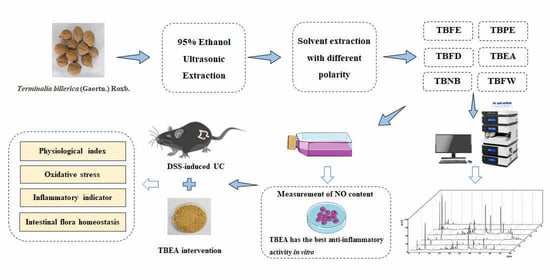
Terminalia bellirica Fruit Extract Alleviates DSS-Induced Ulcerative Colitis by Regulating Gut Microbiota, Inflammatory Mediators, and Cytokines
Abstract
1. Introduction
2. Results
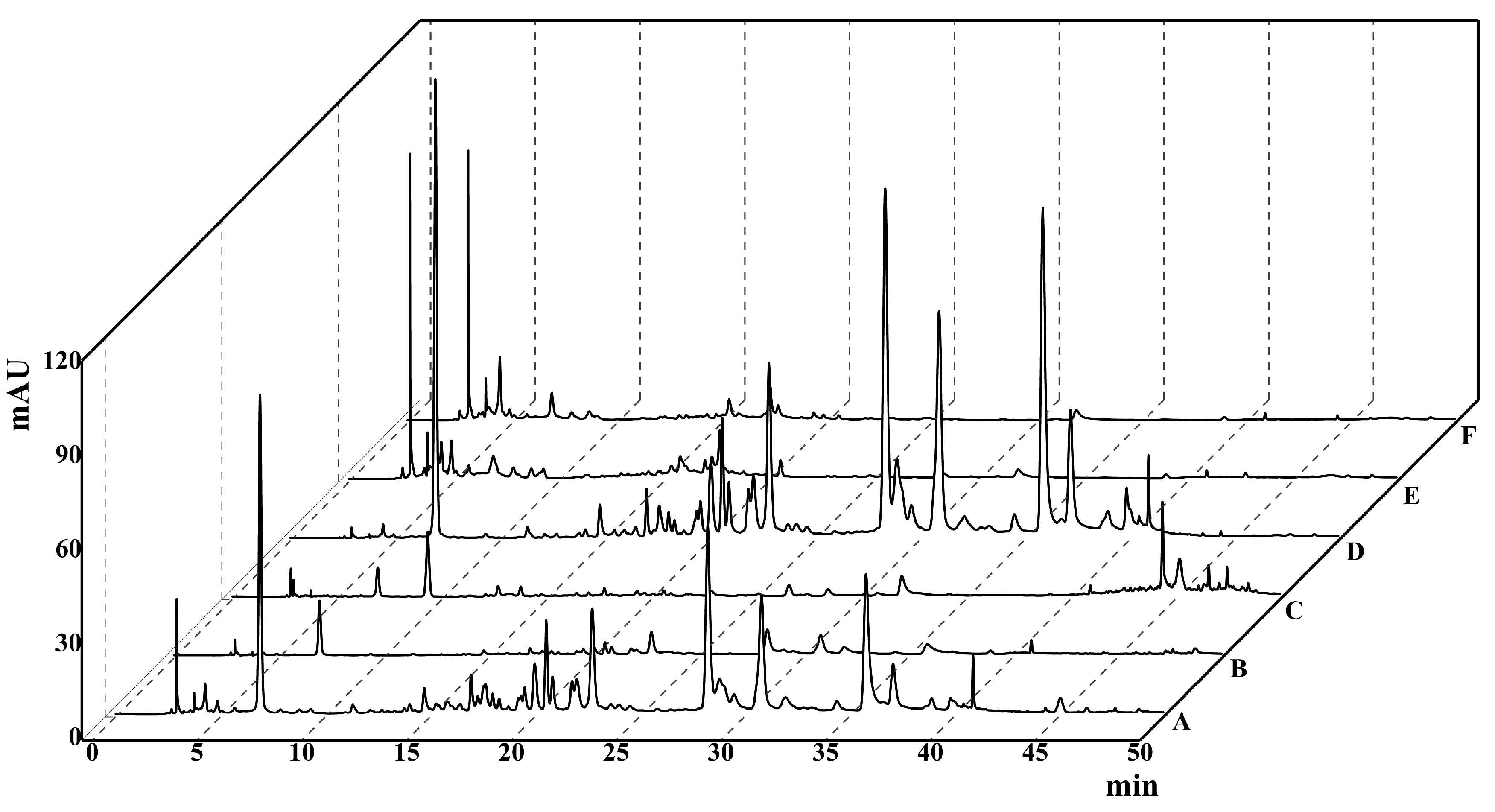
2.1. HPLC Chromatogram
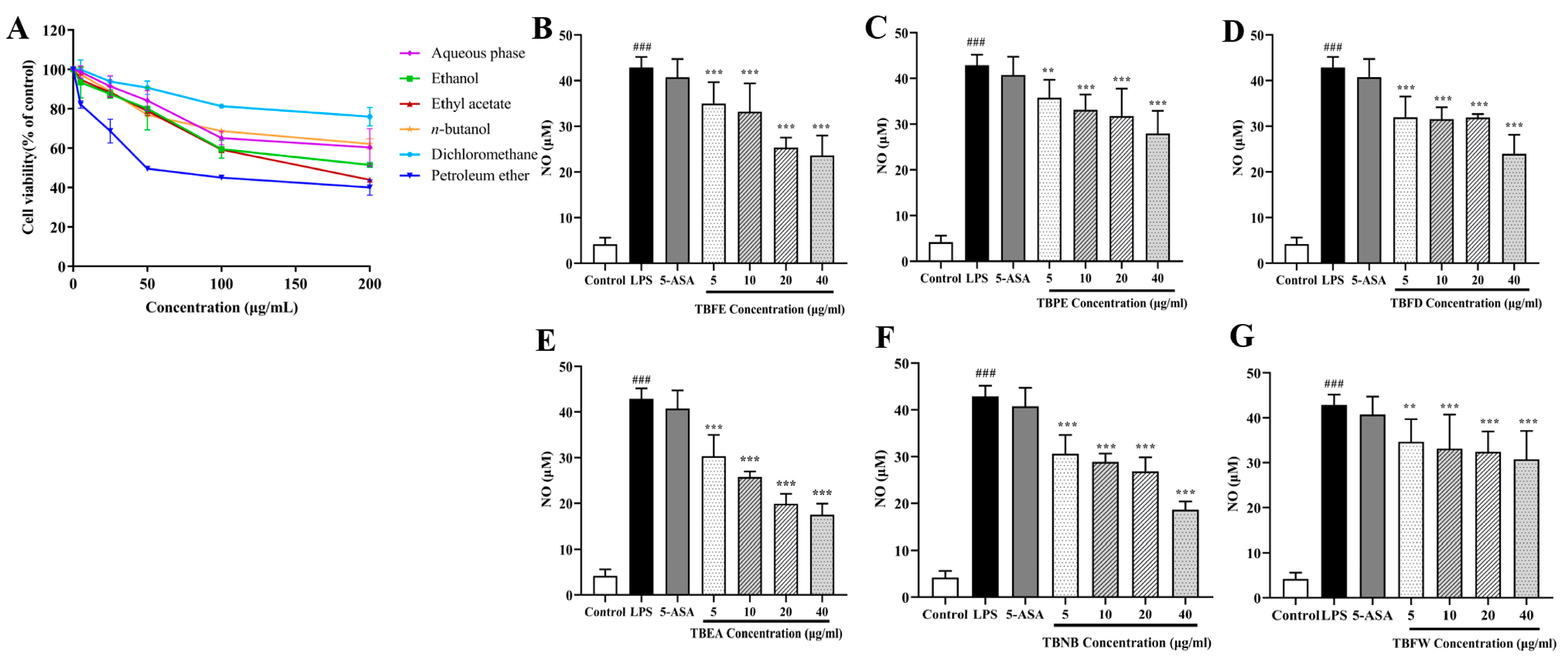
2.2. Effect of TBFE and Five Extract Fractions on Macrophage Viability and In Vitro Anti-Inflammatory Activity
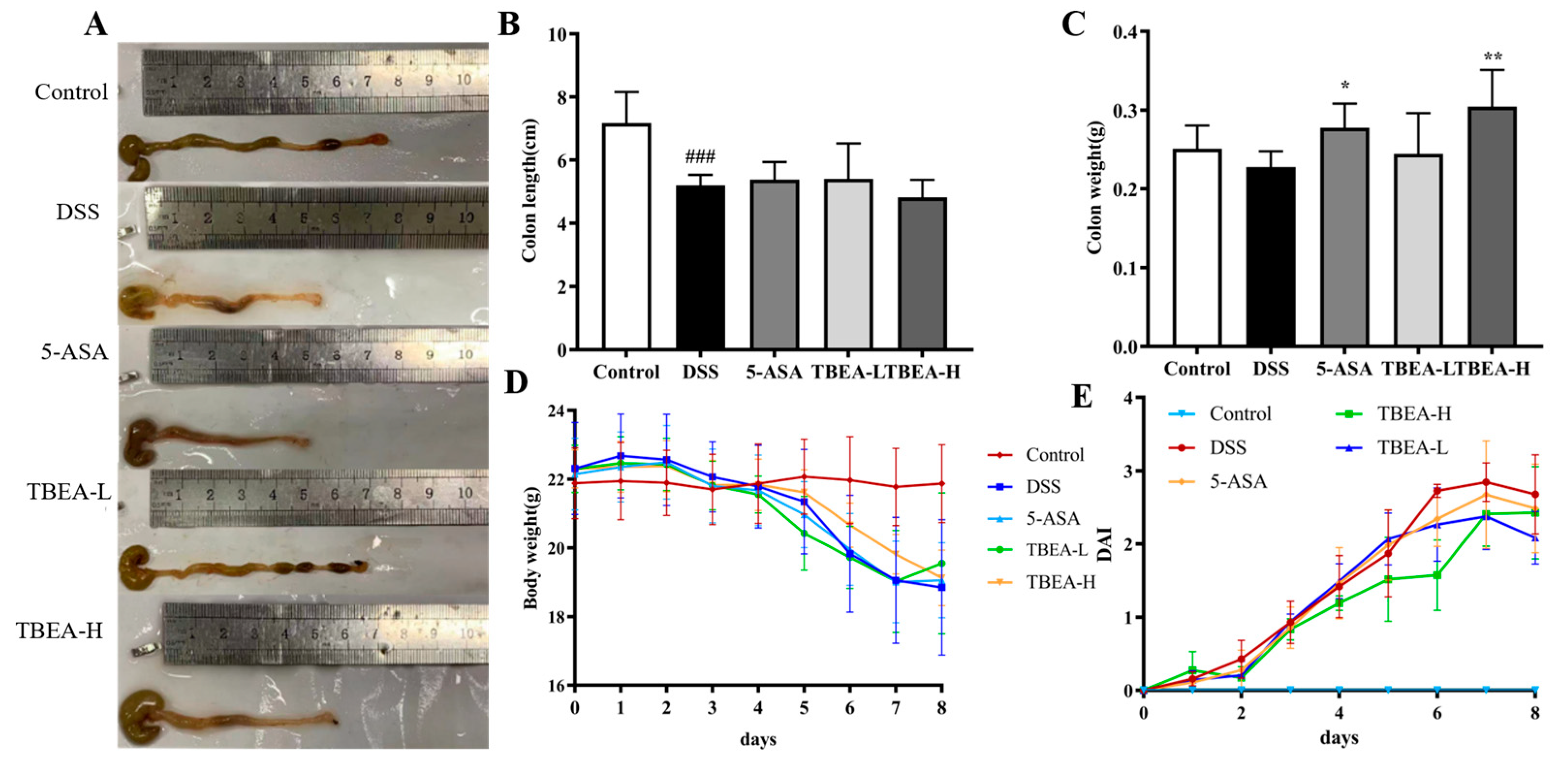
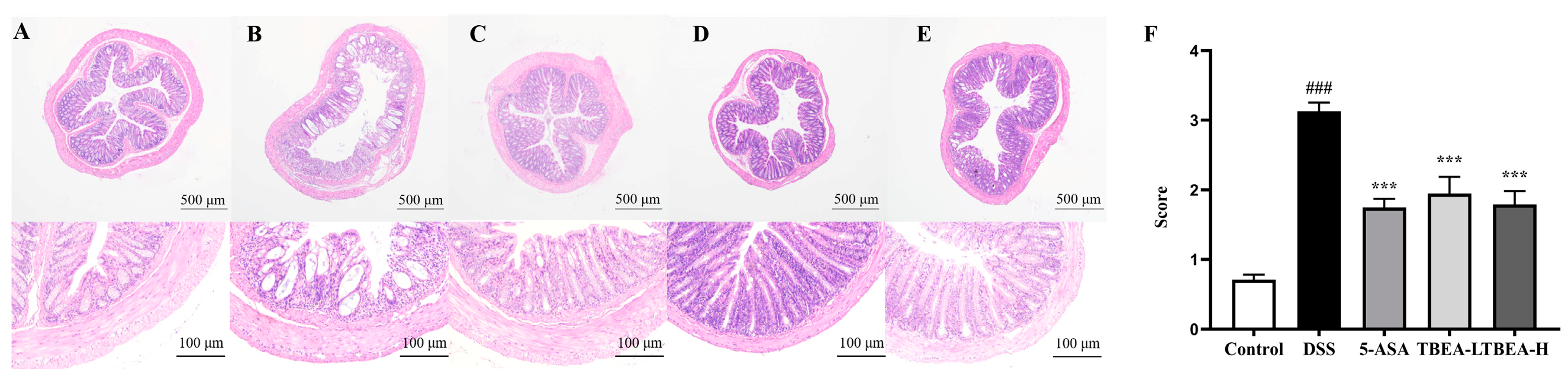
2.3. Amelioration of Physiological Characteristics and Colonic Injury in UC Mice with TBEA
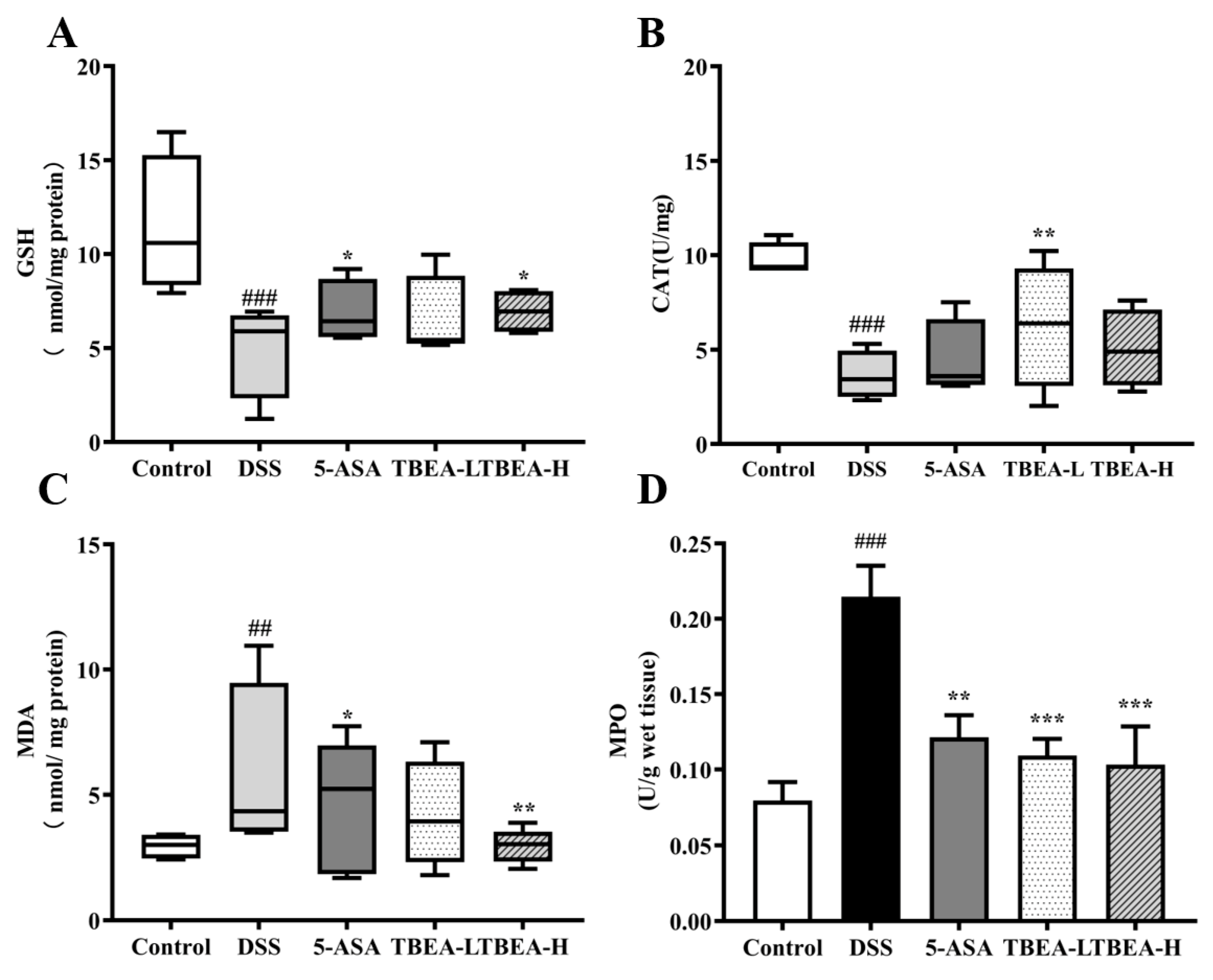
2.4. TBEA Reduces the Level of Oxidative Stress in the Colonic Tissue of UC Mice
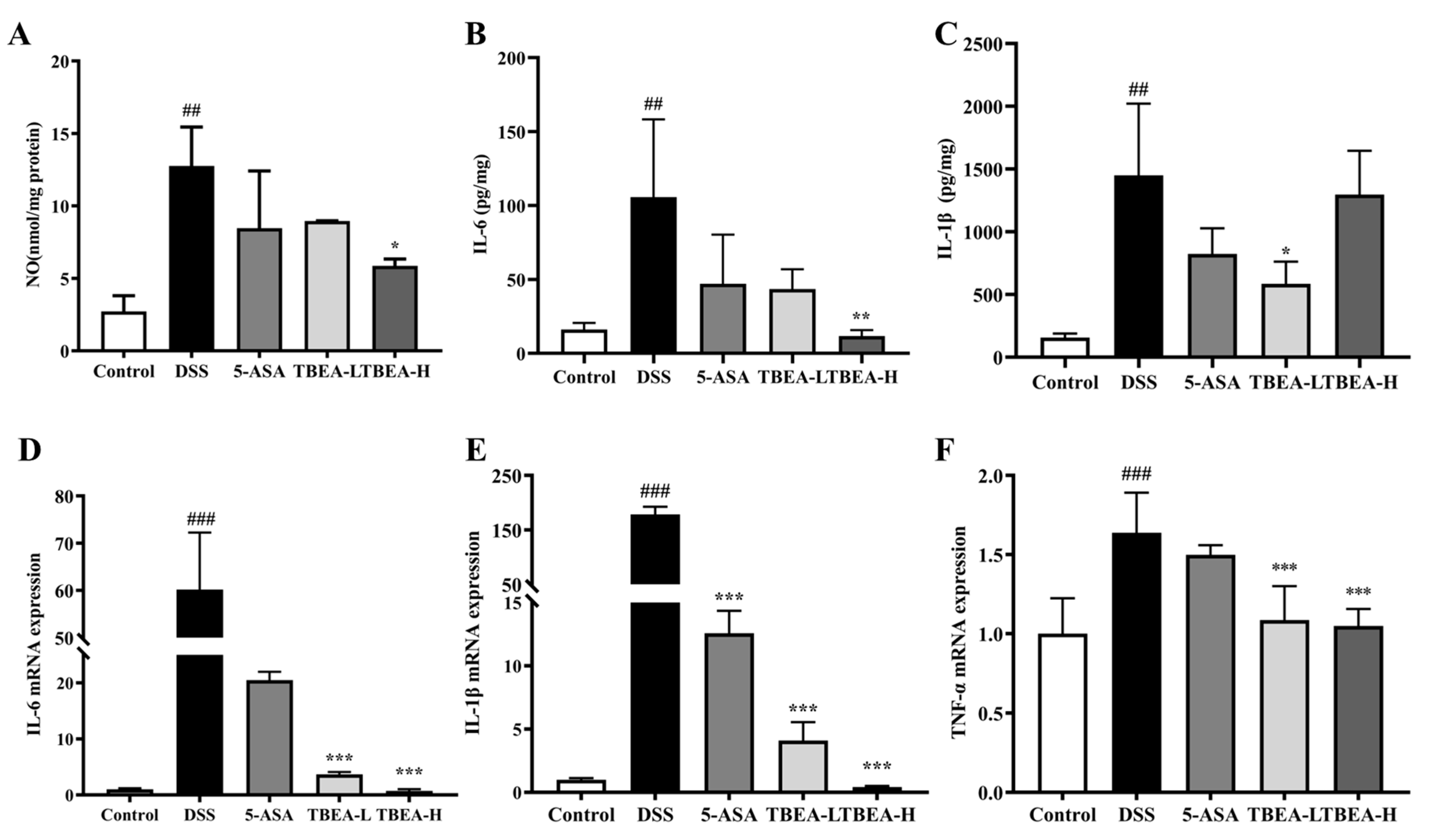
2.5. TBEA Reduces Pro-Inflammatory Cytokine Contents in the Colonic Tissue of UC Mice
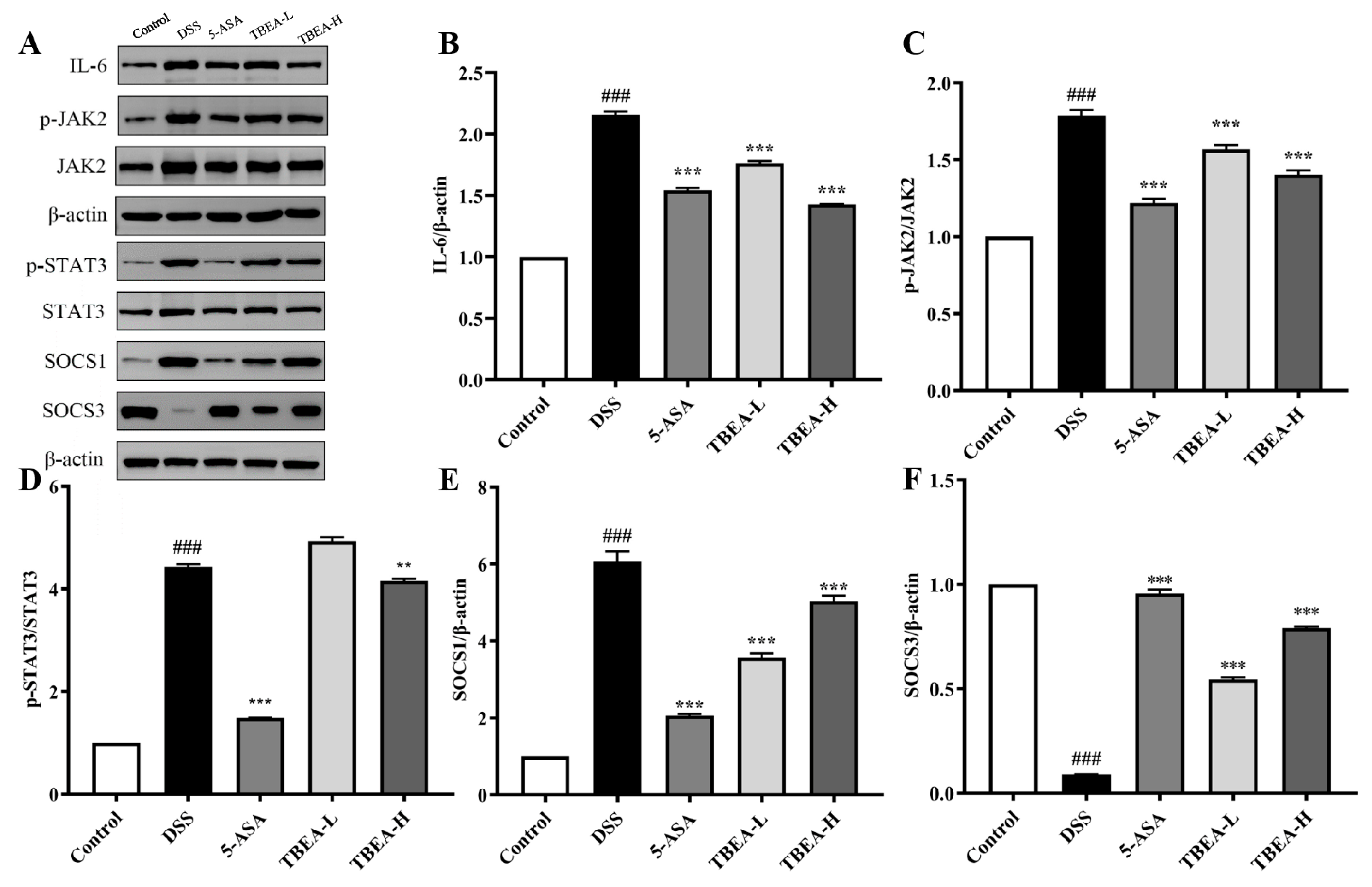
2.6. TBFE Inhibits the IL-6/JAK2/STAT3 Inflammatory Signaling Pathway
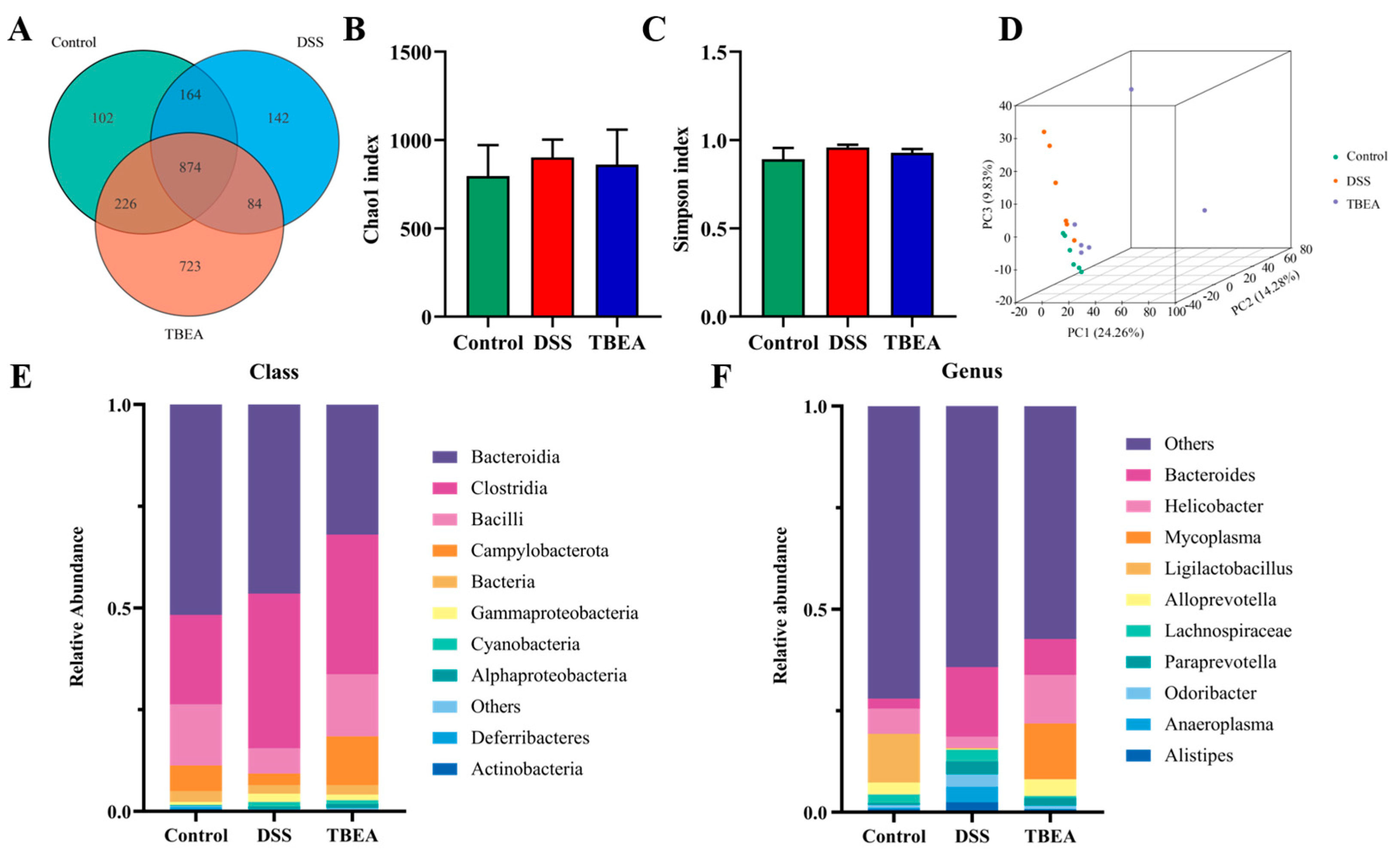
2.7. Influences of TBEA Treatment on Intestinal Flora
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Preparation and Evaluation of the TBF Extracts
4.3. High-Performance Liquid Chromatography Analysis
4.4. Cell Viability Determination
4.5. NO Content Determination
4.6. Animals and Experimental Design
4.7. DAI
4.8. Histological Analysis of the Colon
4.9. Determination of Oxidative Stress-Related Indicators in the Mice Colon
4.10. Measurement of Colonic Inflammatory Factor Levels in Mice
4.11. Analysis of Inflammatory Factors using qPCR
4.12. Western Blot Analysis
4.13. Gut Microbiota Analysis and 16S rDNA Sequencing
4.14. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Al-Horani, R.; Spanudakis, E.; Hamad, B. The market for ulcerative colitis. Nat. Rev. Drug Discov. 2022, 21, 15–16. [Google Scholar] [CrossRef] [PubMed]
- Gajendran, M.; Loganathan, P.; Jimenez, G.; Catinella, A.P.; Ng, N.; Umapathy, C.; Ziade, N.; Hashash, J.G. A comprehensive review and update on ulcerative colitis. Disease-a-Month DM 2019, 65, 100851. [Google Scholar] [CrossRef] [PubMed]
- Ordás, I.; Eckmann, L.; Talamini, M.; Baumgart, D.C.; Sandborn, W.J. Ulcerative colitis. Lancet 2012, 380, 1606–1619. [Google Scholar] [CrossRef] [PubMed]
- Ungaro, R.; Mehandru, S.; Allen, P.B.; Peyrin-Biroulet, L.; Colombel, J.-F. Ulcerative colitis. Lancet 2017, 389, 1756–1770. [Google Scholar] [CrossRef] [PubMed]
