus on the epidermal neuropeptides. Am. J. Physiol. Cell Physiol. 2022, 323, C1757–C1776. [Google Scholar] [CrossRef]
- Ramot, Y.; Oláh, A.; Paus, R. Cover Image: Neuroendocrine treatment of inherited keratin disorders by cannabinoids? Br. J. Dermatol. 2018, 178, 1469. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.; Wortsman, J. Neuroendocrinology of the skin. Endocr. Rev. 2000, 21, 457–487. [Google Scholar] [CrossRef]
- Yang, X.; Cai, M. New Insights into the Mutual Promotion of Rosacea, Anxiety, and Depression from Neuroendocrine Immune Aspects. Clin. Cosmet. Investig. Dermatol. 2023, 16, 1363–1371. [Google Scholar] [CrossRef]
- Jin, R.; Luo, L.; Zheng, J. The Trinity of Skin: Skin Homeostasis as a Neuro-Endocrine-Immune Organ. Life 2022, 12, 725. [Google Scholar] [CrossRef]
- Bocheva, G.; Slominski, R.M.; Slominski, A.T. Neuroendocrine Aspects of Skin Aging. Int. J. Mol. Sci. 2019, 20, 2798. [Google Scholar] [CrossRef]
- Lipina, C.; Hundal, H.S. Modulation of cellular redox homeostasis by the endocannabinoid system. Open Biol. 2016, 6, 150276. [Google Scholar] [CrossRef]
- Ambrożewicz, E.; Wójcik, P.; Wroński, A.; Łuczaj, W.; Jastrząb, A.; Žarković, N.; Skrzydlewska, E. Pathophysiological Alterations of Redox Signaling and Endocannabinoid System in Granulocytes and Plasma of Psoriatic Patients. Cells 2018, 7, 159. [Google Scholar] [CrossRef] [PubMed]
- Belikov, A.V.; Schraven, B.; Simeoni, L. T cells and reactive oxygen species. J. Biomed. Sci. 2015, 22, 85. [Google Scholar] [CrossRef] [PubMed]
- Dawidowicz, A.L.; Olszowy-Tomczyk, M.; Typek, R. CBG, CBD, Δ9-THC, CBN, CBGA, CBDA and Δ9-THCA as antioxidant agents and their intervention abilities in antioxidant action. Fitoterapia 2021, 152, 104915. [Google Scholar] [CrossRef] [PubMed]
- Atalay, S.; Dobrzyńska, I.; Gęgotek, A.; Skrzydlewska, E. Cannabidiol protects keratinocyte cell membranes following exposure to UVB and hydrogen peroxide. Redox Biol. 2020, 36, 101613. [Google Scholar] [CrossRef]
- Kim, J.; Choi, J.Y.; Seo, J.; Choi, I.S. Neuroprotective Effect of Cannabidiol Against Hydrogen Peroxide in Hippocampal Neuron Culture. Cannabis Cannabinoid Res. 2021, 6, 40–47. [Google Scholar] [CrossRef]
- Gęgotek, A.; Atalay, S.; Rogowska-Wrzesińska, A.; Skrzydlewska, E. The Effect of Cannabidiol on UV-Induced Changes in Intracellular Signaling of 3D-Cultured Skin Keratinocytes. Int. J. Mol. Sci 2021, 22, 1501. [Google Scholar] [CrossRef]
- Chen, J.; Hou, C.; Chen, X.; Wang, D.; Yang, P.; He, X.; Zhou, J.; Li, H. Protective effect of cannabidiol on hydrogen peroxide-induced apoptosis, inflammation and oxidative stress in nucleus pulposus cells. Mol. Med. Rep. 2016, 14, 2321–2327. [Google Scholar] [CrossRef]
- Norooznezhad, A.H.; Norooznezhad, F. Cannabinoids: Possible agents for treatment of psoriasis via suppression of angiogenesis and inflammation. Med. Hypotheses 2017, 99, 15–18. [Google Scholar] [CrossRef]
- Wilkinson, J.D.; Williamson, E.M. Cannabinoids inhibit human keratinocyte proliferation through a non-CB1/CB2 mechanism and have a potential therapeutic value in the treatment of psoriasis. J. Dermatol. Sci. 2007, 45, 87–92. [Google Scholar] [CrossRef]
