Evaluation of α-Glucosidase Inhibition and Antihyperglycemic Activity of Extracts Obtained from Leaves and Flowers of Rumex crispus L.
Abstract
1. Introduction
2. Results
2.1. Plant Material and Preparation of the Extracts
2.2. Quantitative Phytochemical Analysis
2.3. α-Glucosidase Inhibition Provided by R. crispus L. and Its Fractions
2.4. Preliminary Identification of Compounds in the Active Extracts of R. crispus L. by UHPLC-MS/MS
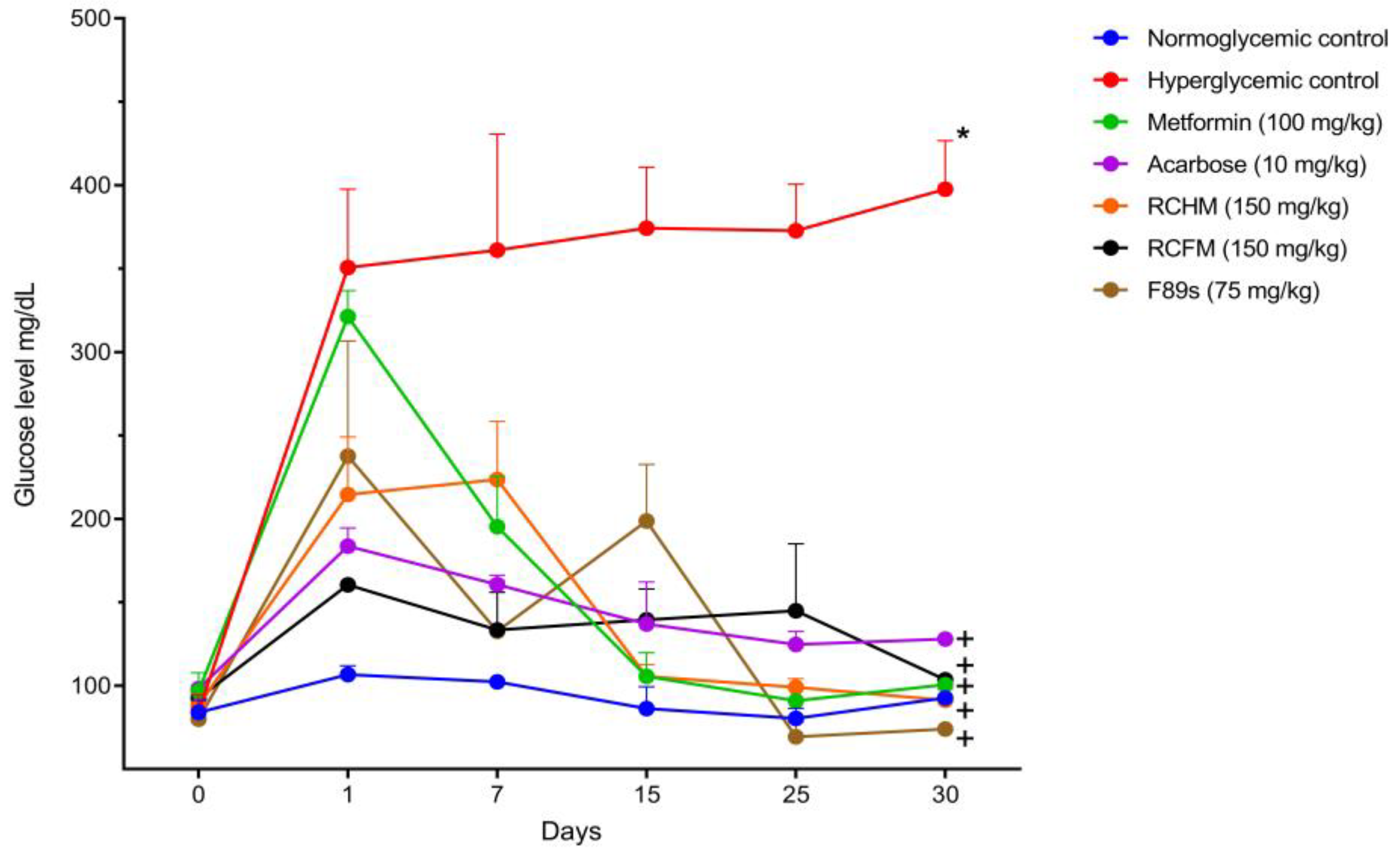
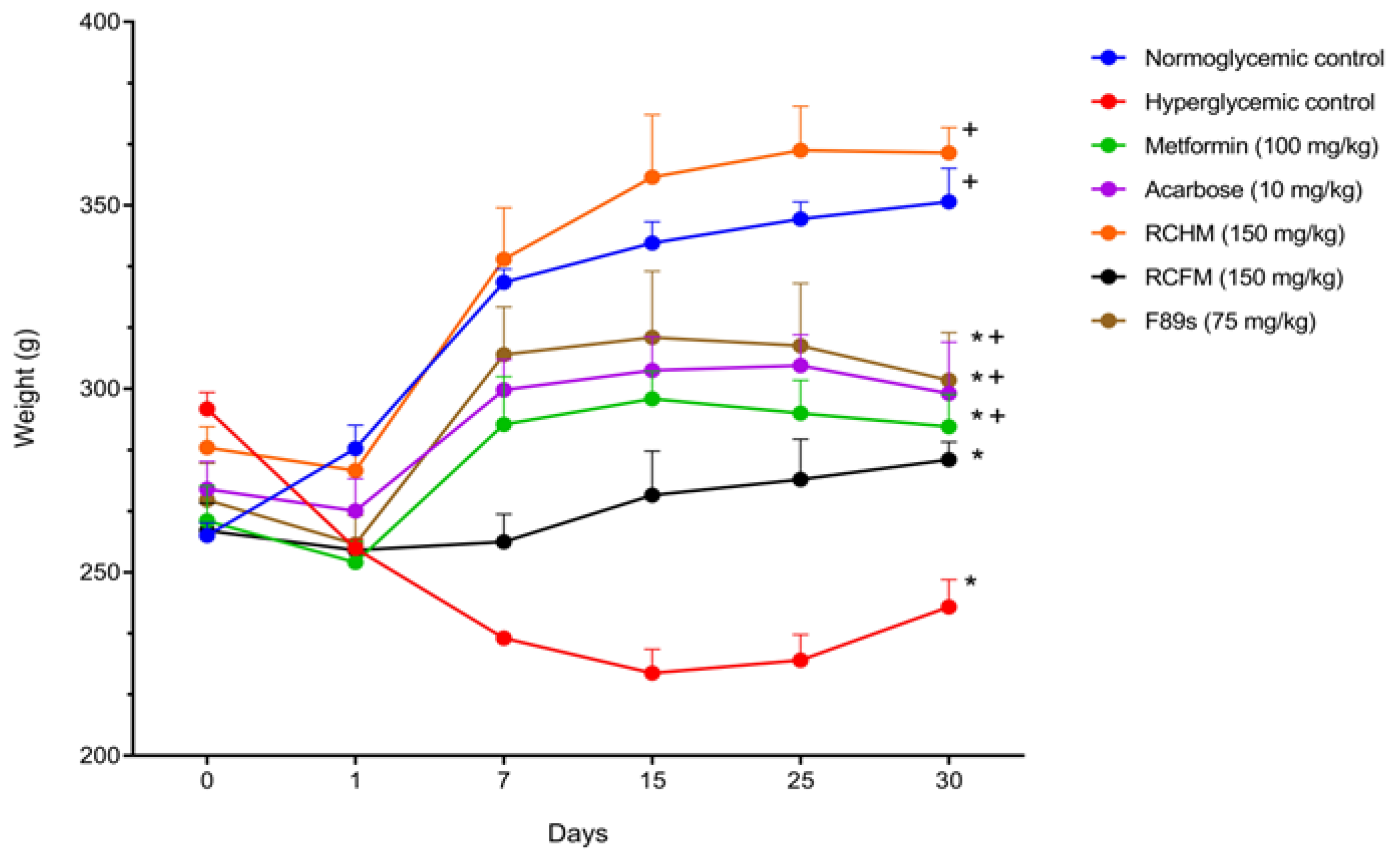
2.5. Effect of R. crispus L. on the Blood Glucose Level and Body Weight of Rats
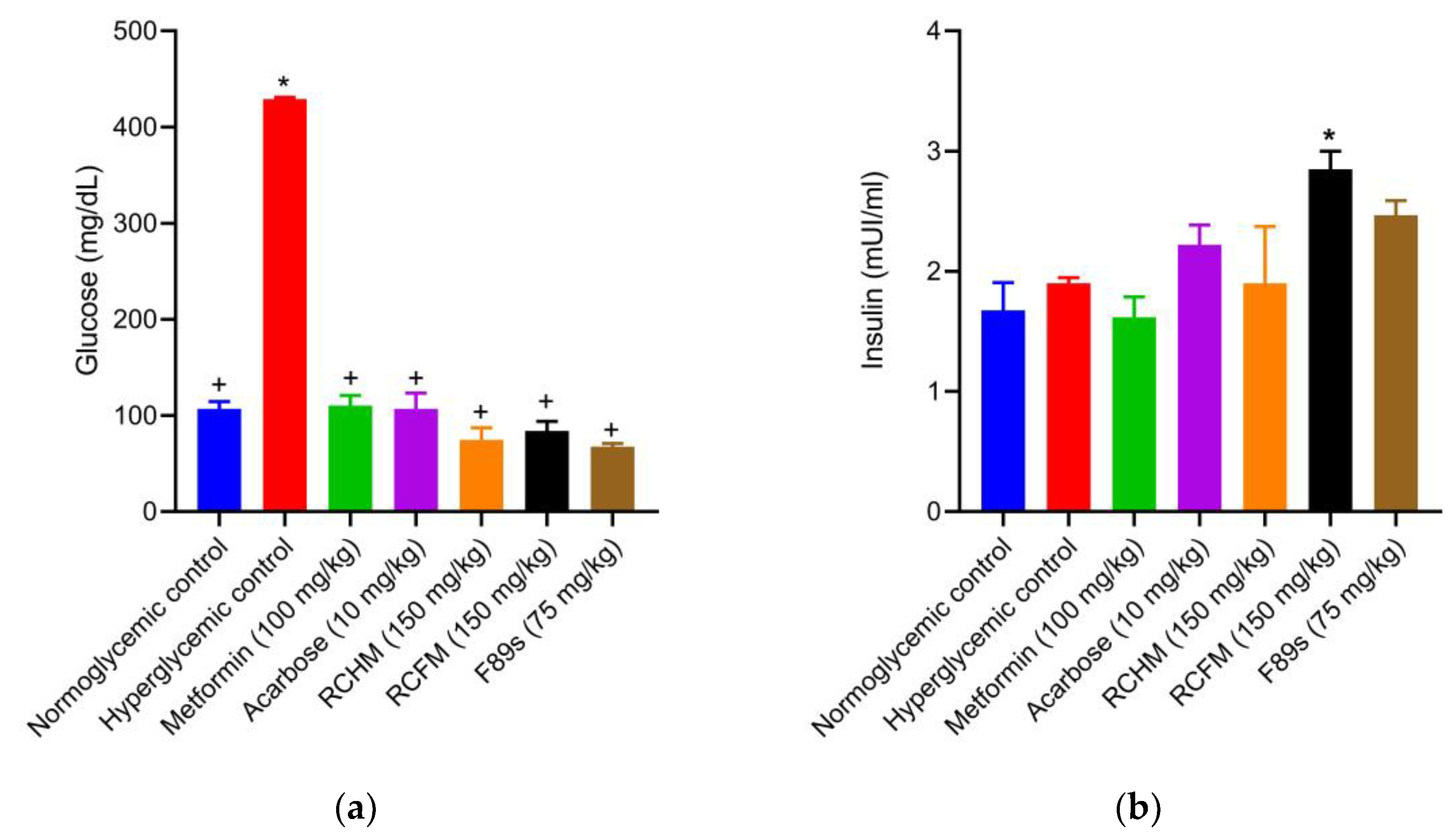
2.6. Effect of R. crispus L. on the Serum Glucose and Insulin Levels
2.7. Effect of R. crispus L. on the Serum Lipid Profile
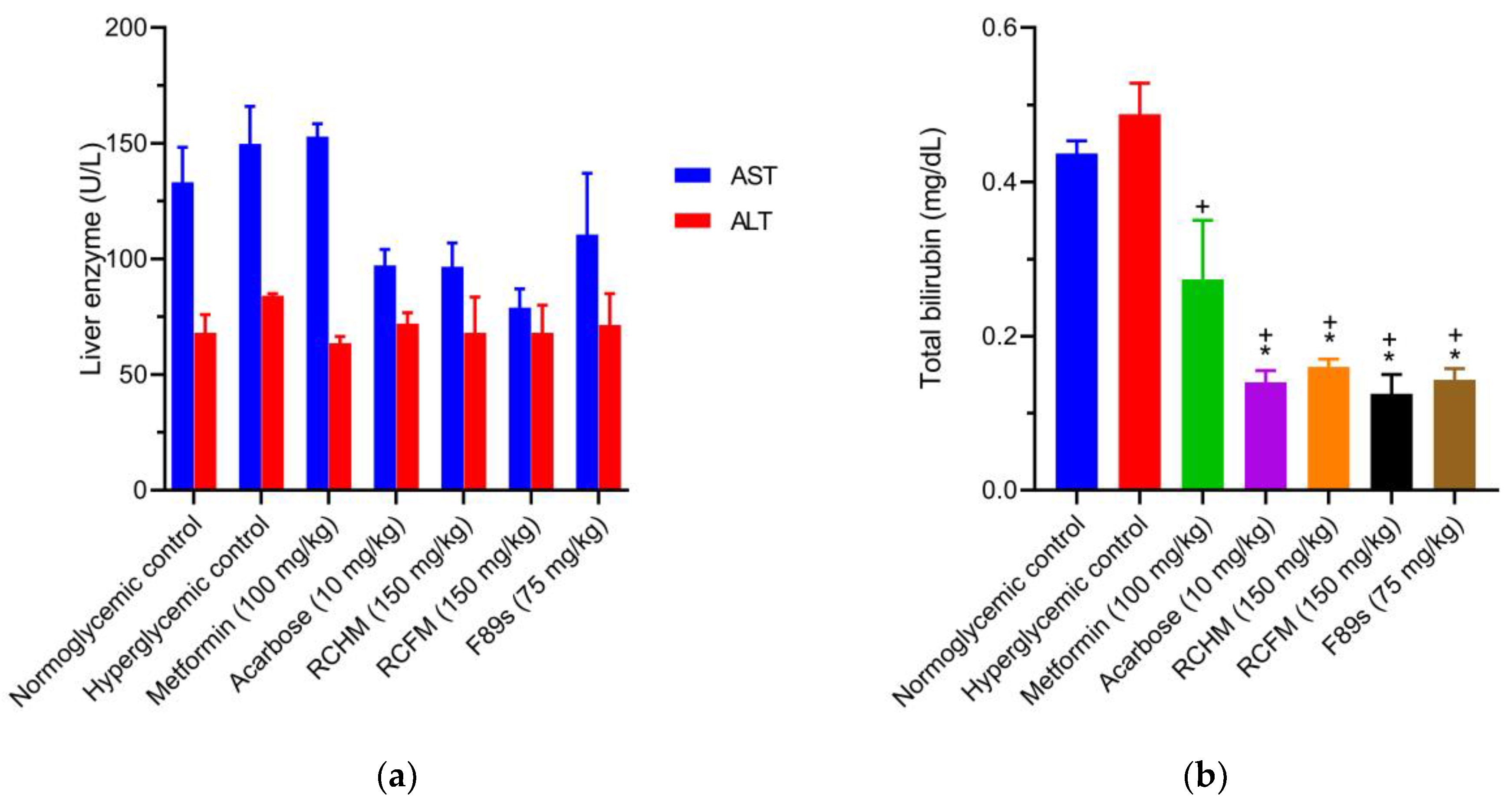
2.8. Effect of R. crispus L. on the Hepatic Profile
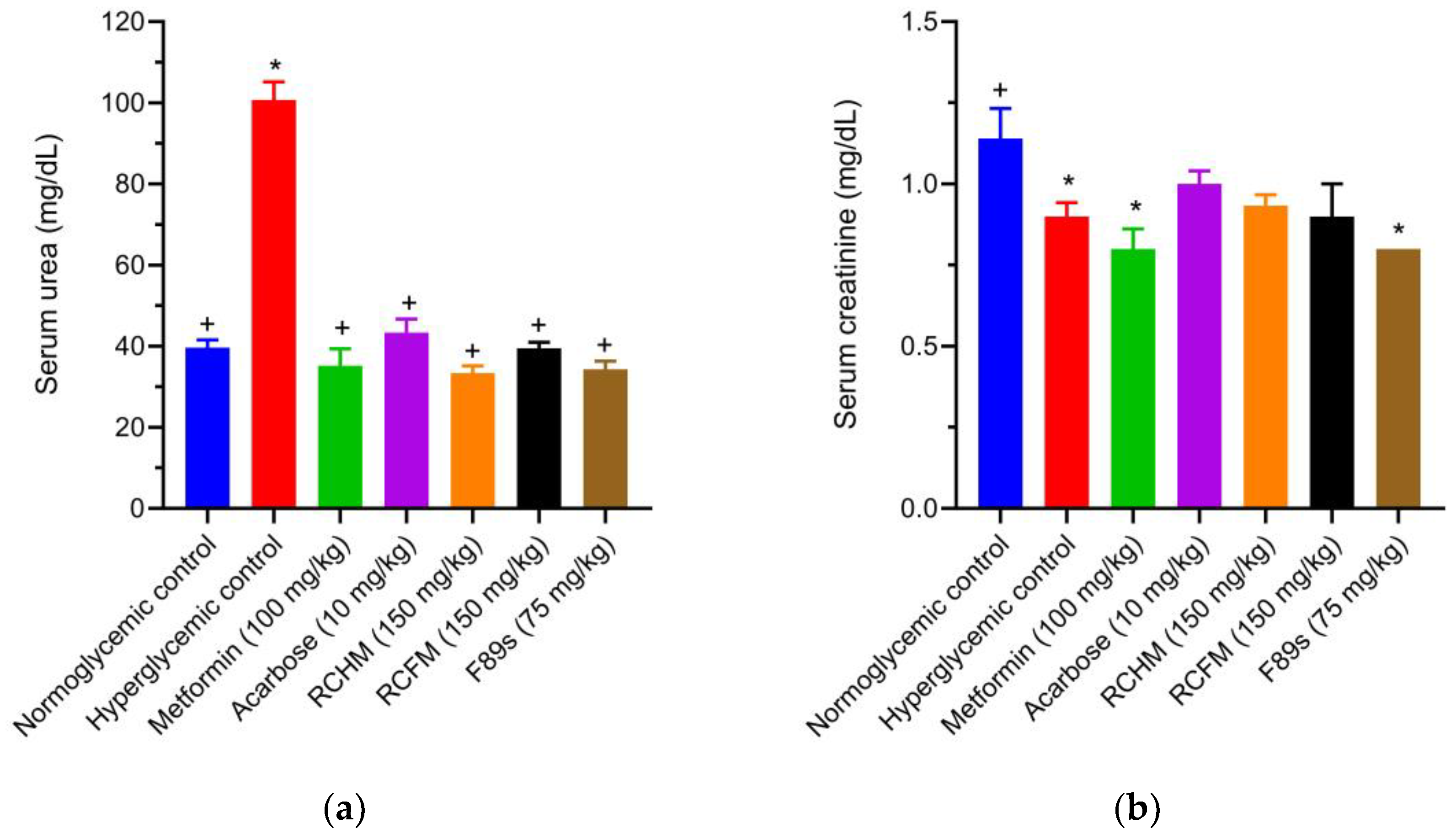
2.9. Effect of R. crispus L. on the Renal Profile
3. Discussion
4. Materials and Methods
4.1. Plant Material and Preparation of the Extracts
4.2. Quantitative Phytochemical Analysis
4.3. Preparation of R. crispus L. Fractions
4.4. α-Glucosidase Inhibitory Assay
4.5. Preliminary Identification of Phenolic Compounds Using UHPLC-MS/MS
4.6. Experimental Animals
4.7. Induction of Hyperglycemia in Rats
4.8. Experimental Design
4.9. Measurement of Body Weight and the Level of Peripheral Glucose
4.10. Biochemical Profile
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- de Paulo Farias, D.; de Araújo, F.F.; Neri-Numa, I.A.; Pastore, G.M. Antidiabetic Potential of Dietary Polyphenols: A Mechanistic Review. Food Res. Int. 2021, 145, 110383. [Google Scholar] [CrossRef] [PubMed]
- Proença, C.; Freitas, M.; Ribeiro, D.; Oliveira, E.F.T.; Sousa, J.L.C.; Tomé, S.M.; Ramos, M.J.; Silva, A.M.S.; Fernandes, P.A.; Fernandes, E. α-Glucosidase Inhibition by Flavonoids: An in Vitro and in Silico Structure–Activity Relationship Study. J. Enzyme Inhib. Med. Chem. 2017, 32, 1216–1228. [Google Scholar] [CrossRef]
- Hossain, U.; Das, A.K.; Ghosh, S.; Sil, P.C. An Overview on the Role of Bioactive α-Glucosidase Inhibitors in Ameliorating Diabetic Complications. Food Chem. Toxicol. 2020, 145, 111738. [Google Scholar] [CrossRef] [PubMed]
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and Regional Diabetes Prevalence Estimates for 2019 and Projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th Edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef] [PubMed]