- Segal, J.P.; LeBlanc, J.-F.; Hart, A.L. Ulcerative colitis: An update. Clin. Med. 2021, 21, 135–139. [Google Scholar] [CrossRef]
- Liu, Y.; Li, B.-G.; Su, Y.-H.; Zhao, R.-X.; Song, P.; Li, H.; Cui, X.-H.; Gao, H.-M.; Zhai, R.-X.; Fu, X.-J.; et al. Potential activity of Traditional Chinese Medicine against Ulcerative colitis: A review. J. Ethnopharmacol. 2022, 289, 115084. [Google Scholar] [CrossRef]
- Che, Q.; Luo, T.; Shi, J.; He, Y.; Xu, D.-L. Mechanisms by Which Traditional Chinese Medicines Influence the Intestinal Flora and Intestinal Barrier. Front. Cell. Infect. Microbiol. 2022, 12, 863779. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Y.; Su, Y.; Fang, X.; Guo, W. The alleviating effect and mechanism of Bilobalide on ulcerative colitis. Food Funct. 2021, 12, 6226–6239. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, L.; Chan, J.C.P.; Wang, X.; Zhao, C.; Xu, Y.; Xiong, W.; Chung, W.C.; Liang, F.; Wang, X.; et al. Chinese herbal medicines in the treatment of ulcerative colitis: A review. Chin. Med. 2022, 17, 43. [Google Scholar] [CrossRef]
- Commission, C.P. Pharmacopoeia of the People’s Republic of China; China Medical Science Press: Beijing, China, 2020; p. 75. [Google Scholar]
- Pfundstein, B.; El Desouky, S.K.; Hull, W.E.; Haubner, R.; Erben, G.; Owen, R.W. Polyphenolic compounds in the fruits of Egyptian medicinal plants (Terminalia bellerica, Terminalia chebula and Terminalia horrida): Characterization, quantitation and determination of antioxidant capacities. Phytochemistry 2010, 71, 1132–1148. [Google Scholar] [CrossRef]
- Nampoothiri, S.V.; Prathapan, A.; Cherian, O.L.; Raghu, K.; Venugopalan, V.; Sundaresan, A. In vitro antioxidant and inhibitory potential of Terminalia bellerica and Emblica officinalis fruits against LDL oxidation and key enzymes linked to type 2 diabetes. Food Chem. Toxicol. 2011, 49, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Qu, L.; Shi, K.; Xu, J.; Liu, C.; Ke, C.; Zhan, X.; Xu, K.; Liu, Y. Atractylenolide-1 targets SPHK1 and B4GALT2 to regulate intestinal metabolism and flora composition to improve inflammation in mice with colitis. Phytomedicine 2022, 98, 153945. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Kishimoto, Y.; Sasaki, M.; Sato, A.; Kamiya, T.; Kondo, K.; Iida, K. Terminalia bellirica (Gaertn.) Roxb. Extract and Gallic Acid Attenuate LPS-Induced Inflammation and Oxidative Stress via MAPK/NF-κB and Akt/AMPK/Nrf2 Pathways. Oxidative Med. Cell. Longev. 2018, 2018, 9364364. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Wang, H.; Zhou, Y.; Zhang, H.; Zhou, H.; Zhang, X. Slimy partners: The mucus barrier and gut microbiome in ulcerative colitis. Exp. Mol. Med. 2021, 53, 772–787. [Google Scholar] [CrossRef] [PubMed]
