LCMS/MS Phytochemical Profiling, Molecular, Pathological, and Immune-Histochemical Studies on the Anticancer Properties of Annona muricata
Abstract
1. Introduction
2. Results and Discussion
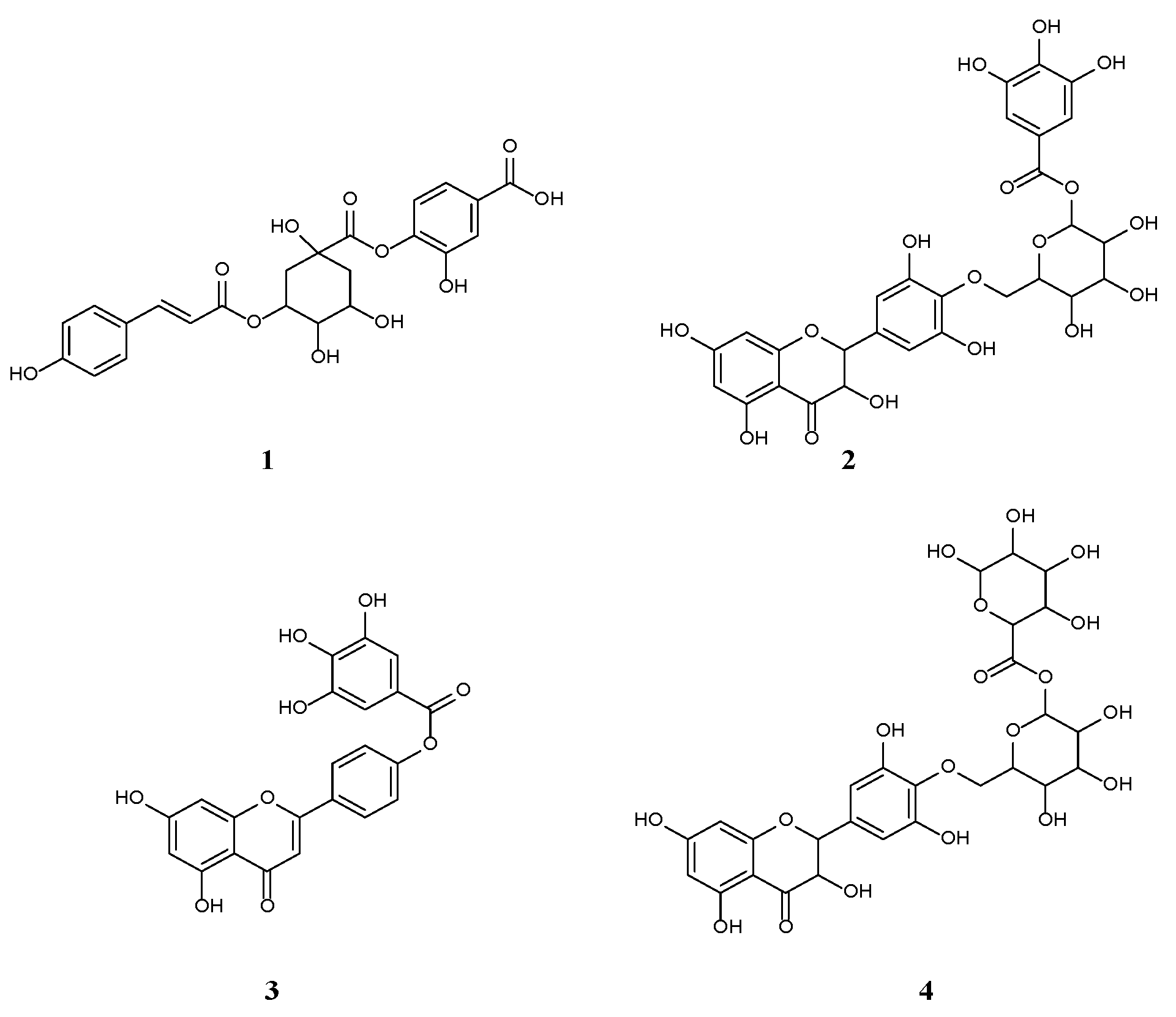
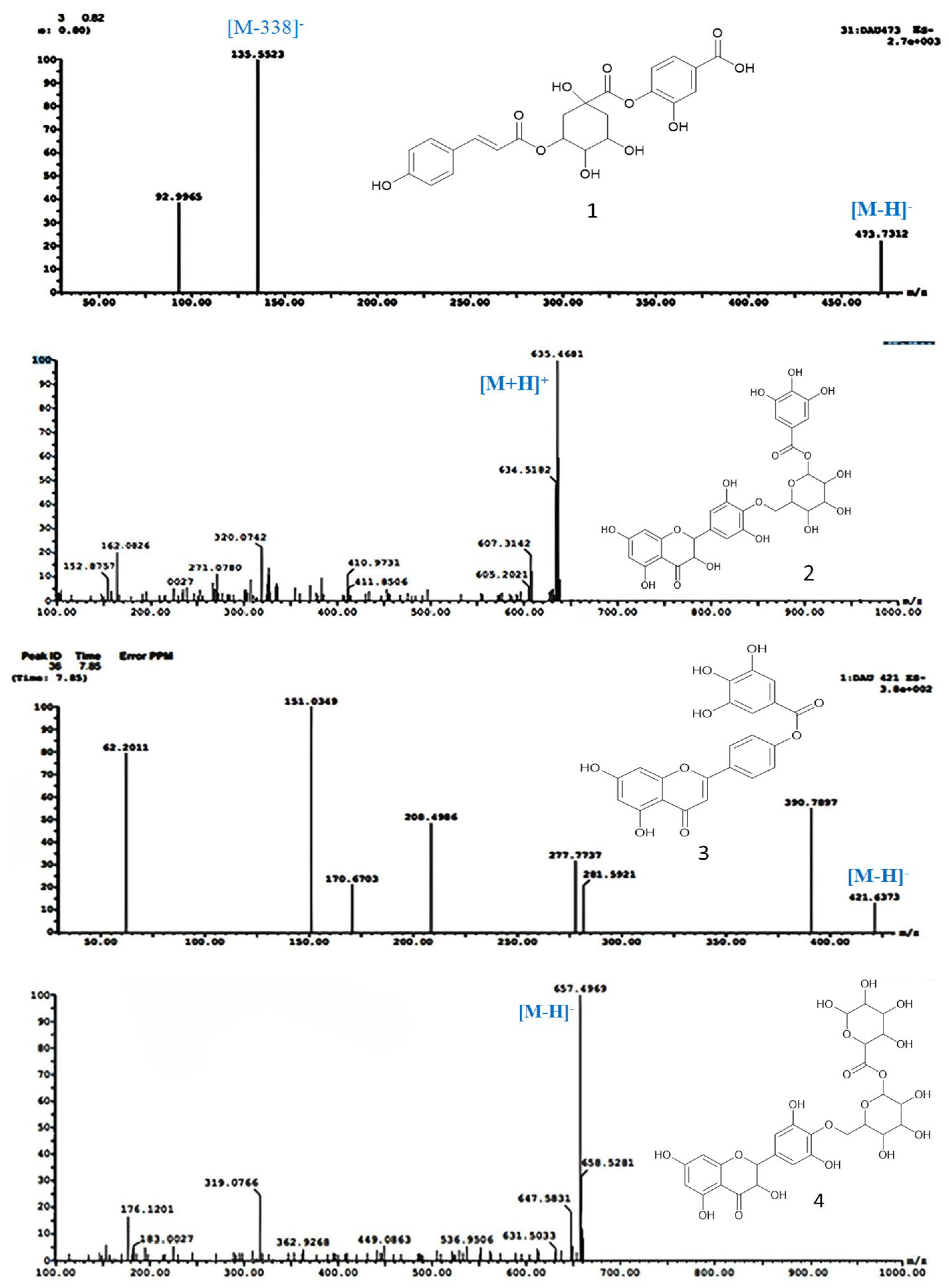
2.1. Characterization of the Phytochemical Constituents
Structural Identification of New Compounds
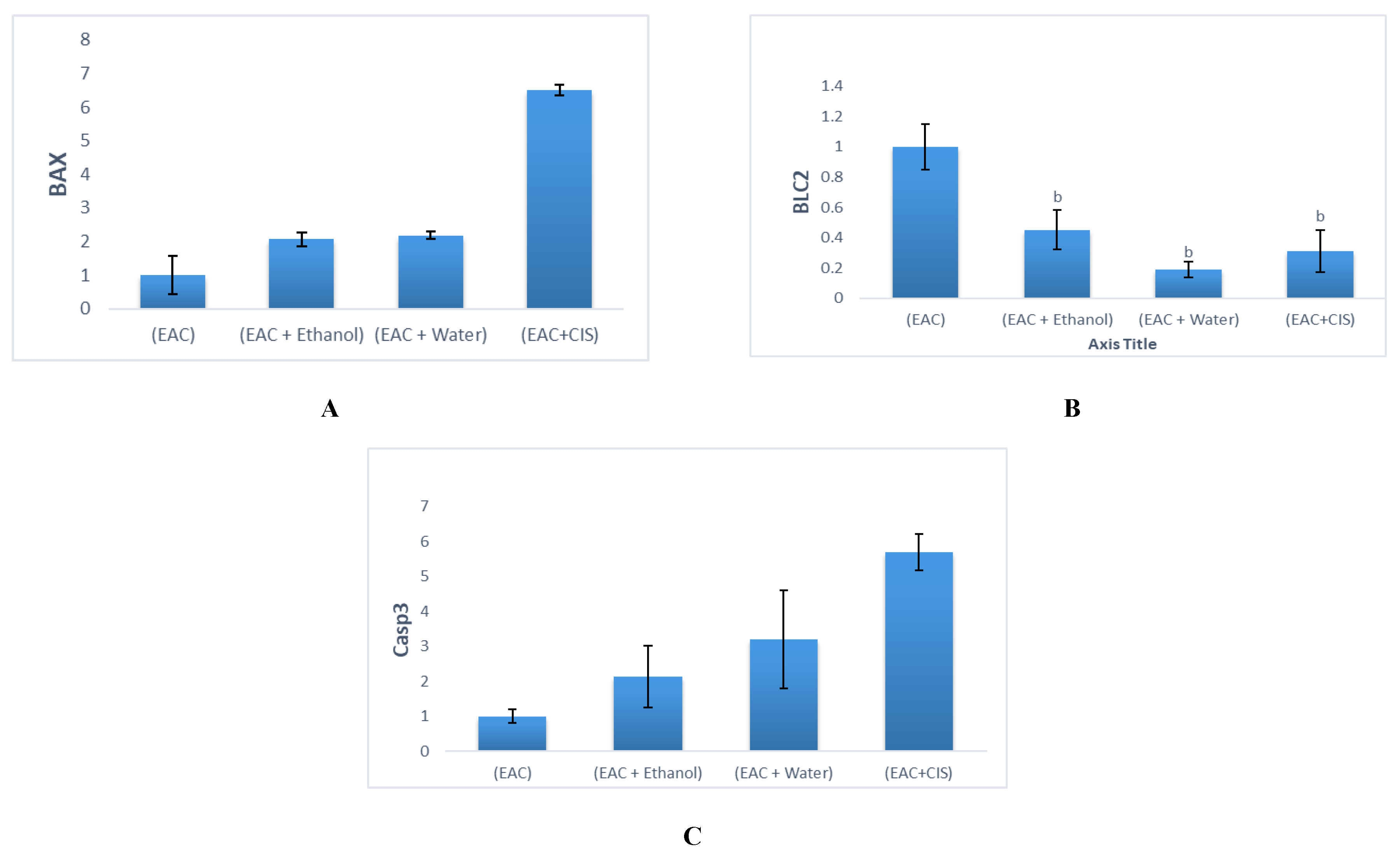
2.2. Molecular Findings
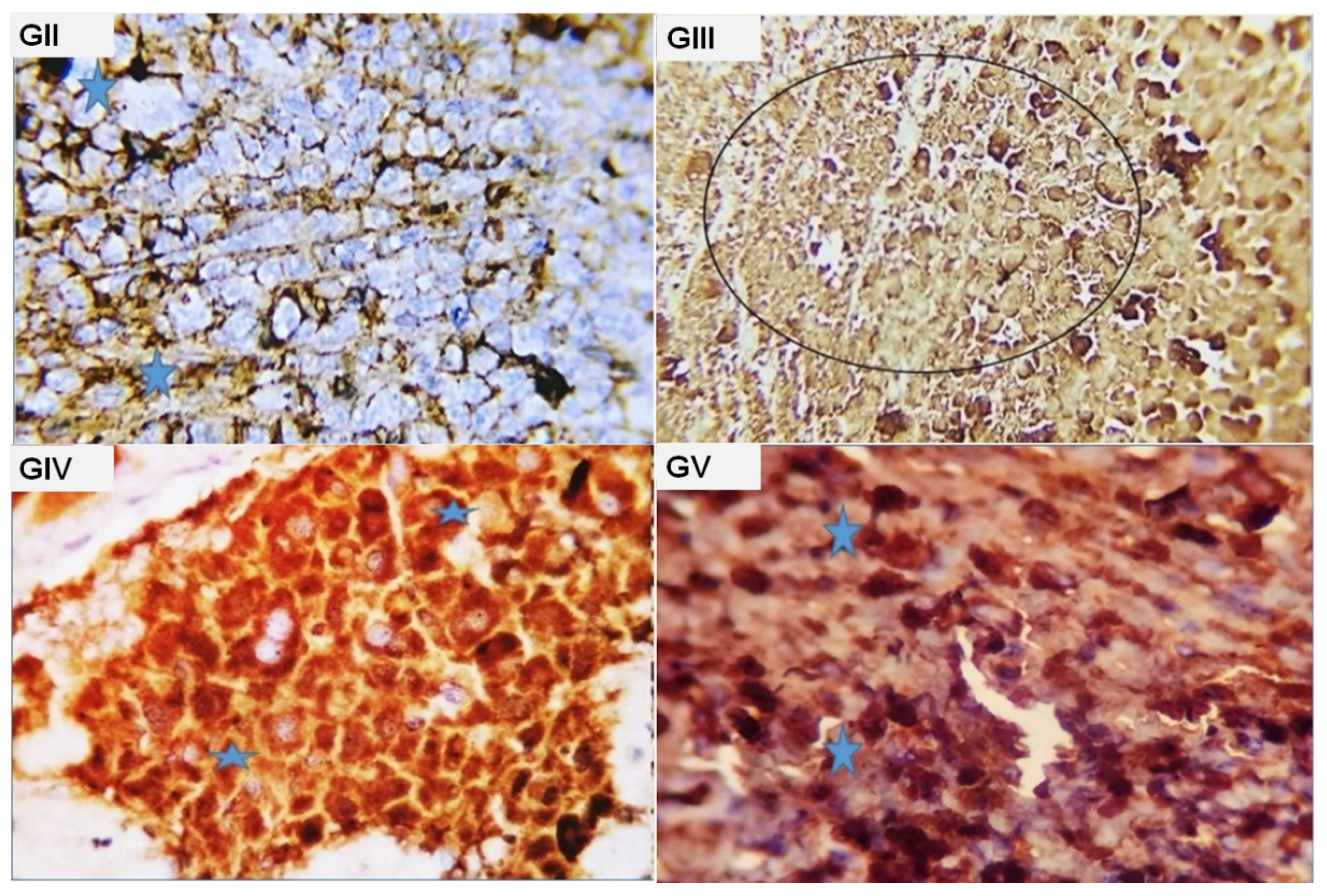
2.3. Histopathological Findings
3. Materials and Methods
3.1. Plant Materials and Extract Preparation
3.2. Extract Preparation
3.2.1. Ethanolic Extraction of the Whole Fruits
3.2.2. Aqueous Extraction of the Fruit Pulps
3.3. UPLC-ESI-MS/MS Analyses of A. muricata Extracts
3.3.1. LC/MS Instrument and Separation Technique
3.3.2. Determination of UPLC-ESI-MS-MS
3.4. Cytotoxic Activity
3.4.1. Experimental Animals
3.4.2. Ehrlich Ascites Carcinoma
3.4.3. Cisplatin
3.4.4. Induction of Cancer by Ehrlich Ascites Carcinoma
3.4.5. Treatment Regimen
3.4.6. Tissue Samples
3.4.7. Molecular Determination
3.4.8. Histopathological Examination
3.4.9. Immunohistochemistry Investigation
3.4.10. Morphometric Analysis
3.5. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Moghadamtousi, S.Z.; Fadaeinasab, M.; Nikzad, S.; Mohan, G.; Ali, H.M.; Kadir, H.A. Annona muricata (Annonaceae): A review of its traditional uses, isolated acetogenins and biological activities. Int. J. Mol. Sci. 2015, 16, 15625–15658. [Google Scholar] [CrossRef] [PubMed]
- Hadisaputri, Y.E.; Habibah, U.; Abdullah, F.F.; Halimah, E.; Mutakin, M.; Megantara, S.; Abdulah, R.; Diantini, A. Antiproliferation activity and apoptotic mechanism of soursop (Annona muricata L.) leaves extract and fractions on MCF7 breast cancer cells. Breast Cancer Targ. Ther. 2021, 447–457. [Google Scholar] [CrossRef] [PubMed]
- Mutakin, M.; Fauziati, R.; Fadhilah, F.N.; Zuhrotun, A.; Amalia, R.; Hadisaputri, Y.E. Pharmacological activities of soursop (Annona muricata Lin.). Molecules 2022, 27, 1201. [Google Scholar] [CrossRef] [PubMed]
- Asare, G.A.; Afriyie, D.; Ngala, R.A.; Abutiate, H.; Doku, D.; Mahmood, S.A.; Rahman, H. Antiproliferative activity of aqueous leaf extract of Annona muricata L. on the prostate, BPH-1 cells, and some target genes. Integr. Cancer Ther. 2015, 14, 65–74. [Google Scholar] [CrossRef]
- Dinda, B.; Dinda, S.; DasSharma, S.; Banik, R.; Chakraborty, A.; Dinda, M. Therapeutic potentials of baicalin and its aglycone, baicalein against inflammatory disorders. Eur. J. Med. Chem. 2017, 131, 68–80. [Google Scholar] [CrossRef]
- Avula, B.; Bae, J.-Y.; Majrashi, T.; Wu, T.-Y.; Wang, Y.-H.; Wang, M.; Ali, Z.; Wu, Y.-C.; Khan, I.A. Targeted and non-targeted analysis of annonaceous alkaloids and acetogenins from Asimina and Annona species using UHPLC-QToF-MS. J. Pharm. Biom. Anal. 2018, 159, 548–566. [Google Scholar] [CrossRef]
- Garcia-Oliveira, P.; Otero, P.; Pereira, A.G.; Chamorro, F.; Carpena, M.; Echave, J.; Fraga-Corral, M.; Simal-Gandara, J.; Prieto, M.A. Status and challenges of plant-anticancer compounds in cancer treatment. Pharmaceuticals 2021, 14, 157. [Google Scholar] [CrossRef]
- Testa, U.; Petrucci, E.; Pasquini, L.; Castelli, G.; Pelosi, E. Ovarian cancers: Genetic abnormalities, tumor heterogeneity and progression, clonal evolution and cancer stem cells. Medicines 2018, 5, 16. [Google Scholar] [CrossRef]
- Kim, S.S.; Cho, H.J.; Kang, J.Y.; Kang, H.K.; Yoo, T.K. Inhibition of androgen receptor expression with small interfering RNA enhances cancer cell apoptosis by suppressing survival factors in androgen insensitive, late stage LNCaP cells. Sci. World J. 2013, 2013. [Google Scholar] [CrossRef]
- Martinou, J.-C.; Youle, R.J. Mitochondria in apoptosis: Bcl-2 family members and mitochondrial dynamics. Devel. Cell 2011, 21, 92–101. [Google Scholar] [CrossRef]
- Bermejo, A.; Figadère, B.; Zafra-Polo, M.-C.; Barrachina, I.; Estornell, E.; Cortes, D. Acetogenins from Annonaceae: Recent progress in isolation, synthesis and mechanisms of action. Nat. Prod. Rep. 2005, 22, 269–303. [Google Scholar] [CrossRef] [PubMed]
- Jacobo-Herrera, N.; Pérez-Plasencia, C.; Castro-Torres, V.A.; Martínez-Vázquez, M.; González-Esquinca, A.R.; Zentella-Dehesa, A. Selective acetogenins and their potential as anticancer agents. Front. Pharm. 2019, 10, 783. [Google Scholar] [CrossRef] [PubMed]