- Chinsembu, K.C. Diabetes Mellitus and Nature’s Pharmacy of Putative Antidiabetic Plants. J. Herb. Med. 2019, 15, 100230. [Google Scholar] [CrossRef]
- Choudhury, H.; Pandey, M.; Hua, C.K.; Mun, C.S.; Jing, J.K.; Kong, L.; Ern, L.Y.; Ashraf, N.A.; Kit, S.W.; Yee, T.S.; et al. An Update on Natural Compounds in the Remedy of Diabetes Mellitus: A Systematic Review. J. Tradit. Complement. Med. 2018, 8, 361–376. [Google Scholar] [CrossRef]
- Choudhari, V.P.; Gore, K.P.; Pawar, A.T. Antidiabetic, Antihyperlipidemic Activities and Herb–Drug Interaction of a Polyherbal Formulation in Streptozotocin Induced Diabetic Rats. J. Ayurveda Integr. Med. 2017, 8, 218–225. [Google Scholar] [CrossRef]
- Salehi, B.; Ata, A.; Anil Kumar, N.V.; Sharopov, F.; Ramírez-Alarcón, K.; Ruiz-Ortega, A.; Ayatollahi, S.A.; Fokou, P.V.T.; Kobarfard, F.; Zakaria, Z.A.; et al. Antidiabetic Potential of Medicinal Plants and Their Active Components. Biomolecules 2019, 9, 551. [Google Scholar] [CrossRef]
- Abbas, G.; Al Harrasi, A.; Hussain, H.; Hamaed, A.; Supuran, C.T. The Management of Diabetes Mellitus-Imperative Role of Natural Products against Dipeptidyl Peptidase-4, α-Glucosidase and Sodium-Dependent Glucose Co-Transporter 2 (SGLT2). Bioorg. Chem. 2019, 86, 305–315. [Google Scholar] [CrossRef]
- Papoutsis, K.; Zhang, J.; Bowyer, M.C.; Brunton, N.; Gibney, E.R.; Lyng, J. Fruit, Vegetables, and Mushrooms for the Preparation of Extracts with α-Amylase and α-Glucosidase Inhibition Properties: A Review. Food Chem. 2021, 338, 128119. [Google Scholar] [CrossRef]
- Vijaykrishnaraj, M.; Wang, K. Dietary Natural Products as a Potential Inhibitor towards Advanced Glycation End Products and Hyperglycemic Complications: A Phytotherapy Approaches. Biomed. Pharmacother. 2021, 144, 112336. [Google Scholar] [CrossRef]
- Wang, T.Y.; Li, Q.; Bi, K.S. Bioactive Flavonoids in Medicinal Plants: Structure, Activity and Biological Fate. Asian J. Pharm. Sci. 2018, 13, 12–23. [Google Scholar] [CrossRef]
- Yang, J.; Wang, X.; Zhang, C.; Ma, L.; Wei, T.; Zhao, Y.; Peng, X. Comparative Study of Inhibition Mechanisms of Structurally Different Flavonoid Compounds on α-Glucosidase and Synergistic Effect with Acarbose. Food Chem. 2021, 347, 129056. [Google Scholar] [CrossRef]
- Mishra, A.P.; Sharifi-Rad, M.; Shariati, M.A.; Mabkhot, Y.N.; Al-Showiman, S.S.; Rauf, A.; Salehi, B.; Župunski, M.; Sharifi-Rad, M.; Gusain, P.; et al. Bioactive Compounds and Health Benefits of Edible Rumex Species–A Review. Cell. Mol. Biol. 2018, 64, 27–34. [Google Scholar] [CrossRef]