- Yao, D.; Dong, M.; Dai, C.; Wu, S. Inflammation and Inflammatory Cytokine Contribute to the Initiation and Development of Ulcerative Colitis and Its Associated Cancer. Inflamm. Bowel Dis. 2019, 25, 1595–1602. [Google Scholar] [CrossRef] [PubMed]
- Feuerstein, J.D.; Moss, A.C.; Farraye, F.A. Ulcerative Colitis. Mayo Clin. Proc. 2019, 94, 1357–1373. [Google Scholar] [CrossRef]
- Akter, S.; Begum, T.; Begum, R.; Tonny, T.; Yasmin, M.; Shifa, S.; Afroze, F. Phytochemical Analysis and Investigation of Anti-Inflammatory and Anti-Ulcer Activity of Terminalia Bellirica Leaves Extract. Int. J. Pharmacogn. 2019, 6, 54–65. [Google Scholar] [CrossRef]
- Pandey, G.; Gupta, S.S.; Bhatia, A.; Sidhu, O.; Rawat, A.; Rao, C.V. Grilling enhances antidiarrheal activity of Terminalia bellerica Roxb. fruits. J. Ethnopharmacol. 2017, 202, 63–66. [Google Scholar] [CrossRef]
- Gupta, A.; Kumar, R.; Bhattacharyya, P.; Bishayee, A.; Pandey, A.K. Terminalia bellirica (Gaertn.) roxb. (Bahera) in health and disease: A systematic and comprehensive review. Phytomedicine Int. J. Phytother. Phytopharm. 2020, 77, 153278. [Google Scholar] [CrossRef]
- Shin, H.-K.; Seo, C.-S.; Jeong, S.-J.; Yoo, S.-R.; Lee, N.-R. Quantitative Analysis and in vitro Anti-inflammatory effects of gallic acid, ellagic acid, and quercetin from radix sanguisorbae. Pharmacogn. Mag. 2016, 12, 104–108. [Google Scholar] [CrossRef]
- BenSaad, L.A.; Kim, K.H.; Quah, C.C.; Kim, W.R.; Shahimi, M. Anti-inflammatory potential of ellagic acid, gallic acid and punicalagin A&B isolated from Punica granatum. BMC Complement. Altern. Med. 2017, 17, 47. [Google Scholar] [CrossRef]
- Wu, Y.; Li, K.; Zeng, M.; Qiao, B.; Zhou, B. Serum Metabolomics Analysis of the Anti-Inflammatory Effects of Gallic Acid on Rats with Acute Inflammation. Front. Pharmacol. 2022, 13, 830439. [Google Scholar] [CrossRef]
- Yu, T.-Y.; Feng, Y.-M.; Kong, W.-S.; Li, S.-N.; Sun, X.-J.; Zhou, G.; Xie, R.-F.; Zhou, X. Gallic acid ameliorates dextran sulfate sodium-induced ulcerative colitis in mice via inhibiting NLRP3 inflammasome. Front. Pharmacol. 2023, 14, 1095721. [Google Scholar] [CrossRef]