- Zafra-Polo, M.C.; González, M.C.; Estornell, E.; Sahpaz, S.; Cortes, D. Acetogenins from Annonaceae, inhibitors of mitochondrial complex I. Phytochemistry 1996, 42, 253–271. [Google Scholar] [CrossRef] [PubMed]
- Champy, P.; Guérineau, V.; Laprévote, O. MALDI-TOF MS profiling of annonaceous acetogenins in Annona muricata products for human consumption. Molecules 2009, 14, 5235–5246. [Google Scholar] [CrossRef] [PubMed]
- Gomes, I.N.F.; Silva-Oliveira, R.J.; Oliveira Silva, V.A.; Rosa, M.N.; Vital, P.S.; Barbosa, M.C.S.; Dos Santos, F.V.; Junqueira, J.G.M.; Severino, V.G.; Oliveira, B.G. Annona coriacea Mart. fractions promote cell cycle arrest and inhibit autophagic flux in human cervical cancer cell lines. Molecules 2019, 24, 3963. [Google Scholar] [CrossRef]
- Melot, A.; Fall, D.; Gleye, C.; Champy, P. Apolar Annonaceous acetogenins from the fruit pulp of Annona muricata. Molecules 2009, 14, 4387–4395. [Google Scholar] [CrossRef]
- Adesanwo, J.K.; Akinloye, A.A.; Otemuyiwa, I.O.; Akinpelu, D.A. Chemical Characteristics and Biological Activities of Annona squamosa Fruit Pod and Seed Extracts. J. Expl. Res. Pharm. 2020, 6, 5–15. [Google Scholar] [CrossRef]
- Le Ven, J.; Schmitz-Afonso, I.; Lewin, G.; Brunelle, A.; Touboul, D.; Champy, P. Identification of the environmental neurotoxins annonaceous acetogenins in an Annona cherimolia Mill. Alcoholic beverage using HPLC-ESI-LTQ-Orbitrap. J. Agri. Food Chem. 2014, 62, 8696–8704. [Google Scholar] [CrossRef]
- Rupprecht, J.K.; Hui, Y.-H.; McLaughlin, J.L. Annonaceous acetogenins: A review. J. Nat. Prod. 1990, 53, 237–278. [Google Scholar] [CrossRef]
- Bonneau, N.; Baloul, L.; ba Ndob, I.B.; Senejoux, F.; Champy, P. The fruit of Annona squamosa L. as a source of environmental neurotoxins: From quantification of squamocin to annotation of Annonaceous acetogenins by LC–MS/MS analysis. Food Chem. 2017, 226, 32–40. [Google Scholar] [CrossRef]
- Ragasa, C.Y.; Galian, R.F.; Shen, C.-C. Chemical constituents of Annona muricata. Der. Pharma Chem. 2014, 6, 382–387. [Google Scholar]
- Gu, Z.-M.; Zhou, D.; Wu, J.; Shi, G.; Zeng, L.; McLaughlin, J.L. Screening for Annonaceous acetogenins in bioactive plant extracts by liquid chromatography/mass spectrometry. J. Nat. Prod. 1997, 60, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Gavamukulya, Y.; Maina, E.N.; Meroka, A.M.; Madivoli, E.S.; El-Shemy, H.A.; Magoma, G.; Wamunyokoli, F. Liquid chromatography single quadrupole mass spectrometry (LC/SQ MS) analysis reveals presence of novel antineoplastic metabolites in ethanolic extracts of fruits and leaves of Annona muricata. Pharm. J. 2019, 11, 660–668. [Google Scholar] [CrossRef]
- Rinaldi, M.V.; Díaz, I.E.; Suffredini, I.B.; Moreno, P.R. Alkaloids and biological activity of beribá (Annona hypoglauca). Rev. Bras. Farm. 2017, 27, 77–83. [Google Scholar] [CrossRef]
- Calixto, N.O.; Cordeiro, M.S.; Giorno, T.; Oliveira, G.G.; Lopes, N.P.; Fernandes, P.D.; Pinto, A.C.; Rezende, C.M. Chemical constituents of Psychotria nemorosa Gardner and antinociceptive activity. J. Braz. Chem. Soc. 2017, 28, 707–723. [Google Scholar] [CrossRef]
- Justino, A.B.; Florentino, R.M.; França, A.; Antonio Filho, C.; Franco, R.R.; Saraiva, A.L.; Fonseca, M.C.; Leite, M.F.; Espindola, F.S. Alkaloid and acetogenin-rich fraction from Annona crassiflora fruit peel inhibits proliferation and migration of human liver cancer HepG2 cells. PLoS ONE 2021, 16, e0250394. [Google Scholar] [CrossRef]
- Rini Vijayan, K.; Raghu, A. Polyphenolic profiling of two Embelia spp. endemic to South Western Ghats of India by liquid chromatography coupled with tandem mass spectrometry analysis. Nat. Prod. Res. 2021, 35, 2628–2632. [Google Scholar] [CrossRef]
- Marzouk, M.M.; Elkhateeb, A.; Latif, R.R.A.; Abdel-Hameed, E.-S.S.; Kawashty, S.A.; Hussein, S.R. C-glycosyl flavonoids-rich extract of Dipcadi erythraeum Webb & Berthel. bulbs: Phytochemical and anticancer evaluations. J. App. Pharm. Sci. 2019, 9, 94–98. [Google Scholar]
- Abu-Reidah, I.M.; Ali-Shtayeh, M.S.; Jamous, R.M.; Arráez-Román, D.; Segura-Carretero, A. HPLC–DAD–ESI-MS/MS screening of bioactive components from Rhus coriaria L. (Sumac) fruits. Food Chem. 2015, 166, 179–191. [Google Scholar] [CrossRef]