- Shafiq, N.; Saleem, M.; Kousar, S.; Sahar, M.; Hussain, S.M.; Jabeen, F. Investigation of genus Rumex for their biologically active constituents. Res. J. Life Sci. Bioinforatics Pharm. Chem. Sci. 2017, 2, 148–165. [Google Scholar] [CrossRef]
- de Rzedowski, G.C.; Rzedowski, J. Flora Fanerogámica Del Valle de México, 1st ed.; Martínez, R.M.M., Ed.; Comisión Nacional para el Conocimiento y Uso de la Biodiversidad: Mexico City, Mexico, 2005; ISBN 978-607-7607-36-6. [Google Scholar]
- Pareek, A.; Kumar, A. Rumex crispus L.—A Plant of Traditional Value. Drug Discov. 2014, 9, 20–23. [Google Scholar]
- Li, J.J.; Li, Y.X.; Li, N.; Zhu, H.T.; Wang, D.; Zhang, Y.J. The Genus Rumex (Polygonaceae): An Ethnobotanical, Phytochemical and Pharmacological Review. Nat. Products Bioprospect. 2022, 12, 21. [Google Scholar] [CrossRef] [PubMed]
- Vasas, A.; Orbán-Gyapai, O.; Hohmann, J. The Genus Rumex: Review of Traditional Uses, Phytochemistry and Pharmacology. J. Ethnopharmacol. 2015, 175, 198–228. [Google Scholar] [CrossRef]
- Buzgaia, N.; Awin, T.; Elabbar, F.; Abdusalam, K.; Lee, S.Y.; Rukayadi, Y.; Abas, F.; Shaari, K. Antibacterial Activity of Arbutus Pavarii Pamp against Methicillin-Resistant Staphylococcus Aureus (MRSA) and UHPLC-MS/MS Profile of the Bioactive Fraction. Plants 2020, 9, 1539. [Google Scholar] [CrossRef]
- Xin, Z.; Ma, S.; Ren, D.; Liu, W.; Han, B.; Zhang, Y.; Xiao, J.; Yi, L.; Deng, B. UPLC-Orbitrap-MS/MS Combined with Chemometrics Establishes Variations in Chemical Components in Green Tea from Yunnan and Hunan Origins. Food Chem. 2018, 266, 534–544. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Liang, F.; Bin, Y.; Li, P.; Duan, C. Screening Non-Colored Phenolics in Red Wines Using Liquid Chromatography/Ultraviolet and Mass Spectrometry/Mass Spectrometry Libraries. Molecules 2007, 12, 679–693. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Zhao, J.; Liu, S.; Song, F.; Liu, Z. The Screening of Potential α-Glucosidase Inhibitors from the Polygonum Multiflorum Extract Using Ultrafiltration Combined with Liquid Chromatography-Tandem Mass Spectrometry. Anal. Methods 2014, 6, 3353–3359. [Google Scholar] [CrossRef]
- Wang, X.; Sun, W.; Sun, H.; Lv, H.; Wu, Z.; Wang, P.; Liu, L.; Cao, H. Analysis of the Constituents in the Rat Plasma after Oral Administration of Yin Chen Hao Tang by UPLC/Q-TOF-MS/MS. J. Pharm. Biomed. Anal. 2008, 46, 477–490. [Google Scholar] [CrossRef]