- Reddy, D.B.; Reddanna, P. Chebulagic acid (CA) attenuates LPS-induced inflammation by suppressing NF-κB and MAPK activation in RAW 264.7 macrophages. Biochem. Biophys. Res. Commun. 2009, 381, 112–117. [Google Scholar] [CrossRef]
- Lu, K.; Iwenofu, O.H.; Mitra, R.; Mo, X.; Dasgupta, P.S.; Basu, S. Chebulinic acid is a safe and effective antiangiogenic agent in collagen-induced arthritis in mice. Arthritis Res. Ther. 2020, 22, 273. [Google Scholar] [CrossRef]
- Gautam, M.K.; Goel, S.; Ghatule, R.R.; Singh, A.; Nath, G.; Goel, R.K. Curative effect of Terminalia chebula extract on acetic acid-induced experimental colitis: Role of antioxidants, free radicals and acute inflammatory marker. Inflammopharmacology 2013, 21, 377–383. [Google Scholar] [CrossRef]
- Cao, Q.; Gao, X.; Lin, Y.; Yue, C.; Wang, Y.; Quan, F.; Zhang, Z.; Liu, X.; Lu, Y.; Zhan, Y.; et al. Thymopentin ameliorates dextran sulfate sodium-induced colitis by triggering the production of IL-22 in both innate and adaptive lymphocytes. Theranostics 2019, 9, 7490–7505. [Google Scholar] [CrossRef]
- Ma, Y.; Hu, C.; Yan, W.; Jiang, H.; Liu, G. Lactobacillus pentosus Increases the Abundance of Akkermansia and Affects the Serum Metabolome to Alleviate DSS-Induced Colitis in a Murine Model. Front. Cell Dev. Biol. 2020, 8, 591408. [Google Scholar] [CrossRef] [PubMed]
- Fay, N.C.; Muthusamy, B.-P.; Nyugen, L.P.; Desai, R.C.; Taverner, A.; MacKay, J.; Seung, M.; Hunter, T.; Liu, K.; Chandalia, A.; et al. A Novel Fusion of IL-10 Engineered to Traffic across Intestinal Epithelium to Treat Colitis. J. Immunol. 2020, 205, 3191–3204. [Google Scholar] [CrossRef] [PubMed]
- Damiani, C.R.; Benetton, C.A.; Stoffel, C.; Bardini, K.C.; Cardoso, V.H.; Di Giunta, G.; Pinho, R.A.; Dal-Pizzol, F.; Streck, E.L. Oxidative stress and metabolism in animal model of colitis induced by dextran sulfate sodium. J. Gastroenterol. Hepatol. 2007, 22, 1846–1851. [Google Scholar] [CrossRef]
- Elmaksoud, H.A.A.; Motawea, M.H.; Desoky, A.A.; Elharrif, M.G.; Ibrahimi, A. Hydroxytyrosol alleviate intestinal inflammation, oxidative stress and apoptosis resulted in ulcerative colitis. Biomed. Pharmacother. 2021, 142, 112073. [Google Scholar] [CrossRef] [PubMed]
- Ren, D.-D.; Chen, K.-C.; Li, S.-S.; Zhang, Y.-T.; Li, Z.-M.; Liu, S.; Sun, Y.-S. Panax quinquefolius polysaccharides ameliorate ulcerative colitis in mice induced by dextran sulfate sodium. Front. Immunol. 2023, 14, 1161625. [Google Scholar] [CrossRef]