- Tan, L.; Jin, Z.; Ge, Y.; Nadeem, H.; Cheng, Z.; Azeem, F.; Zhan, R. Comprehensive ESI-Q TRAP-MS/MS based characterization of metabolome of two mango (Mangifera indica L) cultivars from China. Sci. Rep. 2020, 10, 1–19. [Google Scholar] [CrossRef]
- Mena, P.; Calani, L.; Dall’Asta, C.; Galaverna, G.; García-Viguera, C.; Bruni, R.; Crozier, A.; Del Rio, D. Rapid and comprehensive evaluation of (poly) phenolic compounds in pomegranate (Punica granatum L.) juice by UHPLC-MSn. Molecules 2012, 17, 14821–14840. [Google Scholar] [CrossRef] [PubMed]
- Lachowicz, S.; Oszmiański, J.; Rapak, A.; Ochmian, I. Profile and content of phenolic compounds in leaves, flowers, roots, and stalks of Sanguisorba officinalis L. determined with the LC-DAD-ESI-QTOF-MS/MS analysis and their in vitro antioxidant, antidiabetic, antiproliferative potency. Pharmaceuticals 2020, 13, 191. [Google Scholar] [CrossRef]
- El-Hawary, S.S.; Sobeh, M.; Badr, W.K.; Abdelfattah, M.A.; Ali, Z.Y.; El-Tantawy, M.E.; Rabeh, M.A.; Wink, M. HPLC-PDA-MS/MS profiling of secondary metabolites from Opuntia ficus-indica cladode, peel and fruit pulp extracts and their antioxidant, neuroprotective effect in rats with aluminum chloride induced neurotoxicity. Saudi J. Biol. Sci. 2020, 27, 2829–2838. [Google Scholar] [CrossRef]
- Mancini, S.; Nardo, L.; Gregori, M.; Ribeiro, I.; Mantegazza, F.; Delerue-Matos, C.; Masserini, M.; Grosso, C. Functionalized liposomes and phytosomes loading Annona muricata L. aqueous extract: Potential nanoshuttles for brain-delivery of phenolic compounds. Phytomedicine 2018, 42, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Plazonić, A.; Bucar, F.; Maleš, Ž.; Mornar, A.; Nigović, B.; Kujundžić, N. Identification and quantification of flavonoids and phenolic acids in burr parsley (Caucalis platycarpos L.), using high-performance liquid chromatography with diode array detection and electrospray ionization mass spectrometry. Molecules 2009, 14, 2466–2490. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Price, W.E.; Ashton, J.; Tapsell, L.C.; Johnson, S. Identification and characterization of phenolic compounds in hydromethanolic extracts of sorghum wholegrains by LC-ESI-MSn. Food Chem. 2016, 211, 215–226. [Google Scholar] [CrossRef]
- Zhang, Y.; Shi, P.; Qu, H.; Cheng, Y. Characterization of phenolic compounds in Erigeron breviscapus by liquid chromatography coupled to electrospray ionization mass spectrometry. Rapid Commun. Mass Spectrom. Int. J. Devoted Rapid Dissem. Up Minute Res. Mass Spectrom. 2007, 21, 2971–2984. [Google Scholar] [CrossRef]
- El-Sayed, M.A.; Al-Gendy, A.A.; Hamdan, D.I.; El-Shazly, A.M. Phytoconstituents, LC-ESI-MS profile, antioxidant and antimicrobial activities of Citrus x limon L. Burm. f. cultivar variegated pink lemon. J. Pharm. Sci. Res. 2017, 9, 375. [Google Scholar]
- Saftić, L.; Peršurić, Ž.; Fornal, E.; Pavlešić, T.; Pavelić, S.K. Targeted and untargeted LC-MS polyphenolic profiling and chemometric analysis of propolis from different regions of Croatia. J. Pharm. Biomed. Anal. 2019, 165, 162–172. [Google Scholar] [CrossRef]
- Boukhalkhal, S.; Gourine, N.; Pinto, D.C.; Silva, A.M.; Yousfi, M. UHPLC-DAD-ESI-MSn profiling variability of the phenolic constituents of Artemisia campestris L. populations growing in Algeria. Biocata. Agr. Biotech. 2020, 23, 101483. [Google Scholar] [CrossRef]
- Oliveira-Alves, S.C.; Vendramini-Costa, D.B.; Cazarin, C.B.B.; Júnior, M.R.M.; Ferreira, J.P.B.; Silva, A.B.; Prado, M.A.; Bronze, M.R. Characterization of phenolic compounds in chia (Salvia hispanica L.) seeds, fiber flour and oil. Food Chem. 2017, 232, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Yisimayili, Z.; Abdulla, R.; Tian, Q.; Wang, Y.; Chen, M.; Sun, Z.; Li, Z.; Liu, F.; Aisa, H.A.; Huang, C. A comprehensive study of pomegranate flowers polyphenols and metabolites in rat biological samples by high-performance liquid chromatography quadrupole time-of-flight mass spectrometry. J. Chrom. A 2019, 1604, 460472. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-González, A.; Quispe, C.; Bórquez, J.; Sepúlveda, B.; Riveros, F.; Areche, C.; Nagles, E.; García-Beltrán, O.; Simirgiotis, M.J. UHPLC-ESI-ORBITRAP-MS analysis of the native Mapuche medicinal plant palo negro (Leptocarpha rivularis DC.–Asteraceae) and evaluation of its antioxidant and cholinesterase inhibitory properties. J. Enz. Inh. Med. Chem. 2018, 33, 936–944. [Google Scholar] [CrossRef] [PubMed]