- Ferreres, F.; Grosso, C.; Gil-Izquierdo, A.; Valentão, P.; Azevedo, C.; Andrade, P.B. HPLC-DAD-ESI/MS n Analysis of Phenolic Compounds for Quality Control of Grindelia Robusta Nutt. and Bioactivities. J. Pharm. Biomed. Anal. 2014, 94, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Abidi, J.; Ammar, S.; Ben Brahim, S.; Skalicka-Woźniak, K.; Ghrabi-Gammar, Z.; Bouaziz, M. Use of Ultra-High-Performance Liquid Chromatography Coupled with Quadrupole-Time-of-Flight Mass Spectrometry System as Valuable Tool for an Untargeted Metabolomic Profiling of Rumex tunetanus Flowers and Stems and Contribution to the Antioxidant Activity. J. Pharm. Biomed. Anal. 2019, 162, 66–81. [Google Scholar] [CrossRef]
- He, L.; Zhang, Z.; Lu, L.; Liu, Y.; Li, S.; Wang, J.; Song, Z.; Yan, Z.; Miao, J. Rapid Identification and Quantitative Analysis of the Chemical Constituents in Scutellaria indica L. by UHPLC-QTOF-MS and UHPLC-MS/MS. J. Pharm. Biomed. Anal. 2016, 117, 125–139. [Google Scholar] [CrossRef]
- Zolkeflee, N.K.Z.; Ramli, N.S.; Azlan, A.; Abas, F. In Vitro Anti-Diabetic Activities and UHPLC-ESI-MS/MS Profile of Muntingia Calabura Leaves Extract. Molecules 2022, 27, 287. [Google Scholar] [CrossRef]
- Francescato, L.N.; Debenedetti, S.L.; Schwanz, T.G.; Bassani, V.L.; Henriques, A.T. Identification of Phenolic Compounds in Equisetum Giganteum by LC–ESI-MS/MS and a New Approach to Total Flavonoid Quantification. Talanta 2013, 105, 192–203. [Google Scholar] [CrossRef]
- Farag, M.A.; Kabbash, E.M.; Mediani, A.; Döll, S.; Esatbeyoglu, T.; Afifi, S.M. Comparative Metabolite Fingerprinting of Four Different Cinnamon Species Analyzed via UPLC–MS and GC–MS and Chemometric Tools. Molecules 2022, 27, 2935. [Google Scholar] [CrossRef]
- Feduraev, P.V.; Chupakhina, G.; Maslennikov, P.; Tacenko, N.; Skrypnik, L. Variation in Phenolic Compounds Content and Antioxidant Activity of Different Plant Organs from Rumex crispus L. and Rumex obtusifolius L. at Different Growth Stages. Antioxidants 2019, 8, 237. [Google Scholar] [CrossRef]
- Feduraev, P.V.; Skrypnik, L.N.; Maslennikov, P.V.; Tchoupakhina, G.N.; Tacenko, N.A. The Accumulation Features of Phenolic Compounds in Some Species of Genus Rumex L. Khimiya Rastit. Syr’ya 2017, 3, 123–130. [Google Scholar] [CrossRef][Green Version]