- Wroński, A.; Jarocka-Karpowicz, I.; Stasiewicz, A.; Skrzydlewska, E. Phytocannabinoids in the Pharmacotherapy of Psoriasis. Molecules 2023, 28, 1192. [Google Scholar] [CrossRef]
- Leong, T.T.; Fearon, U.; Veale, D.J. Angiogenesis in psoriasis and psoriatic arthritis: Clues to disease pathogenesis. Curr. Rheumatol. Rep. 2005, 7, 325–329. [Google Scholar] [CrossRef] [PubMed]
- Cintosun, A.; Lara-Corrales, I.; Pope, E. Mechanisms of Cannabinoids and Potential Applicability to Skin Diseases. Clin. Drug Investig. 2020, 40, 293–304. [Google Scholar] [CrossRef] [PubMed]
- Di Meo, C.; Tortolani, D.; Standoli, S.; Angelucci, C.B.; Fanti, F.; Leuti, A.; Sergi, M.; Kadhim, S.; Hsu, E.; Rapino, C.; et al. Effects of Rare Phytocannabinoids on the Endocannabinoid System of Human Keratinocytes. Int. J. Mol. Sci. 2022, 23, 5430. [Google Scholar] [CrossRef] [PubMed]
- Stebulis, J.A.; Johnson, D.R.; Rossetti, R.G.; Burstein, S.H.; Zurier, R.B. Ajulemic acid, a synthetic cannabinoid acid, induces an antiinflammatory profile of eicosanoids in human synovial cells. Life Sci. 2008, 83, 666–670. [Google Scholar] [CrossRef]
- Oláh, A.; Markovics, A.; Szabó-Papp, J.; Szabó, P.T.; Stott, C.; Zouboulis, C.C.; Bíró, T. Differential effectiveness of selected non-psychotropic phytocannabinoids on human sebocyte functions implicates their introduction in dry/seborrhoeic skin and acne treatment. Exp. Dermatol. 2016, 25, 701–707. [Google Scholar] [CrossRef]
- Peyravian, N.; Deo, S.; Daunert, S.; Jimenez, J.J. The Anti-Inflammatory Effects of Cannabidiol (CBD) on Acne. J. Inflamm. Res. 2022, 15, 2795–2801. [Google Scholar] [CrossRef]
- Todurga Seven, Z.G.; Tombulturk, F.K.; Gokdemir, S.; Ozyazgan, S. The Effects of the Heat Shock Protein 90 Inhibitor 17-Allylamino-17-Demethoxygeldanamycin, Cannabinoid Agonist WIN 55,212-2, and Nitric Oxide Synthase Inhibitor Nω-Nitro-L-Arginine Methyl Ester Hydrochloride on the Serotonin and Dry Skin-Induced Itch. Int. Arch. Allergy Immunol. 2022, 183, 443–452. [Google Scholar] [CrossRef]
- Silvestri, C.; Paris, D.; Martella, A.; Melck, D.; Guadagnino, I.; Cawthorne, M.; Motta, A.; Di Marzo, V. Two non-psychoactive cannabinoids reduce intracellular lipid levels and inhibit hepatosteatosis. J. Hepatol. 2015, 62, 1382–1390. [Google Scholar] [CrossRef]
- Abioye, A.; Ayodele, O.; Marinkovic, A.; Patidar, R.; Akinwekomi, A.; Sanyaolu, A. Δ9-Tetrahydrocannabivarin (THCV): A commentary on potential therapeutic benefit for the management of obesity and diabetes. J. Cannabis Res. 2020, 2, 6. [Google Scholar] [CrossRef]
- Xi, Z.X.; Muldoon, P.; Wang, X.F.; Bi, G.H.; Damaj, M.I.; Lichtman, A.H.; Pertwee, R.G.; Gardner, E.L. Δ8-Tetrahydrocannabivarin has potent anti-nicotine effects in several rodent models of nicotine dependence. Br. J. Pharmacol. 2019, 176, 4773–4784. [Google Scholar] [CrossRef]
- Salau, O.; Bagde, A.; Kalvala, A.; Singh, M. Enhancement of transdermal permeation of cannabinoids and their pharmacodynamic evaluation in rats. Int. J. Pharm. 2022, 624, 122016. [Google Scholar] [CrossRef]