- Lantzouraki, D.Z.; Sinanoglou, V.J.; Tsiaka, T.; Proestos, C.; Zoumpoulakis, P. Total phenolic content, antioxidant capacity and phytochemical profiling of grape and pomegranate wines. RSC Adv. 2015, 5, 101683–101692. [Google Scholar] [CrossRef]
- Coria-Téllez, A.V.; Obledo-Vázquez, E.N.; Padilla-Camberos, E.; González-Ávila, M.; Martínez-Velázquez, M. Bioactivity, nutritional property, and rapid chemical characterization of aqueous extract of Annona muricata leaf from Mexico. Trop. J. Pharm. Res. 2019, 18, 611–617. [Google Scholar] [CrossRef]
- Larrazábal-Fuentes, M.J.; Fernández-Galleguillos, C.; Palma-Ramírez, J.; Romero-Parra, J.; Sepúlveda, K.; Galetovic, A.; González, J.; Paredes, A.; Bórquez, J.; Simirgiotis, M.J. Chemical profiling, antioxidant, anticholinesterase, and antiprotozoal potentials of Artemisia copa Phil. (Asteraceae). Front. Pharmacol. 2020, 11, 594174. [Google Scholar] [CrossRef]
- Cristofori, V.; Caruso, D.; Latini, G.; Dell’Agli, M.; Cammilli, C.; Rugini, E.; Bignami, C.; Muleo, R. Fruit quality of Italian pomegranate (Punica granatum L.) autochthonous varieties. Eur. Food Res. Tech. 2011, 232, 397–403. [Google Scholar] [CrossRef]
- Souza, D.O.; dos Santos Sales, V.; de Souza Rodrigues, C.K.; de Oliveira, L.R.; Lemos, I.C.S.; de Araújo Delmondes, G.; Monteiro, Á.B.; do Nascimento, E.P. Phytochemical analysis and central effects of Annona muricata Linnaeus: Possible involvement of the gabaergic and monoaminergic systems. Iran. J. Pharm. Res. 2018, 17, 1306–1317. [Google Scholar]
- Simirgiotis, M.J.; Quispe, C.; Mocan, A.; Villatoro, J.M.; Areche, C.; Bórquez, J.; Sepúlveda, B.; Echiburu-Chau, C. UHPLC high resolution orbitrap metabolomic fingerprinting of the unique species Ophryosporus triangularis Meyen from the Atacama Desert, Northern Chile. Rev. Bras. Farm. 2017, 27, 179–187. [Google Scholar] [CrossRef]
- Izquierdo-Vega, J.A.; Arteaga-Badillo, D.A.; Sánchez-Gutiérrez, M.; Morales-González, J.A.; Vargas-Mendoza, N.; Gómez-Aldapa, C.A.; Castro-Rosas, J.; Delgado-Olivares, L.; Madrigal-Bujaidar, E.; Madrigal-Santillán, E. Organic acids from Roselle (Hibiscus sabdariffa L.)—A brief review of its pharmacological effects. Biomedicines 2020, 8, 100. [Google Scholar] [CrossRef]
- George, V.C.; Kumar, D.N.; Suresh, P.; Kumar, R.A. Antioxidant, DNA protective efficacy and HPLC analysis of Annona muricata (soursop) extracts. J. Food Sci. Tech. 2015, 52, 2328–2335. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, X.; Gao, S.; Qian, K.; Liu, Q.; Yin, X. Research on the neuro-protective compounds in Terminalia chebula Retz extracts in-vivo by UPLC–QTOF-MS. Act. Chrom. 2018, 30, 169–174. [Google Scholar] [CrossRef]
- Beelders, T.; De Beer, D.; Stander, M.A.; Joubert, E. Comprehensive phenolic profiling of Cyclopia genistoides (L.) Vent. by LC-DAD-MS and-MS/MS reveals novel xanthone and benzophenone constituents. Molecules 2014, 19, 11760–11790. [Google Scholar] [CrossRef]
- Bystrom, L.M.; Lewis, B.A.; Brown, D.L.; Rodriguez, E.; Obendorf, R.L. Characterisation of phenolics by LC–UV/Vis, LC–MS/MS and sugars by GC in Melicoccus bijugatus Jacq.‘Montgomery’fruits. Food Chem. 2008, 111, 1017–1024. [Google Scholar] [CrossRef] [PubMed]
- Kammerer, D.; Carle, R.; Schieber, A. Characterization of phenolic acids in black carrots (Daucus carota ssp. sativus var. atrorubens Alef.) by high-performance liquid chromatography/electrospray ionization mass spectrometry. Rapid Commun. Mass Spectr. 2004, 18, 1331–1340. [Google Scholar] [CrossRef] [PubMed]
- Falcão, S.I.; Vale, N.; Gomes, P.; Domingues, M.R.; Freire, C.; Cardoso, S.M.; Vilas-Boas, M. Phenolic profiling of Portuguese propolis by LC–MS spectrometry: Uncommon propolis rich in flavonoid glycosides. Phytoch. Anal. 2013, 24, 309–318. [Google Scholar] [CrossRef]