- Idris, O.A.; Wintola, O.A.; Afolayan, A.J. Phytochemical and Antioxidant Activities of Rumex crispus L. in Treatment of Gastrointestinal Helminths in Eastern Cape Province, South Africa. Asian Pac. J. Trop. Biomed. 2016, 6, 962–966. [Google Scholar] [CrossRef]
- Hong, H.C.; Li, S.L.; Zhang, X.Q.; Ye, W.C.; Zhang, Q.W. Flavonoids with α-Glucosidase Inhibitory Activities and Their Contents in the Leaves of Morus Atropurpurea. Chin. Med. 2013, 8, 19. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Yao, F.; Xue, Q.; Fan, H.; Yang, L.; Li, X.; Sun, L.; Liu, Y. Inhibitory Effects against α-Glucosidase and α-Amylase of the Flavonoids-Rich Extract from Scutellaria Baicalensis Shoots and Interpretation of Structure–Activity Relationship of Its Eight Flavonoids by a Refined Assign-Score Method. Chem. Cent. J. 2018, 12, 82. [Google Scholar] [CrossRef] [PubMed]
- Hamed, Y.S.; Abdin, M.; Rayan, A.M.; Saleem Akhtar, H.M.; Zeng, X. Synergistic Inhibition of Isolated Flavonoids from Moringa Oleifera Leaf on α-Glucosidase Activity. LWT 2021, 141, 111081. [Google Scholar] [CrossRef]
- Chokki, M.; Cudalbeanu, M.; Zongo, C.; Dah-Nouvlessounon, D.; Ghinea, I.O.; Furdui, B.; Raclea, R.; Savadogo, A.; Baba-Moussa, L.; Avamescu, S.M.; et al. Exploring Antioxidant and Enzymes (A-Amylase and B-Glucosidase) Inhibitory Activity of Morinda Lucida and Momordica Charantia Leaves from Benin. Foods 2020, 9, 434. [Google Scholar] [CrossRef]
- Spínola, V.; Llorent-Martínez, E.J.; Castilho, P.C. Inhibition of α-Amylase, α-Glucosidase and Pancreatic Lipase by Phenolic Compounds of Rumex maderensis (Madeira sorrel). Influence of Simulated Gastrointestinal Digestion on Hyperglycaemia-Related Damage Linked with Aldose Reductase Activity and Protein G. LWT Food Sci. Technol. 2020, 118, 108727. [Google Scholar] [CrossRef]
- Minh, T.N.; Van, T.M.; Andriana, Y.; Vinh, L.T.; Hau, D.V.; Duyen, D.H.; de Guzman-Gelani, C. Antioxidant, Xanthine Oxidase, α-Amylase and α-Glucosidase Inhibitory Activities of Bioactive Compounds from Rumex crispus L. Root. Molecules 2019, 24, 3899. [Google Scholar] [CrossRef]
- Savran, A.; Zengin, G.; Aktumsek, A.; Mocan, A.; Glamoćlija, J.; Ćirić, A.; Soković, M. Phenolic Compounds and Biological Effects of Edible: Rumex scutatus and Pseudosempervivum sempervivum: Potential Sources of Natural Agents with Health Benefits. Food Funct. 2016, 7, 3252–3262. [Google Scholar] [CrossRef] [PubMed]