- Zhao, J.; Wu, R.; Wei, P.; Ma, Z.; Pei, H.; Hu, J.; Wen, F.; Wan, L. Ethanol extract of Piper wallichii ameliorates DSS-induced ulcerative colitis in mice: Involvement of TLR4/NF-κB/COX-2 signaling pathway. J. Ethnopharmacol. 2023, 308, 116293. [Google Scholar] [CrossRef] [PubMed]
- Nakase, H.; Sato, N.; Mizuno, N.; Ikawa, Y. The influence of cytokines on the complex pathology of ulcerative colitis. Autoimmun. Rev. 2022, 21, 103017. [Google Scholar] [CrossRef] [PubMed]
- Bergemalm, D.; Andersson, E.; Hultdin, J.; Eriksson, C.; Rush, S.T.; Kalla, R.; Adams, A.T.; Keita, V.; D’amato, M.; Gomollon, F.; et al. Systemic Inflammation in Preclinical Ulcerative Colitis. Gastroenterology 2021, 161, 1526–1539.e9. [Google Scholar] [CrossRef]
- Marafini, I.; Sedda, S.; Dinallo, V.; Monteleone, G. Inflammatory cytokines: From discoveries to therapies in IBD. Expert Opin. Biol. Ther. 2019, 19, 1207–1217. [Google Scholar] [CrossRef]
- Randhawa, P.K.; Singh, K.; Singh, N.; Jaggi, A.S. A Review on Chemical-Induced Inflammatory Bowel Disease Models in Rodents. Korean J. Physiol. Pharmacol. Off. J. Korean Physiol. Soc. Korean Soc. Pharmacol. 2014, 18, 279–288. [Google Scholar] [CrossRef]
- Jiang, S.; Xu, H.; Zhao, C.; Zhong, F.; Li, D. Oyster polysaccharides relieve DSS-induced colitis via anti-inflammatory and maintaining the physiological hypoxia. Int. J. Biol. Macromol. 2023, 238, 124150. [Google Scholar] [CrossRef]
- Wang, N.; Chen, W.; Cui, C.; Zheng, Y.; Yu, Q.; Ren, H.; Liu, Z.; Xu, C.; Zhang, G. The Peanut Skin Procyanidins Attenuate DSS-Induced Ulcerative Colitis in C57BL/6 Mice. Antioxidants 2022, 11, 2098. [Google Scholar] [CrossRef]
- Ye, M.; Joosse, M.E.; Liu, L.; Sun, Y.; Dong, Y.; Cai, C.; Song, Z.; Zhang, J.; Brant, S.R.; Lazarev, M.; et al. Deletion of IL-6 Exacerbates Colitis and Induces Systemic Inflammation in IL-10-Deficient Mice. J. Crohn’s Coliti. 2020, 14, 831–840. [Google Scholar] [CrossRef]
- Hagiwara, S.; Nagata, K.; Kasakura, K.; Sakata, F.; Kishino, S.; Ogawa, J.; Yashiro, T.; Nishiyama, C. The anti-inflammatory effect of the gut lactic acid bacteria-generated metabolite 10-oxo-cis-6,trans-11-octadecadienoic acid on monocytes. Biochem. Biophys. Res. Commun. 2020, 530, 342–347. [Google Scholar] [CrossRef]