- Nuncio-Jáuregui, N.; Nowicka, P.; Munera-Picazo, S.; Hernández, F.; Carbonell-Barrachina, Á.A.; Wojdyło, A. Identification and quantification of major derivatives of ellagic acid and antioxidant properties of thinning and ripe Spanish pomegranates. J. Func. Foods 2015, 12, 354–364. [Google Scholar] [CrossRef]
- Ibrahim, T.A. Chemical composition and biological activity of extracts from Salvia bicolor Desf. growing in Egypt. Molecules 2012, 17, 11315–11334. [Google Scholar] [CrossRef]
- Benayad, Z.; Gómez-Cordovés, C.; Es-Safi, N.E. Characterization of flavonoid glycosides from fenugreek (Trigonella foenum-graecum) crude seeds by HPLC–DAD–ESI/MS analysis. Inter J. Mol. Sci. 2014, 15, 20668–20685. [Google Scholar] [CrossRef]
- Gu, D.; Yang, Y.; Abdulla, R.; Aisa, H.A. Characterization and identification of chemical compositions in the extract of Artemisia rupestris L. by liquid chromatography coupled to quadrupole time-of-flight tandem mass spectrometry. Rap. Comm. Mass. Spect. 2012, 26, 83–100. [Google Scholar] [CrossRef]
- Saldanha, L.L.; Vilegas, W.; Dokkedal, A.L. Characterization of flavonoids and phenolic acids in Myrcia bella cambess. Using FIA-ESI-IT-MSn and HPLC-PAD-ESI-IT-MS combined with NMR. Molecules 2013, 18, 8402–8416. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Hameed, E.-S.S.; Bazaid, S.A.; Salman, M.S. Characterization of the phytochemical constituents of Taif rose and its antioxidant and anticancer activities. BioMed Res. Int. 2013, 2013. [Google Scholar] [CrossRef]
- Šuković, D.; Knežević, B.; Gašić, U.; Sredojević, M.; Ćirić, I.; Todić, S.; Mutić, J.; Tešić, Ž. Phenolic profiles of leaves, grapes and wine of grapevine variety vranac (Vitis vinifera L.) from Montenegro. Foods 2020, 9, 138. [Google Scholar] [CrossRef]
- Ambigaipalan, P.; de Camargo, A.C.; Shahidi, F. Phenolic compounds of pomegranate byproducts (outer skin, mesocarp, divider membrane) and their antioxidant activities. J. Agric. Food Chem. 2016, 64, 6584–6604. [Google Scholar] [CrossRef] [PubMed]
- Al-Yousef, H.M.; Hassan, W.H.; Abdelaziz, S.; Amina, M.; Adel, R.; El-Sayed, M.A. UPLC-ESI-MS/MS profile and antioxidant, cytotoxic, antidiabetic, and antiobesity activities of the aqueous extracts of three different Hibiscus Species. J. Chem. 2020, 6749176. [Google Scholar] [CrossRef]
- Flamini, R. Recent applications of mass spectrometry in the study of grape and wine polyphenols. Int. Sch. Res. Not. 2013, 2013, 813563. [Google Scholar] [CrossRef]
- Hassan, W.H.; Abdelaziz, S.; Al Yousef, H.M. Chemical composition and biological activities of the aqueous fraction of Parkinsonea aculeata L. growing in Saudi Arabia. Arab. J. Chem. 2019, 12, 377–387. [Google Scholar] [CrossRef]
- Zhao, H.-Y.; Fan, M.-X.; Wu, X.; Wang, H.-J.; Yang, J.; Si, N.; Bian, B.-L. Chemical profiling of the Chinese herb formula Xiao-Cheng-Qi decoction using liquid chromatography coupled with electrospray ionization mass spectrometry. J. Chrom. Sci. 2013, 51, 273–285. [Google Scholar] [CrossRef]
- Ozarowski, M.; Piasecka, A.; Paszel-Jaworska, A.; Chaves, D.S.d.A.; Romaniuk, A.; Rybczynska, M.; Gryszczynska, A.; Sawikowska, A.; Kachlicki, P.; Mikolajczak, P.L. Comparison of bioactive compounds content in leaf extracts of Passiflora incarnata, P. caerulea and P. alata and in vitro cytotoxic potential on leukemia cell lines. Rev. Bras. Farm. 2018, 28, 179–191. [Google Scholar] [CrossRef]
- Ben Said, R.; Hamed, A.I.; Mahalel, U.A.; Al-Ayed, A.S.; Kowalczyk, M.; Moldoch, J.; Oleszek, W.; Stochmal, A. Tentative characterization of polyphenolic compounds in the male flowers of Phoenix dactylifera by liquid chromatography coupled with mass spectrometry and DFT. Int. J. Mol. Sci. 2017, 18, 512. [Google Scholar] [CrossRef]
- Li, S.; Lin, Z.; Jiang, H.; Tong, L.; Wang, H.; Chen, S. Rapid identification and assignation of the active ingredients in fufang banbianlian injection using HPLC-DAD-ESI-IT-TOF-MS. J. Chrom. Sci. 2016, 54, 1225–1237. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-H.; Kim, H.-W.; Lee, M.-K.; Kim, Y.J.; Asamenew, G.; Cha, Y.-S.; Kim, J.-B. Phenolic profiling and quantitative determination of common sage (Salvia plebeia R. Br.) by UPLC-DAD-QTOF/MS. Eur. Food Res. Tech. 2018, 244, 1637–1646. [Google Scholar] [CrossRef]