- Elsayed, R.H.; Kamel, E.M.; Mahmoud, A.M.; El-Bassuony, A.A.; Bin-Jumah, M.; Lamsabhi, A.M.; Ahmed, S.A. Rumex dentatus L. Phenolics Ameliorate Hyperglycemia by Modulating Hepatic Key Enzymes of Carbohydrate Metabolism, Oxidative Stress and PPARγ in Diabetic Rats. Food Chem. Toxicol. 2020, 138, 111202. [Google Scholar] [CrossRef]
- Sedaghat, R.; Roghani, M.; Ahmadi, M.; Ahmadi, F. Antihyperglycemic and Antihyperlipidemic Effect of Rumex patientia Seed Preparation in Streptozotocin-Diabetic Rats. Pathophysiology 2011, 18, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Sampietro, D.A.; Catalan, C.A.N.; Vattuone, M.A.; Narwal, S.S. Isolation, Identification and Characterization of Allelochemicals/Natural Products; Taylor & Francis Ltd.,: Mexico City, Mexico, 2009; ISBN 9781578085774. [Google Scholar]
- Pérez-Pérez, E.M.; Vit, P.; Rivas, E.; Sciortino, R.; Sosa, A.; Tejada, D.; Rodríguez-Malaver, A.J. Antioxidant Activity of Four Color Fractions of Bee Pollen from Mérida, Venezuela. Arch. Latinoam. Nutr. 2012, 62, 375–380. [Google Scholar] [PubMed]
- Mahboubi, M.; Kazempour, N.; Nazar, A.R.B. Total Phenolic, Total Flavonoids, Antioxidant and Antimicrobial Activities of Scrophularia Striata Boiss Extracts. Jundishapur J. Nat. Pharm. Prod. 2013, 8, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Herald, T.J.; Gadgil, P.; Perumal, R.; Bean, S.R.; Wilson, J.D. High-Throughput Micro-Plate HCl-Vanillin Assay for Screening Tannin Content in Sorghum Grain. J. Sci. Food Agric. 2014, 94, 2133–2136. [Google Scholar] [CrossRef] [PubMed]
- Makkar, H.P.S.; Siddhuraju, P.; Becker, K. Plant Secondary Metabolites; Methods in Molecular BiologyTM; Humana Press: Totowa, NJ, USA, 2007; Volume 393, ISBN 978-1-58829-993-2. [Google Scholar]
- Xiong, Q.; Wilson, W.K.; Pang, J. The Liebermann–Burchard Reaction: Sulfonation, Desaturation, and Rearrangment of Cholesterol in Acid. Lipids 2007, 42, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Ajanal, M.; Gundkalle, M.; Nayak, S. Estimation of Total Alkaloid in Chitrakadivati by UV-Spectrophotometer. Anc. Sci. Life 2012, 31, 198. [Google Scholar] [CrossRef]
- Salehi, P.; Asghari, B.; Esmaeili, M.A.; Dehghan, H.; Ghazi, I. α-Glucosidase and α-Amylase Inhibitory Effect and Antioxidant Activity of Ten Plant Extracts Traditionally Used in Iran for Diabetes. J. Med. Plants Res. 2013, 7, 257–266. [Google Scholar] [CrossRef]
- Norma Oficial Mexicana NOM-062-ZOO-1999; Especificaciones Técnicas Para La Producción, Cuidado y Uso de Los Animales de Laboratorio. Diario Oficial de la Federación: Mexico City, Mexico, 1999.