- Zheng, S.; Xue, T.; Wang, B.; Guo, H.; Liu, Q. Chinese Medicine in the Treatment of Ulcerative Colitis: The Mechanisms of Signaling Pathway Regulations. Am. J. Chin. Med. 2022, 50, 1781–1798. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.Y.; Sp, N.; Jo, E.S.; Rugamba, A.; Kim, H.D.; Kim, I.H.; Park, J.-C.; Bae, S.W.; Jang, K.-J.; Yang, Y.M. Non-toxic sulfur inhibits LPS-induced inflammation by regulating TLR-4 and JAK2/STAT3 through IL-6 signaling. Mol. Med. Rep. 2021, 24, 485. [Google Scholar] [CrossRef] [PubMed]
- Qin, W.; Luo, H.; Yang, L.; Hu, D.; Jiang, S.P.; Peng, D.Y.; Hu, J.M.; Liu, S.J. Rubia cordifolia L. ameliorates DSS-induced ulcerative colitis in mice through dual inhibition of NLRP3 inflammasome and IL-6/JAK2/STAT3 pathways. Heliyon 2022, 8, e10314. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Li, X.; Wang, Y.; Zhang, Z.; Deng, A.; Wang, W.; Zhang, H.; Qin, H.; Wu, L. Butyric Acid Increases the Therapeutic Effect of EHLJ7 on Ulcerative Colitis by Inhibiting JAK2/STAT3/SOCS1 Signaling Pathway. Front. Pharmacol. 2019, 10, 1553. [Google Scholar] [CrossRef] [PubMed]
- Elhennawy, M.G.; Abdelaleem, E.A.; Zaki, A.A.; Mohamed, W.R. Cinnamaldehyde and hesperetin attenuate TNBS-induced ulcerative colitis in rats through modulation of the JAk2/STAT3/SOCS3 pathway. J. Biochem. Mol. Toxicol. 2021, 35, e22730. [Google Scholar] [CrossRef]
- Li, Y.; Jia, Y.; Cui, T.; Zhang, J. IL-6/STAT3 signaling pathway regulates the proliferation and damage of intestinal epithelial cells in patients with ulcerative colitis via H3K27ac. Exp. Ther. Med. 2021, 22, 890. [Google Scholar] [CrossRef]
- Salas, A.; Hernandez-Rocha, C.; Duijvestein, M.; Faubion, W.; McGovern, D.; Vermeire, S.; Vetrano, S.; Casteele, N.V. JAK–STAT pathway targeting for the treatment of inflammatory bowel disease. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 323–337. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Luan, H.; Jiang, H.; Xu, Y.; Wu, X.; Zhang, Y.; Li, R. Gegen Qinlian decoction relieved DSS-induced ulcerative colitis in mice by modulating Th17/Treg cell homeostasis via suppressing IL-6/JAK2/STAT3 signaling. Phytomedicine 2021, 84, 153519. [Google Scholar] [CrossRef]
- Tao, J.-H.; Duan, J.-A.; Zhang, W.; Jiang, S.; Guo, J.-M.; Wei, D.-D. Polysaccharides from Chrysanthemum morifolium Ramat Ameliorate Colitis Rats via Regulation of the Metabolic Profiling and NF-κ B/TLR4 and IL-6/JAK2/STAT3 Signaling Pathways. Front. Pharmacol. 2018, 9, 746. [Google Scholar] [CrossRef]