- Abdelaziz, S.; Hassan, W.H.; Elhassanny, A.E.; Al-Yousef, H.M.; Elsayed, M.A.; Adel, R. Ultra performance liquid chromatography-tandem mass spectrometeric analysis of ethyl acetate fraction from saudi Lavandula coronopifolia Poir and evaluation of its cytotoxic and antioxidant activities. J. Herbmed Pharm. 2020, 9, 268–276. [Google Scholar] [CrossRef]
- Taamalli, A.; Arráez-Román, D.; Abaza, L.; Iswaldi, I.; Fernández-Gutiérrez, A.; Zarrouk, M.; Segura-Carretero, A. LC-MS-based metabolite profiling of methanolic extracts from the medicinal and aromatic species Mentha pulegium and Origanum majorana. Phytoch. Anal. 2015, 26, 320–330. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, L.; He, Y.Q.; Wang, C.H.; Welbeck, E.W.; Bligh, S.A.; Wang, Z.T. Characterization of fifty-one flavonoids in a Chinese herbal prescription Longdan Xiegan Decoction by high-performance liquid chromatography coupled to electrospray ionization tandem mass spectrometry and photodiode array detection. Rapid Commun. Mass Spect. Int. J. Devoted Rapid Dissem. Up Minute Res. Mass Spectrom. 2008, 22, 1767–1778. [Google Scholar] [CrossRef]
- Bielecka, M.; Pencakowski, B.; Stafiniak, M.; Jakubowski, K.; Rahimmalek, M.; Gharibi, S.; Matkowski, A.; Ślusarczyk, S. Metabolomics and DNA-Based Authentication of Two Traditional Asian Medicinal and Aromatic Species of Salvia subg. Perovskia. Cells 2021, 10, 112. [Google Scholar] [CrossRef]
- Uysal, S.; Zengin, G.; Sinan, K.I.; Ak, G.; Ceylan, R.; Mahomoodally, M.F.; Uysal, A.; Sadeer, N.B.; Jekő, J.; Cziáky, Z. Chemical characterization, cytotoxic, antioxidant, antimicrobial, and enzyme inhibitory effects of different extracts from one sage (Salvia ceratophylla L.) from Turkey: Open a new window on industrial purposes. RSC Adv. 2021, 11, 5295–5310. [Google Scholar] [CrossRef]
- Haq, F.U.; Ali, A.; Akhtar, N.; Aziz, N.; Khan, M.N.; Ahmad, M.; Musharraf, S.G. A high-throughput method for dereplication and assessment of metabolite distribution in Salvia species using LC-MS/MS. J. Adv. Res. 2020, 24, 79–90. [Google Scholar] [CrossRef]
- Friščić, M.; Bucar, F.; Hazler Pilepić, K. LC-PDA-ESI-MSn analysis of phenolic and iridoid compounds from Globularia spp. J. Mass Spec. 2016, 51, 1211–1236. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhang, H.; Zhao, L.; Dong, X.; Li, X.; Chai, Y.; Zhang, G. Rapid separation and identification of phenolic and diterpenoid constituents from Radix Salvia miltiorrhizae by high-performance liquid chromatography diode-array detection, electrospray ionization time-of-flight mass spectrometry and electrospray ionization quadrupole ion trap mass spectrometry. Rapid Commun. Mass Spect. Int. J. Devoted Rapid Dissem. Up Minute Res. Mass Spectrom. 2007, 21, 1855–1865. [Google Scholar]
- Hou, Z.-F.; Xie, Z.-X.; Tu, Y.-Q.; Li, Y. Triterpenes and triterpene glycosides from Salvia tricupis. Indian J. Chem. B 2002, 41, 234–236. [Google Scholar]
- Jia, C.; Zhu, Y.; Zhang, J.; Yang, J.; Xu, C.; Mao, D. Identification of glycoside compounds from tobacco by high performance liquid chromatography/electrospray ionization linear ion-trap tandem mass spectrometry coupled with electrospray ionization orbitrap mass spectrometry. J. Braz. Chem. Soc. 2017, 28, 629–640. [Google Scholar] [CrossRef]
- Park, S.-H.; Kim, M.; Lee, S.; Jung, W.; Kim, B. Therapeutic potential of natural products in treatment of cervical cancer: A review. Nutrients 2021, 13, 154. [Google Scholar] [CrossRef]
- Awad, M.G.; Ali, R.A.; Abd El-Monem, D.D.; El-Magd, M.A. Graviola leaves extract enhances the anticancer effect of cisplatin on various cancer cell lines. Mol. Cell. Toxicol. 2020, 16, 385–399. [Google Scholar] [CrossRef]
- Dai, Y.; Hogan, S.; Schmelz, E.M.; Ju, Y.H.; Canning, C.; Zhou, K. Selective growth inhibition of human breast cancer cells by graviola fruit extract in vitro and in vivo involving downregulation of EGFR expression. Nutr. Cancer 2011, 63, 795–801. [Google Scholar] [CrossRef]
- Torres, M.P.; Rachagani, S.; Purohit, V.; Pandey, P.; Joshi, S.; Moore, E.D.; Johansson, S.L.; Singh, P.K.; Ganti, A.K.; Batra, S.K. Graviola: A novel promising natural-derived drug that inhibits tumorigenicity and metastasis of pancreatic cancer cells in vitro and in vivo through altering cell metabolism. Cancer Lett. 2012, 323, 29–40. [Google Scholar] [CrossRef]