- Facheris, P.; Jeffery, J.; Del Duca, E.; Guttman-Yassky, E. The translational revolution in atopic dermatitis: The paradigm shift from pathogenesis to treatment. Cell Mol. Immunol. 2023, 20, 448–474. [Google Scholar] [CrossRef] [PubMed]
- Kleinman, E.; Laborada, J.; Metterle, L.; Eichenfield, L.F. What’s New in Topicals for Atopic Dermatitis? Am. J. Clin. Dermatol. 2022, 23, 595–603. [Google Scholar] [CrossRef] [PubMed]
- Freitas, E.; Gooderham, M.; Torres, T. New Topical Therapies in Development for Atopic Dermatitis. Drugs 2022, 82, 843–853. [Google Scholar] [CrossRef] [PubMed]
- Deiana, S. Chapter 99-Potential Medical Uses of Cannabigerol: A Brief Overview. In Handbook of Cannabis and Related Pathologies; Preedy, V.R., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 958–967. [Google Scholar]
- Gugliandolo, A.; Pollastro, F.; Grassi, G.; Bramanti, P.; Mazzon, E. In vitro model of neuroinflammation: Efficacy of cannabigerol, a non-psychoactive cannabinoid. Int. J. Mol. Sci. 2018, 19, 1992. [Google Scholar] [CrossRef] [PubMed]
- Anokwuru, C.P.; Makolo, F.L.; Sandasi, M.; Tankeu, S.Y.; Elisha, I.L.; Agoni, C.; Combrinck, S.; Viljoen, A. Cannabigerol: A bibliometric overview and review of research on an important phytocannabinoid. Phytochem. Rev. 2022, 21, 1523–1547. [Google Scholar] [CrossRef]
- Scheau, C.; Badarau, I.A.; Mihai, L.-G.; Scheau, A.-E.; Costache, D.O.; Constantin, C.; Calina, D.; Caruntu, C.; Costache, R.S.; Caruntu, A. Cannabinoids in the Pathophysiology of Skin Inflammation. Molecules 2020, 25, 652. [Google Scholar] [CrossRef]
- Avila, C.; Massick, S.; Kaffenberger, B.H.; Kwatra, S.G.; Bechtel, M. Cannabinoids for the treatment of chronic pruritus: A review. J. Am. Acad. Dermatol. 2020, 82, 1205–1212. [Google Scholar] [CrossRef]
- Sampson, P.B. Phytocannabinoid Pharmacology: Medicinal Properties of Cannabis sativa Constituents Aside from the “Big Two”. J. Nat. Prod. 2021, 84, 142–160. [Google Scholar] [CrossRef]
- Oultram, J.M.J.; Pegler, J.L.; Bowser, T.A.; Ney, L.J.; Eamens, A.L.; Grof, C.P.L. Cannabis sativa: Interdisciplinary Strategies and Avenues for Medical and Commercial Progression Outside of CBD and THC. Biomedicines 2021, 9, 234. [Google Scholar] [CrossRef]
- Karsak, M.; Gaffal, E.; Date, R.; Wang-Eckhardt, L.; Rehnelt, J.; Petrosino, S.; Starowicz, K.; Steuder, R.; Schlicker, E.; Cravatt, B.; et al. Attenuation of Allergic Contact Dermatitis Through the Endocannabinoid System. Science 2007, 316, 1494–1497. [Google Scholar] [CrossRef] [PubMed]
- Petrosino, S.; Cristino, L.; Karsak, M.; Gaffal, E.; Ueda, N.; Tüting, T.; Bisogno, T.; De Filippis, D.; D’Amico, A.; Saturnino, C.; et al. Protective role of palmitoylethanolamide in contact allergic dermatitis. Allergy 2010, 65, 698–711. [Google Scholar] [CrossRef] [PubMed]
- Vaia, M.; Petrosino, S.; De Filippis, D.; Negro, L.; Guarino, A.; Carnuccio, R.; Di Marzo, V.; Iuvone, T. Palmitoylethanolamide reduces inflammation and itch in a mouse model of contact allergic dermatitis. Eur. J. Pharmacol. 2016, 791, 669–674. [Google Scholar] [CrossRef] [PubMed]