- Furman, B.L. Streptozotocin-Induced Diabetic Models in Mice and Rats. Curr. Protoc. 2021, 1, e78. [Google Scholar] [CrossRef]
- Norma Oficial Mexicana NOM-033-ZOO-1995; Sacrificio Humanitario de Los Animales Domésticos y Silvestres. Diario Oficial de la Federación: Mexico City, Mexico, 1995.
| ID | Physical Description of the Extract | Yield (g) | % Yield (w/w) |
|---|---|---|---|
| RCHH | Dark brown and gummy | 3.694 | 0.92 |
| RCHD | Dark brown and gummy | 4.437 | 1.11 |
| RCHM | Brown powder | 19.372 | 4.84 |
| RCFH | Dark brown and gummy | 3.5189 | 0.75 |
| RCFD | Dark brown and gummy | 3.069 | 0.65 |
| RCFM | Brown powder | 52.8 | 11.19 |
| ID | Total Phenolics (mg EGA/g) | Total Polyphenols (mg ETA/g) | Flavonoids (mg EQ/g) | Condensed Tannins (mg EC/g) | Total Sterols (mg EChol/g) | Total Saponins (mg ED/g) | Alkaloids (mg EA/g) |
|---|---|---|---|---|---|---|---|
| RCHH | 12.24 ± 0.66 E | 3.54 ± 0.27 C | 63.18 ± 0.39 C | 23.62 ± 1.38 D | 142.82 ± 1.0 D | 154.14 ± 1.72 F | 2.13 ± 0.23 A |
| RCHD | 54.78 ± 0.83 C | 7.39 ± 0.17 C | 190.57 ± 0.74 A | 122.02 ± 0.59 A | 329.82 ± 5.19 A | 161.05 ± 1.54 E | 0.55 ± 0.22 CD |
| RCHM | 260.76 ± 1.77 B | 108.65 ± 0.46 B | 19.37 ± 0.18 E | 81.19 ± 0.20 C | 13.68 ± 1.02 E | 319.93 ± 0.96 C | 0.76 ± 0.07 BC |
| RCFH | 7.50 ± 0.20 E | 3.02 ± 0.10 C | 26.80 ± 0.22 D | 8.75 ± 0.14 F | 163.78 ± 3.06 C | 289.73 ± 1.62 D | 1.15 ± 0.28 B |
| RCFD | 38.76 ± 0.26 D | 9.11 ± 0.46 C | 135.41 ± 0.60 B | 21.48 ± 0.59 E | 290.54 ± 1.15 B | 374.44 ± 1.54 B | 0.18 ± 0.02 D |
| RCFM | 662.31 ± 4.51 A | 837.44 ± 7.50 A | 8.34 ± 0.16 F | 117.30 ± 0.59 B | 2.09 ± 0.11 F | 727.72 ± 2.72 A | 0.18 ± 0.02 D |
| ID | IC50 (μg/mL) | ID | IC50 (μg/mL) |
|---|---|---|---|
| RCHH | >400 | RCFH | >400 |
| RCHD | >400 | RCFD | >400 |
| RCHM | 112.0 ± 1.23 | RCFM | 7.3 ± 0.17 |
| RCHM-FDCM | 205.5 ± 2.46 | RCFM-FDCM | 15.8 ± 0.15 |
| RCHM-FM | 98.2 ± 0.78 | RCFM-FM | 4.4 ± 0.03 |
| RCHM-SA | >100 | RCFM-SA | 3.89 ± 0.06 |
| RCHM-SM | >100 | RCFM-SM | >4.0 |
| Acarbose | 3698.0 ± 76.5 | F89s | 3.8 ± 0.11 |
| Parameters | 1/IC50 | Total Phenolics | Total Polyphenols | Flavonoids | Condensed Tannins | Total Sterols | Alkaloids |
|---|---|---|---|---|---|---|---|
| Total phenolics | 0.946 | ||||||
| Total polyphenols | 0.997 | 0.968 | |||||
| Flavonoids | −0.459 | −0.522 | −0.487 | ||||
| Condensed Tannins | 0.541 | 0.641 | 0.562 | 0.146 | |||
| Total Sterols | −0.588 | −0.716 | −0.63 | 0.925 | −0.178 | ||
| Alkaloids | −0.434 | −0.49 | −0.447 | −0.204 | −0.504 | −0.081 | |
| Total saponins | 0.914 | 0.895 | 0.92 | −0.501 | 0.35 | −0.568 | −0.62 |
| Peak | RT (min) | Tentative Identification | Formula | Observed m/z | Main Fragments (m/z) | Bibliography |
|---|---|---|---|---|---|---|
| 1 | 0.47 | Aspalathin | C21H24O11 | 451.1211 | 377, 271, 211, 125 | |
| 2 | 0.75 | Gallic acid | C7H6O5 | 169.0124 | 151, 125, 124, 123 | [20,21,22] |
| 3 | 1.94 | D-(+)-catechin | C15H14O6 | 289.071 | 273, 257, 179, 161, 137, 125 | [20,21,23] |
| 4 | 2.42 | Procyanidin A2 | C30H24O12 | 575.1205 | 451, 425, 407, 289, 161, 125 | |
| 5 | 2.73 | (−)-Epicatechin | C15H14O6 | 289.0716 | 273, 243, 161, 137, 125, 123 | [20,21] |
| 6 | 3.08 | Isorhamnetin-3-O-galactoside | C22H22O12 | 477.1034 | 411, 372, 314, 289, 250, 193 | [24] |
| 7 | 4.6 | (-)-Epicatechin-3-O-gallate | C22H18O10 | 441.0829 | 301, 271, 245, 175, 169, 151, 125 | [21] |
| 9 | 4.74 | Quercetin-7-glucuronide | C21H18O13 | 477.0667 | 463, 301, 300, 288, 271, 151, 133, 123, 125 | [25] |
| 8 | 4.74 | Demethylwedelolactone | C15H8O7 | 299.0183 | 271, 255, 151, 133 | |
| 10 | 4.79 | Rutin | C27H30O16 | 609.1491 | 463, 301, 299, 243, 151, 133 | [21,26] |
| 11 | 4.84 | Lyoniside | C27H36O12 | 551.2143 | 521, 289, 215, 187, 171, 133, 125 | |
| 12 | 5.01 | Procyanidin B3 3′-O-gallate | C37H30O16 | 729.1468 | 559, 407, 303, 289, 287, 269, 169, 125 | [26] |