- Zeweil, M.M.; Sadek, K.M.; Taha, N.M.; El-Sayed, Y.; Menshawy, S. Graviola attenuates DMBA-induced breast cancer possibly through augmenting apoptosis and antioxidant pathway and downregulating estrogen receptors. Environ. Sci. Poll. Res. 2019, 26, 15209–15217. [Google Scholar] [CrossRef]
- Syed Najmuddin, S.U.F.; Romli, M.F.; Hamid, M.; Alitheen, N.B.; Nik Abd Rahman, N.M.A. Anti-cancer effect of Annona Muricata Linn Leaves Crude Extract (AMCE) on breast cancer cell line. BMC Complement. Altern. Med. 2016, 16, 311. [Google Scholar] [CrossRef]
- Moghadamtousi, S.Z.; Kadir, H.A.; Paydar, M.; Rouhollahi, E.; Karimian, H. Annona muricata leaves induced apoptosis in A549 cells through mitochondrial-mediated pathway and involvement of NF-κB. BMC Complement. Altern. Med. 2014, 14, 299. [Google Scholar] [CrossRef]
- Chakraborty, T.; Bhuniya, D.; Chatterjee, M.; Rahaman, M.; Singha, D.; Chatterjee, B.N.; Datta, S.; Rana, A.; Samanta, K.; Srivastawa, S. Acanthus ilicifolius plant extract prevents DNA alterations in a transplantable Ehrlich ascites carcinoma-bearing murine model. World J. Gastr. 2007, 13, 6538. [Google Scholar]
- Mansour, M.A.; Salama, A.F.; Ibrahim, W.M.; Shalaan, E.S. Assessment of Autophagy as Possible Mechanism of the Antitumor Effects of Arsenic Trioxide and/or Cisplatin on Ehrlich Ascites Carcinoma Model. Alex. J. Vet. Sci. 2019, 61, 159–167. [Google Scholar] [CrossRef]
- Bassiony, H.; Sabet, S.; Salah El-Din, T.A.; Mohamed, M.M.; El-Ghor, A.A. Magnetite nanoparticles inhibit tumor growth and upregulate the expression of P53/P16 in Ehrlich solid carcinoma bearing mice. PLoS ONE 2014, 9, e111960. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Lu, Q.-B. New combination chemotherapy of cisplatin with an electron-donating compound for treatment of multiple cancers. Sci. Rep. 2021, 11, 788. [Google Scholar] [CrossRef] [PubMed]
- Do Amaral, C.L.; Francescato, H.D.C.; Coimbra, T.M.; Costa, R.S.; Darin, J.D.a.C.; Antunes, L.M.G.; Bianchi, M.D.L.P. Resveratrol attenuates cisplatin-induced nephrotoxicity in rats. Arch. Toxicol. 2008, 82, 363–370. [Google Scholar] [CrossRef]
- Gong, C.; Qian, L.; Yang, H.; Ji, L.-l.; Wei, H.; Zhou, W.-b.; Qi, C.; Wang, C.-h. Hepatotoxicity and pharmacokinetics of cisplatin in combination therapy with a traditional Chinese medicine compound of Zengmian Yiliu granules in ICR mice and SKOV-3-bearing nude mice. BMC Complement. Altern. Med. 2015, 15, 283. [Google Scholar] [CrossRef]
- Niu, C.; Ma, M.; Han, X.; Wang, Z.; Li, H. Hyperin protects against cisplatin-induced liver injury in mice. Acta Cir. Bras. 2017, 32, 633–640. [Google Scholar] [CrossRef]
- Ikitimur-Armutak, E.I.; Sonmez, K.; Akgun-Dar, K.; Sennazli, G.; Kapucu, A.; Yigit, F.; Yilmaz, V.T.; Ulukaya, E. Anticancer effect of a novel palladium–saccharinate complex of terpyridine by inducing apoptosis on Ehrlich ascites carcinoma (EAC) in Balb-C mice. Anticaner Res. 2015, 35, 1491–1497. [Google Scholar]
- Alzergy, A.; Haman, M.R.; Shushni, M.A.; Almagtouf, F.A. Phyto-pharmaceuticals and biological study on graviola (Annona muricata L.) fruit and dietary supplement of graviola sold on the Libyan market as a cancer cure against TCA induce hepatotoxicity in mice. Cancer Biol.Ther. 2018, 8, 1–23. [Google Scholar]
- Samin, B.; Fachrial, E.; Refilda; Chaidir, Z.; Almahdy, A. Protective Effect of Aqueous Extract of Annona muricata Leaves Against Copper Induced Hepatotoxicity in Experimental Rats. Res. J. Pharm. Biol. Chem. Sci. 2016, 7, 880–885. [Google Scholar]
- Shukry, M.; El-Shehawi, A.M.; El-Kholy, W.M.; Elsisy, R.A.; Hamoda, H.S.; Tohamy, H.G.; Abumandour, M.M.; Farrag, F.A. Ameliorative effect of graviola (Annona muricata) on mono sodium glutamate-induced hepatic injury in rats: Antioxidant, apoptotic, anti-inflammatory, lipogenesis markers, and histopathological studies. Animals 2020, 10, 1996. [Google Scholar] [CrossRef]
- Abd El-Kaream, S.A. Biochemical and biophysical study of chemopreventive and chemotherapeutic anti-tumor potential of some Egyptian plant extracts. Biochem. Bioph. Rep. 2019, 18, 100637. [Google Scholar] [CrossRef]
- Prasad, S.K.; Varsha, V.; Devananda, D. Anti-cancer properties of Annona muricata (L.): A Review. Medicinal Plants–Int. J. Phytomed. Relat. Ind. 2019, 11, 123–134. [Google Scholar] [CrossRef]