- Iannotti, F.A.; De Maio, F.; Panza, E.; Appendino, G.; Taglialatela-Scafati, O.; De Petrocellis, L.; Amodeo, P.; Vitale, R.M. Identification and Characterization of Cannabimovone, a Cannabinoid from Cannabis sativa, as a Novel PPARγ Agonist via a Combined Computational and Functional Study. Molecules 2020, 25, 1119. [Google Scholar] [CrossRef]
- Dennis, D.G.; Anand, S.D.; Lopez, A.J.; Petrovčič, J.; Das, A.; Sarlah, D. Synthesis of the Cannabimovone and Cannabifuran Class of Minor Phytocannabinoids and Their Anti-inflammatory Activity. J. Org. Chem. 2022, 87, 6075–6086. [Google Scholar] [CrossRef]
- Ang, S.P.; Sidharthan, S.; Lai, W.; Hussain, N.; Patel, K.V.; Gulati, A.; Orhurhu, V. Cannabinoids as a Potential Alternative to Opioids in the Management of Various Pain Subtypes: Benefits, Limitations, and Risks. Pain Ther. 2023, 12, 355–375. [Google Scholar] [CrossRef]
- Tijani, A.O.; Thakur, D.; Mishra, D.; Frempong, D.; Chukwunyere, U.I.; Puri, A. Delivering therapeutic cannabinoids via skin: Current state and future perspectives. J. Control. Release 2021, 334, 427–451. [Google Scholar] [CrossRef]
| Dermatological Diseases | Cannabinoid | Potential Mechanism of Action | Literature |
|---|---|---|---|
| Psoriasis | CBN CBC Ajulemic acid | Inhibition of excessive keratinocyte divisions; inhibition of the proliferation of keratinocytes; inhibition of the signaling of the Wnt/β-catenin pathway; inhibition of the activity of pro-inflammatory cytokines and other inflammatory mediators; increasing the synthesis of 15d-PGJ2—prostaglandins; the mechanism of action is the suppression of cyclooxygenase-2 (COX-2) | [13,40,41] |
| Acne | CBDV CBC THCV WIN-55,212-2 Ajulemic acid | Reduction in the production of sebum; inhibition of the growth of bacteria C. acnes; regulation of the secretion of sebum; anti-inflammatory and antibacterial properties | [47,48,49,50,51] |
| Atopic Dermatitis (AD) | CBG CBDV | The ability to inhibit the action of enzymes responsible for the synthesis of fatty acids, such as arachidonic acid, antibacterial and antifungal activity; the ability to inhibit the activity of pro-inflammatory cytokines such as interleukin 6 and 17A | [57,58,59,60,61,62] |
| Allergic Contact Dermatitis (ACZ) | CBD THC | Relieving the symptoms of ACZ by reducing inflammation and itching of the skin | [64,65,66] |
| Anti-inflamatory (requires further detailed research) | CBM CBE | Inhibition of pro-inflammatory biomarkers | [67,68] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwiecień, E.; Kowalczuk, D. Therapeutic Potential of Minor Cannabinoids in Dermatological Diseases—A Synthetic Review. Molecules 2023, 28, 6149. https://doi.org/10.3390/molecules28166149
Kwiecień E, Kowalczuk D. Therapeutic Potential of Minor Cannabinoids in Dermatological Diseases—A Synthetic Review. Molecules. 2023; 28(16):6149. https://doi.org/10.3390/molecules28166149
Chicago/Turabian StyleKwiecień, Emilia, and Dorota Kowalczuk. 2023. "Therapeutic Potential of Minor Cannabinoids in Dermatological Diseases—A Synthetic Review" Molecules 28, no. 16: 6149. https://doi.org/10.3390/molecules28166149
APA StyleKwiecień, E., & Kowalczuk, D. (2023). Therapeutic Potential of Minor Cannabinoids in Dermatological Diseases—A Synthetic Review. Molecules, 28(16), 6149. https://doi.org/10.3390/molecules28166149