| 13 | 5.02 | Brucein A | C26H34O11 | 521.2023 | 461, 359, 313, 289, 165, 125 | |
| 14 | 5.25 | Nudiposide | C27H36O12 | 551.2122 | 521, 359, 289, 284, 165, 125 | |
| 15 | 5.5 | Tectoridin | C22H22O11 | 461.1089 | 447, 357, 300, 284, 169 | |
| 16 | 5.84 | Scutellarin | C21H18O12 | 461.0732 | 300, 285, 284, 271, 161, 151, 133 | [27] |
| 17 | 6.08 | Quercitrin | C21H20O11 | 447.0931 | 300, 285, 243, 201, 190, 151 | [28] |
| 18 | 6.09 | Rhein glucoside | C21H18O11 | 445.0776 | 271, 255, 243, 215, 161, 133, 125 | |
| 19 | 6.36 | Isorhamnetin 3-glucoside | C22H22O12 | 477.1041 | 407, 372, 314, 285, 243, 161, 133 | |
| 20 | 6.93 | Aloesin | C19H22O9 | 393.1179 | 300, 299, 285, 270, 255, 227 | |
| 21 | 7.06 | Vitexin | C21H20O10 | 431.0987 | 299, 285, 270, 255, 227, 135 | [21] |
| 22 | 7.37 | Chrysophanol-8-(6-O-galloyl-β-D-glucopyranoside) | C28H24O13 | 567.1156 | 407, 341, 270, 225, 169, 135, 125 | |
| 23 | 7.68 | Prunin | C21H22O10 | 433.1134 | 323, 311, 270, 269, 231, 151, 125 | [21] |
| 24 | 8.96 | 7-Hydroxy-3-(4-hydroxyphenyl)-4-oxo-4H-chromen-8-yl-hexopyranoside | C21H20O10 | 431.0983 | 359, 297, 269, 131 | |
| 25 | 9.05 | Apigenin | C15H10O5 | 269.0443 | 253, 230, 227, 151, 131, 117 | [21,27] |
| 26 | 9.21 | Kaempferol | C15H10O6 | 285.0391 | 253, 229, 227, 151, 137, 117 | [21,29] |
| 27 | 14.74 | Emodin | C15H10O5 | 269.0446 | 241, 165, 137, 133 | [23,30] |
| Normoglycemic Controls | Hyperglycemic Controls | Metformin (100 mg/kg) | Acarbose (10 mg/kg) | RCHM (150 mg/kg) | RCFM (150 mg/kg) | F89s (75 mg/kg) | |
|---|---|---|---|---|---|---|---|
| TC (mg/dL) | 72.20 ± 2.75 | 64.50 ± 2.66 | 61.60 ± 2.97 | 51.8 ± 3.44 * | 52.3 ± 3.76 * | 48.50 ± 5.50 * | 56.70 ± 2.96 * |
| HDL (mg/dL) | 35.60 ± 2.82 + | 14.86 ± 1.58 * | 24.20 ± 1.46 *,+ | 25.5 ± 3.52 *,+ | 17.67 ± 1.45 * | 12.50 ± 2.50 * | 22.00 ± 3.51 * |
| LDL (mg/dL) | 21.67 ± 4.39 | 31.13 ± 0.93 | 30.60 ± 4.02 | 30.33 ± 1.43 | 32.67 ± 5.9 | 31.50 ± 7.50 | 24.50 ± 0.50 |
| TG (mg/dL) | 104.80 ± 12.71 + | 200.50 ± 21.03 * | 83.29 ± 7.89 + | 37.6 ± 2.18 *,+ | 35.67 ± 6.64 *,+ | 35.50 ± 9.50 *,+ | 35.33 ± 2.91 *,+ |
| VLDL (mg/mL) | 20.95 ± 2.54 + | 40.10 ± 4.21 * | 16.66 ± 1.58 + | 6.27 ± 1.3 *,+ | 7.13 ± 1.33 *,+ | 7.10 ± 1.90 *,+ | 7.07 ± 0.58 *,+ |
| AI | 2.50 ± 0.15 + | 4.61 ± 0.80 * | 2.69 ± 0.20 + | 2.29 ± 0.48 + | 3.02 ± 0.36 | 3.95 ± 0.35 | 2.67 ± 0.32 + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aguila-Muñoz, D.G.; Jiménez-Montejo, F.E.; López-López, V.E.; Mendieta-Moctezuma, A.; Rodríguez-Antolín, J.; Cornejo-Garrido, J.; Cruz-López, M.C. Evaluation of α-Glucosidase Inhibition and Antihyperglycemic Activity of Extracts Obtained from Leaves and Flowers of Rumex crispus L. Molecules 2023, 28, 5760. https://doi.org/10.3390/molecules28155760
Aguila-Muñoz DG, Jiménez-Montejo FE, López-López VE, Mendieta-Moctezuma A, Rodríguez-Antolín J, Cornejo-Garrido J, Cruz-López MC. Evaluation of α-Glucosidase Inhibition and Antihyperglycemic Activity of Extracts Obtained from Leaves and Flowers of Rumex crispus L. Molecules. 2023; 28(15):5760. https://doi.org/10.3390/molecules28155760
Chicago/Turabian StyleAguila-Muñoz, Dolores G., Fabiola E. Jiménez-Montejo, Víctor E. López-López, Aarón Mendieta-Moctezuma, Jorge Rodríguez-Antolín, Jorge Cornejo-Garrido, and María C. Cruz-López. 2023. "Evaluation of α-Glucosidase Inhibition and Antihyperglycemic Activity of Extracts Obtained from Leaves and Flowers of Rumex crispus L." Molecules 28, no. 15: 5760. https://doi.org/10.3390/molecules28155760
APA StyleAguila-Muñoz, D. G., Jiménez-Montejo, F. E., López-López, V. E., Mendieta-Moctezuma, A., Rodríguez-Antolín, J., Cornejo-Garrido, J., & Cruz-López, M. C. (2023). Evaluation of α-Glucosidase Inhibition and Antihyperglycemic Activity of Extracts Obtained from Leaves and Flowers of Rumex crispus L. Molecules, 28(15), 5760. https://doi.org/10.3390/molecules28155760