- De Sousa, O.V.; Vieira, G.D.-V.; De Pinho, J.d.J.R.; Yamamoto, C.H.; Alves, M.S. Antinociceptive and anti-inflammatory activities of the ethanol extract of Annona muricata L. leaves in animal models. Int. J. Mol. Sci. 2010, 11, 2067–2078. [Google Scholar] [CrossRef] [PubMed]
- Syahida, M.; Maskat, M.; Suri, R.; Mamot, S.; Hadijah, H. Soursop (Annona muricata L.): Blood hematology and serum biochemistry of sprague-dawley rats. Intern. Food Res. J. 2012, 19, 955. [Google Scholar]
- Abd Eldaim, M.A.; Tousson, E.; Soliman, M.M.; El Sayed, I.E.T.; Abdel Aleem, A.A.H.; Elsharkawy, H.N. Grape seed extract ameliorated Ehrlich solid tumor-induced hepatic tissue and DNA damage with reduction of PCNA and P53 protein expression in mice. Environ. Sci. Pollut. Res. Int. 2021, 28, 44226–44238. [Google Scholar] [CrossRef]
- El-Naggar, S.A. Lack of the beneficial effects of mirazid (Commiphora molmol) when administered with chemotherapeutic agents on Ehrlich ascetic carcinoma bearing mice. Biol. Res. 2011, 5, 193–199. [Google Scholar]
- Suvarna, S.; Layton, C.; Bancroft, J. The Hematoxylins and Eosin. Bancroft’s Theory and Practice of Histological Techniques, 7th ed.; Churchill Livingstone: London, UK, 2013; pp. 172–186. [Google Scholar]
- Hsu, S.-M.; Raine, L.; Fanger, H. A comparative study of the peroxidase-antiperoxidase method and an avidin-biotin complex method for studying polypeptide hormones with radioimmunoassay antibodies. Am. J. Clin. Pathol. 1981, 75, 734–738. [Google Scholar] [CrossRef]
- Carson, H.J.; Reddy, V.; Taxy, J.B. Proliferation markers and prognosis in Merkel cell carcinoma. J. Cutan. Pathol. 1998, 25, 16–19. [Google Scholar] [CrossRef] [PubMed]
- Harlow, E.; Lane, D. A Laboratory Manual; Cold Spring Harbor Laboratory: New York, NY, USA, 1988; p. 579. [Google Scholar]
- Hashish, H.; Kamal, R. Effect of curcumin on the expression of Caspase-3 and Bcl-2 in the spleen of diabetic rats. J. Exp. Clin. Anat. 2015, 14, 18–23. [Google Scholar] [CrossRef]

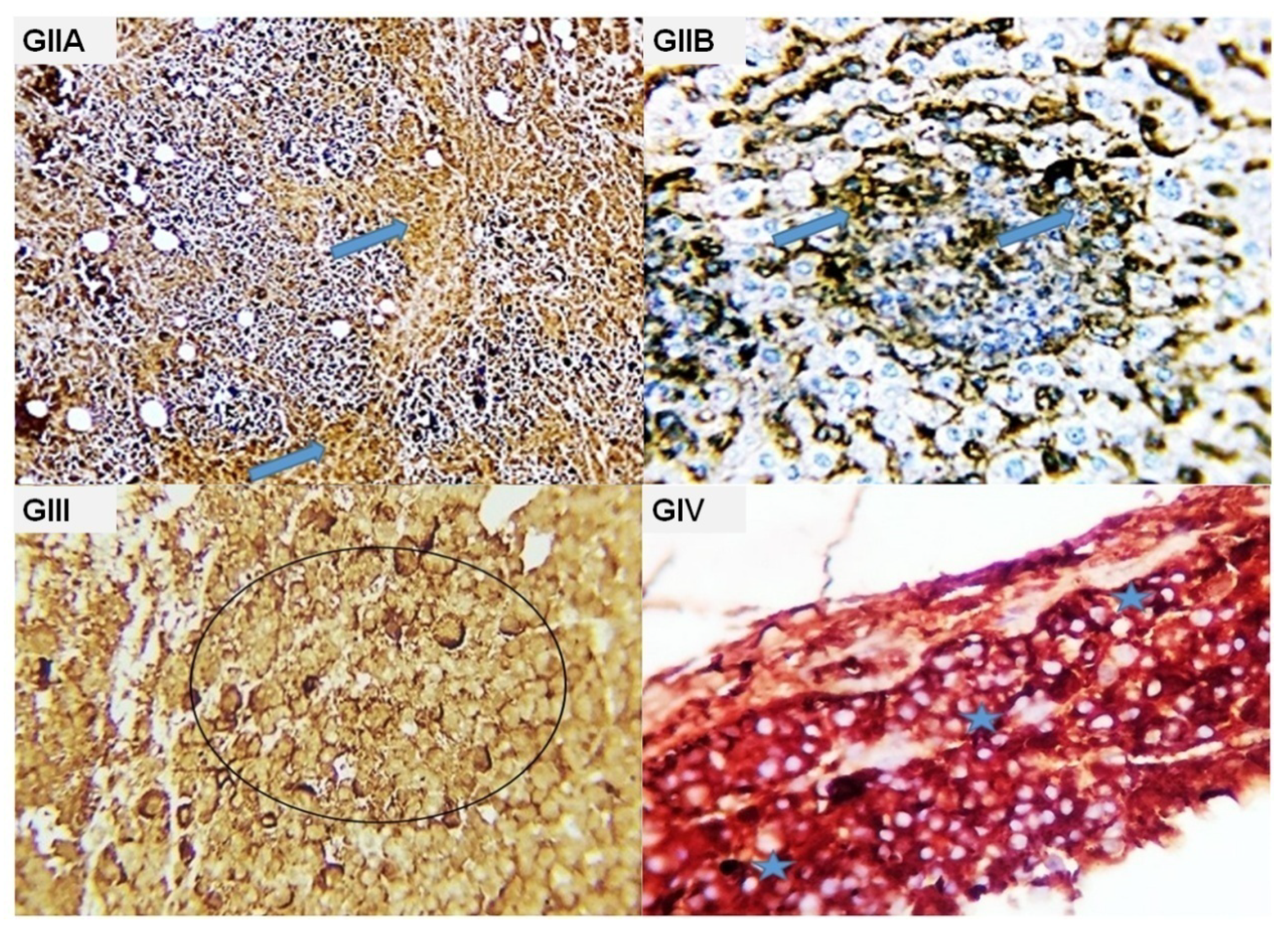
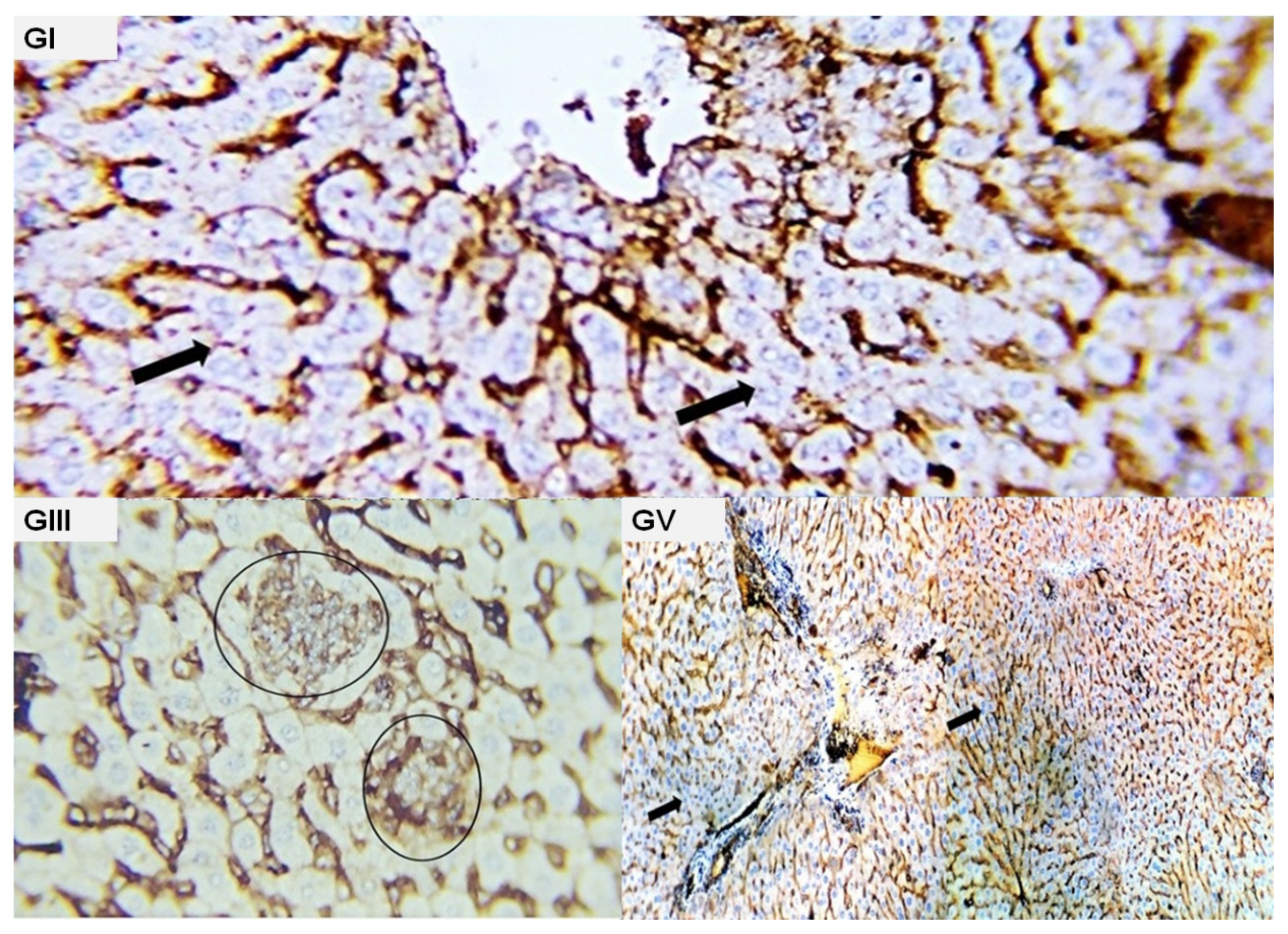
| Parameters | GII (EAC) | GIII (EAC + Cisplatin) | GIV (EAC + Water) | GV (EAC + Ethanol) |
|---|---|---|---|---|
| BAX | 1.00 ± 0.58 | 6.51 ± 0.71 b | 2.19 ± 0.11 | 2.07 ± 0.21 |
| Bcl-2 | 1.00 ± 0.15 | 0.31 ± 0.09 b | 0.19 ± 0.05 b | 0.45 ± 0.13 b |
| Casp-3 | 1.00 ± 0.20 | 5.71 ± 1.88 b | 3.21 ± 1.40 | 2.13 ± 0.88 |
| Parameters | P53 | CK |
|---|---|---|
| Groups | ||
| Ethanol extract | 2.44 ± 0.12 ab# | 2.78 ± 0.12 ab# |
| Water extract | 18.58 ± 0.48 * | 16.40 ± 0.24 * |
| Cisplatin | 19.35 ± 0.31 * | 17.66 ± 0.41 * |
| Control −ve (GI) | 2.24 ± 0.36 | 3.97 ± 0.69 |
| Control +ve(GII) | 19.05 ± 0.64 * | 19.44 ± 0.61 * |
| p value | 0.0001 | 0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdallah, R.H.; Al-Saleem, M.S.M.; Abdel-Mageed, W.M.; Al-Attar, A.-S.R.; Shehata, Y.M.; Abdel-Fattah, D.M.; Atta, R.M. LCMS/MS Phytochemical Profiling, Molecular, Pathological, and Immune-Histochemical Studies on the Anticancer Properties of Annona muricata. Molecules 2023, 28, 5744. https://doi.org/10.3390/molecules28155744
Abdallah RH, Al-Saleem MSM, Abdel-Mageed WM, Al-Attar A-SR, Shehata YM, Abdel-Fattah DM, Atta RM. LCMS/MS Phytochemical Profiling, Molecular, Pathological, and Immune-Histochemical Studies on the Anticancer Properties of Annona muricata. Molecules. 2023; 28(15):5744. https://doi.org/10.3390/molecules28155744
Chicago/Turabian StyleAbdallah, Rehab H., Muneera S. M. Al-Saleem, Wael M. Abdel-Mageed, Al-Sayed R. Al-Attar, Youssef M. Shehata, Doaa M. Abdel-Fattah, and Rahnaa M. Atta. 2023. "LCMS/MS Phytochemical Profiling, Molecular, Pathological, and Immune-Histochemical Studies on the Anticancer Properties of Annona muricata" Molecules 28, no. 15: 5744. https://doi.org/10.3390/molecules28155744
APA StyleAbdallah, R. H., Al-Saleem, M. S. M., Abdel-Mageed, W. M., Al-Attar, A.-S. R., Shehata, Y. M., Abdel-Fattah, D. M., & Atta, R. M. (2023). LCMS/MS Phytochemical Profiling, Molecular, Pathological, and Immune-Histochemical Studies on the Anticancer Properties of Annona muricata. Molecules, 28(15), 5744. https://doi.org/10.3390/molecules28155744