- Wan, X.; Bi, J.; Gao, X.; Tian, F.; Wang, X.; Li, N.; Li, J. Partial Enteral Nutrition Preserves Elements of Gut Barrier Function, Including Innate Immunity, Intestinal Alkaline Phosphatase (IAP) Level, and Intestinal Microbiota in Mice. Nutrients 2015, 7, 6294–6312. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Liu, W.; Lin, Y.; Zhang, S.; Zou, B.; Xiao, D.; Lin, L.; Zhong, Y.; Zheng, H.; Liao, Q.; et al. Compound polysaccharides ameliorate experimental colitis by modulating gut microbiota composition and function. J. Gastroenterol. Hepatol. 2019, 34, 1554–1562. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.-X.; Shao, M.-J.; Qi, Q.; Xu, Y.-S.; Yang, X.-Q.; Zhu, F.-H.; He, S.-J.; He, P.-L.; Feng, C.-L.; Wu, Y.-W.; et al. Artemisinin analogue SM934 ameliorates DSS-induced mouse ulcerative colitis via suppressing neutrophils and macrophages. Acta Pharmacol. Sin. 2018, 39, 1633–1644. [Google Scholar] [CrossRef]
- Xiong, T.; Zheng, X.; Zhang, K.; Wu, H.; Dong, Y.; Zhou, F.; Cheng, B.; Li, L.; Xu, W.; Su, J.; et al. Ganluyin ameliorates DSS-induced ulcerative colitis by inhibiting the enteric-origin LPS/TLR4/NF-κB pathway. J. Ethnopharmacol. 2022, 289, 115001. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-K.; Chen, T.-X.; Wang, W.; Xu, L.-L.; Zhang, Y.-Q.; Jin, Z.; Liu, Y.-B.; Tang, Y.-Z. Aesculetin exhibited anti-inflammatory activities through inhibiting NF-κB and MAPKs pathway in vitro and in vivo. J. Ethnopharmacol. 2022, 296, 115489. [Google Scholar] [CrossRef]
- Xie, Q.; Li, H.; Ma, R.; Ren, M.; Li, Y.; Li, J.; Chen, H.; Chen, Z.; Gong, D.; Wang, J. Effect of Coptis chinensis franch and Magnolia officinalis on intestinal flora and intestinal barrier in a TNBS-induced ulcerative colitis rats model. Phytomedicine 2022, 97, 153927. [Google Scholar] [CrossRef]
- Zeng, J.; Zhang, D.; Wan, X.; Bai, Y.; Yuan, C.; Wang, T.; Yuan, D.; Zhang, C.; Liu, C. Chlorogenic Acid Suppresses miR-155 and Ameliorates Ulcerative Colitis through the NF-κB/NLRP3 Inflammasome Pathway. Mol. Nutr. Food Res. 2020, 64, e2000452. [Google Scholar] [CrossRef]
- Mo, J.; Ni, J.; Zhang, M.; Xu, Y.; Li, Y.; Karim, N.; Chen, W. Mulberry Anthocyanins Ameliorate DSS-Induced Ulcerative Colitis by Improving Intestinal Barrier Function and Modulating Gut Microbiota. Antioxidants 2022, 11, 1674. [Google Scholar] [CrossRef]


| Score | Rate of Weight Change | Stool Formation | Occult Blood Status |
|---|---|---|---|
| 0 | 0% | Normal | No color development within 2 min |
| 1 | 1–5% | Soften | Change from light green to green after 10 s |
| 2 | 5–10% | Loose | Change from light green to blue–brown |
| 3 | 10–15% | Unshaped | Change from blue–brown to black–brown |
| 4 | >15% | Diarrhea | Immediately turns blue–black–brown |
| Score | Inflammatory Factor Infiltration | Epithelial Cell Integrity |
|---|---|---|
| 0 | Normal | Normal |
| 1 | Local inflammatory infiltration of crypts | Partial loss of cup cells |
| 2 | Inflammatory infiltration to the base of the crypt | Partial disappearance of the crypt |
| 3 | Inflammatory infiltration into the mucosa | Large absence of crypt fossa |
| 4 | Inflammatory infiltration into the submucosa | The disappearance of crypt fossa |
| Target Gene | Upstream Primer (5′-3′) | Downstream Primer (5′-3′) |
|---|---|---|
| IL-6 | GAGTCACAGAAGGAGTGGCTAAGGA | CGCACTAGGTTTGCCGAGTAGATCT |
| IL-1β | TGCCACCTTTTGACAGTGATG | CATCTCGGAGCCTGTAGTGC |
| TNF-α | GCATGGTGGTGGTTGTTTCTGACGAT | GCTTCTGTTGGACACCTGGAGACA |
| β-actin | GCAGGAGTACGATGAGTCCG | ACGCAGCTCAGTAACAGTCC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.-Y.; Cui, Y.; Dong, W.-R.; Liu, T.-T.; Zhou, G.; Chen, Y.-X. Terminalia bellirica Fruit Extract Alleviates DSS-Induced Ulcerative Colitis by Regulating Gut Microbiota, Inflammatory Mediators, and Cytokines. Molecules 2023, 28, 5783. https://doi.org/10.3390/molecules28155783
Li Y-Y, Cui Y, Dong W-R, Liu T-T, Zhou G, Chen Y-X. Terminalia bellirica Fruit Extract Alleviates DSS-Induced Ulcerative Colitis by Regulating Gut Microbiota, Inflammatory Mediators, and Cytokines. Molecules. 2023; 28(15):5783. https://doi.org/10.3390/molecules28155783
Chicago/Turabian StyleLi, Yao-Yao, Yu Cui, Wan-Rong Dong, Tian-Tian Liu, Gao Zhou, and Yu-Xin Chen. 2023. "Terminalia bellirica Fruit Extract Alleviates DSS-Induced Ulcerative Colitis by Regulating Gut Microbiota, Inflammatory Mediators, and Cytokines" Molecules 28, no. 15: 5783. https://doi.org/10.3390/molecules28155783
APA StyleLi, Y.-Y., Cui, Y., Dong, W.-R., Liu, T.-T., Zhou, G., & Chen, Y.-X. (2023). Terminalia bellirica Fruit Extract Alleviates DSS-Induced Ulcerative Colitis by Regulating Gut Microbiota, Inflammatory Mediators, and Cytokines. Molecules, 28(15), 5783. https://doi.org/10.3390/molecules28155783